Abstract
The human tongue muscle hyoglossus (HG) muscle is active in oro-motor behaviors encompassing a wide range of tongue movement speeds. Here we test the hypothesis that the human HG is composed of “uncommon” myosin heavy chain (MHC) isoforms MHCembryonic, MHCneonatal and MHCslow tonic as has been reported for other head and neck muscles active during kinematically diverse behaviors. Following reaction of human HG with antibodies specific for MHCI, MHCIIA, MHCII, MHCembryonic, MHCextraocular, MHCneonatal and MHCslow tonic only antibodies to MHCI, MHCIIA and MHCIIA-X label more than occasional muscle fibers. These antibodies describe five phenotypes with prevalence MHCIIA>MHCI>MHCI-IIX>MHCI-IIA>MHCIIX. In MHC composition, the human HG is thus similar to human appendicular muscles, the human tongue muscle styloglossus and many human head and neck muscles but different from human masseter and extraocular muscles which contain five or more MHC isoforms.
Keywords: Tongue, Myosin Heavy Chain, Muscle, Immunohistochemistry
Introduction
The tongue muscle hyoglossus (HG) is active in drinking, oral transport, respiration, suckling, swallowing and in humans speech production (1-4), motor behaviors that in humans encompass a wide range of tongue movement speeds (5-8). Muscle fiber contractile properties are correlated with myosin heavy chain (MHC) composition (9,10), and it has been hypothesized that head and neck muscles with complex functional demands will have complex patterns of MHC isoform expression (11; see also 12). We currently lack physiological and molecular studies of the human HG, and thus the extent to which human HG participation in different tongue movements is associated with diversity in HG contractile properties is not known.
Contractile properties of muscle fibers may be modified by the expression of specific MHC isoforms with unique contractile properties and by the hybridization of multiple MHC isoforms in individual muscle fibers thereby creating fibers with intermediate contractile properties (e.g.,13-15). Recent studies suggest evidence for both of these strategies of MHC expression in adult human head and neck extrafusal muscle fibers. Human extraocular muscles, for example, express MHCembryonic, MHCneonatal, MHCαcardiac, MHCextraocular (MHCeom), and MHCslow tonic in addition to MHCI(βcardiac), MHCIIA and MHCIIX isoforms common in human appendicular muscles (16-18). Some adult human masticatory muscles and suprahyoid muscles are also reported to be composed of five (e.g., masseter, 19) or seven (e.g., anterior digastric, mylohyoid; 20, 21) MHC isoforms with a majority of muscle fibers hybrids of common (MHCI, MHCIIA, MHCIIX) and “uncommon” MHC isoforms (e.g, MHCneonatal, MHCαcardiac, MHCslow tonic, 20, 21). Human head and neck muscle MHC composition may thus differ from human appendicular muscles which are composed exclusively of MHCI, MHCIIA and MHCIIX and, with the exception of MHCIIA-MHCIIX, typically have limited MHC hybridization (22-25).
In contrast to extraocular, masticatory and suprahyoid muscles, there has been limited investigation of MHC in human tongue muscles. Stal et al (26) demonstrated MHCI, MHCIIA and probable MHCIIX in human intrinsic tongue muscles (i.e., muscles with origin and insertion in the tongue body) but did not test for other MHC isoforms or for hybridization of MHC isoforms in individual fibers. In our recent study of the extrinsic human tongue muscle styloglossus (27) we did not find evidence for MHCembryonic, MHCneonatal or MHCslow tonic in more than occasional fibers or evidence for a large percent of MHCI-II hybrid fibers (<25% fibers phenotype MHCI-II). The presence of uncommon MHC as not been investigated in other human tongue muscles. Here we test the hypothesis that, despite activation of the human HG during kinematically diverse oromotor behaviors, the human HG does not contain appreciable uncommon MHCembryonic, MHCeom, MHCneonatal and MHCslow tonic.
Materials and Methods
Subjects and Tissue Preparation
Tissue for immunohistochemical study was taken from the left or right HG within 9 hours post-mortem from three subjects with no known neuromuscular disease: a 63 year old female (designated HG1), an 80 year old male (HG2), and a 56 year old male (HG3) (all from the Emory University School of Medicine Body Donor Program, EUSMBDP; all tissue used in this study is IRB-exempt). HG tissue was taken proximal to the decussation of HG with the styloglossus and proximal to the entry of the HG into the tongue body to avoid the inclusion of fibers of other tongue muscles which interdigitate with the HG in the posterior tongue body (e.g., 28). Tissue was sampled from both anterior and posterior regions of the HG to minimize spatial bias. Additional tissue for immunohistochemical control studies and for gel electrophoresis was obtained within 12 hours post-mortem from a human fetal tongue (designated FT; National Disease Research Interchange), from the HG (HG4, tissue sampled as above) and biceps brachii (BB) of an 86 year old female and from the medial rectus (MR) of a 63 year old female (all from EUSMBDP). All tissue samples were immediately mounted onto tongue depressors with OCT tissue tek, quick-frozen in isopentane cooled by liquid nitrogen, and stored at -80°C.
Immunohistochemical Methods
For each muscle sample, serial 12-μm thick cross-sections were cut on a cryostat at -23°C and mounted onto gelatin-subbed slides. Because histological techniques do not allow confident determination of MHC composition and MHC phenotype in muscles containing hybrid fibers and uncommon MHC, serial sections of each muscle were reacted with the following antibodies (Ab) to MHC: Ab 2F7 (Developmental Studies Hybridoma Bank, DSHB, Iowa City, IA) which in rats and likely baboons is specific for MHCIIA (29); Ab 4A6 (DSHB), which in rabbits and likely baboons and humans is specific for MHCeom (18, 30); Ab A4.74 (DSHB), which in humans is reported to be specific for MHCIIA (19, 31, 32) or MHCIIA > MHCIIX (26, 33); Ab A4.84 (DSHB), which in humans is specific for MHCI(βcardiac) (31, 34); Ab F1.652 (DSHB) which in humans is specific for MHCembryonic (35); Ab NCL-MHCd (Vector Laboratories, Burlingame, CA) which in mammals is likely specific for MHCembryonic (36); Ab MY-32 (Sigma-Aldrich, St Louis, MO) which in rats and humans reacts with MHCII isoforms and likely MHCneonatal (37, 38); Ab N2.261 (DSHB) which in humans reacts with MHCI, MHCαcardiac, MHCIIA, and MHCeom (31, 39) or is thought to be specific for MHCneonatal (20); Ab NCL-MHCn (Vector Laboratories, Burlingame, CA) which in mammals is specific for MHCneonatal (19, 40); Ab S46 (DSHB) which reacts with putative MHCslow tonic in adult human muscle spindles and human extraocular muscles but does not cross react with MHCαcardiac or MHCI(βcardiac) in adult catarrhine primate muscle (41); and Ab SC-71 (ATCC) which in humans is thought to be specific for MHCIIA (34, 42), to label MHCIIA>MHCIIX (19, 33)or to label MHCIIA and MHCIIX (43). Ab MY-32 was reacted at dilution of 1:400, Abs 2F7, SC-71 and S46 were reacted at dilution of 1:25 and Abs A4.74, A4.84, F1.652, NCL-MHCd, NCL-MHCn, and N2.261 were reacted at dilution of 1:5. On additional serial sections the anti-laminin Ab D-18 (DSHB, 1:5 dilution) was co-reacted with Abs MHC-MHCd, NCL-MHCn and S46 to aid in muscle fiber visualization (see 26).
Tissue was reacted following the protocol of Eason et al (44). Briefly, tissue sections were incubated in a blocking solution composed of 2% normal goat serum, 0.03% Triton-X, and 0.1 M Tris-HCl (T-NGS) at room temperature for 1 hour, followed by incubation overnight with primary antibody in blocking solution in a humid chamber at -4 °C. Tissue was then washed in Tris-HCl buffer and incubated with secondary antibody (for Ab 4A6, peroxidase-conjugated goat IGM fraction to mouse immunoglobulins, Capel dilution 1:100; for all other Abs, peroxidase-conjugated goat IGG fraction to mouse immunoglobulins, Capel; dilution 1:100) for 1 hour at room temperature. A standard DAB reaction was used to visualize label (0.5 mg DAB/mL, 0.1 M PBS, 0.03% H2O2). Slides were then washed with H20 for 1 minute, dehydrated, and coverslipped in permount.
Tissue sections were viewed on an Olympus BX51 microscope at 100×, 200× and 400× magnification. Images were collected with Neurolucida software (Microbrightfield, Burlington, VT) and a MicroFire digital microscope camera (Optronics, Goleta, CA) and stored onto computer (Dell Optiplex GX270, 1280 × 1024 pixel resolution).
Tests of Ab Specificity and Ab Criteria for MHC Phenotype Classification
In the light of discordant reports of MHC Ab specificities in human muscle (see Methods above) we conducted control studies to minimize the possibility of false-positive attribution of MHC due to Ab cross-reactivity. We reacted Abs with human biceps brachii muscle as a positive control for MHCI, MHCIIA and MHCIIX and a negative control for MHCembryonic, MHCeom, MHCneonatal and MHCslow tonic (see 39, 45); human fetal tongue as a positive control for MHCembryonic and MHCneonatal; and human MR as a positive control for MHCeom and MHCslow tonic (see 16, 18). For some MHC we also compared reaction of two Abs on control and HG tissue to determine optimal immunohistochemical criteria for HG MHC phenotype characterization. Results of these control studies are described by MHC isoform.
MHCembryonic: Abs NCL-MHCd and F1.652
Abs NCL-MHCd and F1.652 labeled muscle fibers in human fetal tongue but did not label muscle fibers in adult human BB (Figure 1B, 1E; data shown for NCL-MHCd). Both Abs have been shown to react with an early developmental MHC, likely MHCembryonic (35, 36). Reaction with either Ab NCL-MHCd or Ab F1.652 is here considered positive for MHCembryonic.
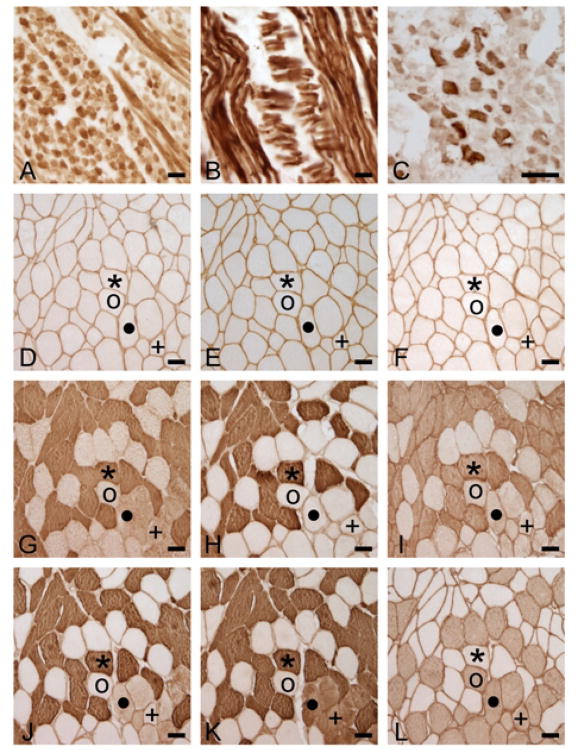
Figure 1.
Reaction of fetal tongue (A, B), adult medial rectus (C) and adult biceps brachii (E-L) with antibody (Ab) NCL-MHCn (A, D), Ab NCL-MHCd (B,E), Ab 4A6 (C,F), Ab N2.261 (G), Ab SC-71 (H), Ab 2F7 (I), Ab A4.74 (J), Ab MY-32 (K) and Ab A4.84 (L). Note reaction of Abs NCL-MHCn and NCL-MHCd to fetal tissue but not to biceps brachii, reaction of Ab N2.261 to biceps brachii MHCII fibers (Ab MY-32+/NCL-MHCn-) and reaction of Abs SC-71 and 2F7 to a subset of MHCII fibers in biceps brachii. Symbols *, o, •, +, denote the same fibers on serial sections. Calibration bar = 50μm.
MHCeom: Ab 4A6
Ab 4A6 labeled muscle fibers in human MR but did not label any muscle fibers in the BB (Figure 1C, 1F). Here reaction with Ab 4A6 is considered positive for MHCeom.
MHCneonatal: Abs NCL-MHCn and N2.261
Ab NCL-MHCn labeled muscle fibers in fetal human tongue but did not label muscle fibers in adult biceps brachii (Figure 1). In contrast, Ab N2.261 labeled muscle fibers in human fetal tongue, Ab MY-32+/NCL-MHCn- (i.e., MHCII) fibers in biceps brachii (Figure 1G) and MHCII (strongly) and MHCI (moderately) fibers in HG (Figure 2E). Because of cross-reaction of Ab N2.261 with MHCII and MHCI isoforms (see also 18, 31, 39), in this study reaction with Ab NCL-MHCn is considered positive for MHCneonatal.
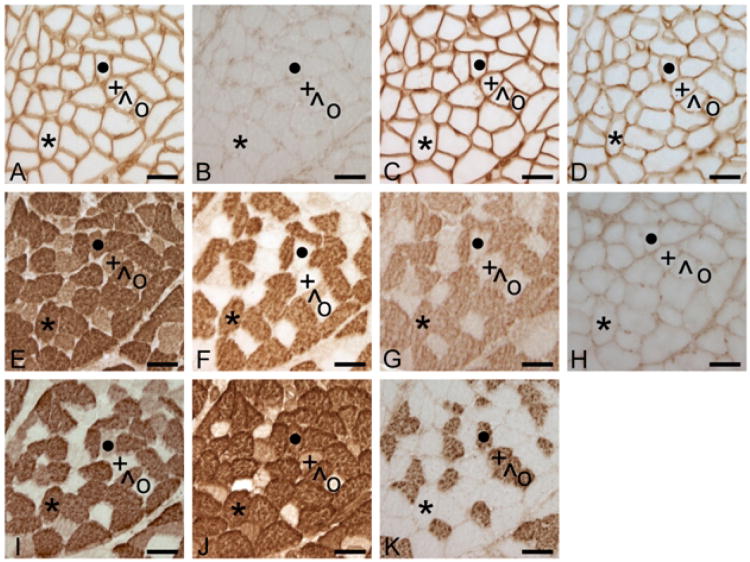
Figure 2.
Serial sections from the human HG (HG1). From A to K, sections are reacted with Ab NCL-MHCn (anti-MHCneonatal), Ab F1.652 (anti-MHCembryonic), Ab NCL-MHCd (anti-MHCembryonic), Ab S46 (anti-MHCslow tonic), Ab N2.261, (anti-MHCII, -MHCI, -MHCeom, -MHCαcardiac), Ab SC-71 (anti-MHCIIa), Ab 2F7 (anti-MHCIIa), Ab 4A6 (anti-MHCextraocular), Ab A4.74 (anti-MHCIIA>MHCIIX), Ab MY-32 (anti-MHCII) and Ab A4.84 (anti-MHCI). Note absence of reaction with Abs to uncommon MHCembryonic, MHCextraocular, MHCneonatal and MHCslow tonic. Also note reaction of Ab N2.261 reacts with all HG fibers and of Abs SC-71 and 2F7 with a subset of MHCII (Ab MY-32+/Ab NCL-MHCn-) fibers. Symbols identify the same muscle fibers in serial sections. + = fiber with profile MHCI-IIX, ˙ = MHCI-IIX, * = MHCIIA, ^ = MHCI-IIA, ° = MHCI. Sections A, C, D and H also reacted with anti-laminin Ab D-18. Calibration bar = 50μm.
MHCslow tonic: Ab S46
Ab S46 labeled muscle fibers in EOM and muscle spindles in BB but did not label extrafusal muscle fibers in BB (data not shown; see also 41). Here reaction with Ab S46 is considered positive for MHCslow tonic.
MHCII: Abs MY-32 and A4.74
Abs MY-32 and A4.74 reacted with control BB (Figure 1J, 1K). Among HG fibers used in this study weak, moderate or strong reaction of either Ab was present in 945 muscle fibers (Figure 2I, 2J). Of these 930 fibers (98.4%) were labeled with both Abs, but 15 fibers (1.6%) were labeled exclusively by Ab MY-32 (Figure 2). Absence of Ab NCL-MHCn reaction in all HG fibers positive for Ab MY-32 suggests that Ab MY-32 label is not due to cross-reactivity with MHCneonatal. We therefore attribute the reaction of Ab MY-32 but not Ab A4.74 in these fibers to the greater sensitivity of Ab MY-32 for MHCIIX. In our study, a reaction profile of Ab MY-32+/Ab NCL-MHCn- is considered positive for MHCII without distinction of MHCIIA and MHCIIX.
MHCIIA: Abs SC-71 and 2F7
Abs SC-71 and 2F7 each labeled a subset of MHCII fibers in the BB (Figure 1H, 1I). Among HG fibers used in this study, Abs SC-71 and Ab 2F7 had similar patterns of reaction: of 682 fibers labeled by either Ab, 676 fibers (99.1%) were labeled by both Abs (Figure 2F, 2G). Four fibers (0.6%) were labeled by Ab 2F7 but not Ab SC-71 and two fibers (0.3%) were labeled by Ab SC-71 but not Ab 2F7. With the exception of one fiber, all fibers positive for Abs SC-71 or 2F7 were also positive for Ab MY-32. Reaction of Abs 2F7 or SC-71 is here considered positive for MHCIIA. Fibers with reaction profiles Ab 2F7+/Ab SC-71+/Ab MY-32+, Ab SC-71+/Ab MY-32+ and Ab 2F7+/MY-32+ are categorized as MHCIIA, although the presence of MHCIIX cannot be ruled out in these fibers.
Based on our control studies, we used Abs 4A6, A4.84, F1.652, MY-32, NCL-MHCd, NCL-MHCn, SC-71, S46 and 2F7 to determine HG MHC composition.
MHC Composition, Phenotype Analysis and Statistics
MHC Composition
Most Abs either labeled many or no muscle fibers, but with some Abs only occasional fibers were labeled. For reactions with these Abs we counted every labeled muscle fiber and expressed this number relative to the total number of muscle fibers in our samples, determined for each subject on a serial section reacted with Abs NCL-MHCn and D-18 (Physical Fractionator software, Stereo Investigator, Microbrightfield, Vermont; second estimated Schmitz-Hof CE (46) of 0.029 for HG1, 0.027 for HG2 and 0.037 for HG3). Perimeters of fibers positive for Abs F1.652, NCL-MHCd, NCL-MHCn and S46 were traced at 400× magnification in Neurolucida software (Microbrightfield, Vermont; MicroFire digital microscope camera, Dell Optiplex GX270, 1280 × 1024 pixel resolution) and measurement of diameter were calculated by the program (the few positive fibers sectioned obliquely were not measured). The diameter measurement used is defined as the feret max of the fiber, which is calculated as the maximum distance between two parallel lines that enclose the muscle fiber (Neurolucida).
MHC Phenotype Analysis
Hyoglossus muscle fiber MHC phenotype was determined by analyzing 1349 muscle fibers from three individuals (475 from HG1, 464 from HG2, 410 from HG3). For each individual, fibers were sampled from anterior and posterior HG regions in fascicles with consistent morphology through all tissue sections. Even with this criterion, a few fibers could not be followed in all sections and thus were excluded from analysis. Individual fibers were scored as positive or negative for MHCI, MHCIIA, MHCII(A/X), MHCembryonic, MHCeom, MHCneonatal and MHCslow tonic and MHC phenotype was determined based on reaction profile (Table 1). Fiber perimeters were traced and fiber cross-sectional area, perimeter and diameter were calculated in Neurolucida software
Table I. Immunohistochemical Criteria for Myosin Heavy Chain (MHC) Phenotype Classification.
| MHC Phenotype | Aba2F7 | Ab A4.84 | Ab NCL-MHCnb | Ab MY-32 | Ab SC-71 |
|---|---|---|---|---|---|
| MHCI | − | + | − | − | − |
| MHCIIAc | + | − | − | + | + |
| MHCIIX | − | − | − | + | − |
| MHCI-IIAd | + | + | − | + | + |
| MHCI-IIAe | − | + | − | + | + |
| MHCI-IIAf | + | + | − | + | − |
| MHCI-IIAg | + | + | − | − | − |
| MHCI-IIX | − | + | − | + | − |
Ab = Antibody
All fibers used for phenotype analysis were negative for Ab NCL-MHCn
Classified as Phenotype MHCIIA but cannot rule out the presence of MHCIIX with Abs used.
Classified as Phenotype MHCI-IIA but cannot rule out the presence of MHCIIX with Abs used.
Two fibers had this profile.
Three fibers had this profile.
One fiber had this profile.
Graphs of the distribution of MHC phenotypes and fiber measurements were generated in Statistica (ver 6, Statsoft). Fiber counts per 1000 were calculated for each phenotype. Ninety-five percent confidence intervals were estimated for the prevalence rates using exact methods based on the Poisson distribution. Repeated-measures analyses using mixed linear models were performed for perimeter, diameter and area utilizing SAS Proc Mixed (version 9, SAS Institute, Cary NC). These models provide separate estimates of the means by phenotype. A compound symmetry variance-covariance form in repeated measurements was assumed for each outcome, and robust estimates of the standard errors of parameters were used to do statistical tests and construct 95% confidence intervals. The model-based means are unbiased with unbalanced and missing data so long as the missing data are non-informative (missing at random). Statistical tests were 2-sided. A P-value less than 0.05 was considered statistically significant.
Electrophoretic Immunoblotting
Tissue obtained from HG2, HG4, BB, MR and FT was used for immunoblot test of MHCembryonic, MHCneonatal and MHCslow tonic. Approximately 15-50 mgs of muscle tissue was placed in 300 ul of non-denaturing lysis buffer (50 mM Tris, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, with 0.02% NaN3 and 0.1% protease inhibitors and homogenized for 30 seconds with a Tissuemiser at 15,000 rpm in an ice bath and cleared by centrifugation at 14,000 × g for 10 minutes. Protein content was assayed by bicinchronic acid assay (BCA; Pierce, Rockford, IL) according to the manufacturer's instructions. Protein was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis through a 7.5% acrylamide resolving gel at 200 V for 60 minutes. Equivalent amounts of protein were loaded in each lane at levels that produced reaction in positive control tissue (8.20-8.36 μg/lane for test of MHCembryonic and MHCneonatal, 19.80-19.92 μg/lane for test of MHCslow tonic). Migration of MHC was determined by running a 204 kDa molecular weight standard (Bio-Rad kaleidoscope prestained standard; Bio-Rad Laboratories, Hercules, CA) on each gel. Gels were transferred to a PVDF membrane using a semi-wet transfer system. Membranes were blocked for 60 minutes in 0.5% blocking buffer prior to incubation with primary Abs F1.652, NCL-MHCd, NCL-MHCn, N2.261 or S46 (1:500 dilution). Well-rinsed membranes were conjugated with secondary Alexa Fluor 680 goat anti-mouse IgG (1:2,000 dilution) and visualized by Odyssey Infrared Imaging System (Li-Cor, Lincoln, NE).
Results
Immunohistochemical Identification of MHC Isoforms
MHCI, MHCIIA, MHCII
Abs 2F7, A4.84, MY-32 and SC-71 each labeled many fibers in all HG samples (Figure 2).
MHCembryonic, MHCeom MHCneonatal and MHCslow tonic
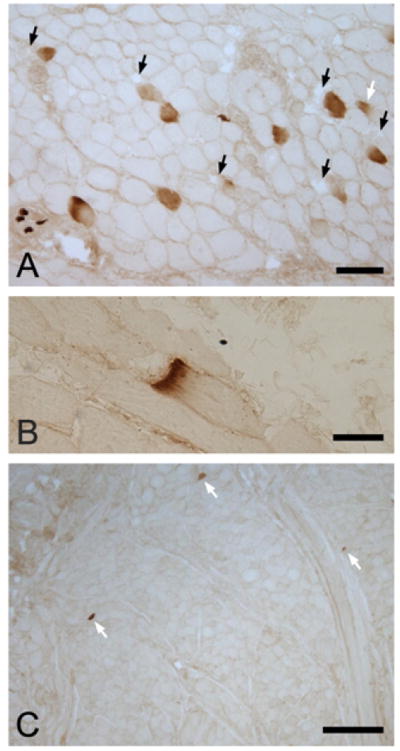
Abs 4A6, F1.652, NCL-MHCd, NCL-MHCn and S46 labeled few or no fibers in HG tissue sections (Figure 2). No fibers were positive for Ab 4A6 (anti-MHCeom) and few were positive for Abs F1.652 or NCL-MHCd (anti-MHCembryonic, <0.14% fibers, Table 2) or for Ab S46 (anti-MHCslow tonic, <0.18%) (Figure 2; Table 2). Ab NCL-MHCn (anti-MHCneonatal) labeled from 0.36 to 1.35% of muscle fibers (Table 2; Figure 3). Most fibers labeled with Abs to MHCembryonic, MHCneonatal or MHCslow tonic were within the size range of muscle fibers composed of MHCI, MHCIIA and MHCIIX (≥23 μm diameter, see below): for MHCembryonic, 71% of fibers were ≥23 μm diameter (data for Ab NCL-MHCd), for MHCslow tonic 97.4% of fibers were ≥23 μm diameter, and for MHCneonatal 74% of fibers were ≥23 μm diameter. Many Ab NCL-MHCn-positive fibers were adjacent to fascicle borders or to spaces devoid of muscle fibers (Figure 3A). In occasional fibers sectioned obliquely, reaction with Ab NCL-MHCn was typically localized to the fiber termination (Figure 3B).
Table II. Number and Percent of Fibers with Uncommon Myosin Heavy Chain.
| Total No of Fibersa | No. (%) Fibers Labeled with Ab NCL-MHCd | No. (%) Fibers Labeled with Ab F1.652 | No. (%) Fibers Labeled with Ab NCL-MHCn | No. (%) Fibers Labeled with Ab S46 | |
|---|---|---|---|---|---|
| HT1 | 8921 | 11 (0.12) | 3 (0.03) | 60 (0.67) | 5 (0.06) |
| HT2 | 24788 | 31 (0.13) | 14 (0.06) | 89 (0.36) | 41 (0.17) |
| HT3 | 13088 | 0 (0.0) | 2 (0.02 | 177 (1.35) | 9 (0.07) |
Total fiber counts made using Physical Fractionator, Stereo Investigator; second estimated Schmitz-Hof CE (45) of 0.029 for HG1, 0.027 for HG2 and 0.037 for HG3).
Figure 3.
Occasional fibers labeled with Ab NCL- label of MHCneonatal in human HG.
Muscle Fiber Phenotype Classification and Size Measures
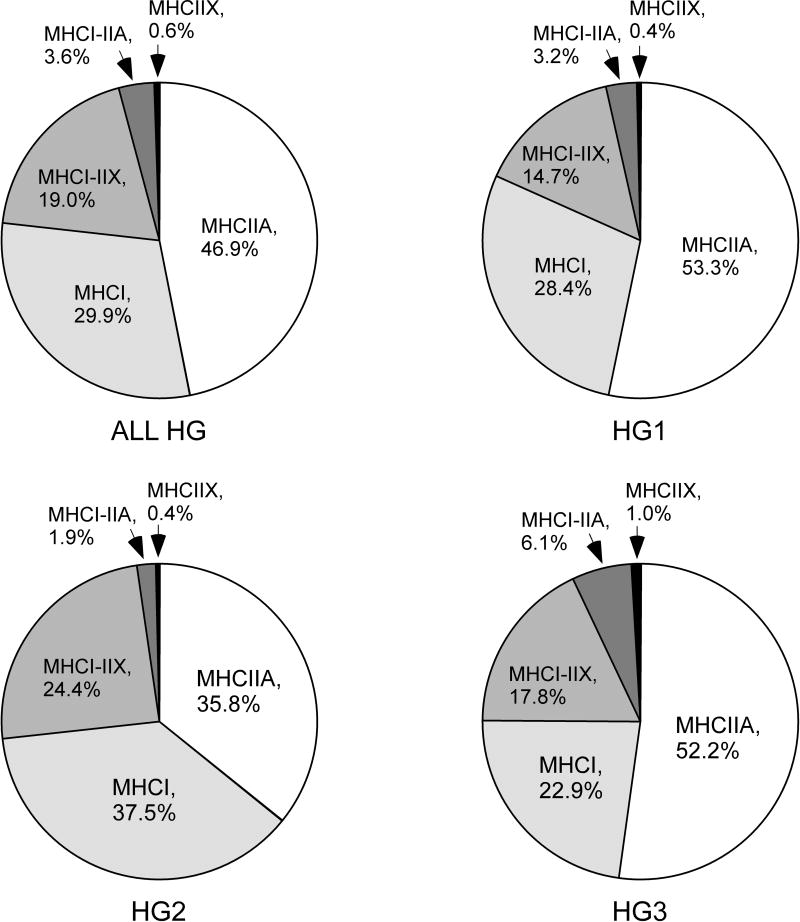
The prevalence and morphometry of muscle fiber phenotypes was determined from 1349 muscle fibers from HG1 (n=475), HG2 (n=464) and HG3 (n=410). These fibers only contained MHCI, MHCIIA and MHCIIX. Table 3 provides a summary of the fiber counts/1000 for each phenotype. The most frequent fiber type was MHCIIA, followed by MHCI, MHCI-IIX, MHCI-IIA and MHCIIX (Figure 4).
Table III. Fiber Counts/1000 (95% Confidence Interval) from the Human Hyoglossus Muscle by Myosin Heavy Chain (MHC) Phenotype.
| Phenotype | All Subjects | HG1 | HG2 | HG3 |
|---|---|---|---|---|
| MHCI | 299 (270 to 329) | 284 (238 to 336) | 375 (322 to 435) | 229 (185 to 281) |
| MHCI-IIA | 36 (27 to 48) | 32 (18 to 52) | 19 (9 to 37) | 61 (40 to 90) |
| MHCI-IIX | 190 (167 to 215) | 147 (115 to 186) | 244 (201 to 293) | 178 (140 to 224) |
| MHCIIA | 469 (433 to 507) | 533 (469 to 603) | 358 (305 to 417) | 522 (454 to 597) |
| MHCIIX | 6 (3 to 12) | 4 (1 to 15) | 4 (1 to 15) | 10 (3 to 25) |
Figure 4.
Pie charts showing the prevalence of fibers of phenotypes MHCI, MHCIIa, MHCI-MHCIIa, MHCI-IIx and MHCIIx.
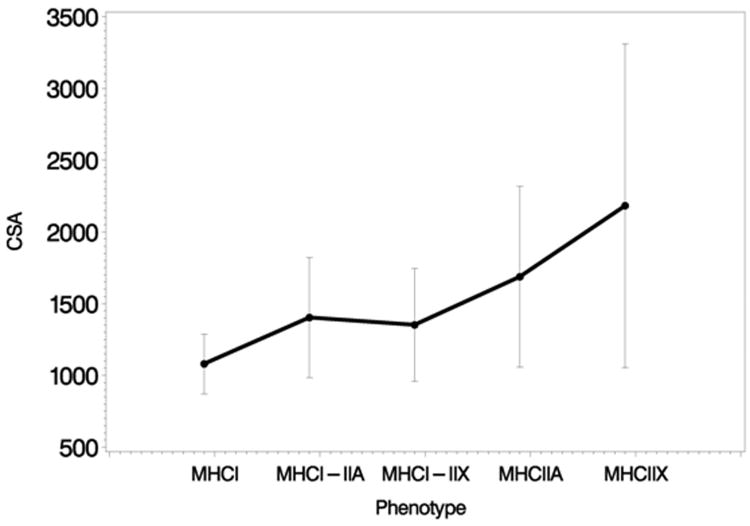
Mean cross-sectional area increased across the 5 phenotype groups (MHCI, MHCI-IIA, MHCI-IIX, MHCIIA and MHCIIX) (p = 0.027, test for linear trend; Figure 5). The diameter also increased across the 5 phenotype groups (p = 0.0013, test for linear trend). A similar linear trend was identified for perimeter (p = 0.0055).
Figure 5.
Cross sectional area (CSA) of fibers with phenotype MHCI, MHCIIX, MHCIIX, MHCI-IIA, and MHCI-IIX. Note increase in CSA by phenotype in anterior but not posterior HG.
Immunoblot Identification of MHC Isoforms
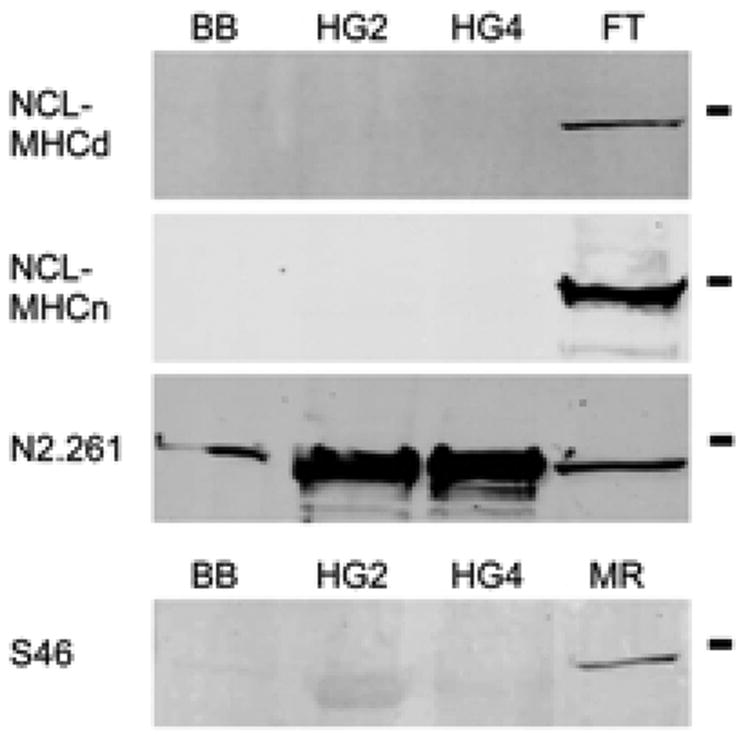
MHCembryonic, MHCneonatal and MHCslow tonic were demonstrated in positive control tissue (FT and MR) but were not demonstrated in human HG or BB (Figure 6).
Figure 6.
Immunoblots of human biceps brachii, HG2, HG4 and fetal tongue (top three panels) and biceps brachii, HG2, HG4 and medial rectus (bottom panel). Abs NCL-MHCd and NCL-MHCn react with fetal tongue but not biceps or HG. Ab N2.261 reacts with all tissue. Ab S46 reacts exclusively with medial rectus.
Discussion
Summary
The primary finding of this study is that the human HG is composed of MHCI, MHCIIA, and MHCIIX isoforms and does not contain MHCembryonic, MHCneonatal or MHCslow tonic in more than occasional fibers. A secondary finding is that the majority of HG muscle fibers are composed of either slow (MHCI) or fast (MHCIIA, possibly MHCIIA-IIX) isoforms, and that slow-fast hybrids account for <26% of muscle fibers. In MHC phenotype composition, the human HG is thus similar to human appendicular muscles and many human head and neck muscles but different from a handful of human head and neck muscles which express MHCembryonic, MHCneonatal and MHCslow tonic in an appreciable number of fibers.
Comparison to other human tongue muscles
The present findings are generally similar to reports of MHC composition and muscle fiber Type (by ATPase) in other human tongue muscles. In our study of the human styloglossus (27), we found few or no fibers positive for MHCembryonic or MHCneonatal. MHC phenotype prevalence was also ranked MHCII>MHCI>MHCI-II (although hybrid fibers were less common, MHCI-II, 17%) and ranking of fiber size by phenotype (MHCII>MHCI-II>MHCI) was the same as in the current study. In a previous study, appreciable MHCslow tonic was not present in human tongue muscles genioglossus, HG, superior longitudinalis, transversus, and verticalis (41). In human intrinsic tongue muscles, Stal et al (26) reported positive reaction with Abs to MHCII and MHCI as well as regional variation of fiber Type prevalence, with prevalence of Type IIA highest in anterior tongue regions (57-59%) and of Type I highest in posterior regions (46-58%). Prevalence of Type IM/IIC fibers (which reacted with Abs for both MHCI and MHCII) was 4-8% in anterior and middle regions but 20% in posterior regions. Thus in comparison to anterior and middle intrinsic tongue muscles, posterior intrinsic muscles, the HG and the styloglossus share a relatively high prevalence of slow-fast hybrid fibers and thus presumably increased diversity in muscle fiber contractile properties. Saigusa et al (46) noted greater prevalence of Type I fibers in posterior (51%) versus anterior (31.3%) genioglossus regions but did not report IM/IIC. Size measures for the HG (average diameter, 56.4 μm) are similar to those for styloglossus (27) and posterior intrinsic tongue muscles (47 μm) (26), but much greater than measures for the genioglossus (by region, 19.2-25.7 μm) (46).
Comparison to other human cranial muscles
The extent of MHC diversity in human cranial muscles is currently a subject of some contention (e.g., 20, 21, 41, 44, 47, 48). In human extraocular muscle, MHCeom, MHCαcardiac, MHCneonatal and MHCslow tonic are each expressed in many muscle fibers (16, 17, 18). Appreciable expression (i.e., each MHC expressed in >5% fibers) is reported for MHCneonatal in the masseter (44, 47), medial pterygoid (47), sternohyoid (47), and temporalis (44, 49) and for MHCαcardiac in the digastric (47), geniohyoid, (47), lateral pterygoid (44, 47), masseter (47, 51), medial pterygoid (47) and temporalis (44, 47). In other muscles tested however, studies report few or no fibers positive for MHCneonatal (e.g., digastric (44, 47), cricopharyngeus, (48), geniohyoid (44), interarytenoid (52), levator veli palatini (53), palatopharyngeus (53), pharyngeal constrictor (48), posterior cricoarytenoid, (38), lateral pterygoid (44), sternohyoid (54), tensor veli palatini (53), styloglossus (27), thyroarytenoid (54), uvula (53), zygomaticus major/minor (55)), few or no fibers positive for MHCαcardiac (e.g., anterior digastric (44), levator veli palatini (53), masseter (44, 55), mylohyoid (47), obicularis oris (55), palatopharyngeus (53), posterior cricoarytenoid, (38), tensor veli palatini (53), uvula (53) zygomaticus major/minor (55)), few or no fibers positive for MHCslow tonic (e.g., anterior digastric (41), genioglossus (41), geniohyoid (41) hyoglossus (41), intrinsic tongue muscles (41), levator veli palatini (53), masseter (55), mylohyoid (41), palatopharyngeus (53), posterior cricoarytenoid (38), styloglossus (41), tensor veli palatini (53), thyroarytenoid (54), zygomaticus major/minor (55)), and few or no fibers positive for MHCembryonic (e.g., digastric (44), levator veli palatini (53), masseter (44, 55), mylohyoid (20), obicularis oris (55), palatopharyngeus (53), lateral pterygoid (44), tensor veli palatini (53), uvula (53) and zygomaticus major/minor (55)). A conclusion from these studies is that appreciable expression of less common MHC (i.e., MHCαcardiac, MHCembryonic, MHCneonatal and MHCslow tonic) is a feature of only a few human head and neck muscles (primarily human extraocular muscles and muscles of mastication).
In contrast to this conclusion are recent reports of abundant MHCαcardiac and MHCslow tonic in human digastric (21) and abundant MHCαcardiac, MHCneonatal and MHCslow tonic in human mylohyoid cricopharyngeus, inferior pharyngeal constrictor and upper esophagus (20, 56). Because discordant reports of MHC composition in the same muscles may reflect differing specificities of Abs used in different studies (e.g, Ab BA-G5 to identify MHCαcardiac, Ab ALD-58 to identify MHCslow tonic and Ab N2.261 to identify MHCneonatal in 20, 21, 56 versus Abs F88112F8 and 249-5A4 to identify MHCαcardiac, Ab S46 to identify MHCslow tonic, and Abs NN5 and NCL-MHCn to identify MHCneonatal in 41, 47, 48, 59) we tested Abs on control tissue sections to establish Ab specificity in our hands. Whereas Abs F1.652, NCL-MHCd and NCL-MHCn reacted with fetal but not adult control tissue, Ab N2.261 reacted with MHCII in adult BB and with MHCII>MHCI in adult HG (Figures 1, 2; see also 31, 39). In adult human tissue, Ab 4A6 reacted exclusively with putative MHCeom in human extraocular muscle and Ab S46 reacted exclusively with putative MHCslow tonic in extraocular muscle and muscle spindles (we did not test for MHCαcardiac). Based on these findings, we tested for MHCembryonic, MHCneonatal and MHCslow tonic in HG with Abs 4A6, F1.652, NCL-MHCd, NCL-MHCn and S46; no or few fibers were labeled with these Abs. We conclude that there is no appreciable MHCeom, MHCembryonic, MHCneonatal or MHCslow tonic in the human HG and that, despite the activation of the HG in behaviors with different tongue movement speeds and diverse tongue shape changes, expression of multiple, uncommon MHC is not required for normal HG function. We also suggest that recent reports of abundant MHCneonatal and MHCslow tonic (and possibly MHCαcardiac) in human digastric, mylohyoid, cricopharyngeus, inferior pharyngeal constrictor and upper esophagus may reflect the false-positive attribution of uncommon MHC due to Ab cross-reactivity with other MHC isoforms (i.e., Ab N2.261 with MHCII>I (31, 39), ALD58 with MHCI and MHCαcardiac (41, see also 57) and possibly Ab BA-G5 with MHCI (58)).
Limited expression of MHCembryonic, MHCneonatal and MHCslow tonic in human HG
Antibody S46 to MHCslow tonic labeled a very few fibers (0.06-0.17%) in the human HG. Ab S46-positive fibers were isolated and were not associated with obvious muscle spindle structures. Isolated extrafusal fibers positive for MHCslow tonic have also been reported in a number of human cranial muscles including anterior digastric, intrinsic tongue muscles, mylohyoid and thyroarytenoid (41, 54). We have no explanation for the limited expression of MHCslow tonic in these muscles. Previously we found no MHCslow tonic in HG from two different individuals (41).
In adult vertebrates, developmental MHC is present in muscles in a number of pathological (e.g. 61, 62, 63) and experimental (e.g., 64, 65, 66) conditions. Expression of developmental MHC in these conditions is typically considered to be associated with muscle fiber atrophy, denervation, regeneration and/or remodeling (see 61, 65, 67); muscle fibers positive for developmental MHC range in size, but commonly are very small (i.e. 63, 66). Additionally in normal adult vertebrate muscle, MHCembryonic may be expressed at myotendinous junctions (35; for expression at myotendinous junctions following muscle loading see 68) and MHCneonatal at muscle fiber terminations (69; for expression at myotendinous junctions following muscle stretch see 70). Expression of MHCneonatal at muscle fiber terminations has been most extensively studied in the avian pectoralis (e.g., 70, 71), a muscle of in-series design (i.e., primarily comprised of short, tapered muscle fibers that overlap from muscle origin to insertion). Reflecting the elongated tapered morphology of constituent muscle fibers, most MHCneonatal-positive fibers are very small and react for MHCneonatal in many serial tissue sections (71).
In the human HG, fibers positive for MHCembryonic were few (0.0%-0.13%) and predominantly of normal size suggesting, by criteria of MHCembryonic and fiber size, very limited muscle fiber regeneration or remodeling in our HG sample. Muscle fibers positive for MHCneonatal were more common (0.35% to 1.35%) but also predominantly of normal size. Although we cannot rule out a pathological basis for MHCneonatal in ou sample, the normal size of most MHCneonatal-positive fibers, the location of MHCneonatal fibers adjacent to fascicle borders and regions devoid of muscle fibers and the terminal localization of MHCneonatal in most obliquely-sectioned muscle fibers suggests that MHCneonatal in the human HG is primarily expressed at muscle fiber terminations (see 53 for a similar suggestion in the human interarytenoid muscle). To our knowledge the architecture of human HG muscle fibers has not been described, but from these findings we hypothesize that most human HG muscle fibers have blunt terminations. Blunt terminations are present in >99% of fibers in the human tongue muscle superior longitudinalis (72). We also suggest that findings of occasional fibers positive for MHCneonatal and low levels of MHCneonatal mRNA in other non-pathological head and neck muscles (e.g. 48, 49, 51, 73) may at least partially reflect the expression and sampling of MHCneonatal at muscle fiber terminations and not solely the specialization of muscle fiber phenotype.
Acknowledgments
This work was supported by grant DC005017 from the National Institute on Deafness and Other Communication Disorders to Dr. Alan J. Sokoloff. The authors would also like to thank Mr. Kirk Easley, MApStat, for help with statistical analyses, Dr. Dominique Weber for hybridoma culture, Ms. Karen Jensen at DSHB for Ab production, Dr. Shawn Hochman and Mr. Michael Sawchuk for assistance with stereology, and Ms. Betty Yang for laboratory assistance. Human tissue was kindly provided by the Emory University School of Medicine Body Donor Program or purchased from the National Disease Research Interchange.
Footnotes
supported by grant DC005017 from the National Institute on Deafness and Other Communication Disorders to A. Sokoloff.
References
- 1.Car A, Amri M. Activity of neurons located in the region of the hypoglossal motor nucleus during swallowing in sheep. Exp Brain Res. 1987;69:175–82. doi: 10.1007/BF00247040. [DOI] [PubMed] [Google Scholar]
- 2.Bailey E, Fregosi RF. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J Appl Physiol. 2004;96:440–9. doi: 10.1152/japplphysiol.00733.2003. [DOI] [PubMed] [Google Scholar]
- 3.Baer TA, Alfonso PJ, Honda K. Electromyography of the tongue muscles during vowels in/epvp/environment. Ann Bull RILP. 1988;22:7–19. [Google Scholar]
- 4.Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool. 1998;280:327–43. doi: 10.1002/(sici)1097-010x(19980401)280:5<327::aid-jez2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Hirose H, Kiritani S. Velocity of articulatory movements in normal; and dysarthric subject. Ann Bull RILP. 1979;13:105–112. doi: 10.1159/000265651. [DOI] [PubMed] [Google Scholar]
- 6.Cheng CF, Peng CL, Chiou HY, Tsai CY. Dentofacial morphology and tongue function during swallowing. Am J Orthod Dentofacial Orthop. 2002;122:491–9. doi: 10.1067/mod.2002.128865. [DOI] [PubMed] [Google Scholar]
- 7.Shcherbatyy V, Liu ZJ. Internal kinematics of the tongue during feeding in pigs. Anat Rec. 2007;290:1288–99. doi: 10.1002/ar.20582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tasko SM, Kent RD, Westbury JR. Variability in tongue movement kinematics during normal liquid swallowing. Dysphagia. 2002;17:126–38. doi: 10.1007/s00455-001-0112-6. [DOI] [PubMed] [Google Scholar]
- 9.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 10.Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73:195–262. doi: 10.1016/s0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- 11.Hoh JF. Laryngeal muscle fibre types. Acta Physiol Scand. 2005;183:133–149. doi: 10.1111/j.1365-201X.2004.01402.x. [DOI] [PubMed] [Google Scholar]
- 12.Butler-Browne GS, Eriksson PO, Laurent C, Thornell LE. Adult human masseter muscle fibers express myosin isozymes characteristic of development. Muscle & Nerve. 1988;11:610–620. doi: 10.1002/mus.880110614. [DOI] [PubMed] [Google Scholar]
- 13.Reiser PJ, Moss RL, Giulian GG, Greaser M. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985;260:9077–80. [PubMed] [Google Scholar]
- 14.Galler S, Hilber K, Pette D. Stretch activation and myosin heavy chain isoforms of rat, rabbit and human skeletal muscle fibres. J Muscle Res Cell Motil. 1997;18:441–448. doi: 10.1023/a:1018646814843. [DOI] [PubMed] [Google Scholar]
- 15.D'Antona G, Megighian A, Bortolotto S, Pellegrino MA, Marchese-Ragona R, Staffieri A, Botinelli R, Regianni C. Contractile properties and myosin heavy chain isoform composition in single fibres of human laryngeal muscles. J Muscle Res Cell Motil. 2002;23:187–195. doi: 10.1023/a:1020963021105. [DOI] [PubMed] [Google Scholar]
- 16.Bormioli SP, Torresan P, Sartore S, Moschini GB, Schiaffino S. Immunohistochemical identification of slow-tonic fibers in human extrinsic eye muscles. Invest Ophthalmol Vis Sci. 1979;18:303–6. [PubMed] [Google Scholar]
- 17.Wieczorek DF, Periasamy M, Butler-Brown GS, Whalen RG, Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kjellgren D, Thornell LE, Andersen J, Pedrosa-Domellof Myosin heavy chain isoforms in human extraocular muscles. Invest Ophthalmol Vis Sci. 2003;44:1419–1425. doi: 10.1167/iovs.02-0638. [DOI] [PubMed] [Google Scholar]
- 19.Yu F, Stal P, Thornell LE, Larsson L. Human single masseter muscle fibers contain unique combinations of myosin and myosin binding protein C isoforms. J Muscle Res Cell Motil. 2002;23:317–326. doi: 10.1023/a:1022061706126. [DOI] [PubMed] [Google Scholar]
- 20.Mu L, Su H, Wang J, Han Y, Sanders I. Adult human mylohyoid muscle fibers express slow-tonic, alpha-cardiac, and developmental myosin heavy-chain isoforms. Anat Rec A Discov Mol Cell Evol Biol. 2004;279:749–60. doi: 10.1002/ar.a.20065. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Han Y, Su H, Mu L. Expression of unique and developmental myosin heavy chain isoforms in adult human digastric muscle. J Histochem Cytochem. 2004;52:851–859. doi: 10.1369/jhc.3A6136.2004. [DOI] [PubMed] [Google Scholar]
- 22.Smerdu V, Karsch-Mizrachi I, Campione M, Leinwand L, Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol. 1994;267:C1723–8. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- 23.Williamson DL, Gallagher PM, Carroll CC, Raue U, Trappe SW. Reduction in hybrid single muscle fiber proportions with resistance training in humans. J Appl Physiol. 2001;91:1955–1961. doi: 10.1152/jappl.2001.91.5.1955. [DOI] [PubMed] [Google Scholar]
- 24.Andersen JL, Terzis G, Kryger A. Increase in the degree of coexpression of myosin heavy chain isoforms in skeletal muscle fibers of the very old. Muscle Nerve. 1999;22:449–54. doi: 10.1002/(sici)1097-4598(199904)22:4<449::aid-mus4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Zawadowska B, Majerczak J, Semik D, Karasinski J, Kolodziejski L, Kilarski WM, Duda K, Zoladz JA. Characteristics of myosin profile in human vastus lateralis muscle in relation to training background. Folia Histochem Cytobiol. 2004;42:181–90. [PubMed] [Google Scholar]
- 26.Stal P, Marklund S, Thornell LE, De Paul R, Eriksson PO. Fibre composition of human intrinsic tongue muscles. Cells Tissues Organs. 2003;173:147–61. doi: 10.1159/000069470. [DOI] [PubMed] [Google Scholar]
- 27.Sokoloff AJ, Yang B, Li H, Burkholder TJ. Immunohistochemical characterization of slow and fast myosin heavy chain composition of muscle fibres in the styloglossus muscle of the human and macaque (Macaca rhesus) Arch Oral Biol. 2007;52:533–43. doi: 10.1016/j.archoralbio.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abd-El-Malek S. Observations on the morphology of the human tongue. J Anat. 1939;73:201–210. [PMC free article] [PubMed] [Google Scholar]
- 29.Lucas CA, Kang LHD, Hoh JFY. Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem Biophys Res Comm. 2000;272:303–308. doi: 10.1006/bbrc.2000.2768. [DOI] [PubMed] [Google Scholar]
- 30.Lucas CA, Hoh JFY. Extraocular fast myosin heavy chain expression in the levator palpebrae and retractor bulbi muscles. Invest Opthal & Vis Sci. 1997;38:2817–2825. [PubMed] [Google Scholar]
- 31.Hughes SM, Cho M, Karsch-Mizrachi I, Travis M, Silberstein L, Leinwand LA, Blau HM. Three slow myosin heavy chains sequentially expressed in developing mammalian skeletal muscle. Dev Biol. 1993;158:183–99. doi: 10.1006/dbio.1993.1178. [DOI] [PubMed] [Google Scholar]
- 32.Tikunov BA, Mancini D, Levine S. Changes in myofibrillar protein composition of human diaphragm elicited by congestive heart failure. J Mol Cell Cardiol. 1996;28:2537–41. doi: 10.1006/jmcc.1996.0245. [DOI] [PubMed] [Google Scholar]
- 33.Smerdu V, Soukup T. Demonstration of myosin heavy chain isoforms in rat and humans: the specificity of seven available monoclonal antibodies used in immunohistochemical and immunoblotting methods. Eur J Histochem. 2008;52:179–90. doi: 10.4081/1210. [DOI] [PubMed] [Google Scholar]
- 34.Li ZB, Lehar M, Nakagawa H, Hoh JFY, Flint PW. Differential expression of myosin heavy chain isoforms between abductor and adductor muscles in the human larynx. Otolaryngol Head Neck Surg. 2004;130:217–222. doi: 10.1016/j.otohns.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Cho M, Hughes SM, Karsch-Mizrachi I, Travis M, Leinwand LA, Blau HM. Fast myosin heavy chains expressed in secondary mammalian muscle fibers at the time of their inception. J Cell Sci. 1994;107:2361–71. doi: 10.1242/jcs.107.9.2361. [DOI] [PubMed] [Google Scholar]
- 36.Brueckner JK, Itkis O, Porter JD. Spatial and temporal patterns of myosin heavy chain expression in developing rat extraocular muscle. J Muscle Res Cell Motil. 1996;17:297–312. doi: 10.1007/BF00240928. [DOI] [PubMed] [Google Scholar]
- 37.Harris AJ, Fitzsimonrs B, McEwan JC. Neural control of the sequence of expression of myosin heavy chain isoforms in foetal mammalian muscles. Development. 1989;107:751–769. doi: 10.1242/dev.107.4.751. [DOI] [PubMed] [Google Scholar]
- 38.Brandon CA, Rosen C, Georgelis G, Horton MJ, Mooney MP, Sciote JJ. Muscle fiber type composition and effects of vocal fold immobilization on the two compartments of the human posterior cricoarytenoid: a case study of four patients. J Voice. 2003;17:63–75. doi: 10.1016/s0892-1997(03)00027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu JX, Eriksson PO, Thornell LE, Pedrosa-Domellof F. Myosin heavy chain composition of muscle spindles in human biceps brachii. J Histochem Cytochem. 2002;50:171–83. doi: 10.1177/002215540205000205. [DOI] [PubMed] [Google Scholar]
- 40.Ecob-Prince M, Hill M, Brown W. Immunocytochemical demonstration of myosin heavy chain expression in human muscle. J Neurol Sci. 1989;91:71–78. doi: 10.1016/0022-510x(89)90076-2. [DOI] [PubMed] [Google Scholar]
- 41.Sokoloff AJ, Li H, Burkholder TJ. Limited expression of slow tonic myosin heavy chain in human cranial muscles. Muscle Nerve. 2007;36:183–9. doi: 10.1002/mus.20797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano AL, Perez M, Lucia A, Chicharro JL, Quiroz-Rothe E, Rivero JLL. Immunolabelling, histochemistry and in situ hybridisation in human skeletal muscle fibres to detect myosin heavy chain expression at the protein and mRNA level. J Anat. 2001;199:329–337. doi: 10.1046/j.1469-7580.2001.19930329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bamman MM, Clarke MS, Feeback DL, Talmadge RJ, Stevens BR, Lieberman SA, Greenisen Ml. Impact of resistance exercise during bed rest on skeletal muscle sarcopenia and myosin isoform distribution. J Appl Physiol. 1998;84:157–63. doi: 10.1152/jappl.1998.84.1.157. [DOI] [PubMed] [Google Scholar]
- 44.Eason JM, Schwartz G, Shirley KA, English AW. Investigation of sexual dimorphism in the rabbit masseter muscle showing different effects of androgen deprivation in adult and young adult animals. Arch Oral Biol. 2000;45:683–690. doi: 10.1016/s0003-9969(00)00030-3. [DOI] [PubMed] [Google Scholar]
- 45.Monemi M, Kadi F, Liu JX, Thornell LE, Eriksson PO. Adverse changes in fibre type and myosin heavy chain compositions of human jaw muscle vs. limb muscle during ageing. Acta Physiol Scand. 1999;167:339–45. doi: 10.1046/j.1365-201x.1999.00624.x. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz C, Hof PR. Recommendations for straightforward and rigorous methods of counting neurons based on a computer simulation approach. J Chem Neuroanat. 2000;20:93–114. doi: 10.1016/s0891-0618(00)00066-1. [DOI] [PubMed] [Google Scholar]
- 47.Saigusa H, Niimi S, Yamashita K, Gotoh T, Kumada M. Morphological and histochemical studies of the genioglossus muscle. Ann Otol Rhinol Laryngol. 2001;110:779–84. doi: 10.1177/000348940111000815. [DOI] [PubMed] [Google Scholar]
- 48.Korfage JA, Brugman P, Van Eijden TM. Intermuscular and intramuscular differences in myosin heavy chain composition of the human masticatory muscles. J Neurol Sci. 2000;178:95–106. doi: 10.1016/s0022-510x(00)00372-5. [DOI] [PubMed] [Google Scholar]
- 49.Sundman E, Ansved T, Margolin G, Kuylenstierna R, Eriksson LI. Fiber-type composition and fiber size of the human cricopharyngeal muscle and the pharyngeal constrictor muscle. Acta Anaesthesiol Scand. 2004;48:423–9. doi: 10.1111/j.1399-6576.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 50.Korfage JA, Van Eijden TM. Regional differences in fibre type composition in the human temporalis muscle. J Anat. 1999;194:355–62. doi: 10.1046/j.1469-7580.1999.19430355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindman R, Stål PS. Abnormal palatopharyngeal muscle morphology in sleep-disordered breathing. J Neurol Sci. 2002;195:11–23. doi: 10.1016/s0022-510x(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 52.Kirkeby S, Garbarsch C. Histochemical studies of the masseter, the temporal and small zygomaticomandibular, and the temporomandibular masticatory muscles from aged male and female humans. Fiber types and myosin isoforms Cranio. 2001;19:174–82. doi: 10.1080/08869634.2001.11746167. [DOI] [PubMed] [Google Scholar]
- 53.Tellis CM, Rosen C, Thekdi A, Sciote JJ. Anatomy and fiber type composition of human interarytenoid muscle. Ann Otol Rhinol Laryngol. 2004;113:97–107. doi: 10.1177/000348940411300203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stål PS, Lindman R. Characterisation of human soft palate muscles with respect to fibre types, myosins and capillary supply. J Anat. 2000;197:275–90. doi: 10.1046/j.1469-7580.2000.19720275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brandon CA, Rosen C, Georgelis G, Horton MJ, Mooney MP, Sciote JJ. Staining of human thyroarytenoid muscle with myosin antibodies reveals some unique extrafusal fibers, but no muscle spindles. J Voice. 2003;17:245–54. doi: 10.1016/s0892-1997(03)00013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stål P, Eriksson PO, Schiaffino S, Butler-Browne GS, Thornell LE. Differences in myosin composition between human oro-facial, masticatory and limb muscles: enzyme-, immunohisto- and biochemical studies. J Muscle Res Cell Motil. 1994;15:517–34. doi: 10.1007/BF00121158. [DOI] [PubMed] [Google Scholar]
- 57.Mu L, Su H, Wang J, Sanders I. Myosin heavy chain-based fiber types in the adult human cricopharyngeus muscle. Muscle Nerve. 2007;35:637–48. doi: 10.1002/mus.20741. [DOI] [PubMed] [Google Scholar]
- 58.Shafiq SA, Shimizu T, Fischman DA. Heterogeneity of type 1 skeletal muscle fibers revealed by monoclonal antibody to slow myosin. Muscle Nerve. 1984;7:380–387. doi: 10.1002/mus.880070507. [DOI] [PubMed] [Google Scholar]
- 59.Putman CT, Conjard A, Peuker H, Pette DJ. Alpha-cardiac-like myosin heavy chain MHCI alpha is not upregulated in transforming rat muscle. Muscle Res Cell Motil. 1999;20:155–62. doi: 10.1023/a:1005430115402. [DOI] [PubMed] [Google Scholar]
- 60.Monemi M, Liu JX, Thornell LE, Eriksson PO. Myosin heavy chain composition of the human lateral pterygoid and digastric muscles in young adults and elderly. J Muscle Res Cell Motil. 2000;21:303–12. doi: 10.1023/a:1005632624826. [DOI] [PubMed] [Google Scholar]
- 61.Biral D, Scarpini E, Angelini C, Salviati G, Margreth A. Myosin heavy chain composition of muscle fibers in spinal muscular atrophy. Muscle Nerve. 1989;12:43–51. doi: 10.1002/mus.880120109. [DOI] [PubMed] [Google Scholar]
- 62.Winter A, Bornemann A. NCAM, vimentin and neonatal myosin heavy chain expression in human muscle diseases. Neuropathol Appl Neurobiol. 1999;25:417–24. doi: 10.1046/j.1365-2990.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- 63.Pontén EM, Stål PS. Decreased capillarization and a shift to fast myosin heavy chain IIx in the biceps brachii muscle from young adults with spastic paresis. J Neurol Sci. 2007;253:25–33. doi: 10.1016/j.jns.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Yang S, Alnaqeeb M, Simpson H, Goldspink G. Cloning and characterization of an IGF-1 isoform expressed in skeletal muscle subjected to stretch. J Muscle Res Cell Motil. 1996;17:487–95. doi: 10.1007/BF00123364. [DOI] [PubMed] [Google Scholar]
- 65.Williams P, Simpson H, Kenwright J, Goldspink G. Muscle fibre damage and regeneration resulting from surgical limb distraction. Cells Tissues Organs. 2001;169:395–400. doi: 10.1159/000047907. [DOI] [PubMed] [Google Scholar]
- 66.Kadi F, Thornell LE. Training affects myosin heavy chain phenotype in the trapezius muscle of women. Histochem Cell Biol. 1999;112:73–8. doi: 10.1007/s004180050393. [DOI] [PubMed] [Google Scholar]
- 67.Snow LM, McLoon LK, Thompson LV. Adult and developmental myosin heavy chain isoforms in soleus muscle of aging Fischer Brown Norway rat. Anat Rec A Discov Mol Cell Evol Biol. 2005;286:866–73. doi: 10.1002/ar.a.20218. [DOI] [PubMed] [Google Scholar]
- 68.St Pierre BA, Tidball JG. Macrophage activation and muscle remodeling at myotendinous junctions after modifications in muscle loading. Am J Pathol. 1994;145:1463–71. [PMC free article] [PubMed] [Google Scholar]
- 69.Rosser BW, Farrar CM, Crellin NK, Andersen LB, Bandman E. Repression of myosin isoforms in developing and denervated skeletal muscle fibers originates near motor endplates. Dev Dyn. 2000;217:50–61. doi: 10.1002/(SICI)1097-0177(200001)217:1<50::AID-DVDY5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 70.Aärimaa V, Rantanen J, Best T, Schultz E, Corr D, Kalimo H. Mild eccentric stretch injury in skeletal muscle causes transient effects on tensile load and cell proliferation. Scand J Med Sci Sports. 2004;14:367–72. doi: 10.1111/j.1600-0838.2004.403.x. [DOI] [PubMed] [Google Scholar]
- 71.Rosser BW, Waldbillig DM, Lovo SD, Armstrong JD, Bandman E. Myosin heavy chain expression within the tapered ends of skeletal muscle fibers. Anat Rec. 1995;242:462–70. doi: 10.1002/ar.1092420404. [DOI] [PubMed] [Google Scholar]
- 72.Slaughter K, Li H, Sokoloff AJ. Neuromuscular organization of the superior longitudinalis muscle in the human tongue. 1. Motor endplate morphology and muscle fiber architecture. Cells Tissues Organs. 2005;181:51–64. doi: 10.1159/000089968. [DOI] [PubMed] [Google Scholar]
- 73.Horton MJ, Rosen C, Close JM, Sciote JJ. Quantification of myosin heavy chain RNA in human laryngeal muscles: differential expression in the vertical and horizontal posterior cricoarytenoid and thyroarytenoid. Laryngoscope. 2008;118:472–7. doi: 10.1097/MLG.0b013e31815c1a93. [DOI] [PMC free article] [PubMed] [Google Scholar]