Abstract
Objective: The aim of this study was to test photodynamic therapy (PDT) as an alternative approach to biofilm disruption on dental hard tissue, We evaluated the effect of methylene blue and a 660 nm diode laser on the viability and architecture of Gram-positive and Gram-negative bacterial biofilms. Materials and methods: Ten human teeth were inoculated with bioluminescent Pseudomonas aeruginosa or Enterococcus faecalis to form 3 day biofilms in prepared root canals. Bioluminescence imaging was used to serially quantify and evaluate the bacterial viability, and scanning electron microscopic (SEM) imaging was used to assess architecture and morphology of bacterial biofilm before and after PDT employing methylene blue and 40 mW, 660 nm diode laser light delivered into the root canal via a 300 μm fiber for 240 sec, resulting in a total energy of 9.6 J. The data were statistically analyzed with analysis of variance (ANOVA) followed by Tukey test. Results: The bacterial reduction showed a dose dependence; as the light energy increased, the bioluminescence decreased in both planktonic suspension and in biofilms. The SEM analysis showed a significant reduction of biofilm on the surface. PDT promoted disruption of the biofilm and the number of adherent bacteria was reduced. Conclusions: The photodynamic effect seems to disrupt the biofilm by acting both on bacterial cells and on the extracellular matrix.
Introduction
Recently, many in vitro and in vivo studies have highlighted the potential of photodynamic therapy (PDT) to treat localized microbial infections, especially those causing oral disease.1–6 PDT is a photochemical antimicrobial strategy that involves the combination of a nontoxic photosensitizer (PS) and a harmless visible light source.1 The excited PS reacts with molecular oxygen to produce highly reactive oxygen species, which induce injury and death of microorganisms.2,3 It has been established that PS, which possess a cationic charge, can rapidly bind to or penetrate into bacterial cells and, therefore, these compounds demonstrate a high degree of selectivity for killing microorganisms and have little toxicity toward host mammalian cells.4,5 PDT has an efficient killing effect upon different classes of microorganisms, such as Gram-positive, Gram-negative bacteria and yeasts.7,8 Several studies have reported the PDT inactivation of different planktonic microorganisms.9–11 However, the PDT efficacy for the inactivation of microorganisms organized in biofilms differs from that observed in planktonic cultures, and biofilms are considered more resistant.
PDT has been studied as a promising approach to eradicate oral pathogenic bacteria6,7 that cause diseases such as periodontitis,12 peri-implantitis,13 and dental cavities.14 The oral cavity has complex microflora, because of the different environments associated with different anatomical sites in both hard and soft tissues. An example is the presence of anaerobic microorganisms in the subgingival area caused by low oxygen supply. More than 1000 different species have been identified in the mouth, and most of them can be found attached to surfaces forming biofilms.15,16
Dental prosthetic materials have developed greatly since the 1970s, and they aim to mimic dental hard tissues in order to repair the physiological and esthetic oral functions. All of these biomaterials as well as the natural tissues in the mouth, such as dental enamel and cementum are open environments for bacterial adherence and biofilm formation. In addition to the oral infections caused by biofilm bacteria, these pathogens also represent a threat for systemic infections as found in infective endocarditis (IE), which represents a major cost burden for healthcare services.17 The American Heart Association (AHA) has identified risk factors for IE including the use of prosthetic cardiac valves or prosthetic materials used to repair cardiac valves, congenital heart disease, and cardiac transplantation, and the AHA has recommended antibiotic prophylaxis for all dental procedures that involve possible bleeding, as manipulation of gingival tissue or the periapical region of teeth and any type of perforation in the oral mucosa. Furthermore, in 1997, the AHA recognized that most cases of IE were not related to invasive procedures but were the result of frequent and transient bacteremia caused by routine daily activities, such as brushing and flossing teeth.18
In view of the growing problem of bacterial resistance to conventional antimicrobials, the use of an alternative bactericidal approach to which bacteria are not likely to develop resistance would be valuable. The current treatment for dental plaque-related diseases in the oral cavity involves, first, the mechanical removal of all accessible contamination, and, second, the use of topical and/or systemic antimicrobial medications.19 PDT may be a suitable antimicrobial approach that can overcome biofilm and antimicrobial-related resistance.
The aim of this study was to test PDT as an antimicrobial approach to disrupt biofilm formed on a dental hard tissue. We evaluated the effect of the phenothiazinium PS, methylene blue (MB), and a 660 nm diode laser on bacterial viability, and biofilm architecture as well as microorganism morphology. We used a mixed biofilm composed Enterococcus faecalis, a Gram-positive bacteria, and Pseudomonas aeruginosa, a Gram-negative bacteria, which are well known to have different sensitivities to PDT.
Materials and Methods
Biofilm growth and bacterial strain
P. aeruginosa (XEN5) that had been engineered to be stably bioluminescent by transformation with a transposon containing the entire Photorhabdus luminescens lux operon20 was kindly donated by XenogenCorp. (Alameda, CA) and was used for real-time monitoring of bacterial reduction inside the root canal. E. faecalis (ATCC® 1494), both in a single species biofilm, and in a mixed biofilm with P. aeruginosa (XEN5) were used for scanning electron microscopy (SEM) analysis to evaluate the effects of PDT on biofilm architecture and cellular morphology.
The bacteria were grown in brain heart infusion (BHI) broth at 37°C with shaking (150 rpm) to form a stationary growth phase suspension of 1×10(9) cells/mL. Ten microliters of the microorganism suspension was poured inside the root canals of 10 human single-rooted anterior teeth, which had been previously endodontically prepared with rotatory instrumentation up to file # 30.4 (working length of ∼15 mm) and sterilized by autoclaving for 15 min at 121°C. After inoculation, each tooth was placed inside a 1.5 mL microcentrifuge tube that was subsequently sealed and kept upright and incubated, with the bacterial suspension, for 72 h at 37°C, with shaking to allow biofilm formation. To facilitate the biofilm formation, the BHI broth was changed every 24 h.
Growing a biofilm in root canal
After 72 h, bioluminescence imaging of each tooth (in the P. aeruginosa group) inside its transparent microcentrifuge tube was performed with a low-light intensified camera (Hamamatsu Photonics KK, Bridgewater, NJ). The use of this imaging system has been described in detail in previous work.21 Briefly, bioluminescence signal was accumulated for 2 min at 35 sensitivity level and a maximum setting on the image intensifier control module. Using ARGUS software (Siemens Medical Solutions, Erlangen, Germany) the luminescence image was presented as a false-color image superimposed on top of the gray scale reference image. The image-processing component of the software gave mean pixel values from the luminescence images on defined areas covering each tooth on a 256 gray scale. For bioluminescence comparison, all images were recorded at same bit range. These images served to confirm the level of infection and to obtain the initial signal from the bacteria inside the root canals.
Antimicrobial photodynamic therapy
To estimate the effect of PDT on planktonic bacteria, suspensions of P. aeruginosa in stationary phase were diluted in phosphate-buffered saline (PBS) to a cell density of 109/mL, and 1 mL aliquots were added to wells of a 24 well plate and the relative light unit values were read in a luminescence plate reader (MicroBeta Trilux 1450, PerkinElmer Life And Analytical Sciences, Inc., Wellesley, MA). Bacteria were incubated with 1 mL of MB (Sigma-Aldrich, Milwaukee, MI) (60 μM dissolved in distilled water) for 10 min followed by illumination with 660 nm light from a diode laser (MMOptics, São Paulo, Brazil) for defined times corresponding to the delivery of 2.4, 4.8, 7.2, and 9.6 J of total energy.
At each stage, the luminescence values were measured. To evaluate the effect of PDT on biofilms grown on root canal dentine tissue, all liquid content inside the root canal was removed with a pipette, and the canals were filled with 10 μL of MB 60 μM and allowed to incubate for 2 min followed by a second bioluminescence imaging to quantify any dark toxicity of the PS. Thereafter, the illumination was performed with a 300 mm diameter fiber-coupled diode laser (MMOptics, São Paulo, Brazil). The diode laser delivered 660 nm light at a total power of 40 mW out of the fiber. The fiber was initially placed in the apical portion (bottom) of the root canal, and spiral movements, from apical to cervical, were manually performed to ensure the even diffusion of the light inside the canal lumen. These movements were repeated ∼10 times/min, and after each minute, a bioluminescence image was captured. The irradiation was performed for 4 min, total energy out of the fiber of 9.6 J (2.4 J/min) and bioluminescence imaging was performed after each minute until the end of the treatment.1,3,5
SEM images
E. faecalis (ATCC 29212) and a mixed biofilm composed of E. faecalis and P. aeruginosas, simulating a situation in which Gram-positive and Gram-negative bacteria (known to be more resistant to PDT) were coexistent in a multi-species biofilm model. The growing conditions were the same as described previoiusly, with a 72 h culture time on the surface of bisected teeth (see subsequent description) and on a glass slide.
To grow the biofilm on dental tissue, an anterior single canal tooth was previously sectioned longitudinally in two halves (∼15 mm in length) and cleaned with 37% H3PO4 (FGM, Blumenau, SC Brazil) for 1 min plus 2.5% NaOCl (Biodinamica, Londrina, PR Brazil) for 5 min and sterilized by autoclaving (121°C for 15 min). The samples were then randomly divided into two groups. Group one received MB solution for 2 min and group two also received MB solution for 2 min plus 9.6 J (4 min), 660 nm light, from the same diode laser equipment, using the same parameters described. After that, the samples were washed with saline solution to remove the cells nonadherent to the biofilm, and then fixed for SEM. The species were incubated with increasing concentrations of ethanol for 30 min, dried at 37°C for 24 h, and placed on a mounting base. Finally, the samples were coated with gold and examined under a SEM.
The SEM images were analyzed using the software ImageJ (National Institute of Health, Bethesda, MD) to measure the cell diameter and biofilm area (in pixels) after MB incubation and after PDT. To calculate the cell diameter, 20 randomly selected cells were measured in each image.22 The biofilm was measured selecting the total area of each image covered by the biomass on the initial image and after the PDT treatment; to confirm these data, the area uncovered by the biofilm was also compared on the initial and final images.
The measures from cell diameter and biofilm areas were tested for significant differences by using analysis of variance (ANOVA) followed by Tukey tests. p<0.05 was considered statistically significant. Statistical comparisons between means were performed with software Origin, version 8.5 (OriginLab, Northampton, MA).
Results
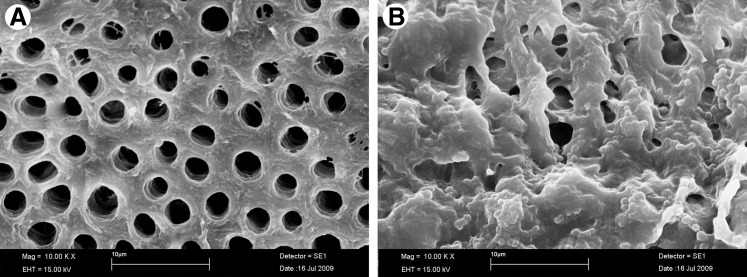
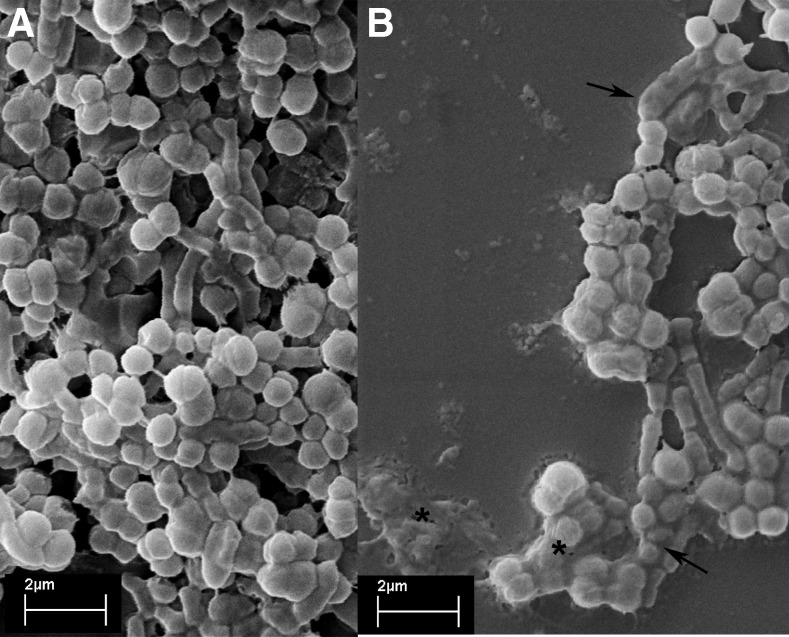
The addition of 10 μL of a suspension containing 109 cells of P. aeruginosa into the root canals followed by 3-day incubation at 37°C reliably and reproducibly produced a bioluminescent biofilm that could be imaged through the entire width of the tooth. There were minor variations from tooth to tooth in the pattern of the detected luminescence, which was probably the result of differences in the geometry of the root canal system. The presence of a microbial biofilm rather than planktonic bacteria was demonstrated by the failure of irrigation with saline to significantly diminish the luminescence signal (data not shown). Also, the images (Fig. 1) comparing a clean dentine surface and a 3-day incubated surface with E. faecalis clearly demonstrated the biofilm formation.
FIG. 1.
(A) Scanning electron microscopic (SEM) image of dentine cleaned with H3PO4 and NaOCl and sterilized and (B) with a 3-day biofilm of Enterococcus faecalis incubated with 60.M of methylene blue (MB) for 2 min without irradiation.
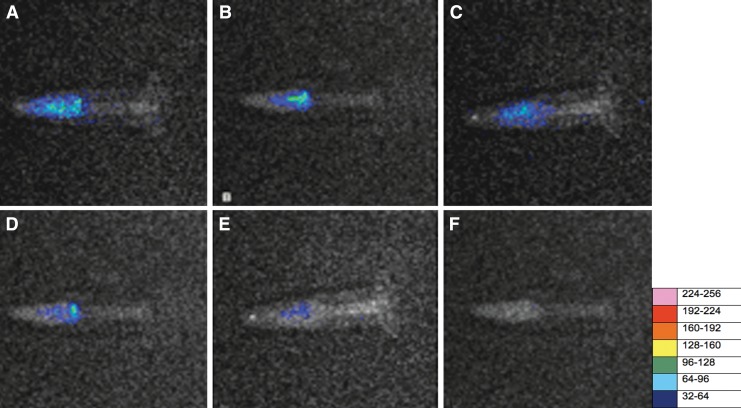
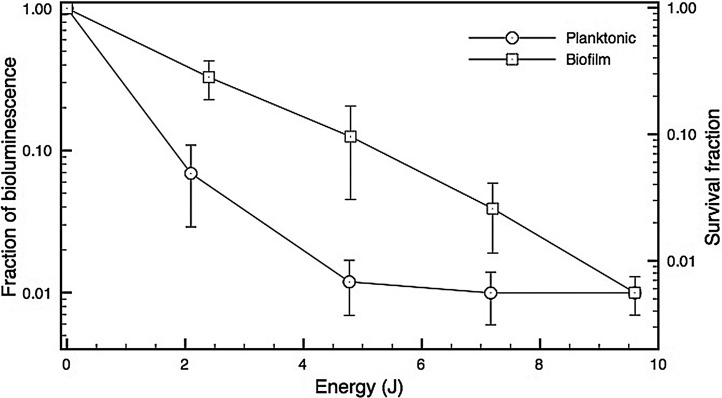
Our results showed only a slight reduction in bioluminescence signal from the bacteria in the root canal biofilm after 2 min of incubation with MB solution in the absence of light, because of its low inherent toxicity (0.1 log). By contrast, after illumination, the reduction of the signal was dramatic. Representative bioluminescent images from before and after irradiation, for all energies tested over the biofilm incubated with MB, are presented in Fig. 2 (panel A–F). When the effects of PDT on planktonic bacteria and on biofilms were compared (Fig. 3), the results showed that the energy necessary to achieve reduction of bioluminescence signal in planktonic bacteria was lower than that required to achieve the same results on biofilm (p=0.03).
FIG. 2.
Representative bioluminescence images captured of teeth infected with 3 day Pseudomonas. aeruginosa biofilms. The teeth received either: (A) no treatment; (B) 2 min of methylene blue (MB) incubation in dark conditions, or (C) after 2.4 J of illumination, (D) after 4.8 J of illumination, (E) after 7.2 J of illumination, or (F) after 9.6 J of illumination.
FIG. 3.
Comparative loss of bacterial viability as measured by fraction of bioluminescence signal remaining after incubation with 60 μM methylene blue (MB) for 2 min and after photodynamic therapy (PDT) using 660-nm light on a Pseudomonas. aeruginosa suspension or intracanal biofilm.
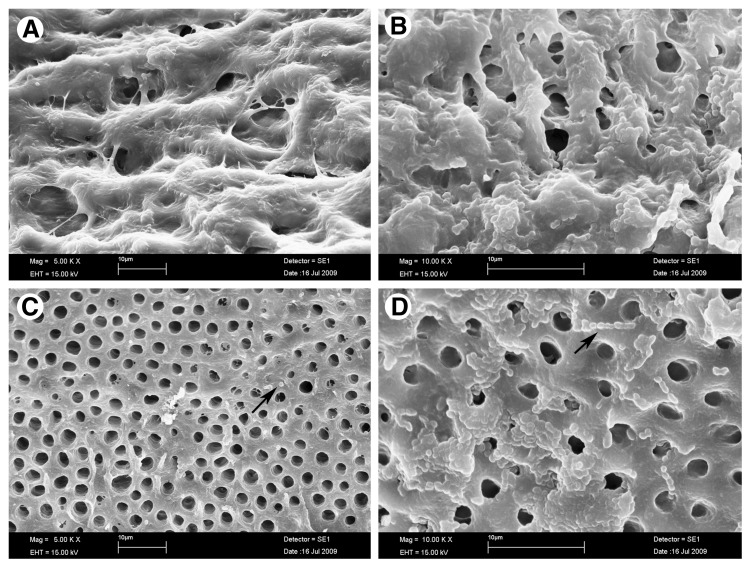
The SEM analysis with E. faecalis 3 day biofilms showed a significant reduction of surface biofilm after PDT. Cellular aggregates surrounded by extracellular matrix could be observed in the biofilms produced by E. faecalis incubated with MB in the dark (Fig. 4, panels A and B). These biofilms are very similar to those of untreated controls (data not shown). PDT promoted disruption of the biofilm as can be seen in panels C and D. The presence of adherent bacteria and extracellular matrix were markedly reduced, and very few aggregated colonies were observed in the biofilm after PDT. As expected for E. faecalis, most of the remaining bacteria were presented as single cells or organized in short chains. There were changes on bacterial morphology (cocci shaped, size or chain aggregation) and the biofilm area was significantly reduced by 88.7% (initial area of 694 Mpx. and final area of 282 Mpx. – p<0.02 compared with the SEM image of biofilm after MB dark incubation [measured by the ImageJ software]).
FIG. 4.
Scanning electron microscopic (SEM) image of a 3 day old biofilm of Enterococcus faecalis incubated with 60 μM of methylene blue (MB) for 2 min (A and B) without irradiation and (C and D) after photodynamic therapy (PDT) using 660 nm diode laser. Images A and C show a 5000x amplification, and images B and D show a 10000x magnification. Arrows indicate the presence of single bacteria or bacteria in association in small chains. Comparing the area covered by the biofilm in image B and image D, there was a reduction of 88.7% of the biomass.
In the mixed biofilms, as expected, the reduction of E. faecalis (Gram+ bacteria) was higher than that observed for P. aeruginosa (Gram- bacteria), but it was possible to observe a definite alteration that was caused by PDT, resulting in disruption of the biofilm. There was a reduction of 59.3% in the area covered by the biofilm (initial area of 50 Mpx. and final image of 20.5 Mpx. – p=0.016) compared with the initial biofilm image on Fig. 5A, and additional changes in cell morphology could be observed (indicating probable membrane disruption); the alterations included loss of cocci or bacilli shape, and the presence of grooves on the cell surface. Features could be seen that suggested the presence of cell membrane shriveling, and draining of the intracellular components (Fig. 5A and B).
FIG. 5.
Scanning electron microscopic (SEM) images of a 3 day biofilm of Enterococcus. faecalis and Pseudomonas aeruginosa (A) before and (B) after photodynamic therapy (PDT) using 660 nm diode laser. In panel B the lost biofilm structure can be seen. Arrows point to changes in cellular morphology and alterations in cellular size. Asterisks show structures suggesting cellular rupture and grooves on cell membrane especially for E. faecalis (cocci shaped bacteria).
Discussion
In this study, we examined biofilms formed by two bacteria (a Gram+ cocci E. faecalis and a Gram- bacilli P. aeruginosa) employing SEM and real-time bioluminescent imaging. We tested a photochemical strategy (methylene blue PDT) aiming to disrupt biofilm and reduce bacterial numbers. The results demonstrated that both species of bacteria could be reduced by PDT with MB and a 660 nm low-power diode laser in the two models used, the single and the mixed biofilm environment.
E. faecalis is a Gram-positive microorganism commonly detected in asymptomatic, persistent endodontic infections. Its prevalence in such infections ranges from 24% to 77%.5 These data can be explained by various survival and virulence factors found in E. faecalis, including its ability to compete with other microorganisms, invade dentinal tubules, form biofilm, and resist nutritional deprivation.23 P. aeruginosa was selected for this investigation based on its strong bioluminescence activity added to its propensity to form biofilms, and also there is pre-existing data classifying this microorganism24 as an endodontic infectious species. Their morphology (Gram- rods 2–3 mm in length) is highly similar to other Gram-negative rods commonly found in endodontic infections.25 Because bioluminescence imaging is a nondestructive method, the comparative evaluation of more than one procedure is possible. Sequential images could be obtained for each tooth, avoiding conventional methods that could interfere with biofilm structure.1
The purpose of this study was to test PDT as an alternative approach against biofilm formed on dental hard tissue. The bacterial reduction showed energy-dose dependence; as the light energy increased, the bioluminescence signal decreased in both planktonic suspension and biofilm. Usually to describe the light irradiation parameters on PDT, the literature uses fluence (power×time/area) instead of total energy. In this study, we opted to use total energy (power×time), as it is difficult to calculate the area of a root canal, and the use of an optical fiber with spiral movements did not allow irradiation of the entire canal walls, but only a small area on each movement.
The bacterial reduction showed that the photosensitivity of planktonic bacteria was higher than bacteria in a biofilm. The energy used for inactivation of biofilm was higher than that required to inactivate planktonic bacterial suspension. According to Sharma et al., this difference could be for several reasons: cells in biofilm differ from their planktonic counterparts in a number of ways, such as cell wall composition, rate of growth, and presence of a self-produced extracellular polymeric matrix composed mainly of a large polysaccharide referred to as polysaccharide intercellular adhesins (PIA), which may hinder the uptake of the PS and also obstruct the penetration of light, thereby reducing the efficiency of the photodynamic process.
The SEM analysis showed large cellular aggregates enclosed by an extracellular matrix in all the biofilms treated with MB in dark conditions (Fig. 4 A and B). The images of the biofilm (E. faecalis) after PDT show a reduction in the number of adherent bacteria grouped in very few aggregated colonies; most of them were single cells or organized in short chains. The images were very similar to the biofilm images obtained by Di Poto et al.26 and Sharma et al.27 even though, in these studies, the authors had worked with staphylococcal biofilms. The significant reduction of the Gram-positive E. faecalis biofilm (89%) was higher than the reduction obtained with the Gram-negative P. aeruginosa. It is well known that Gram-positive bacteria are more easily killed by PDT than Gram negative species, most likely because of the difference in membrane structure between the two classes.7
Our results with the bioluminescent Gram-negative bacteria biofilm reduction are lower than those reported by Sharma et al.27 and Zanin et al.19 who used, respectively, staphylococcal and streptococcal biofilms (Gram+ bacteria), and in addition to morphological differences among the species, this fact could be the result of different experimental conditions, the diverse nature of the biofilm, and/or substrate interaction. The results with mixed biofilm confirm this statement, as it is possible to see on Fig. 5.
The most common substrate for biofilm study is polystyrene as the material comprising 96 or 24 well plates, but other biomaterials have been employed such as anionic hydrogel copolymers used to build intraocular lenses.28 In this study, we used dentine from human root canal surface as a substrate. The organic substrate makes it possible to obtain a more adherent biofilm, and therefore enhance the PDT challenge. It also mimics the clinical condition in a more realistic manner, and experiments conducted on polycarbonate or glass substrate may not provide a true indication of the biofilm–substrate interaction.29
Dentin is a tissue composed of an organic fraction, which is mainly collagen, and an inorganic fraction, essentially carbonated hydroxyapatite. Certain bacteria can attach to dentin collagen through the expression of surface adhesins, and then go on to form biofilms.30 PDT has shown to be a suitable approach to disrupt and inactivate biofilms adherent to biological tissue, if the target can be reached by the light and the PS. The PDT effect seems to act on both cells, promoting killing and membrane changes, and also on the extracellular matrix, disturbing the microbiological environment and facilitating the elimination of the remaining structures by irrigation.
Therefore, the use of PDT against oral infections could be an interesting approach for the treatment of diseases related to biofilm presence, such as dental caries, periodontal pockets, and root canal infections.
Conclusions
In this article, we examined the effects of PDT with MB and a 660 nm diode laser on biofilms grown on dental tissue. PDT successfully reduced the survival of P. aeruginosa and E. faecalis cells in biofilms. The bacterial reduction showed energy dependence, and the energy used for inactivation of biofilm was higher than that required for planktonic suspension. The photodynamic effect seemed to act both on cells, promoting killing and membrane alterations, and on the extracellular matrix, disrupting biofilm structure.
Acknowledgments
Dr. Hamblin was supported by United States National Institutes of Health (US NIH) grant R01AI050875. M.S. Ribeiro thanks FAPESP (Fundação de Amparo e Pesquisa do Estado de São Paulo) and CNPq (Conselho Nacional de Pesquisa e Desenvolvimento) for financial support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Garcez A.S. Ribeiro M.S. Tegos G.P. Núñez S.C. Jorge A.O. Hamblin M.R. Antimicrobial photodynamic therapy combined with conventional endodontic treatment to eliminate root canal biofilm infection. Lasers Surg. Med. 2007;39:59–66. doi: 10.1002/lsm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foschi F. Fontana C.R. Ruggiero K. Riahi R. Vera A. Doukas A.G. Pagonis T.C. Kent R. Stashenko P.P. Soukos N.S. Photodynamic inactivation of Enterococcus faecalis in dental root canals in vitro. Lasers Surg. Med. 2007;39:782–787. doi: 10.1002/lsm.20579. [DOI] [PubMed] [Google Scholar]
- 3.Garcez A.S. Nuñez S.C. Hamblin M.R. Ribeiro M.S. Antimicrobial effects of photodynamic therapy on patients with necrotic pulps and periapical lesion. J. Endod. 2008;34:138–142. doi: 10.1016/j.joen.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonseca M.B. Júnior P.O. Pallota R.C. Filho H.F. Denardin O.V. Rapoport A. Dedivitis R.A. Veronezi J.F. Genovese W.J. Ricardo A.L. Photodynamic therapy for root canals infected with Enterococcus faecalis. Photomed. Laser Surg. 2008;26:209–213. doi: 10.1089/pho.2007.2124. [DOI] [PubMed] [Google Scholar]
- 5.Silva Garcez A. Nunez S.C. Lage–Marques J.L. Jorge A.O. Ribeiro M.S. Efficiency of NaOCl and laser-assisted photosensitization on the reduction of Enterococcus faecalis in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;102:e93–e98. doi: 10.1016/j.tripleo.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Bonsor S.J. Nichol R. Reid T.M. Pearson G.J. Microbiological evaluation of photo-activated disinfection in endodontics (an in vivo study) Br. Dent. J. 2006;25:337–341. doi: 10.1038/sj.bdj.4813371. [DOI] [PubMed] [Google Scholar]
- 7.Dahl T.A. Midden W.R. Hartman P.E. Comparison of killing of gram-negative and gram-positive bacteria by pure singlet oxygen. J. Bacteriol. 1989;171:2188–2194. doi: 10.1128/jb.171.4.2188-2194.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedberg J.S. Skema C. Baum E.D. Burdick J. Vinogradov S.A. Wilson D.F. Horan A.D. Nachamkin I. In vitro effects of photodynamic therapy on Aspergillus fumigatus. J. Antimicrob. Chemother. 2001;48:105–107. doi: 10.1093/jac/48.1.105. [DOI] [PubMed] [Google Scholar]
- 9.Tegos G.P. Anbe M. Yang C. Demidova T.N. Satti M. Mroz P. Janjua S. Gad F. Hamblin M.R. Protease-stable polycationic photosensitizer conjugates between polyethyleneimine and chlorin(e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob. Agents Chemother. 2006;50:1402–1410. doi: 10.1128/AAC.50.4.1402-1410.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Phoenix D.A. Sayed Z. Hussain S. Harris F. Wainwright M. The phototoxicity of phenothiazinium derivates against Escherichia coli and Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2003;39:17–22. doi: 10.1016/S0928-8244(03)00173-1. [DOI] [PubMed] [Google Scholar]
- 11.Gois M.M. Kurachi C. Santana E.J.B. Mima E.G.O. Spolidório D.M.P. Pelino J.E.P. Salvador Bagnato V. Susceptibility of Staphylococcus aureus to porphyrin-mediated photodynamic antimicrobial chemotherapy: an in vitro study. Lasers Med. Sci. 2009;25:391–395. doi: 10.1007/s10103-009-0705-0. [DOI] [PubMed] [Google Scholar]
- 12.Noro Filho G.A. Casarin R.C. Casati M.Z. Giovani E.M. PDT in non-surgical treatment of periodontitis in HIV patients: a split-mouth, randomized clinical trial. Lasers Surg. Med. 2012;44:296–302. doi: 10.1002/lsm.22016. [DOI] [PubMed] [Google Scholar]
- 13.Hayek R.R.A. Araújo N.S. Gioso M.A. Ferreira J. Baptista–Sobrinho C.A. Yamada A.M. Ribeiro M.S. Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J. Periodontol. 2005;76:1275–1281. doi: 10.1902/jop.2005.76.8.1275. [DOI] [PubMed] [Google Scholar]
- 14.Baptista A. Kato I.T. Prates R.A. Suzuki L.C. Raele M.P. Freitas A.Z. Ribeiro M.S. Antimicrobial photodynamic therapy as a strategy to arrest enamel demineralization: a short-term study on incipient caries in a rat model. Photochem. Photobiol. 2012;88:584–589. doi: 10.1111/j.1751-1097.2011.01059.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilson M. Lethal photosensitization o oral bactéria and its potential application in the photodynamic therapy of oral infections. J. Photochem. Photobiol. B: biol. 2004;3:412–418. doi: 10.1039/b211266c. [DOI] [PubMed] [Google Scholar]
- 16.Zanin I.C. Lobo M.M. Rodrigues L.K. Pimenta L.A. Höfling J.F. Gonçalves R.B. Photosensitization of in vitro biofilms by toluidine blue O combined with a light-emitting diode. Eur. J, Oral Sci. 2006;114:64–69. doi: 10.1111/j.1600-0722.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- 17.Farbod F. Kanaan H. Farbod J. Infective endocarditis and antibiotic prophylaxis prior to dental/oral procedures: latest revision to the guidelines by the American Heart Association published April 2007. Int. J. Oral Maxillofac. Surg. 2009;38:626–631. doi: 10.1016/j.ijom.2009.03.717. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez–Rodriguez F. Rivera R. Suarez–Gonzalez J. Gonzalez–Claudio G. Prevention of infective endocarditis: a review of the American Heart Association guidelines. Bol. Assoc. Med. P. R. 2008;100:25–28. [PubMed] [Google Scholar]
- 19.Zanin I.C. Gonçalves R.B. Junior A.B. Hope C.K. Pratten J. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J. Antimicrob. Chemother. 2005;56:324–330. doi: 10.1093/jac/dki232. [DOI] [PubMed] [Google Scholar]
- 20.Maoz A. Mayr R. Bresolin G. Neuhaus K. Francis K.P. Scherer S. Sensitive in situ monitoring of a recombinant bioluminescent Yersinia enterocolitica reporter mutant in real time on Camembert cheese. Appl. Environ. Microbiol. 2002;68:5737–5740. doi: 10.1128/AEM.68.11.5737-5740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcez A.S. Nuñez S.C. Lage–Marques J.L. Hamblin M.R. Ribeiro M.S. Photonic real-time monitoring of bacterial reduction in root canals by genetically engineered bacteria after chemomechanical endodontic therapy. Braz. Dent. J. 2007;18:202–207. doi: 10.1590/s0103-64402007000300005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcez A.S. Núñez S.C. Baptista M.S. Daghastanli N.A. Itri R. Hamblin M.R. Ribeiro M.S. Antimicrobial mechanisms behind photodynamic effect in the presence of hydrogen peroxide. Photochem. Photobiol. Sci. 2011;10:483–490. doi: 10.1039/c0pp00082e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stuart C.H. Schwartz S.A. Beeson T.J. Owatz C.B. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006;32:93–98. doi: 10.1016/j.joen.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 24.Ranta K. Haapasalo M. Ranta H. Monoinfection of root canal with Pseudomonas aeruginosa. Endod. Dent. Traumatol. 1988;4:269–272. doi: 10.1111/j.1600-9657.1988.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 25.Haapasalo M. Ranta H. Ranta K.T. Facultative gram-negative enteric rods in persistent periapical infections. Acta Odontol. Scand. 1983;41:19–22. doi: 10.3109/00016358309162299. [DOI] [PubMed] [Google Scholar]
- 26.Di Poto A. Sbarra M.S. Provenza G. Visai L. Speziale P. The effect of photodynamic treatment combined with antibiotic action or host defence mechanisms on Staphylococcus aureus biofilms. Biomaterials. 2009;30:3158–3166. doi: 10.1016/j.biomaterials.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 27.Sharma M. Visai L. Bragheri F. Cristiani I. Gupta P.K. Speziale P. Toluidine blue-mediated photodynamic effects on staphylococcal biofilms. Antimicrob. Agents Chemother. 2008;52:299–305. doi: 10.1128/AAC.00988-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsons C. McCoy C.P. Gorman S.P. Jones D.S. Bell S.E. Brady C. McGlinchey S.M. Anti-infective photodynamic biomaterials for the prevention of intraocular lens-associated infectious endophthalmitis. Biomaterials. 2009;30:597–602. doi: 10.1016/j.biomaterials.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Stepanović S. Vuković D. Hola V. Di Bonaventura G. Djukić S. Cirković I. Ruzicka F. Biofilm formation by Salmonella spp. and Listeria monocytogenes on plastic surface. Lett. Appl. Microbiol. 2004;38:428–432. doi: 10.1111/j.1472-765X.2004.01513.x. [DOI] [PubMed] [Google Scholar]
- 30.Shen Y. Qian W. Chung C. Olsen I. Haapasalo M. Evaluation of the effect of two chlorhexidine preparations on biofilm bacteria in vitro: a three-dimensional quantitative analysis. J. Endod. 2009;35:981–985. doi: 10.1016/j.joen.2009.04.030. [DOI] [PubMed] [Google Scholar]