Abstract
The atypical antipsychotic olanzapine is often associated with serious metabolic side effects including weight gain and increased visceral fat. These adverse events are a considerable clinical problem and the mechanisms underlying them are multifactorial and poorly understood. Growing evidence suggests that the gut microbiota has a key role in energy regulation and disease states such as obesity. Moreover, we recently showed that chronic olanzapine altered the composition of the gut microbiome in the rat. It is thus possible that treatments that alter gut microbiota composition could ameliorate olanzapine-induced weight gain and associated metabolic syndrome. To this end, we investigated the impact of antibiotic-induced alteration of the gut microbiota on the metabolic effects associated with chronic olanzapine treatment in female rats. Animals received vehicle or olanzapine (2 mg kg−1 per day) for 21 days, intraperitoneal injection, two times daily. Animals were also coadministered vehicle or an antibiotic cocktail consisting of neomycin (250 mg kg−1 per day), metronidazole (50 mg kg−1 per day) and polymyxin B (9 mg kg−1 per day) by oral gavage, daily, beginning 5 days before olanzapine treatment. The antibiotic cocktail drastically altered the microbiota of olanzapine-treated rats, and olanzapine alone was also associated with an altered microbiota. Coadministration of the antibiotic cocktail in olanzapine-treated rats attenuated: body weight gain, uterine fat deposition, macrophage infiltration of adipose tissue, plasma free fatty acid levels, all of which were increased by olanzapine alone. These results suggest that the gut microbiome has a role in the cycle of metabolic dysfunction associated with olanzapine, and could represent a novel therapeutic target for preventing antipsychotic-induced metabolic disease.
Keywords: antipsychotics, metabolism, microbiota, obesity, olanzapine, weight gain
Introduction
Olanzapine (OLZ) and other atypical antipsychotics are associated with a constellation of serious metabolic side effects including weight gain, increased visceral fat and glucose dysregulation.1, 2 These adverse effects lead to comorbidities such as Type 2 diabetes mellitus and cardiovascular disease, as well as contributing to the poor treatment adherence rates seen in schizophrenia.3, 4
Overall, cardiometabolic disease is the major cause of morbidity and mortality in patients with schizophrenia.5, 6 Thus, tackling the causes of metabolic disease in schizophrenia is a clinical imperative, and ways to attenuate the metabolic effects of antipsychotics is a top priority in this regard.7 At present, a limited number of interventions exist for tackling antipsychotic-induced metabolic dysfunction and available agents, such as the antidiabetic drug metformin, are inadequate.8 Hence, novel approaches to prevent or attenuate these adverse effects are desperately strived for.
The mechanisms underlying antipsychotic-induced weight gain and metabolic dysfunction are not fully understood but involve both central and peripheral mechanisms which converge to produce metabolic dysfunction.9, 10 Initial increases in body weight are primarily driven by increases in appetitive drive11 due to antagonism of multiple central receptors including 5-HT2c, histamine H1 and dopamine D2.12, 13, 14
OLZ, and other antipsychotics, can however cause metabolic dysregulation independently of effects on body weight.15 In particular, increased visceral fat mass, a key component in the development of metabolic disease, has been seen in the absence of overt weight gain following OLZ treatment in both clinical and preclinical studies.16, 17
The gut microbiota comprises the approximately 100 trillion bacteria (as well as fungi, archaea and viruses) that have coevolved with the human host to live symbiotically in the gastrointestinal tract.18 Recently, the gut microbiota has received increasing attention as its potential role in several disease states has emerged, spurred on by technological advances in methods to monitor and evaluate the microbiota composition.19
The critical role played by the gut microbiota in normal weight gain and fat deposition was demonstrated in seminal studies by Gordon and co-workers using germ-free mice (mice lacking any microbiota).20, 21 Germ-free mice have 40% less total body fat than conventionally raised mice, and are resistant to diet-induced obesity.20, 21 The same group also found that obese mice have an ‘obese-associated microbiome' consisting of proportional shifts in the two most abundant phyla of bacteria—firmicutes (increased) and bacteriodetes (decreased). A link between obesity and the composition of the gut flora in humans has also been found.22, 23
The gut microbiota is therefore now recognised as an exciting therapeutic target24, 25 and investigations of agents such as antibiotics and probiotics in metabolic disease and obesity have produced encouraging results in animal models.26, 27
Recently, our lab found an altered faecal microbiota profile in rats chronically treated with OLZ, suggesting that the gut microbiota may be directly or indirectly involved in certain aspects of OLZ's metabolic effects.28 To investigate this possibility further, in the present study we used a cocktail of broad-spectrum antibiotics to examine if marked alteration of the gut microbiota would affect the metabolic side effects induced by OLZ.
Materials and methods
Animals
Female Sprague–Dawley rats, 6 weeks old and weighing approximately 200 g were used (Harlan, Derby, UK). Female rats are used as they have been shown by us and others to better reflect the elevated weight gain induced by atypical antipsychotics clinically.28, 29 Animals were allowed to habituate to the facility for 10 days. Animals were housed 5 per cage (56 × 38 × 17 cm3) and allowed access to standard chow and water ad libitum. Animals were maintained on a 12 h light–dark cycle, lights on 0730 hours. All experiments were approved by the Animal Experimentation Ethics Committee (AEEC) of University College Cork and carried out in accordance with the Cruelty to Animals Act 1876 and European Directive 86/609/EEC on the protection of animals used for experimental and other scientific purposes.
OLZ administration
OLZ (Discovery Fine Chemicals, Wimborne, UK) was dissolved in a minimal amount of glacial acetic acid (approx. 0.1 ml), made to volume with sterile water and pH adjusted to 6.0 with 0.1 M NaOH. Animals received 2 mg kg−1 per day. Vehicle (VEH) consisted of sterile water acidified with 0.1 ml of glacial acetic acid and pH adjusted to 6.0 with 0.1 M NaOH. Drug solutions were prepared daily and administered via intraperitoneal injection, two times daily, for 21 days, with the first injection between 0900 and 1000 hours and the second between 1600 and 1700 hours. Dose and regimen of OLZ treatment was selected based on previous studies from our group and others, in which they were found to best represent the clinical setting in terms of inducing side effects.28, 29
Antibiotic cocktail administration
Neomycin (250 mg kg−1), metronidazole (50 mg kg−1) (Discovery Fine Chemicals) and polymyxin B (9 mg kg−1) (Sigma-Aldrich, Buchs, Switzerland) were dissolved in sterile water and sonicated for 10 min to ensure complete dissolution. The antibiotic cocktail (ABX) was administered once daily per os in a volume of 4 ml kg−1 and was prepared fresh daily. Antibiotics and their doses were chosen to target the entire gut microbiota and were also based on published studies demonstrating that this cocktail effectively sterilises the gastrointestinal tract of rats.30
Treatment groups
Animals received either VEH or ABX for 5 days before the commencement of OLZ or VEH treatment and on all subsequent days. After the initial 5 days animals received OLZ (2 mg kg−1 per day) or VEH for 21 days. Hence, there were four treatment groups: (1) VEH+VEH; (2) VEH+ABX; (3) OLZ+VEH and (4) OLZ +ABX. Groups were weight matched before study commencement to eliminate any bias of baseline weight. N=9/10.
Animal behaviour
To assess possible effects of sedation, animals performed a 30 min locomotor test 2 days before being killed. The test was performed under low lighting (100 lx) to minimise any freezing effect. The animals were placed in a rectangular container (60 × 50 × 40 cm3) and behaviour was recorded via an overhead camera. Data were analysed using a tracking software system (Ethovision, Noldus, The Netherlands). During the locomotor test, faecal output was recorded as a measure of gastrointestinal health.
Sample collection
All animals were fasted overnight (16 h) before being killed. Periuterine fat was quickly and carefully dissected and weighed to the nearest 0.001 g. Trunk blood was collected in ethylenediamine tetraacetic acid-coated tubes and centrifuged at 6000 r.p.m. for 15 min at 4 °C. Plasma supernatant was then aliquoted and frozen. A sample of periuterine fat and frontal lobe of the liver were snap frozen. All samples were stored at −80 °C for later analysis.
Gut microbiota analysis
For analysis of the microbial community composition, total DNA was extracted from faecal pellets collected directly from the rats one day before being killed (n=6) using the QIAamp DNA stool mini kit according to the manufacturer's instructions (Qiagen, West Sussex, UK) coupled with an initial bead-beating step. Universal 16s rRNA primers, designed to amplify from highly conserved regions corresponding to those flanking the V4 region were used for Taq-based polymerase chain reaction amplification: forward primer F1 (5′-AYTGGGYDTAAAGNG-3′); reverse primers R1 (5′-TACCRGGGTHTCTAATCC-3′), R2 (5′-TACCAGAGTATCTAATTC-3′), R3 (5′-CTACDSRGGTMTCTAATC-3′) and R4 (5′-TACNVGGGTATCTAATC-3′) (RDP's Pyrosequencing Pipeline: http://pyro.cme.msu.edu/pyro/help.jsp). Sequencing was performed on a Roche 454 GS-FLX using Titanium chemistry by the Teagasc454 Sequencing Platform (454 Life Sciences, Branford, CT, USA). Resulting raw sequences reads were quality trimmed as described previously.31 Trimmed FASTA sequences were then BLASTed32 against a previously published 16s rRNA-specific database33 using default parameters. The resulting BLAST output was parsed using MEGAN.34 MEGAN assigns reads to National Center for Biotechnology Information taxonomies by using the Lowest Common Ancestor algorithm. Bit scores were used from within MEGAN for filtering the results before tree construction and summarisation. A bit score of 86 was selected as previously used for 16s ribosomal sequence data.33 Phylum and family counts for each subject were extracted from MEGAN. Clustering and alpha diversities were generated with the MOTHUR software package (provided free of charge by Dr Patrick Schloss from the Department of Microbiology & Immunology at the University of Michigan, MI, USA).
Plasma analysis
Insulin was measured using a commercially available enzyme-linked immunosorbent assay (Mercodia, Uppsala, Sweden). Glucose was measured using a colorimetric assay (Bioassays Systems, Hayward, CA, USA). The quantitative insulin sensitivity check index (QUICKI) was calculated as the inverse log of the sum of fasting plasma insulin and fasting plasma glucose.35 Plasma free fatty acids (FFAs) were measured using a commercially available colorimetric assay (Bioassay Systems). All samples were analysed in duplicate.
Gene expression analysis
Total RNA was extracted using a commercially available kit (Qiagen, Valencia, CA, USA). mRNA was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) in a G-Storm thermocycler (G-Storm, Ringmer, East Sussex, UK). Gene expression was analysed by qualitative real-time polymerase chain reaction using TaqMan Gene expression assays and the AB7300 system (Applied Biosystems). The expression of each gene was normalised to β-actin. All samples were analysed in triplicate.
Statistical analysis
Data are expressed as mean±s.e.m. Body weight change was analysed using two-way repeated measures analysis of variance and a Greenhouse–Geisser sphericity correction was applied. Two-way analysis of variance was used for the analysis of periuterine fat, gene expression and plasma analysis. Where a significant overall effect was observed, further analysis was carried with Fisher's least significant difference test. A P-value <0.05 was considered statistically significant.
Results
Gut microbiota
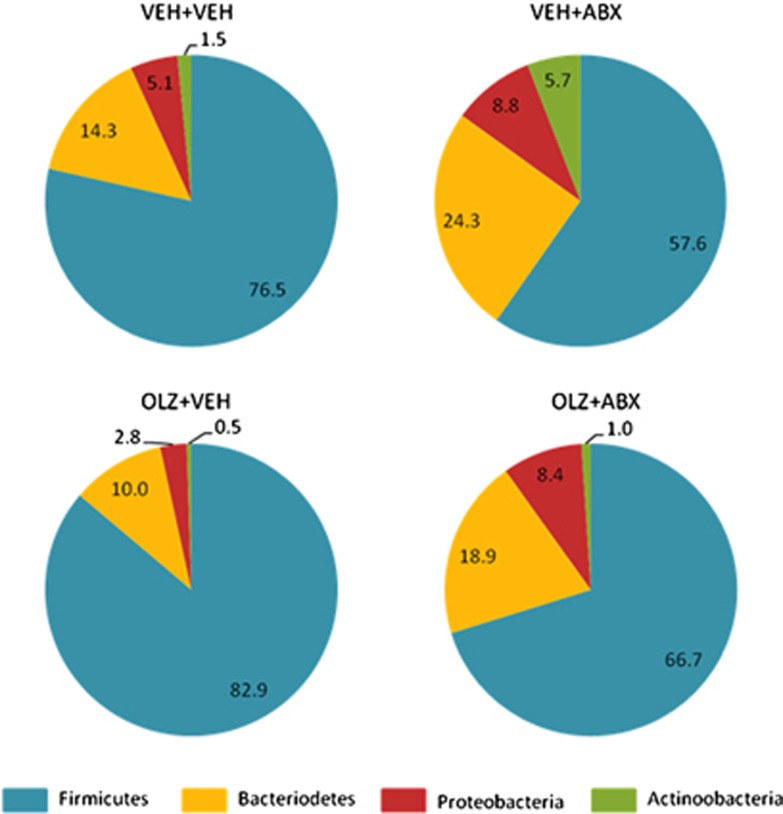
As expected, administration of ABX resulted in considerable changes in the abundance of all major bacterial phyla (Figure 1).
Figure 1.
Effect of olanzapine (OLZ) coadministered with vehicle (VEH) or an antibiotic cocktail (ABX) on the faecal microbiota of female rats. N=6.
OLZ treatment resulted in a trend for increased abundance of the major phyla Firmicutes (82.9% versus 76.5%) and reductions in the phyla Bacteriodetes (10.0% versus 14.3%) compared with VEH+VEH-treated animals (Figure 1). Intriguingly, coadministration of ABX resulted in the opposite trend in the OLZ+ABX group, with reduced Firmicutes (66.7% versus 82.9%) and increased Bacteriodetes (18.9% versus 10.0%) compared with OLZ+VEH-treated animals (Figure 1).
Body weight
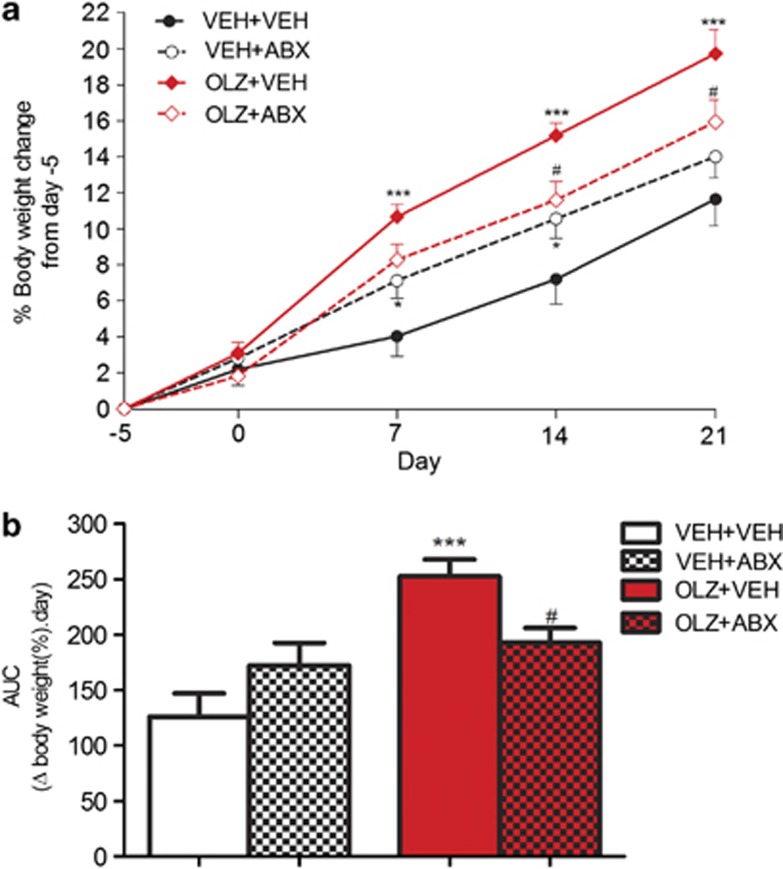
OLZ administration had a significant effect on body weight change (F(1,34)=14.91, P<0.001) and there was significant OLZ × ABX interaction (F(1,34)=8.71, P<0.01). An OLZ-induced increase in body weight was evident on days 7, 14 and 21 (P<0.001). Animals receiving OLZ+ABX had significantly lower weight gain compared with OLZ+VEH on days 14 and 21 (P<0.05) (Figure 2a).
Figure 2.
(a) Effect of olanzapine (OLZ) 2 mg kg−1 coadministered with vehicle (VEH) or an antibiotic cocktail (ABX) on body weight gain in female rats. (b) Area under the curve analysis. *P<0.05, ***P<0.001 versus VEH+VEH, #P<0.05 versus OLZ+VEH. N=9/10. Data represent mean±s.e.m.
Area under the curve analysis shows animals receiving OLZ+VEH had significantly greater weight gain overall compared with VEH+VEH (P<0.001) and OLZ+ABX-treated rats (P<0.05) (Figure 2b).
Food intake
Animals receiving OLZ+VEH had significantly increased food intake during the first 2 weeks of OLZ treatment. OLZ-induced increase in food intake was not affected by antibiotic coadministration (Supplementary Figure 1).
Locomotor activity and faecal output
OLZ administration was associated with reduced locomotor activity that was not affected by coadministration of ABX. ABX was also associated with reduced locomotor activity (Supplementary Figure 2). There were no differences in faecal output (number of pellets produced in 30 min) between any of the groups (data not shown).
Adipose tissue
Uterine fat weight
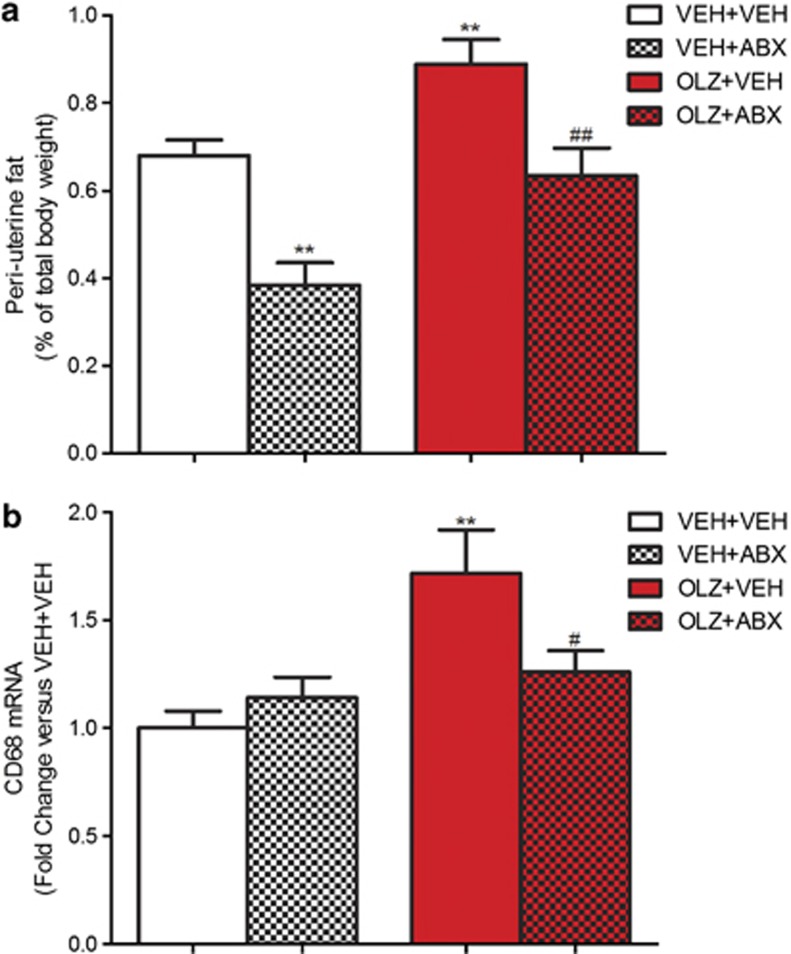
OLZ administration had a significant effect on periuterine fat mass (F(1,34)=22.91, P<0.001). ABX treatment also had a significant effect (F(1,34)=23.66, P<0.001).
Post hoc analysis revealed that animals receiving OLZ+VEH had increased levels of periuterine fat compared with VEH+VEH-treated rats (P<0.01). OLZ+ABX-treated animals had significantly lower uterine fat compared with OLZ+VEH group (P<0.05) (Figure 3a).
Figure 3.
Effect of olanzapine (OLZ) 2 mg kg−1 and an antibiotic cocktail (ABX), alone and combined, on (a) periuterine fat percentage and (b) CD68 mRNA expression in adipose tissue. **P<0.01 versus vehicle (VEH)+VEH group. #P<0.05, ##P<0.01 versus OLZ+VEH group. N=9/10. All data represent mean±s.e.m.
CD68 expression
CD68 expression was significantly increased by OLZ administration (F(1,34)=10.10, P<0.01) and there was a significant OLZ × ABX interaction (F(1,34)=5.18, P<0.05). Further analysis showed that the animals receiving OLZ+VEH had significantly increased expression of CD68 compared with VEH+VEH-treated rats (P<0.01). While OLZ+ABX-treated rats had significantly lower expression compared with the OLZ+VEH group (P<0.05) (Figure 3b).
Plasma profile
Free fatty acids
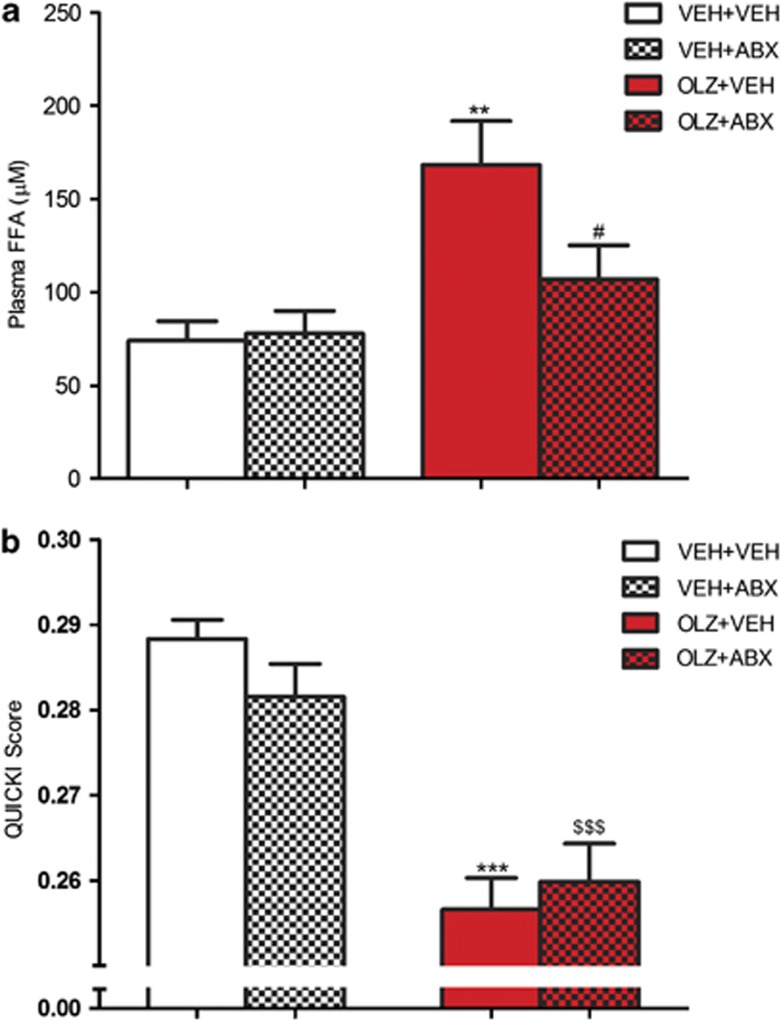
OLZ treatment had a significant effect on plasma FFAs (F(1,32)=6.393, P<0.01). Further analysis showed that animals receiving OLZ+VEH had significantly elevated levels of FFAs compared with the VEH+VEH group (P<0.01) OLZ (2 mg kg−1). This increase was not seen in rats receiving OLZ+ABX, which had reduced levels compared with OLZ+VEH-treated animals (P<0.05) (Figure 4a).
Figure 4.
Effect of olanzapine (OLZ) 2 mg kg−1 and an antibiotic cocktail (ABX), alone and combined, on (a) plasma free fatty acid concentration and (b) quantitative insulin sensitivity check index (QUICKI). **P<0.01, ***P<0.001 compared with vehicle (VEH)+VEH group. #P<0.05 compared with OLZ+VEH; $$$P<0.001 compared with VEH+ABX-treated animals. N=9/10. Data represent mean±s.e.m.
QUICKI
The QUICKI score was significantly affected by OLZ administration (F(1,32)=53.45, P<0.001). Post hoc analysis revealed that the animals receiving OLZ+VEH had lower QUICKI scores compared with the VEH+VEH-treated rats (P<0.001). Furthermore, the animals that received OLZ+ABX had reduced QUICKI scores compared with the VEH+ABX-treated rats (P<0.001) (Figure 4b).
Liver gene expression
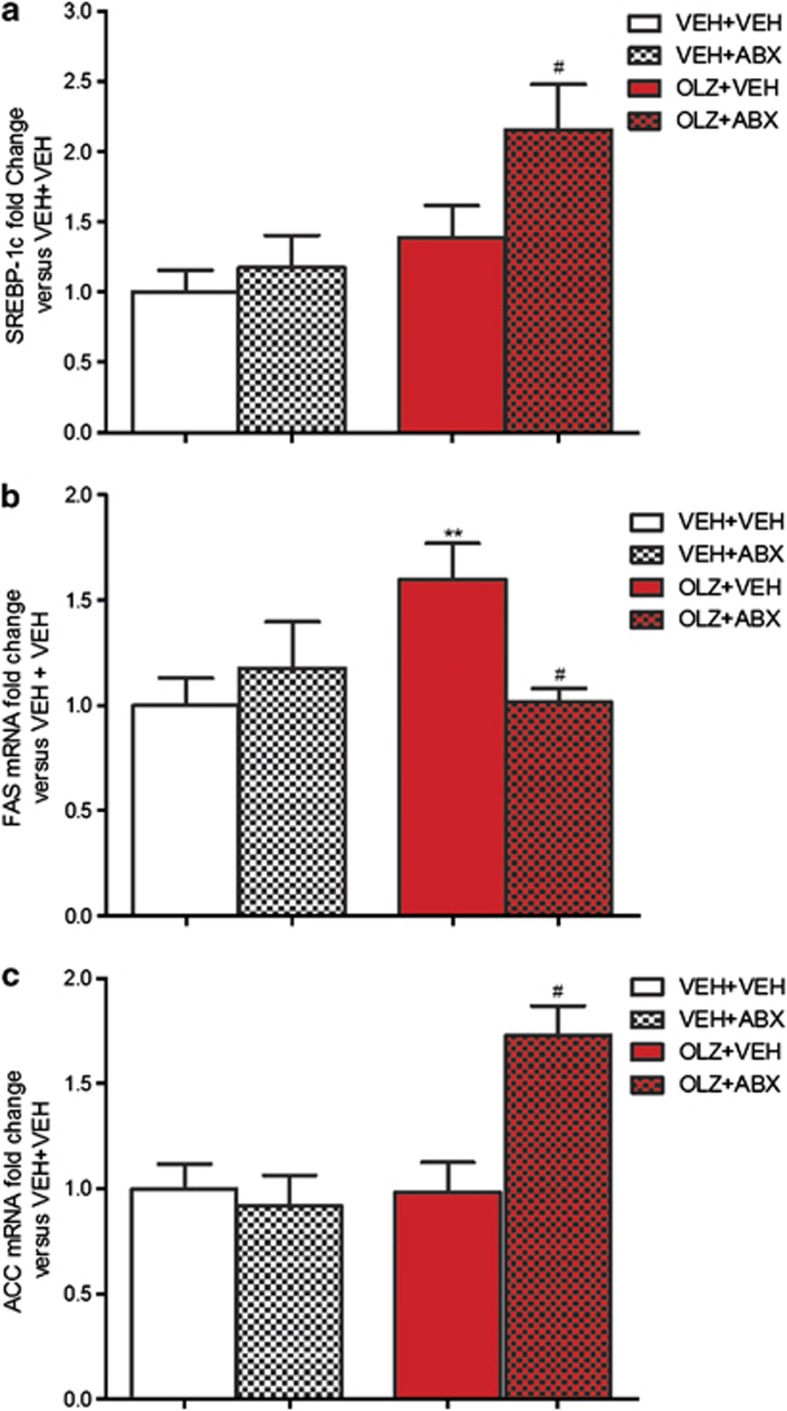
OLZ had a significant overall effect on sterol-regulatory element binding protein-1c expression in the liver (F1,34)=4.44, P<0.01). OLZ+ABX-treated animals had increased expression compared with both OLZ+VEH- and VEH+ABX-treated rats (P<0.05) (Figure 5a).
Figure 5.
Effect of olanzapine (OLZ) 2 mg kg−1 and an antibiotic cocktail (ABX), alone and combined, on (a) sterol-regulatory element binding protein (SREBP)-1c; (b) fatty acid synthase (FAS); and (c) acetyl-CoA-carboxylase (ACC) mRNA expression in hepatic tissue. **P<0.01 versus vehicle (VEH)+VEH group; #P<0.05 versus OLZ+VEH group. N=9/10. Data represent mean±s.e.m.
Analysis of the hepatic expression of the lipogenic enzyme fatty acid synthase (FAS) revealed a significant OLZ × ABX interaction (F(1,34)=5.87, P<0.05). Further analysis revealed OLZ+VEH-treated animals had increased expression compared with VEH+VEH-treated rats (P<0.01). This increase was not observed in animals receiving OLZ+ABX, which displayed reduced expression compared with the OLZ+VEH group (Figure 5b).
The hepatic expression of another lipogenic enzyme, acetyl Co-A carboxylase (ACC), was significantly affected by both OLZ administration (F(1,32)=5.86, P<0.05) and ABX treatment F(1,32)=8.51, P<0.01) and there was a significant OLZ × ABX interaction F(1,32)=9.17, P<0.01). OLZ+ABX-treated rats displayed significantly increased expression of acetyl Co-A carboxylase compared with the OLZ+VEH and VEH+ABX groups (P<0.01) (Figure 5c).
Discussion
As expected, OLZ induced rapid body weight gain and significant accretion of visceral fat in line with several previous reports.28, 36 OLZ was also associated with an altered microbiota, with a shift in the abundance of the major phyla observed. The faecal microbiota of OLZ-treated rats showed a trend for increases in the phyla Firmicutes and concomitant decreases in Bacteriodetes. This same shift has previously been associated with an obese phenotype in animals and humans23, 37 and was in line with our previous findings.28
These alterations in the microbiota were likely a consequence of metabolic dysfunction induced by OLZ rather than a direct effect. That said, it is now clear that the microbiota is an important part of metabolic processes in both health and disease states.38, 39 Thus, an aberrant gut flora may be viewed as a further aspect in the cycle of metabolic dysfunction associated with OLZ.
In the present study, we markedly altered the microbiota of OLZ-treated rats using a cocktail of broad-spectrum antibiotics. Although not quantitatively assessed, this cocktail has been previously shown to ablate the gut microbiota of rats before surgery,30 and 454 pyrosequencing revealed that the ABX was associated with considerable qualitative shifts in the gut flora in this study (Figure 1).
We found that this radical alteration of the gut flora attenuated certain clinically relevant side effects of OLZ in the rat, including weight gain and visceral fat accumulation. This novel finding supports recent work showing antibiotic treatment can prevent weight gain in diet-induced obesity models in mice.26, 27
Moreover, coadministration of the ABX resulted in an opposite shift in the microbiota to those observed in the rats receiving only OLZ. The physiological relevance of shifts at the phyla level as observed in this study remain unclear, as several metabolic functions are conserved across diverse species and correlating changes to a trait as globally pervasive as energy regulation is a considerable challenge.40 Yet, it is conceivable that different species confer different effects on the host. Whether OLZ-induced alterations in the flora are secondary to metabolic changes or indeed have an aetiological role in them remains an unanswered question and one that future studies should address. It may be the case that even before OLZ treatment patients may have a ‘susceptible' microbiota that puts them at a greater risk of developing metabolic disorders once treatment starts. However, the rats used in our study were not models of schizophrenia and one would expect that they had a healthy microbiota. This indicates that the OLZ alone and not the disease, in this case, led to the altered microbiota and metabolic dysfunction.
The mechanisms underlying the prevention of body weight gain observed in this study appear to be independent of food intake as the ABX did not reduce food consumption over the course of OLZ treatment. The reduction in body weight was therefore most likely accounted for by a reduction in fat mass, as the antibiotic cocktail prevented increases in uterine fat. This is particularly relevant to the clinical setting as increased visceral fat is a key determinant in the development of insulin resistance and the metabolic syndrome.41
Interestingly, reduced fat mass was also seen in animals receiving antibiotics only, in line with germ-free studies.20 Although this may seem paradoxical, as these animals also displayed a trend for increased body weight, it is worth noting that this phenomenon has been utilised in the agricultural food industry for many decades, as low-dose antibiotics have been used and abused as growth promoters to produce larger, leaner animals.42
A crucial step in linking increased adipose mass and metabolic disease is the recruitment of macrophages, which infiltrate the fat tissue and together with the adipocytes release proinflammatory cytokines.43 We found increased expression of CD68, a macrophage marker, in the adipose tissue of animals receiving OLZ but not in those coadministered with the ABX. The reason for macrophage infiltration in obesity is not clear, although it may be the result of adipose tissue hypertrophy, which causes adipocyte death that in turn leads to the release of signals that recruit macrophages.44 Thus, by preventing increases in fat mass, the ABX may have indirectly prevented macrophage infiltration.
Obesity is also associated with adipocyte dysfunction, which results in increased release of FFA as the storage capacity of adipocytes is reduced in the face of continued energy storage. Increased levels of circulating FFA were found in association with OLZ treatment in line with previous reports of both patients45 and animals,46 and moreover these increases were prevented by antibiotic treatment. Increased plasma levels of FFA contribute to metabolic dysfunction as storage in ectopic sites promotes insulin resistance, and increased delivery to the liver stimulates lipogenic enzymes.47
Antibiotic treatment prevented OLZ-induced increases in gene expression of one such lipogenic enzyme, FAS. FAS drives de novo lipogenesis in the liver (conversion of carbohydrates to triglycerides, which are then stored in adipose tissue), and hence reduced expression of this enzyme induced by the ABX likely has a role in preventing fat deposition in the animals coadministered with the ABX. In support of this possibility, the gut microbiota has previously been shown to influence the expression of a number of lipogenic genes, including FAS, at least in part through effects on nutrient absorption.48
Increased expression of sterol-regulatory element binding protein-1c and acetyl Co-A carboxylase-1 were, however, only observed in animals receiving both OLZ and antibiotics. This is contrary to what one might expect, and may be the result of a positive feedback mechanisms due to an altered nutrient absorption (such as reduced short-chain fatty acid production in the antibiotic-treated animals) or the fact that the animals were fasted overnight, which is known to decrease sterol-regulatory element binding protein-1c expression in the rat.49
Thus, both direct and indirect effects of alterations to the gut microbiota likely have a role in the widespread metabolic benefits observed in this study. The effects on the OLZ-induced changes to the inter-related factors of FFA levels, lipogenic gene expression and visceral fat deposition highlights that the gut microbiota can influence the full cycle of metabolic dysfunction associated with OLZ, and lends support to the hypothesis that the gut microbiota may be a viable therapeutic target for antipsychotic-induced weight gain.
We used the QUICKI as a measure of insulin sensitivity as this model is viewed as the most appropriate for animal models, especially when estimating whole-body insulin resistance.50 Although OLZ resulted in reduced insulin sensitivity as reported previously,51, 52 the ABX could not prevent the development of insulin resistance. This may have been due to direct effects of OLZ, which can cause insulin resistance acutely via direct effects on insulin secretion.53
Another mechanism potentially involved in the observed effects of the antibiotics include pharmacokinetics, as considerable alteration of the gut flora potentially impacts drug absorption and metabolism. Hence, active levels of the drug may have been diminished, and moreover, interventions involving the microbiota, if ever applied clinically, could conceivably impact on the efficacy of the antipsychotic, a concern that may warrant investigation in the future.
Of course, widespread use of broad-spectrum antibiotics as an adjunctive therapy cannot be advocated because of the well-known problem of antibiotic resistance. However, the emergence of probiotic, prebiotic and also synbiotic therapies for a variety of medical conditions, including obesity,54 encourages the development of such strategies for not only second-generation antipsychotic-induced weight gain but also obesity and metabolic syndrome in general. These therapies could be administered concomitantly with the second-generation antipsychotic therapy, for instance, to potentially prevent the shift in flora, altered metabolic profile and altered energy extraction from food that may occur in patients. Further understanding of host–microbe interactions and the effect of specific alterations to the gut on host metabolism, such as the recent finding that administration of the bacteria Akkermansia muciniphila reversed high-fat diet-induced metabolic disorders,55 may allow for more clinically suitable microbial approaches to be investigated.
Taken together, these findings do however offer an exciting proof of principle that manipulation of the gut flora potentially represents a new therapeutic strategy for tackling the serious clinical problem of antipsychotic-induced metabolic dysfunction.
Acknowledgments
We thank Patrick Fitzgerald for his assistance and staff of the biological services unit for technical support, and also Lisa Quigley for technical assistance. The Alimentary Pharmabiotic Centre is a research centre funded by Science Foundation Ireland (SFI), through the Irish Government's National Development Plan. The authors and their work were supported by SFI (grant nos.: 02/CE/B124 and 07/CE/B1368).
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Albaugh VL, Judson JG, She P, Lang CH, Maresca KP, Joyal JL, et al. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol Psychiatry. 2011;16:569–581. doi: 10.1038/mp.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomer JW. Metabolic risk during antipsychotic treatment. Clin Ther. 2004;26:1936–1946. doi: 10.1016/j.clinthera.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302:1765–1773. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farwell WR, Stump TE, Wang J, Tafesse E, L'Italien G, Tierney WM. Weight gain and new onset diabetes associated with olanzapine and risperidone. J Gen Intern Med. 2004;19:1200–1205. doi: 10.1111/j.1525-1497.2004.40126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley Dl, Morley KI. Systematic review of early cardiometabolic outcomes of the first treated episode of psychosis. Arch Gen Psychiatry. 2011;68:609–616. doi: 10.1001/archgenpsychiatry.2011.2. [DOI] [PubMed] [Google Scholar]
- Osby U, Correia N, Brandt L, Ekbom A, Sparen P. Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr Res. 2000;45:21–28. doi: 10.1016/s0920-9964(99)00191-7. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Lonnqvist J, Wahlbeck K, Klaukka T, Niskanen L, Tanskanen A, et al. No mental health without physical health. Lancet. 2011;377:611. doi: 10.1016/S0140-6736(11)60211-0. [DOI] [PubMed] [Google Scholar]
- Maayan L, Vakhrusheva J, Correll CU. Effectiveness of medications used to attenuate antipsychotic-related weight gain and metabolic abnormalities: a systematic review and meta-analysis. Neuropsychopharmacology. 2010;35:1520–1530. doi: 10.1038/npp.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyda HN, Tse L, Procyshyn RM, Honer WG, Barr AM. Preclinical models of antipsychotic drug-induced metabolic side effects. Trends Pharmacolog Sci. 2010;31:484–497. doi: 10.1016/j.tips.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19 (Suppl 1:1–93. doi: 10.2165/00023210-200519001-00001. [DOI] [PubMed] [Google Scholar]
- Davoodi N, Kalinichev M, Korneev SA, Clifton PG. Hyperphagia and increased meal size are responsible for weight gain in rats treated sub-chronically with olanzapine. Psychopharmacology (Berl) 2009;203:693–702. doi: 10.1007/s00213-008-1415-1. [DOI] [PubMed] [Google Scholar]
- Kirk SL, Glazebrook J, Grayson B, Neill JC, Reynolds GP. Olanzapine-induced weight gain in the rat: role of 5-HT2C and histamine H1 receptors. Psychopharmacology (Berl) 2009;207:119–125. doi: 10.1007/s00213-009-1639-8. [DOI] [PubMed] [Google Scholar]
- Matsui-Sakata A, Ohtani H, Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet. 2005;20:368–378. doi: 10.2133/dmpk.20.368. [DOI] [PubMed] [Google Scholar]
- Reynolds GP. Pharmacogenetic aspects of antipsychotic drug-induced weight gain—a critical review. Clin Psychopharmacol Neurosci. 2012;10:71–77. doi: 10.9758/cpn.2012.10.2.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minet-Ringuet J, Even PC, Goubern M, Tome D, de Beaurepaire R. Long term treatment with olanzapine mixed with the food in male rats induces body fat deposition with no increase in body weight and no thermogenic alteration. Appetite. 2006;46:254–262. doi: 10.1016/j.appet.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Victoriano M, Hermier D, Even PC, Fromentin G, Huneau JF, Tome D, et al. Early perturbation in feeding behaviour and energy homeostasy in olanzapine-treated rats. Psychopharmacology (Berl) 2009;206:167–176. doi: 10.1007/s00213-009-1593-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z-J, Yao Z-J, Liu WEN, Fang QUN, Reynolds GP. Effects of antipsychotics on fat deposition and changes in leptin and insulin levels: magnetic resonance imaging study of previously untreated people with schizophrenia. Br J Psychiatry. 2004;184:58–62. doi: 10.1192/bjp.184.1.58. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host–bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Fraher MH, O'Toole PW, Quigley EMM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat Rev Gastroenterol Hepatol. 2012;9:312–322. doi: 10.1038/nrgastro.2012.44. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104:979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri M, Moreno LA, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obes. 2009;33:758–767. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–U7. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneveld J, Turnbaugh PJ, Lozupone C, Ley RE, Hamady M, Gordon JI, et al. Host–bacterial coevolution and the search for new drug targets. Curr Opin Chem Biol. 2008;12:109–114. doi: 10.1016/j.cbpa.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W, Li HK, Zhao LP, Nicholson JK. Gut microbiota: a potential new territory for drug targeting. Nat Rev Drug Discov. 2008;7:123–129. doi: 10.1038/nrd2505. [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Neyrinck AM, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- Murphy EF, Cotter PD, Hogan A, O'Sullivan O, Joyce A, Fouhy F, et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 2012;62:220–226. doi: 10.1136/gutjnl-2011-300705. [DOI] [PubMed] [Google Scholar]
- Davey KJ, O'Mahony SM, Schellekens H, O'Sullivan O, Bienenstock J, Cotter PD, et al. Gender-dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. 2012;221:155–169. doi: 10.1007/s00213-011-2555-2. [DOI] [PubMed] [Google Scholar]
- Cooper GD, Pickavance LC, Wilding JP, Halford JC, Goudie AJ. A parametric analysis of olanzapine-induced weight gain in female rats. Psychopharmacology (Berl) 2005;181:80–89. doi: 10.1007/s00213-005-2224-4. [DOI] [PubMed] [Google Scholar]
- Juno RJ, Knott AW, Jarboe MD, Profitt SA, Erwin CR, Warner BW. Characterization of small bowel resection and intestinal adaptation in germ-free rats. Surgery. 2003;134:582–589. doi: 10.1016/s0039-6060(03)00281-2. [DOI] [PubMed] [Google Scholar]
- Claesson C, Nilsson LE, Kronvall G, Walder M, Sorberg M. Antimicrobial activity of tigecycline and comparative agents against clinical isolates of staphylococci and enterococci from ICUs and general hospital wards at three Swedish university hospitals. Scand J Infect Dis. 2009;41:171–181. doi: 10.1080/00365540902721368. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urich T, Lanzen A, Qi J, Huson DH, Schleper C, Schuster SC. Simultaneous assessment of soil microbial community structure and function through analysis of the meta-transcriptome. PLoS One. 2008;3:e2527. doi: 10.1371/journal.pone.0002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–386. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Anjum N, Dickinson K, Marshall KM, Peltola LM, Vickers S, et al. The distinct effects of subchronic antipsychotic drug treatment on macronutrient selection, body weight, adiposity, and metabolism in female rats. Psychopharmacology (Berl) 2007;194:221–231. doi: 10.1007/s00213-007-0833-9. [DOI] [PubMed] [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA [Article] 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F. Programming of host metabolism by the gut microbiota. Ann Nutr Metab. 2011;58 (Suppl:244–252. doi: 10.1159/000328042. [DOI] [PubMed] [Google Scholar]
- Burcelin R, Serino M, Chabo C, Blasco-Baque V, Amar J. Gut microbiota and diabetes: from pathogenesis to therapeutic perspective. Acta Diabetol. 2011;48:257–273. doi: 10.1007/s00592-011-0333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JH, Antoine JM, Azpiroz F, Bourdet-Sicard R, Brandtzaeg P, Calder PC, et al. PASSCLAIM—gut health and immunity. Eur J Nutr. 2004;43:118–173. doi: 10.1007/s00394-004-1205-4. [DOI] [PubMed] [Google Scholar]
- Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93 (Suppl 1:S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamphues J. Antibiotic growth promoters in animal nutrition. Berliner Und Munchener Tierarztliche Wochenschrift. 1999;112:370–379. [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res [Article] 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Wang C-j, Zhang Z-j, Sun J, Zhang X-b, Mou X-d, Zhang X-r, et al. Serum free fatty acids and glucose metabolism, insulin resistance in schizophrenia with chronic antipsychotics. Biol Psychiatry. 2006;60:1309–1313. doi: 10.1016/j.biopsych.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Jassim G, Skrede S, Jesus Vazquez M, Wergedal H, Vik-Mo AO, Lunder N, et al. Acute effects of orexigenic antipsychotic drugs on lipid and carbohydrate metabolism in rat. Psychopharmacology. 2012;219:783–794. doi: 10.1007/s00213-011-2397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegorier J-P, CdL May, Girard J. Control of Gene Expression by Fatty Acids. J Nutr. 2004;134:2444S–2449SS. doi: 10.1093/jn/134.9.2444S. [DOI] [PubMed] [Google Scholar]
- Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des [Review] 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- Gosmain Y, Dif N, Berbe V, Loizon E, Rieusset J, Vidal H, et al. Regulation of SREBP-1 expression and transcriptional action on HKII and FAS genes during fasting and refeeding in rat tissues. J Lipid Res. 2005;46:697–705. doi: 10.1194/jlr.M400261-JLR200. [DOI] [PubMed] [Google Scholar]
- Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague–Dawley rats. Am J Physiol Endocrinol Metab. 2008;295:E1269–E1276. doi: 10.1152/ajpendo.90207.2008. [DOI] [PubMed] [Google Scholar]
- Houseknecht KL, Robertson AS, Zavadoski W, Gibbs EM, Johnson DE, Rollema H. Acute effects of atypical antipsychotics on whole-body insulin resistance in rats: implications for adverse metabolic effects. Neuropsychopharmacology. 2007;32:289–297. doi: 10.1038/sj.npp.1301209. [DOI] [PubMed] [Google Scholar]
- Smith RC, Lindenmayer JP, Davis JM, Kelly E, Viviano TF, Cornwell J, et al. Effects of olanzapine and risperidone on glucose metabolism and insulin sensitivity in chronic schizophrenic patients with long-term antipsychotic treatment: a randomized 5-month study. J Clin Psychiatry. 2009;70:1501–1513. doi: 10.4088/JCP.08m04446yel. [DOI] [PubMed] [Google Scholar]
- Chintoh AF, Mann SW, Lam L, Lam C, Cohn TA, Fletcher PJ, et al. Insulin resistance and decreased glucose-stimulated insulin secretion after acute olanzapine administration. J Clin Psychopharmacol. 2008;28:494–499. doi: 10.1097/JCP.0b013e318184b4c5. [DOI] [PubMed] [Google Scholar]
- Mallappa RH, Rokana N, Duary RK, Panwar H, Batish VK, Grover S. Management of metabolic syndrome through probiotic and prebiotic interventions. Indian J Endocrinol Metab. 2012;16:20–27. doi: 10.4103/2230-8210.91178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.