Abstract
Although recent studies have shown that immunological processes play an important role in the pathophysiology of mood disorders, immune activation may only be present in specific subgroups of patients. Our study aimed to examine whether immune activation was associated with (a) the presence of manic symptoms and (b) the onset of manic symptoms during 2 years of follow-up in depressed patients. Patients with a depressive disorder at baseline (N=957) and healthy controls (N=430) were selected from the Netherlands Study of Depression and Anxiety. Assessments included lifetime manic symptoms at baseline and two-year follow up, as well as C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) at baseline. Within depressed patients, immune activation was not related to the presence or absence of lifetime manic symptoms at baseline. However, CRP levels were strongly elevated in depressed men who developed manic symptoms compared with those who did not develop manic symptoms over 2 years (P<0.001, Cohen's d=0.89). IL-6 and TNF-α were also higher in depressed men with an onset of manic symptoms, but this association was not significant. However, we found that the onset of manic symptoms was particularly high in men with multiple elevated levels of inflammatory markers. Depressed men who developed manic symptoms during follow-up had increased immunological activity (especially CRP) compared with depressed men who did not develop manic symptoms. Further research should explore whether a treatment approach focusing on inflammatory processes may be more effective in this specific subgroup of depressed patients.
Keywords: C-reactive protein, depression, immune activation, interleukin-6, manic symptoms, tumor necrosis factor alpha
Introduction
The underlying pathophysiology of mood disorders, such as unipolar and bipolar depression, is receiving growing interest from researchers. The ‘monocyte-T-cell theory of mood disorders',1, 2 for example, considers activation of the immune response system as the driving force behind mood disorders. This theory implies that there is a disbalance in immune regulatory processes, resulting in a more pro-inflammatory state. As pro-inflammatory cytokines are capable of destabilizing brain function,3 the brain may become more vulnerable to stress, causing the onset of mood disorders. Pro-inflammatory cytokines can act as neuromodulators affecting both the neuroendocrine and neurochemical pathways, thereby causing mood symptoms.4
Two recent meta-analyses have reported that unipolar depressed patients showed increased levels of inflammatory markers (for example, C-reactive protein (CRP), interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α)), compared with healthy controls.5, 6 However, these pooled studies showed heterogeneous results, with most studies reporting higher levels of proinflammatory cytokines,7, 8, 9 but with others presenting normal10, 11 or even lower12 levels in unipolar depressed patients vs healthy controls. Although these contradicting findings may partly reflect methodological differences across studies (for example, type of assessment,6 small sample sizes6 or selection of covariates6, 13, 14, 15, 16, 17, 18), it has also been hypothesized that immune activation may only play a role in specific subgroups of unipolar depression.19, 20 For example, Vogelzangs et al.19 reported that men, but not women, with a current depressive disorder showed significantly increased levels of CRP compared with healthy controls, especially in those with late-onset disorders. Moreover, a recent meta-analysis found that inflammation activation appeared to be more often present in patients with atypical depression features.20
As previous studies have found strong associations between immune activation and bipolar disorder,21, 22, 23 it may also be important to consider the impact of (subthreshold) manic symptoms on the association between immune activation and depressive disorder. Although the prevalence of manic symptoms is around 40% in patients with unipolar depression,24, 25 and, in addition, 20.6% of unipolar depressed patients are likely to develop manic symptoms during their course of depression,26 no previous study has examined the association between immune activation and manic symptoms in depressed patients. Furthermore, in the recent DSM-5, the ‘mixed episode' in major depression is replaced with a ‘mixed features' specifier for manic, hypomanic and major depressive episode.27 Clinical studies have shown that depressed patients with manic symptoms are less likely to respond to regular treatment in unipolar depression (for example, use of antidepressants and psychotherapy),28, 29 and, in addition, evidence suggests that increased inflammatory cytokines prior to treatment may predict non-response.30, 31 As both non-response and the onset of manic symptoms are associated with a more severe and chronic course of depression, it could be hypothesized that high levels of cytokines not only underlie non-response but also predict the onset of manic symptoms in depressed patients. Taken together, these findings suggest that the role of inflammatory markers may be more pronounced in depressed patients with manic symptoms than in patients without these symptoms.
Therefore, the present study aimed to examine whether immune activation was associated with (a) the presence of manic symptoms and (b) the onset of manic symptoms during 2 years of follow-up in patients with a depressive disorder. To avoid the limitations of previous studies, this study included a large sample of currently depressed patients (N=939) in whom manic symptoms were thoroughly assessed, as well as healthy controls (N=430). Because previous research has found sex differences in the association between inflammatory markers and depressive disorders,6, 19 the results for men and women were shown separately. In addition, we were able to explore the role of potential confounders, such as sociodemographics, childhood trauma,32 lifestyle factors and disease-related factors. This is important, as these factors are related to inflammatory markers13, 14, 15, 16, 17, 33, 34 as well as depression with and without manic symptoms.18, 35, 36 Furthermore, we have explored the additive effect of the different inflammatory markers, which, to our knowledge, no other study has done before.
Subjects and methods
Sample and measures for depression and manic symptoms
Data were derived from the Netherlands Study of Depression and Anxiety (NESDA), an ongoing cohort study (N=2981, age 18–65 years) including 2329 persons with a lifetime diagnosis of a depressive and/or anxiety disorder, as well as 652 healthy controls at the baseline assessment. Participants were recruited from the community (19%), general practice (54%) and secondary mental health care (27%). Patients with a primary clinical diagnosis of bipolar disorder, obsessive compulsive disorder, severe substance use disorder and psychotic disorder were excluded from the study. The research protocol was approved by the ethical committee of the participating universities and all the participants provided written informed consent. For a detailed description about the NESDA study, see Penninx et al.37
For the present study, we used data from the baseline (cross-sectional analyses) as well as 2-year follow-up (prospective analyses) assessment (overall response rate: 87.1% see Lamers et al.38 also for determinants of non-response). During the baseline interview, the presence of depressive (major depressive disorder, dysthymia) and anxiety disorders (social phobia, generalized anxiety disorder, panic disorder and agoraphobia) was established using the Composite International Diagnostic Interview (CIDI) according to DSM-IV criteria.39 The CIDI is a highly reliable instrument with high inter-rater reliability rates.40 The severity of depression was measured by the 28-item self-report Inventory of Depressive symptoms (IDS).41 To detect the presence of lifetime manic symptoms, the Mood Disorder Questionnaire (MDQ)42 was used at baseline as well as 2-year follow-up. The MDQ is a 15-item self-report questionnaire comprising 13 dichotomous items on the lifetime presence or absence of manic symptoms as well as two additional questions regarding the clustering of symptoms in time and severity of related problems. We considered manic symptoms to be present when a patient reported seven or more positive answers from the thirteen items, irrespective of the answers on the two additional questions. Our group recently showed that this strategy is adequate in detecting a recent (hypo)manic episode (sensitivity=0.83 and specificity=0.82).43
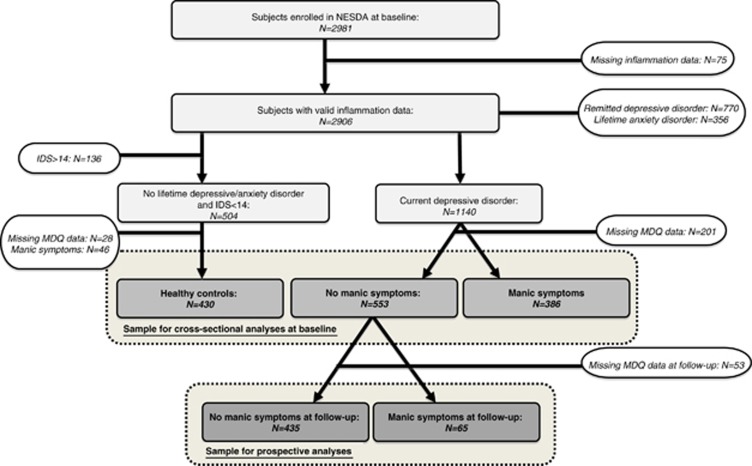
Figure 1 shows the flow chart presenting the procedures to select participants for our cross-sectional and prospective analyses. For the cross-sectional analyses (based on the baseline assessment), the following groups were distinguished: (a) healthy controls (reference group I: N=430) without any lifetime depressive or anxiety disorder, without depressive symptoms (IDS score lower than 14) and without manic symptoms (MDQ score lower than 7); (b) patients with a current (<6 months) depressive disorder, but without lifetime manic symptoms (reference group II: N=533); and (c) patients with a current (<6 months) depressive disorder and lifetime manic symptoms(N=368). For the prospective analyses we considered the absence (reference group: N=435) or presence (N=65) of manic symptoms at the 2-year follow-up assessment in patients with a current depressive disorder and without lifetime manic symptoms at baseline.
Figure 1.
Flow-chart for the cross-sectional and prospective analyses.
Inflammatory markers
Inflammatory markers were evaluated at the baseline measurement and included CRP, IL-6 and TNF-α. Fasting blood samples of the participants were obtained at approximately 0800 hours and kept frozen at −80 °C. CRP and IL-6 were assayed at the Clinical Chemistry department of the VU University Medical Center. High-sensitivity CRP plasma levels were measured in duplicate by an in-house ELISA based on purified protein and polyclonal anti-CRP antibodies (Dako, Glostrup, Denmark). Intra- and inter-assay coefficients of variation were 5% and 10%, respectively. Plasma IL-6 levels were measured in duplicate by a high-sensitivity enzyme-linked immunosorbent assay (PeliKine Compact ELISA, Sanquin, Amsterdam, The Netherlands). Intra- and inter-assay coefficients of variation were 8% and 12%, respectively. Plasma TNF-α levels were assayed in duplicate at Good Biomarker Science (Leiden, The Netherlands), using a high-sensitivity solid-phase ELISA (Quantikine HS Human TNF-α Immunoassay, R&D Systems, Minneapolis, MN, USA). Intra- and inter-assay coefficients of variation were 10% and 15%, respectively.
Covariates
On the basis of previously reported associations with depression, manic symptoms and inflammatory markers, sociodemographics,33, 34, 35, 36 childhood trauma,32 lifestyle factors,6, 13, 14, 15 and disease-related characteristics6, 16, 17, 18 were selected as covariates. Sociodemographics included age and years of education. Lifestyle factors included smoking status (never, former or current, assessed by self-report), alcohol intake (no (<1 drink per week), moderate (women: 1–14 drinks, men: 1–21 drinks per week) or heavy (women>14 drinks, men>21 drinks per week) alcohol intake). Body mass index (BMI; weight in kilograms divided by height in meters squared) and physical activity measured with the International Physical Activity Questionnaire44 in MET-minutes (metabolic equivalent; total effort expended in different activities over a week). Childhood trauma was assessed by the Childhood Trauma NEMESIS Questionnaire, which shows a high similarity with the Childhood Trauma Interview, which is a reliable and valid method for brief assessment of multiple dimensions of childhood interpersonal trauma.45 Then a cumulative index was calculated as the sum of experienced number and frequency of childhood trauma for each participant (range 0–8). Disease-related characteristics included the presence of cardiovascular disease (assessed by self-report and appropriate medication use, based on an article by Vogelzangs et al.46) and the presence of diabetes (fasting plasma glucose level ⩾7.0 mmol l−1 or use of antidiabetic medication (A10)). Medication use was classified according to World Health Organization Anatomical Therapeutic Chemical classification.47 Furthermore, self-reported chronic diseases for which persons were receiving treatment were assessed, including lung disease, osteoarthritis or rheumatic disease, cancer, ulcer, intestinal problems, liver disease, epilepsy and thyroid gland disease. Medication included use of statins (C10AA, C10B), anti-inflammatory medication (M01A, M01B, A07EB and A07EC) and antidepressants (selective serotonin reuptake inhibitors (N06AB), serotonin-norepinephrine reuptake inhibitors (N06AX16 and N06AX21), tricyclic (N06AA) and tetracyclic antidepressants (N06AX03, N06AX05 and N06AX11)).
Statistical analyses
All data were analyzed with SPSS version 20.0 (SPSS, Chicago, IL, USA). Baseline characteristics for men and women were compared using Pearson's chi-square tests for dichotomous and categorical variables, independent T-tests for continuous variables. For subsequent analyses, CRP, IL-6 and TNF-α were ln-transformed to normalize distributions and presented back-transformed. The results are shown for men and women separately, as previous analyses on the same data set19 demonstrated that only depressed men, but not depressed women, had elevated inflammatory markers compared with healthy controls. To justify sex stratification in our analyses on manic symptoms, we have also tested sex interactions by including a sex by manic symptoms interaction term.
To examine whether inflammatory markers (CRP, IL-6 and TNF-α) differed for depressed patients with manic symptoms compared with healthy controls (reference group I) and depressed patients without manic symptoms (reference group II), analyses of (co)variance were used. The same analyses were used to determine whether inflammatory markers were associated with the onset of manic symptoms during 2 years of follow-up. Results of unadjusted analyses as well as analyses adjusted for sociodemographics (basic adjustment) and additionally adjusted for childhood trauma, lifestyle and disease-related factors (full adjustment) were presented. As a set of sensitivity analyses, analyses were also adjusted for use of antidepressants. Finally we investigated the additional effect of the different inflammatory markers on the association with manic symptoms by dividing our patients into four groups (with 0, 1, 2 or all 3 inflammatory markers in the highest quartile) and comparing the percentage of manic symptoms between these groups.
Results
Table 1 shows the baseline characteristics across healthy controls, depressed patients without lifetime manic symptoms and depressed patients with lifetime manic symptoms in men (N=476) and women (N=893) separately. In both men and women, significant differences were found for education level, childhood trauma, smoking status, alcohol use, number of chronic diseases and use of antidepressants. Differences in anti-inflammatory medication were only significant for men, whereas those in cardiovascular disease were significant only in women. Pearson's correlations between inflammatory markers were modest, likely reflecting only partial biological overlap, and were generally higher in men (CRP–IL-6: r=0.33; CRP–TNF-α: r=0.12; IL-6–TNF-α: r=0.11) than in women (CRP–IL-6: r=0.005; CRP–TNF-α: r=0.02; IL-6–TNF-α: r=0.04).
Table 1. Baseline characteristics for men and women separately.
|
Men (n=476) |
Women (n=893) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Healthy controls (n=167) | Depressive disorder without lifetime manic symptoms (n=163) | Depressive disorder with lifetime manic symptoms (n=146) | Pa | Healthy controls (n=263) | Depressive disorder without lifetime manic symptoms (n=390) | Depressive disorder with lifetime manic symptoms (n=240) | Pa | |
| Sociodemographics | ||||||||
| Age (years), mean (s.d.) | 42.9 (15.2) | 44.5 (10.8) | 43.6 (11.6) | 0.50 | 40.5 (14.3) | 41.2 (12.5) | 39.8 (12.2) | 0.43 |
| Education (years), mean (s.d.) | 13.2 (3.3) | 11.9 (3.2) | 11.4 (3.0) | <0.001 | 13.1 (3.0) | 12.1 (3.4) | 11.8 (3.2) | <0.001 |
| Childhood trauma index, mean (s.d.) | 0.4 (1.3) | 1.4 (1.9) | 2.2 (2.2) | <0.001 | 0.5 (1.1) | 2.1 (2.3) | 2.8 (2.5) | <0.001 |
| Lifestyle factors | ||||||||
| Smoking status | 0.004 | <0.001 | ||||||
| Never, n (%) | 57 (34.1) | 37 (22.7) | 36 (24.7) | 118 (44.9) | 134 (34.4) | 55 (22.9) | ||
| Former, n (%) | 65 (38.9) | 59 (36.2) | 42 (25.3) | 89 (33.8) | 118 (30.3) | 62 (25.8) | ||
| Current, n (%) | 45 (26.9) | 67 (41.1) | 68 (46.6) | 56 (21.3) | 138 (35.4) | 123 (51.2) | ||
| Alcohol intake | 0.009 | <0.001 | ||||||
| No alcohol use, n (%) | 13 (7.8) | 24 (14.7) | 28 (19.2) | 33 (3.7) | 106 (27.2) | 43 (17.9) | ||
| Moderate alcohol use, n (%) | 141 (84.4) | 124 (76.1) | 98 (67.1) | 197 (74.9) | 245 (62.8) | 158 (65.8) | ||
| Heavy alcohol use, n (%) | 13 (7.8) | 15 (9.2) | 20 (13.7) | 33 (12.5) | 39 (10.0) | 39 (16.2) | ||
| Body Mass Index, mean (s.d.) | 25.5 (4.3) | 26.4 (4.6) | 26.2 (4.5) | 0.15 | 24.8 (4.8) | 25.6 (5.7) | 25.2 (5.6) | 0.16 |
| Physical activity (MET-min per week), mean (s.d.) | 4128 (3460) | 3533 (3211) | 3475 (3384) | 0.16 | 3723 (2730) | 3502 (3131) | 3417 (2933) | 0.48 |
| Disease-related factors | ||||||||
| Cardiovascular disease, n (%) | 14 (8.4) | 16 (9.8) | 9 (6.2) | 0.50 | 4 (1.5) | 22 (5.6) | 8 (3.3) | 0.02 |
| Diabetes, n (%) | 12 (7.2) | 7 (4.3) | 9 (6.2) | 0.53 | 4 (1.5) | 12 (3.1) | 4 (1.7) | 0.33 |
| Number of other chronic diseases, mean (s.d.) | 0.6 (1.0) | 1.0 (1.1) | 1.1 (1.3) | 0.001 | 0.6 (0.8) | 1.0 (1.1) | 1.0 (1.0) | <0.001 |
| Statin use, n (%) | 17 (10.2) | 27 (16.6) | 12 (8.2) | 0.06 | 8 (3.0) | 17 (4.4) | 12 (5.0) | 0.52 |
| Anti-inflammatory medication use, n (%) | 2 (1.2) | 10 (6.1) | 6 (4.1) | 0.06 | 4 (1.5) | 18 (4.6) | 13 (5.4) | 0.05 |
| Antidepressant use | <0.001 | <0.001 | ||||||
| No antidepressant, n (%) | 167 (100.0) | 94 (57.7) | 91 (62.3) | 263 (100.0) | 207 (53.1) | 144 (60.0) | ||
| SSRI, n (%) | 0 (0.0) | 45 (27.6) | 31 (21.2) | 0 (0.0) | 129 (33.1) | 71 (29.6) | ||
| SNRI, n (%) | 0 (0.0) | 13 (8.0) | 14 (9.6) | 0 (0.0) | 28 (7.2) | 12 (5.0) | ||
| TCA, n (%) | 0 (0.0) | 8 (4.9) | 2 (1.4) | 0 (0.0) | 18 (4.6) | 5 (2.1) | ||
| TeCA, n (%) | 0 (0.0) | 3 (1.8) | 8 (5.5) | 0 (0.0) | 8 (2.1) | 8 (3.3) | ||
Abbreviations: IQR, interquartile range; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant; TeCA, tetracyclic antidepressant.
Based on χ2-test for dichotomous and categorical variables testing the difference between men and women; for inflammatory markers the Mann–Whitney U-test was used.
Table 2 shows the unadjusted and adjusted mean levels of CRP, IL-6 and TNF-α across healthy controls, depressed patients without manic symptoms and depressed patients with manic symptoms at baseline. All results were presented for men and women separately, as sex interactions were significant for CRP (P=0.01) and IL-6 (P=0.01), but not for TNF-α (P=0.88). Levels of CRP and IL-6 were significantly higher in patients with and without manic symptoms compared with controls. These associations remained significant after basic adjustment, but not after adjustment for childhood trauma, lifestyle and disease-related factors. In addition, no significant differences were found for depressed patients with vs without manic symptoms in the unadjusted or adjusted analyses. Baseline depression with or without manic symptoms was not related to TNF-α in men or any of the inflammatory markers in women.
Table 2. Cross-sectional associations between inflammation and psychopathology at baseline (N=1369).
|
Patients with depressive disorder (N=939) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Healthy controls (N=430) |
No lifetime manic symptoms at baseline (N=553) |
Lifetime manic symptoms at baseline (N=386) |
|||||||
| Mean | (s.e.) | Mean | (s.e.) | Pa | Mean | (s.e.) | Pa | Pb | |
| CRP (mg l−1)c | Reference group I | Reference group II | |||||||
| Men | N=167 | N=163 | N=146 | ||||||
| Unadjusted | 0.80 | (1.08) | 1.33 | (1.11) | 0.001 | 1.29 | (1.10) | 0.002 | 1.00 |
| Basic adjustmentd | 0.88 | (1.09) | 1.27 | (1.09) | 0.004 | 1.22 | (1.10) | 0.01 | 0.77 |
| Full adjustmente | 1.02 | (1.09) | 1.21 | (1.08) | 0.15 | 1.10 | (1.09) | 0.54 | 0.43 |
| Women | N=263 | N=390 | N=240 | ||||||
| Unadjusted | 1.28 | (1.07) | 1.37 | (1.07) | 1.00 | 1.31 | (1.08) | 1.00 | 1.00 |
| Basic adjustmentd | 1.36 | (1.08) | 1.35 | (1.06) | 0.97 | 1.26 | (1.08) | 0.52 | 0.50 |
| Full adjustmente | 1.39 | (1.08) | 1.33 | (1.06) | 0.68 | 1.26 | (1.08) | 0.39 | 0.55 |
| IL-6 (pg ml−1)c | |||||||||
| Men | N=167 | N=163 | N=146 | ||||||
| Unadjusted | 0.66 | (1.07) | 0.88 | (1.08) | 0.01 | 0.94 | (1.06) | 0.002 | 1.00 |
| Basic adjustmentd | 0.79 | (1.07) | 0.85 | (1.07) | 0.04 | 0.91 | (1.07) | 0.009 | 0.54 |
| Full adjustmente | 0.75 | (1.07) | 0.83 | (1.07) | 0.33 | 0.86 | (1.07) | 0.19 | 0.66 |
| Women | N=263 | N=390 | N=240 | ||||||
| Unadjusted | 0.74 | (1.06) | 0.78 | (1.05) | 1.00 | 0.70 | (1.07) | 1.00 | 0.66 |
| Basic adjustmentd | 0.76 | (1.06) | 0.77 | (1.05) | 0.95 | 0.70 | (1.06) | 0.29 | 0.22 |
| Full adjustmente | 0.79 | (1.06) | 0.75 | (1.05) | 0.52 | 0.69 | (1.06) | 0.16 | 0.30 |
| TNF-α (pg/ml)c | |||||||||
| Men | N=167 | N=163 | N=146 | ||||||
| Unadjusted | 0.85 | (1.05) | 0.88 | (1.04) | 1.00 | 0.88 | (1.05) | 1.00 | 1.00 |
| Basic adjustmentd | 0.87 | (1.04) | 0.87 | (1.04) | 0.94 | 0.87 | (1.05) | 0.95 | 0.90 |
| Full adjustmente | 0.89 | (1.05) | 0.86 | (1.04) | 0.61 | 0.86 | (1.05) | 0.58 | 0.93 |
| Women | N=263 | N=390 | N=240 | ||||||
| Unadjusted | 0.81 | (1.04) | 0.82 | (1.03) | 1.00 | 0.86 | (1.05) | 0.98 | 1.00 |
| Basic adjustmentd | 0.82 | (1.04) | 0.81 | (1.03) | 0.84 | 0.85 | (1.04) | 0.53 | 0.38 |
| Full adjustmente | 0.83 | (1.04) | 0.80 | (1.03) | 0.53 | 0.86 | (1.05) | 0.64 | 0.23 |
Compared with reference group I (healthy controls).
Compared with reference group II (persons with depressive disorder and without manic symptoms).
To normalize inflammation parameters, CRP, IL-6 and TNF-α were ln-transformed; for interpretation the means and standard errors were back transformed.
Based on analyses of covariance adjusted for age and education.
Based on analyses of covariance additionally adjusted for childhood trauma, smoking status, alcohol intake, body mass index, physical activity, diabetes, cardiovascular disease, number of other chronic diseases, anti-inflammatory drug and statin use.
Then, we examined whether baseline inflammatory markers were associated with the onset of manic symptoms during 2 years of follow-up in a sample of depressed patients without manic symptoms at baseline (see Table 3). Even after full adjustment, baseline CRP levels were significantly higher (Cohen's d=0.85) in depressed men with manic symptoms at follow-up compared with patients with no manic symptoms at follow-up. This association remained significant after adjustment for use of antidepressants at baseline and depression status at follow-up. Depressed men with an onset of manic symptoms had also higher levels of IL-6 and TNF-α compared with depressed men without an onset of manic symptoms, but these associations were not statistically significant (full adjustment, IL-6: P=0.34; TNF-α: P=0.25).
Table 3. Prospective associations between inflammation and the onset of manic symptoms at 2-year follow-up (N=500).
|
Patients with depressive disorder (N=500) |
|||||
|---|---|---|---|---|---|
|
No onset of manic symptoms (N=435) |
Onset of manic symptoms (N=65) |
||||
| Mean | (s.e.) | Mean | (s.e.) | P | |
| CRP (mg l−1)a | Reference group | ||||
| Men | N=128 | N=21 | |||
| Unadjusted | 1.17 | (1.12) | 3.81 | (1.31) | <0.001 |
| Basic adjustmentb | 1.11 | (1.10) | 3.24 | (1.27) | <0.001 |
| Full adjustmentc | 1.21 | (1.10) | 3.06 | (1.28) | 0.001 |
| Women | N=307 | N=44 | |||
| Unadjusted | 1.34 | (1.08) | 1.18 | (1.18) | 1.00 |
| Basic adjustmentb | 1.31 | (1.07) | 1.03 | (1.20) | 0.22 |
| Full adjustmentc | 1.35 | (1.07) | 1.12 | (1.20) | 0.34 |
| IL-6 (pg/ml)a | |||||
| Men | N=128 | N=21 | |||
| Unadjusted | 0.84 | (1.10) | 1.18 | (1.18) | 0.42 |
| Basic adjustmentb | 0.81 | (1.09) | 1.07 | (1.23) | 0.22 |
| Full adjustmentc | 0.85 | (1.09) | 1.08 | (1.25) | 0.35 |
| Women | N=307 | N=44 | |||
| Unadjusted | 0.77 | (1.06) | 0.66 | (1.17) | 0.93 |
| Basic adjustmentb | 0.76 | (1.05) | 0.62 | (1.15) | 0.18 |
| Full adjustmentc | 0.77 | (1.05) | 0.66 | (1.16) | 0.32 |
| TNF-α (pg/ml)a | |||||
| Men | N=128 | N=21 | |||
| Unadjusted | 0.86 | (1.05) | 1.08 | (1.09) | 0.27 |
| Basic adjustmentb | 0.85 | (1.05) | 1.03 | (1.13) | 0.15 |
| Full adjustmentc | 0.87 | (1.05) | 1.01 | (1.13) | 0.24 |
| Women | N=307 | N=44 | |||
| Unadjusted | 0.81 | (1.04) | 0.81 | (1.09) | 1.00 |
| Basic adjustmentb | 0.80 | (1.04) | 0.80 | (1.10) | 0.93 |
| Full adjustmentc | 0.81 | (1.03) | 0.81 | (1.10) | 0.93 |
To normalize inflammation-parameters, CRP, IL-6 and TNF-α were ln-transformed; for interpretation the means and standard errors were back transformed.
Based on analyses of covariance adjusted for age and education.
Based on analyses of covariance additionally adjusted for childhood trauma, smoking status, alcohol intake, body mass index, physical activity, diabetes, cardiovascular disease, number of other chronic diseases, anti-inflammatory drug and statin use.
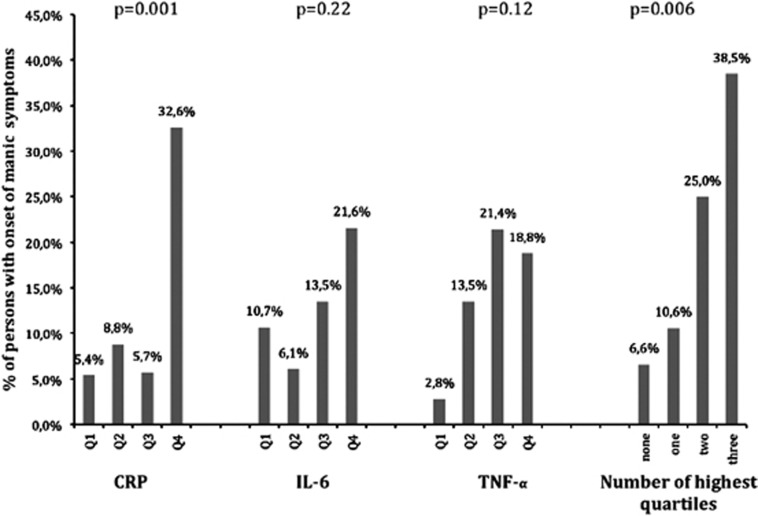
The shapes of the prospective associations were illustrated with graphs plotting the onset of manic symptoms across groups with increasing levels (quartiles) of inflammatory markers (see Figure 2). A non-linear association was found for CRP, as the percentage of patients with an onset of manic symptoms was only increased in patients in the highest quartile of CRP, which was significantly higher than in the three groups with lower levels of CRP (Q4 vs Q1: P=0.002; Q4 vs Q2: P=0.013; Q4 vs Q3: P=0.003). Although the associations of IL-6 and TNF-α were not significant, the onset of manic symptoms was higher in depressed men with higher levels of these markers. This could indicate that these markers, in addition to CRP, may contribute to the onset of manic symptoms. This was also demonstrated by the last graph in Figure 2, showing that the onset of manic symptoms gradually increased with the number of inflammatory markers in the highest quartiles (range: 0–3).
Figure 2.
Shape of relation between inflammatory markers and linear relationship between the number of highest quartiles and onset of manic symptoms in men. Q1–Q4: quartiles of inflammatory markers. P-values in figure are the overall P-values based on χ2-tests.
Discussion
To our knowledge, this study is the first in examining the association between immune activation and (1) the presence and (2) the onset of manic symptoms in a large sample of depressed patients. Our findings showed substantial differences in the cross-sectional inflammatory marker levels across depressed patients with and without manic symptoms vs healthy controls. However, after complete adjustment for covariates, these differences were not significant. In the prospective analyses CRP was a strong predictor of the onset of manic symptoms during 2 years of follow-up in depressed men. IL-6 and TNF-α levels were also higher in depressed men with an onset of manic symptoms, but this association was not significant. When combining these inflammatory markers, we found that the onset of manic symptoms was significantly higher in men with multiple elevated levels of inflammatory markers.
Prospective findings
Our findings showed that increased levels of CRP were a strong risk factor for the onset of manic symptoms in depressed men. This association was independent of the effects of sociodemographics, childhood trauma, lifestyle and disease-related factors. Our findings also suggest that IL-6 and TNF-α may be additional risk factors for the onset of manic symptoms as the development of manic symptoms was particularly increased among those with multiple elevated inflammatory markers. This may indicate that increased levels of inflammatory markers in patients with unipolar depression reflects a biological vulnerability for future conversion to bipolar disorder and may therefore be a risk factor for the onset of manic symptoms in patients with unipolar depression. A recent study supports this theory, as it was shown that offspring of patients with bipolar disorder had a more pronounced pro-inflammatory gene expression signature than offspring of persons without psychopathology.48 Another recent study confirms our findings, since they have found a positive association between hypomanic and manic symptoms and CRP in adolescents.49
Another important finding was that we found a clear statistically significant relationship for CRP with the onset of manic symptoms, while for IL-6 and TNF-α this relationship was statistically not significant. So it seems that these markers are not as strongly associated to manic symptoms as CRP, although the cumulative measurement of the three markers does have a strong association with the onset of manic symptoms. There is growing evidence that especially CRP is associated with manic symptoms,50, 51 whereas there are discrepancies between studies investigating cytokines and mania.22 We hypothesized that it may be better to consider immune dysregulation, instead of immune activation. Induction of CRP in hepatocytes is principally regulated by IL-6;52 it could be that, due to the immune dysregulation that occurs in depressed patients, the hepatocytes overreact to normal or lightly increased IL-6 levels and therefore produce excessively high levels of CRP. We know that the inter-relationship between the different inflammatory markers is complex; it could be that CRP is a more resumptive measurement of inflammation activation, as CRP levels are regulated by cytokines. Future research should focus on the underlying pathways and try to unravel the relationships of these inflammatory markers.
Cross-sectional findings
The present study showed that depressed men with and without manic symptoms had substantially higher serum levels of CRP and IL-6, but not TNF-α, than healthy controls. This corroborates the findings of some previous studies reporting on immune activation in patients with unipolar depression7, 8 and bipolar depression.53 However, we did not find differences in inflammatory levels between depressed patients with and without manic symptoms. This confirms recent findings from a study by Su et al.,54 who did not find statistically significant differences in CRP, IL-6 and TNF-α levels between unipolar and bipolar depressed men. But our finding contradicts with two other studies50, 51 reporting on significantly higher levels of CRP during mania compared to other mood states in BD. However, these two studies considered patients who were experiencing manic symptoms at the moment of immunological assessment, whereas our study focussed on lifetime manic symptoms. Consequently, a large proportion of our sample probably experienced manic symptoms in the past and not at baseline measurement. More research is needed to determine whether inflammatory activation is only related to recent and not to previous manic symptoms.
Possible mechanisms
Several mechanisms may explain the association between the activated immune system and manic symptoms. First, sleep is a powerful regulator of the immune system and prolonged loss of sleep induces increasing cytokine and CRP levels.55 Other studies also showed that manic patients often (69–99%) experience reduced need for sleep,56 and that sleep disturbances often escalate just before a (hypo)manic episode.57, 58, 59 Consequently, it could be that the onset of manic symptoms is caused by the increased immunological activity resulting from sleep disturbances. Another mechanism that relates proinflammatory cytokines to mood disorders is their capacity to induce the indoleamine-2,3-dioxygenase enzyme, which catalyzes the synthesis of kynurenine from tryptophan,60, 61, 62 leading to degradation of tryptophan, which can cause depressive symptoms by reducing the availability of this precursor for the synthesis of serotonin and melatonin.60, 61 Moreover, activation of indoleamine-2,3-dioxygenase leads to the production of detrimental tryptophan catobolites with neurotoxic effects, inducing a chronically depressed state.63 As previous studies also showed that significantly more tryptophan was broken down to kynurenine in manic patients,64, 65 the degradation of tryptophan may disrupt the patients' euthymic state by causing either depressive or manic symptoms.
Implications
From a clinical point of view, it is important to examine whether immune activation predicts the development of manic symptomatology in patients with depression. The identification of manic symptomatology is often delayed in clinical practice,66 which is problematic as depressed patients with manic symptoms are usually more therapy-resistant28 and are more likely to commit suicide attempts.29 This clinical relevance of bipolarity in unipolar depression has motivated the DSM-5 work group to include bipolar disorders not only but also a mixed features specifier for major depressive disorder in the new version of the DSM.27 This emphasizes the importance of the search for a biomarker that can detect a predisposition for bipolar disorder and it is tempting, but not completely appropriate, to consider CRP as such a biomarker. Interestingly, recent findings suggest that patients with high inflammatory activity may respond less to antidepressants and better to anti-inflammatory medication.67, 68, 69 Depression is a heterogeneous concept and studies investigating immune activation in mood disorders are important to further differentiate particular subgroups in which immune activation plays an etiological role or has clinical implications. It is likely that the monocyte-T-cell theory of mood disorders does not apply to all depressed patients, and our findings add to this rapidly growing body of literature by showing that higher levels of inflammatory markers are found among depressed men who are more at risk to become bipolar.
Strengths and limitations
The strengths of this study are its large sample size, the possibility to address cross-sectional as well as longitudinal associations, the use of clinical diagnoses of depression instead of self-report questionnaires, the assessment of multiple inflammatory markers and adequate adjustment for a comprehensive set of potential confounders. However, some limitations have to be recognized. Despite the large sample size, the group of patients with manic symptoms at follow-up was still small (N=65). Second, we used a 2-year interval between baseline measurements and follow-up, and future studies should ideally look at shorter follow-up periods or more frequent measurements to further determine the nature of these longitudinal associations. Furthermore, all patients with a clinical diagnosis of bipolar disorder at baseline were excluded. It would be interesting to further examine the inflammatory activation in patients who have more severe, clinically established bipolar disorder, and not only those who experienced manic symptoms assessed by the MDQ. Another limitation of the MDQ is that it only measures lifetime manic symptoms and, therefore, it was impossible to determine whether these symptoms were actually present at baseline assessment or only previously. Finally, the modest overall associations between depression, manic symptoms and immune activation may be explained by the fact that we have used circulating levels of inflammatory markers that vary substantially within individuals. When peripheral cytokines are used, it is a temporary measurement of the immune system, and one cannot automatically assume that this reflects immune activity in the central nervous system. Future research should therefore also consider more proximal factors, such as the genetic activation of monocytes, which can be measured in mRNA known activator genes of monocytes and microglia activation in the brain.
Conclusion
Although immune activation was not related to the presence of lifetime manic symptoms in depressed patients, we did find that depressed patients with and without lifetime manic symptoms had substantially higher levels of inflammatory markers compared to healthy controls. But after full adjustment, these differences were not significant anymore. However, we did find that CRP levels were significantly increased in depressed men with an onset of manic symptoms during 2 years of follow-up. The onset of manic symptoms was particularly high in men with multiple elevated levels of inflammatory markers, which may indicate that a subgroup of depressed patients with increased immunological activity is at greater risk for incident manic symptoms. Further research should explore whether a treatment approach focussing on inflammatory processes may be more effective in the treatment of specific subgroups of depressed patients.
Acknowledgments
The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organization for Health Research and Development (Zon-Mw, grant number 10-000-1002) and is supported by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Arkin, Leiden University Medical Center, GGZ Rivierduinen, University Medical Center Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Scientific Institute for Quality of Health Care (IQ Healthcare), Netherlands Institute for Health Services Research (Nivel) and the Netherlands Institute of Mental Health and Addiction (TRIMBOS)). Assaying of inflammatory markers was supported by the Neuroscience Campus Amsterdam and VIDI project by Penninx.
The authors declare no conflict of interest.
References
- Maes M, Smith R, Scharpe S. The monocyte-T-lymphocyte hypothesis of major depression. Psychoneuroendocrinology. 1995;20:111–116. doi: 10.1016/0306-4530(94)00066-j. [DOI] [PubMed] [Google Scholar]
- Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuro-psychopharmacol Biol Psychiatry. 2011;35:664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Skwerer RG, Barna B, Gulledge AD, Valenzuela R, Butkus A, et al. Depression, immunocompetence, and prostaglandins of the E series. Psychiatry Res. 1986;17:41–47. doi: 10.1016/0165-1781(86)90040-5. [DOI] [PubMed] [Google Scholar]
- Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Prog Neuro-psychopharmacol Biol Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1 and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Häfner S, Baghai TC, Eser D, Schüle C, Rupprecht R, Bondy B. C-reactive protein is associated with polymorphisms of the angiotensin-covering enzyme in major depressed patients. J Psychiatr Res. 2008;42:163–165. doi: 10.1016/j.jpsychires.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Lehman BJ, Kiefe CI, Seeman TE. Relationship of early life stress and psychological functioning to adult C-reactive protein in the Coronary Artery Risk Development in Young Adults Study. Biol Pscychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Beekman ATF, Deeg DJH, BWJH Penninx, Dik MG, Hack CE, et al. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Caska M, Hendrickson BE, Rourke MA, Ho J, Ali S. Depression and inflammation in patients with coronary heart disease: findings from the heart and soul study. J Psychiatr Res. 2007;62:314–320. doi: 10.1016/j.biopsych.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques-Deak AH, Neto FL, Dominguez WV, Solis AC, Kurcgant D, Sato F, et al. Cytokine profiles in women with different subtypes of major depressive disorder. J Psychiatr Res. 2007;41:152–159. doi: 10.1016/j.jpsychires.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Brambilla F, Monteleone P, Maj M. Interleukin-1-beta and tumor necrosis factor-alpha in children with major depressive disorder or dysthymia. J Affect Disord. 2004;78:273–277. doi: 10.1016/S0165-0327(02)00315-4. [DOI] [PubMed] [Google Scholar]
- Allam E, Delacruz K, Ghoneima A, Sun J, Windsor L. Effects of tobacco on cytokine expression from human endothelial cells. Oral Dis. 2012;19:660–665. doi: 10.1111/odi.12050. [DOI] [PubMed] [Google Scholar]
- Diaz LE, Montero A, Gonzales-Gross M, Vallejo AI, Romeo J, Marcos A. Influence of alcohol consumption on immunological status: a review. Eur J Clin Nutr. 2002;56:550–553. doi: 10.1038/sj.ejcn.1601486. [DOI] [PubMed] [Google Scholar]
- Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Timmerman KL, et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39:1714–1719. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- Greenfield JR, Campbell LV. Relationship between inflammation, insulin resistance and type 2 diabetes: cause or effect. Curr Diabetes Rev. 2006;2:195–211. doi: 10.2174/157339906776818532. [DOI] [PubMed] [Google Scholar]
- Huth C, Heid IM, Vollmert C, Gieger C, Grallert H, Wolford JK, et al. IL6 gene promoter polymorphisms and type 2 diabetes: joint analysis of individual participants' data from 21 studies. Diabetes. 2006;55:2915–2921. doi: 10.2337/db06-0600. [DOI] [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. Am J Cardiol. 2005;95:317–321. doi: 10.1016/j.amjcard.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Duivis HE, Beekman ATF, Kluft C, Neuteboom J, Hoogendijk W, et al. Association of depressive disorders, depression characteristics and antidepressant medication with inflammation. Transl Psychiatry. 2012;2:e79. doi: 10.1038/tp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BWJH, Milaneschi Y, Lamers F, Vogelzangs N. Understanding somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Medicine. 2013;11:129. doi: 10.1186/1741-7015-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboyer M, Soreca I, Scott J, Frye M, Henry C, Tamouza R, et al. Review: Can bipolar disorder be viewed as a multi-system inflammatory disease. J Affect Disord. 2012;141:1–10. doi: 10.1016/j.jad.2011.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomology, pathopyshiology, comorbidity, and treatment of bipolar disorder. J Clin Psychiatry. 2009;70:1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between proinflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007;104:91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Zimmerman P, Bruck T, Nocon A, Pfister H, Lieb R, Wittchen H, et al. Heterogeneity of DSM-IV major depressive disorder as a consequence of subtreshold bipolarity. Arch Gen Psychiatry. 2009;66:1341–1352. doi: 10.1001/archgenpsychiatry.2009.158. [DOI] [PubMed] [Google Scholar]
- Angst J, Cui L, Swendsen J, Rothen S, Cravchik A, Kessler RC, et al. Major depressive disorder with subtreshold bipolarity in the National Comorbidity Survey Replication. Am J Psychiatry. 2010;167:1194–1201. doi: 10.1176/appi.ajp.2010.09071011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boschloo L, Spijker AT, Hoencamp E, Kupka R, Schoevers RA, Nolen WA, et al. Predictors of the onset of manic symptomatology in patients with a major depressive episode: a prospective analysis of data from the Netherlands Study of Depression and Anxiety (submitted).
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders5th edn.American Psychiatric Association: Washington, DC; 2013 [Google Scholar]
- Seemüller F, Severus E, Möller HJ, Riedel M. Antidepressants and suicidality in younger adults; is bipolar illness the missing link. Acta Psychiatr Scand. 2009;119:166–167. doi: 10.1111/j.1600-0447.2008.01328.x. [DOI] [PubMed] [Google Scholar]
- Angst J, Gamma A, Benazzi F, Ajdadic V, Eich D, Rossler W. Toward a re-definition of subtreshold bipolarity: epidemiology and proposed criteria for bipolar-II, minor bipolar disorders, and hypomania. J Affect Disord. 2013;73:133–146. doi: 10.1016/s0165-0327(02)00322-1. [DOI] [PubMed] [Google Scholar]
- Lanquillon S, Krieg JC, Ling-Abu-Schach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shlik J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Hovens JGFM GiltayEJ, Wiersma JE, Spinhoven P, BWJH Penninx, Zitman FG. Impact of childhood life events and trauma on the course of depressive and anxiety disorders. Acta Psychiatr Scand. 2012;126:198–207. doi: 10.1111/j.1600-0447.2011.01828.x. [DOI] [PubMed] [Google Scholar]
- Ghazeeri G, Abdullah L, Abbas O. Immunological differences in women compared with men: overview and contributing factors. Am J Reprod Immunol. 2011;66:163–169. doi: 10.1111/j.1600-0897.2011.01052.x. [DOI] [PubMed] [Google Scholar]
- Cannon JG. Cytokines in aging and muscle homeostasis. J Gerontol Biol Sci Med Sci. 1995;50 (Spec No):120–123. doi: 10.1093/gerona/50a.special_issue.120. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Khess CR, Munda SK, Bakhla AK, Praharaj SK, Kumar M. Sex difference in symptomatology of manic episode. Compr Psychiatry. 2011;52:288–292. doi: 10.1016/j.comppsych.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Chazelle E, Lemone C, Morgan K, Kelleher CC, Chastang JF, Niedhammer I. Explanations of educational differences in major depression and generalised anxiety disorder in the Irish population. J Affect Disord. 2011;134:304–314. doi: 10.1016/j.jad.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Beekman AT, Smit JH, Zitman FG, Nolen WA, Spinhoven P, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121–140. doi: 10.1002/mpr.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Hoogendoorn A, Smit J, van Dyck R, Zitman FG, Nolen WA, et al. Sociodemographic and psychiatric determinants of attrition in the Netherlands Study of Depression and Anxiety (NESDA) Compr Psychiatry. 2012;53:63–70. doi: 10.1016/j.comppsych.2011.01.011. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders4th edn.American Psychiatric Association: Washington, DC; 2001 [Google Scholar]
- Wittchen HU, Robins LN, Cottler LB, Sartorius N, Burke JD, Regier D.Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicentre WHO/ADAMHA field trials Br J Psychiatry 1991159546–553.658. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychosometric properties. Psychol Med. 1996;28:57–84. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Hirschfield RM, Holzer C, Calabrese JR, Weissman M, Reed M, Davies M, et al. Validity of the mood disorder questionnaire: a general population study. Am J Psychiatry. 2003;160:178–180. doi: 10.1176/appi.ajp.160.1.178. [DOI] [PubMed] [Google Scholar]
- Boschloo L, Nolen WA, Spijker AT, Hoencamp E, Kupka R, Penninx WB, et al. The Mood Disorder Questionnaire (MDQ) for detecting (hypo)manic episodes: its validity and impact of recall bias. J Affect Disord. 2013;151:203–208. doi: 10.1016/j.jad.2013.05.078. [DOI] [PubMed] [Google Scholar]
- Craig CL, Marshall AL, Sjostrom M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire; 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Fink LA, Bernstein D, Handelsman L, Foote J, Lovejoy M. Initial reliability and validity of the childhood trauma interview: a new multidimensional measure of childhood interpersonal trauma. Am J Psychiatry. 1995;152:1329–1335. doi: 10.1176/ajp.152.9.1329. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Seldenrijk A, Beekman AT, van Hout HP, de Jonge P, Penninx BW. Cardiovascular disease in persons with depressive and anxiety disorders. J Affect Disord. 2010;125:241–248. doi: 10.1016/j.jad.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology . Anatomical Therapeutic Chemical Classification. World Health Organization: Geneva; 2007. [Google Scholar]
- Padmos RC, Hilligers MH, Knijf EM, Vonk R, Bouvy A, Staal FJ, et al. A discriminating messenger RNA signature for bipolar disorder formed by aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry. 2008;65:395–407. doi: 10.1001/archpsyc.65.4.395. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Collinger KA, Lotrich F, Marsland AL, Gill MK, Axelson DA, et al. Preliminary findings regarding proinflammatory markers and brain-derived neutrophic factor among adolescents with bipolar spectrum disorders. J Child Adolesc Psychopharmacol. 2011;21:479–484. doi: 10.1089/cap.2011.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. Elevated serum levels of C-reactive protein are associated with mania symptoms in outpatients with bipolar disorder. Prog Neuro-psychopharmacol Biol Psychiatry. 2007;31:952–955. doi: 10.1016/j.pnpbp.2007.02.018. [DOI] [PubMed] [Google Scholar]
- Cunha AB, Andreazza AC, Gomes FA, Frey BN, da Silveira LE, Concalves CA, et al. Investigation of serumhigh-sensitive C-reactive protein levels across all mood states in bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2008;248:300–304. doi: 10.1007/s00406-007-0797-0. [DOI] [PubMed] [Google Scholar]
- Kushner I, Jiang SL, Zhang D, Lozanski G, Samols D. Do post-transcriptional mechanisms participate in induction of C-reactive protein and serum amyloid A by IL-6 and IL-1. Ann NY Acad Sci. 1995;762:102–107. doi: 10.1111/j.1749-6632.1995.tb32318.x. [DOI] [PubMed] [Google Scholar]
- Wadee AA, Kuschke RH, Wood LA, Berk M, Ichim L, Maes M. Serological observations in patients suffering from acute manic episodes. Hum Psychopharmacol. 2002;17:175–179. doi: 10.1002/hup.390. [DOI] [PubMed] [Google Scholar]
- Su SC, Sun MT, Wen MJ, Lin CJ, Chen YC, Hung YJ. Brain-derived neutrophic factor, adiponectin, and proinflammatory markers in various subtypes of depression in young men. Int J Psychiatry Med. 2011;42:211–226. doi: 10.2190/PM.42.3.a. [DOI] [PubMed] [Google Scholar]
- Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory and metabolic consequences of sleep deprivation. Progr Carciovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony and regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8:160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Albert PS, Rosenthal NE, Wehr TA. Relationship between sleep and mood in patients with rapid-cycling bipolar disorder. Psychiatry Res. 1996;63:161–168. doi: 10.1016/0165-1781(96)02854-5. [DOI] [PubMed] [Google Scholar]
- Barbini B, Bertelli S, Colombo C, Smeraldi E. Sleep loss, a possible factor in augmenting manic episode. Psychiatry Res. 1996;65:121–125. doi: 10.1016/s0165-1781(96)02909-5. [DOI] [PubMed] [Google Scholar]
- Schrocksnadel K, Wirleitner B, Winkler C, Fusch D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006;364:82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Saito K, Markey SP. Human macrophages convert L-tryptophan into the neurotoxin quinolic acid. Biochem J. 1992;293:633–635. doi: 10.1042/bj2830633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. Tryptophan catabolism a T-cell tolerance: immunosuppression by starvation. Immunol Today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwartz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Pscychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Miller CL, Llenos JC, Dulay JR, Weis S. Upregulation of the initiating step of the kynurenine pathway in postmortem anterior cingulated cortex from individuals with schizophrenia and bipolar disorder. Brain Res. 2006;1073–1074:25–57. doi: 10.1016/j.brainres.2005.12.056. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Park SH, Scharpe S, Steinbusch HW, et al. Tryptophan breakdown pathway in bipolar mania. J Affect Disord. 2007;102:65–72. doi: 10.1016/j.jad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Hirschfeld RM. Bipolar depression: the real challenge. Eur Neuropsychopharmacol. 2004;14 (Suppl 2:S83–S88. doi: 10.1016/j.euroneuro.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, et al. A randomized controlled trial of the tumor necrosis factor antagonist Infliximab for treatment-resistant depression. Arch Gen Psychiatry. 2012;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacol. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- Eller T, Vasar V, Shilk J, Maron E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:445–450. doi: 10.1016/j.pnpbp.2007.09.015. [DOI] [PubMed] [Google Scholar]