Abstract
Estradiol is thought to play a critical role in the increased vulnerability to psychostimulant abuse in women. Sex differences in the ability of estradiol to influence cocaine self-administration in adult rats has been hypothesized to depend upon pubertal estradiol exposure. The current study investigated whether the presence of gonadal hormones during puberty affected cocaine self-administration behavior and its sensitivity to adult estradiol treatment in male and female Sprague-Dawley rats. Subjects were gonadectomized or SHAM-operated at postnatal day (PD) 22, and received either OIL or estradiol benzoate (EB) during the approximate time of puberty (PD27 to PD 37). Adult rats were subsequently treated with either EB or OIL 30 minutes before cocaine self-administration (0.3 mg/kg/inf) in order to examine the effects of pubertal manipulations on the estradiol sensitivity of acquisition on a fixed ratio (FR)1 schedule, total intake on a FR5 schedule and motivation on a progressive ratio schedule. Adult EB treatment only affected cocaine self-administration in females, which is consistent with previous research. Adult EB treatment enhanced acquisition in all females irrespective of puberty manipulations. All females, except those treated with EB during puberty, displayed increased cocaine intake following adult EB treatment. Adult EB treatment only enhanced motivation in females that were intact during puberty, whereas those treated with EB during puberty showed reduced motivation. Therefore, the sensitivities of different self-administration behaviors to adult estradiol treatment are organized independently in females, with pubertal estradiol exerting a greater influence over motivational processes, and negligible effects on learning/acquisition.
Keywords: estradiol, puberty, organization, cocaine self-administration, sex differences, acquisition, motivation
INTRODUCTION
Men and women are differentially vulnerable to drugs of abuse. Even though more men than women use cocaine and psychotherapeutics, more women show dependence for these substances (Back et al., 2010; Cotto et al., 2010; Elman et al., 2001). The subjective effects of psychostimulants, like euphoria, desire, increased energy and intellectual efficiency, vary across the menstrual cycle. Thus, women report effects that are comparable to those of men when they are in the follicular phase and reduced during the luteal phase (Justice and de Wit, 1999; Justice and De Wit, 2000; Justice and de Wit, 2000). During the follicular phase, estradiol administration enhances, whereas progesterone attenuates, the subjective effects of psychostimulants (Justice and de Wit, 2000). In men, the effects of estradiol on psychostimulant responses have not been examined and the effects of progesterone are ambiguous (Evans and Foltin, 2005; Evans, 2007; Fox et al., 2013; Sofuoglu et al., 2004). Furthermore, it is unclear whether the effects of ovarian hormones on subjective effects of drugs translate into different patterns of psychostimulant use between men and women (Reed et al., 2011; Sofuoglu et al., 2004).
In contrast, estradiol has been shown to have significant effects on drug self-administration in preclinical models, with the majority occurring selectively in females. Estradiol treatment facilitates acquisition of cocaine self-administration, increases cocaine intake and enhances motivation for cocaine in ovariectomized female rats, but has no apparent effects in males (Hu and Becker, 2008; Jackson et al., 2006; Lynch and Carroll, 2001). Male rats are quite capable of responding to estradiol, as it is one of the primary signals required for both the organization and activation of their reproductive behavior (McCarthy, 2008; Ogawa et al., 2000). Current views on sexual differentiation of the brain are largely based on the differentiation of reproductive behavior in males and females, which is driven in large part by perinatal testosterone exposure in males (but not females) and further refinement by sex-specific gonadal hormone profiles during puberty (McCarthy, 2008; Mccarthy and Arnold, 2011; Sisk and Zehr, 2005). During the perinatal period, estradiol primarily functions as a masculinizing hormone, whereas it becomes crucial for female differentiation during puberty (McCarthy, 2008; Stewart and Cygan, 1980).
We have previously proposed that the expression of sex differences in motivated behaviors in adulthood are at least partly mediated by ovarian hormones secreted during puberty, which are essential for feminizing the mesocorticolimbic dopamine system (Becker, 2009). The current experiment was conducted to test whether pubertal estradiol exposure is necessary and/or sufficient for the subsequent feminization of cocaine self-administration behavior in adult rats. We predicted that the self-administration behavior of females would only be sensitive to adult estradiol treatment if they had also been exposed to estradiol during puberty. Conversely, we predicted that pubertal estradiol would not be sufficient to feminize behavior in males, possibly due to the prior masculinizing effects of perinatal testosterone/estradiol.
METHODS
Animals
Sprague-Dawley rats purchased from Charles Rivers (Portage, MI) were gonadectomized (OVX/CAST) at postnatal day (PD) 22, or remained intact through puberty (pINTACT) (n = 51 per sex). Animals were housed in same-sex groups in standard laboratory cages (2–3 per cage) and maintained on a 14:10 (light:dark) reversed light cycle (lights off at 08:00) and provided free access to rat chow (Teklad Global 14% protein rodent maintenance diet) and carbon-filtered water in a temperature- and humidity-controlled vivarium. All experimental procedures were conducted between 09:00 and 16:00 and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were preapproved by the University of Michigan Committee on the Use and Care of Animals.
OVX/CAST animals received subcutaneous (s.c.) injections of either estradiol benzoate (EB) or the peanut oil vehicle (OIL) from PD27-37 (approximate time of puberty in females). Treatments consisted of OIL or a ramping EB regime (5, 10 and 15mg/kg, s.c.) delivered over 3 days for 3 cycles with one day off between cycles. These groups were referred to as puberty EB (pEB) or puberty OIL (pOIL). The pINTACT animals were treated with OIL during PD27-37 and were subsequently OVX/CAST at PD67. Animals were weighed daily and the age at vaginal opening (for females) or preputial separation (for males) was determined. Estrogen reduces weight gain in both males and females and accelerates vaginal opening, so these measures were used to validate the effectiveness of pubertal gonadectomies and EB treatments.
Catheter Surgeries
At PD74, all animals were fitted with indwelling jugular catheters connected to a back port. Catheters were constructed by gluing silastic tubing (Silastic tubing, 0.51 mm I.D. × 0.94 mm O.D., Dow Corning, Midland, MI) to an external guide cannula (22 Gauge guide cannula; Plastics One, Roanoke, VA) using cranioplastic cement. A polypropylene mesh was secured to the bottom of the cannula using this same cement. Rats received an injection of Buprenorphine (0.02 mg/kg, s.c.) 30 minutes before they were anesthetized with isoflurane (5% isoflurane in oxygen). The free end of the silastic tubing of the catheter apparatus was inserted into the right jugular vein of the animal and secured using 4.0 silk sutures around the tubing and the venous tissue. The catheter port exited dorsally from the animal. After successful implantation, the animal’s catheter was flushed with 0.2 ml each of heparin (30 U/ml in 0.9% sterile saline) and gentamicin (3 mg/kg) to prevent clotting and infection, respectively. A dummy stylet was then inserted into the port opening. Two days after surgery, catheters were flushed with 0.2 ml of heparin (30 U/ml in 0.9% sterile saline) and gentamicin (3 mg/kg), and each day after that with gentamicin and heparin (3 mg/kg and 20 U/ml, resp.). Catheter patency was checked weekly using a solution of Pentothal® (thiopental sodium, 15mg/ml, 0.15–0.25ml in sterile water). Animals were allowed to recover for 5–7 days before the start of cocaine self-administration.
Cocaine self-administration
Half of the animals in each puberty group (pOIL, pEB and pINTACT) were treated with OIL or EB (5mg/kg, s.c.) 30 minutes before the start of each daily self-administration session, resulting in 12 final treatment groups: 2 sexes (male or female) × 3 puberty treatments (pOIL, pEB or pINTACT) × 2 adult treatments (aOIL or aEB). Group treatments and assignments are described in Table 1. Self-administration and adult hormone treatments were administered 5 days a week, followed by 2 days off as in previous experiments from this laboratory where EB enhanced cocaine-taking behavior (Hu et al., 2004; Jackson et al., 2006).
Table 1.
Group names and corresponding timing of the hormone treatments
| PD22 | puberty | adult | group name | ||||
|---|---|---|---|---|---|---|---|
| PD 27-37 | PD 67 | SA | |||||
|
|
|||||||
| Male (N = 51) | INTACT | OIL (N = 13) | CAST | OIL | male | pINTACT-aOIL | (N = 7) |
|
|
|||||||
| EB | male | pINTACT-aEB | (N = 6) | ||||
|
|
|||||||
| CAST | OIL (N = 20) | OIL | male | pOIL-aOIL | (N = 9) | ||
|
|
|||||||
| EB | male | pOIL-aEB | (N = 11) | ||||
|
|
|||||||
| EB (N = 18) | OIL | male | pEB-aOIL | (N = 9) | |||
|
|
|||||||
| EB | male | pEB-aEB | (N = 9) | ||||
|
|
|||||||
| Female (N = 51) | INTACT | OIL (N = 13) | OVX | OIL | female | pINTACT-aOIL | (N = 7) |
|
|
|||||||
| EB | female | pINTACT-aEB | (N = 6) | ||||
|
|
|||||||
| OVX | OIL (N = 20) | OIL | female | pOIL-aOIL | (N = 10) | ||
|
|
|||||||
| EB | female | pOIL-aEB | (N = 10) | ||||
|
|
|||||||
| EB (N = 18) | OIL | female | pEB-aOIL | (N = 8) | |||
|
|
|||||||
| EB | female | pEB-aEB | (N = 10) | ||||
PD: Postnatal Day, EB: Estradiol Benzoate, CAST: castration, OVX: ovariectomy, SA: self-administration.
Self-administration was performed in standard operant chambers (Med Associates, Inc., Georgia, VT) where the animals could nose poke into the active hole for cocaine or in an inactive hole, which had in no consequences. Rats were connected to the infusion syringe via a swivel mounted to a counter-balanced arm, which allowed animals to move freely in the testing environment. Animals were weighed prior to each self-administration session.
Subjects self-administered cocaine (0.3 mg/kg/inf) on a fixed ratio (FR) 1 schedule of reinforcement for the first 13 days. On the FR1 schedule, animals were required to perform one nose poke in the active hole in order to receive an infusion. Nose pokes in the inactive hole had no consequences. The FR1 tests were two hours long, but terminated early once the animal received 100 infusions. Acquisition of cocaine self-administration was defined as three consecutive days with two times the number of active to inactive nose pokes. Animals were then tested on a progressive ratio (PR) schedule of reinforcement to determine breaking points (BP). The PR requirement escalated through an exponential series: 1, 3, 6, 9, 12, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, 268…. adapted from Richardson and Roberts (Richardson and Roberts, 1996). A higher BP is an indication that the animal is more motivated for cocaine, as they are willing to work harder to obtain each subsequent infusion. The number of infusions, nose pokes in the active and inactive holes and the last completed ratio (BP) were recorded. The PR session terminated after 6 hours, or earlier if 1 hour elapsed without receiving an infusion.
Following the PR test, animals were transitioned to a FR5 schedule of reinforcement with daily tests consisting of 3 × 40 minute active sessions (i.e., house light illuminated indicating drug availability) separated by 2 × 15 minute inactive sessions (i.e., house light turned off signaling drug unavailability). There was also a 40-second delay following each infusion, during which time nose pokes in the active hole were recorded but did not result in any consequences. To investigate if chronic cocaine self-administration affected the motivation to take cocaine, animals were retested on the PR schedule after 18 days on the FR5 schedule.
Statistics
Statistical analyses were performed with SPSS (version 18.0). Weight gain for the different periods was analyzed with repeated-measures ANOVA with day as within-subject variable and sex, puberty-treatment and adult treatment (when applicable) as between subject variables. The effects of puberty manipulations on the proportion of individuals displaying vaginal opening or preputial separation were examined by Pearson’s Chi Square analysis, whereas differences in the timing of vaginal opening were analyzed by unpaired t-tests. Acquisition of cocaine self-administration was defined as the first day the animal had twice as many nose-pokes in the active hole compared to the inactive hole for three consecutive days. The percentage of animals that acquired within each group was analyzed using a Kaplan-Meier survival analysis. For the FR1 and FR5 the total number of infusions were analyzed with an ANOVA with sex, puberty-treatment and adult-treatment as between subject variables. For the PR data, breaking points (BP) were analyzed using repeated-measures ANOVA with day as within-subject variable and sex, puberty treatment and adult treatment as between subject variables. Sphericity assumed modeling, with Greenhouse-Geisser and Huynh-Feldt adjustments, was applied (Quintana and Maxwell, 1994).
RESULTS
Acquisition of cocaine self-administration on FR1 schedule
Adult EB treatment enhanced acquisition of cocaine self-administration in all female groups irrespective of hormone treatment during puberty (Fig. 1A). Therefore, data were combined into four groups based on sex and adult hormone treatment for survival analyses. Females treated with EB as adults (aEB) acquired cocaine self-administration significantly earlier aOIL females (χ2=4.53, p=0.033, Cohen’s d=0.67, r=0.32), whereas there was no effect of adult EB treatment in males. The mean latencies for acquisition ± SEM for each group were as follows: 4.1 ± 0.5 days (aOIL females), 2.4 ± 0.5 days (aEB females), 3.9 ± 0.5 days (aOIL males) and 3.7 ± 0.6 days (aEB males). There were no group differences in the total number of infusions of cocaine on the FR1 schedule of reinforcement.
Figure 1. Adult estradiol treatment only enhances acquisition in females and this effect is independent of pubertal hormone manipulations.
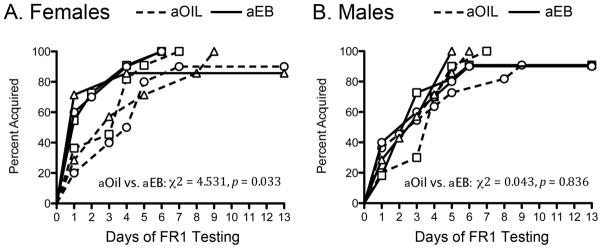
Survival curves of acquisition in females (A) and males (B) (pINTACT: triangles, pOIL: squares, pEB: circles).
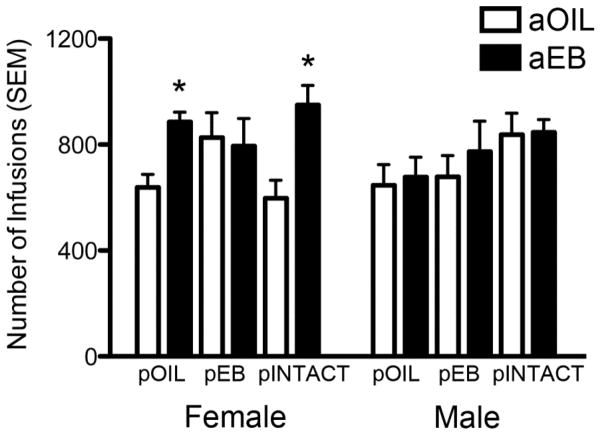
Total infusions on FR5 schedule
A significant main effect of adult hormone treatment on total cocaine intake on the FR5 schedule was found (Fig. 2; F1,90=6.97, p=0.01; η2=0.06). aEB treatment increased cocaine intake in pOIL females and pINTACT females, relative to aOIL counterparts (p=0.019, Cohen’s d=1.81, r=0.67; and p=0.008, Cohen’s d=1.95, r=0.70, respectively). aEB treatment had no effect on the number of infusions in pEB females, counter to our prediction. Neither pubertal nor adult hormone treatment affected cocaine intake in males on the FR5 schedule of reinforcement.
Figure 2. Adult estradiol increases cocaine intake in females, but not males, and this effect is modulated by pubertal hormone exposure.
*p≤ 0.05, significant difference between aOIL and aEB treatment within respective sex and puberty treatment.
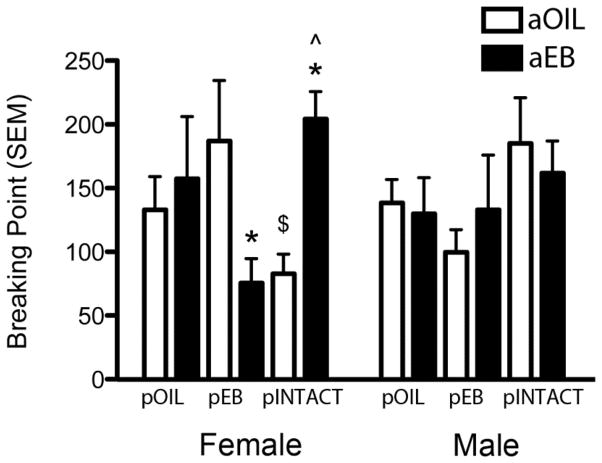
Motivation for cocaine on the PR schedule
There were no significant main effects on BP; however, there was a significant interaction between sex, puberty treatment and adult treatment (F2,90=5.72, p=0.005; ηP2=0.11). There was also a significant effect of test (early vs. late PR; F1,90=68.00, p≤0.001; ηP2=0.43).
Early PR test
Puberty treatments significantly influenced the response to adult EB treatment in females. Females that were exposed to EB both during puberty and adulthood exhibited a reduced motivation to take cocaine compared to females that received OIL during puberty (pEB/aEB vs. pOIL/aEB; p=0.049, Cohen’s d=1.01, r=0.45). No differences were found between groups that received OIL or EB during adulthood. A sex difference was found in pEB/aEB animals, with males showing a higher BP than females (p=0.048, Cohen’s d=0.81, r=0.37).
Late PR test
Compared to their aOIL counterparts, adult EB treatment significantly reduced BP in females that received EB during puberty, and increased BP in females that were intact during puberty (Fig. 3; p=0.015, Cohen’s d=1.07, r=0.47, and p=0.023, Cohen’s d=2.59, r=0.79, resp.). The differential effect of adult EB in these groups also resulted in their being significantly different from another (p=0.029, Cohen’s d=2.28, r=0.75). There was a significant sex difference in the pINTACT/aOIL animals, with males having a higher BP than female pINTACT/aOIL (p=0.045, Cohen’s d=1.40, r=0.57).
Figure 3. Adult estradiol treatment only enhances motivation to self-administer cocaine in females that were intact during puberty.
*p≤ 0.05, significant difference between aOIL and aEB treatment within respective sex and puberty treatment; $p≤ 0.05, significant difference between males and females within the same treatment group; ^ p≤ 0.05, significant difference from pEB group within the same sex and adult treatment.
Early vs. late PR tests
The motivation to take cocaine increased with the animals’ experience taking cocaine on the FR5 schedule as would be expected, and BP’s for most groups were significantly higher during the second PR test (p<0.05) This was true for all groups except for female pINTACT/aOIL, female pEB/aEB, male pEB/aOIL and male pEB/aEB.
Effects of treatments on physiological measures
Physiological measures were taken to assess the effects of treatments on ontogeny of physiological parameters of growth as well as endocrine status. Significant group differences are presented in table 1. There were main effects of sex (F1,96=20.33, p≤0.001; η2=0.14) and puberty treatment (F2,96=11.85, p≤0.001; η2=0.17) on weight gain during PD27 to 39. In general, males gained more weight than similarly treated females. Pubertal EB treatment significantly reduced weight gain in both sexes. There was a main effect of sex (F1,96=257.30, p≤0.001; η2=0.68) and an interaction between sex and puberty treatment (F2,96=23.46, p≤0.001; η2=0.12) on weight gain following the termination of puberty treatments (PD39-60). Males gained more weight than similarly treated females. pINTACT females grew less than OVX females, whereas pINTACT males gained more weight than CAST males. There were also main effects of sex (F1,90=52.51, p≤0.001; η2=0.21), puberty treatment (F2,90=3.11, p=0.049; η2=0.02) and adult treatment (F1,90=80.84, p≤0.001; η2=0.32) on weight gain during the self-administration period. Adult EB treatment significantly attenuated weight gain in all groups irrespective of puberty treatment and males generally gained more weight than similarly treated females.
Puberty treatments had a significant effect on the number of animals showing vaginal opening (VO) or preputial separation (PS) (χ2 =51.0, p<0.001 for both males and females). The following numbers of animals showed VO/PS: 0/20 (pOIL females), 18/18 (pEB females), 13/13 (pINTACT females), 0/20 (pOIL males) 0/18 (pEB males) and 13/13 (pINTACT males). pEB females opened earlier than the pINTACT group (t1,29=5.08, p<0.001, Cohen’s d=1.79, r=0.67).
There were main effects of sex (F1,90=16.87, p≤0.001; η2=0.07), adult treatment (F1,90=108.63, p≤0.001; η2=0.47), and an interaction between these factors (F1,90=7.87, p=0.006; η2=0.03) on relative adrenal weights. Treatment with EB during adulthood increased the relative adrenal weight in both males and females, with females showing higher adrenal weights than males. There were significant main effects of puberty treatment (F1,45=7.22, p=0.002; η2=0.05), and adult treatment (F1,45=231.04, p≤0.002; η2=0.79) on the relative uterine weights. EB treatment during adulthood increased adrenal weights in all groups.
DISCUSSION
The results of this experiment confirm that estradiol treatment only affects cocaine self-administration in adult females and that pubertal estradiol may be necessary, but is not sufficient, for this subsequent sensitivity to estradiol. Furthermore, the effects of adult estradiol treatment on acquisition, intake and the motivation to self-administer cocaine were differentially sensitive to pubertal estradiol manipulations in females. Our initial hypothesis was based on the assumption that estrogen sensitivity of cocaine self-administration behaviors would be uniformly organized during development. The dissociation of estrogen effects on acquisition, intake and motivation by our pubertal manipulations supports a more complex model in which these different facets of behavior are independently regulated during development, although possibly still in a coordinated fashion. The etiology of estrogen sensitivity may differ for these behaviors due to their reliance on different underlying brain structures, which could be organized during distinct development periods or employ divergent signaling mechanisms.
Estradiol effects on acquisition of cocaine self-administration
Estrogen replacement to OVX females is known to enhance acquisition of cocaine self-administration (Hu et al., 2004; Jackson et al., 2006; Lynch and Carroll, 2001). Our data show that this estrogen effect is evident in adult females irrespective of their gonadal status or estradiol exposure during puberty. Additionally, prepubertal CAST and estradiol treatment did not render acquisition of cocaine self-administration sensitive to estrogen in adult males. Collectively, these data suggest that the ability of adult estradiol exposure to enhance acquisition is organized prior to puberty. Perinatal testosterone/estradiol signaling renders the neural substrates of acquisition in the male brain insensitive to subsequent estradiol, whereas these substrates retain their estrogen sensitivity in females not exposed to these hormones perinatally. It is also possible that the neural substrates of acquisition are organized independently from gonadal hormones, such as through sex-determining genes or other environmental influences (Arnold, 2011). Treating females with testosterone/estradiol during the perinatal period or blocking these potential hormone effects in males via neonatal CAST or other pharmacological means would distinguish between these possibilities.
There are no published data that directly address the issue of where in the brain estradiol may be acting to enhance acquisition of cocaine self-administration. Acquisition requires the ability to discriminate the effects of cocaine and associate them with a specific response in a particular location. Given the significant learning component inherent in acquisition, estradiol may be acting in the cortex or hippocampus to enhance this component of cocaine self-administration. Both of these structures contain estrogen receptors (Butler et al., 1999; Shughrue et al., 1997), with expression in the cortex being sexually dimorphic and especially pronounced early in development (Wilson et al., 2011). Long-term OVX has been shown to abolish some estrogen responses in the hippocampus, but not cortex (Bohacek et al., 2008; Wu et al., 2011). Thus, the currently available information would suggest that the cortex may be the primary site of action for estradiol’s effects on acquisition of cocaine self-administration.
Estradiol effects on cocaine intake
Estradiol can increase cocaine intake in OVX females (Hu and Becker, 2008; Hu et al., 2004; Jackson et al., 2006); however, we did not see any group differences in the number of infusions earned over the first 13 days while animals were self-administering on the FR1 schedule. In the present study, all animals had stable patterns of self-administration by the time they were put on the FR5 schedule, which might explain why we were able to detect an estradiol effect on intake during this later stage of testing. However, it should be mentioned that this effect of estradiol was relatively modest and only detectable when considering the cumulative number of infusions over the entire 18 days of self-administration on the FR5 schedule. The ability of estradiol to increase cocaine intake was only present in pINTACT and pOIL females. These results demonstrate that females do not require ovarian hormones during puberty in order for estradiol to increase cocaine intake in adulthood. The lack of an estrogen effect in pEB females is similar to the response profile of males, which suggests that pubertal estradiol exposure may actually masculinize the neural substrates underlying this effect. It is possible that pubertal estradiol can either masculinize or feminize behavior depending upon concentration, pattern of exposure or interactions with other factors (e.g., progesterone).
Estradiol effects on motivation to self-administer cocaine
Estradiol enhances motivation to self-administer cocaine in OVX females and intact females reach higher cocaine BP during estrus compared to other stages of the reproductive cycle, suggesting that long-term estrogen exposure is required for this effect (Lynch, 2008; Ramoa et al.; Roberts et al., 1989). Our data show that females’ motivation was only sensitive to adult estradiol administration if they were intact or treated with estradiol during puberty. Interestingly, while both pINTACT and pEB females were sensitive to adult estradiol treatment, their motivation was respectively increased and decreased by hormone treatment. Thus, pubertal estradiol may feminize motivated behavior (i.e., render it sensitive to adult estradiol), but additional factors may determine the final behavioral response. In males, the motivation to self-administer cocaine was unaffected by adult estradiol treatment irrespective of our pubertal manipulations.
The ability of estradiol to enhance motivation for cocaine in adult females has largely been attributed to its ability to increase drug-induced dopamine overflow in the striatum (Becker et al., 1984; Becker and Rudick, 1999). However, estradiol can also modulate the firing rate and rhythmicity of dopamine neurons within the substantia nigra and ventral tegmental area by altering negative feedback mechanisms, which can reduce dopamine neurotransmission (Chiodo and Caggiula, 1980; Torres-Hernández and González-Vegas, 2005; Zhang et al., 2008). Thus, it is possible that in the intact female, pubertal estradiol acts with other factors (e.g., progesterone) to completely feminize the adult estradiol response, whereas prepubertal OVX and estradiol treatment might only feminize the negative feedback mechanisms leading to reduced dopamine neurotransmission and lower cocaine BP following adult estradiol administration. It is also probable that the effects of estradiol on motivation for cocaine involve downstream changes in dopamine receptors, dopamine transport or metabolism, not to mention myriad effects on other neurochemical systems in the striatum and other brain regions.
Efficacy of estradiol treatments
Treatment effects on the various physiological measures confirm that both the pubertal and adult estradiol treatments exerted effects in both males and females. The comparable patterns of weight gain and sexual maturation in pEB and pINTACT females suggests that our pubertal estradiol regime was sufficient to support normal developmental changes and most likely not supra physiological. The pattern of ovarian hormone secretion leading up to puberty has not been well characterized. However, feeding patterns and weight gain show cyclic fluctuations weeks before vaginal opening (Sieck et al., 1977) and several cycles of estradiol exposure may be required for the normal induction of puberty (Greenstein, 1992). Therefore, we chose to administer estradiol in a cyclic regime with ramping doses followed by withdrawal. It is possible that even lower doses, a different temporal pattern, or co-administration of other factors (e.g., progesterone) would produce more reliable feminization of motivated behavior.
Additionally, the lack of behavioral effects of prepubertal CAST and estradiol treatment in males should be interpreted with caution. The reduction in weight gain induced by exogenous estradiol during the pubertal and adult treatment periods and their adrenal hypertrophy confirm that males can respond to estradiol. Therefore, the lack of an estradiol effect on behavior would suggest that this hormone does not contribute to the organization of their self-administration behavior, perhaps due to the prior organizing effects of perinatal testosterone on relevant neurocircuitry. However, it is also possible that our negative findings relate to the specific estradiol treatment paradigm, which may not be optimal for identifying estrogen effects in males.
Summary/Conclusion
In the current study we show that several of the effects of adult estradiol treatment on cocaine self-administration behavior are modulated by exposure to gonadal hormones during puberty in female rats, but not males. Aspects of cocaine self-administration behavior, such as acquisition and motivation, are differentially regulated by gonadal hormone exposure during puberty in females. Sensitivity of acquisition to adult estradiol treatment did not depend on gonadal hormones during puberty, whereas the sensitivity of motivation to estradiol was affected by the gonadal status and hormone exposure during puberty. We conclude that sexual differentiation of the neural substrates of drug taking behaviors are not bi-modally distributed to become ‘male’ or ‘female’, as multiple developmental events influence the pattern of behavior and the sensitivity to estradiol potentially contributing to both sex differences and individual differences in drug taking behaviors.
Table 2.
Physiological effects of pubertal and adult estradiol treatment.
| Puberty Trt: Adult Trt: |
OIL | EB | SHAH | ||||
|---|---|---|---|---|---|---|---|
| OIL | EB | OIL | EB | DIL | EB | ||
| 3 n E £ |
A Weight PD27-39 (.g> | 70.7 £1.41 A-$ | 61.4 (2.2) $ | 67.1 (2.0) $ | |||
| A Weight PD39-60 (.g} | 104.2 [2.6) * 3 | 102.1 [1.91 # $ | 76.3 (3.0) $ | ||||
| Age at VO | - | 31,3 (0.3) # | 33.2 (0.4) | ||||
| A Weight During SA (g) | 62.6 (5.6) $ | 10,4 (3.4) * if $ | S3.3 (8.0) $ | 20,2 (3.0) * $ | 66.7 (6.S) $ | 43,8 (5.1) * | |
| Adrenals (mg/100 g BW) | 13.4 (0.4) | 22.3 (1.4) * $ | 13.9 (0.8) | 23.4 (1.5) * $ | 12.6 (1.3) | 22.9 (2.4) * | |
| Uterus (mg/100 g BW) | 12.2 (1.2) | 84.8 (8.5) * # | 22.4(1.9) | 101.0 (10.7) * | 25.1(2.7) | 126.3 (5.0) * | |
| vi ta t |
A Weight PD27-39 (g) | 79,0 (2.2) * | 68.2 (2.5) | 77,0 (3.1) * | |||
| A Weight PD39-60 (g) | 135.6 [3.71 * | 130.1 [3.2) # | 148.2 [5.3) | ||||
| Age at PS | - | - | 47.5 (0.5) | ||||
| A Weight During 5A (g) | 92.1 (8.3) | 51.5 (10.3) * | 91.7 (5.0) | 52.2 (8.4) * | 90.2 (9.1) | 65.3 (8.1) 41 | |
| Adrenal (mg/100 g BW) | 11.9 (0.4) | 16.5 (1.1) * | 13.7 (1.0) | 18.2 (1.5) * | 11.5 (0.9) | 18.9 (1.1) 41 | |
p< 0.05, significant difference between adult EB and OIL treatment (within the same sex and puberty treatment).
p< 0.05, significant difference from pINTACT (within the same sex and adult treatment).
p< 0.05, significant sex difference (within the same puberty and adult treatment).
p<. 0.05, significant difference between pOIL and pEB (within the same sex and adult treatment).
Highlights.
Developmental effects of estradiol on cocaine self-administration were examined.
Adult estradiol affected self-administration behavior in females, but not males.
Pubertal estradiol differentially affected cocaine responses to adult estradiol.
Acquisition and motivation were independently regulated during development.
Acknowledgments
This research was supported by NIDA grants DA012677 (JBB) and DA007268 (T32 Training Grant). The authors thank Brandon Luma for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold AP. The end of gonad-centric sex determination in mammals. Trends in Genetics. 2011 doi: 10.1016/j.tig.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SE, Payne RL, Simpson AN, Brady KT. Gender and prescription opioids: Findings from the National Survey on Drug Use and Health. Addict Behav. 2010;35:1001–1007. doi: 10.1016/j.addbeh.2010.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. Sexual differentiation of motivation: a novel mechanism? Hormones and Behavior. 2009;55:646–654. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Beer ME, Robinson TE. Striatal dopamine release stimulated by amphetamine or potassium: influence of ovarian hormones and the light-dark cycle. Brain Research. 1984;311:157–160. doi: 10.1016/0006-8993(84)91410-0. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN. Rapid effects of estrogen or progesterone on the amphetamine-induced increase in striatal dopamine are enhanced by estrogen priming: a microdialysis study. Pharmacology Biochemistry and Behavior. 1999;64:53–57. doi: 10.1016/s0091-3057(99)00091-x. [DOI] [PubMed] [Google Scholar]
- Bohacek J, Bearl AM, Daniel JM. Long-term ovarian hormone deprivation alters the ability of subsequent oestradiol replacement to regulate choline acetyltransferase protein levels in the hippocampus and prefrontal cortex of middle-aged rats. Journal of Neuroendocrinology. 2008;20:1023–1027. doi: 10.1111/j.1365-2826.2008.01752.x. [DOI] [PubMed] [Google Scholar]
- Butler JA, Kalló I, Sjöberg M, Coen CW. Evidence for extensive distribution of oestrogen receptor alpha-immunoreactivity in the cerebral cortex of adult rats. Journal of Neuroendocrinology. 1999;11:325–329. doi: 10.1046/j.1365-2826.1999.00346.x. [DOI] [PubMed] [Google Scholar]
- Cotto JH, Davis E, Dowling GJ, Elcano JC, Staton AB, Weiss SRB. Gender effects on drug use, abuse, and dependence: a special analysis of results from the National Survey on Drug Use and Health. Gend Med. 2010;7:402–13. doi: 10.1016/j.genm.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am J Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Evans S, Foltin R. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2005;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Experimental and Clinical Psychopharmacology. 2007;15:418–426. doi: 10.1037/1064-1297.15.5.418. [DOI] [PubMed] [Google Scholar]
- Fox HC, Sofuoglu M, Morgan PT, Tuit KL, Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: Impact of gender and cue type. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein BD. Effects of rat alpha-fetoprotein administration on estradiol free fraction, the onset of puberty, and neural and uterine nuclear estrogen receptors. Endocrinology. 1992;130:3184–90. doi: 10.1210/endo.130.6.1375894. [DOI] [PubMed] [Google Scholar]
- Hu M, Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug and alcohol dependence. 2008;94:56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–38. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Justice AJ, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacol Biochem Behav. 2000;66:509–15. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000;71:51–9. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197:237–246. doi: 10.1007/s00213-007-1028-0. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Regulation of drug intake. Experimental and Clinical Psychopharmacology. 2001;9:131–143. doi: 10.1037//1064-1297.9.2.131. [DOI] [PubMed] [Google Scholar]
- McCarthy M. Estradiol and the developing brain. Physiological reviews. 2008;88:91. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nature neuroscience. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO) Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14737–14741. doi: 10.1073/pnas.250473597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintana SM, Maxwell SE. A Monte Carlo comparison of seven ε-adjustment procedures in repeated measures designs with small sample sizes. Journal of Educational and Behavioral Statistics. 1994;19:57–71. [Google Scholar]
- Ramoa CP, Doyle SE, Naim DW, Lynch WJ. Estradiol as a Mechanism for Sex Differences in the Development of an Addicted Phenotype following Extended Access Cocaine Self-Administration. Neuropsychopharmacology. doi: 10.1038/npp.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Evans SM, Bedi G, Rubin E, Foltin RW. The effects of oral micronized progesterone on smoked cocaine self-administration in women. Hormones and Behavior. 2011;59:227–235. doi: 10.1016/j.yhbeh.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Roberts D, Bennett S, Vickers G. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. The Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Nance DM, Ramaley JA, Taylor AN, Gorski RA. Prepubertal cyclicity in feeding behavior and body weight regulation in the female rat. Physiol Behav. 1977;18:299–305. doi: 10.1016/0031-9384(77)90137-8. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Frontiers in Neuroendocrinology. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Mitchell E, Kosten TR. Effects of progesterone treatment on cocaine responses in male and female cocaine users. Pharmacology Biochemistry and Behavior. 2004;78:699–705. doi: 10.1016/j.pbb.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Stewart J, Cygan D. Ovarian hormones act early in development to feminize adult open-field behavior in the rat. Horm Behav. 1980;14:20–32. doi: 10.1016/0018-506x(80)90012-4. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Westberry JM, Trout AL. Estrogen receptor-alpha gene expression in the cortex: sex differences during development and in adulthood. Hormones and Behavior. 2011;59:353–357. doi: 10.1016/j.yhbeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WW, Adelman JP, Maylie J. Ovarian hormone deficiency reduces intrinsic excitability and abolishes acute estrogen sensitivity in hippocampal CA1 pyramidal neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:2638–2648. doi: 10.1523/JNEUROSCI.6081-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]