Abstract
Background
Head and neck squamous cell carcinomas (HNSCC) are characterized by exophytic or endophytic growth. We hypothesized that the growth pattern predicts outcome and associates with distinct clinical and immunological profiles.
Patients and Methods
Tumors obtained from 60 HNSCC patients treated with surgery and adjuvant radiotherapy were identified as exophytic or endophytic. Recurrence free survival (RFS) at 42 months was determined. In a subsets of 30 patients (22 exophytic and 8 endophytic) tumor stroma and parenchyma were evaluated for infiltrating CD4+ and CD8+ T, dendritic, myeloid and FOXP3+ regulatory T cells (Treg) and expression of immunosuppressive cytokines by immunohistochemistry. The localization and frequency of positive cells were determined microscopically and analyzed by hierarchical clustering to distinguish exophytic vs. endophytic tumors.
Results
34/60 patients had exophytic and 26/60 endophytic tumors. No differences in clinicopathologic data, disease progression or RFS were seen between the two cohorts. Infiltrates of CD3+CD8+ T cells were larger in endophytic than exophytic tumors, while FOXP3+ Treg; TGF-β+, IL-10+, Arg-1+, CD11b+ cells were equally prominent in both. FOXP3+ Treg accumulated in endophytic tumor nests, while exophytic tumor stroma was enriched in IL-10+ cells (both at p<0.05). Hierarchical clustering based on immunophenotyping failed to identify different clusters in these two tumor types. However, CD68+ macrophages and FOXP3+ Treg showed a distinct distribution.
Conclusions
The HNSCC growth pattern did not predict RFS. Although higher numbers and differences in localization of immunosuppressive cells in endophytic vs. exophytic tumors were observed, no significant relationship was established between the growth pattern and the immune profile of infiltrating lymphocytes.
Keywords: Head and neck squamous cell carcinoma (HNSCC), exophytic growth, endophytic growth, immunohistochemistry, recurrence free survival (RFS)
Introduction
HNSCC represent the 6th most common cancer worldwide [1, 2]. Today, HNSCC is considered curable when diagnosed early. However, almost 50% of patients diagnosed with early stage HNSCC will eventually recur or develop metastatic disease [3]. According to the American Joint Committee on Cancer (AJCC) staging manual, the most important prognostic factors in HNSCC are the location and tumor size, the presence of metastases in regional lymph nodes as well as the overall clinical status of the patient [3,4].
HNSCC lesions are characterized by two distinct growth patterns: exophytic and endophytic [5-7]. Exophytic tumors grow out from the mucosal surface in cauliflower-like clusters, and rapidly become symptomatic. Thus, these tumors are usually diagnosed earlier in the course of the disease. In contrast, endophytic tumors crawl under the mucosa, carpeting the interior of the larynx. The submucosal tumor is hidden from view and maybe quite extensive. This type of cancer is more difficult to diagnose, and consequently therapy of endophytic tumors is usually delayed as compared to exophytic tumors.
Numerous studies have suggested that the growth pattern of HNSCC may play a role in prognosis and responses to treatment in HNSCC [8-11]. Investigating responses to chemotherapy of untreated patients with advanced HNSCC, Boheim et al. reported that exophytic neoplasms responded better than those with the endophytic growth pattern [12]. It also has been reported that the exophytic tumors are more sensitive to radiotherapy (RT) compared to endophytic tumors [13, 14]. Furthermore, it has been suggested that endophytic tumors have a higher propensity to metastasize to cervical lymph nodes than exophytic tumors [15]. In aggregate, these studies suggested that prognosis was less favorable for endophytic than exophytic HNSCC. In contrast, Nigauri et al reported that the pattern of tumor growth was not a useful prognostic factor in advanced HNSCC [16].
It appears that the role of the exophytic vs. endophytic tumor growth pattern in prognosis and response to treatment of HNSCC and remains debatable. Further, no information is available about immune cells infiltrating these two tumor types. Cells of the immune system can inhibit tumor growth and its progression through the recognition and elimination of malignant cells, and recent evidence suggests that tumor infiltration by CD4+ and CD8+ T cells may be associated with favorable disease outcome [17-19]. However, human tumors, including HNSCC, are also able to promote the generation and accumulation of immunosupressive cells that block anti-tumor immunity [20-22]. Specifically, regulatory T cells (Treg) and myeloid derived suppressor cells (MDSC) which accumulate in human tumors and in the peripheral circulation down-regulate activities of CD4+ and CD8+ effector T cells, natural killer (NK) cells, dendritic cells (DC) and macrophages [23, 24]. Recent studies reported a higher frequency of Treg (CD4+CD25high Foxp3+) in the tumor tissue or peripheral blood in a variety of cancers, including HNSCC [23]. While some studies suggest that Treg accumulations in the tumor are associated with poor prognosis [25], others demonstrate that the elevated CD8+/Treg ratio correlates with prolonged survival [26]. Finally, the presence of dendritic cells (S-100, CD11c+) in the tumor was found to be associated with improved prognosis [27]. Accumulated evidence suggests tumor infiltrating immune cells are important for establishing prognosis.
In view of inconsistent literature reports, we considered the possibility that the tumor growth pattern could serve as a prognostic marker in patients with advanced HNSCC. In addition, we hypothesized that accumulations and nature of immune cells in the tumor were distinct in tumors with exophytic vs. endophytic growth patterns. If so, then the composition and/or frequency of immune infiltrates could explain in part the different morphology presented by these neoplasms. To the best of our knowledge, this is the first study reporting on the differences in the immune cell frequency and phenotype between exophytic and enodphytic HNSCC.
Patients and Methods
Patients
Patients with histologically confirmed, locally advanced HNSCC were seen in the the Otolaryngology University Clinic in Poznan, Poland between 2006-2009. All patients signed the informed consent. Ethics Committee at the University of Medical Sciences in Poznan approved the use of specimens for research purposes (#116/2006). Patients were examined by two physicians and the diagnosis of HNSCC was confirmed by pathology. After visual and microscopic examinations, 36 patients were found to have exophytic and 24 endophytic tumors. 54/60 tumors were localized in the larynx. Clinicopathologic and demographic data for the patients are listed in the Table 1. Tumor samples for immunohistochemistry (IHC) were available from 30 patients. The clinicopathologic data for this patient subgroup were similar to those for the entire patient cohort (not shown). All patients were routinely scheduled for postoperative RT which was initiated within 4-6 weeks after surgery. None were treated with chemotherapy. A median total dose of radiation (60 Gy) was applied in daily fractions of 2 Gy to the primary tumor and the pathologically involved neck region, using 6MV photons. Patients were seen every 3 months for the first 3 years after treatment and then every six months. The follow up included ENT physical examination and ultrasound of both neck sides performed at the time of each visit. The follow up time period ranged from 3 to 47 months. Response evaluation criteria in solid tumors (RECIST) were used for evaluation of the disease status.
Table 1.
Demographic and clinicopathological characteristics of the HNSCC patients enrolled in the study (n=60).
| Exophytic n=36 (60%) | Endophytic n=24 (40%) | |
|---|---|---|
| Median age at diagnosis | ||
| <60 yrs | 13(36%) | 11(46%) |
| >60 yrs | 23 (64%) | 13(54%) |
| Range | 48-75 | 47-78 |
| Gender | ||
| Male | 34 (94%) | 21(87%) |
| Female | 2 (6%) | 3 (13%) |
| Tumor stage | ||
| T3 | 19 (53%) | 16 (67%) |
| T4 | 17 (47%) | 8 (33%) |
| Tumor Grade | ||
| G2 | 29 (80%) | 19 (79%) |
| G3 | 7(20%) | 5 (21%) |
| Nodal status | ||
| N0 | 13 (36%) | 6 (25 %) |
| N1 | 10 (28%) | 5(21%) |
| N2 | 10 (28%) | 12 (54 %) |
| N3 | 3 (8%) | 0 |
| M status | ||
| M0 | 36(100%) | 23(96%) |
| M1 | 0 | 1(4%) |
| Recurrence | ||
| Yes | 11(30%) | 10(42%) |
| No | 25(70%) | 14 (58%) |
| Bacterial isolates | ||
| None | 10(27%) | 4(16%) |
| Gram - | 3 (8%) | 9(38%) |
| Gram + | 21 (58%) | 6 (25%) |
| not determined | 2(5%) | 5(21%) |
Histopathology
The tumor specimens were fixed in 4% formalin overnight, embedded in paraffin, sectioned, and stained with hematoxylin and eosin (H&E). For each tumor H&E sections were available to differentiate tumor nests from the tumor stroma, density of inflammatory infiltrates and the localization of immune cells in tumor vs. the tumor stroma. Stained sections of each tumor were examined by a pathologist. Among 36 exophytic tumors 24 were keratinizing and 12 non-keratinizing, while 24 endophytic tumors were equally divided into 12 keratinizing and 12 non-keratinizing types. The TNM staging categories were determined according to the AJCC/IUAC – (2000) - TNM Classification and Stage Grouping.
Microbiology
Smears for bacterial typing were taken from all patients during surgery. Bacterial flora in each tumor was determined in the Laboratory of Microbiology in Poznan, Poland using standard isolation and culture techniques.
Antibodies used and immunohistochemistry
Primary antibodies used for immunohistochemistry (IHC) are listed in Table 2. Secondary biotynylated antibodies were included in the LSAB2 kit (DAKO). IHC was performed by the avidin-biotin complex (ABC) method as recommended by the manufacturer. Working dilutions for primary antibodies were determined in preliminary titration experiments performed with sections of human tonsils or appendix. Appendix and tonsils were also used as control tissues. In negative controls staging with primary antibodies was omitted. The IHC procedure and controls used in our laboratory for staining of paraffin sections was previously described [28].
Table 2. Antibodies used for immunohistochemistry.
| Specificity of antibodies1 | Company | Dilution |
|---|---|---|
|
|
|
|
| CD3 (mouse) | DAKO | 1:100 |
| CD8 (mouse) | ABCAM | 1:50 |
| CD4 (mouse) | DAKO | 1:100 |
| CD25 (mouse) | ABCAM | 1:50 |
| CD11b (rabbit) | ABCAM | 1:100 |
| CD11c (rabbit) | ABCAM | 1:100 |
| S-100 (rabbit) | DAKO | 1:100 |
| FOXP3 (rat) | eBiosciences | 1:100 |
| TGF-β(rabbit) | ABCAM | 1:50 |
| IL-10 (rabbit) | ABCAM | 1:100 |
| HLA-DR(mouse) | ABCAM | 1:50 |
| Arginase-1(rabbit) | Santa Cruz | 1:50 |
| CD68 (mouse) | DAKO | 1:50 |
| CD34 (mouse) | ABCAM | 1:50 |
|
| ||
The species in which antibodies were made is given in parentheses.
Immunohistochemical analysis
Sections were microscopically evaluated for expression of the following markers on tumor infiltrating cells: CD3, CD4, CD8, CD25, CD11c, CD11b, FOXP3, CD34, S100, HLA DR, TGF-β, IL-10, Arginase-1 (Arg-1). Samples were examined by two independent investigators (M.H) and (G.D). Positive cells (≈650) were separately counted in the tumor stroma and in tumor nests at the mag. × 400, using five randomly selected high power fields (HPF), each encompassing an area of 0.2 mm2. Measurments were recorded as the number of positive cells per surface unit of tissue in mm2. Cell counts obtained by each investigator were pooled and mean values were calculated. The sections were scored as follows: (0) no staining; (+) less than 25% positive cells; (++) more than 50% positive cells; (+++) more than 75% positive cells.
Statistical analysis
Endophytic tumors were compared to exophytic tumors for clinical, pathologic and immune measurements by the Wilcoxon test. If the data were categorical, Fisher's exact test or the chi-square test was used. The patients' clinical data were analyzed and correlated with the four-year recurrence-free survival (RFS). The log rank test was used to examine survival differences. Correlations between expression of individual biomarkers as well as time RFS in patients with exophytic vs. endophytic tumors were analyzed by the Spearman's rank correlation test. Hierarchical clustering of immune measurements was conducted for exploratory analysis of differential growth pattern influences.
Results
The tumor growth pattern and disease
Figure 1 illustrates differences in appearance of tumors with exophytic vs. endophytic growth pattern. Demographic and cliniopathologic features of patients with exophytic vs. endophytic tumors were similar as listed in Table 1. Histologically, 70% of exophytic tumors were keratinizing vs. 50% of endophytic tumors (NSD). Although not significant, there was a trend toward higher N stages in endophytic tumors, with 76% of these cases presenting with nodal involvement vs. 64% of patients with exophytic tumors. The only potentially significant differences were seen in the type of bacterial flora in the tumor, as exophytic tumors tended to yield mostly Gram + bacterial isolates (i.e., streptococcus species) as shown in Table 3. In terms of symptoms such as hoarsness, otalgia and obstruction or pain when swallowing, the two cohorts were comparable (Table 4). At diagnosis, tumors were found in the neck in 68-71% of all cases, and the median time to seeking medical attention was 7 months for exophytic vs. 13 months for endophytic tumors (NSD).
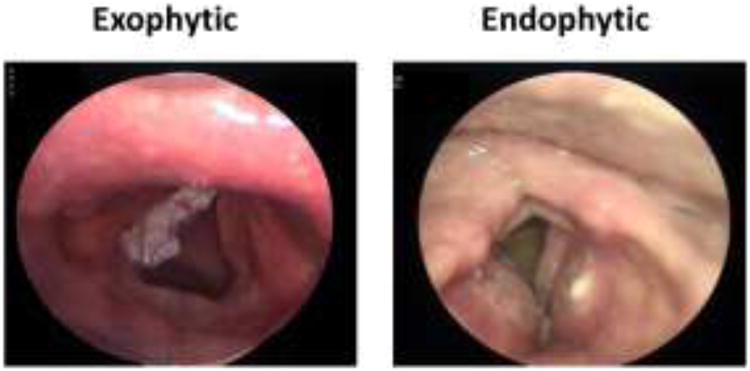
Fig. 1.
Comparison between exophytic and endophytic type of tumor growth in microlaryngoscopy modo Kleinsasser. Exophytic tumors grow out from the mucosal surface in cauliflower-like clusters, while endophytic tumors crawl under the mucosa, carpeting the inferior of the distinct local structure.
Table 3.
Characteristics of bacterial flora in tumors of the patients enrolled in this study (n=60).
| kind of bacteria: | Bacterial isolates | Exophytic n=36 | Endophytic n=24 |
|---|---|---|---|
| Gram negative | Pseudomonas aeruginosa, | +++ | |
| Prevotella bucae, Prevotella den. | + | ||
| Klebsiella oxytoca | + | ||
| Gemella morbillorum | ++ | ||
| Morganella morganii, Pseu.aerug. | ++ | ||
| Pasteurella multocida | + | + | |
| Enterobacter cloacae | + | ||
|
| |||
| Gram positive | Streptococcus sanguis | ++++++ | + |
| Staphylococcus aureus | ++++ | ++ | |
| Enterococcus gallinarum | ++ | + | |
| Streptococcus agalactie | +++++++ | ++ | |
| Enterococcus faecalis | ++ | ||
| Fungus | Candida albicans | ++++ | |
| None | ++++++++++ | ++++ | |
| Not determined | ++ | ||
Table 4.
Correlations between clinical symptoms at the time of diagnosis and the tumor growth pattern.
| Type of growth pattern | |||
|---|---|---|---|
|
|
|||
| Symptoms | |||
|
|
|||
| Exophytic n=36 | Endophytic n=24 | p value | |
|
|
|
|
|
| Palpable tumor in the neck | 23 (68%) | 17 (71%) | NSD |
| Hoarseness | 33 (92%) | 24 (100%) | |
| (duration in months) | (0-48) | (8-24) | NSD |
| Otalgia | 23 (65%) | 15 (62%) | |
| (duration in months) | (3-14) | (4-12) | NSD |
| Obstruction and pain when Swallowing | 15 (41%) | 19 (59%) | NSD |
| Average time to diagnosis | (0-40) | (0-48) | |
| Range in months | 7 | 13 | NSD |
|
| |||
The risk factors commonly associated with head and neck cancer development: age, sex, alcohol consumption and smoking were not different for patients with exophytic vs. endophytic tumors. Among the cohort of 60 patients, nearly all were males whose median age was 60 years. The percent of patients older or younger than 60 years was similar for both cohorts (Table 1). All the enrolled patients were smokers who used from 21 to 136 pack years at the time of diagnosis. All were alcohol abusers who consumed from 150g to 250g ethanol per week. The HPV status of the patients was not determined.
Head and neck tumors are frequently positive for bacterial flora, and in this cohort of patients, 66% of exophytic and 63% of endophytic tumors were positive for bacteria (Table 1). The former contained 3 × more Gram+ bacteria than the latter (Table 3). The bacterial isolates from exophytic tumors included a greater variety of Gram+ species and were positive for candida (Table 3).
The tumor growth pattern and prognosis
All 60 patients underwent surgical resection with a curative intent and were treated the SOC regimen of adjuvant radiation therapy. Among 54 patients with the known time to recurrence, 21 had tumor recurrence, all within 2 years, except for one patient who recurred at 40 months. The median follow up period for the 33 patients who were disease-free was 31 months after surgery (range 3-47 months). The tumor growth pattern was not associated with RFS (Figure 2).
Fig. 2.
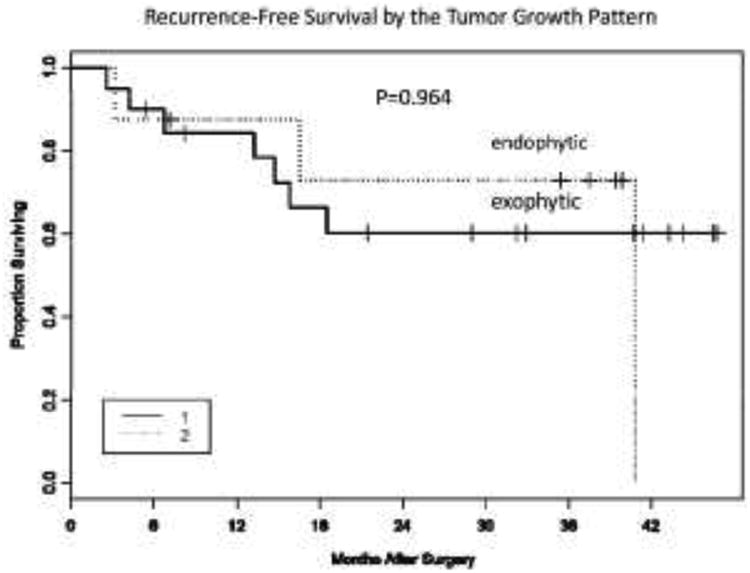
Recurrence-free survival by the tumor growth pattern. Kaplan-Meier curves were used to determine recurrence-free survival (RFS) of HNSCC patients (n=60) with exophytic vs. endophytic tumors.
Lymph node metastasis at the time of surgery proved to be a significant correlate of RFS for the entire cohort (p = .0295) as expected (Figure 3a). However, when we prepared Kaplan-Meier plots that cross the node status and the growth pattern, effects upon RFS appeared to be dictated by the node status and not the growth pattern (Figure 3b). Also, no significant differences in RFS were observed when exophytic vs. endophytic tumors were compared with respect to tumor size or differentiation or patient's sex and age (data not shown).
Fig. 3.
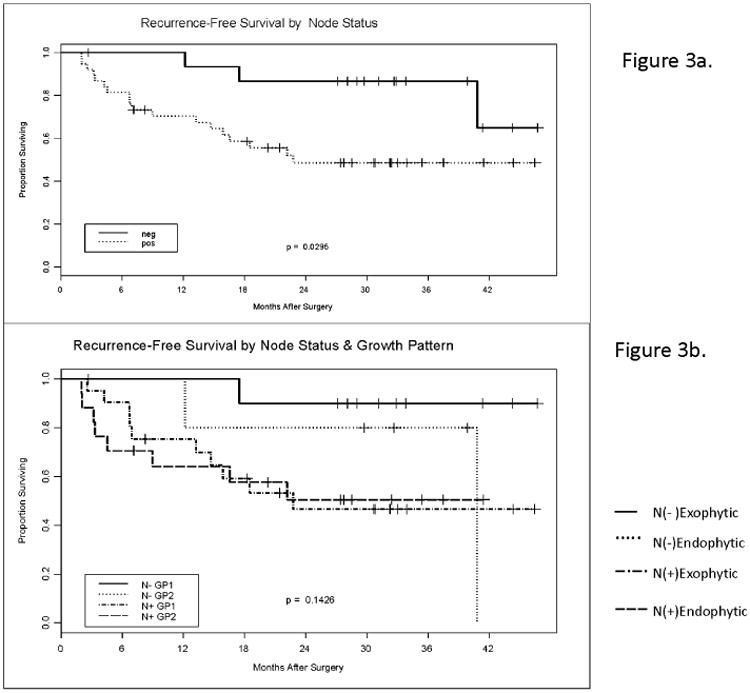
Recurrence-free survival by nodal involvement and the tumor growth pattern. (a) Kaplan-Meier curves for RFS of HNSCC patients (n=60) by their nodal status. (b) Kaplan-Meier curves for recurrence-free survival by the nodal status and the tumor growth pattern.
The tumor growth pattern and immune cell infiltrates
In a subset of 30 patients, who did not differ from the other half of the cohort in respect to any of the dermatographic or clinicopathologic characteristics, we performed immunohistochemistry on paraffin-embedded tumor specimens. An initial evaluation of H&E obtained sections revealed that endophytic tumors were characterized by more pronounced inflammatory infiltrates in the tumor stroma (Figure 4). These infiltrates in endophytic tumor stroma consisted largely of CD3+CD8+ T lymphocytes. Further, small blood vessels were more numerous in endophytic than exophytic tumors (Figure 4).
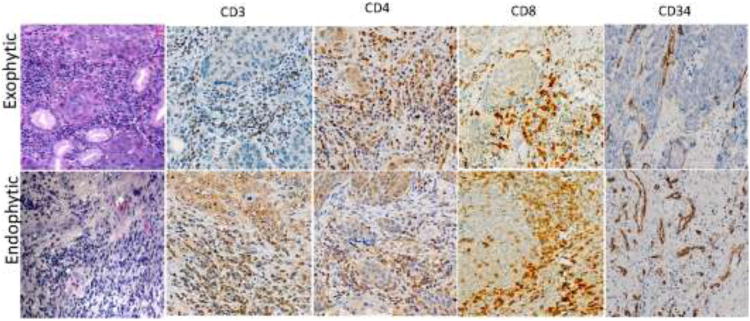
Fig. 4.
Immune infiltrates in exophytic vs. endophytic HNSCC. H&E staining and immunohistochemistry of paraffin embedded tissue sections of representative tumors at × 400 mag. In addition to inflammatory cells (CD3+, CD4+, CD8+), staining for CD34+ cells indicates localization of blood vessels.
Immune characteristics of exophytic vs. endophytic tumors
The IHC analysis of tumors obtained from a subgroup of 30 patients showed that immune cell infiltrates were more prominent in the tumor stroma than in the tumor nests (Figure 5a). Absolute numbers of immune cells positive for various markers were significantly higher in the tumor stroma than in tumor nests. CD3+CD8+ T lymphocytes many of which were CD25+ were more numerous in the stroma than in tumor nests. In contrast, fewer CD4+ T cells were present in these tumors and were equally distributed between the stroma and tumor nests. Of special interest, cells responsible for immune suppression (FOXP3+ Treg, CD11b+ myeloid cells, TGF-β+, IL-10+, Arg-1+) were a predominant component of infiltrates into the tumor stroma and tumor nests (Figure 5a) of these tumors. Relatively few CD11c+ dendritic cells (DC) were observed.
Fig. 5.
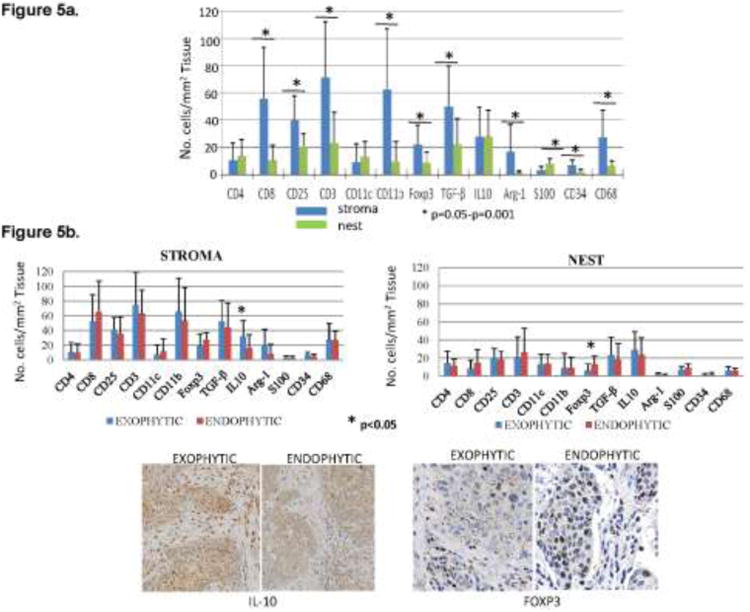
Inflammatory cells in the tumor stroma vs. tumor nests. (a) Numbers of immune cells positive for various surface markers in the tumor stroma and tumor nests (n=30). Asterisks indicate significant differences at p<0.05 to p<0.001. (b) Absolute numbers of immune cells positively stained for different markers in the stroma and nests in tumors with exophytic (n=22) and endophytic (n=8) tumors. On the right, representative tumor sections showing the presence of IL-10+ and FOXP3+ cells in the tumor stroma or tumor nests. Mag ×200 for IL-10+ and ×400 for FOXP3 staining.
Comparing exophytic with endophytic tumors by IHC for numbers of immune cells in the tumor stroma vs. tumor nests, we found no differences in the density and the cellular content of inflammatory infiltrates (Figure 5b). The only significant differences detected were in the absolute numbers of FOXP3+ and IL-10+ immune cells. The former were more numerous in the tumor nests of many, but not all, endophytic tumors (Figure 5b), and the latter in the stroma of exophytic tumors. There were other quantitative differences in the phenotype of infiltrating cells observed between the two tumor types, but because of substantial immunologic heterogeneity within this small group of specimens, none were statistically significant (Figure 5b).
Because both FOXP3+ transcription-factor and IL-10 are expressed by immunosuppressive cells in the tumor microenvironment, and because we observed that numbers of TGF-β+, Arg-1+ and CD11b+ immune cells were also elevated in some of the tumors, we next performed the hierarchical clustering analysis for exophytic and endophytic tumors (Figure 6). This analysis revealed no significant differences in clustering of cells bearing the immunosuppression-associated markers in the stroma or nests of tumors with exophytic vs. endophytic growth patterns.
Fig. 6.
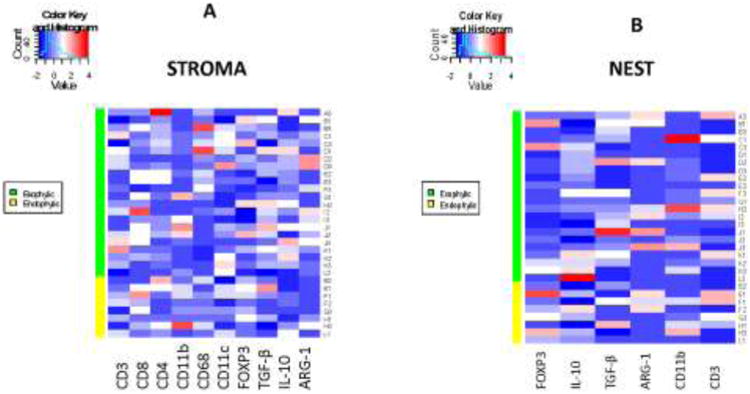
Hierarchal clustering for numbers of selected infiltrating immune cells found in the tumor stroma or tumor nests in exophytic and endophytic tumors.
Finally, despite the absence of significant evidence for qualitative and quantitative differences in tumor-infiltrating immune cells, we observed several interesting differences in tissue distribution of these cells. Specifically, as shown in Figure 7, there was a strong tendency for CD68+ macrophages to accumulate in tumor nests of exophytic tumors, while in endophytic, tumors these cells were largely found in the tumor stroma. In endophytic tumors, FOXP3+ cells were not only more numerous in tumor nests but were localized to the periphery of tumor nests, while in exophytic tumors, these FOXP3+ Treg were fewer and randomly scattered throughout the stroma (Figure 7). These differences in distribution within the tumors of immune cell subsets involved in the regulation of anti-tumor immune responses suggest that exophytic and endophytic tumors might differently modulate localization and perhaps functions of infiltrating immune cells.
Fig. 7.
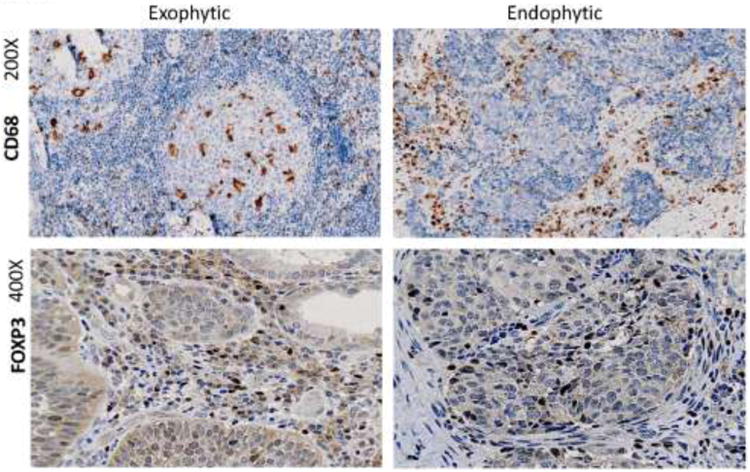
Localization of inflammatory cells in tumor tissues in exophytic and endophytic tumors. Representative immunohistochemistry staining of paraffin embedded tissue sections for CD68+ (mag ×200) and FOXP3+ cells (mag ×400).
Discussion
Although head and neck squamous cell carcinomas are considered to be a single histophathological entity, these carcinomas often behave differently. Not infrequently, tumors that have identical histophathology differ in their aggressiveness and clinical behavior [8, 29]. Differences in the tumor growth pattern or tumor anatomical location as well as the type and density of inflammatory infiltrates into the tumor might be some of the factors that determine its biologic behavior. The type of primary tumor growth pattern has been recognized as an important predictor for risk of nodal metastasis in some tumors, such as those of oral cavity, tongue and larynx [9, 10, 13]. Some of the earlier studies have suggested that endophytic tumors have worse prognosis than exophytic tumors [14, 30, 31]. Endophytic tumors tend to be diagnosed later than exophytic tumors, often are in a more advanced stage at the time treatment is initiated and thus have a worse prognosis. The goal of the present study was to evaluate the clinicopathologic characteristics of advanced-stage laryngeal carcinomas with the exophytic vs. endophytic growth pattern and relate the clinicopathologic data with DFS after radiation therapy. The two cohorts comprised patients with exophytic and endophytic tumors in an equal number. The risk factors, demographic, pathologic and clinical data as well as therapies received were comparable in these two groups. The follow-up after therapy and tumor recurrence were carefully documented. The Kaplan-Meir analysis showed no significant difference in DFS between the two groups. Thus, patients with endophytic tumors, who were diagnosed later than those with exophytic tumors, tended to recur earlier. They also tended to have more advanced nodal involvement, a poor prognostic factor in HNSCC [3]. However, with respect to tumor size or differentiation exophytic and endophytic tumors were comparable. Interestingly, in the exophytic group, tumors contained Gram + bacteria 3× more often than did endophytic tumors. This suggests that differences in the tumor microenvironment support distinct bacterial flora and thus contribute to molecular and metabolic pathways dominating in the tumor. Altogether, there were detectable differences in several characteristics between these tumors which, without reaching statistical significance, suggested to us that endophytic and exophytic tumors might represent biologically-distinct entities.
It is well accepted today that tumors can shape their environment and control their own survival. Tumor infiltrating immune cells are an important component of the tumor microenvironment [19, 32]. Not surprisingly, more invasive and less well-differentiated tumors have the potential to create a strongly immunosuppressive microenvironment which promotes the generation of inducible regulatory T cells (iTreg) and myeloid-derived suppressor cells (MDSC). Accumulations of anti-tumor effector T cells in the tumor have been linked to improved survival in several types of human cancer, including HNSCC [19, 32]. Phenotypic and functional features of these immune cells are thought to influence tumor growth and progression [33]. We, therefore, compared immune infiltrates present in endophytic and exophytic tumors, expecting to find differences that could explain differential growth patterns these tumors assume. Surprisingly, the differences in the density and phenotype of infiltrating cells, while present, were by and large not statistically significant with few exceptions. Thus, endophytic tumors were more intensely infiltrated by CD8+ cells, concentrated FOXP3+ Treg to the tumor nests and confined the localization of activated CD68+ macrophages to the tumor stroma. Exophytic tumors, in contrast, had numerous activated CD68+ macrophages in tumor nests, Treg localized almost entirely to the tumor stroma and increased numbers of IL-10+ cells infiltrating the stroma.
These distinct localization patterns, seen in the vast majority of tumor samples examined, support the conclusion that immune responses are regulated differently in tumors with exophytic vs. endophytic growth patterns. They also suggest that immunophenotypic features of these tumors might provide at least a partial explanation for differences in their biologic behavior. While the numbers of specimens compared in our study were relatively few, the immunologic data suggests a need for additional explorations of the interactions between the host immune system and the tumor growth pattern in patients with HNSCC.
Ethical standards. All human studies have been approved by the appropriate ethics committee and have therefore been perormed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. All subjects gave their informed consent prior to their inclusion in this study.
Acknowledgments
Supported in part by NIH grant PO1 CA109688 to TLW
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest and that the infomation is original and has not been submitted or published in any other journal.
References
- 1.Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Brockstein BE. Management of recurrent head and neck cancer: recent progress and future directions. Drugs. 2011;71:1551–1559. doi: 10.2165/11592540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Chin D, Boyle GM, Porceddu S, Theile DR, Parsons PG, Coman WB. Head and neck cancer: past, present and future. Exp Rev Anticancer Ther. 2006;6:1111–1118. doi: 10.1586/14737140.6.7.1111. [DOI] [PubMed] [Google Scholar]
- 5.Jakobsson PA, Eneroth CM, Killander D, Moberger G, Martensson B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol Ther Phys Biol. 1973;12:1–8. doi: 10.3109/02841867309131085. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz T, Hosal AS, Gedikoglu G, Kaya S. Prognostic significance of histopathological parameters in cancer of the larynx. Eur Arch Otorhinolaryngol. 1999;256:139–144. doi: 10.1007/s004050050127. [DOI] [PubMed] [Google Scholar]
- 7.Sotiriou C, Lothaire P, Dequanter D, Cardoso F, Awada A. Molecular profiling of head and neck tumors. Curr Opin Oncol. 2004;16:211–214. doi: 10.1097/00001622-200405000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Patel SG, Shah JP. TNM staging of cancers of the head and neck: striving for uniformity among diversity. CA Cancer J Clin. 2005;55:242–258. doi: 10.3322/canjclin.55.4.242. [DOI] [PubMed] [Google Scholar]
- 9.Spiro RH, Guillamondegui O, Jr, Paulino AF, Huvos AG. Pattern of invasion and margin assessment in patients with oral tongue cancer. Head Neck. 1999;21:408–413. doi: 10.1002/(sici)1097-0347(199908)21:5<408::aid-hed5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Hussein MR, Cullen K. Molecular biomarkers in HNSCC: prognostic and therapeutic implications. Expert Rev Anticancer Ther. 2001;1:116–124. doi: 10.1586/14737140.1.1.116. [DOI] [PubMed] [Google Scholar]
- 11.Yilmaz T, Gedikoglu G, Gursel B. The relationship between tumor thickness and clinical and histopathologic parameters in cancer of the larynx. Otolaryngol Head Neck Surg. 2003;129:192–198. doi: 10.1016/S0194-5998(03)00712-5. [DOI] [PubMed] [Google Scholar]
- 12.Boheim K, Boheim C, Rauchegger H. Induction chemotherapy with cis-platinum in head and neck tumors. First clinical and histopathologic findings. Arch Otorhinolaryngol. 1981;233:31–40. [PubMed] [Google Scholar]
- 13.Pedruzzi PA, Kowalski LP, Nishimoto IN, Oliveira BV, Tironi F, Ramos GH. Analysis of prognostic factors in patients with oropharyngeal squamous cell carcinoma treated with radiotherapy alone or in combination with systemic chemotherapy. Arch Otolaryngol Head Neck Surg. 2008;134:1196–1204. doi: 10.1001/archotol.134.11.1196. [DOI] [PubMed] [Google Scholar]
- 14.Shintani S, Matsuura H, Hasegawa Y, Nakayama B, Fujimoto Y. The relationship of shape of tumor invasion to depth of invasion and cervical lymph node metastasis in squamous cell carcinoma of the tongue. Oncology. 1997;54:463–467. doi: 10.1159/000227604. [DOI] [PubMed] [Google Scholar]
- 15.Kurokawa H, Yamashita Y, Murata T, Yoshikawa T, Tokudome S, Miura K, Kajiyama M. Histological grading of malignancy correlates with regional lymph node metastasis and survival of patients with oral squamous cell carcinoma. Fukuoka Igaku Zasshi. 1998;89:225–231. [PubMed] [Google Scholar]
- 16.Nigauri T, Kamata SE, Kawabata K, Nakamizo M, Hoki K, Mitani H, Nagahashi T, Yokoshima K, Yoshimoto S. Prognostic factors of lateral wall oropharyngeal squamous cell carcinoma--retrospective study of 79 patients. Nihon Jibiinkoka Gakkai Kaiho. 1996;99:1190–1199. doi: 10.3950/jibiinkoka.99.1190. [DOI] [PubMed] [Google Scholar]
- 17.Whiteside TL. Medical Intelligence Unit. R.G. Landes Co; Austin, TX: 1993. Tumor-infiltrating lymphocytes in human malignancies. [Google Scholar]
- 18.Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 19.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 20.Distel LV, Fickenscher R, Dietel K, Hung A, Iro H, Zenk J, Nkenke E, Buttner M, Niedobitek G, Grabenbauer GG. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009;45:e167–e174. doi: 10.1016/j.oraloncology.2009.05.640. [DOI] [PubMed] [Google Scholar]
- 21.Whiteside TL. Immunobiology and immunotherapy of head and neck cancer. Curr Oncol Rep. 2001;3:46–55. doi: 10.1007/s11912-001-0042-3. [DOI] [PubMed] [Google Scholar]
- 22.Whiteside TL. Tumor-induced death of immune cells: its mechanisms and consequences. Sem Cancer Biol. 2002;12:43–50. doi: 10.1006/scbi.2001.0402. [DOI] [PubMed] [Google Scholar]
- 23.Strauss L, Bergmann C, Gooding W, Johnson JT, Whiteside TL. The frequency and suppressor function of CD4+CD25highFoxp3+ T cells in the peripheral circulation of patients with squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13:6301–6311. doi: 10.1158/1078-0432.CCR-07-1403. [DOI] [PubMed] [Google Scholar]
- 24.Ferrone S, Whiteside TL. Tumor microenvironment and immune escape. Surg Oncol Clin N Am. 2007;16:755–774. doi: 10.1016/j.soc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 26.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 27.Reichert TE, Scheuer C, Day R, Wagner W, Whiteside TL. The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer. 2001;91:2136–2147. [PubMed] [Google Scholar]
- 28.Szczepanski MJ, Czystowska M, Szajnik M, Harasymczuk M, Boyiadzis M, Kruk-Zagajewska A, Szyfter W, Zeromski J, Whiteside TL. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–3113. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amin MB, Gomez JA, Young RH. Urothelial transitional cell carcinoma with endophytic growth patterns: a discussion of patterns of invasion and problems associated with assessment of invasion in 18 cases. Am J Surg Pathol. 1997;21:1057–1068. doi: 10.1097/00000478-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Stoliarov VI, Kolosov AE, Dovgaliuk AZ. The prognostic significance of the anatomical forms of esophageal cancer. Vopr Onkol. 1990;36:1067–1071. [PubMed] [Google Scholar]
- 31.Steinhart H, Kleinsasser O. Growth and spread of squamous cell carcinoma of the floor of the mouth. Eur Arch Otorhinolaryngol. 1993;250:358–361. doi: 10.1007/BF00188386. [DOI] [PubMed] [Google Scholar]
- 32.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292–302. doi: 10.1186/1471-2407-9-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whiteside TL, Parmiani G. Tumor-infiltrating lymphocytes: their phenotype, functions and clinical use. Cancer Immunol Immunother. 1994;39:15–21. doi: 10.1007/BF01517175. [DOI] [PMC free article] [PubMed] [Google Scholar]