Abstract
Background
Atrial fibrillation (AF) guideline recommendations for antiarrhythmic drugs (AADs) are based upon the effectiveness and safety of the AAD in patients with selected, concomitant heart disease. It is unknown to what extent these recommendations are being implemented in clinical practice.
Methods
Using commercial health claims, patients with AF were identified and then categorized into mutually exclusive, guideline-established subgroups based upon their most serious concurrent heart disease: heart failure, coronary artery disease (CAD), hypertension, no heart disease. AAD use following the first AF encounter and the identified concurrent heart disease encounter was determined from prescription claims, and this was compared with guideline recommendations.
Results
From January 2006 through December 2010, a total of 331,274 AF patients aged <65 years were identified: 18% heart failure, 23% CAD, 33% hypertension, and 25% no heart disease. Of these, 78,877 (24%) patients filled ≥1 qualifying AAD prescription. The median age was 57 years (interquartile range 52, 61), and 69% were male. A total of 74,191 patients had AADs after both the AF and concurrent heart disease encounters: 27% with heart failure, 25% with CAD, 21% with hypertension, and 19% with no heart disease. In the heart failure and CAD subgroups, 45% and 31% of AADs were inconsistent with first- or second-line guideline recommendations, respectively.
Conclusion
More than one-third of the AADs used in AF patients with CAD or heart failure did not conform to guideline recommendations. This highlights the potential need for increased clinician education and intervention to improve the safe use of AADs for AF management.
Keywords: atrial fibrillation, antiarrhythmic drugs, evidence-based medicine, clinical practice guidelines, adherence
Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia that affects up to 6.1 million people in the United States, with estimates of AF prevalence increasing to 12 million by 2050.1,2 One method for maintaining sinus rhythm and reducing recurrence of AF is the use of antiarrhythmic drugs (AADs). These drugs can be harmful if not used appropriately; therefore, clinical practice guidelines were developed to provide specific, evidence-based recommendations to improve the safe and effective use of these drugs. For maintenance of sinus rhythm, AAD selection recommendations are based upon whether a patient has concomitant heart failure, coronary artery disease (CAD), or hypertension because adverse events, especially proarrhythmias, can vary by extent and type of heart disease.3,4 Little is known about how well these AAD recommendations are incorporated into clinical practice.
The purpose of this study was to determine the frequency of use of different AADs in patients with AF overall and within clinical practice guideline subsets of patients with heart failure, CAD, and hypertension and without heart disease. Conformity to clinical practice guideline recommendations was determined.
Methods
Data source
The Thomas Reuters MarketScan® Commercial Claims and Encounters Database was used to identify the study cohort. This database consists of inpatient, outpatient, and prescription claims data from U.S. employers and health plans for employees and their spouses and dependents. The MarketScan® databases have been used for more than 450 publications of health care utilization and outcomes in a variety of diseases, including atrial fibrillation.5–7 Data from all patients with an inpatient or outpatient encounter that included a diagnosis of AF (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9] code 427.31) between January 1, 2006 and December 31, 2010 were obtained. The database does not include Medicare claims data, and therefore, does not include patients aged ≥65 years.
Selection of overall study cohort
For this study, only patients with individual-level and pharmacy benefit data were included. The first inpatient or outpatient encounter with a diagnosis of AF was identified and considered the index AF encounter. If the first AF encounter was 30 days before or after a cardiothoracic surgical procedure (ICD-9 codes 35.x to 39.x), then a subsequent AF encounter (if present) was used, provided that it was not within 30 days of another cardiothoracic surgical procedure. Patients aged <30 years at the time of the index AF encounter were excluded due to low incidence of AF in these patients without congenital heart disease; also, patients with ventricular arrhythmias diagnosed at any time were excluded due to use of AADs outside of AF guideline recommendations in these patients (ICD-9 codes 427.1x, 427.41, 427.42, and 427.5x).
Selection of subgroups
Subgroups were determined using the American Heart Association/American College of Cardiology/Heart Rhythm Society clinical practice guidelines in which specific AAD recommendations are based upon the presence of heart failure (regardless of the left ventricular ejection fraction), CAD (with or without prior myocardial infarction), hypertension (with and without substantial left ventricular hypertrophy [LVH]), or none of these.3 According to the guideline recommendations, for patients with more than one of these coexisting diseases, clinicians should select AADs based upon the most serious condition (heart failure > CAD > hypertension with LVH > hypertension without LVH).3 Therefore, in this study, subgroups were identified by first searching for any prior, concomitant, or subsequent inpatient or outpatient encounter with a diagnosis of heart failure (ICD-9 codes 428.xx, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 398.91) or cardiomyopathy (ICD-9 codes 425.0, 425.1, 425.2, 425.3, 425.5, 425.7, 425.8, and 425.9).8 If a patient was ever diagnosed with heart failure, that patient was assigned to the heart failure subgroup. Also, if the date of that diagnosis was after the AF index encounter, then it became the subgroup index date. If the diagnosis date was before the AF index encounter, then the AF index date was used as the subgroup index date.
For the remaining patients (those without a heart failure diagnosis), the same process was applied for CAD diagnosis (ICD-9 codes 410–414 and 429.2) and then for hypertension diagnosis (ICD-9 codes 402.00, 402.10, 402.90, and any prior hypertension code along with 429.3, 401.x, 403.xx, 404.00, 404.02, 404.10, 404.12, 404.90, 404.92, 405.xx, and 437.2).8 For patients with hypertension, an attempt was made to differentiate between those with and without left ventricular hypertrophy; however, due to the lack of specific and reliable claims codes for this condition, we could not reliably classify patients into the 2 guideline-recommended subcategories of hypertension.
All remaining patients (those without heart failure, CAD, or hypertension) were classified as not having any of the selected heart diseases, and the AF index encounter date remained their subgroup index date.
Selection of AADs of interest
National Drug Codes (NDCs) for oral formulations of Vaughan Williams Class Ia (quinidine, procainamide, disopyramide), Class Ic (propafenone, flecainide), and Class III (amiodarone, dofetilide, sotalol, and dronedarone) AADs that may be used for the treatment of AF were identified using the 2010 Thomas Reuters RED BOOK®. AAD use was defined by prescription claims for ≥30-day supplies of all selected AADs at any time after the index AF encounter. Prescription claims for ≥30-day supplies of selected AADs at any time after the index dates for heart failure, CAD, hypertension, and no heart disease subgroups were also identified.
Statistical analysis
From the overall study cohort, characteristics of patients taking one or more AADs during the study period were compared with those of patients not taking AADs. Characteristics for comparison included age, sex, geographic region, type of health plan, inpatient versus outpatient index AF encounter, year of AF index encounter, and concomitant atrial flutter. Categorical variables were compared using a chi-square test, and continuous variables were compared using the Wilcoxon rank sum test. The number of different AADs used by each patient during the study period was determined. Trends in use by calendar quarter overall and by age subgroups (patients aged <60 years versus 60–64 years) were determined and were graphically displayed.
From the overall study cohort, the number of patients in each of the 4 subgroups of interest was also determined, and characteristics of these patients were described. The number and proportions of patients taking one or more AADs during the study period were determined for each subgroup. In addition, the number and proportions of AADs used were determined by subgroup. Trends in use by calendar quarter were determined and were graphically displayed. Conformity to guideline recommendations within each subgroup was determined by calculating the proportion of AADs that were used and that were also included as first-line or second-line choices in the guideline recommendations for that subgroup.
Study Funding
This work was funded in part through grant KM1 CA156687 from the National Institutes of Health. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
A total of 331,274 patients with an inpatient or outpatient diagnosis of AF met all of the inclusion criteria and none of the exclusion criteria. Of these, a total of 78,877 (24%) patients had a prescription claim for a ≥30-day supply of one or more AADs after their index AF encounter. During the median follow-up of 465 (interquartile range [IQR] 211, 877) days, most patients (86%) received only one AAD, 12% received 2, and 2% received 3 or more. The median time from AF encounter to first prescription claim for an AAD was 29 (IQR 9, 89) days. Overall, the use of Class Ia AADs occurred in only 0.4% of patients. Given the low usage, these drugs were not presented in results, tables, or figures. Of the 78,877 patients identified with an AAD, 71,286 (90%) had ≥1 subsequent AF encounter. Characteristics of patients with and without AADs are presented in Table I. Compared with patients who did not receive AADs, patients who received AADs more often were men, were from the South or North Central United States, were inpatients, and had concomitant atrial flutter.
Table I.
Characteristics of the Overall Population
| Characteristic | No AAD (n=252,397) | AAD (n=78,877) | P-value |
|---|---|---|---|
| Age, yrs, median (IQR) | 56 (50, 61) | 57 (52, 61) | <0.001 |
| Sex | <0.001 | ||
| Male | 156,838 (62) | 54,547(69) | |
| Female | 95,559 (38) | 24,330 (31) | |
| Region | <0.001 | ||
| Northeast | 43,981 (17) | 8984 (11) | |
| North Central | 70,589 (28) | 23,934 (30) | |
| South | 94,558 (38) | 32,440 (41) | |
| West | 39,216 (16) | 12,221 (16) | |
| Unknown | 4053 (2) | 1298 (2) | |
| Type of health plan | <0.001 | ||
| HMO | 40,348 (17) | 11,835 (15) | |
| PPO | 157,824 (64) | 50,359 (66) | |
| POS | 20,731 (9) | 6917 (9) | |
| Other | 26,188 (11) | 7810 (10) | |
| Index AF encounter | <0.001 | ||
| Inpatient | 43,061 (17) | 15,874 (20) | |
| Outpatient | 209,336 (83) | 63,003 (80) | |
| Year of index AF encounter | <0.001 | ||
| 2006 | 45,918 (18) | 20,147 (26) | |
| 2007 | 35,844 (14) | 12,103 (15) | |
| 2008 | 59,738 (24) | 18,573 (24) | |
| 2009 | 57,233 (23) | 15,488 (20) | |
| 2010 | 53,664 (21) | 12,566 (16) | |
| Atrial flutter before/during index AF encounter | 7557 (3) | 4723 (6) | <0.001 |
Data are presented as n (%) unless otherwise specified.
AAD indicates antiarrhythmic drug; AF, atrial fibrillation; HMO, health maintenance organization; IQR, interquartile range; POS, point of service; PPO, preferred provider organization.
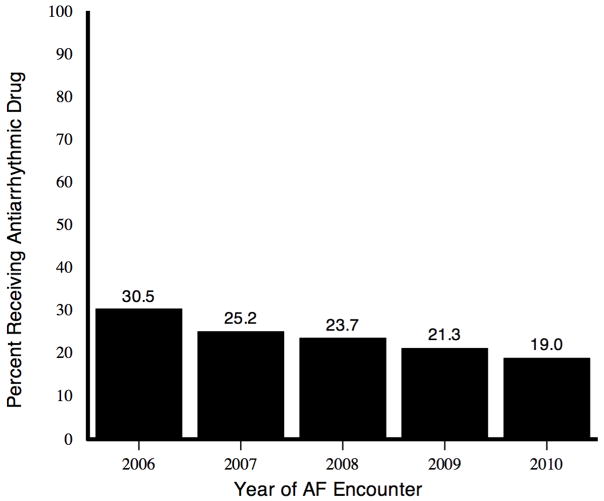
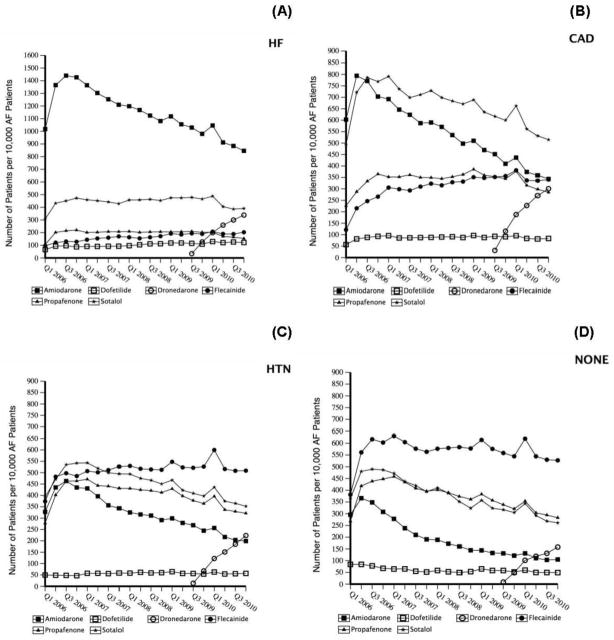
The proportion of AF patients identified in each calendar year who subsequently received an AAD decreased from 2006 through 2010 (Figure 1). Likewise, the overall number of patients receiving AADs per 10,000 patients with AF per calendar quarter decreased from 2006 through 2010, with sotalol and flecainide use surpassing amiodarone use in 2008 and 2009, respectively (Figure 2A). Trends in AAD use were different among those aged <60 years compared with those aged 60–64 years (Figures 2B and 2C) at their index AF encounter.
Figure 1. Proportion of patients identified with AF in each calendar year who subsequently received one or more AADs.
AAD indicates antiarrhythmic drug; AF, atrial fibrillation.
Figure 2. Trends in AAD use in overall AF population and by age subgroups.
A. Number of patients taking AADs during each calendar quarter per 10,000 patients with AF diagnosis.
B. Number of patients aged <60 years taking AADs during each calendar quarter per 10,000 patients aged <60 years with AF diagnosis.
C. Number of patients aged 60–64 years taking AADs during each calendar quarter per 10,000 patients aged 60–64 years with AF diagnosis.
AF indicates atrial fibrillation; Q, quarter.
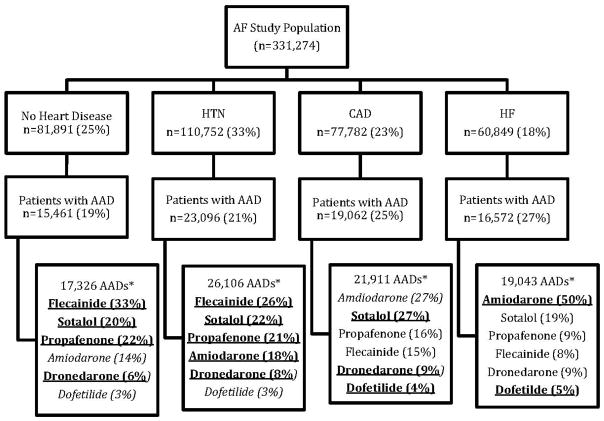
After dividing the study population into subgroups based upon inpatient or outpatient diagnoses for comorbidities (heart failure, CAD, hypertension, or none of these) that are specified in clinical practice guidelines to guide AAD selection, a total of 74,191 patients (22%) had an outpatient prescription claim for a ≥30-day supply of one or more AADs after the first subgroup index encounter date. The distribution of patients into these subgroups is shown in Figure 3. Characteristics of patients within each subgroup are presented in Table II.
Figure 3. AAD use by guideline-specified subgroups.
*Underlined/bolded AAD drugs indicate first-line, guideline-recommended drugs.4 Italicized AAD drugs indicate second-line, guideline-recommended drugs.4 Patients could have taken one or more AADs (successively, not simultaneously).
AAD indicates antiarrhythmic drug; AF, atrial fibrillation; CAD, coronary artery disease; HF, heart failure; HTN, hypertension.
Table II.
Characteristics of Patients Receiving AADs within Subgroups
| Characteristic | Heart Failure (n=16,572) | CAD (n=19,062) | Hypertension (n=23,096) | No Heart Disease (n=15,461) |
|---|---|---|---|---|
| Age, yrs, median (IQR) | 58 (53, 62) | 59 (54, 62) | 57 (52, 61) | 55 (47, 60) |
| Sex | ||||
| Male | 11,391 (69) | 14,096 (74) | 15,172 (66) | 10,579 (68) |
| Female | 5181 (31) | 4966 (26) | 7924 (34) | 4882 (32) |
| Region | ||||
| Northeast | 1775 (11) | 2280 (12) | 2553 (11) | 1805 (12) |
| North Central | 5091 (31) | 5868 (31) | 6973 (30) | 4672 (30) |
| South | 7073 (43) | 8293 (44) | 9518 (1) | 5690 (37) |
| West | 2373 (14) | 2356 (12) | 3663 (16) | 3068 (20) |
| Unknown | 260 (2) | 265 (1) | 389 (2) | 226 (2) |
| Type of health plan | ||||
| HMO | 2443 (15) | 2593 (14) | 3404 (15) | 2536 (17) |
| PPO | 10,647 (66) | 12,368 (67) | 14,944 (66) | 9662 (64) |
| POS | 1393 (9) | 1679 (9) | 1987 (9) | 1362 (9) |
| Other | 1704 (11) | 1968 (11) | 2177 (10) | 1485 (10) |
| Index AF encounter | ||||
| Inpatient | 5980 (36) | 4070 (21) | 4113 (18) | 1917 (12) |
| Outpatient | 10,592 (64) | 14,992 (79) | 18,983 (82) | 13,544 (88) |
| Year of index AF encounter | ||||
| 2006 | 2576 (16) | 3398 (18) | 3792 (16) | 3920 (25) |
| 2007 | 2652 (16) | 3170 (17) | 3657 (16) | 2151 (14) |
| 2008 | 3831 (23) | 4363 (23) | 5434 (24) | 3510 (23) |
| 2009 | 4134 (25) | 4467 (23) | 5418 (24) | 2963 (19) |
| 2010 | 3379 (20) | 3664 (19) | 4795 (21) | 2917 (19) |
| Time from index date to first AAD prescription fill, median number of days (IQR) | 25 (8, 71) | 25 (8, 69) | 26 (8, 74) | 26 (8, 72) |
| Proportion of days with any AAD following index date, median (IQR) | 53% (18, 96) | 64% (20, 99) | 71% (24, 100) | 67% (21, 99) |
| Proportion of days with initial AAD following index date, median (IQR) | 47% (16 94) | 57% (17, 98) | 64% (20, 99) | 61% (18 99) |
| Prior prescription for the initial AAD | 7293 (44) | 9404 (49) | 10,748 (47) | 6164 (40) |
Data are presented as n (%) unless otherwise specified.
AAD indicates antiarrhythmic drug; AF, atrial fibrillation; CAD, coronary artery disease; HMO, health maintenance organization; IQR, interquartile range; POS, point of service; PPO, preferred provider organization.
AF and heart failure
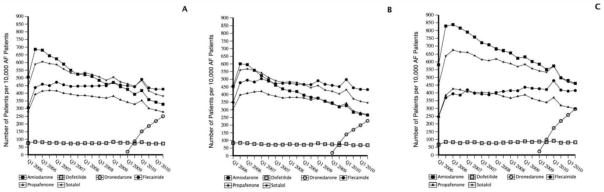
Of the 60,849 patients with atrial fibrillation and heart failure, 16,572 (27%) received a total of 19,043 AADs. Only 55% of the AAD use in this subgroup conformed to guideline recommendations (specifically, 50% of AADs were amiodarone and 5% of AADs were dofetilide). The use of sotalol (19%), dronedarone (9%), propafenone (9%), and flecainide (8%) does not conform to guideline recommendations. AAD use by drug and calendar quarter is shown in Figure 4A.
Figure 4. Number of patients taking AADs per 10,000 AF patients with coexisting disease.
AAD indicates antiarrhythmic drug; AF, atrial fibrillation; CAD, coronary artery disease; HF, heart failure; HTN, hypertension; Q, quarter; NONE, none of the specified heart diseases.
AF and CAD
Of the 77,782 patients with AF and CAD, 19,062 (25%) received a total of 21,911 AADs. Only 40% of the AAD use conformed to first-line guideline recommendations (27% sotalol, 9% dronedarone, and 4% dofetilide). Amiodarone, a second-line recommendation, accounted for 27% of the AADs used. Propafenone (16%) and flecainide (15%), which are not recommended in patients with CAD, accounted for 31% of the AAD use. AAD use by drug and calendar quarter is shown in Figure 4B. A total of 5070 (27%) of the patients identified in this subgroup had a myocardial infarction as their qualifying CAD diagnosis for inclusion in the group. Of these, 294 (6%) received propafenone or flecainide.
AF and hypertension
Of the 110,752 patients with AF and hypertension, 23,096 (21%) received a total of 26,106 AADs. Of these patients, only 4.5% had one or more coded claims indicating the presence of LVH. The most frequently used AADs were flecainide (26%), sotalol (22%), propafenone (21%), and amiodarone (18%). All of these high-use AADs are consistent with clinical practice guideline recommendations in the absence of definitive information on the presence of LVH. AAD use by drug and calendar quarter is shown in Figure 4C.
AF without heart failure, CAD, or hypertension
A total of 81,891 patients with AF did not have heart failure, CAD, or hypertension. Of these patients, 15,461 (19%) received 17,326 AADs. The most frequently used drugs were flecainide (33%), propafenone (22%), and sotalol (20%), which are all acceptable according to clinical practice guidelines. A total of 23% of AADs were non–guideline-recommended, first-line AADs (amiodarone in 14% and dofetilide in 3%). AAD use by drug and calendar quarter is shown in Figure 4D.
Discussion
Given the potential risks of AADs, clinical practice guidelines for AF management were developed to provide clinicians with AAD recommendations that minimize the potential risk. However, little is known about AAD use in clinical practice in the United States and whether it conforms to guideline recommendations. In this study, we found that 45% of AAD use in patients with concomitant heart failure and 31% of AAD use in patients with CAD did not conform to first- or second-line guideline recommendations. While this study only provides a snapshot of AAD use based upon claims data, the high rate of nonconformity is concerning. Additional research is needed to better understand the reasons for this lack of conformity to guideline recommendations.
For AF management, much of the recent focus has been on evaluating and comparing a rate- versus rhythm-control strategy, with relatively little attention being given to evaluating or comparing the specific drugs used in those strategies.9–13 Clinical practice guidelines for AF management provide AAD recommendations based upon concomitant heart disease (heart failure, CAD, or hypertension) because the risks of some AADs may outweigh potential benefits in patients with structural heart disease.3,4 For example, several AADs, such as Class Ic drugs and dronedarone, can worsen heart failure and/or have been shown to increase the risk of adverse effects or poor outcomes in patients with heart failure.3,14 Also, Class Ic drugs have been shown to have a higher risk of potentially life-threatening proarrhythmias in patients with CAD and are considered contraindicated in these patients.15,16 Therefore, there are fewer AAD options for patients with heart failure or CAD.
In applying the guideline recommendations to clinical practice, clinicians are instructed to select an AAD from those recommended for the most severe type of concomitant heart disease that is present (heart failure > CAD > hypertension). For example, a patient with heart failure and hypertension would only be eligible for those AADs recommended for a patient with heart failure. In this study, we applied this hierarchical process and categorized patients by their most severe type of concomitant heart disease to mimic clinical practice. We then looked at the actual AADs that were prescribed and compared them with the recommended AADs for each subgroup. As expected, there was little discrepancy between clinical practice and guideline recommendations in patients with no structural heart disease because these patients’ AAD options are more extensive. It was only within the heart failure and CAD subgroups that we found extensive AAD use that was not recommended by the guidelines.
From the published results of 7 AF registries, 21% to 48% of patients with AF received AADs at enrollment or during the first year after enrollment.17–25 The types of AADs that were used varied depending upon the years of enrollment and the types of patients with AF that were included. In these studies, little information was found regarding adherence to guideline recommendations in the selection of AADs. One study in Germany reported that <0.2% of patients with left ventricular dysfunction or severe CAD received Class Ic AADs.22 One study in the United States and Canada reported that use of Class Ic drugs was less likely in patients with heart failure and CAD.25 One non-US, worldwide study reported that 20% of patients with structural heart disease received Class Ic drugs.17 In addition, 3 studies were identified that looked at AAD prescription patterns in the United States in the 1990s and 2000s; these studies showed that 10% to 13% of patients with AF received AADs. Drug use in subgroups of patients with other heart diseases was not assessed.26–28 In one of these studies, Class Ic drugs were the most commonly prescribed AADs in patients who were aged <60 years, indicating that these drugs may be preferred in younger patients with seemingly less concomitant heart disease.26 Therefore, the results of our study provide the most detailed and contemporary picture of current AAD use in younger AF patients in the United States and have identified several areas of concern that warrant further investigation to improve care.
Our study is also the first to include a snapshot of the use of dronedarone in the United States. Dronedarone was introduced to the market in the third quarter of 2009. We found a substantial uptake in dronedarone use through the end of our study period in all subgroups. Despite being available for <30% of the study period, dronedarone accounted for 9% of all AAD use in patients with heart failure, 9% of AAD use in patients with CAD, 8% of AAD use in patients with hypertension, and 6% of AAD use in patients without any of these selected cardiac diseases. Because our study period ended in November 2010—before increasing reports of dronedarone’s safety concerns and publication of the results from the Permanent Atrial Fibrillation Outcome Study Using Dronedarone on Top of Standard Therapy (PALLAS) trial—additional research is needed to determine whether use of dronedarone, including potentially inappropriate use, continues.29,30
There are several limitations to this study. This study relies on the use of coded diagnoses during inpatient and outpatient encounters and prescription claims rather than confirmed diagnoses from clinicians or confirmed medication adherence from patients. There may have been mitigating factors not captured by claims data that resulted in the selection of a drug that did not conform to guideline recommendations, including incorrectly coded diagnosis, contraindications to or prior failure of all other AADs, patient preferences, and ambiguity in the guideline recommendations as to the specific definition of heart failure or CAD. Physicians must consider numerous factors in drug selection, and many of those factors were unavailable in this study, which prevented us from assessing potential reasons for selection of drugs that were not recommended by the clinical practice guidelines. Lastly, in patients with hypertension, we were unable to differentiate between those with or without LVH. Guideline-recommended AADs differ between these 2 subgroups, and as such, there may have been patients who received non–guideline-conforming AADs that we were unable to identify.
Conclusion
More than one-third of AF patients with CAD or heart failure received an AAD that did not conform to guideline recommendations. This extensive use of potentially inappropriate AADs highlights the need for more detailed analyses of AAD drug selection, especially in patients with concomitant heart failure and CAD, and the potential need for increased clinician education and intervention to improve the safe use of AAD therapy for AF management.
Footnotes
Disclosures
NM Allen LaPointe, GD Sanders, ED Peterson, and SM Al-Khatib: All disclosures are listed at https://www.dcri.org/about-us/conflict-of-interest
Y Lokhnygina: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114(2):119–25. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 3.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123(10):e269–367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 4.Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (Updating the 2006 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57(2):223–42. doi: 10.1016/j.jacc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Naccarelli GV, Johnston SS, Dalal M, et al. Rates and implications for hospitalization of patients ≥65 years of age with atrial fibrillation/flutter. Am J Cardiol. 2012;109(4):543–9. doi: 10.1016/j.amjcard.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds MR, Gunnarsson CL, Hunter TD, et al. Health outcomes with catheter ablation or antiarrhythmic drug therapy in atrial fibrillation: results of a propensity-matched analysis. Circ Cardiovasc Qual Outcomes. 2012;5(2):171–81. doi: 10.1161/CIRCOUTCOMES.111.963108. [DOI] [PubMed] [Google Scholar]
- 7.Truven Health Analytics website. [Accessed July 11, 2013];Databases and Online Tools. http://www.truvenhealth.com/your_healthcare_focus/pharmaceutical_and_medical_device/data_databases_and_online_tools.aspx.
- 8.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–9. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 9.Ionescu-Ittu R, Abrahamowicz M, Jackevicius CA, et al. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch Intern Med. 2012;172(13):997–1004. doi: 10.1001/archinternmed.2012.2266. [DOI] [PubMed] [Google Scholar]
- 10.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358(25):2667–77. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 11.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–33. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 12.Allen Lapointe NM, Sun JL, Kaplan S, et al. Rhythm versus rate control in the contemporary management of atrial fibrillation in-hospital. Am J Cardiol. 2008;101(8):1134–41. doi: 10.1016/j.amjcard.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 13.AFFIRM First Antiarrhythmic Drug Substudy Investigators. Maintenance of sinus rhythm in patients with atrial fibrillation: an AFFIRM substudy of the first antiarrhythmic drug. J Am Coll Cardiol. 2003;42(1):20–9. doi: 10.1016/s0735-1097(03)00559-x. [DOI] [PubMed] [Google Scholar]
- 14.Kober L, Torp-Pedersen C, McMurray JJ, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–87. doi: 10.1056/NEJMoa0800456. [DOI] [PubMed] [Google Scholar]
- 15.Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med. 1989;321(6):406–12. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg HM, Dwyer EM, Jr, Hochman JS, et al. Interaction of ischaemia and encainide/flecainide treatment: a proposed mechanism for the increased mortality in CAST I. Br Heart J. 1995;74(6):631–5. doi: 10.1136/hrt.74.6.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alam M, Bandeali SJ, Shahzad SA, et al. Real-life global survey evaluating patients with atrial fibrillation (REALISE-AF): results of an international observational registry. Expert Rev Cardiovasc Ther. 2012;10(3):283–91. doi: 10.1586/erc.12.8. [DOI] [PubMed] [Google Scholar]
- 18.Andrade JG, Connolly SJ, Dorian P, et al. Antiarrhythmic use from 1991 to 2007: insights from the Canadian Registry of Atrial Fibrillation (CARAF I and II) Heart Rhythm. 2010;7(9):1171–7. doi: 10.1016/j.hrthm.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Camm AJ, Breithardt G, Crijns H, et al. Real-life observations of clinical outcomes with rhythm- and rate-control therapies for atrial fibrillation RECORDAF (Registry on Cardiac Rhythm Disorders Assessing the Control of Atrial Fibrillation) J Am Coll Cardiol. 2011;58(5):493–501. doi: 10.1016/j.jacc.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Chiang CE, Naditch-Brule L, Murin J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. 2012;5(4):632–9. doi: 10.1161/CIRCEP.112.970749. [DOI] [PubMed] [Google Scholar]
- 21.Meiltz A, Zimmermann M, Urban P, et al. Atrial fibrillation management by practice cardiologists: a prospective survey on the adherence to guidelines in the real world. Europace. 2008;10(6):674–80. doi: 10.1093/europace/eun086. [DOI] [PubMed] [Google Scholar]
- 22.Nabauer M, Gerth A, Limbourg T, et al. The Registry of the German Competence NETwork on Atrial Fibrillation: patient characteristics and initial management. Europace. 2009;11(4):423–34. doi: 10.1093/europace/eun369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiffel JA, Kowey PR, Myerburg R, et al. Practice patterns among United States cardiologists for managing adults with atrial fibrillation (from the AFFECTS Registry) Am J Cardiol. 2010;105(8):1122–9. doi: 10.1016/j.amjcard.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 24.Steg PG, Alam S, Chiang CE, et al. Symptoms, functional status and quality of life in patients with controlled and uncontrolled atrial fibrillation: data from the RealiseAF cross-sectional international registry. Heart. 2012;98(3):195–201. doi: 10.1136/heartjnl-2011-300550. [DOI] [PubMed] [Google Scholar]
- 25.Zimetbaum P, Ho KK, Olshansky B, et al. Variation in the utilization of antiarrhythmic drugs in patients with new-onset atrial fibrillation. Am J Cardiol. 2003;91(1):81–3. doi: 10.1016/s0002-9149(02)03004-7. [DOI] [PubMed] [Google Scholar]
- 26.Allen LaPointe NM, Governale L, Watkins J, et al. Outpatient use of anticoagulants, rate-controlling drugs, and antiarrhythmic drugs for atrial fibrillation. Am Heart J. 2007;154(5):893–8. doi: 10.1016/j.ahj.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 27.Fang MC, Stafford RS, Ruskin JN, et al. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164(1):55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- 28.Kashyap A, Li C. Trends in utilization of management strategies for newly diagnosed atrial fibrillation patients in the United States: 1999 to 2008. J Pharm Pract. 2012;25(2):151–9. doi: 10.1177/0897190011424803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.In brief: FDA warning on dronedarone (Multaq) Med Lett Drug Ther. 2011;53(1359):17. [PubMed] [Google Scholar]
- 30.Connolly SJ, Camm AJ, Halperin JL, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011;365(24):2268–76. doi: 10.1056/NEJMoa1109867. [DOI] [PubMed] [Google Scholar]