Abstract
The dynorphin opioid peptides control glutamate neurotransmission in the hippocampus. Alcohol-induced dysregulation of this circuit may lead to impairments in spatial learning and memory. This study examines whether changes in the hippocampal dynorphin and glutamate systems are related, and contribute to impairment of spatial learning and memory in a rat model of cognitive deficit associated with alcohol binge drinking. Hippocampal dynorphins (radioimmunoassay) and glutamate (in vivo microdialysis) were analyzed in Wistar rats exposed to repeated moderate-dose ethanol bouts that impair spatial learning and memory in the Water Maze Task (WMT). The highly selective, long-acting κ-opioid receptor (KOR) antagonist nor-binaltorphimine (nor-BNI) was administered systemically or into the hippocampal CA3 region to test a role of dynorphins in alcohol-induced dysregulations in glutamate neurotransmission and behavior in the WMT. The ethanol treatment impaired learning and memory, upregulated dynorphins and increased glutamate overflow in the CA3 region. Administration of nor-BNI after cessation of ethanol exposure reversed ethanol-induced changes in glutamate neurotransmission in animals exposed to ethanol and normalized their performance in the WMT. The findings suggest that impairments of spatial learning and memory by binge-like ethanol exposure are mediated through the KOR activation by upregulated dynorphins resulting in elevation in glutamate levels. Selective KOR antagonists may correct alcohol-induced pathological processes, thus representing a novel pharmacotherapy for treating of ethanol-related cognitive deficits.
Keywords: alcohol, dynorphin, glutamate, κ-opioid receptor, learning, memory
Introduction
A substantial part of the population in western countries consumes moderate to large amounts of alcohol, which can result in acute and delayed impairments in cognitive and executive functions—operations that guide complex behavior over time through planning, decision-making and response control.1, 2, 3, 4, 5 These impairments lead to medical and social problems including dementia, violence and decreased work productivity.4, 6, 7, 8 Total alcohol consumption and binge drinking pattern, characterized by consumption of large amount of alcohol within a limited time, are risk factors for dementia later in life.9, 10, 11 Binge drinking during adolescence causes impairment in a spatial working memory and other cognitive tasks.7, 12, 13, 14 Alcohol-induced deficits in cognition are critical factors underlying the habitual drug seeking and taking that characterize addiction and dependence.15, 16
The mechanisms of alcohol-induced cognitive impairments remain unknown but they may involve neurodegeneration and/or aberrant neurotransmission. Several studies indicate that cognitive effects of alcohol are mediated through the dysregulation of the glutamate system in the hippocampus and prefrontal cortex (PFC).6, 7, 8 Glutamate neurotransmission in the hippocampus is tonically controlled by the dynorphin opioid peptides17, 18 that have been implicated in cognitive decline.19, 20, 21, 22, 23, 24 Administration of synthetic dynorphin into the dorsal hippocampus impairs spatial learning in rats.19 Dynorphins contribute to age-related and stress-induced deficits in learning and memory.20, 21, 22 In elderly humans, prodynorphin (PDYN) gene polymorphisms have a role in memory function.23 In individuals with Alzheimer's disease, dynorphins are elevated in the PFC and this increase correlates with neuropathological lesions.24 In human alcohol-dependent subjects, dynorphins are upregulated in the hippocampus and PFC that may underlie, in part, cognitive impairments including the dysfunctions in cognitive control of addictive behavior and disrupted inhibitory control.25
In this study, we investigated whether dynorphin levels are altered in the rat model of cognitive deficits induced by binge-like ethanol exposure26, 27 and whether blockade of κ-opioid receptor (KOR), a target for dynorphins, prevents alcohol-induced cognitive impairments. In this model, repeated, intermittent intragastric administration of moderate dose of ethanol (3.4 g kg−1; once daily by gavage for 6 days) produces blood alcohol concentrations (that is, between 110 and 150 mg dl−1) similar to those produced by binge ethanol intoxications in humans (that is, between 80 and 200 mg dl−1),28, 29, 30 and impairs spatial learning and memory in the Barnes maze task 3–6 days following the ethanol bouts. The dose used is substantially lower than those used in the previous studies (from 7.6–15 g kg−1 per day).8, 31, 32, 33 Ethanol exposure in this and other models alters basal glutamate dynamics in the hippocampus resulting in augmentation of the basal glutamate release.27, 34 Because dynorphins inhibit glutamate release in the hippocampus,17, 18 we investigated a role of these peptides in the ethanol-induced alterations in glutamate neurotransmission that may be critical for impairments of spatial learning and memory.
Methods and materials
Drugs
Nor-Binaltorphimine (nor-BNI; 17,17′-(dicyclopropylmethyl)-6,6′,7,7′-6,6′-imino-7,7′-bimorphinan-3,4′,14,14′-tetrol) was obtained from Tocris Bioscience, Bristol, UK.
Behavioral experiments
Animals
Male, Wistar rats (B&K Universal, Sollentuna, Sweden), 12–14 weeks old (390–420 g) were housed at a 12-h light/dark cycle (lights on at 0800 hours) with food and water available ad libitum. Experiments took place between 1000 and 1500 hours. The experimental procedures followed the recommendations of the Swedish animal protection legislation and were approved by the local Animal Ethics Committee.
Surgery and cannulation
Rats were anaesthetized with a combination of Ketalar (ketamine hydrochloride 50 mg ml−1, 0.2 ml, subcutaneously, Parke Davis, Barcelona, Spain) and Hypnorm (fluanisone 10 mg ml−1 and fentanyl 0.2 mg ml−1; 0.2 ml, intraperitonealy, Janssen, Beerse, Belgium). The animals were implanted with steel guide cannulae (custom made, outside diameter=0.4 mm) aimed at the CA3 region of the dorsal hippocampus (coordinates based on Paxinos and Watson atlas:35 anterior–posterior—3.3 mm from bregma, lateral—3.2 mm to the midsagittal suture line and ventral—3.5 mm from the skull surface). During surgery, the body temperature of the animal was maintained at 37 °C using a temperature controller (CMA/105, CMA/Microdialysis, Stockholm, Sweden). After surgery, a dummy cannula was put into the guide cannula and the rats were allowed at least 5 days of recovery. Animals with intracranial cannulae were individually housed in standard Macrolon (type 3, Sweden). The position of the cannula was verified histologically after the experiments following an intrahippocampal infusion of 0.3 μl methylene blue.
Forced intragastric ethanol administration
Rats were treated with ethanol (40% v/v; 10 ml kg−1) or tap water once per day for 6 days intragastrically through gavages. In the acutely treated naïve rats (3.4 g kg−1 of ethanol), and after the last ethanol treatment in animals exposed to ethanol binges for 6 days, blood alcohol concentrations reached the peak values of 112±27 mg dl−1 and 153±27 mg dl−1, respectively, in 3–4 h and then decreased to the basal levels by 8 h in both animal groups.26, 27 No significant differences in weight gain between the ethanol- and water-treated rats, and no signs of inflammatory reaction on the lining of the stomach examined visually after the experiments were evident.26, 27 No signs of spontaneous ethanol withdrawal were observed in rats treated with ethanol.26, 27
The water maze task
A circular water tank, 180 cm in diameter and 45 cm high (AB Lomma Plastprodukter, Arlöv, Sweden), was used in the water maze task (WMT) procedure. The water tank was filled with tap water (22 °C) and the transparent plexiglas escape platform invisible in clean water was submerged 1 cm below the water surface. The water tank was located in a room with several visual cues that were kept constant throughout the experiment.36
Acquisition test
During training, the submerged escape platform was located in the center of the southeast quadrant. The rats were given four trials per day for five consecutive days. In each trial, the rat was placed in the water facing the pool wall at one of four selected starting points (north, south, east or west).36 After each trial, the rat was placed at a different starting point, thus eliminating the use of a simple response strategy. Latency to escape to the platform in seconds, swim path in centimeters, average swim speed in cm s−1, percent of time spent in near board zone, percent of time spent in quadrant with platform, proximity to the platform (mean distance to the platform in cm during the trial) and percent of swimming time with slow swim speed (less than 5 cm s−1) were analyzed.
Probe test
Four hours after the last trial, spatial memory was examined. For this purpose, the platform was removed from the pool and the animals were allowed to swim freely for 60 s. The rat was released into the water tank from a position opposite to that of the quadrant where the platform had been during the training sessions. The latency to the first crossing and the time spent in the different quadrants and zones were analyzed.
Experiment 1
The objective was to assess if repeated administration of ethanol (3.4 g kg−1, intragastrically, daily for 6 days) affects spatial learning. Animals were treated with water (n=27) or ethanol (n=42) as described above. The WMT training was initiated 2 days after the last ethanol treatment.
Experiment 2
To investigate whether the effects of repeated ethanol administration are mediated through the KOR, animals were treated with the KOR-antagonist nor-BNI.35, 37, 38, 39, 40, 41 Rats treated with either water or ethanol solution (see Experiment 1) for 6 days, were randomly assigned to two groups, treated once with saline (5 ml kg−1, s.c.) or nor-BNI (6 mg kg−1, dissolved in saline; s.c.) 2 h after the last ethanol administration. The WMT training was initiated 2 days after the last ethanol treatment.
Experiment 3
To examine if impairment produced by ethanol is mediated through the activation of the KOR in the hippocampus, artificial CSF (aCSF; 0.3 μl per side) or nor-BNI (10 nmol per side in 0.3 μl of aCSF) was injected once into the hippocampal CA3 region. Nor-BNI was infused bilaterally over 30 s using a microinfusion pump (CMA 100). The injection needles (0.2 mm outside diameter) were left in the guide cannulae for an additional 30 s after infusion. The WMT training was initiated 2 days after the last ethanol treatment.
Microdialysis experiments
Animals
Male, Wistar rats (Charles River Laboratories, Germantown, MD, USA) (250–300 g at the beginning of the experiment) were housed in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care and experiments were reviewed by the National Institute on Drug Abuse Intramural Research Program Institutional Care and Use Committee under National Institute on Drug Abuse (National Institutes of Health) guidelines.
Surgical procedures
Standard stereotaxic procedures were used to implant unilateral microdialysis guide cannulae (CMA/11; CMA/Microdialysis, Acton, MA, USA) in the CA3 region of the dorsal hippocampus (for coordinates see above) of animals anesthetized intraperitonealy with 3 ml kg−1 Equithesin.
Treatment
Following 5 days of recovery, rats were treated via gastric gavage with either 3.4 g kg−1 dose of ethanol or water for 6 days as described above. Animals were injected with either saline or nor-BNI (6 mg kg−1, s.c.) 2 h after last ethanol administration.
Microdialysis procedures
Microdialysis experiments42 were commenced 2 days after treatment with ethanol. Before measurements, each probe was flushed overnight with 0.3 μl min−1 aCSF containing 145 mM NaCl, 2.8 mM KCl, 1.2 mM MgCl2, 1.2 mM CaCl2, 0.25 mM ascorbic acid and 5.4 mM D-glucose, adjusted to pH 7.2 with high-performance liquid chromatography-grade NaOH or H3PO4. During the experiment, fresh aCSF was perfused at 1 μl min−1. After 60 min of equilibration, 5-min dialysate sample collection commenced. After baseline determination (30 min), the aCSF was changed to that containing 60 mM KCl, and, after 30 min of equilibration, six consecutive samples were collected. The perfusion solution was then changed to regular aCSF and six additional baseline samples were collected following a 30 min equilibration period.
Probe location confirmation
For anatomical confirmation, animals were euthanized by Equithesin and probe placement was assessed on 25-μm serial coronal cryostat sections. Only data obtained from animals with histologically correct placements were used for subsequent analysis.
Glutamate determination
Amino acid content was quantified using a capillary electrophoresis P/ACE MDQ system (Beckman, Fullerton, CA, USA) coupled to an external ZETALIF laser-induced fluorescence detector (Picometrics, Toulouse, France) with a specially developed automatic derivatization procedure suitable for unattended derivatization and injection of the samples by the P/ACE MDQ8.42
Dynorphin RIA
The procedure was described elsewhere.43 Briefly, tissue extracts in 1 M acetic acid were run through a SP-Sephadex ion exchange C-25 column, and peptides were eluted and analyzed by RIA.
Data analysis
Data were analyzed by one or two-way analysis of variance (ANOVA), with group and time as independent factors. When appropriate, Student's t-test and Newman–Keuls test were performed for post hoc comparison. The data are presented as mean±s.e.m. The accepted value of significance was set<0.05.
Results
Effects of ethanol treatment on spatial learning and memory in the WMT
Experiment 1
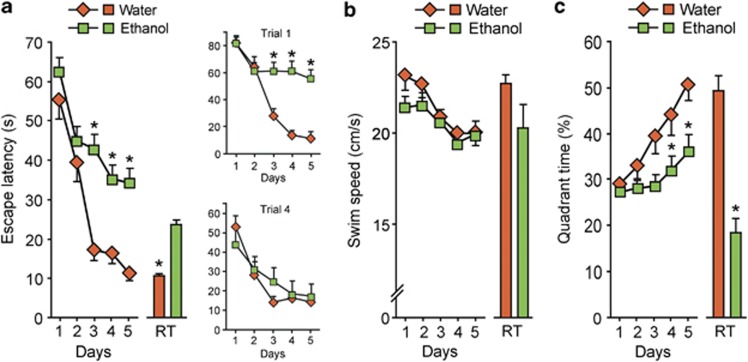
Spatial learning and memory analyzed on the WMT are hippocampally dependent and sensitive to ethanol treatment.8, 33, 44 Escape latencies (averaged for four daily trials) significantly decreased over the five blocks of training in water-treated group while this decline was affected by ethanol treatment (Figure 1a). The differences were significant on day 3 to day 5 of training. Effects of ethanol were not attributed to the decrease in swim speed because ethanol treatment did not change this parameter (Figure 1b). Analysis of the spatial distribution of swimming revealed that the increase in quadrant selectivity over the five blocks of training observed in the water-treated group was significantly reduced by ethanol treatment (Figure 1c). Latency to reach the position of the platform was increased, and percentage of time in the correct quadrant was reduced by ethanol treatment in the probe tests conducted 4 h after the final training block (Figures 1a and c). Between-trial analysis revealed that impairment of learning (latency to escape) in ethanol-treated rats was evident for the first but not for the last daily trials (Figure 1a; treatment × time interaction for the first trial (upper right panel), (F(4,112)=4.2, P<0.05); for the last trial (lower right panel), nonsignificant) suggesting that ethanol-induced cognitive impairment is related to the retrieval deficit. Experiment with the visible platform revealed no differences between ethanol- (n=6) and water- (n=6) treated rats (ANOVA: time × treatment interaction, P=0.7) in the latency to navigate to the platform. Thus, the ethanol treatment did not affect the sensory and motor systems (abilities to navigate using local and distal cues and swim) or the motivation to escape from water.
Figure 1.
Effects of ethanol administration on rat performance in the WMT. Rats were treated daily for 6 days with water or ethanol followed by the WMT initiated in 2 days after the last water/ethanol administration. (a) Escape latencies (s); the upper and lower right panels show escape latencies (s) for the first and last daily trials. ANOVA: water, time effect F(4, 888)=10.3, P<0.01; ethanol, treatment × time: F(4, 888)=3.68, P<0.05. (b) Swim speed (cm s−1). ANOVA: ethanol, F(4, 888)=0.77, P=0.42. (c) Time in the platform associated quadrant (% to total time of swimming). ANOVA: water, time effect F(4, 888)=14.6, P<0.01; ethanol, treatment × time: F(4, 888)=2.66, P<0.05. The data for the retention trials (RT) are shown as bar graphs associated with and on the same scale as the acquisition curves. Data are shown as mean±s.e.m. n=42 and 27 rats treated with ethanol and water, respectively. *P<0.05; the Newman–Keuls test was used.
Ethanol effects on the hippocampal dynorphins
To evaluate whether ethanol exposure affects dynorphin neurotransmission, we analyzed the levels of these peptides in the hippocampus. Analysis was conducted on day 3 and day 7 after cessation of ethanol exposure that corresponded to the first and last day of learning and memory trials (Figure 2). Dynorphin A was significantly increased in the day and remained elevated on day 7 (for both points, P<0.05), whereas the increase in dynorphin B reached significance on day 7 (P<0.05).
Figure 2.
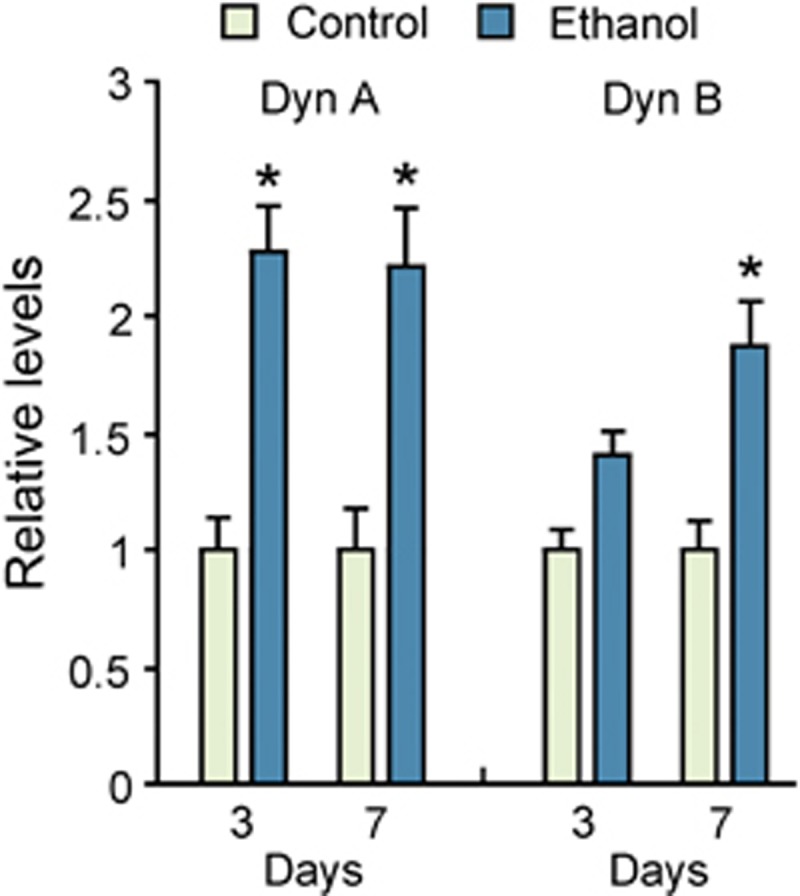
Dynorphins are upregulated in the hippocampus of rats exposed to ethanol. Rats were treated daily for 6 days with water or ethanol. The brain samples were taken on the day 3 or day 7 after the last ethanol/water administration. Two-way ANOVA performed separately for Dyn A and Dyn B revealed significant time effect (F(1, 28)=7.0; P<0.05) and significant group effect ((F1.28)=57.8; P<0.01) with no significant group × time interaction for Dyn A, and significant group effect (F(1, 28)=9.8; P<0.01) with no time effect and group × time interaction for Dyn B. The data are presented as means±s.e.m. of relative levels in the ethanol-treated animals compared with the water-treated controls that showed Dyn A levels on day 3 and day 7: 4.68±0.64 and 3.12±0.55 fmol per mg tissue; Dyn B levels on the day 3 and day 7: 1.21±0.11 and 2.19±0.27 fmol per mg tissue), which were all set to 1; n=8 in each group. *P<0.05; the Student's t-test was used.
Effects of KOR antagonist nor-BNI on learning and memory impairment induced by ethanol administration
To examine whether KOR activation mediates ethanol effects on spatial learning and memory, we blocked KOR using nor-BNI, the highly selective KOR antagonist that exerts effects lasting for at least 1 month.37, 38, 39, 41
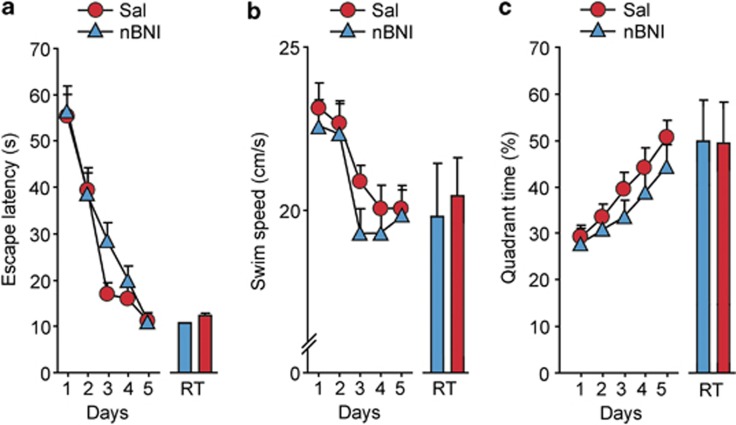
In the control rats (received water, intragastrically), nor-BNI (6 mg kg−1; s.c.) administered 2 days before the WMT training failed to modify their performance in the WMT (Figure 3). Virtually identical data were obtained after intrahippocampal administration of aCSF (n=6) or nor-BNI (10 nmol per 0.3 μl per side bilaterally; n=6). Thus, endogenous dynorphins are not likely involved in spatial learning and memory formation under physiological conditions.
Figure 3.
Effects of nor-BNI on performance of control rats in the WMT. Rats were treated daily for 6 days with water followed by the WMT initiated 2 days after the last water administration. Nor-BNI (6 mg kg−1; s.c.) or saline were administered 2 h after the last water administration (that is, 2 days before the first WMT training block). Acquisition trials: (a) latency to escape (s) mean for the four daily trials, (b) swim speed (cm s−1), (c) quadrant selectivity as the percentage to the total swimming time. The data for the retention trials are shown as bar graphs associated with and on the same scale as the acquisition curves. Repeated measures ANOVA did not reveal treatment × time interaction in latency to escape, swim speed and quadrant selectivity between nor-BNI and saline-treated groups. Data are shown as means±s.e.m.: n=8 rats for each saline and nor-BNI treatment.
Experiment 2
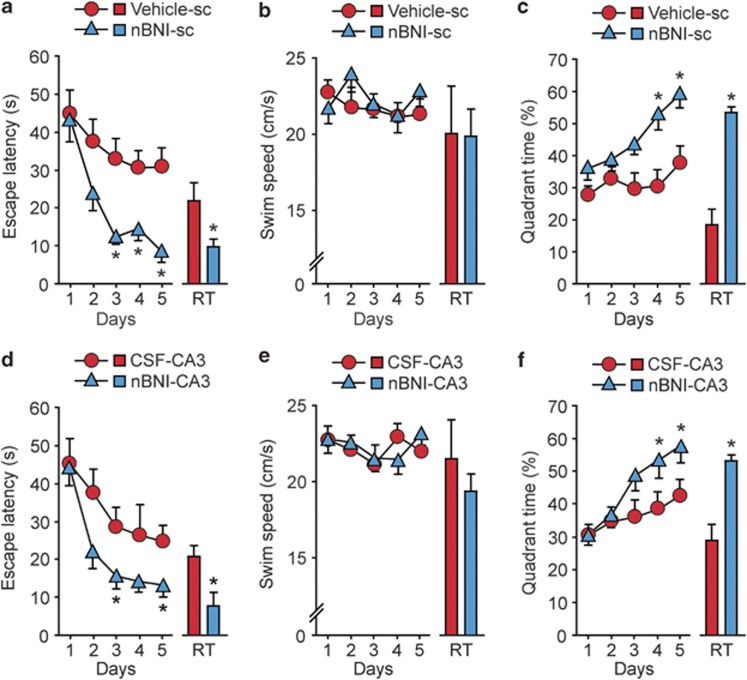
The ethanol-treated rats administered s.c. with nor-BNI 2 days before the WMT training were superior in spatial learning performance compared with the ethanol-saline group; the latencies to locate the platform were markedly shortened in the ethanol-nor-BNI group (Figure 4a). The differences in the latencies were significant on day 3 to day 5 of training. No effects of nor-BNI on swim speed were evident (Figure 4b). Quadrant selectivity returned to normal in nor-BNI-treated rats but not in saline-treated rats (Figure 4c). In the probe test, the latency to reach the position of the platform and the percentage of time spent in the correct quadrant were normalized in the ethanol-treated rats by nor-BNI administration (Figures 4a and c).
Figure 4.
The selective, long-acting opioid KOR-antagonist nor-BNI (nBNI) normalizes rat performance impaired by ethanol in the WMT. Rats were treated for 6 days with water or ethanol followed by the WMT initiated 2 days after the last ethanol administration. The effects of (a–c) systemic (6 mg kg−1; sc) or (d–f) intrahippocampal (CA3 region; 10 nmol per 0.3 μl per site, bilaterally) administration of nor-BNI on ethanol-induced cognitive impairment in rats. Either the nor-BNI or vehicle (saline or aCSF) was administered 2 h after the last ethanol treatment (that is, 2 days before the first WMT training block). Acquisition trials: (a, d) mean values (s) of escape latency for the four daily trials; ANOVA: (a) ethanol-nor-BNI group, treatment × time: F (4, 328)=2.66, P<0.05; (d) ethanol-nor-BNI group, treatment × time: F(4, 162)=4.52, P<0.05). Swim speed: (b, e) the mean values in cm s−1. Quadrant selectivity: (c, f) the percentage of the total swimming time; ANOVA: (c) ethanol-nor-BNI group, treatment × time: F(4, 328)=2.34, P<0.05; (f) ethanol-nor-BNI group, treatment × time: F(4, 162)=2.15, P<0.05). The data for the retention trials are shown as bar graphs associated with and on the same scale as the acquisition curves. In (a–c): n=18 and 16 rats in the saline and nor-BNI-treated groups, respectively. In (d–f), n=8 and 7 in the aCSF- and nor-BNI-treated groups, respectively. The data are shown as means±s.e.m.; *P<0.05; the Newman–Keuls test was used.
Experiment 3
The dorsal CA3 area is critically involved in spatial learning.45, 46, 47 Nor-BNI or aCSF was microinjected into the dorsal CA3 area 2 days before the first WMT training block. Intrahippocampal nor-BNI significantly improved learning in ethanol-treated rats as was evident from the decrease in latencies to locate the platform compared with aCSF-treated rats on day 3 and day 5 (Figure 4d). No effects of nor-BNI on swim speed were found (Figure 4e). Nor-BNI-treated rats also displayed enhanced quadrant selectivity on day 4 and day 5 (Figure 4f) and a significant improvement in memory function in a probe test manifested as a normalization of both percentage time in correct quadrant and latency to reach the position of the platform (Figures 4d and f). The nor-BNI effects were significant on day 3 to/and day 5 of training, that is in 5–7 days after the last ethanol administration, suggesting that they were not mediated through ethanol withdrawal.
Nor-BNI effects on ethanol-evoked alterations in hippocampal glutamate transmission
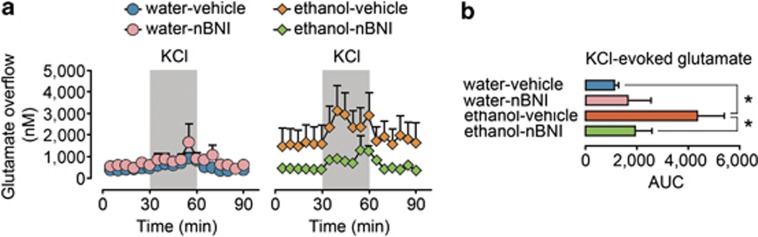
We previously demonstrated that ethanol treatment regimen used in the present study augments basal and depolarization-induced dialysate glutamate levels in the CA3 area due to elevated glutamate release.27 To evaluate whether KOR is involved in ethanol-induced alterations in glutamate neurotransmission, we examined the effects of nor-BNI pretreatment on these alterations. Ethanol treatment increased both basal and potassium-induced glutamate overflow in the CA3 region (Figures 5a and b) in average by 400% compared with controls, from 388±97 nM to 1576±770 nM and from 683±120 nM to 2748±869 nM for basal and stimulated overflow, respectively. Two-way ANOVA revealed a significant ethanol treatment effect (F(3,24)=5.58, P<0.05) and ethanol × nor-BNI treatment interaction (F(3,24)=4.31, P=0.05) for stimulated glutamate levels, whereas for basal levels they were not significant (F(3,24)=1.01, P>0.05 and F(3,24)=1.51, P>0.05, respectively). Analysis of stimulated glutamate levels revealed a significant effect of ethanol in saline-treated (F(1,13)=9.85, P<0.01), but not in nor-BNI-treated animals (F(1,10)=0.04, P>0.05). Correspondingly, effect of nor-BNI pretreatment was significant in ethanol-treated animals (F(1,14)=5.54, P<0.05), but not in controls (F(1,9)=0.52, P>0.05). There were no differences between pretreatment groups in basal or depolarization-induced GABA overflow (data not shown). Thus, ethanol treatment augmented potassium-induced glutamate release, whereas pretreatment with nor-BNI blocked this increase.
Figure 5.
The nor-BNI (nBNI) pretreatment reverses the effects of ethanol on the basal- and potassium-induced dialysate concentrations of glutamate in the CA3 area of the hippocampus. (a) The time pattern of the basal- and potassium-stimulated extracellular glutamate concentration in the CA3 area of the hippocampus of the water- and ethanol-treated rats. The left panel shows the effects of vehicle (n=6) or nor-BNI (n=4) treatment on the water-exposed rats, and the right panel shows the effects of vehicle (n=7) or nor-BNI (n=7) treatment on the ethanol-exposed rats. The nor-BNI treatment normalized the glutamate concentrations in the ethanol-exposed rats but failed to influence those of the water-exposed rats. (b) The bar graphs of the area under the curve (AUC) values for the stimulated glutamate levels. *P⩽0.05; the significance of the differences between the water-vehicle and ethanol-vehicle treatment group; and between the ethanol-nor-BNI and ethanol-vehicle treatment group.
Discussion
Postmortem analysis of human alcohol-dependent subjects identified upregulation of the dynorphin opioid peptides in the hippocampus and dl-PFC.25, 48 These changes may be caused by many years of alcohol drinking and withdrawal, and may contribute to alcohol-associated impairments of cognitive and executive functions. Consistently, the present study found that dynorphins are upregulated in the hippocampus of rats exposed to ethanol that produced spatial memory impairment. The critical finding was that the highly selective long-acting KOR-antagonist nor-BNI,37, 38, 39, 40, 41 administered after cessation of ethanol exposure reversed cognitive deficits and normalized elevated glutamate levels in ethanol-treated rats. Nor-BNI was effective under both systemic and intrahippocampal administration suggesting that ethanol actions on glutamate overflow and spatial memory were mediated through the hippocampal dynorphin-KOR-dependent mechanism. No effects of nor-BNI on learning and memory and glutamate levels were evident in naïve animals, suggesting that this mechanism is not activated under normal conditions.
In the hippocampus, synthetic dynorphins act through presynaptic KOR to inhibit Ca2+-dependent glutamate secretion thereby inhibiting synaptic transmission and reducing neural plasticity.17, 18, 49, 50 These effects may potentially underlie inhibitory actions of KOR agonists on spatial memory.17, 18, 19, 20, 21 Dialysis studies, however, do not support this hypothesis. Thus, synthetic dynorphin increased glutamate levels in the hippocampus51 and spinal cord,52 and dynorphin upregulation followed by ethanol treatment resulted in elevation of hippocampal glutamate concentrations (the present study).
Under normal conditions endogenous dynorphins may control glutamate release in the hippocampus via two processes, by acting through KOR localized on glutamate synaptic terminals and by inhibiting GABA interneurons thereby disinhibiting glutamate release from dentate granule cells.18, 49, 50 At least two factors are critical for the maintenance of the balance between these two processes: (a) local dynorphin concentrations in the vicinity of the KOR localized to glutamate terminals or to GABA interneurons, and (b) status of KOR-activated signal transduction pathways in the two neuronal types. Ethanol intake may differentially alter peptide concentrations at these locations resulting in selective activation of glutamate release.
Effects of KOR activation on glutamate and GABA release are mediated through mechanisms, which may differ in their sensitivity towards addictive substances.53 In the hippocampus, the preferential desensitization of KOR-activated pathways in glutamate terminals resulting from ethanol treatment could lead to increased glutamate release with no changes in GABA secretion. Another possibility is that ethanol treatment may alter the interactions of the KOR with Gs proteins, the association of the opioid receptors with Galphas54 or the shift from inhibitory to stimulatory mode as has been observed in the chronic morphine treatment.55 Activation of the Gs-mediated signaling would induce an excitatory response and stimulate glutamate release. These mechanisms are supported by electrophysiological experiments demonstrating that KOR-opioids can evoke direct receptor-mediated excitatory effects on CA3 pyramidal neurons56, 57, 58 and may mediate the alcohol-induced impairment of learning and memory.
Increases in glutamate concentrations in the synaptic cleft through the increase in its release59 or decrease in its uptake60 may impair spatial memory.61 Elevated glutamate may enable both the induction of LTD through spillover activation of extrasynaptically localized NMDA receptors and the expression of LTD by facilitating endocytosis of postsynaptic AMPA receptors, thereby leading to the impairment of the retrieval of spatial memory.61, 62 This mechanism apparently underlies the decline in spatial memory induced by acute stress and may be relevant for the ethanol-induced dynorphin-mediated cognitive impairment.
It has been hypothesized that neuropathological alterations including reduction in brain weight and volume, and loss of neurons, glia and white matter induced by alcohol neurotoxicity underlie cognitive deficits in human alcoholics and heavy binge drinkers.5, 7, 10, 63, 64, 65 In animals, forced administration of intoxicating doses of ethanol induces neurodegenerative processes8, 66 that are considered to be the cause of learning and memory impairments.67, 68 Functional alterations in the hippocampus including rewiring in neuronal networks and change in efficacy of synaptic transmission may represent another mechanism of alcohol-induced cognitive deficits. Specifically, repeated moderate-dose ethanol bouts may dysregulate hippocampal neurotransmission by targeting the dynorphin/KOR system controlling glutamate release leading to deficits in learning and memory. The fact that learning and memory acquisition are normalized by KOR antagonist administered after the cessation of ethanol administration supports the functional view. The critical consequence of the dynorphin actions is the elevation of glutamate levels that may result in the development of LTD and impairments in the retrieval of spatial memory as described for the stress-induced cognitive deficit.61, 62
This study suggests that both dynorphin and glutamate systems mediate effects of alcohol on learning and memory impairment. Although KOR activation has been identified as a critical event, the molecular mechanism of alcohol-induced dynorphin upregulation and causal relationships between dynorphin upregulation, increased glutamate overflow and learning and memory impairment have not been established, which is a limitation of the work. In the future studies, it would be important to address these issues and examine whether dysregulated interaction of the two neurotransmitter systems may contribute to decline in spatial memory in other neuropathological conditions including acute and chronic stress in which dynorphins may also be involved.69
Activation of the dynorphin system in the hippocampus and dl-PFC in human alcoholics may be a part of the functional mechanism of cognitive deficits that may include the loss of cognitive control over alcohol drinking and seeking behavior.25, 48 Indeed, these brain areas are implicated in the encoding and retrieval of drug-related memories that lead to drug craving and drug use,70, 71 whereas variations in the PDYN and/or KOR genes are associated with the risk for alcohol dependence,72, 73 negative craving for alcohol74 and memory in the elderly.23 Importantly, nor-BNI decreases ethanol self-administration in ethanol-dependent but not in nondependent rats supporting the hypothesis that dynorphins and KOR are dysregulated in alcohol addiction and contribute to the increased drinking in dependent rats.75, 76 Furthermore, KOR activation was found to induce an impulsive phenotype that may contribute to the initiation of alcohol abuse and relapse in dependent individuals.77
In summary, our findings propose the functional mechanism of spatial learning and memory impairment induced by binge-like alcohol exposure. This mechanism involves the dynorphin/KOR system, whose activation augments glutamate neurotransmission and hence produces cognitive deficits. Selective KOR antagonists may correct alcohol-dysregulated neurotransmission, thus representing a novel pharmacotherapy for treating alcohol-related cognitive deficits.
Acknowledgments
We thank Dr Tzvetomira Philipova for technical assistance. This work was supported by grants from the Swedish Council for Working Life and Social Research (FAS), Swedish Science Research Council, Swedish Research Council FORMAS, AFA Forsäkring, Alcohol Research Council of the Swedish Retailing Monopoly and Uppsala University.
This article is dedicated to the memory of Tony Shippenberg.
The authors declare no conflict of interest.
References
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, et al. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol. 2009;44:372–381. doi: 10.1093/alcalc/agp030. [DOI] [PubMed] [Google Scholar]
- Park SQ, Kahnt T, Beck A, Cohen MX, Dolan RJ, Wrase J, et al. Prefrontal cortex fails to learn from reward prediction errors in alcohol dependence. J Neurosci. 2010;30:7749–7753. doi: 10.1523/JNEUROSCI.5587-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber WR, Jenkins RL, Parsons OA. Recovery of visual-spatial learning and memory in chronic alcoholics. J Clin Psychol. 1981;37:192–197. doi: 10.1002/1097-4679(198101)37:1<192::aid-jclp2270370140>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Cary MS. Alcohol-related dementia: validation of diagnostic criteria. Am J Geriatr Psychiatry. 2003;11:441–447. [PubMed] [Google Scholar]
- Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Duka T. Review. Cognitive and emotional consequences of binge drinking: role of amygdala and prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 2008;363:3169–3179. doi: 10.1098/rstb.2008.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Frankola K, Goldstein A, Thorsell A, Singley E, et al. Alcohol-induced neurodegeneration, suppression of transforming growth factor-beta, and cognitive impairment in rats: prevention by group II metabotropic glutamate receptor activation. Biol Psychiatry. 2010;67:823–830. doi: 10.1016/j.biopsych.2009.12.018. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr., Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289:1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- Saunders PA, Copeland JRM, Dewey ME, Davidson IA, Mcwilliam C, Sharma V, et al. Heavy drinking as a risk factor for depression and dementia in elderly men - findings from the liverpool longitudinal community study. Brit J Psychiat. 1991;159:213–216. doi: 10.1192/bjp.159.2.213. [DOI] [PubMed] [Google Scholar]
- Jarvenpaa T, Rinne JO, Koskenvuo M, Raiha I, Kaprio J. Binge drinking in midlife and dementia risk. Epidemiology. 2005;16:766–771. doi: 10.1097/01.ede.0000181307.30826.6c. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24:164–171. [PubMed] [Google Scholar]
- Duka T, Gentry J, Malcolm R, Ripley TL, Borlikova G, Stephens DN, et al. Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res. 2004;28:233–246. doi: 10.1097/01.alc.0000113780.41701.81. [DOI] [PubMed] [Google Scholar]
- Colsher PL, Wallace RB. Elderly men with histories of heavy drinking: correlates and consequences. J Stud Alcohol. 1990;51:528–535. doi: 10.15288/jsa.1990.51.528. [DOI] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Gillan CM, Smith DG, De Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16:81–91. doi: 10.1016/j.tics.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Zalutsky RA, Nicoll RA. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993;362:423–427. doi: 10.1038/362423a0. [DOI] [PubMed] [Google Scholar]
- Wagner JJ, Terman GW, Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993;363:451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin J, Nylander I, Georgieva J, Schott PA, Ogren SO, Terenius L. Hippocampal dynorphin B injections impair spatial learning in rats: a kappa-opioid receptor-mediated effect. Neuroscience. 1998;85:375–382. doi: 10.1016/s0306-4522(97)00605-2. [DOI] [PubMed] [Google Scholar]
- Jiang HK, Owyang VV, Hong JS, Gallagher M. Elevated dynorphin in the hippocampal formation of aged rats: relation to cognitive impairment on a spatial learning task. Proc Natl Acad Sci USA. 1989;86:2948–2951. doi: 10.1073/pnas.86.8.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen XV, Masse J, Kumar A, Vijitruth R, Kulik C, Liu M, et al. Prodynorphin knockout mice demonstrate diminished age-associated impairment in spatial water maze performance. Behav Brain Res. 2005;161:254–262. doi: 10.1016/j.bbr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Carey AN, Lyons AM, Shay CF, Dunton O, McLaughlin JP. Endogenous kappa opioid activation mediates stress-induced deficits in learning and memory. J Neurosci. 2009;29:4293–4300. doi: 10.1523/JNEUROSCI.6146-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch H, Wagner M, Bilkei-Gorzo A, Toliat MR, Pentzek M, Fuchs A, et al. Gene polymorphisms in prodynorphin (PDYN) are associated with episodic memory in the elderly. J Neural Transm. 2009;116:897–903. doi: 10.1007/s00702-009-0238-5. [DOI] [PubMed] [Google Scholar]
- Yakovleva T, Marinova Z, Kuzmin A, Seidah NG, Haroutunian V, Terenius L, et al. Dysregulation of dynorphins in Alzheimer disease. Neurobiol Aging. 2007;28:1700–1708. doi: 10.1016/j.neurobiolaging.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Bazov I, Kononenko O, Watanabe H, Kuntic V, Sarkisyan D, Taqi MM, et al. The endogenous opioid system in human alcoholics: molecular adaptations in brain areas involved in cognitive control of addiction. Addict Biol. 2013;18:161–169. doi: 10.1111/j.1369-1600.2011.00366.x. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Liljequist S, Meis J, Chefer V, Shippenberg T, Bakalkin G. Repeated moderate-dose ethanol bouts impair cognitive function in Wistar rats. Addict Biol. 2012;17:132–140. doi: 10.1111/j.1369-1600.2010.00224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer V, Meis J, Wang G, Kuzmin A, Bakalkin G, Shippenberg T. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addict Biol. 2011;16:229–237. doi: 10.1111/j.1369-1600.2010.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedford D, O'Farrell A, Howell F. Blood alcohol levels in persons who died from accidents and suicide. Ir Med J. 2006;99:80–83. [PubMed] [Google Scholar]
- Jones AW. Ultra-rapid rate of ethanol elimination from blood in drunken drivers with extremely high blood-alcohol concentrations. Int J Legal Med. 2008;122:129–134. doi: 10.1007/s00414-007-0181-7. [DOI] [PubMed] [Google Scholar]
- Lamminpaa A, Hoppu K. First-order alcohol elimination in severe alcohol intoxication in an adolescent: a case report. Am J Emerg Med. 2009;27:128 e5–6. doi: 10.1016/j.ajem.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical-dependence upon ethanol and associated behavioral-changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Faingold CL. The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Curr Protoc Neurosci. 2008;Chapter 9:Unit 9 28. doi: 10.1002/0471142301.ns0928s44. [DOI] [PubMed] [Google Scholar]
- Obernier JA, White AM, Swartzwelder HS, Crews FT. Cognitive deficits and CNS damage after a 4-day binge ethanol exposure in rats. Pharmacol Biochem Behav. 2002;72:521–532. doi: 10.1016/s0091-3057(02)00715-3. [DOI] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Effect of repeated ethanol withdrawal on glutamate microdialysate in the hippocampus. Alcohol Clin Exp Res. 1999;23:1698–1703. doi: 10.1111/j.1530-0277.1999.tb04063.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.(eds.).. The rat brain in stereotaxic coordinatesFourth EditionAcademic Press: New York, USA; 1998 [Google Scholar]
- Ogren SO, Kehr J, Schott PA. Effects of ventral hippocampal galanin on spatial learning and on in vivo acetylcholine release in the rat. Neuroscience. 1996;75:1127–1140. doi: 10.1016/0306-4522(96)00215-1. [DOI] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F. Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Exp Ther. 1992;260:1237–1243. [PubMed] [Google Scholar]
- Jones DNC, Holtzman SG. Long-term kappa-opioid receptor blockade following nor-binaltorphimine. Eur J Pharmacol. 1992;215:345–348. doi: 10.1016/0014-2999(92)90055-9. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Negus SS, Butelman ER, De Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- Chang AC, Takemori AE, Portoghese PS. 2-(3,4-Dichlorophenyl)-N-Methyl-N-[(1s)1-(3-Isothiocyanatophenyl)-2-(1-Pyrrolidinyl)ethyl]acetamide - an opioid receptor affinity label that produces selective and long-lasting kappa antagonism in mice. J Med Chem. 1994;37:1547–1549. doi: 10.1021/jm00037a001. [DOI] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Dose- and time-dependent bimodal effects of kappa-opioid agonists on locomotor activity in mice. J Pharmacol Exp Ther. 2000;295:1031–1042. [PubMed] [Google Scholar]
- Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis. Curr Protoc Neurosci. 2009;Chapter 7:Unit7 1. doi: 10.1002/0471142301.ns0701s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensson-Nylander I, Nyberg F, Ragnarsson U, Terenius L. A general procedure for analysis of proenkephalin B derived opioid peptides. Regul Pept. 1985;11:65–76. doi: 10.1016/0167-0115(85)90032-1. [DOI] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Langston RF, Stevenson CH, Wilson CL, Saunders I, Wood ER. The role of hippocampal subregions in memory for stimulus associations. Behav Brain Res. 2010;215:275–291. doi: 10.1016/j.bbr.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Gilbert PE, Brushfield AM. The role of the CA3 hippocampal subregion in spatial memory: a process oriented behavioral assessment. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:774–781. doi: 10.1016/j.pnpbp.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP. Behavioral functions of the CA3 subregion of the hippocampus. Learn Mem. 2007;14:771–781. doi: 10.1101/lm.688207. [DOI] [PubMed] [Google Scholar]
- Taqi MM, Bazov I, Watanabe H, Sheedy D, Harper C, Alkass K, et al. Prodynorphin CpG-SNPs associated with alcohol dependence: elevated methylation in the brain of human alcoholics. Addict Biol. 2011;16:499–509. doi: 10.1111/j.1369-1600.2011.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Terman GW, Simmons ML, Milner TA, Kunkel DD, Schwartzkroin PA, et al. Dynorphin opioids present in dentate granule cells may function as retrograde inhibitory neurotransmitters. J Neurosci. 1994;14:3736–3750. doi: 10.1523/JNEUROSCI.14-06-03736.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman GW, Drake CT, Simmons ML, Milner TA, Chavkin C. Opioid modulation of recurrent excitation in the hippocampal dentate gyrus. J Neurosci. 2000;20:4379–4388. doi: 10.1523/JNEUROSCI.20-12-04379.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden AI. Dynorphin increases extracellular levels of excitatory amino acids in the brain through a non-opioid mechanism. J Neurosci. 1992;12:425–429. doi: 10.1523/JNEUROSCI.12-02-00425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skilling SR, Sun X, Kurtz HJ, Larson AA. Selective potentiation of NMDA-induced activity and release of excitatory amino acids by dynorphin: possible roles in paralysis and neurotoxicity. Brain Res. 1992;575:272–278. doi: 10.1016/0006-8993(92)90090-v. [DOI] [PubMed] [Google Scholar]
- Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Modulation of opioid analgesia, tolerance and dependence by G(s)-coupled, GM1 ganglioside-regulated opioid receptor functions. Trends Pharmacol Sci. 1998;19:358–365. doi: 10.1016/s0165-6147(98)01241-3. [DOI] [PubMed] [Google Scholar]
- Rivera M, Gintzler AR. Differential effect of chronic morphine on mRNA encoding adenylyl cyclase isoforms: relevance to physiological sequela of tolerance/dependence. Mol Brain Res. 1998;54:165–169. doi: 10.1016/s0169-328x(97)00303-3. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Chavkin C, Valentino RJ, Siggins GR. Dynorphin-A alters the excitability of pyramidal neurons of the rat hippocampus in vitro. Life Sci. 1983;33 (Suppl 1:533–536. doi: 10.1016/0024-3205(83)90558-1. [DOI] [PubMed] [Google Scholar]
- Moore SD, Madamba SG, Schweitzer P, Siggins GR. Voltage-dependent effects of opioid peptides on hippocampal CA3 pyramidal neurons in vitro. J Neurosci. 1994;14:809–820. doi: 10.1523/JNEUROSCI.14-02-00809.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, Schrader LA. Activation of kappa opioid receptors increases intrinsic excitability of dentate gyrus granule cells. J Physiol. 2011;589 (Pt 14:3517–3532. doi: 10.1113/jphysiol.2011.211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA. 2005;102:19204–19207. doi: 10.1073/pnas.0507572102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CH, Huang CC, Hsu KS. Behavioral stress enhances hippocampal CA1 long-term depression through the blockade of the glutamate uptake. J Neurosci. 2005;25:4288–4293. doi: 10.1523/JNEUROSCI.0406-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TP, Howland JG, Robillard JM, Ge Y, Yu W, Titterness AK, et al. Hippocampal long-term depression mediates acute stress-induced spatial memory retrieval impairment. Proc Natl Acad Sci USA. 2007;104:11471–11476. doi: 10.1073/pnas.0702308104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri H, Tanaka K, Manabe T. Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eur J Neurosci. 2001;14:547–553. doi: 10.1046/j.0953-816x.2001.01664.x. [DOI] [PubMed] [Google Scholar]
- Harper C, Dixon G, Sheedy D, Garrick T. Neuropathological alterations in alcoholic brains. Studies arising from the New South Wales Tissue Resource Centre. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:951–961. doi: 10.1016/S0278-5846(03)00155-6. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G. Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res. 2006;30:1845–1855. doi: 10.1111/j.1530-0277.2006.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79:983–998. doi: 10.1016/s0306-4522(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Walker DW, Freund G. Impairment of shuttle box avoidance learning following prolonged alcohol consumption in rats. Physiol Behav. 1971;7:773–778. doi: 10.1016/0031-9384(71)90147-8. [DOI] [PubMed] [Google Scholar]
- Arendt T, Allen Y, Sinden J, Schugens MM, Marchbanks RM, Lantos PL, et al. Cholinergic-rich brain transplants reverse alcohol-induced memory deficits. Nature. 1988;332:448–450. doi: 10.1038/332448a0. [DOI] [PubMed] [Google Scholar]
- Melis F, Stancampiano R, Imperato A, Carta G, Fadda F. Chronic ethanol consumption in rats: correlation between memory performance and hippocampal acetylcholine release in vivo. Neuroscience. 1996;74:155–159. doi: 10.1016/0306-4522(96)00109-1. [DOI] [PubMed] [Google Scholar]
- Chavkin C. Dynorphin-still an extraordinarily potent opioid peptide. Mol Pharmacol. 2013;83:729–736. doi: 10.1124/mol.112.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann N Y Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Xuei X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L, et al. Association of the kappa-opioid system with alcohol dependence. Mol Psychiatry. 2006;11:1016–1024. doi: 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Wang J, Tian HJ, Pochareddy S, Xuei XL, Wetherill L, et al. A regulatory variation in OPRK1, the gene encoding the kappa-opioid receptor, is associated with alcohol dependence. Hum Mol Genet. 2008;17:1783–1789. doi: 10.1093/hmg/ddn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpyak VM, Winham SJ, Preuss UW, Zill P, Cunningham JM, Walker DL, et al. Association of the PDYN gene with alcohol dependence and the propensity to drink in negative emotional states. Int J Neuropsychopharmacol. 2013;16:975–985. doi: 10.1017/S1461145712001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, et al. The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors Biol Psychiatry 2013. e-pub 2013/04/25. [DOI] [PMC free article] [PubMed]
- Walker BM, Kissler JL.Dissociable effects of kappa-opioid receptor activation on impulsive phenotypes in Wistar rats Neuropsychopharmacology 2013. e-pub 2013/05/22. [DOI] [PMC free article] [PubMed]