Abstract
Mothers who breastfeed typically exhibit lower levels of depressive symptomatology than mothers who do not. However, very few studies have investigated the directionality of this relationship. Of the prospective studies published, all but one focus exclusively on whether maternal depression reduces rates of subsequent breastfeeding. This study again examines this relationship, but also the reverse—that breastfeeding might predict lower levels of later depression. Using multilevel modeling, we investigated the relationship between breastfeeding and self-reported depressive symptomatology in 205 women followed prenatally and at 3, 6, 12, and 24 months after birth. Consistent with previous research, women with prenatal depressive symptomatology weaned their infants 2.3 months earlier, on average, than women without such symptomatology. We also found, however, that women who breastfed more frequently at 3 months postpartum showed greater subsequent declines in depressive symptomatology over time compared to women who breastfed less frequently, resulting in lower absolute levels of depressive symptoms by 24 months postpartum, controlling for important confounds. In sum, these findings are consistent with a bidirectional association between breastfeeding and depression, with prenatal depression predicting less breastfeeding soon after birth and breastfeeding predicting declines in maternal depression up to 2 years after birth. We discuss mechanisms that could potentially explain these associations and avenues for future research.
Keywords: Depression, Affective disorders, Breastfeeding, Pregnancy, Motherhood
Depression is the leading cause of disability in women (Nobel 2005) and is the most prevalent of all childbearing-related illnesses, affecting approximately 13 % of women worldwide within the first 12 weeks after giving birth (O'Hara and Swain 1996) and roughly one in five women within the first postpartum year (Gaynes et al. 2005). Depression has a number of negative consequences for mothers and infants. For example, depression in mothers can disrupt parenting behaviors (Field 2010; Paulson et al. 2006), leading to long-term negative consequences for cognitive, emotional, and behavioral development of children (see Grace et al. 2003, for a review). Further, maternal distress resulting from depression adversely impacts broader family relationships and can lead to marital discord (Zelkowitz and Milet 1996). Therefore, identifying factors that are protective against maternal depression, especially if they are modifiable, is a high research priority.
One underexplored factor related to maternal depression is breastfeeding. Although many cross-sectional studies have found lower rates of depressive symptomatology among women who breastfeed (see Dennis and McQueen 2009, for a review), very little longitudinal research exists that can begin to address the directionality of this association. Are women with depression less likely to breastfeed, are women who breastfeed less likely to become depressed, or both? Yet another possibility is that a third variable accounts for the association between depression and postpartum depression. These four logical possibilities have not been systematically tested by most research to date. With the exception of one study described below, studies have focused on testing whether maternal depression predicts reduced breastfeeding, overlooking the possibility that breastfeeding might play a role in prevention of maternal depressive symptomatology.
Previous studies have found that women with prenatal depressive symptomatology are less likely to initiate breastfeeding and tend to wean their infants sooner than women without prenatal depressive symptomatology (Green and Murray 1994; Seimyr et al. 2004). In addition, women with depressive symptomatology in the early postpartum go on to wean their infants several months sooner than women without depressive symptomatology postpartum (Dennis and McQueen 2007; Galler et al. 1999; Taveras et al. 2003). These studies report a clear directional sequence whereby depressive symptomatology precedes and predicts subsequent infant feeding outcomes. Critically, however, the observation that depressive symptomatology can precede and predict later breastfeeding does not preclude the possibility of an additional reverse causal pathway, specifically that early breastfeeding could help protect some women against depressive symptomatology. Several lines of evidence have recently emerged that add plausibility to the notion that breastfeeding could offer such protective benefits.
Possible benefits of breastfeeding for mothers
The benefits of breastfeeding for the infant are widely recognized (Ip et al. 2007, for a review), however, scientists and health professionals are just beginning to appreciate the long-lasting and multiple effects that breastfeeding can have on mothers (see Hahn-Holbrook et al. 2013; Rea 2004, for reviews). Breastfeeding is associated with reduced maternal risk for developing type 2 diabetes (Stuebe et al. 2005), cardiovascular disease (Schwarz et al. 2009), and breast cancer (Bernier et al. 2000). Breastfeeding triggers the release of the neuropeptide hormone oxytocin (Uvnäs-Moberg and Eriksson 1996), which has been linked to reductions in stress (Light et al. 2000; Nissen et al. 1998) and depressive symptomatology in postpartum mothers (Skrundz et al. 2011). In line with these findings, women currently using both breast and formula infant feeding methods report less negative mood if they are randomly assigned to breastfeed their infant in the laboratory than if they are randomly assigned to formula feed their infant (Mezzacappa and Katlin 2002). Further, breastfeeding is associated with infants with easier temperaments (Jones et al. 2004; Merjonen et al. 2011), and fewer health problems in infants and mothers over the long-term (Ip et al. 2007) both of which could have positive downstream consequences for maternal mental health (Beck 1996a). Taken together, these findings suggest that breastfeeding could be protective against maternal depression.
A search of the existing literature produced only one study that has tested whether breastfeeding is predictive of subsequent depressive symptomatology. In a sample of 594 new mothers, women who were exclusively breastfeeding at 1 week postpartum were not any more or less likely to score above the depressive symptomatology cutoff on the Edinburgh Postnatal Depression Scale (EPDS) scale at 4 or 8 weeks than women who were exclusively formula feeding at 1 week (Dennis and McQueen 2007). A limitation of this study is that mothers in the sample had only been breastfeeding for 1 week, which is before full lactation is typically established, making it difficult to fully test whether breastfeeding predicts later depressive symptomatology. Furthermore, some physical health benefits of breastfeeding do not emerge until months or even years after birth. Therefore, the time frame used in this study may have been too short to detect any long-term benefits of early lactation on maternal mental health.
Several other studies have reported results consistent with the hypothesis that breastfeeding is protective against subsequent depressive symptomatology, although they were not explicitly designed to address the question of whether breastfeeding predicts subsequent depressive symptomatology. For example, studies have found that having breastfed (vs. never having breastfed) is associated with fewer cases of subsequent depressive symptomatology (Hannah et al. 1992, but see Chaudron et al. 2001 for a null result), as is continuing to breastfeed between 1 and 5 months (vs. weaning between 1 and 5 months) (Nishioka et al. 2011). Critically, however, these studies did not control for levels of depressive symptomatology at baseline or during pregnancy, leaving open the possibility that women who breastfed were simply less likely to be depressed from the outset. Further, past studies have not taken into account potential confounds such as age, income, education, preterm birth, or social support. These factors are relevant because they could potentially produce specious associations between breastfeeding and depressive symptomatology, given that higher rates of depression and lower rates of breastfeeding have been reported in women who are young, economically disadvantaged, less educated, deliver preterm, or report poor social support (Baranowski, et al. 1983; Beck 1996b; Beck 2001; Cooper et al. 1993; Merewood, et al. 2006; Scott et al. 2006).
Study goals
There were three primary goals of this study. The first was to test whether early breastfeeding behaviors predicted reduced incidence of later depressive symptomatology in mothers. The second was to test whether depressive symptoms in pregnancy or postpartum predicted later breastfeeding behaviors. The third was to attempt to rule out third variables that could account for observed associations between breastfeeding and depression. To achieve these objectives, we utilized a dataset from a longitudinal study that included assessments of women's depressive symptomatology during pregnancy and women's breastfeeding behavior and depressive symptomatology at 3, 6, 12, and 24 months after birth.
Methods
Participants
Two-hundred fifty-four women who had been enrolled in a larger longitudinal study of pregnancy in southern California participated. To be eligible to participate, women had to be over the age of 18, English-speaking, nonsmoking, have a singleton pregnancy, and an absence of any medical condition that could dysregulate neuroendocrine function. The final sample included in this study was comprised of the 205 women who had data on depressive symptomatology and infant feeding practices for at least two time points after birth and depressive symptomatology data for at least one time point during pregnancy. Women included in the final sample tended to be slightly older, more likely to be married, and had higher incomes than women who were not included. Means and standard deviations of demographic variables for the final sample are presented in Table 1. Forty-eight percent of the sample self-identified as White, 27 % as Latina or Hispanic, 11 % as Asian, 11 % as multiethnic, and 2 % as Black or African American. The mean age of participants was 29 years and the average household income was $62,000/year. Over half of the sample had an associate degree or higher and a large majority was married (94 %).
Table 1.
Characteristics of the Sample as a Whole and as a Function of Breastfeeding Behavior at 3 Months Postpartum
| Breastfeeding behavior at 3 months | Breastfeeding frequency at 3 months | |||||
|---|---|---|---|---|---|---|
| Total Sample | Any Breastfeeding | Exclusive Breastfeeding | No Breastfeeding | High | Low | |
| N=137 | N=83 | N=63 | N=100 | N=100 | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 29.1 (5.0) | 30 (5) a | 30.1a (4.2) | 28 (6) b | 31 (4) | 29 (5) |
| Income (in dollars) | 62 K (33 K) | 68 K (33 K)a | 71Ka (30 K) | 48 K (30 K)b | 71 K (34 K) | 66 K (33 K) |
| Education | 2.5 (0.9) | 2.7 (0.9) a | 3.0a (.08) | 1.9 (0.6) b | 2.9 (0.9)a | 2.7 (1.0)b |
| # of children | 1.8 (0.9) | 1.7 (0.8) | 1.2 (0.4) | 1.8 (0.9) | 1.8 (0.8) | 1.9 (1) |
| Social Support | 4.2 (0.8) | 4.3 (0.6) | 4.4 (0.6) | 4.2 (0.8) | 4.26 (0.5) | 4.26 (0.7) |
| % Married | 94 % | 97 % a | 98%a | 88 % b | 98 % | 96 % |
| % Working | 37 % | 34 % | 24 % a | 44%b | 35 % | 35 % |
| % Preterm | 6 % | 3 % a | 0%a | 14 % b | 2 % | 3 % |
| % Prenatal Depression | 25 % | 22 % a | 17%a | 33 % b | 22 % | 23 % |
| % Asian | 11 % | 84 % | 81 % | 16 % | 43 % | 57 % |
| % Latina | 27 % | 50 % | 24 % | 50 % | 38 % | 62 % |
| % White | 48 % | 73 % | 64 % | 27 % | 40 % | 60 % |
Variables within columns that differ in superscripts a and b represent significant differences between groups. Breastfeeding frequency was treated as a continuous variable in the statistical analyses; however, a median split was preformed for the purposes of this table. Education values represent an ordinal level variable coded as 1 = high school or less, 2 = associates degree, 3 = college degree, 4 = graduate degree
Procedures
Pregnant participants were recruited by research nurses during the first trimester of pregnancy and were then followed over the first 2 years after birth. During pregnancy, demographic information and measures of depressive symptomatology were collected by trained interviewers. After the birth of the child, mothers came into the laboratory at 3 months (M=13.2 weeks, range 11–18 weeks), 6 months (M= 26.5 weeks, 20–37 weeks), 12 months (M=52.9 weeks, range 49–65 weeks), and 24 months (M=108.6 weeks, range 100–117 weeks) and reported on their depressive symptomatology and breastfeeding behaviors.
Measures
Depressive symptomatology
Prenatal depressive symptomatology was assessed via the brief version of the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff 1977). Validation reports show higher associations with the Structured Interview of the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised (DSM-III-R) when items are rescored into bivariate form (Santer and Coyne 1997). Therefore, responses of 0 or 1 were recoded as 0 and responses of 2 or 3 were recoded as 1. These bivariate scores were then added together to create a score that ranged from 0 and 9, with a suggested cutoff score of 4 or more indicating the presence of depressive symptomatology. This measure has good internal consistency (Kuder–Richardson formula 20, 0.87) and the bivariate scores correlate highly with the original scale scores (r=0.97). The CES-D was administered at five time points during pregnancy (at 15, 20, 25, 31, and 37 weeks gestation). Participants were considered to have reported prenatal depressive symptomatology if they scored above the CES-D cutoff at any one of the assessments during pregnancy. Twenty-six percent of women (54/205) scored above the CES-D cutoff at least once during pregnancy. Although this rate of depressive symptoms is high, a meta-analysis suggests that prevalence rates of depression during pregnancy are generally high: 7.4 % during the first trimester, 12.8 % during the second trimester, and 12.0 % during the third trimester (Bennett et al. 2004). Rates of prenatal depression symptomatology in this study were also probably higher than previous estimates because there were multiple assessments of depressive symptomatology (Gaynes et al. 2005). Therefore, there were multiple opportunities for women to be categorized as having prenatal depressive symptomatology.
Depressive symptomatology after birth was measured at 3, 6, 12, and 24 months using the 10-item EPDS (Cox et al. 1987). Participants indicated how often they experienced a symptom of depression in the past week on a four-point scale. A cutoff score of 10 or more is suggested by the creators of the EPDS for cases of minor depression (hereafter referred to as depressive symptomatology) and has been validated by other studies (see Matthey et al. 2006, for a review). The continuous EPDS scores (values ranged from 0 to 30) (hereafter referred to as a continuous EPDS score) were also used if cutoff scores yielded null results. This approach of using both cutoff and continuous measures of depressive symptomatology was taken because although continuous scores are generally preferable in statistical modeling because they provide more variability, cutoff scores have been validated in identifying women with depression (Matthey et al. 2006).
Breastfeeding
At 3, 6, 12, and 24months postpartum, mothers were asked the following questions about their current daily infant feeding practices: (1) whether they engaged in any breastfeeding or were not breastfeeding at all (hereafter termed any breastfeeding) and if they were currently breastfeeding, (2) the frequency of infant feedings per day by breast milk (hereafter termed breastfeeding frequency, (3) the total percentage of the child's diet that was made up by breast milk, and (4) the percentage of the child's daily breast milk intake that was expressed by breast pump and then given by bottle vs. the percentage of breast milk given directly via infant suckling. This last variable was used to create a ratio score of breastfeeding via breast pumping vs. infant suckling (hereafter termed ratio of breast pumping vs. suckling) to determine whether the mode of breast milk expression was predictive of later depressive symptomatology. We defined exclusive breastfeeding as a woman's report that 100 % of her child's diet was comprised of breast milk and defined exclusive formula feeding as a woman's report that 0 % of her child's diet was comprised of breast milk, as per the standard definitions recommended by Labbok and Krasovec (1990).
If women weaned their child between assessments, they were asked retrospectively about when in the intervening period they had ceased breastfeeding. A total breastfeeding duration score was then calculated based on how many months mothers had been engaged in any breastfeeding (based on both perspective and retrospective assessments) in the first 2 years after birth (scores ranged from 0 to 24 months). In cases in which prospective and retrospective reports of breastfeeding duration conflicted, prospective reports were privileged and cessation of breastfeeding was placed just after the last prospective report of breastfeeding.
Women were also asked retrospectively at 3 months post-partum if they had initiated breastfeeding. However, only four women in our sample reported that they had never initiated any breastfeeding; therefore, we were not able to include breastfeeding initiation as a variable in the analyses.
Covariates
The following potential confounds were assessed: maternal age, income, education, marital status, parity, preterm birth, maternal employment, ethnicity, and social support. They were chosen because, in most cases, they had been identified in previous research as predictors of postpartum depression (i.e., prenatal depression, income, education, marital status, and social support; Beck 1996b; Beck 2001; Cooper et al. 1993) or of breastfeeding (i.e.,maternal age, education, pretermbirth, maternal employment, social support, and ethnicity; Baranowski, et al. 1983; Merewood, et al. 2006; Scott et al. 2006). Preterm birth, defined medically as birth at less than 37 weeks' gestation, was assessed from birth records and was coded as a dichotomous variable (0 = term, 1 = preterm). Self-reported demographic covariates included maternal age, household income (in dollars earned annually), employment status (0 = not engaged in paid employment,1 = engaged in paid employment), education (ordinal variable, coded as 1 = high school or less, 2 = associates degree, 3 = college degree, 4 = graduate degree), marital status (0 = not married, 1 = married), parity (0 = primiparous, 1 = multiparous), and ethnicity, which was coded into three dummy coding schemes (Caucasian vs. non-Caucasian, Latina vs. non-Latina, and Asian vs. non-Asian), each of which was entered into models separately so contrasts could be made between the ethnic group in question compared with all other groups. Not enough of the sample self-identified as Black or African American (2 %) to allow for analysis. At each visit after birth, mothers were asked whether they were currently engaging in paid employment (no = 0, yes = 1).
Social support was measured with the 19-item Medical Outcomes Study (MOS) Social Support Survey (Sherbourne and Stewart 1991). This instrument measures participant's perceptions of being socially supported and includes subscales for affectionate support, emotional/informational support, positive social interaction support, and tangible support. All items are averaged to create an overall measure of perceived support. In the present study, the Cronbach's alpha coefficient for the overall measure was high (0.96).
Some women in our sample (N=16) did admit to smoking at T1 during pregnancy, despite the fact that smokers were not eligible for the study. However, smoking in pregnancy was unrelated to any of our key breastfeeding or depression variables and, therefore, was not included in the analyses. We did not have information on whether women smoked postpartum.
Data analytic strategy
Multilevel modeling was used to test the potentially bidirectional relationship between breastfeeding and postpartum depression. If breastfeeding is protective against subsequent depressive symptomatology, it is expected that women who breastfeed at 3 months postpartum will show less depressive symptomatology over time than women who do not breastfeed at 3 months. Furthermore, if there is a dose–response relationship between breastfeeding and later depressive symptomatology, then relatively frequent breastfeeding at 3 months will predict less depressive symptomatology over time compared with infrequent breastfeeding at 3 months. Conversely, if depressive symptomatology leads to less breastfeeding, it was expected that women who are depressed during pregnancy or postpartum will be less likely to be breastfeeding at 3 months and may wean more quickly between 3 and 24 months. Finally, if the relationships between postpartum depression and breastfeeding are not accounted for by a third variable, then the effects should remain significant when covariates are included in the models.
Multilevel modeling was used for these analyses because it allows for the determination of between-person differences among within-person trajectories and offers advantages over other statistical tools for the evaluation of longitudinal data as it accounts for shared variance on within-individual measurements and can accommodate missing values (Raudenbush and Bryk 2002). Time postpartum was entered as a continuous variable and linear interaction terms were used to test for changes as a function of predictors over time. Linear, quadratic, and unstructured models were tested to model changes in breastfeeding and depression over time; however, linear models were used throughout because these were a good fit for all dependent variables and helped to maintain consistency between results. Unstructured error terms were included in all models to account for the shared variance in the outcome variables over multiple observations. All covariates were entered into multilevel models as time unvarying/fixed predictors, with the exception of working outside the home, which was allowed to vary over time. Models were first run without any covariates to test for direct effects of breastfeeding and depression and then all covariates were added into the models together to test whether any observed association remained significant after controlling for those possible confounds. Multilevel analyses were conducted in STATA version 11 using the xtgee function. All other analyses were conducted in SPSS version 18.
Several tests did not require multilevel modeling. For example, linear regression was used to test whether depressive symptoms predicted total breastfeed duration (total months women engaged in any breastfeeding). A logistic regression model was used to test whether prenatal depression predicted exclusive breastfeeding at 3 months because too few women were exclusively breastfeeding at 6 (5 %), 12 (0 %), and 24 (0 %) months to allow us to assess changes in this variable over time. Further, initial analyses examining whether potential covariates (prenatal depressive symptomatology, age, income, education, marital status, parity, work outside the home, ethnicity, preterm birth, and social support) were associated with breastfeeding and/or depression were conducted using linear regression (in the case of continuous dependent variables), logistic regression (in the case of categorical dependent variables), or Analysis of Variance (ANOVA) (in the case of categorical dependent variables with more than two groups).
See Tables 1 and 2 for associations between potential covariates and breastfeeding and depression, respectively. Four covariates emerged as candidate third variables because they significantly (p<0.05) predicted both reduced breastfeeding and more depressive symptomatology: prenatal depressive symptomatology, marital status, income, and education. These variables were included in the final models. In addition, any covariate that significantly predicted a breastfeeding variable (age, preterm birth, Latina ethnicity, and working outside the home) or depression variable (social support) was also included as a covariate in order to provide a more stringent test of the associations between breastfeeding and depressive symptomatology.
Table 2.
Characteristics of the sample as a function of depressive symptomatology at 3 months postpartum
| Prenatal depressive symptoms | Depressive symptomatology at 3 months | Continuous EPDS scores at 3 months | ||||
|---|---|---|---|---|---|---|
| Yes (N=52) | No (N=153) | Yes (N=40) | No (N=160) | Higher (N=120) | Lower (N=80) | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 29.0 (6.2) | 29.1 (4.9) | 28 (6) | 29(5) | 28.3 (5.8) | 29.6 (4.8) |
| Income (in $) | 50K (35K)a | 66K (32K)b | 48K (33K)a | 66K (33K)b | 52K (34K)a | 69K (31K)b |
| Education | 2.2 (0.9)a | 2.6 (1.0)b | 2.3 (1.0) | 2.8 (1.0) | 2.3 (1.0)a | 2.7 (0.9)b |
| No. of children | 1.8 (0.9) | 1.79 (0.9) | 1.8 (0.9) | 1.9 (0.9) | 1.9 (0.8) | 1.74 (0.8) |
| Social support | 3.9 (0.9)a | 4.3 (0.6)b | 4.0(0.8)a | 4.3(0.6)a | 4.1 (0.7)a | 4.4 (0.6)b |
| % Married | 87 %a | 97 %b | 89 % | 95 % | 91 % | 97 % |
| % Working | 29 % | 40 % | 33 % | 39 % | 34 % | 41 % |
| % Preterm | 6 % | 7 % | 10 % | 5 % | 9 %a | 5 %b |
| % Prenatal depression | – | – | 60 %a | 17 %b | 45 %a | 13 %b |
| % Asian | 21 % | 79 % | 22 % | 78 % | 26 % | 74 % |
| % Latina | 17 % | 83 % | 18 % | 82 % | 34 % | 56 % |
| % White | 26 % | 74 % | 19 % | 81 % | 36 % | 64 % |
Variables within columns that differ in superscripts a and b represent significant differences (p<.05) between groups. Continuous EPDS scores were treated as a continuous variable in the statistical analyses; however, a mean split (<5.2, >5.2) was preformed for the purposes of this table. Education values represent an ordinal-level variable coded as 1 = high school or less, 2 = associates degree, 3 = college degree, and 4 = graduate degree
Results
Modeling change in dependent variables over time
Before examining predictors, models of depression and breastfeeding over time were estimated for descriptive purposes. Forty-four percent of the variability in depressive symptomatology over the 2-year time period was the result of within-person changes, and 56 % was the result of between-person differences. Depressive symptomatology tended to decline slightly, though nonsignificantly, over time (Coeff=−0.14, SE=0.11, t ratio=−1.32, p<0.2). The frequencies of women with depressive symptomatology in our sample was 19.5 % (40 of 200) at 3 months, 15.1 % (31 of 205) at 6 months, 13.7 % (28 of 183) at 12 months, and 15 % at 24 months (24 of 160).
Only 22 % of the variance in any breastfeeding and 24 % of the variance in breastfeeding frequency was accounted for by with-person changes, while over 80 % of the variance was accounted for by between-person differences (mainly due to differences in base rates of breastfeeding at 3 months postpartum). There were significant declines in breastfeeding rates over time, as is expected (any breastfeeding: Coeff=−0.22, SE=0.01, t ratio=−21.71, p< 0.001; breastfeeding frequency: Coeff=−1.57, SE=0.07, t ratio=−23.47, p<0.001). Sixty-nine percent (137/200) of mothers engaged in any breastfeeding at 3 months postpartum; however, this percentage dropped to 53.2 % at 6 months (109/205), 24 % at 12 months (49/200), and 4 % at 24 months (7/191). Exclusive breastfeeding dropped off dramatically from 43 % at 3 months (63/200) to 5 % at 6 months (10/205). No one in our sample was exclusively breastfeeding at 12 or 24 months. The average breastfeeding frequency was 4.75 times per day at 3 months, 3.06 times per day at 6 months, 0.93 times per day at 12 months, and 0.13 times per day at 24 months.
Breastfeeding predicting depressive symptomatology
Any breastfeeding vs. no breastfeeding
Baseline rates of depressive symptomatology at 3 months postpartum did not differ as a function of any breastfeeding at 3 months postpartum (p>0.7); however, the interaction between any breastfeeding at 3 months and time trended in the predicted direction (without covariates: Coeff=−0.04, SE=0.022, t ratio=−1.84, p=0.07; with covariates: Coeff= −0.04, SE=0.021, t ratio=−1.74, p=0.08). Post hoc analyses also suggested a trend wherein absolute levels of depressive symptomatology were lower at 24 months in women who had been breastfeeding at 3 months than women who had not (Coeff=−0.10, SE=0.06, t ratio=−1.82, p=0.07). Absolute levels of depressive symptoms at 6 and 12 months did not differ as a function of early breastfeeding.
More frequent breastfeeding vs. less frequent breastfeeding
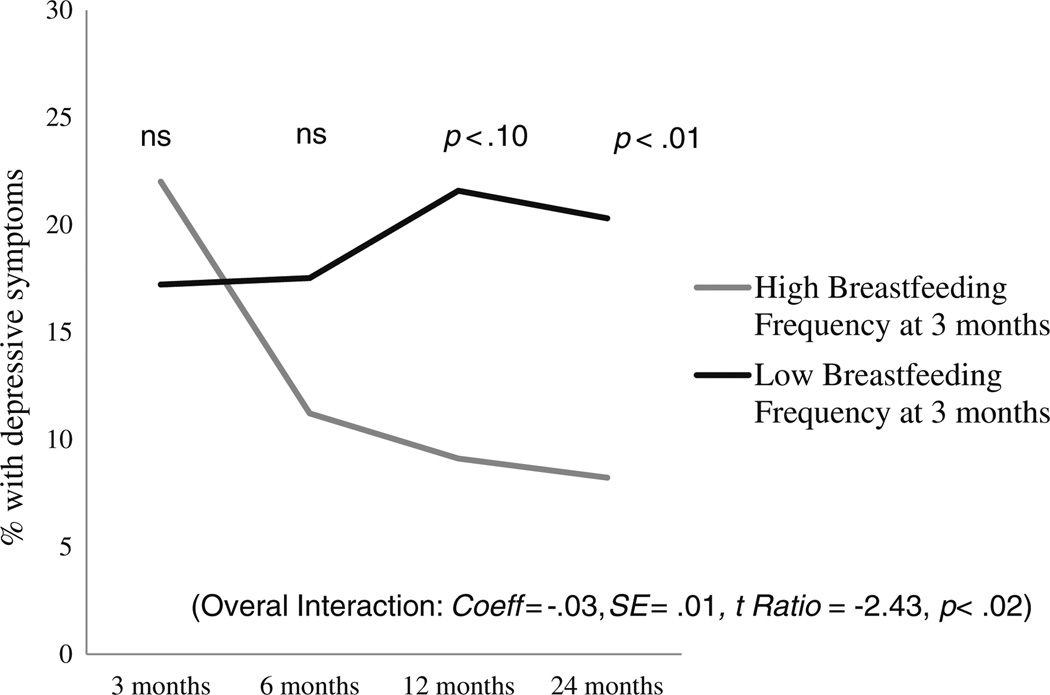
Breastfeeding frequency at 3 months postpartum was unrelated to depressive symptomatology at 3 months (p> 0.5), although there was a significant interaction between breastfeeding frequency at 3 months and change in depressive symptomatology over the 2-year period, both before (Coeff=−0.03, SE=0.01, t ratio=−2.40, p<0.02) and after covariates were entered into the model (Coeff=−0.03, SE= 0.01, t ratio=2.43, p<0.02) (Fig. 1). Women who breastfed more frequently at 3 months postpartum showed greater declines in subsequent depressive symptomatology than women who breastfeed less frequently at 3 months. This decline resulted in lower absolute levels of depressive symptoms by 24 months postpartum (Coeff=−0.06, SE=0.03, t ratio=−2.20, p<0.05), although this effect was dampened when covariates were entered into the model (Coeff=−0.04, SE=0.03, t ratio=−1.45, p=0.14). Absolute levels of depression did not differ at 6 or 12 months as a function of breastfeeding frequency at 3 months.
Fig. 1.
Effect of breastfeeding frequency at 3 months on future depressive symptomatology. This figure shows the percentages of women who had an EPDS score of 10 or above at 3, 6, 12, and 24 months postpartum as a function of high vs. low frequency of breastfeeding at 3 months postpartum. High and low breastfeeding frequencies were graphed at levels one standard deviation above (nine times a day) and below (four times a day) the mean, respectively. Women who were breastfeeding at a high frequency at 3 months postpartum had greater declines in rates of depressive symptoms than women breastfeeding at a low frequency at 3 months (Coeff=−0.03, SE=0.01, t ratio=−2.43, p<0.02). Note: although a linear model was used to test this interaction, an unrestricted model was plotted here, which gives a more accurate reflection of the actual rates of depressive symptoms in the data
This effect did not appear to be driven solely by differences between breastfeeding vs. nonbreastfeeding women. Namely, when women who were not engaged in any breastfeeding at 3 months were removed from the analyses (N=63, leaving 137 women remaining), breastfeeding frequency at 3 months still predicted declines in depressive symptoms over time (without covariates: Coeff=−0.05, SE =0.02, t ratio=−2.61, p<0.01; with covariates: Coeff= −0.05, SE=0.02, t ratio=−2.64, p<0.01) and lower absolute levels of depressive symptoms by 24 months (without covariates: Coeff=−0.11, SE=0.05, t ratio=−2.36, p<0.05; with covariates: Coeff=−0.09, SE=0.05, t ratio=−2.02, p< 0.05). Again, no differences in absolute levels were observed at 6 or 12 months as a function of breastfeeding frequency at 3 months.
Breast pumping vs. infant suckling
The effect of breastfeeding frequency on depressive symptomatology did not differ as a function of mode of breast milk expression. More frequent bouts of lactation, independent of the ratio of breast pumping vs. suckling (p>0.1), predicted declines in depressive symptoms over the 2-year period (without covariates: Coeff=−0.04, SE=0.013, t ratio=−3.00, p<0.01; with covariates: Coeff=−0.04, SE= 0.013, t ratio=−3.06, p<0.01) and lower absolute levels of depressive symptoms by 24 months (without covariates: Coeff=−0.08, SE=0.04, t ratio=−2.26, p<0.025; with covariates: Coeff=−0.07, SE=0.03, t ratio=−2.06, p<0.05).
Exclusive breastfeeding vs. exclusive formula feeding
Exclusive breastfeeding (vs. exclusive formula feeding) at 3 months did not predict depressive symptomatology at 3 months (p>0.8) or changes in depressive symptomatology over time (p<0.2), and covariates had no effect on the pattern of these results. However, it should be noted that there was a nonsignificant trend for the interaction between percentage of child's diet made up by breast milk and time: women who were feeding their infant a diet comprised of a relatively higher percentage of breast milk at 3 months tended to show greater declines in depressive symptoms over time (without covariates: Coeff=−0.02, SE=0.01, t ratio=−1.90, p=0.06; with covariates: Coeff=−0.02, SE=0.01, t ratio=−1.89, p=0.06). No differences in absolute levels emerged.
Depressive symptomatology predicting breastfeeding
Prenatal depressive symptomatology
Consistent with prior research, women who reported depressive symptomatology prenatally had shorter durations of breastfeeding (β=−0.18, p=0.02) than women who did not report any depressive symptomatology prenatally, an effect that remained significant with covariates included in the model (β=−0.16, p=0.03). When quantified, prenatally depressed moms weaned an estimated 2.3 months (SE= 1.04 months) sooner than women who did not report depressive symptomatology prenatally. Prenatal depression also predicted lower rates of exclusive breastfeeding at 3 months postpartum (B=−0.83, SE=0.40, p=0.038), an effect that remained significant when covariates were entered into the model (B=−1.18, SE=0.53, p<0.028).
To further explore this relationship, multilevel modeling was used to test if prenatal depressive symptomatology predicted changes in any breastfeeding or breastfeeding frequency between 3 and 24 months. Women with prenatal depressive symptomatology (vs. without) were less likely to be breastfeeding (vs. not breastfeeding) at 3 months postpartum (Coeff=−0.17, SE=0.06, t ratio=−2.78, p<0.01), but prenatal depression did not predict breastfeeding cessation between 3 and 24 months (Coeff=0.03, p=0.11). Likewise, prenatal depressive symptomatology predicted fewer breastfeeding bouts per day at 3 months postpartum (Coeff= −0.89, SE=0.41, t ratio=−2.19, p<0.05) but no interaction over time (p>0.25). When covariates were included into the models, prenatal depressive symptomatology remained a significant predictor of any breastfeeding at 3 months (intercept: Coefficient=−0.14, SE=0.06, t ratio=−2.39, p< 0.01), although the relationship with breastfeeding frequency at 3 months was reduced to a trend (intercept: Coefficient =−0.74, SE=0.40, t ratio=−1.88, p=0.06). Using average CES-D score in pregnancy, as opposed to cutoff scores, to predict breastfeeding behavior produced a similar pattern of results.
Postpartum depressive symptomatology
Depressive symptomatology at 3 months postpartum was not related to any of our breastfeeding outcome variables (intercepts all p>0.20; interaction terms all p>0.30). Quadratic and unrestricted models were also run and yielded null results, as did using continuous EPDS scores as the dependent measure. Exclusive breastfeeding at 3 months was also unrelated to depressive symptoms at 3 months. The statistical significance of these results did not change by adding covariates to the models.
Discussion
The results of this study are consistent with a bidirectional relationship between breastfeeding and depression, with prenatal depressive symptomatology predicting less breastfeeding postpartum and early breastfeeding predicting less depressive symptomatology later in the postpartum. Women who experienced depressive symptomatology prenatally weaned their infants an average of 2.3 months sooner than those who did not report prenatal depressive symptomatology, an effect that remained after controlling for potential confounds. The relationship between prenatal depressive symptomatology and later breastfeeding seemed to be driven by weaning taking place between 0 and 3 months postpartum; that is, prenatal depressive symptomatology predicted fewer cases of breastfeeding and less frequent breastfeeding at 3 months but did not predict breastfeeding cessation or steeper declines in breastfeeding frequency between 3 and 24 months.
Breastfeeding at 3 months postpartum also predicted lower risk of subsequent depressive symptomatology. There was a trend for women who engaged in any amount of breastfeeding at 3 months to have greater declines in subsequent depressive symptomatology. A more dramatic effect was observed when a dose–response model was tested of breastfeeding frequency on subsequent depression risk. Namely, more frequent breastfeeding at 3 months postpartum was associated with greater declines in subsequent rates of depressive symptomatology as compared with less frequent breastfeeding at 3 months. Further, women who breastfed more frequently at 3 months postpartum had lower absolute levels of depressive symptomatology by 24 months postpartum than women who breastfed less frequently at 3 months. Exclusive breastfeeding, however, did not predict less depressive symptomatology, although using a percentage of child's diet made up by breast milk as a continuous variable revealed a trend in the predicted direction (p=0.06). None of the aforementioned associations between breastfeeding and depression could be accounted for by candidate confounds or third variables (alternative explanations) including age, education, income, Latina ethnicity, work outside the home, marital status, preterm birth, social support, or, in the case of breastfeeding, predicting later depression, prenatal depressive symptomatology.
Depressive symptomatology predicting less breastfeeding
The finding that prenatal depressive symptomatology predicts earlier weaning and less frequent breastfeeding at 3 months is consistent with a few previous studies (Green and Murray 1994; Seimyr et al. 2004). Unlike previous research, however, postpartum depression did not predict later breastfeeding behavior in this study. One factor that may account for this null result is the fact that the first assessment of postpartum depressive symptomatology in this study was taken at 3 months. All of the previous longitudinal studies that have found significant negative associations between postpartum depression and subsequent breastfeeding behavior have measured depression earlier (1 week: Dennis and McQueen 2007; 2 weeks: Taveras et al. 2003; 7 weeks: Galler et al. 1999; 2006; 2 months: Seimyr et al. 2004). It is possible that depressive symptomatology predicts early weaning in the first few months when breastfeeding problems like nipple pain, difficulty latching, and poor milk letdown are more common (Riordan 2005). Once breastfeeding is established by 3 months, depressive symptomatology may become a less potent predictor of subsequent breastfeeding outcomes. This interpretation is also consistent with the findings in this study that prenatal depressive symptomatology predicted less breastfeeding at 3 months but did not predict changes in breastfeeding between 3 and 24 months.
Although no studies of which we are aware have explicitly tested for mediators underlying the association between depression and later breastfeeding outcomes, researchers have found that women with depressive symptomatology report breastfeeding problems (Edhborg et al. 2005; Tamminen 1988), more failed breastfeeding attempts (Fergerson et al. 2002), and less breastfeeding self-efficacy (Dai and Dennis 2003). Given this, it seems plausible that symptoms common to depression-like negative mood, poor self-esteem, and anxiety could lead depressed women to perceive common breastfeeding hurdles such as pain, difficultly latching, or worries that the infant is not getting enough milk, as less surmountable or more serious than their nondepressed peers (see Dennis and McQueen 2007, for a discussion). Likewise, anxiety can interfere with milk supply and the milk letdown reflex (Dewey 2001), which could lead depressed mothers to have more breastfeeding problems (Stuebe et al. 2012). In addition, depressed mothers tend to be less sensitive to infant cues (Murray et al. 1996), which may lead to problems in infant latching and the establishment of breastfeeding routines. Finally, many antidepressant medications are not recommended for breastfeeding mothers, leading some depressed women to opt to formula feed and not to disrupt medical treatment. These potential mediators of the association between depression and later breastfeeding outcomes should be explicitly tested in future research.
Breastfeeding predicting reduced risk of depressive symptomatology
This study reports the first evidence that early breastfeeding might provide some protection against later depressive symptomatology for mothers. These results should be interpreted with caution until replicated and additional confounds are ruled out. However, if robust, the link between breastfeeding and reduced risk for depressive symptomatology could have clinical implications. For example, interventions targeted at increasing breastfeeding rates, and specifically, breastfeeding frequency, might decrease mothers' risk for subsequent depression years after birth.
The only other published study that has addressed the question of whether early breastfeeding is predictive of later depression reported a null result (Dennis and McQueen 2007); however, it is worth noting that the results of the current study do not actually contradict but rather they are, in fact, consistent with those of this earlier study. That study found that women who were breastfeeding exclusively at 1 week postpartum were not less likely to report depressive symptomatology at 1 month or 2 months postpartum compared to women who were not breastfeeding at all at 1 week. This finding is consistent with the results of the current study indicating that breastfeeding was not associated with depressive symptomatology in the early postpartum, but instead emerge slowly over time, resulting in lower rates of depressive symptoms only at 2 years postpartum. Further, it was breastfeeding frequency in this study, not exclusive breastfeeding, which predicted declines in depressive symptoms over 2 years postpartum. It is possible that whereas exclusive breastfeeding vs. nonexclusive breastfeeding is a key distinction to make when assessing the impact of breastfeeding on infant health, breastfeeding exclusivity may be a less important distinction when assessing the impact of breastfeeding on maternal health. Studies suggest that the spacing and frequency of breastfeeding bouts, more so than exclusivity, is the key mediator of the hormonal changes associated with lactation for mothers (Labbok 2001). There was a large amount of variability in the frequency of breastfeeding bouts of women who were exclusively breastfeeding at 3 months in our sample (range five to 17 breastfeeding bouts a day). Therefore, future investigations on the impact of lactation on mothers might include measures of breastfeeding frequency, duration, spacing, and time since last breastfeeding bout, in addition to reports of breastfeeding exclusivity.
Because this is the first study to report this striking association, we hope it will inspire replication attempts and investigations of possible mediating mechanisms. To aid future research endeavors, we briefly discuss three classes of mediators (direct pathways, infant-mediated pathways, and third variables) that could potentially underlie a time-lagged relationship between breastfeeding and reduced risk of depressive symptomatology.
Direct pathways
Hormonal changes triggered by breastfeeding initiate a cascade of biological changes in the maternal brain and body (see Uvnäs-Moberg and Eriksson 1996) that could act as direct pathways through which breastfeeding influences maternal depression. For example, the act of breastfeeding releases the hormone oxytocin (Uvnäs-Moberg and Eriksson 1996), which has been found in lower levels in women with depressive symptomatology postpartum than women without such symptoms (Skrundz et al. 2011). A logical problem for this hypothesis, however, is the fact that the breastfeeding women in our sample did not have lower rates of depressive symptomatology than nonbreastfeeding women at 3 months postpartum, the time at which differences between the two groups of women in oxytocin and other hormones (e.g., prolactin, estrogen, and progesterone) would be largest (Riordan 2005; Uvnäs-Moberg and Eriksson 1996). One possibility that reconciles this problem is that there could be additive effects of hormone exposures associated with breastfeeding that take longer than 3 months to accrue to sufficient levels to impact depression.
A related process has been proposed to explain the observed long-term physical health benefits of breastfeeding for mothers. A hypothesis, dubbed the “reset hypothesis”, proposes that breastfeeding causes myriad metabolic changes that help “reset” the body after pregnancy and have long-term impacts on women's health (Stuebe and Rich- Edwards 2009), reducing the risk for type 2 diabetes (Stuebe and Rich-Edwards 2009) and cardiovascular disease (Schwarz et al. 2009). Given that large meta-analyses have identified depression as a predictor of both type 2 diabetes (Pan et al. 2010) and cardiovascular disease (Rugulies 2002), there is the possibility that a shared biological mechanism, such as reduced chronic inflation (Kendall-Tackett 2007; Dantzer et al. 2008; Groer and Davis 2006; Jaedicke et al. 2009), underlies the association between these physical diseases, breastfeeding, and the reduced risk for depressive symptomatology observed in the current study. The possibility that reduced inflammation or other long-term biological changes link breastfeeding to reduced risk for depression is an area ripe for further investigation.
Another potential mediator linking breastfeeding to reduced depression risk is the role that breastfeeding could play in promoting maternal bonding. The relationship between breastfeeding and maternal bonding is surprisingly understudied (see Jansen et al. 2008, for a review); however, there are theoretical reasons to think that breastfeeding could facilitate maternal bonding. Specifically, breastfeeding triggers the release of the hormone oxytocin, which has been linked to enhanced maternal caregiving in humans (Feldman et al. 2007) and animals (Kendrick 2000). One study found that mothers who had breastfed for at least 1 week displayed fewer negative interactions (as rated by observers) with their babies 1 year after birth, but not 4 months postpartum (Else-Quest et al. 2003), which is similar to the time-lagged effects of breastfeeding on depressive symptomatology observed in the current study. One could hypothesize that mothers who have stronger bonds with their infants might find parenting less stressful or more rewarding than less bonded mothers and, therefore, could be at reduced risk for depressive symptomatology throughout their child's development. The idea that bonding could be the mediator between breastfeeding and depression is a speculation at this point but deserves further empirical attention.
Indirect pathways
Another possibility is that early breastfeeding protects against later maternal depression indirectly though the benefits it confers to the infant. It is widely recognized that breast-fed infants have fewer health problems than formula-fed infants (see Ip et al. 2007, for a review). Given that having a child with health problems is difficult and increases maternal distress (Beck 1996b), breastfeeding might decrease the risk of maternal depression indirectly by leading to healthier infants. Additionally, early breastfeeding has been associated with infants with easier temperaments over the long-term (Field et al. 2002; Merjonen et al. 2011; Worobey 1992), and infants with easier temperaments tend to have mothers who are less likely to get depressed (Beck 1996a). It is worth noting, however, that other studies have also found that infants with easier temperaments are more likely to be breastfed (Vandiver 1997) and that the association between breastfeeding and infant temperament becomes negligible after the first half of the infant's life (Neigel et al. 2008). Nonetheless, these indirect pathways warrant further empirical attention.
Third variable explanations
Another possibility is that an unaccounted for third variable covaries with both breastfeeding frequency and later vulnerability for depressive symptomatology. There are a number of psychological, social, and environmental factors that could influence breastfeeding and depressive symptomatology that were not addressed in this study. Future research might attempt to replicate our results while adjusting for variables such as maternal personality, attachment style, life stress, and access to health care. Nonetheless, the analyses we reported here did account for factors commonly associated with breastfeeding and depression (age, income, education, marital status, work outside the home, preterm birth, ethnicity, social support, and prenatal depressive symptomatology), none of which accounted for the observed relationship between early breastfeeding frequency and later depression. In addition, the finding that early breastfeeding frequency moderates the relationship with latter depressive symptomatology strengthens the possibility that it is a characteristic of breastfeeding, rather than some difference associated with the decision to breastfeed vs. formula feed, which accounts for the fact that early breastfeeding predicted reduced risk for depressive symptomatology in our sample. Whatever the mechanism explaining the link between breastfeeding and later depressive symptomatology, these results point to a novel predictor of future depression symptoms (early breastfeeding frequency) that could be of benefit to clinicians or health professionals who are working toward depression prevention or intervention.
Limitations and strengths
As with previous studies on this topic, causality cannot be inferred from correlational evidence, even when the dataset is longitudinal. It was not possible to randomly assign women to be depressed or to breastfeed for obvious ethical and practical reasons. One promising research design for the future is a randomized controlled trial examining the effects of a breastfeeding promotion program (vs. no program). Such a study could measure the variables noted above and test whether increases in breastfeeding could reduce rates of maternal depression. A second limitation of this study was that depressive symptomatology was assessed with self-report questionnaires. Future studies should include diagnostic interviews to ascertain clinically significant mood disorders. However, even subclinical levels of depressive symptomatology appear to have effects on breastfeeding, which is known to be a positive health behavior. Finally, women in this sample were largely White, upper–middle class, and married; therefore, further study is needed on women from diverse ethnic, socioeconomic, and family backgrounds before generalizations can be drawn to the wider population. Balancing these limitations, are a number of strengths of the present study including a prospective design, multilevel modeling approach, tests of bidirectional effects, and a well-characterized cohort, all of which allowed us to provide needed clues concerning to the nature of the relationship between breastfeeding and depression.
Conclusion
The question of whether breastfeeding is protective against subsequent depressive symptomatology is important because if the answer is yes, breastfeeding could be a target for preventing a disorder that has substantial negative consequences for both maternal and infant health. The present findings suggest that the relationship between breastfeeding and depression is complex and bidirectional, with early breastfeeding predicting fewer cases of depressive symptomatology and early depressive symptomatology predicting less breastfeeding. The premise that women with depression are less likely to breastfeed has more support in the existing literature than the reverse, but only two studies, including the study we report here, have addressed the latter hypothesis. This research highlights the need for further inquiry into both causal pathways linking breastfeeding and depression, especially given that breastfeeding has substantial benefits for infant health, andmaternal depression has substantial negative consequences for both maternal and infant health.
Contributor Information
Jennifer Hahn-Holbrook, Email: jhahn@psych.ucla.edu, Department of Psychology and the Institute for Society and Genetics, University of California, Los Angeles, Los Angeles, CA, USA.
Martie G. Haselton, Department of Psychology and Communications Studies and the Institute for Society and Genetics, University of California, Los Angeles, Los Angeles, CA, USA
Christine Dunkel Schetter, Department of Psychology and the Institute for Society and Genetics, University of California, Los Angeles, Los Angeles, CA, USA; Department of Psychology, University of California, Los Angeles, Los Angeles, CA, USA.
Laura M. Glynn, University of California, Irvine, Irvine, CA, USA
References
- Baranowski T, Bee DE, Rassin DK, Richardson CJ, Brown JP, Guenther N, Nader PR. Social support, social influence, ethnicity and the breastfeeding decision. Soc Sci Med. 1983;17:1599–1611. doi: 10.1016/0277-9536(83)90306-4. [DOI] [PubMed] [Google Scholar]
- Beck CT. A meta-analysis of the relationship between postpartum depression and infant temperament. Nurs Res. 1996a;45:225–230. doi: 10.1097/00006199-199607000-00006. [DOI] [PubMed] [Google Scholar]
- Beck CT. A meta-analysis of predictors of postpartum depression. Nurs Res. 1996b;45:297–303. doi: 10.1097/00006199-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275–285. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Bernier MO, Plu-Bureau G, Bossard N, Ayzac L, Thalabard JC. Breastfeeding and risk of breast cancer: a metaanalysis of published studies. Hum Reprod Updat. 2000;6(4):374–386. doi: 10.1093/humupd/6.4.374. [DOI] [PubMed] [Google Scholar]
- Chaudron LH, Klein MH, Remington P, Palta M, Allen C, Essex MJ. Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol. 2001;22:103–112. doi: 10.3109/01674820109049960. [DOI] [PubMed] [Google Scholar]
- Cooper PJ, Murray L, Stein A. Psychosocial factors associated with the early termination of breast-feeding. J Psychosom Res. 1993;37:171–176. doi: 10.1016/0022-3999(93)90084-s. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item postpartum depression scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Dai X, Dennis CL. Translation and validation of the breastfeeding self-efficacy scale into Chinese. J Midwifery Womens Health. 2003;48:350–356. doi: 10.1016/s1526-9523(03)00283-6. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Conner JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis C-L, McQueen K. Does maternal postpartum depressive symptomatology influence infant feeding outcomes? Acta Paediatr. 2007;96:590–594. doi: 10.1111/j.1651-2227.2007.00184.x. [DOI] [PubMed] [Google Scholar]
- Dennis C-L, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123:e736–e751. doi: 10.1542/peds.2008-1629. [DOI] [PubMed] [Google Scholar]
- Dewey KG. Maternal and fetal stress are associated with impaired lactogenesis in humans. J Nutr. 2001;131:30125–30155. doi: 10.1093/jn/131.11.3012S. [DOI] [PubMed] [Google Scholar]
- Edhborg M, Friberg M, Lundh W, Widstrom A. “Struggling with life”: narratives from women with signs of postpartum depression. Scand J Public Health. 2005;33:261–267. doi: 10.1080/14034940510005725. [DOI] [PubMed] [Google Scholar]
- Else-Quest NM, Shibley Hyde J, Clark R. Breastfeeding, bonding, and the mother–infant relationship. Merrill-Palmer Q. 2003;49:495–517. [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocinological foundation for human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Fergerson SS, Jamieson DJ, Lindsay M. Diagnosing postpartum depression: can we do better? Am J Obstet Gynecol. 2002;186:899–902. doi: 10.1067/mob.2002.123404. [DOI] [PubMed] [Google Scholar]
- Field T. Postpartum depression effects on early interactions, parenting and safety practices: a review. Infant Behav Dev. 2010;33:1–6. doi: 10.1016/j.infbeh.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T, Hernandez-Reif M, Feijo L. Breastfeeding in depressed mother–infant dyads. Early Child Dev Care. 2002;172:539–545. [Google Scholar]
- Galler J, Harrison RH, Biggs MA, Ramsey F, Forde V. Maternal moods predict breastfeeding in Barbados. J Dev Behav Pediatr. 1999;20:80–87. doi: 10.1097/00004703-199904000-00002. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Reprod Technol Assess. 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child and cognitive development and behavior: a review and critical analysis of the literature. Arch Women's Ment Heal. 2003;6:263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- Green J, Murray D. The use of the Edinburgh Postnatal Depression Scale in research to explore the relationship between antenatal and postnatal dysphoria. In: Cox J, Holden J, editors. Perinatal psychiatry: use and misuse of the Edinburgh Postnatal Depression Scale. London: Gaskell; 1994. pp. 180–198. [Google Scholar]
- Groer MW, Davis MW. Cytokines, infections, stress, and dysphoric moods in breastfeeders and formula feeders. J Obstet Gynecol Neonatal Nurs. 2006;35:599–607. doi: 10.1111/j.1552-6909.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- Hahn-Holbrook J, Dunkel Schetter C, Haselton M. Breastfeeding and maternal mental and physical health: Is breast best for mom too? In: Spiers M, Geller P, Kloss J, editors. Women's Health Psychology. New Jersey: Wiley; 2013. [Google Scholar]
- Hannah P, Adams D, Lee A, Glover V, Sandler M. Links between early post-partum mood and postnatal depression. Br J Psychiatry. 1992;160:777–780. doi: 10.1192/bjp.160.6.777. [DOI] [PubMed] [Google Scholar]
- Ip S, Chung M, Raman G, Chew P, Magula N, DeVine D, Trikalinos T, Lau J. Tufts-New England Medical Center Evidence-based Practice. Rockville, MD: Center for Healthcare Research and Quality; 2007. Breastfeeding and maternal and infant health outcomes in developed countries (AHRQ 07-E007) [PMC free article] [PubMed] [Google Scholar]
- Jaedicke KM, Fuhrmann MD, Stefanski V. Lactation modifies stress-induced immune changes in laboratory rats. Brain Behav Immun. 2009;23:700–708. doi: 10.1016/j.bbi.2009.02.005. [DOI] [PubMed] [Google Scholar]
- Jansen J, de Weerth C, Riksen-Walraven M. Breastfeeding and the mother–infant relationship—a review. Dev Rev. 2008;28:503–521. [Google Scholar]
- Jones NA, McFall BA, Diego MA. Patterns of brain electrical activity in infants of depressed mothers who breastfeed and bottle feed: the mediating role of infant temperament. Biol Psychol. 2004;67:103–124. doi: 10.1016/j.biopsycho.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett KM. A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. Int Breastfeed J. 2007;2:2–41. doi: 10.1186/1746-4358-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick KM. Oxytocin, motherhood and bonding. Exp Physiol. 2000;85:111–124. doi: 10.1111/j.1469-445x.2000.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Labbok M. Effects of breastfeeding on the mother. Pediatr Clin N Am. 2001;48:143–158. doi: 10.1016/s0031-3955(05)70290-x. [DOI] [PubMed] [Google Scholar]
- Labbok M, Krasocvec K. Towards consistency in breastfeeding definitions. Stud Fam Plan. 1990;21:226–230. [PubMed] [Google Scholar]
- Light KC, Smith TE, Johns JM, Brownley KA, Hofheimer JA, Amico JA. Oxytocin responsivity in mothers of infants: a preliminary study of relationships with blood pressure during laboratory stress and normal ambulatory activity. Heal Psychol. 2000;19:560–567. doi: 10.1037//0278-6133.19.6.560. [DOI] [PubMed] [Google Scholar]
- Matthey S, Henshaw C, Elliot S, Barnett B. Variability in use of cut-off scores and formats on the Edinburgh postnatal depression scale: implications for clinical and research practice. Br J Psychiatry. 2006;9:309–315. doi: 10.1007/s00737-006-0152-x. [DOI] [PubMed] [Google Scholar]
- Merewood A, Brooks D, Bauchner H, MacAuley L, Metha S. Maternal birthplace and breastfeeding initiation among term and preterm infants: a statewide assessment for Massachusetts. Pediatrics. 2006;118:1048–1054. doi: 10.1542/peds.2005-2637. [DOI] [PubMed] [Google Scholar]
- Merjonen P, Jakela M, Palkki-Raback L, Hintsanen M, Raitakari OT, Viikari J, Keltikangas-Jarvinen L. Breastfeeding and off-spring hostility in adulthood. Psychother Psychosom. 2011;80:371–373. doi: 10.1159/000324748. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Katlin ES. Breast-feeding is associated with reduced perceived stress and negative mood in mothers. Heal Psychol. 2002;21(2):187–193. [PubMed] [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, Cooper P. The impact of postnatal depression and associated adversity on early mother–child interactions and later infant outcomes. Child Development. 1996;67:2512–2526. [PubMed] [Google Scholar]
- Neigel S, Ystrom E, Hagtvet K, Vollrath M. Difficult temperament, breastfeeding and their mutual prospective effects: the Norwegian mother and child cohort study. J Dev Behav Pediatr. 2008;29:458–462. doi: 10.1097/dbp.0b013e3181877a88. [DOI] [PubMed] [Google Scholar]
- Nishioka E, Haruna M, Ota E, Matsuzaki M, Murayama R, Yoshimura K, Murashima S. A prospective study of the relationship between breastfeeding and postpartum depressive symptoms appearing at 1–5 months after delivery. J Affect Disord. 2011;133(3):553–559. doi: 10.1016/j.jad.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Nissen E, Gustavsson P, Widström AM, Uvnäs-Moberg K. Oxytocin, prolactin, milk production and their relationship with personality traits in women after vaginal delivery or Cesarean section. J Psychosom Obstet Gynecol. 1998;19:49–58. doi: 10.3109/01674829809044221. [DOI] [PubMed] [Google Scholar]
- Nobel RE. Depression in women. Metab Clin Exp. 2005;54:49–52. doi: 10.1016/j.metabol.2005.01.014. [DOI] [PubMed] [Google Scholar]
- O'Hara M, Swain A. Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- Pan A, Lucas M, Sun Q, van Dam RM, Franco OH, Manson JE, Willett WC, Ascherio A. Bidirectional association between depression and type 2 diabetes mellitus in women. Arch Intern Med. 2010;170:1884–1891. doi: 10.1001/archinternmed.2010.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson JF, Dauber S, Leiferman JA. Individual and combined effects of postpartum depression in mothers and fathers on parenting behaviors. Pediatrics. 2006;118:659–668. doi: 10.1542/peds.2005-2948. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis method. London: Sage; 2002. [Google Scholar]
- Rea MF. Benefits of breastfeeding and women's health. J Pediatr. 2004;80:142–146. doi: 10.2223/1247. [DOI] [PubMed] [Google Scholar]
- Riordan J. Breastfeeding and human lactation. 3rd edn. Sudbury: Jones and Bartlett; 2005. [Google Scholar]
- Rugulies R. Depression as a predictor for coronary heart disease: a review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- Santer DA, Coyne JC. Shortening the CES-D to improve its ability to detect cases of depression. Psychol Assess. 1997;9:233–243. [Google Scholar]
- Schwarz EB, Ray RM, Stuebe AM, Allison MA, Ness RB, Freiberg MS, Cauley JA. Duration of lactation and risk factors for maternal cardiovascular disease. Obstet Gynecol. 2009;113:974–982. doi: 10.1097/01.AOG.0000346884.67796.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JA, Binns CW, Oddy WH. Predictors of breastfeeding duration: evidence from a cohort study. Pediatrics. 2006;117:646–655. doi: 10.1542/peds.2005-1991. [DOI] [PubMed] [Google Scholar]
- Seimyr L, Edhborg M, Lundh W, Sjogren B. In the shadow of maternal depressed mood: experiences of parenthood during the first year after childbirth. J Psychosom Obstet Gynecol. 2004;25:23–34. doi: 10.1080/01674820410001737414. [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32:705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36:1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26(1):81–88. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Rich-Edwards JW, Willett WC, Manson JE, Michels KB. Duration of lactation and incidence of type 2 diabetes. JAMA. 2005;294:2601–2610. doi: 10.1001/jama.294.20.2601. [DOI] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Pedersen CA, Propper C, Meltzer-Brody S. Failed lactation and perinatal depression: common problems with shared neuroendocrine mechanisms? J Women's Heal. 2012;21:264–272. doi: 10.1089/jwh.2011.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamminen T. The impact of mother's depression on her nursing experiences and attitudes during breastfeeding. Acta Paediatr Scand Suppl. 1988;77:87–94. doi: 10.1111/j.1651-2227.1988.tb10864.x. [DOI] [PubMed] [Google Scholar]
- Taveras EM, Capra AM, Braveman PA, Jensvold NG, Escobar GJ, Lieu TA. Clinician support and psychosocial risk factors associated with breastfeeding discontinuation. Pediatrics. 2003;112:108–115. doi: 10.1542/peds.112.1.108. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Eriksson M. Breastfeeding: physiological, endocrine and behavioral adaptations caused by oxytocin and local neurogenic activity in the nipple and mammary gland. Acta Peadiatr. 1996;85:525–530. doi: 10.1111/j.1651-2227.1996.tb14078.x. [DOI] [PubMed] [Google Scholar]
- Vandiver TA. Relationship of mother's perceptions and behavior to the duration of breastfeeding. Psychol Rep. 1997;80:1375–1384. doi: 10.2466/pr0.1997.80.3c.1375. [DOI] [PubMed] [Google Scholar]
- Worobey J. Development milestones related to feeding status: evidence from the Child Health Supplement to the 1981: National Health Interview Survey. J Hum Nutr Diet. 1992;5:363–369. [Google Scholar]
- Zelkowitz P, Milet TH. Postpartum psychiatric disorders: their relationship to psychological adjustment and marital satisfaction in the spouses. J Abnorm Psychol. 1996;105:281–285. doi: 10.1037//0021-843x.105.2.281. [DOI] [PubMed] [Google Scholar]