Abstract
C-reactive protein (CRP) is made in liver and its serum concentration increases in inflammation. Measurement of serum CRP is recommended for use as an indicator of inflammation and predictor of atherosclerosis. Cholesterol-lowering drugs statins also lower CRP. To evaluate statin-mediated CRP reduction and to reassess clinical usefulness of CRP, we investigated regulation of CRP gene expression. Here, we show that pravastatin and simvastatin prevent the induction of CRP expression in human hepatoma Hep3B cells exposed to proinflammatory cytokines IL-6 and IL-1β· The nitric oxide (NO) donor, sodium nitroprusside, also prevented the induction of CRP expression while the CRP inducers IL-6 and IL-1β were present with the cells. The effect of NO on CRP expression was at the level of transcription. These findings suggest that the decrease in CRP level in vivo after statin-treatment does not necessarily reflect absence of inflammation, and that NO-releasing drugs have the potential to reduce serum CRP levels. Thus, the measurement of serum CRP levels alone in individuals on statin/NO-therapy is not as useful as was imagined.
Keywords: C-reactive protein, Inflammation, Statin, Nitric oxide, Atherosclerosis
1. Introduction
C-reactive protein (CRP) is an acute phase protein whose serum concentration increases even under chronic inflammatory conditions, such as atherosclerosis (Agrawal, 2005; Black et al., 2004). CRP starts functioning in vivo probably after binding to ligands like phosphocholine-containing substances (Agrawal et al., 2002), such as modified low-density lipoproteins (Chang et al., 2002; Bhakdi et al., 2004), and extracellular matrix proteins, such as fibronectin (Suresh et al., 2004). Any role of CRP in the pathogenesis of atherosclerosis is not certain, although elevated serum CRP is considered as a predictor of cardiovascular diseases (Libby and Ridker, 2004).
CRP is primarily produced by hepatocytes (Kushner and Feldmann, 1978) and can be experimentally induced in human hepatoma Hep3B cells by treatment with proinflammatory cytokines IL-6 and IL-1β (Ganapathi et al., 1991). In these cells, induction of CRP expression by (IL-6 + IL-1β) is further enhanced by dexamethasone (Dex) (Ganapathi et al., 1991). Hep3B cells cultured in the presence of proinflamma-tory mediators represent an alternative to animal models of inflammation to investigate the mechanism of regulation of CRP gene expression.
In Hep3B cells, transcription factors C/EBPβ (Li and Goldman, 1996; Agrawal et al., 2001, 2003a; Cha-Molstad et al., 2000), STAT3 (Zhang et al., 1996) and NF-κB (Agrawal et al., 2003b) participate in the induction of CRP expression. On the CRP promoter, transcription factor C/EBPβ binds to a site at –52, STAT3 binds to a site at –108 and NF-κB binds to a site at –69 (Li and Goldman, 1996; Zhang et al., 1996; Voleti and Agrawal, 2004). A second C/EBP-site is located at position –219, however, the first 157 bp of the CRP promoter are sufficient for synergistic induction of CRP expression by IL-6 and IL-1β (Li and Goldman, 1996; Zhang et al., 1995).
Statins that lower cholesterol levels have also been shown to lower CRP levels in human blood (Nissen et al., 2005; Ridker et al., 2005). Statins enhance nitric oxide (NO) production from many cell types (Kaesemeyer et al., 1999; Harris et al., 2004), and since NO regulates expression of a number of genes in the hepatocytes (Bogdan, 2001; Davis et al., 2001), we explored the possible role of NO donors, and of statins, in CRP expression in Hep3B cells.
2. Materials and methods
2.1. Cell culture, ELISA, RNA isolation and Northern blot
Hep3B cells were cultured in 100 mm dish containing 5 ml growth media and subjected to serum starvation overnight for cytokine, sodium nitroprusside (SNP) and statin treatments as described previously (Agrawal et al., 2001). The confluency of cells was approximately 60% at the time of treatments. IL-6 and IL-1β (R&D) were used at concentrations of 10 ng/ml and 1 ng/ml, respectively. Dex (Sigma) was used at 1 μM. SNP (Fisher Scientific), pravastatin sodium salt (Wako Pure Chemical Industries Ltd.) and simvastatin sodium salt (Calbiochem) treatments were started 45 min prior to cytokine treatment. For CRP ELISA (Suresh et al., 2004), RNA isolation and luciferase assay, the cells were treated with cytokines for 72, 40 and 24 h, respectively. Total cellular RNA was isolated using RNeasy Mini Kit (Qiagen) and subjected to Northern blot exactly as described previously (Agrawal et al., 2003b). EcoRI-cut CRP cDNA clone (Agrawal et al., 2002) in the plasmid p91023 and GAPDH cDNA (Sigma) were used as probes in Northern blot.
2.2. Determination of NO production
NO production was determined using the Greiss reaction to monitor nitrite levels in cell culture media (Green et al., 1982). One hundred microliters of Griess reagent (1% sulfanilamide and 0.1% naphthylethylenediamide in 5% phosphoric acid) was mixed with 50 μl of culture medium. OD550 was measured using a microplate reader. Nitrite concentrations were calculated by comparison with OD550 values of standard solutions of sodium nitrite (1–1000 nM).
2.3. Transfection and luciferase transactivation assay
For transient transfections, cells were plated into six-well plates and transfected using FuGENE 6 reagent (Roche) as described previously (Agrawal et al., 2003b). Luciferase reporter-CRP promoter constructs were used at 1 μg plasmid per well. SNP and cytokine treatments were started 16 h post-transfection. After 40 h of transfection, luciferase assays were performed as described previously (Agrawal et al., 2003b). Luciferase activity was measured in a luminometer (Molecular Devices).
2.4. Engineering of CRP promoter-luciferase reporter constructs
The wild-type (WT) CRP promoter construct, Luc-157 WT, has been described earlier (Agrawal et al., 2001). Luc-300 WT construct was prepared according to a published method (Kleemann et al., 2003). Briefly, genomic DNA (Promega) was used to amplify a fragment corresponding to nucleotides –300/–1 of the CRP promoter, using the primers 5′-CCTAGATCTAGAGCTACCTCCTCCTGCCTGG and 5′-CCGACGCGTACCCAGATGGCCACTCGTTTAATATGTTACC. Primers were designed to contain the BglII and MluI restriction sites, respectively. The PCR product was cloned into the luciferase reporter vector pGL2 basic (Promega) and the DNA sequence was confirmed. These two WT constructs were used as templates for mutagenesis. Constructs containing mutated κB and STAT3 site were generated using the QuickChange site-directed mutagenesis kit (Stratagene). The κB-site was mutated by substituting –72AAAATT–67 with –72TTAATA–67 using mutagenic primers: 5′-GCGCCACTATGTAAATTATTAACCAACATTGCTTGTTGGGGC and 5′-GCCCCAACAAGCAATGTTGGTTAATAATTTACATAGTGGCGC. The STAT3-site was mutated by substituting –111TCCCGA–106 with –111GATATC–106 using mutagenic primers 5′-GCTTCCCCTCTGATATCAGCTCTGACACCTG and 5′-CAGGTGTCAGAGCTGATATCAGAGGGGAAGC. Mutations were verified by sequencing. Plasmids were purified using maxiprep plasmid isolation kit (Eppendorf).
3. Results
3.1. Pravastatin and simvastatin prevent (IL-6 + IL-1β)-induced CRP production
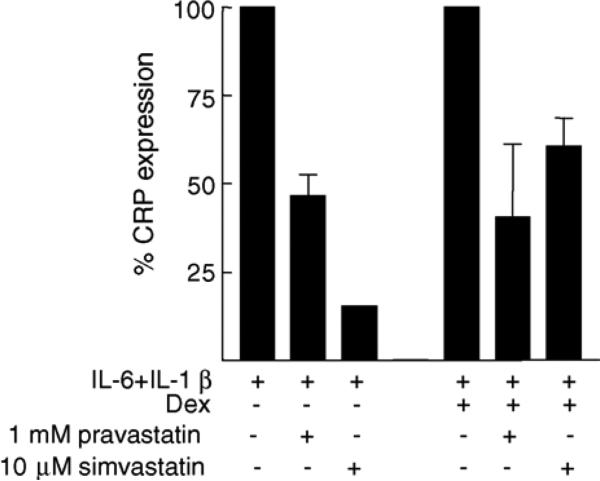
Production of CRP by Hep3B cells in response to (IL-6 + IL-1β) or (IL-6 + IL-1β + Dex) was reduced when the cells were co-treated with pravastatin or simvastatin (Fig. 1). Requirement of higher concentrations of pravastatin for the observed inhibition of CRP expression indicated that the pravastatin we used here had poor accessibility to the cells and was not suitable for cell culture experiments. The inhibitory effect of statins was independent of the action of Dex.
Fig. 1.
Statins prevent production of CRP by Hep3B cells while the cells are exposed to proinflammatory cytokines IL-6 and IL-1β. CRP expression in the absence of statins is plotted as 100%.
3.2. NO prevents (IL-6 + IL-1β)-induced CRP production
Treatment of Hep3B cells (Fig. 2) with the NO donor SNP (Yamamoto and Bing, 2000) alone did not affect CRP production. Pretreatment of cells with SNP prevented almost completely the induction of CRP production in response to (IL-6 + IL-1β + Dex). The suppressing effect of NO on CRP expression was SNP-dose-dependent (Fig. 3A), as determined by ELISA. Increasing concentrations of SNP produced increasing concentrations of NO in the cell culture medium (Fig. 3B). Another NO donor, S-nitroso-N-acetylpenicillamine (SNAP), also inhibited (IL-6 + IL-1β + Dex)-induced CRP expression (data not shown).
Fig. 2.
NO prevents production of CRP, as measured by ELISA, by Hep3B cells while the cells are exposed to proinflammatory cytokines. SNP was used at 200 μM. A representative experiment is presented.
Fig. 3.
NO-dose-dependent suppression of CRP production. A representative experiment is presented. (A) CRP ELISA, treatment with increasing concentration of SNP in the presence of a fixed concentration of IL-6 + IL-1β + Dex. CRP expression without SNP-treatment was used to calculate percent inhibition. (B) Increased nitrite production in the culture media in response to increase in SNP.
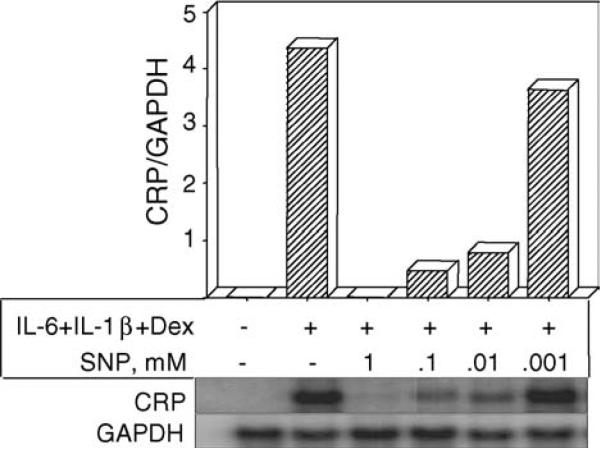
3.3. Effect of NO on CRP mRNA accumulation
Employing Northern blot, the accumulation of CRP mRNA in Hep3B cells was measured in response to (IL-6 + IL-1β + Dex)-treatment in the absence and presence of various concentrations of SNP. The inhibition of CRP expression by NO was at the level of transcription as indicated by the dose-dependent decrease in CRP mRNA accumulation (Fig. 4). A dose of 1 mM SNP was not toxic to the cells as seen by GAPDH expression and trypan blue staining. As little as 1 μM SNP was sufficient to observe the inhibition of CRP expression, both at the protein level and at the mRNA level.
Fig. 4.
NO decreases CRP mRNA accumulation in Hep3B cells in the presence of IL-6 and IL-1β. Northern blot on total RNA was analyzed in a phosphorimager. Ratio of CRP to GAPDH mRNA accumulation is shown on the y-axis. A representative experiment is presented.
3.4. The STAT3-site and the κB-site do not mediate the inhibition of CRP expression by NO
Luciferase-transactivation assays were performed utilizing CRP promoter constructs with the mutated STAT3-site and mutated κB-site (Luc-300 m-κB, Luc-300 m-ST, Luc-157 m-κB and Luc-157 m-ST) to evaluate their role in mediating the inhibitory effect of NO on CRP expression (Fig. 5). SNP-treatment of the cells inhibited (IL-6 + IL-1 ± Dex)-induced transactivation of all the mutated constructs, as SNP did on the WT promoters. Thus, the suppressing effect of NO on CRP expression was not through the STAT3 and κB sites. We could not employ mutagenesis to determine the role of the C/EBP-site because mutation of the C/EBP-site abolished all responses to cytokines (Cha-Molstad et al., 2000; Agrawal et al., 2001). By comparing 300 and 157 bp WT promoters, we ruled out involvement of the CEBP-site located at position –219 in mediating NO effects.
Fig. 5.
The STAT3-site and the κB-site do not mediate inhibition of CRP expression by NO. In all the promoter constructs, basal luciferase activity is shown as 1 and the luciferase activity in treated-cells is plotted as fold-induction over basal activity. The average ± S.E.M. of three experiments is shown.
4. Discussion
Statins have been shown to lower CRP levels independently of their cholesterol-lowering activity (Nissen et al., 2005; Ridker et al., 2005). Since statins are known to generate NO and since NO modulates gene expression (Kaesemeyer et al., 1999; Harris et al., 2004; Bogdan, 2001; Davis et al., 2001), we investigated the effects of statins and NO on CRP expression in Hep3B cells. Our major findings were: (1) pravastatin and simvastatin prevented induction of CRP expression in Hep3B cells exposed to proinflammatory molecules IL-6 + IL-1β. We have not yet determined whether the statins’ effect was mediated through NO generation. (2) NO donor SNP also prevented induction of CRP expression in response to IL-6 + IL-1β. These findings suggest that the lowering of CRP by statins is not an indication of a decrease in the extent of proinflammatory cytokines and that the measurement of cytokines, but not of CRP, is required to assess anti-inflammatory outcome of statins.
In the presence of statins, the unresponsiveness of Hep3B cells to proinflammatory cytokines, resulting in the loss of CRP production, was not totally unexpected. It has been demonstrated previously that statins inhibit CRP production by IL-1-treated human CRP-transgenic mice, by IL-1-treated and IL-6-treated primary human hepatocytes, and by IL-1-treated human hepatoma cells (Kleemann et al., 2004; Verschuren et al., 2005; Arnaud et al., 2005). We interpret these data, generated from in vitro and in vivo models of inflammation, to conclude that even if inflammation persists, CRP is not produced due to the direct inhibitory action of statins on CRP-producing cells. We present this concept in a schematic diagram (Fig. 6) showing the reduction of CRP expression in response to statin/NO while the proinflammatory mediators may still be present with the hepatocytes.
Fig. 6.
Schematic diagram of our in vitro experiment: statins and NO reduce CRP production while the proinflammatory mediators are still present in the vicinity of CRP-producing hepatoma cells.
Earlier, it was proposed that the lowering of CRP production in response to statin-treatment might be due to the ability of statins to induce NO (Kaesemeyer and Caldwell, 2000). An inverse relationship between CRP and NO concentrations was shown in population-based studies (Braga et al., 1996; Cleland et al., 2000; Fichtlscherer et al., 2004). We have shown here the role of exogenously supplied NO in CRP expression by Hep3B cells exposed to cytokines. Hep3B cells, however, are capable of producing endogenous NO and under certain conditions, e.g. hypoxia, IFNγ-treatment, the production of NO by Hep3B cells is enhanced (Yoshioka et al., 1997; Imagawa et al., 2002). Both NO and CRP are produced by liver in inflammation (Curran et al., 1989; Geller et al., 1993). Additionally, NO is produced by a variety of mammalian cells and is produced in numerous physiological and pathological conditions including during inflammation. Thus, it is likely that endogenous NO may also be participating in regulating CRP expression.
Our results support the hypotheses that NO might down-regulate CRP expression in vivo (Kaesemeyer and Caldwell, 2000; McCarty, 2004) and that the strategies to lower plasma CRP might be effective by improving NO bioavailability (Fichtlscherer et al., 2004). The contribution of CRP in the development of atherosclerosis has not been documented yet, but if a deleterious role of CRP is proposed, our findings raise the possibility of utilizing NO-releasing drugs to lower CRP expression. If the manipulation of NO is feasible, then the dose of statins sufficient to lower cholesterol levels need not be modified. NO-releasing aspirins have been developed for use to improve protection for the heart without the unwanted effects on the stomach (Wallace et al., 2002; Napoli et al., 2002). We suggest that, until a role of CRP in the pathogenesis of atherosclerosis is found, the NO-aspirin should be used with caution because it may lower CRP levels.
IL-6 and IL-1 are the main inducers of CRP expression (Castell et al., 1990; Ganapathi et al., 1991). Many other molecules, such as IL-4, IL-11, TNF, TGFβ and Dex have also been reported to participate in regulating CRP expression. IL-4 and TNF decrease IL-6-induced CRP expression (Gabay et al., 1999; Yap et al., 1991). IL-11 has been shown to increase CRP in a study with women participants (Gordon et al., 1996). TGFβ affects CRP expression at the post-translational level (Taylor et al., 1990). Our data indicate that NO is also a major player and is as important as IL-6 and IL-1 in regulating CRP expression.
One mechanism by which NO regulates gene expression is by directly influencing transcription factors. It is shown previously that p50–p50/C/EBPβ complex participates through a nonconsensus κB-site on the CRP promoter in inducing CRP expression (Agrawal et al., 2001), and the fenofibrate-mediated inhibition of IL-1-induced CRP expression is due to the decrease in the formation of p50–p50/C/EBPβ complexes (Kleemann et al., 2003). We have not yet measured such complexes in SNP-treated cells. STAT3 was also shown to mediate the inhibitory effect of fenofibrates on CRP expression (Gervois et al., 2004). However, we found that neither the STAT3-site nor the κB-site was involved in mediating the NO effects. At present, we do not conclude about the mechanism of NO-mediated inhibition solely based on the luciferase assays.
The mechanism of statin/NO-mediated reduction in CRP mRNA levels is not clear, but the implications of our findings remain as significant for the evaluation of statin-mediated lowering of CRP in the presence of inflammation. We favour the advice that the CRP values to evaluate cardiovascular complications should be considered carefully (Munford, 2001; Kushner, 2002; Pepys, 2005).
Acknowledgements
We are grateful to Dr. I. Kushner and Dr. D. Samols for Luc-157 WT and Dr. G.J. Darlington for Hep3B cells. We are also endebted to Ms. Mahua Chakraborthy for constructing the CRP promoter Luc-300 WT.
References
- Agrawal A, Cha-Molstad H, Samols D, Kushner I. Transactivation of C-reactive protein by IL-6 requires synergistic interactions of CCAAT/enhancer binding protein β (C/EBPβ) and Rel p50. J. Immunol. 2001;166:2378–2384. doi: 10.4049/jimmunol.166.4.2378. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Simpson MJ, Black S, Carey MP, Samols D. A C-reactive protein mutant that does not bind to phosphocholine and pneumococcal C-polysaccharide. J. Immunol. 2002;169:3217–3222. doi: 10.4049/jimmunol.169.6.3217. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Samols D, Kushner I. Transcription factor c-Rel enhances C-reactive protein expression by facilitating the binding of C/EBPβ to the promoter. Mol. Immunol. 2003a;40:373–380. doi: 10.1016/s0161-5890(03)00148-2. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Cha-Molstad H, Samols D, Kushner I. Over-expressed NF-κB can participate in endogenous C-reactive protein induction, and enhances the effects of C/EBPβ and signal transducer and activator of transcription-3. Immunology. 2003b;108:539–547. doi: 10.1046/j.1365-2567.2003.01608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agrawal A. CRP after 2004. Mol. Immunol. 2005;42:927–930. doi: 10.1016/j.molimm.2004.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud C, Burger F, Steffens S, Veillard NR, Nguyen TH, Trono D, Mach F. Statins reduce interleukin-6-induced C-reactive protein in human hepatocytes: new evidence for direct anti-inflammatory effects of statins. Arterioscler. Thromb. Vasc. Biol. 2005;25:1–6. doi: 10.1161/01.ATV.0000163840.63685.0c. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Torzewski M, Paprotka K, Schmitt S, Barsoom H, Suriyaphol P, Han SR, Lackner KJ, Husmann M. Possible protective role of C-reactive protein in atherogenesis: complement activation by modified lipoproteins halts before detrimental terminal sequence. Circulation. 2004;109:1870–1876. doi: 10.1161/01.CIR.0000124228.08972.26. [DOI] [PubMed] [Google Scholar]
- Black S, Kushner I, Samols D. C-reactive protein. J. Biol. Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the regulation of gene expression. Trends Cell Biol. 2001;11:66–75. doi: 10.1016/s0962-8924(00)01900-0. [DOI] [PubMed] [Google Scholar]
- Braga M, Gianotti L, Cestari A, Vignali A, Pellegatta F, Dolci A, Di Carlo V. Gut function and immune and inflammatory responses in patients preoperatively fed with supplemented enteral formulas. Arch. Surg. 1996;131:1257–1264. doi: 10.1001/archsurg.1996.01430240011001. [DOI] [PubMed] [Google Scholar]
- Castell JV, Gomez-Lechon MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990;12:1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- Cha-Molstad H, Agrawal A, Zhang D, Samols D, Kushner I. The rel family member p50 mediates cytokine-induced C-reactive protein expression by a novel mechanism. J. Immunol. 2000;165:4592–4597. doi: 10.4049/jimmunol.165.8.4592. [DOI] [PubMed] [Google Scholar]
- Chang MK, Binder CJ, Torzewski M, Witztum JL. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. U.S.A. 2002;99:13043–13048. doi: 10.1073/pnas.192399699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland SJ, Sattar N, Petrie JR, Forouhi NG, Elliott HL, Connell JMC. Endothelial dysfunction as a possible link between C-reactive protein levels and cardiovascular disease. Clin. Sci. 2000;98:531–535. [PubMed] [Google Scholar]
- Curran RD, Billiar TR, Stuehr DJ, Hofmann K, Simmons RL. Hepatocytes produce nitrogen oxides from l-arginine in response to inflammatory products of Kupffer cells. J. Exp. Med. 1989;170:1769–1774. doi: 10.1084/jem.170.5.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annu. Rev. Pharmacol. Toxicol. 2001;41:203–236. doi: 10.1146/annurev.pharmtox.41.1.203. [DOI] [PubMed] [Google Scholar]
- Fichtlscherer S, Breuer S, Schächinger V, Dimmeler S, Zeiher AM. C-reactive protein levels determine systemic nitric oxide bioavailability in patients with coronary artery disease. Eur. Heart J. 2004;25:1412–1418. doi: 10.1016/j.ehj.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Gabay C, Porter B, Guenette D, Billir B, Arend WP. Interleukin-4 (IL-4) and IL-13 enhance the effect of IL-1β on production of IL-1 receptor antagonist by human primary hepatocytes and hepatoma HepG2 cells: differential effect on C-reactive protein production. Blood. 1999;93:1299–1307. [PubMed] [Google Scholar]
- Ganapathi MK, Rzewnicki D, Samols D, Jiang SL, Kushner I. Effect of combinations of cytokines and hormones on synthesis of serum amyloid A and C-reactive protein in Hep3B cells. J. Immunol. 1991;147:1261–1265. [PubMed] [Google Scholar]
- Geller DA, Nussler AK, Di Silvio M, Lowenstein CJ, Shapiro RA, Wang SC, Simmons RL, Billiar TR. Cytokines, endotoxins and glucocorticocoids regulate the expression of inducible nitric oxide synthase in hepatocytes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:522–526. doi: 10.1073/pnas.90.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervois P, Kleemann R, Pilon A, Percevault F, Koenig W, Staels B, Kooistra T. Global suppression of IL-6-induced acute phase response gene expression after chronic in vivo treatment with the peroxisome proliferator-activated receptor-α activator fenofibrate. J. Biol. Chem. 2004;279:16154–16160. doi: 10.1074/jbc.M400346200. [DOI] [PubMed] [Google Scholar]
- Gordon MS, McCaskill-Stevens WJ, Battiato LA, Loewy J, Loesch D, Breeden E, Hoffman R, Beach KJ, Kuca B, Kaye J, Sledge GW., Jr. A phase I trial of recombinant human interleukin-11 (Neumega rhIL-11 growth factor) in women with breast cancer receiving chemotherapy. Blood. 1996;87:3615–3624. [PubMed] [Google Scholar]
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Harris MB, Blackstone MA, Sood SG, Li C, Goolsby JM, Venema VJ, Kemp BE, Venema RC. Acute activation and phosphorylation of endothelial nitric oxide synthase by HMG-CoA reductase inhibitors. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H560–H566. doi: 10.1152/ajpheart.00214.2004. [DOI] [PubMed] [Google Scholar]
- Imagawa S, Tarumoto T, Suzuki N, Mukai HY, Hasegawa Y, Higuchi M, Neichi T, Ozawa K, Yamamoto M, Nagasawa T. l-Arginine rescues decreased erythropoietin gene expression by stimulating GATA-2 with L-NMMA. Kidney Int. 2002;61:396–404. doi: 10.1046/j.1523-1755.2002.00152.x. [DOI] [PubMed] [Google Scholar]
- Kaesemeyer WH, Caldwell RB, Huang J, Caldwell RW. Pravastatin sodium activates endothelial nitric oxide synthase independent of its cholesterol-lowering actions. J. Am. Coll. Cardiol. 1999;33:234–241. doi: 10.1016/s0735-1097(98)00514-2. [DOI] [PubMed] [Google Scholar]
- Kaesemeyer WH, Caldwell RW. Atherosclerosis and nitric oxide production. Circulation. 2000;102:E90. doi: 10.1161/01.cir.102.11.e90. [DOI] [PubMed] [Google Scholar]
- Kleemann R, Gervois PP, Verschuren L, Staels B, Princen HMG, Kooistra T. Fibrates down-regulate IL-1-stimulated C-reactive protein gene expression in hepatocytes by reducing nuclear p50-NF-κB-C/EBPβ complex formation. Blood. 2003;101:545–551. doi: 10.1182/blood-2002-06-1762. [DOI] [PubMed] [Google Scholar]
- Kleemann R, Verschuren L, De Rooij BJ, Lindeman J, De Maat MM, Szalai AJ, Princen HMG, Kooistra T. Evidence for anti-inflammatory activity of statins and PPARα activators in human C-reactive protein transgenic mice in vivo and in cultured human hepatocytes in vitro. Blood. 2004;103:4188–4194. doi: 10.1182/blood-2003-11-3791. [DOI] [PubMed] [Google Scholar]
- Kushner I, Feldmann G. Control of the acute phase response: demonstration of C-reactive protein synthesis and secretion by hepatocytes during acute inflammation in the rabbit. J. Exp. Med. 1978;148:466–477. doi: 10.1084/jem.148.2.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner I. C-reactive protein and atherosclerosis. Science. 2002;297:520–521. doi: 10.1126/science.297.5581.520. [DOI] [PubMed] [Google Scholar]
- Li SP, Goldman ND. Regulation of human C-reactive protein gene expression by two synergistic IL-6 responsive elements. Biochemistry. 1996;35:9060–9068. doi: 10.1021/bi953033d. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am. J. Med. 2004;116:9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- McCarty MF. AMPK activation may suppress hepatic production of C-reactive protein by stimulating nitric oxide synthase. Med. Hypothesis. 2004;63:328–333. doi: 10.1016/j.mehy.2004.01.044. [DOI] [PubMed] [Google Scholar]
- Munford RS. Statins and the acute-phase response. N. Engl. J. Med. 2001;344:2016–2018. doi: 10.1056/NEJM200106283442609. [DOI] [PubMed] [Google Scholar]
- Napoli C, Ackah E, De Nigris F, Soldato PD, D'Armiento FP, Crimi E, Condorelli M, Sessa WC. Chronic treatment with nitric oxide-releasing aspirin reduces plasma low-density lipoprotein oxidation and oxidative stress, arterial oxidation-specific epitopes, and atherogenesis in hypercholesterolemic mice. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12467–12470. doi: 10.1073/pnas.192244499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, Orazem J, Magorien RD, O'Shaughnessy C, Ganz P. Statin therapy, LDL cholesterol, C-reactive protein and coronary artery disease. N. Engl. J. Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- Pepys MB. CRP or not CRP? That is the question. Arterioscler. Thromb. Vasc. Biol. 2005;25:1091–1094. doi: 10.1161/01.ATV.0000169644.88847.28. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- Suresh MV, Singh SK, Agrawal A. Interaction of calcium-bound C-reactive protein with fibronectin is controlled by pH: in vivo implications. J. Biol. Chem. 2004;279:52552–52557. doi: 10.1074/jbc.M409054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AW, Ku NO, Mortensen RF. Regulation of cytokine-induced human C-reactive protein production by transforming growth factor-β. J. Immunol. 1990;145:2507–2513. [PubMed] [Google Scholar]
- Verschuren L, Kleemann R, Offerman EH, Szalai AJ, Emeis SJ, Princen HMG, Kooistra T. Effect of low dose atorvastatin versus diet-induced cholesterol lowering on atherosclerotic lesion progression and inflammation in apolipoprotein E*3-leiden transgenic mice. Arterioscler. Thromb. Vasc. Biol. 2005;25:161–167. doi: 10.1161/01.ATV.0000148866.29829.19. [DOI] [PubMed] [Google Scholar]
- Voleti B, Agrawal A. Localization of an NF-κB site on the C-reactive protein proximal promoter. Circulation. 2004;110:III327. [Google Scholar]
- Wallace JL, Ignarro LJ, Fiorucci S. Potential cardioprotective actions of NO-releasing aspirin. Nat. Rev. Drug Disc. 2002;1:375–382. doi: 10.1038/nrd794. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Bing RJ. Nitric oxide donors. Proc. Soc. Exp. Biol. Med. 2000;225:200–206. doi: 10.1046/j.1525-1373.2000.22525.x. [DOI] [PubMed] [Google Scholar]
- Yap SH, Moshage HJ, Hazenberg BPC, Roelofs HMJ, Bijzet J, Limburg PC, Aarden LA, Van Rijswijk MH. Tumor necrosis factor (TNF) inhibits interleukin (IL)-1 and/or IL-6 stimulated synthesis of C-reactive protein (CRP) and serum amyloid A (SAA) in primary cultures of human hepatocytes. Biochim. Biophys. Acta. 1991;1091:405–408. doi: 10.1016/0167-4889(91)90207-e. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Thompson J, Miller MJS, Fisher JW. Inducible nitric oxide synthase expression and erythropoietin production in human hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 1997;232:702–706. doi: 10.1006/bbrc.1997.6323. [DOI] [PubMed] [Google Scholar]
- Zhang D, Jiang SL, Rzewnicki D, Samols D, Kushner I. The effect of interleukin-1 on C-reactive protein expression in Hep3B cells is exerted at the transcriptional level. Biochem. J. 1995;310:143–148. doi: 10.1042/bj3100143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Sun M, Samols D, Kushner I. STAT3 participates in transcriptional activation of the C-reactive protein gene by interleukin-6. J. Biol. Chem. 1996;271:9503–9509. doi: 10.1074/jbc.271.16.9503. [DOI] [PubMed] [Google Scholar]