Abstract
The prevalence of age-related diseases is increased in individuals with post-traumatic stress disorder (PTSD). However, the underlying biological mechanisms are still unclear. N-glycosylation is an age-dependent process, identified as a biomarker for physiological aging (GlycoAge Test). To investigate whether traumatic stress accelerates the aging process, we analyzed the N-glycosylation profile in n=13 individuals with PTSD, n=9 trauma-exposed individuals and in n=10 low-stress control subjects. Individuals with PTSD and trauma-exposed individuals presented an upward shift in the GlycoAge Test, equivalent to an advancement of the aging process by 15 additional years. Trauma-exposed individuals presented an intermediate N-glycosylation profile positioned between severely traumatized individuals with PTSD and low-stress control subjects. In conclusion, our data suggest that cumulative exposure to traumatic stressors accelerates the process of physiological aging.
Keywords: aging, GlycoAge test, N-glycosylation, PTSD, stress
Introduction
In the aftermath of traumatic events, the probability of developing post-traumatic stress disorder (PTSD), which is characterized by a cluster of intrusive symptoms, avoidance behavior and hyper-arousal,1 increases with the number of different traumatic event types experienced.2, 3 This cumulative effect of traumatic load is presumably based on the development of a neuronal fear network: the connectivity between nodes is strengthened and the network is enlarged by every additional traumatic event experienced.4
In addition to psychological suffering, traumatic stress also increases the risk of developing somatic illnesses, such as cardiovascular, inflammatory and autoimmune diseases.5 As a result, trauma exposure is associated with premature mortality and an increased risk for carcinogenesis.6, 7, 8 One underlying biological mechanism might be that chronic stress is associated with an accelerating process of cellular aging.9 Individuals with PTSD not only show typical age-related diseases10, 11 and report an older subjective age than controls,12, 13 but they even show characteristics indicative of accelerated aging. For instance, naive T lymphocytes decrease with age and are the lowest in centenarians,14 and individuals with PTSD also show a reduction in naïve T lymphocytes.15 Next, the proportion of memory T lymphocytes increases with normal aging,16, 17 and individuals with PTSD also display a higher proportion of memory T lymphocytes compared with age-matched controls.15 Gene expression of the pro-inflammatory cytokine interleukin-6 is increased after menopause and andropause in elderly adults18—and the concentration19 and spontaneous production20 of interleukin-6 is enhanced in individuals with PTSD. C-reactive protein, which is a marker for low-grade inflammation, is elevated in women over the age of 60 years21and also in individuals with PTSD.22 On a bio-molecular level, DNA damage increases with age,23 and DNA damage, detected as DNA single-strand breaks, also accumulates in individuals with PTSD.24 Contrary to expectations, elevated levels of DNA damage are not related to impaired DNA repair, but rather seem to accelerate DNA repair capacity, as neither old people23 nor individuals with PTSD show deficits in DNA repair capacity;24 yet, DNA repair processes might still be altered in individuals with PTSD and also with age,25 and this should be investigated in more detail in future studies. Finally, telomeres, which stabilize the end of chromosomes, gradually shortened by advancing age in many cell types,26 and telomere shortening is again also associated with childhood maltreatment27 and PTSD.28
N-glycosylation is an enzymatic process that produces sugar chains covalently linked to macromolecules like proteins and lipids. N-glycans present on proteins are oligosaccharides covalently attached to protein at asparagine (Asn) residues by an N-glycosidic bond mediating numerous biological functions. In contrast to proteins, N-glycans are not controlled by the genome, but secondary gene products and can be altered by environmental influences.29 Previous studies identified nine prominent N-glycan structures in human plasma, named peak 1 through peak 9.30 The concentration of N-glycans in human plasma varies with age,31 and whereas N-glycan peak 1 (agalactosylated core-α-1,6-fucosylated biantennary; NGA2F) increases with age, N-glycan peak 6 (bigalactosylated core-α-1,6-fucosylated biantennary; NA2F) decreases.32 The log ratio of these two N-glycans [log10 (peak1/peak6)], called GlycoAge Test, is a biomarker for physiological aging and gives information beyond chronological age.33 Interestingly, juvenile patients with Cockayne syndrome, a progeroid disease that is due to a genetic deficiency in transcription-coupled nucleotide excision repair,34 show high values in the GlycoAge Test.33
We hypothesize that trauma exposure might lead to accelerated aging. We examined the N-glycosylation profile in the plasma of individuals with PTSD, of trauma-exposed individuals who were not diagnosed with PTSD and of a low-stress control group. We hypothesized that individuals with PTSD would present higher values in the GlycoAge Test, compared with the low-stress control group. Further, we hypothesized that trauma-exposed individuals should be positioned between individuals with PTSD and low-stress controls.
Materials and Methods
Participants
We analyzed the N-glycosylation profile of 13 individuals (2 women, 11 men) with PTSD (DSM IV-TR),1 in comparison with a high-stress (trauma-exposed individuals: n=9; 3 women, 6 men) and a low-stress (n=10; 8 women, 2 men) control group. Individuals with PTSD were refugees living in Germany (4 from Africa, 1 Balkan and Eastern Europe, 8 Middle East and Afghanistan) with a history of severe war and torture experiences. Survivors of multiple traumata commonly also exhibit depressive symptoms. In individuals with PTSD, the average scores on the Hamilton rating scale (HAM-D)35 ranged from 0 to 39, with 7 of the 13 patients reaching a threshold of >26.36
As non-PTSD subjects differed substantially in the number of traumatic experiences, we divided this group by median split into a high-stress (Trauma-exposed: 4 from Africa, 1 Balkan and Eastern Europe, 4 Middle East and Afghanistan) group with substantial trauma exposure (traumatic load: M=1.18, s.d.=0.63) and a low-stress group (3 from Africa, 2 Balkan and Eastern Europe, 5 Middle East and Afghanistan) without substantial trauma exposure (traumatic load: M=0.23, s.d.=0.09). The traumatic load in individuals with PTSD was significantly higher (traumatic load: M=1.61, s.d.=0.49; F(2,29)=25.88; P<0.0001). Traumatic load was specified by the proportion of specific war and torture experiences, assessed by the Vivo checklist of war, detention and torture events37 and general traumatic event types, assessed by the Clinical Administered PTSD Scale (CAPS).38
Twelve subjects reported taking psychotropic medication: eight individuals with PTSD (2 benzodiazepines, 5 antidepressants, 1 neuroleptics, 1 lithium), three trauma-exposed individuals (1 benzodiazepines, 2 antidepressants) and one control subject (benzodiazepines). To control for possible effects of medication, we compared the N-glycosylation profile of individuals with PTSD with (n=8) and without medication (n=6).
Individuals with PTSD were recruited by the Center of Excellence for Psychotraumatology. Control subjects were recruited through advertisements, which were posted in town and university.
Exclusion criteria were comorbid psychological disorders (other than major depression episode (MDE)), acute infections and chronic inflammatory diseases. MDE was not used as an exclusion criterion because the comorbidity of MDE in individuals with PTSD is high,39 and MDE symptomatology overlaps with PTSD pathology in a wide range (for example, diminished interest, restricted range of affect, difficulty with falling or staying asleep, lack of concentration).
Psycho-diagnostic interviews took place at the Center of Excellence for Psychotraumatology, University of Konstanz, Germany. Blood analyses were performed in the Laboratory for Molecular Toxicology, University of Konstanz, Germany, and the Department for Molecular Biomedical Research, Ghent University, Belgium. The Ethics Committee of the University of Konstanz approved the study. All study subjects signed an informed consent and received a remuneration of 30 €.
Psycho-diagnostic interview
Psycho-diagnostic interviews were conducted by trained clinical psychologists specialized in the field of trauma. Whenever participants were not fluent in German or English, interviews were conducted with the help of trained interpreters. Traumatic events, PTSD diagnosis and PTSD symptom severity were assessed with the CAPS.38 Traumatic experiences were assessed using the Vivo checklist of war, detention and torture experiences.37 The Hamilton depression rating scale (HAM-D)35 was used for quantification of depressive symptoms. To exclude potential comorbid psychiatric disorders, the Mini International Neuropsychiatric Interview (MINI)40 was applied.
Clinical characteristics
Groups did not differ with respect to age. Individuals with PTSD experienced significantly more different traumatic event types and showed higher traumatic load compared with low-stress controls. PTSD symptom severity (CAPS score) and depressive symptoms (HAMD-D score) were highest in individuals with PTSD. Trauma-exposed individuals were between the two groups (Table 1).
Table 1. Mean and s.d. of clinical characteristics, in n=10 low-stress control subjects, n=9 trauma-exposed individuals and n=13 individuals with PTSD.
| Variables |
Low-stress Controls |
Trauma-exposed |
PTSD |
Statistics | P-value |
|---|---|---|---|---|---|
| M (s.d.) | M (s.d.) | M (s.d.) | |||
| Age | 30.20 (8.94) | 38.67 (14.05) | 34.62 (6.80) | F(2,29)=1.73 | 0.19 |
| CAPS | 0.5 (1.58) | 26.00 (23.54) | 89.62 (18.20) | F(2,29)=84.11 | <0.0001 |
| Number different event types (CAPS) | 3.2 (1.69) | 7.56 (3.36) | 8.85 (1.99) | F(2,29)=16.76 | <0.0001 |
| Traumatic loada | 0.23 (0.09) | 1.18 (0.63) | 1.61 (0.49) | F(2,29)=25.88 | <0.0001 |
| HAM-D score | 2.60 (6.13) | 9.00 (12.16) | 26.62 (10.53) | F(2,29)=18.14 | <0.0001 |
Abbreviations: CAPS, clinical administered PTSD scale); M, mean; s.d., standard deviation.
Analysis of N-glycosylation
To standardize the time of blood collection, blood was always collected at 10:00 a.m. using Coagulation 9 NC Monovettes (Sarstedt, Germany). Blood samples were coded to guarantee blinding of the laboratory staff. In a first step, blood plasma was isolated and quick-frozen at −80 °C at the Laboratory for Molecular Toxicology, University of Konstanz. In a second step, frozen plasma samples were shipped on dry ice to the Department for Molecular Biomedical Research, Ghent University, Belgium for N-glycosylation analysis. N-glycosylation was analyzed in 2 μl of plasma.
Nine N-glycan structures (peak 1—peak 9), which were identified to be most prominent in previous studies,30 and the GlycoAge Test [log10 (peak1/peak6)],33 were measured using a DNA sequencing equipment (3130 Genetic Analyzer; Applied Biosystems, Grand Island, NY, USA), fluorophore-assisted carbohydrate electrophoresis (DSA-FACE).41
Statistical analysis
Statistical analyses were performed using R 2.11.0,42 using an alpha level of 0.05. Group differences in clinical characteristics and in the N-glycosylation profile were analyzed using ANOVAs. Age, use of tobacco, psychotropic medication and gender were included separately as covariates into the model. The Akaike Information Criterion (AIC) was used for evaluating the appropriateness of models,43 with the model without covariates having the lowest AIC. In the case of significant effects, t-tests were calculated for post hoc comparison. Residuals in all models were tested for deviations from the normal distribution. Residuals in the models of the GlycoAge Test, the N-glycan peak 2, the N-glycan peak 8 and the N-glycan peak 9 were not normally distributed; therefore, these variables were re-analyzed using nonparametric statistics (Kruskal–Wallis test; for post hoc comparison Mann–Whitney-U test). Correlations were analyzed using the Kendall Tau rank correlation.
Results
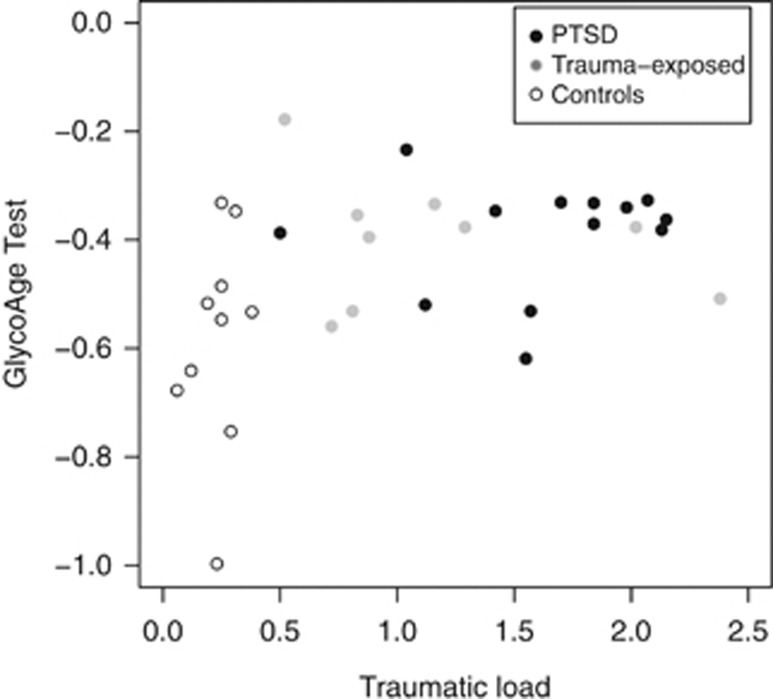
Individuals with PTSD and trauma-exposed individuals presented higher values in the GlycoAge Test compared with low-stress controls (χ2=7.00; P=.03; Table 2). There were significant group differences between the low-stress control group and individuals with PTSD (W=25; P=0.01). Trauma-exposed individuals differed neither significantly from individuals with PTSD (W=70; P=0.47) nor from controls (W=22; P=0.06), but were positioned in-between these two groups. Interestingly, traumatic load was positively correlated with the GlycoAge Test (τ =0.41; P=0.02, Figure 1).
Table 2. N-Glycans in PTSD, trauma-exposed and low-stress control subjects.
| Variables |
Low-stress controls (n=10 ) |
Trauma-exposed (n=9) |
PTSD (n=13) |
Statistics | P-value |
|---|---|---|---|---|---|
| M (s.d.) | M (s.d.) | M(s.d.) | |||
| Peak 1 | 6.46 (2.72) | 8.28 (2.35) | 8.48 (2.03) | F(2,29)=2.36 | 0.11 |
| Peak 2 | 1.04 (0.51) | 1.35 (0.34) | 1.41 (0.44) | χ2=6.04 | 0.05 |
| Peak 3 | 6.99 (1.78) | 7.17 (1.88) | 7.69 (2.20) | F(2,29)=0.28 | 0.69 |
| Peak 4 | 6.19 (1.43) | 6.41 (1.31) | 6.13 (1.42) | F(2,29)=0.11 | 0.89 |
| Peak 5 | 40.31 (5.24) | 40.80 (5.37) | 40.35 (4.72) | F(2,29)=0.03 | 0.97 |
| Peak 6 | 22.77 (1.78) | 20.30 (2.87) | 20.49 (3.35) | F(2,29)=2.44 | 0.11 |
| Peak 7 | 6.39 (1.58) | 5.63 (1.26) | 4.91 (0.73) | F(2,29)=4.35 | 0.02 |
| Peak 8 | 6.91 (1.75) | 6.34 (1.96) | 6.91 (1.75) | χ2=0.52 | 0.77 |
| Peak 9 | 1.77 (0.70) | 2.48 (1.11) | 1.97 (1.25) | χ2=2.47 | 0.29 |
| GlycoAge Test | −0.58 (0.20) | −0.40 (0.12) | −0.39 (0.10) | χ2=7.00 | 0.03 |
Abbreviations: M, mean; s.d., standard deviation; χ2, Kruskal–Wallis test. Bold P-values indicate significance on an alpha level of 0.05 (two-sided).
Figure 1.
Scatterplot between the GlycoAge Test and Traumatic load in n=10 low-stress controls, n=9 trauma-exposed and n=13 PTSD subjects. Higher values in the GlycoAge Test were positively correlated with Traumatic load.
With respect to a possible effect of gender, we included gender as an additional factor into the model and results did not change. Further, there was no significant main effect of gender F(1,28)=0.24; P=0.63 and no significant interaction between group × gender F(2,26)=2.05; P=.15 in the GlycoAge Test.
Individuals with PTSD with psychotropic medication (n=7; median=−0.33; range=−0.53–−0.23) did not differ significantly in the GlycoAge Test (W=22.0; P=0.41) from individuals with PTSD without medication (n=6; median=−0.51; range=−0.62 to −0.34).
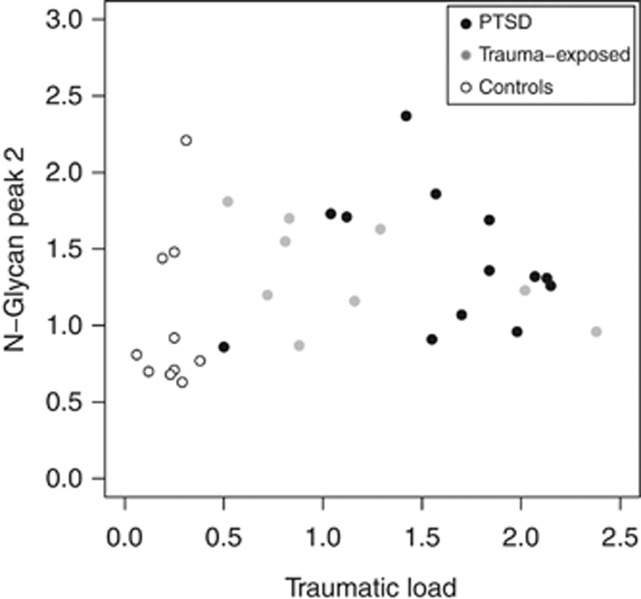
In addition to the effect on GlycoAge Test, also in the N-glycan peak 2 individuals with PTSD presented significantly higher values (χ2=6.04; P=0.05). In post hoc tests, individuals with PTSD (W=30; P=0.03) and trauma-exposed individuals (W=20; P=0.04) differed significantly from low-stress controls. There were no significant differences between PTSD and trauma-exposed individuals (W=65.5; P=0.66; Table 2 and Figure 2).
Figure 2.
Scatterplots in the N-glycan peak 2 from n=10 low-stress controls, n=9 trauma-exposed and n=13 PTSD subjects. Individuals with PTSD demonstrated significant higher values in the N-glycan peak 2, compared with low-stress controls. Trauma-exposed individuals were positioned between PTSD and the low-stress control group.
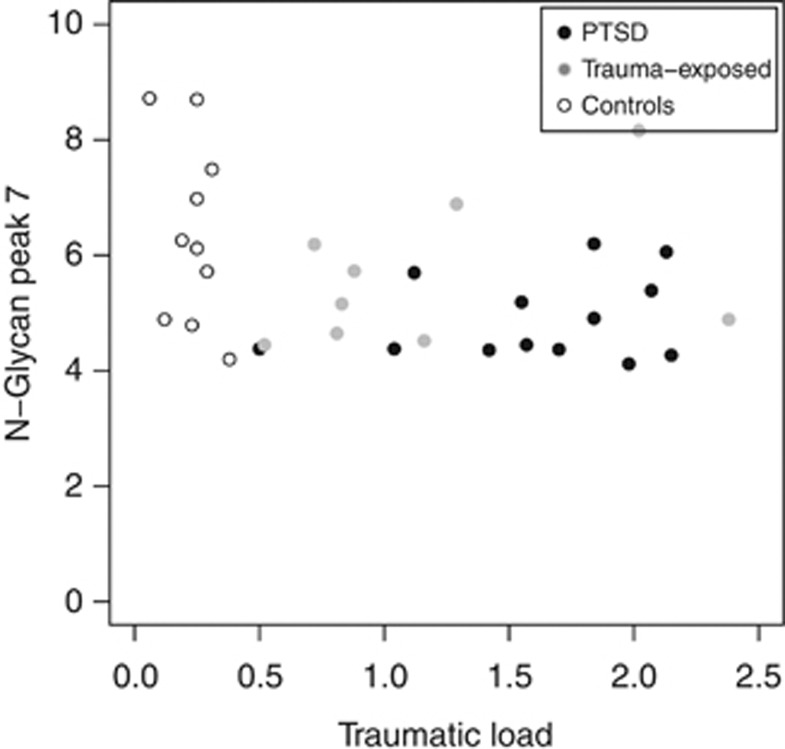
Further, individuals with PTSD, surprisingly, presented reduced values in N-glycan peak 7 (F(2,29)=4.35; P=0.02). Individuals with PTSD differed significantly from the low-stress control group (t(11.9)=2.75; P=0.02), and trauma-exposed individuals were positioned in-between, but did not differ significantly from the PTSD or the low-stress control group (Table 2 and Figure 3).
Figure 3.
Scatterplots in the N-glycan peak 7 from n=10 low-stress controls, n=9 trauma-exposed and n=13 PTSD subjects. Individuals with PTSD demonstrated significant lower values in the N-glycan peak 7, compared with low-stress controls. Trauma-exposed individuals were positioned between PTSD and the low-stress control group.
However, as we did not have a priori hypotheses for the N-glycans peak 2 and peak 7, but rather made exploratory analyses, we corrected these results for multiple comparisons. After a stepwise Holm correction,44 group differences in the N-glycan peak 2 and in the N-glycan peak 7 did not remain significant.
The N-glycosylation of peaks 1, 3, 4, 5, 6, 8 and 9 did not differ significantly between individuals with PTSD and low- or high-stress controls (Table 2).
Discussion
Individuals with PTSD and trauma-exposed individuals showed increased values in the GlycoAge Test, which is a biomarker for physiological aging.33 In the general population, values in the GlycoAge Test are stable until 40 years of age and then increase continuously, with the highest values being observed at the age of 90–99 years.33 The GlycoAge Test profile in individuals with PTSD and trauma-exposed individuals with a mean age of 35 years is similar to the GlycoAge Test profile in people with a mean age of about 50 years, in a Belgian population.33 This shift in N-glycosylation strongly indicates that traumatic stress may accelerate the process of physiological aging.
The N-glycosylation profile depends not only on age, but also on sex.44 However, we did not find a significant influence of gender or group × gender in the GlycoAge Test. Reasons may be that our sample included also relatively young people in the age between 15 and 30 years old, who were not present in the study of Ding et al.,45 as well as of course traumatized participants, which was not the focus of Ding et al.45 Further, as our sample size was limited and men and women were not equally distributed between groups, further research is needed to focus on gender specific alterations in individuals with PTSD.
Given that there are interactive effects between stress, age and immune functions,46 it is noteworthy that the N-glycosylation profile in older people is associated with a state of IgG-mediated low-grade inflammation.47 Low-grade inflammation and changes in the immunoglobulin regulation, in turn, have been reported in individuals with PTSD as well.48 Alterations in N-glycosylation may therefore not only be a marker of physiological aging but might actually contribute to the pathogenesis of aging itself47 via inflammation processes, which can be caused by traumatic stress.49
Further, individuals with PTSD presented higher values in the N-glycan peak 2, although these results did not remain significant after statistical corrections. The N-glycans peak 1 and peak 2 are reported to increase gradually with age, whereas the N-glycan peak 6 is reported to decrease with age.32 We see the same picture in individuals with PTSD, even though group differences in the N-glycan peak 1 and peak 6 were not statistically significant (Table 2).
Interestingly, we found a reduction in the N-glycan peak 7 in individuals with PTSD, although this effect did not withstand statistical corrections. However, we report this effect because this finding might be interesting for future studies. To the best of our knowledge, there has been only one study on the N-glycan peak 7 so far, which was found to be decreased in patients with hepatocellular carcinoma compared with liver cirrhosis patients, with a negative correlation between the N-glycan peak 7 and tumor development.30 At the present state of knowledge, it is difficult to pinpoint a mechanistic link that could explain the concordant N-glycan peak 7 effect in these two quite diverse disease conditions.
Limitation of the study: there was no possibility to control for unfavorable environments (unhealthy food, poor medication, etc.), which might influence the effect of accelerated aging. However, interestingly, traumatic load was positively correlated with the GlycoAge Test (τ=0.41; P=0.02, Figure 1). This argues against the hypothesis that exposure to unfavorable living conditions is a main driver of this effect; on the other hand, trauma exposure in itself is an unfavorable environment. Thus, the influence cannot be controlled for.
In conclusion, we present evidence that traumatic stress leads to an accelerated process of physiological aging, as revealed by a shift in the N-glycosylation profile that is typical of persons with higher age, which is possibly mediated by a state of low-grade inflammation.
Acknowledgments
We thank the German Research Foundation (DFG; grant number Ko 3895/1), the European Refugee Fund, UGent, FWO and Markage for financial support. We thank Heike Riedke for coordination and medical assistance, and Sylviane Dewaele, Monika Schulz and Judy Salzwedel for technical assistance. This research was conducted at the University of Konstanz. Iris-Tatjana Kolassa is now at the University of Ulm.
The authors declare no conflict of interest.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) American Psychiatric Association: Washington, DC, USA; 2000. [Google Scholar]
- Neuner F, Schauer M, Karunakara U, Klaschik C, Robert C, Elbert T. Psychological trauma and evidence for enhanced vulnerability for posttraumatic stress disorder through previous trauma among West Nile refugees. BMC Psychiatry. 2004;4:34. doi: 10.1186/1471-244X-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I-T, Kolassa S, Ertl V, Papassotiropoulos A, De Quervain DJ-F. The risk of posttraumatic stress disorder after trauma depends on traumatic load and the catechol-o-methyltransferase Val(158)Met polymorphism. Biol Psychiatry. 2010;67:304–308. doi: 10.1016/j.biopsych.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Kolassa I-T, Elbert T. Structural and functional neuroplasticity in relation to traumatic Stress. Curr Dir Psychol Sci. 2007;16:321–325. [Google Scholar]
- Boscarino J. Posttraumatic Stress Disorder and Physical Illness: Results from Clinical and Epidemiologic Studies. Ann N Y Acad Sci. 2004;1032:141–153. doi: 10.1196/annals.1314.011. [DOI] [PubMed] [Google Scholar]
- Brown DW, Anda RF, Felitti VJ, Edwards VJ, Malarcher AM, Croft JB, et al. Adverse childhood experiences are associated with the risk of lung cancer: a prospective cohort study. BMC Public Health. 2010;10:20. doi: 10.1186/1471-2458-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaesmer H, Brähler E, Gündel H, Riedel-Heller SG. The association of traumatic experiences and posttraumatic stress disorder with physical morbidity in old age: A German population-based study. Psychosom Med. 2011;73:401–406. doi: 10.1097/PSY.0b013e31821b47e8. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Johnson SL, Andrige RR, Yang EV, Di Gregoria MP, et al. Basal cell carcinoma: stressful life events and the tumor environment. Arch Gen Psychiatry. 2012;69:618–626. doi: 10.1001/archgenpsychiatry.2011.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging. Hormones. 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Sareen J, Cox BJ, Stein MB, Afifi TO, Fleet C, Asmundson GJG. Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med. 2007;69:242–248. doi: 10.1097/PSY.0b013e31803146d8. [DOI] [PubMed] [Google Scholar]
- Boscarino Ja, Forsberg CW, Goldberg J. A twin study of the association between PTSD symptoms and rheumatoid arthritis. Psychosomatic Medicine. 2010;72:481–486. doi: 10.1097/PSY.0b013e3181d9a80c. [DOI] [PubMed] [Google Scholar]
- Solomon Z, Helvitz H, Zerach G. Subjective age, PTSD and physical health among war veterans. Aging Ment Health. 2009;13:405–413. doi: 10.1080/13607860802459856. [DOI] [PubMed] [Google Scholar]
- Solomon Z, Ohry A.The toll of war captivity: vulnerability, resilience, and premature agingIn: Martz E (ed).Rehabilitation After War and Conflict: Community and Individual Perspectives Springer: New York, NY, USA; 2010361–388. [Google Scholar]
- Fagnoni FF, Vescovini R, Passeri G, Bologna G, Pedrazzoni M, Lavagetto G, et al. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- Sommershof A, Aichinger H, Engler H, Adenauer H, Catani C, Boneberg E-M, et al. Substantial reduction of naïve and regulatory T cells following traumatic stress. Brain Behav Immun. 2009;23:1117–1124. doi: 10.1016/j.bbi.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Miller R. Aging and immune function: cellular and biochemical analyses. Exp Gerontol. 1994;29:21–35. doi: 10.1016/0531-5565(94)90060-4. [DOI] [PubMed] [Google Scholar]
- Effros RB. Human Genetics ' 98: aging and senescence replicative senescence in the immune system: impact of the Hayflick limit on T-cell function in the elderly. Am J Hum Genet. 1998;62:1003–1007. doi: 10.1086/301845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Ann Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Gill J, Page GG. Low cortisol, high DHEA, and high levels of stimulated TNF- α, and IL-6 in women with PTSD. J Trauma Stress. 2008;21:530–539. doi: 10.1002/jts.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gola H, Engler H, Sommershof A, Adenauer H, Kolassa S, Schedlowski M, et al. Posttraumatic stress disorder is associated with an enhanced spontaneous production of pro-inflammatory cytokines by peripheral blood mononuclear cells. BMC Psychiatry. 2013;13:40. doi: 10.1186/1471-244X-13-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J, Chae JS, Kang R, Kwon N, Lee SH, Lee JH. Effect of age on atherogenicity of LDL and inflammatory markers in healthy women. Nutr Metab Cardiovasc Dis. 2012;12:191–193. doi: 10.1016/j.numecd.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Völzke H, Wallaschofski H, John U, Freyberger HJ, et al. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J Psych Res. 2010;44:15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Humphreys V, Martin RM, Ratcliffe B, Duthie S, Wood S, Gunnell D, et al. Age-related increases in DNA repair and antioxidant protection: a comparison of the Boyd Orr Cohort of elderly subjects with a younger population sample. Age Ageing. 2007;36:521–526. doi: 10.1093/ageing/afm107. [DOI] [PubMed] [Google Scholar]
- Morath J, Moreno-Villanueva M, Hamuni G, Kolassa S, Ruf-Leuschner M, Schauer M, et al. Effects of psychotherapy on DNA strand break accumulation originating from traumatic stress. A randomized controlled trial. Submitted. [DOI] [PubMed]
- Harris G, Holmes A, Sabovljev SA, Cramp WA, Hedges M, Hornsey S, et al. Sensitivity to X-irradiation of peripheral blood lymphocytes from ageing donors. Int J Radiat Biol. 1986;50:685–694. doi: 10.1080/09553008614551091. [DOI] [PubMed] [Google Scholar]
- Frenck RW, Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci USA. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LH, Kao H-T, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: an overview. Biol Psychiatry. 2012;73:15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, Maguen S, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70:465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, Esko J, Freeze H, Stanley P, Bertozzi C, Hart G, et al. Essentials of GlycoBiology2nd edn.Cold Spring Harbor Laboratory Press: New York, USA; 2009 [PubMed] [Google Scholar]
- Liu X-E, Desmyter L, Gao C-F, Laroy W, Dewaele S, Vanhooren V, et al. N-glycomic changes in hepatocellular carcinoma patients with liver cirrhosis induced by hepatitis B virus. Hepatology. 2007;46:1426–1435. doi: 10.1002/hep.21855. [DOI] [PubMed] [Google Scholar]
- Knezevic A, Gornik O, Polasek O, Pucic M, Redzic I, Novokmet M, et al. Effects of aging, body mass index, plasma lipid profiles, and smoking on human plasma N-glycans. Glycobiology. 2010;20:959–969. doi: 10.1093/glycob/cwq051. [DOI] [PubMed] [Google Scholar]
- Vanhooren V, Desmyter L, Liu X-E, Cardelli M, Franceschi C, Federico A, et al. N-glycomic changes in serum proteins during human aging. Rejuvenation Res. 2007;10:521–531. doi: 10.1089/rej.2007.0556. [DOI] [PubMed] [Google Scholar]
- Vanhooren V, Dewaele S, Libert C, Engelborghs S, De Deyn PP, Toussaint O, et al. Serum N-glycan profile shift during human ageing. Exp Gerontol. 2010;45:738–743. doi: 10.1016/j.exger.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J DNA Damage, Aging, and Cancer. New Eng J Med. 2009;361:1475–1485. doi: 10.1056/NEJMra0804615. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrios G, Bulbena A.The Hamilton depression scale and the numerical description of the symptoms of depressionIn Bech P, Coppen A (eds).The Hamilton Scales Springer: Heidelberg, Germany; 199080–92. [DOI] [PubMed] [Google Scholar]
- Schauer M, Neuner F, Elbert T. Vivo Event Checklist for War, Detention, and Torture Experiences. In: Narrative Exposure Therapy (net). A Short-term intervention for traumatic stress disorders. Hogrefe & Huber Publishers: Cambridge/Göttingen, UK; 2011. pp. 77–79. [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, et al. The development of a clinician-administered PTSD scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson C. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V, Lecrubier Y, Sheehan K.H, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Vanhooren V, Laroy W, Libert C, Chen C. N-glycan profiling in the study of human aging. Biogerontology. 2008;9:351–356. doi: 10.1007/s10522-008-9140-z. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria; 2010. [Google Scholar]
- Burnham KP, Anderson DR. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach. Springer: New York, USA; 2002. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian J Stat. 1979;6:65–70. [Google Scholar]
- Ding N, Nie H, Sun X, Sun W, Qu Y, Liu X, et al. Human serum N-glycan profiles are age and sex dependent. Age Ageing. 2011;40:568–575. doi: 10.1093/ageing/afr084. [DOI] [PubMed] [Google Scholar]
- Graham JE, Christian LM, Kiecolt-Glaser JK. Stress, age, and immune function: toward a lifespan approach. J Behav Med. 2006;29:389–400. doi: 10.1007/s10865-006-9057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'olio F, Vanhooren V, Chen CC, Slagboom PE, Wuhrer M, Franceschi C. N-glycomic biomarkers of biological aging and longevity: A link with inflammaging. Ageing Res Rev. 2012;12:685–698. doi: 10.1016/j.arr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, Page G. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45:262–277. doi: 10.1111/j.1744-6163.2009.00229.x. [DOI] [PubMed] [Google Scholar]
- O'Donovan A, Neylan TC, Metzler T, Cohen BE. Lifetime exposure to traumatic psychological stress is associated with elevated inflammation in the Heart and Soul Study. Brain Behav Immun. 2012;26:642–649. doi: 10.1016/j.bbi.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]