Abstract
Maintenance of genomic integrity is essential for adult tissue homeostasis and defects in the DNA-damage response (DDR) machinery are linked to numerous pathologies including, cancer. Here we present evidence that the DDR exerts tumor suppressor activity in gliomas. We show that genes encoding components of the DDR pathway are frequently altered in human gliomas and that loss of elements of the ATM/Chk2/p53 cascade accelerates tumor formation in a glioma mouse model. We demonstrate that Chk2 is required for glioma response to ionizing radiation in vivo and is necessary for DNA-damage checkpoints in the neuronal stem cell compartment. Finally, we observed that the DDR is constitutively activated in a subset of human GBMs, and such activation correlates with regions of hypoxia.
Keywords: GBM, DDR, Chk2, ATM, Ionizing radiation
Introduction
The gliomas are a large group of brain cancers that include astrocytomas oligodendrogliomas and oligoastrocytomas. Glioblastoma multiforme (GBM), the highest grade of malignant astrocytomas (WHO grade IV), is the most common and lethal primary central nervous system (CNS) tumor in adults. Despite the recent advances in treatment modalities, GBMs patients generally respond poorly to all therapeutic approaches and prognosis remains dismal. On a molecular basis, a decade of studies, including the most recent large-scale genomic analysis (The Cancer Genome Atlas Research Network, 2008; Parsons et al., 2008), has underlined the complexity and heterogeneity of genetic events that characterize the glioblastoma genome, but the mechanisms responsible for the variability in radiation response remain elusive.
GBMs are divided in two subtypes on the basis of clinical history: “primary GBMs” that arise de novo, with no evidences of precursor lesions, and “secondary GBMs”, evolving from a lower grade tumor over time. Histologically, GBMs are characterized by tumor cells invading adjacent normal brain parenchyma, vascular proliferation and by the presence of area of necrosis and hemorrhage. Necrotic regions are typically surrounded by dense cellular zones, normally referred as to pseudopalisades. The origin of these structures is still currently debated but they are of central importance to the malignant behavior of GBMs (Brat et al., 2004).
Preservation of genomic integrity is an essential process for cell homeostasis and defects in the DNA-damage response (DDR), a complex network of proteins required for cell-cycle checkpoint and DNA repair, have been associated with tumorigenesis. In response to DNA damage, cells activate the sensor kinases ATM, ATR and DNA-PK (Durocher and Jackson, 2001; Shiloh, 2003) that in turn phosphorylate multiple downstream substrates, including the effector kinases Chk1 and Chk2 (Bartek and Lukas, 2003; Stracker et al., 2009), resulting in cell-cycle checkpoint initiation and/or apoptosis. Markers of a constitutively active DDR have been described in many types of malignant lesions in different tissues (Bartkova et al., 2005; Gorgoulis et al., 2005; Nuciforo et al., 2007). The DDR acts as a barrier against tumor progression, where precancerous lesions must inactivate p53 or other elements of the DDR to proceed to a more aggressive status (Bartkova et al., 2005; Bartkova et al., 2006). A systematic approach to analyze the putative tumor suppressor activity of the DDR in gliomas has not been undertaken. Furthermore, given that radiation is the standard therapy for GBM patients, understanding the tumor-specific abnormalities of the DDR machinery may be of important value to design patient-specific therapies.
In this study we performed a detailed analysis of the genetic alteration in GBMs of genes encoding DDR components using the data available through The Cancer Genome Atlas (TCGA) consortium. We further focused on the tumor suppressor activity of the ATM/Chk2/p53 pathway in a glioma mouse model and we investigated the contribution of loss of the Chk2 checkpoint kinase to the radiation resistance of GBMs.
Results
Genetic loss of function of the DDR in human GBMs
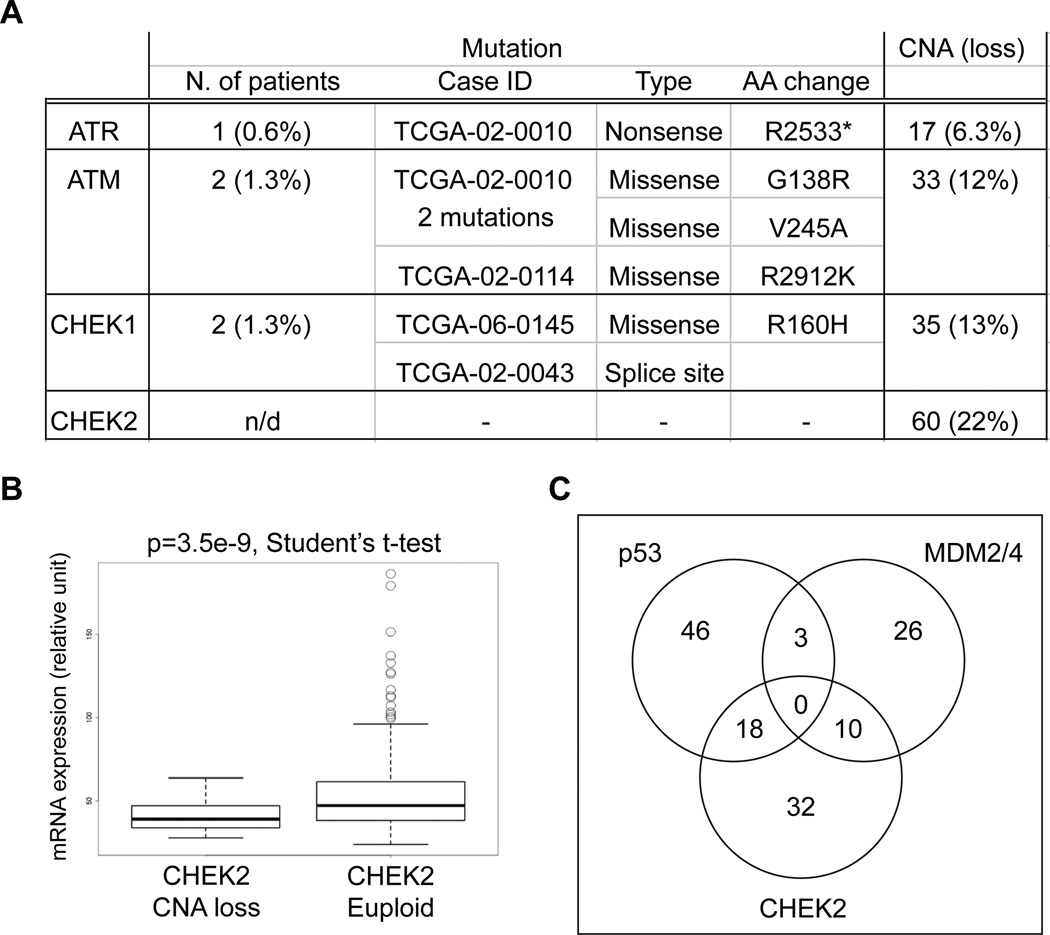
Recent large scale genomic analyses of GBM have identified a diversity of recurrent mutations and chromosomal copy number alterations (CNA) affecting genes involved in multiple signaling pathways (The Cancer Genome Atlas Research Network, 2008; Parsons et al., 2008). We now performed an analysis of TCGA dataset for GBM, looking at genes encoding key components of the DDR and we found that 3.2% of tumors showed somatic mutations in either ATR, ATM, or CHEK1 (Figure 1A and Table S1 available online). The exact frequency of CHEK2 mutations is unknown at this time because many of the mutation initially described in the TCGA dataset have not been reconfirmed by secondary analysis (Table S1 available online).
Figure 1. CHEK2 is commonly altered in GBM.
(A) Table of somatic mutations (n=158) (see also Table S1) and copy number alteration (CNA; only chromosomal copy loss are shown) (n=272) assessed in primary GBM samples of TCGA. CHEK2 mutation frequency couldn’t be determined due to technical reasons (see the text for details). (B) CHEK2 expression is significantly decreased in tumors with copy loss (p=3.5e-9, Student’s t-test). (C) Venn diagram representing event of loss/mutation of TP53, amplification of MDM2/4 and loss/mutation of CHEK2 (unaltered = 126).
Genomic loss of at least one copy of either ATR, ATM, CHEK1 or CHEK2 was found in 36% of GBM samples in TCGA. Loss among the four genes was significantly higher than background as determined by random sampling (p=0.0146, 10,000 iterations, see Supplemental Experimental Procedures). Among these four DDR components analyzed, CHEK2 was the most commonly lost (22%) and copy number loss was associated with significantly lower gene expression (Figure 1B). Only a small percentage of patients that had CHEK2 deletions presented concomitant alterations of the other DDR components under investigation: 13.3% (8/60) for ATM, 10% (6/60) for ATR and 11.6% (7/60) for CHEK1 (Table S2). On the other hand, approximately 50% of GBM patients with CHEK2 alterations also carried defect in the p53 signaling pathway (such as TP53 mutation/loss or amplifications of MDM2/4, both well-known p53 inhibitors), suggesting that these genetic events are not mutually exclusive in gliomas (Figure 1C).
The ATM/Chk2/p53 loss of function accelerates GBM formation in mice
The frequent alterations of DDR components in human GBMs suggested a putative tumor suppressor activity of this pathway in the brain. Therefore, to directly demonstrate a role of loss of DDR core elements (ATM, CHK2 and p53) in glioma formation, we employed the RCAS/tv-a system, that uses a specific avian leukosis virus (RCAS) to mediate gene transfer into somatic cells transgenic for its receptor (tv-a). In this study we used Nestin-tv-a (Ntv-a) mice that express the tv-a receptor under the control of the nestin promoter, a marker of neural/glial stem and progenitor cells (Holland et al., 1998). Newborn mice were injected with cells producing the RCAS retroviruses carrying the Platelet-Derived Growth Factor-B (PDGFB) (Shih et al., 2004).
In Ntv-a mice, loss of either both copies or a single copy of Chk2 significantly accelerates PDGF-induced glioma formation, with the wild-type mice having an average survival of over 90 days, Chk2 null mice of 34 days (p < 0.0001, Log-rank test) and heterozygous of 55 days (p = 0.0036, Log-rank test) (Figure 2A). Notably loss of heterozigosity (LOH) wasn’t detected in Chk2+/− gliomas (Figure S1A), however 2 of 4 tumors analyzed showed lower expression of Chk2 mRNA when compared to the normal contralateral side of the brain (Figure S1B), suggesting that other mechanisms than genomic loss can lead to inactivation of the remaining wild-type allele.
Figure 2. The ATM/Chk2/p53 cascade prevents GBM formation in mice.
(A) and (B) Kaplan-Meier survival curves of PDGF-induced gliomas in Nestin-tva mice of the indicated genotypes; Log-rank p values have been measured with the Mantle-Cox test. (C) Top panel, H&E staining of a RCAS/PDGF-induced GBM (left), inlet shows pseudopalisading necrosis (black arrows) and microvascular proliferation (yellow arrows) (right); Bottom panel, IHC staining with anti-Olig2 (left) and anti-GFAP (right) of the GBM showed above. Scale bars, 100 µm. (D) Graph showing the percentage of Grade II-IV tumors for the indicated genotypes. (E) Table showing the number of Grade IV tumors (GBM)/total tumors; p values were measured vs. wild type mice using the Fisher’s exact test. See also figure S1.
Chk2 is known to be required for the p53-dependent apoptotic response to radiation (Hirao et al., 2002; Hirao et al., 2000; Takai et al., 2002). In order to analyze the role of p53 in Chk2 mediated tumor suppression, we crossed Ntv-a Chk2−/− mice with p53−/− mice and noted that PDGF-induced gliomas arise with a similar latency in both genetic backgrounds (Figure 2A). Furthermore, the loss of a single p53 allele did not further accelerate glioma formation when Chk2 is already partially inactivated (Chk2+/−). Both p53+/− and Chk2+/−; p53+/− genetic backgrounds showed a PDGF-induced glioma-free survival very similar to the Chk2+/− background, suggesting that Chk2 and p53 are epistatic in the suppression of glioma formation.
ATM kinase is required for Chk2 activation and acts in concert and in parallel with Chk2 in DNA damage checkpoint modulation (Matsuoka et al., 1998; Stracker et al., 2007), therefore we determined if ATM was also required to suppress PDGF-induced gliomagenesis. Indeed, loss of a single copy or both copies of ATM significantly accelerates PDGF-induced glioma formation, with Atm+/+ mice having an average survival of 91 days, Atm+/− of 61 days (p = 0.0023, Log-rank test) and Atm−/− of 58.5 days (p = 0.019, Log-rank test) (Figure 2B). No LOH was detected in Atm+/− gliomas (Figure S1C).
Gliomas of all genotypes expressed Olig2 throughout the tumor mass, while GFAP positive cells were restricted to the area adjacent to tumor vessels (Figure 2C, bottom panels) and along the tumor/normal border (data not shown). The tumors of the different genotypes were then classified using the World Health Organization (WHO) grading system: grade IV, also known as GBM (presence of microvascular proliferation, MVP, and pseudopalisading necrosis), grade III (presence of MVP only) and grade II (absence of both MVP and pseudopalisading necrosis) (Figure S1D). GBMs arise with higher frequency in Chk2 null (72%) (p < 0.0001, Fisher’s exact test), p53 null (100%) (p = 0.0029, Fisher’s exact test) and Atm null mice (83%) (p = 0.0002, Fisher’s exact test) compared to wild-type mice (19%) (Figure 2D and 2E), suggesting that the ATM/Chk2/p53 pathway is required to constrain the tumor progression to a more malignant phenotype. Moreover, despite having a similar glioma free survival, p53+/− and Chk2+/−; p53+/− mice develop GBMs with a higher frequency than the Chk2+/− mice (75% and 85% vs. 40%). These data suggest that loss of Chk2 does not completely phenocopy p53 loss in regards to tumor formation, and may explain the concurrent alteration of TP53 and CHEK2 in a subset of human GBMs.
Loss of Chk2 potentiates GBMs radiation resistance in vivo
Radiotherapy and chemotherapy are the standard treatments for GBMs patients, however the treatment outcomes are variable and most patients respond poorly to these treatments. Ionizing radiation (IR) and radiomimetic drugs lead to the formation of double-strand DNA breaks (DSBs) that are responsible for the establishment of the DDR. To determine if loss of Chk2 contributes to the poor response of glioma to IR, we analyzed the outcome of radiation in the Chk2−/− tumor bearing mice. As controls we used both wild-type and Ink4a/Arf null mice (Dai et al., 2001), since deletion of the INK4a/ARF locus is one of the most frequent events that leads to p53 pathway inactivation in human glioma.
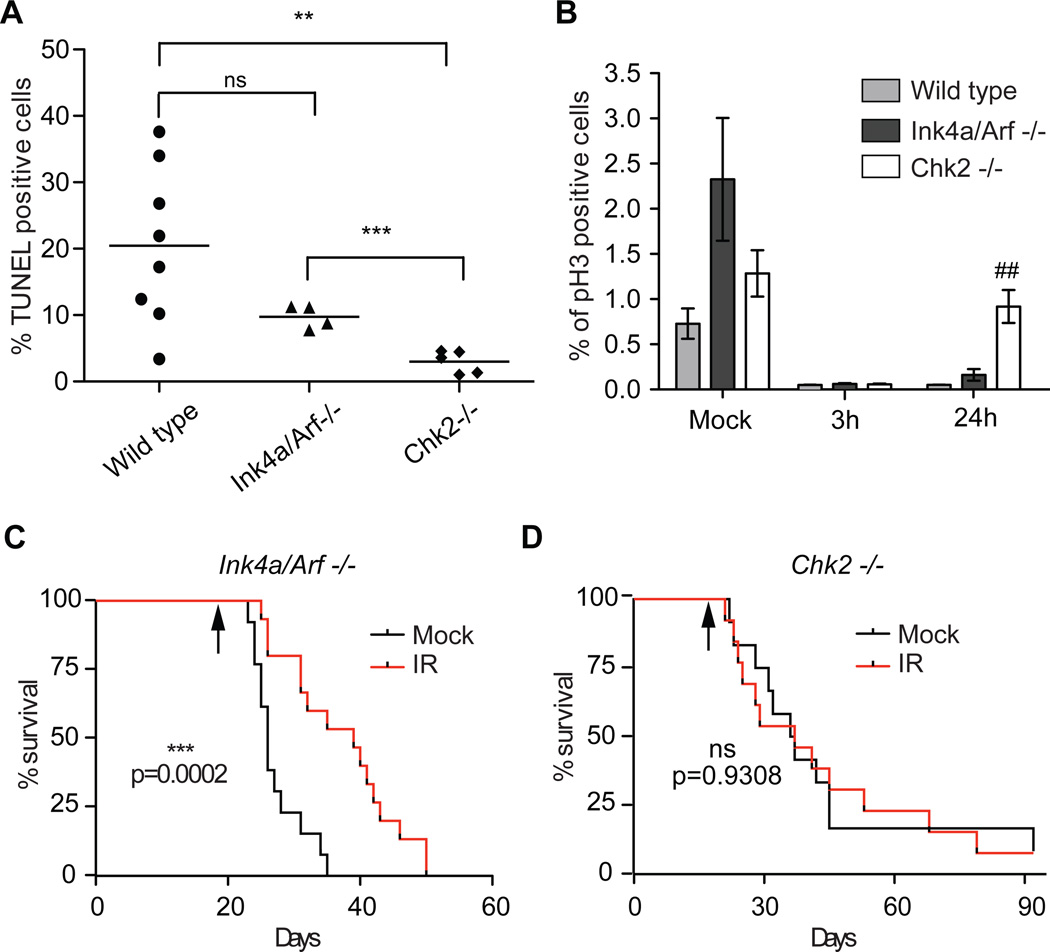
To examine the IR response of glioma in vivo, glioma-bearing mice were exposed to 10 Gy IR and apoptosis and G2/M arrest were measured after 24h. As shown in figure 3A, upon IR Ink4a/Arf null mice showed a non-significant reduction of TUNEL positive tumor cells compared to wild-type mice (10% and 20% respectively, p> 0.05, Student t-test). By contrast, Chk2 null mice showed significantly reduced numbers of apoptotic cells (approximately 3%), compared both to wild-type mice (p= 0.0023, Student t-test) and Ink4a/Arf null (p= 0.0007, Student t-test). Moreover, while the number of dividing tumor cells (phospho-Histone H3 (pH3) are nearly undetectable 3h post-IR in all the different genetic background analyzed, only the Chk2 tumors have a similar number of dividing cells to the control non-irradiated mice 24h post-IR (Figure 3B).
Figure 3. Chk2 is required for radiation response in gliomas in vivo.
Percentage of (A) TUNEL (24h post 10Gy IR) and (B) pH3 (3h and 24h post 10Gy IR) positive glioma cells in tumor-bearing mice of the indicated genotype; Student t-test (A) ** p=0.0023, *** p=0.007 and (B) ## p=0.0089 (Chk2−/− vs. WT). Data are presented as mean ± SD of 3 tumors for each group. (C) and (D) Kaplan-Meier survival curves of RCAS/PDGF-induced gliomas in Ntv-a and Ink4a/Arf−/− and Ntv-a Chk2−/− mice, respectively; black arrows indicate the day of exposure to 10Gy IR. Log-rank p values have been measured with the Mantle-Cox test.
We then examined the long-term effect of ionizing radiation in the Chk2−/− and Ink4a/Arf−/− GBM mouse model. Of note, we did not include wild-type mice in this experiment due to their much lower tumor incidence and longer survival. Newborn mice were injected with RCAS/PDGF and 18 days post injection randomized into two groups. One group was locally irradiated to the head with 10Gy. A small cohort of mice was also sacrificed at the time of irradiation and the presence of tumor lesions was confirmed by H&E in animal analyzed (5/5) in both genetic backgrounds (data not shown). The exposure to IR resulted in a prolonged survival in the Ink4a/Arf−/− (approximately 15 days, p= 0.0002 Log Rank) but did not have any effect on the Chk2−/− mice. These data are consistent with the lower apoptotic index and higher proliferation rate that we observed in Ntv-a Chk2−/− tumor-bearing mice exposed to IR, strongly suggesting that Chk2 plays an important role in the radiation response in glioma.
Cell-cycle checkpoint defects in Chk2 null glioma neurospheres and normal neuronal stem cells (NSC) of immature postnatal mice
We further investigated the integrity of cell-cycle checkpoints in Chk2 null gliomas using tumor neurospheres (tumor cells cultured in the bFGF and EGF) derived from primary glioblastomas. These cell cultures more closely resemble the phenotype and genotype of the tumors than do serum cultured cell lines (Lee et al., 2006). Moreover, GBM cells propagated as neurospheres (both from human and mouse) contain stem-like subpopulations that are able to generate tumors when transplanted into mice, and exhibit a more accurate infiltrating growth pattern seen in primary tumors (Bleau et al., 2009; Galli et al., 2004). As we have previously shown (Bleau et al., 2009; Charles et al., 2010), tumor neurospheres derived from N-tva mice injected with RCAS/PDGF, when transplanted into syngeneic mice, are able to generate tumors that histo-pathologically resemble the primary tumors (Figure S2A), suggesting that they could be used as a reliable in vitro tumor model for our studies.
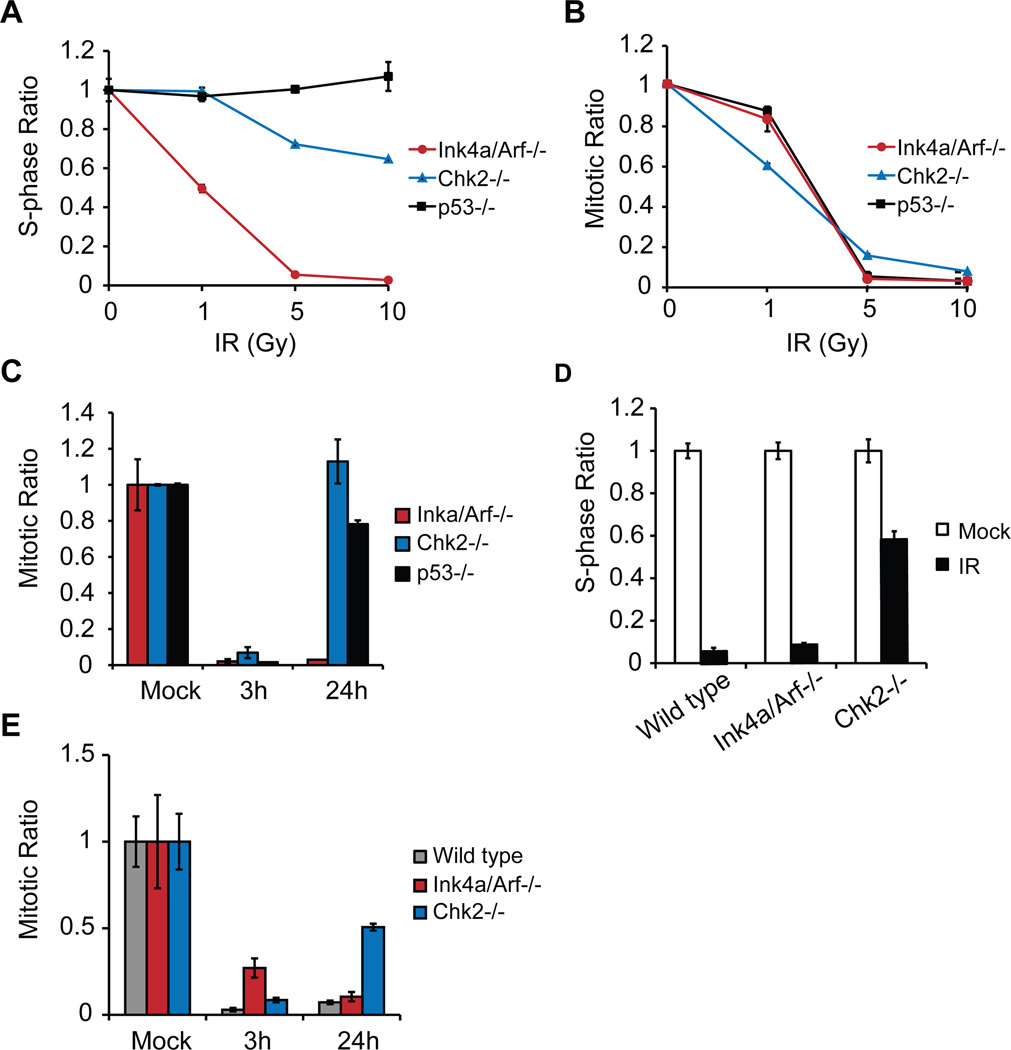
The G1/S checkpoint, which prevents cells from entering S phase, is predominantly regulated by p53 (Kuerbitz et al., 1992) and is defective in ATM null cells (Xu and Baltimore, 1996). Despite its well-known function in ATM-dependent IR-induced p53 activation, the role of Chk2 in the G1/S checkpoint is still controversial (Hirao et al., 2002; Jack et al., 2002; Stracker et al., 2008; Takai et al., 2002). To examine the G1/S checkpoint, glioma neurospheres were exposed to increasing doses of IR (1, 5 and 10Gy), and the percentage of cells in S phase 18 hr post-treatment was measured by BrdU incorporation. In the Ink4a/Arf−/− glioma neurospheres, the percentage of BrdU positive cells at the highest IR dose (10Gy) dropped to approximately 10% of the mock irradiated cells (Figure 4A). The same result was obtained with wild-type tumor neurospheres (data not shown). By contrast over 60% of the cells was still incorporating BrdU in Chk2−/− glioma neurospheres, clearly indicating a defect in the G1/S checkpoint in these cells. As expected p53−/− glioma neurospheres are completely devoid of such a checkpoint and maintain the ability to incorporate BrdU even at the highest radiation dose analyzed (Figure 4A).
Figure 4. Cell-cycle checkpoints are defective in Chk2 null tumor neurospheres and normal neuronal stem cells (NSC).
(A) The S-phase ratios (%BrdU-positive of treated/average %BrdU-positive untreated) and (B) the mitotic ratios (%pH3-positive of treated /average %pH3-positive untreated) of IR-treated tumor neurospheres (18h and 3h post-treatment, respectively) are plotted. The wedges denote increasing ionizing radiation (IR) doses (0, 1, 5 or 10 Gy). (C) Mitotic ratios of 10 Gy IR-treated tumor neurospheres at the indicated time points. (D) Sphase ratios of mock (white bar) or 10 Gy IR-treated (black bar) NSCs of the indicated genotype. See also figure S2. (E) Mitotic ratios of 10 Gy IR-treated NSCs at the indicated time points. Results are presented as mean ± SD from a representative of two experiments, performed in triplicate.
The role of Chk2 in the G2/M checkpoint has also been debated (Hirao et al., 2002; Hirao et al., 2000; Takai et al., 2002). As described above, Chk2 null gliomas exposed to IR show a higher mitotic index (measured as % of pH3 positive cells) than either wild-type or Ink4a/Arf null gliomas (Figure 3B), suggesting a possible defect in the G2/M checkpoint. To analyze the integrity of the G2/M checkpoint, glioma neurospheres were exposed to increasing doses of IR (1, 5 and 10Gy), and the percentage of pH3 positive cells was measured 3 hr post-treatment. Ink4a/Arf−/−, Chk2−/− and p53−/− cells showed similar kinetics in the reduction of pH3-positive cells, becoming almost undetectable at 10Gy IR (Figure 4B). These results are consistent with our in vivo observations in glioma-bearing mice exposed to IR, suggesting that the activation of the G2/M checkpoint was intact both in vitro and in vivo. Moreover, while all the cells in the Chk2−/− glioma neurospheres cultures, and to a similar extent the p53−/− cells, re-entered the cell cycle by 24 hr post-treatment, only 5% of the cells of the Ink4a/Arf−/− (Figure 4C) or wild-type (data not shown) did so. These data clearly indicate that Chk2 is required for the maintenance of the G2/M checkpoint in gliomas, but not for its activation, either in vitro or in vivo.
In order to exclude the possibility that the defects in cell-cycle checkpoints in Chk2 null glioma and tumor neurospheres were due to additional mutations acquired during the tumor formation or in vitro cell culture artifacts (caused by multiple passages of tumor cells), we analyzed the integrity of checkpoints in freshly isolated neuronal stem cells (NSCs), derived from wild-type, Chk2−/− and Ink4a/Arf−/− newborn mice. The G1/S and G2/M checkpoints were tested in these cultures by BrdU incorporation and pH3-positive cell sorting. 18h after exposure to 10Gy IR in both wild-type and Ink4a/Arf−/− resulted in less than 10% of cells incorporating BrdU, while 60% of Chk2−/− NSCs were still synthesizing DNA (Figure 4D and Figure S2B-D). Furthermore, all genotypes responded to 10Gy IR by a 90–95% reduction of pH3-positive cells, but only the Chk2−/− NSCs re-entered the cell cycle by 24h after exposure to IR (Figure 4E). These data indicate that Chk2 is required for the activation of the G1/S and for the maintenance of G2/M checkpoint in the neuronal stem cell compartment of postnatal mice.
Aberrant constitutive activation of DDR in GBM specimens
Markers of a constitutively active DDR have been described in multiple human epithelial tumors (Bartkova et al., 2005; Gorgoulis et al., 2005; Nuciforo et al., 2007), but it has been shown to be absent in other malignant lesions, such as testicular germ cell tumors (Bartkova et al., 2007). To analyze a possible aberrant activation of the DDR in GBMs specimens, we performed IHC on formalin-fixed, paraffin embedded tissues, using antibodies against phosphorylated S139 of histone H2AX (γH2AX) and phosphorylated T68 Chk2 (Chk2 pT68). A granular (focal) nuclear staining pattern of 53BP1, indicating the formation of DNA double-strand breaks, was also considered a marker (53BP1 foci) for an activated DDR (see figure S3A for representative micrographs).
We stained 14 newly diagnosed GBMs samples from patients not previously exposed to radiation or chemotherapy. Approximately 80% (11/14) of the samples showed nuclear γH2AX staining in a small fraction of cells, ranging from 0.5% to 8.4% of total cells (Figure 5A). No γH2AX accumulation was found in 10 normal tissue samples of different area of the brain (data not shown). Chk2 pT68 was detected in a lower percentage of tumors than γH2AX, approximately 60% (8/14), and in a number of cells varying from 0.2% to 2.5% (Figure 5A and 5B) (p = 0.0069, two sided Student’s paired t-test on the % of γH2AX cells vs. % of Chk2 pT68 cells). Consistent with the γH2AX observations, no Chk2 pT68 staining was present in normal tissues (data not shown). Moreover, 14% of tumors analyzed (2/14) presented nuclear 53BP1 foci and notably, those samples were the ones with a high percentage of γH2AX positive cells (Figure 5A).
Figure 5. Constitutive activation of DDR in human GBM.
(A) Summary of IHC results. γH2AX and Chk2 pT68 were quantified as described in Experimental Procedures. 53BP1 staining was scored as follow: weak (+), moderate (++) and strong (+++). n/a = not applicable (absence of pseudopalisades necrosis). (B) Plot of the % of total γH2AX and Chk2 pT68 positive cells. (C-H) Micrographs of pseudopalisading necrosis of GBMs stained with H&E, anti-HIF1α, anti Ki-67, anti-γH2AX, anti-Chk2 pT68 and anti-53bp1, respectively (see also Figure S3). Inlet in (E) shows nuclear accumulation of HIF1α in cells of the pseudopalisades rim. Scale bars, 100 µm. (I) Plot of the % of positive cells in tumor bulk and pseudopalisades for γH2AX (top panel) and for Chk2 pT68 (bottom panel).** p< 0.01 and * p< 0.05 (see text for more details).
Several tumors analyzed presented regions of pseudopalisading necrosis. These structures, which represent the histologic definition of GBMs, are characterized by hyper cellular area surrounding a necrotic center (Figure 5C). Consistent with previous reports (Brat et al., 2004), pseudopalisading cells in these tumors showed a lower proliferating index (measured by Ki-67 staining) as compared to the tumor bulk and also showed nuclear accumulation of HIF1-α (Figure 5D and 5E), indicating higher hypoxic levels in these cells. Although only a small percentage of total number of cells presented an activated DDR, when compared to the adjacent tumor cells, the pseudopalisade rim showed a significantly higher percentage of γH2AX (p = 0.0071, two-sided paired Student’s t-test) (Figure 5F, 5I top panel and S3B) and of Chk2 pT68 positive cells (p = 0.0297, two-sided paired Student’s t-test) (Figure 5G, 5I bottom panel and S3B). These data suggest that the DDR activation in GBM may be hypoxia related.
Discussion
ATM/CHEK2 alteration in human gliomas
It has been previously proposed that the DDR acts as an inducible barrier to tumorigenesis, and defects in elements of the DDR have been associated with different human pathologies, including cancer (Jackson and Bartek, 2009). Here we show that the ATM/Chk2/p53 cascade, key components of the DDR, exert an important tumor suppressor activity in the brain.
Mutations of the ATM gene are responsible for the ataxia-telangiectasia (A-T) disease, an autosomal recessive disorder that is characterized by early onset progressive cerebellar ataxia and high incidence of lymphoid tumors (approximately 30%) (see for review Lavin, 2008). With regard to the central nervous system tumors, some cases of primary brain tumors, including gliomas, have also been reported in A-T patients (Miyagi et al., 1995). CHEK2, one of the main ATM downstream effectors, has been proposed to act as a multiorgan cancer susceptibility gene (Cybulski et al., 2004). While mutations in CHEK2 do not appear to account for the cancer-predisposing Li-Fraumeni Syndrome as originally hypothesized, rare germline mutations have been identified in several types of familial cancers (prostate, breast, ovarian, colorectal, kidney, thyroid, bladder cancers and leukemias) and rare somatic mutations have also been detected in a variety of human tumors (Antoni et al., 2007). Previous studies reported no (Ino et al., 2000) or low frequency of CHEK2 mutations (approximately 6%) (Sallinen et al., 2005) in small cohorts of human glioma patients. Moreover, Wang and colleagues recently reported a significant down-regulation of CHEK2 expression in a group of glioma specimen compared to normal control, and this reduction in expression was partially due to promoter methylation (Wang et al., 2009). Our analysis of TCGA showed that a high number of patients (22%) present single copy loss of the chromosomal region containing CHEK2, with a significant reduction of CHEK2 mRNA expression, suggesting that it might represent an important tumor suppressor in a subset of glioma patients. Evaluation of CHEK2 mutations by resequencing, including TCGA data, is complicated by the presence of paralogs in the genome assembly. Therefore, although five CHEK2 somatic missense mutations have been reported in the TCGA dataset (Table S1 available on line), at this time we consider the mutation rate to be undetermined. We further observed that in human gliomas, CHEK2 alterations could co-occur with TP53 alterations (either TP53 mutations/loss or MDM2 amplifications). This finding correlates with our mouse studies that demonstrate that although p53 and Chk2 are epistatic in terms of glioma-free survival, loss of Chk2 could lead to the development of more aggressive gliomas when combined with p53 defects.
The ATM/Chk2/p53 axis suppress PDGF-induced glioma formation in mice
PDGF-pathway activation, that is primarily ligand-driven, has been recently shown to represent one of the three major signal transduction pathways that characterize different GBM subclasses, together with EGFR activation and NF1 loss, (Brennan et al., 2009; Verhaak et al., 2010). The RCAS/PDGF glioma model has been extensively used to study diverse aspects of glioma biology and has been shown to produce tumors that closely resemble human GBMs (Huse and Holland, 2009; Hambardzumyan et al., 2008). Using this somatic cell gene transfer methodology we showed that the ATM/Chk2/p53 pathway is required for glioma tumor suppression in mice, and loss of any of those genes both shortens tumor latency and leads to a more aggressive phenotype, increasing the frequency of high-grade tumors (GBMs). The role of the ATM/Chk2/p53 pathway on tumor suppression can be attributed to its influence on the induction of apoptosis as well as on the regulation of DNA damage dependent checkpoints. Genetic contexts in which apoptosis and checkpoints are individually defective are not strongly predisposed to malignancy. For example, p53515C/515C mice are defective in apoptosis, but retain p53-dependent G1/S checkpoint functions. Relative to p53−/− mice, in which both apoptosis and G1/S checkpoint control are abrogated, tumor latency in p53515C/515C mice is markedly increased (Liu et al., 2004). Similarly, in Mre11ATLD1/ATLD1 Chk2−/− mice, p53-dependent apoptosis is abrogated, but G1/S checkpoint control is largely intact (Stracker et al., 2008). Hence, concomitant loss of checkpoint control and apoptotic regulation appears to undermine tumor suppression to a significantly greater extent than disruption of either function singly. The clear dependence on Chk2 for G1/S checkpoint control in NSCs contrasts the situation observed in other Chk2−/− murine cell types. This unique dependence on Chk2 has two general implications. First, it supports the view that increased susceptibility to GBM in Chk2−/− mice may reflect the tumor suppressive effects of the G1/S checkpoint in addition to Chk2’s role in promoting apoptosis. Presumably, both functions are required to mitigate the oncogenic effects of spontaneous DNA damage in developing GBM. Second, the data further support the view that radioresistance in Chk2 proficient GBM may reflect the protective effects of the G1/S checkpoint.
Activation of DDR in GBMs correlates with hypoxia
The current model of DDR activation in human tumors proposes that the activation of DNA damage checkpoints acts as a barrier against progression of tumors beyond their early, preinvasive stage, and represent a strong selective pressure for mutation in p53 or other DDR components (Bartek et al., 2007). In agreement with this hypothesis, Bartkova and colleagues have shown in a recent report a more pronounced DDR activation in low grade glioma than what they observed in a set of GBM specimen (Bartkova et al., 2010), suggesting the existence of a selective pressure to lose DDR activity in the progression from low to high grade tumors. Constitutive activation and aberrant loss of DDR components has been shown in other tumor types, such as lung and breast cancer (Bartkova et al., 2007). In GBM tissues, we observed a small percentage of cells presenting markers of an active DDR (such as γH2AX and Chk2 pT68), but notably this activation correlates with region of hypoxia. H2AX is phosphorylated in a context-dependent manner by at least 3 different kinases (ATM, ATR and DNA-PK) of the PI3K-related kinase family (PI3KK) (Hammond et al., 2003; Stiff et al., 2004; Ward and Chen, 2001). Although our TCGA analysis showed copy number alterations in either ATM or ATR, we cannot exclude that the activity of these kinases is partially maintained or, alternatively, partially compensated by other PI3KK kinases, at least to some extent. This residual kinase activity might also be responsible for the phosphorylation of Chk2, that per se is homozygously lost in only 0.35% (1/272) of the patients in the TCGA analysis (data not shown). Although it appears there is a selective pressure to reduce the activity of the ATM/Chk2 kinases in GBM, this pathway might still maintain the ability to be partially activated under higher cellular stress condition such as hypoxia.
Proof of activation of the ATM/ATR signaling pathways by hypoxia has been recently reported in vitro (Bencokova et al., 2009; Freiberg et al., 2006; Gibson et al., 2005) and it was previously hypothesized to happen in human tumors (Bartkova et al., 2005). ATM can be phosphorylated and activated during hypoxia in the absence of DNA damage as detected by either comet assay or 53BP1 focus formation (Freiberg et al., 2006). Subsequently Chk2 is also phosphorylated and activated in an ATM-dependent manner (Bencokova et al., 2009; Gibson et al., 2006). Interestingly, cells in the pseudopalisade rim do not show focal staining of 53BP1, underlining the absence of detectable DNA damage. By contrast, 53BP1 is much less expressed in those cells as compared to the tumor bulk (Figure 5A, 5H and S3B). Several DNA repair factors (such as RAD51, BRCA1 and 53BP1) are down-regulated in hypoxic conditions in vitro, and it has been proposed that this could lead to genomic instability (Bencokova et al., 2009; Bristow and Hill, 2008). 53BP1 is an important component of both the DDR and also of the DNA repair process. A reduction in 53BP1 levels is sufficient to compromise the maintenance of chromosomal structure and number (Ward et al., 2005), therefore low levels of 53BP1 might contribute to hypoxia-induced genomic instability in gliomas.
Clinical perspective
Standard therapy for GBMs includes resection of the tumor mass followed by concurrent radiotherapy and chemotherapy using the alkylating agent temozolomide (TMZ). Nonetheless, many GBMs patients are refractory to radiotherapy and chemotherapy treatments. Understanding the mechanism of this resistance will be instrumental for the development of new treatment modalities for gliomas. Previous studies reported that Chk2 gene silencing prevented a TMZ-induced G2 arrest (Hirose et al., 2005), whereas pharmacological targeting of Chk1 increased TMZ cytotoxicity (Hirose et al., 2001). Moreover, dual pharmacological inhibition of Chk1 and Chk2 kinases has been shown to revert the radioresistance of a sub-population of glioma cells in vitro (Bao et al., 2006).
In this report we demonstrate that genetic loss of Chk2 protects glioma cells from IR-induced apoptosis in vivo and prevents the activation of DNA damage-induced cell-cycle checkpoints. Most importantly, this lack of response to IR also abolishes the IR-mediated survival benefit observed in Chk2-proficient glioma-bearing mice. Our results somehow differ from what Bao and colleagues had previously shown (Bao et al., 2006). In their experimental settings, they observed an increase in radiosensitivity of glioma stem-like cells upon treatment with the sponge alkaloid debromohymenialdisine (DBH) that inhibits both the Chk1 and Chk2 kinases. It is possible that the phenotypes they observed are primary related to the DBH effect on Chk1 activity. Even though our data point to an indispensible role of Chk2 in IR response in gliomas, we believe that it might be too early to conclude whether this evidence can be generalized to other components of the DDR, considering the high complexity of this signaling pathway. Our data do indicate that specific pharmacological inhibition of Chk2 may not be an effective treatment for glioma patients, though genetic or pharmacological inactivation of other elements of the DDR, such as Chk1, might results in increased sensitivity to IR or other treatment modalities that inflict DNA damage.
Experimental Procedures
Human tissue specimens
Following informed consent, tumor samples classified as GBM based on World Health Organization (WHO) criteria were obtained from patients undergoing surgical treatment in accordance with MSKCC Review Board. Tissues were processed as described below. Normal human brain tissues were purchased from Millipore (#TMA3001-4) and Analytical Biological Services (Wilmington, USA).
Histology, Immunohistochemistry and TUNEL assay
Tissues were fixed in 10% neutral buffered formalin and subsequently embedded in paraffin, following standard procedures. Immunohistochemical staining was performed on 5µm sections of formalin-fixed/paraffin-embedded tissues, using a Discovery XT automated staining processor (Ventana Medical Systems, Inc.). The following antibodies were diluted in PBS 2%BSA as follow: γH2AX (Upstate, #07-164) 1:100, anti-phospho Chk2 (Thr68) (Cell Signaling, #2661) 1:100, anti-53bp1 (Novus, #NB100-304) 1:800, anti-HIF1α (Chemicon, #AB3883) 1:100, Ki-67 (Dako, #M7240) 1:100, anti-GFAP (Dako, #Z0334) 1:2000, anti-Olig2 (Millipore, #AB9610) 1:400, anti-phospho-Histone H3 (Ser10) (Millipore, #06-750) 1:800. Images from each tumor were acquired with a Nikon Eclipse E400 microscope connected to a Nikon Digital Slight camera system.
The TUNEL assay was performed with a terminal transferase recombinant kit (Roche, #333-574-001) on an automated staining processor (Discovery XT, Ventana Medical Systems, Inc.).
For quantification of immunohistochemical and TUNEL staining, images were acquired with a Zeiss Imager Z1 microscope using an automated stage, and subsequently analyzed using the HistoQuest image analysis software (Tissue Gnostics, Austria). Briefly, the HistoQuest software use a specific algorithm to identify the nucleus of each cells, it measures the DAB intensity staining (brown nuclei) and gives you the percentage of positive cells (brown nuclei/total nuclei). With exception of the necrotic cells inside the pseudopalisades, no TUNEL-positive cells were detected in the mock irradiated samples (data not shown). Because the number and the size of the pseudopalisades highly variable in different tumors, these areas were excluded from the quantification of TUNEL-positive cells in the irradiated samples.
Mice
Nestin-tva mice have been previously described (Holland et al., 1998). p53−/− mice were obtained from The Jackson Laboratory. All animal experiments were done in accordance with protocols approved by the Institutional Animal Care and Use Committee of Memorial Sloan-Kettering Cancer Center and followed National Institutes of Health guidelines for animal welfare. Genotyping primers will be provided on request.
Cell culture, transfection and neurosphere preparation
DF1 cells (ATCC) were grown at 39°C in DMEM (ATCC) containing 10% FBS (PAA, The cell culture company, USA). DF1 cells were transfected with the RCAS-PDGF viral plasmid, using Fugene 6 Transfection reagent (Roche), accordingly to manufacture protocol.
Mouse tumor neurospheres and normal neuronal stem cells (derived from the whole brain of newborn mice (p1-p3)) were prepared by enzymatic dissociation and low-speed centrifugations as previously described (Bleau et al., 2008). Normal brain cortex or tumors were dissected and collected in 1× Earle’s balanced salt solution (EBSS) (Gibco, USA). The tissue was enzymatically digested in Earle’s balanced salt solution containing 12% papain and 10 µg/ml DNase at 37°C for 15 min, with subsequent inactivation using ovomucoid (1mg/mL) (Worthington, Lakewood, NJ, USA). The cell suspension was consecutively washed and resuspended to obtain a single cell suspension. For neurosphere culture, cells were seeded at 5x104 cells/ml and grown in neurosphere medium from NeuroCult (Stem Cell Technologies Inc, Vancouver, Canada) according to the manufacturer. The medium consists in NSC Basal medium, mouse NSC Proliferation Supplements, 10 ng/ml EGF, 20 ng/ml basic-FGF and 1mg/ml Heparin. Fresh medium was replaced into the cultures every 48–72 hours, and tumor stem-like cells were propagated as neurospheres by serial dilution.
Generation of murine gliomas
Ntv-a mice, and procedures for RCAS-mediated gliomagenesis have been described previously (Holland et al., 1998; Holland and Varmus, 1998). After injection of the RCAS-PDGF virus during the newborn period, mice were aged until they developed symptoms of disease (lethargy, poor grooming, weight loss, macrocephaly).
Cell-cycle analysis
Cell-cycle checkpoints analysis were performed as described (Theunissen and Petrini, 2006). For the G1/S checkpoint studies, BrdU (10µM) was added in the culture medium for 2h, before ethanol fixation. Staining was performed using an anti-BrdU-FITC (BD Pharmingen). For the G2/M checkpoint studies we used the anti-phospho-Histone H3 (Ser10) described above. Samples were acquired using a Becton-Dickinson FACSCalibur flow cytometer and analyzed with the Flowjo software.
Radiation
Neurospheres cultures were irradiated using a Cs-137 source (dose rate 200 cGy/min- Shepherd Mark-1). Tumor-bearing mice were irradiated using a Cs-137 source (Gammacell 40 Exactor, MDS Nordion). For survival analysis, mice were sedated with ketamine and xylazine, and irradiation of the head was done using a X-RAD 320 from Precision X-Ray at 115 cGy/min (the rest of the mouse was shielded with a lead jig).
Statistical analysis
Results were subjected to statistical analysis using GraphPad Prism v5.0 software. Survival curves were analyzed using the Kaplan-Meier method, with groups compared by respective median survival of number of days taken to reach 50% morbidity; Log-rank p valued was measured using the Mantel-Cox test. Two-tailed t-test has been used for other analysis.
Supplementary Material
Significance.
Glioblastoma mutliforme (GBM) is the most aggressive and lethal tumor of the central nervous system in the adult. Despite decades of efforts, GBMs patients remain refractory to standard therapies and have a very low survival rate. Understanding the mechanisms responsible for this poor treatment response is one of the primary goals in the brain tumor research field. Here we show that some of the elements of the DNA Damage Response (DDR) pathway are required to suppress tumor formation in a glioma mouse model. Furthermore we demonstrate that Chk2, one of the key components of the DDR, is required for glioma radiation sensitivity, suggesting that the integrity of this pathway is instrumental both for tumor suppression and for irradiation response.
Acknowledgements
We thank Robert Finney, Quancho Zhang and Eletha Carbajal for technical support. We thank Diane Domingo for the flow cytometric analysis. We thank members of Holland lab for discussion. We gratefully acknowledge the TCGA Consortium for providing samples, tissues, data processing and making data and results available. The results published here are in part based upon data generated by The Cancer Genome Atlas pilot project established by the NCI and NHGRI. Information about TCGA and the investigators and institutions who constitute the TCGA research network can be found at http://cancergenome.nih.gov. This work was supported by “Fondazione Italiana Ricerca Cancro” and “American Brain Tumor Association” Research Fellowship in memory of Justin Porter (to M.S.), “National Brain Tumor Society” Grant Award (to E.C.H.). J.H.P. is supported by NIH grants. K.H is a Howard Hughes Medical Institute Medical Research Training Fellow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antoni L, Sodha N, Collins I, Garrett MD. CHK2 kinase: cancer susceptibility and cancer therapy - two sides of the same coin? Nat Rev Cancer. 2007;7:925–936. doi: 10.1038/nrc2251. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene. 2007;26:7773–7779. doi: 10.1038/sj.onc.1210881. [DOI] [PubMed] [Google Scholar]
- Bartek J, Lukas J. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell. 2003;3:421–429. doi: 10.1016/s1535-6108(03)00110-7. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Hamerlik P, Stockhausen MT, Ehrmann J, Hlobilkova A, Laursen H, Kalita O, Kolar Z, Poulsen HS, Broholm H, et al. Replication stress and oxidative damage contribute to aberrant constitutive activation of DNA damage signalling in human gliomas. Oncogene. 2010 doi: 10.1038/onc.2010.249. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Koed K, Kramer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. doi: 10.1038/nature03482. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Horejsi Z, Sehested M, Nesland JM, Rajpert-De Meyts E, Skakkebaek NE, Stucki M, Jackson S, Lukas J, Bartek J. DNA damage response mediators MDC1 and 53BP1: constitutive activation and aberrant loss in breast and lung cancer, but not in testicular germ cell tumours. Oncogene. 2007;26:7414–7422. doi: 10.1038/sj.onc.1210553. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- Bencokova Z, Kaufmann MR, Pires IM, Lecane PS, Giaccia AJ, Hammond EM. ATM activation and signaling under hypoxic conditions. Mol Cell Biol. 2009;29:526–537. doi: 10.1128/MCB.01301-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4:226–235. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau AM, Howard BM, Taylor LA, Gursel D, Greenfield JP, Lim Tung HY, Holland EC, Boockvar JA. New strategy for the analysis of phenotypic marker antigens in brain tumor-derived neurospheres in mice and humans. Neurosurg Focus. 2008;24:E28. doi: 10.3171/FOC/2008/24/3-4/E27. [DOI] [PubMed] [Google Scholar]
- Brat DJ, Castellano-Sanchez AA, Hunter SB, Pecot M, Cohen C, Hammond EH, Devi SN, Kaur B, Van Meir EG. Pseudopalisades in glioblastoma are hypoxic, express extracellular matrix proteases, and are formed by an actively migrating cell population. Cancer Res. 2004;64:920–927. doi: 10.1158/0008-5472.can-03-2073. [DOI] [PubMed] [Google Scholar]
- Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4:e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristow RG, Hill RP. Hypoxia and metabolism. Hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008;8:180–192. doi: 10.1038/nrc2344. [DOI] [PubMed] [Google Scholar]
- Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulski C, Gorski B, Huzarski T, Masojc B, Mierzejewski M, Debniak T, Teodorczyk U, Byrski T, Gronwald J, Matyjasik J, et al. CHEK2 is a multiorgan cancer susceptibility gene. Am J Hum Genet. 2004;75:1131–1135. doi: 10.1086/426403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durocher D, Jackson SP. DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell Biol. 2001;13:225–231. doi: 10.1016/s0955-0674(00)00201-5. [DOI] [PubMed] [Google Scholar]
- Freiberg RA, Hammond EM, Dorie MJ, Welford SM, Giaccia AJ. DNA damage during reoxygenation elicits a Chk2-dependent checkpoint response. Mol Cell Biol. 2006;26:1598–1609. doi: 10.1128/MCB.26.5.1598-1609.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Gibson SL, Bindra RS, Glazer PM. Hypoxia-induced phosphorylation of Chk2 in an ataxia telangiectasia mutated-dependent manner. Cancer Res. 2005;65:10734–10741. doi: 10.1158/0008-5472.CAN-05-1160. [DOI] [PubMed] [Google Scholar]
- Gibson SL, Bindra RS, Glazer PM. CHK2-dependent phosphorylation of BRCA1 in hypoxia. Radiat Res. 2006;166:646–651. doi: 10.1667/RR0660.1. [DOI] [PubMed] [Google Scholar]
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr., Kastrinakis NG, Levy B, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–913. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- Hambardzumyan D, Squatrito M, Carbajal E, Holland EC. Glioma formation, cancer stem cells, and akt signaling. Stem Cell Rev. 2008;4:203–210. doi: 10.1007/s12015-008-9021-5. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Dorie MJ, Giaccia AJ. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem. 2003;278:12207–12213. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- Hirao A, Cheung A, Duncan G, Girard PM, Elia AJ, Wakeham A, Okada H, Sarkissian T, Wong JA, Sakai T, et al. Chk2 is a tumor suppressor that regulates apoptosis in both an ataxia telangiectasia mutated (ATM)-dependent and an ATM-independent manner. Mol Cell Biol. 2002;22:6521–6532. doi: 10.1128/MCB.22.18.6521-6532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- Hirose Y, Berger MS, Pieper RO. Abrogation of the Chk1-mediated G(2) checkpoint pathway potentiates temozolomide-induced toxicity in a p53-independent manner in human glioblastoma cells. Cancer Res. 2001;61:5843–5849. [PubMed] [Google Scholar]
- Hirose Y, Katayama M, Mirzoeva OK, Berger MS, Pieper RO. Akt activation suppresses Chk2-mediated, methylating agent-induced G2 arrest and protects from temozolomide-induced mitotic catastrophe and cellular senescence. Cancer Res. 2005;65:4861–4869. doi: 10.1158/0008-5472.CAN-04-2633. [DOI] [PubMed] [Google Scholar]
- Holland EC, Hively WP, DePinho RA, Varmus HE. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 1998;12:3675–3685. doi: 10.1101/gad.12.23.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Varmus HE. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc Natl Acad Sci U S A. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse JT, Holland EC. Genetically engineered mouse models of brain cancer and the promise of preclinical testing. Brain Pathol. 2009;19:132–143. doi: 10.1111/j.1750-3639.2008.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino Y, Wahrer DC, Bell DW, Haber DA, Louis DN. Mutation analysis of the hCHK2 gene in primary human malignant gliomas. Neurogenetics. 2000;3:45–46. doi: 10.1007/pl00022979. [DOI] [PubMed] [Google Scholar]
- Jack MT, Woo RA, Hirao A, Cheung A, Mak TW, Lee PW. Chk2 is dispensable for p53-mediated G1 arrest but is required for a latent p53-mediated apoptotic response. Proc Natl Acad Sci U S A. 2002;99:9825–9829. doi: 10.1073/pnas.152053599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992;89:7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin MF. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat Rev Mol Cell Biol. 2008;9:759–769. doi: 10.1038/nrm2514. [DOI] [PubMed] [Google Scholar]
- Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Liu G, Parant JM, Lang G, Chau P, Chavez-Reyes A, El-Naggar AK, Multani A, Chang S, Lozano G. Chromosome stability, in the absence of apoptosis, is critical for suppression of tumorigenesis in Trp53 mutant mice. Nat Genet. 2004;36:63–68. doi: 10.1038/ng1282. [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- Miyagi K, Mukawa J, Kinjo N, Horikawa K, Mekaru S, Nakasone S, Koga H, Higa Y, Naito M. Astrocytoma linked to familial ataxia-telangiectasia. Acta Neurochir (Wien) 1995;135:87–92. doi: 10.1007/BF02307420. [DOI] [PubMed] [Google Scholar]
- Nuciforo PG, Luise C, Capra M, Pelosi G, d'Adda di Fagagna F. Complex engagement of DNA damage response pathways in human cancer and in lung tumor progression. Carcinogenesis. 2007;28:2082–2088. doi: 10.1093/carcin/bgm108. [DOI] [PubMed] [Google Scholar]
- Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallinen SL, Ikonen T, Haapasalo H, Schleutker J. CHEK2 mutations in primary glioblastomas. J Neurooncol. 2005;74:93–95. doi: 10.1007/s11060-005-5953-7. [DOI] [PubMed] [Google Scholar]
- Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64:4783–4789. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- Shiloh Y. ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- Stracker TH, Couto SS, Cordon-Cardo C, Matos T, Petrini JH. Chk2 suppresses the oncogenic potential of DNA replication-associated DNA damage. Mol Cell. 2008;31:21–32. doi: 10.1016/j.molcel.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracker TH, Morales M, Couto SS, Hussein H, Petrini JH. The carboxy terminus of NBS1 is required for induction of apoptosis by the MRE11 complex. Nature. 2007;447:218–221. doi: 10.1038/nature05740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracker TH, Usui T, Petrini JH. Taking the time to make important decisions: The checkpoint effector kinases Chk1 and Chk2 and the DNA damage response. DNA Repair (Amst) 2009 doi: 10.1016/j.dnarep.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai H, Naka K, Okada Y, Watanabe M, Harada N, Saito S, Anderson CW, Appella E, Nakanishi M, Suzuki H, et al. Chk2-deficient mice exhibit radioresistance and defective p53-mediated transcription. EMBO J. 2002;21:5195–5205. doi: 10.1093/emboj/cdf506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen JW, Petrini JH. Methods for studying the cellular response to DNA damage: influence of the Mre11 complex on chromosome metabolism. Methods Enzymol. 2006;409:251–284. doi: 10.1016/S0076-6879(05)09015-4. [DOI] [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang S, Shen L, Chen Y, Zhang X, Zhou J, Wang Z, Hu C, Yue W. Chk2 down-regulation by promoter hypermethylation in human bulk gliomas. Life Sci. 2009 doi: 10.1016/j.lfs.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Ward IM, Difilippantonio S, Minn K, Mueller MD, Molina JR, Yu X, Frisk CS, Ried T, Nussenzweig A, Chen J. 53BP1 cooperates with p53 and functions as a haploinsufficient tumor suppressor in mice. Mol Cell Biol. 2005;25:10079–10086. doi: 10.1128/MCB.25.22.10079-10086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Baltimore D. Dual roles of ATM in the cellular response to radiation and in cell growth control. Genes Dev. 1996;10:2401–2410. doi: 10.1101/gad.10.19.2401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.