Abstract
Here we present a protocol for generating transgenic embryos in Xenopus laevis and Xenopus tropicalis. The method includes three steps: (1) The preparation of high-speed egg extracts, which facilitates the replacement of protamines in sperm nuclei with nucleosomes and decondenses the chromatin of sperm nuclei; (2) The isolation of sperm nuclei; and (3) The mixing of sperm nuclei, restriction enzyme, and high-speed extract in vitro, following by nuclear transplantation into unfertilized eggs to generate the transgenic embryos. This procedure generates non-mosaic transgenic embryos at high frequency and efficiency.
Keywords: Xenopus, Restriction enzyme mediated integration, Transgenesis
1. Introduction
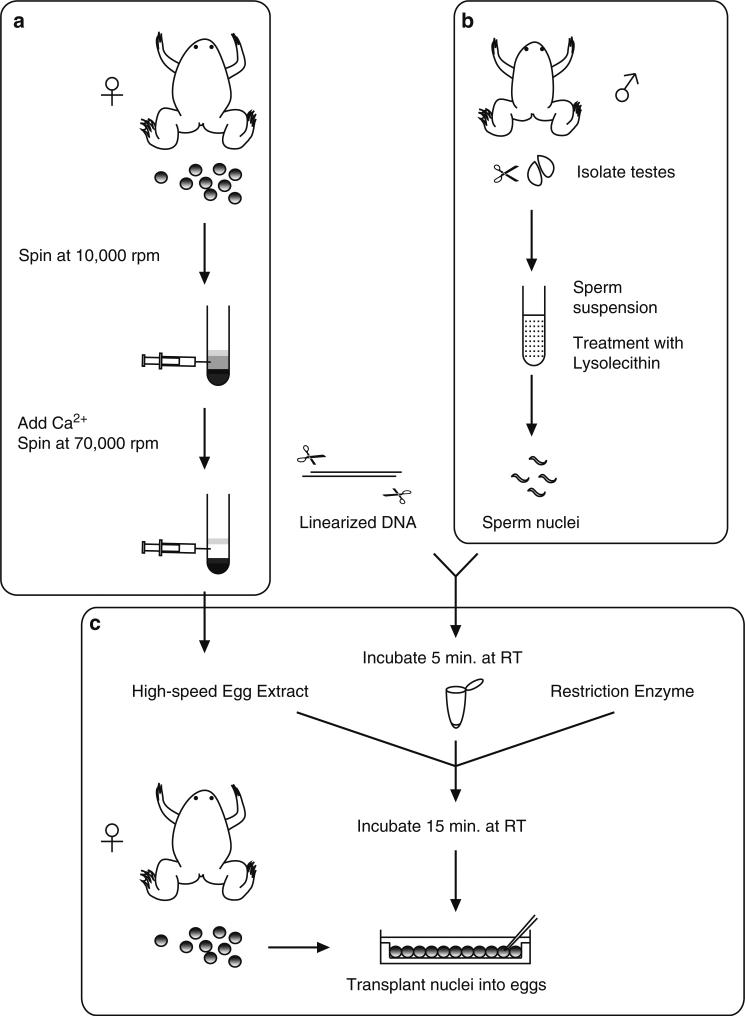
The transgenesis protocol described here can be divided into three parts, (a) preparation of egg extracts, (b) sperm nuclei preparation, and (c) nuclear transplantation (Fig. 1). A crude egg extract is prepared using a low-speed centrifugation step. These extracts are driven into the interphase stage of the cell cycle by addition of calcium. A high-speed centrifugation is then performed to generate an interphase cytosolic fraction containing proteins required for the efficient decondensation of the sperm nuclei (Fig. 1a). In addition, sperm nuclei are prepared from isolated sperm by treatment with lysolecithin, which causes a gentle permeabilization of the sperm plasma membrane (Fig. 1b). The nuclear transplantation procedure involves (1) incubation of linearized plasmid DNA with sperm nuclei, (2) decondensation of sperm nuclei by addition of a high-speed egg extract containing a small amount of the restriction enzyme, and (3) the reaction mix is diluted and transplanted into unfertilized eggs (Fig. 1c). The egg extract partially decondenses sperm chromatin and the restriction enzyme stimulates recombination by creating double-strand breaks, facilitating integration of DNA into the genome (1).
Fig. 1.
Transgenesis procedure includes: (a) Preparation of egg extracts; (b) Sperm nuclei preparation; and (c) Nuclear transplantation. The egg extracts and sperm nuclei can be stored at –80°C. (a) Calcium is added to allow the crude egg extract (which are held in meiotic arrest) to progress to interphase, and a high-speed centrifugation is performed to obtain the cytosolic fraction. (b) Testes are macerated and filtered, and then the sperm suspension is treated with lysolecithin to disrupt the plasma membrane of the cells. (c) Sperm nuclei are incubated with linearized DNA for a brief period of time. High-speed egg extracts and a restriction enzyme are added. The egg extracts partially decondenses chromosomes and the restriction enzyme stimulates recombination by creating double-strand breaks, facilitating integration of DNA into the genome. Diluted nuclei are transplanted into unfertilized egg.
There are several advantages of the REMI method of transgenesis over other methods. First and foremost is that it allows the production of fully transgenic, non-mosaic embryos, without the need of propagating the transgene through the germline, since the transgene integrates into the genome prior or soon after fertilization. Second is its high efficiency. Within an afternoon, one can produce hundreds of fully transgenic embryos using this method, which can be analyzed and studied over the subsequent hours, days, or months. In particular, this method allows the rapid assessment of genomic regulatory regions (promoter and/or enhancer regions), the rapid labeling of tissues and/or subcellular structures in living embryos, and the rapid assessment of phenotypes resulting from misexpression experiments under tight temporal and spatial control. Thus, using this method, one can quickly and easily perform experiments on F0 transgenic animals. However, there are some disadvantages as well, particularly if the experiments rely solely on the analysis of F0 transgenic animals. Firstly, each F0 transgenic embryo is unique. Therefore analysis amongst F0 transgenic embryos will result in significant variability in transgene expression, due to differences in integration site and copy number. For this reason, F0 transgenic experiments should be limited to a quick assessment of expression patterns of promoters/enhancers or missexpresssion experiments. One should not perform these experiments at the exclusion of generating transgenic lines, which will provide results that are considerably more uniform and clean (2). In cases where misexpression experiments are lethal, the experiments will need to be designed using a binary system, such as the GAL4/UAS system (2). Another disadvantage to the REMI method of transgenesis is that the transgenes integrate as large concatemers (1). Thus this method should not be used to generate transgenic lines containing site specific or transposon elements, such as cre/lox, flp/frt, Tol2, etc. The close apposition of such elements in the concatemer insertion will cause havoc following the inducement of site-specific recombination and/or transposition in such lines.
2. Materials
2.1. High-Speed Egg Extract Preparation (Xenopus laevis Protocol, Also Used for Xenopus tropicalis Protocol)
Ultracentrifuge (Beckman Optima) and Rotor (SW 40Ti, Beckman) with Ultra clear tube, 14 × 95 mm (Beckman 344060).
Ultracentrifuge (Beckman optima TLX) and Rotor (TLA100.3) with Thickwall polycarbonate tube (Beckman 349622).
1× Marc's Modified Ringer (MMR): 100 mM NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 5 mM HEPES, pH 7.5. Prepare a 10× stock, and adjust pH with NaOH to 7.5. Sterilize 10× solutions by autoclaving.
20× Extract buffer (XB) salt stock: 2 M KCl, 20 mM MgCl2, 2 mM CaCl2, filter-sterilize and store at 4°C.
Extract buffer (XB): 1× XB salts (100 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2 ; from 20× XB salts stock solution), 50 mM sucrose (1.5 M stock; filter-sterilize and store in aliquots at –20°C), 10 mM HEPES (1 M stock, titrated with KOH so that pH is 7.7 when diluted to 10 mM, should require about 5.5 mL of 10 N KOH for 100 mL, dilution drastically changes the pH of HEPES, so pH must be monitored after dilution; filter-sterilize and store in aliquots at –20°C). Prepare about 100 mL.
2% (w/v) l-Cysteine hydrochloride 1-hydrate (Sigma, C7880): Made up in 1× XB salts before use and titrated to pH 7.8 with NaOH. Prepare about 300 mL.
CSF-XB: 1× XB salts (100 mM KCl, 0.1 mM CaCl2, 1 mM MgCl2), 1 mM MgCl2 (in addition to MgCl2 present in XB salts; final concentration 2 mM), 10 mM HEPES, pH 7.7, 50 mM sucrose, 5 mM EGTA, pH 7.7. Prepare 50 mL.
Protease inhibitors: Mixture of leupeptin (Roche, 1 017 101), chymostatin (Roche, 1004 638), and pepstatin (Roche 253 286), each dissolved to a final concentration of 10 mg/mL in dimethyl sulfoxide (DMSO). Store in small aliquots at –20°C.
1 M CaCl2 (filter-sterilize and store at 4°C).
Energy mix (store in aliquots at –20°C): 150 mM creatine phosphate (Roche, 621 714), 20 mM ATP (Roche, 519 979), 20 mM MgCl2, store in 0.1 mL aliquots at –20°C.
Pregnant mare serum gonadotropin (PMSG): 100 U/mL PMSG (P.G.600 ®, Intervet, Inc., 021825). Dissolve in water and stored at –20°C.
Human chorionic gonadotropin (HCG): 1,000 U/mL HCG (CHORULON ®, Intervet, Inc., 057176). Dissolve in water and stored at 4°C.
2.2. Sperm Nuclei Preparation (Xenopus laevis and Xenopus tropicalis Protocol)
Centrifuge and rotor (e.g., Sorvall HB-4 Swinging bucket rotor).
14 mL tube (Falcon 2059).
Dissection tools; scissors and forceps.
Funnel.
Cheesecloth.
Fluorescence microscope.
Hemacytometer.
1× MMR. Prepare as described in Subheading 2.1, item 3.
0.1% Tricaine Methanesulfonate (MS222, aminobenzoic acid ethyl ester, Sigma A-5040), 0.1% sodium bicarbonate. Dissolve in water.
2× Nuclear preparation butter (NPB): 500 mM sucrose (1.5 M stock; filter-sterilize and store aliquots at –20°C), 30 mM HEPES (1 M stock; titrate with KOH so that pH 7.7 is at 15 mM, filter-sterilize and store aliquots at –20°C), 1 mM spermidine trihydrochloride (Sigma S-2501; 10 mM stock; filter-sterilize and store aliquots at –20°C), 0.4 mM spermine tetrahydrochloride (Sigma S-1141; 10 mM stock; filter-sterilize and store aliquots at –20°C), 2 mM dithiothreitol (Sigma D-0632; 100 mM stock; filter-sterilize and store aliquots at –20°C), 2 mM EDTA (500 mM EDTA, pH 8.0 stock; auto-clave and store at room temperature). On the day of the sperm nuclei preparation, make up 25 mL of 2× NPB for 1–2 males from the stock solutions.
1× NPB: Make up 30 mL of 1× NPB by mixing 15 mL of 2× NPB with 15 mL of water and store on ice.
Lysolecithin: 100 μL of 10 mg/mL l-α-lyso-Lecithin, Egg Yolk (Calbiochem, 440154); dissolve at room temperature just before use. Store solid stock at –20°C. Discard the stock powder if it becomes sticky. Digitonin can be used instead of Lysolecitin. Digitonin is more specific for the plasma membrane leaving the nuclear membranes intact.
Bovine serum albumin (BSA): 10% (w/v) BSA (fraction V, Sigma A-7906). Make up 5 mL in water on the day of the sperm nuclei preparation.
1× NPB, 3% BSA: Mix 5 mL 2× NPB, 3 mL 10% BSA, and 2 mL water and store on ice.
1× NPB, 0.3% BSA: Mix 2.5 mL 2× NPB, 0.15 mL 10% BSA, and 2.35 mL water and store on ice.
100% glycerol.
Sperm storage buffer: 1× NPB, 30% glycerol, 0.3% BSA. Make up by mixing the following solutions, 250 μL of 2× NPB, 15 μL of 10% BSA, 150 μL of 100% glycerol, and 85 μL water.
Sperm dilution buffer (SDB): 250 mM sucrose, 75 mM KCl, 0.5 mM spermidine trihydrochloride, 0.2 mM spermine tetrahydrochloride. Add about 80 μL of 0.1 N NaOH per 20 mL solution to titrate to pH 7.3–7.5 and store 0.5–1 mL aliquots at –20°C.
Hoechst No. 33342 (Sigma B-2261): 10 mg/mL stock in dH2 O, store in a lighttight vessel at –20°C.
PMSG: 100 U/mL PMSG (P.G.600 ®, Intervet, Inc., 021825). Dissolve in water and stored at –20°C.
HCG: 500 U/mL (X. tropicalis) or 1,000 U/mL (X. laevis) HCG (CHORULON ®, Intervet, Inc., 057176). Dissolve in water and stored at 4°C.
2.3 Nuclear Transplantation Reagents and Equipment (X. laevis Protocol)
2% l-Cysteine hydrochloride 1-hydrate in 1× MMR (titrate to pH 8.0 with NaOH). Make up freshly.
1× MMR as described in Subheading 2.1, item 3.
100 mM MgCl2.
SDB. Prepare as described in Subheading 2.2, item 18.
0.4× MMR, 6% (w/v) Ficoll (Sigma, F-4375), 10 μg/mL gentamycin (a 10 mg/mL stock solution is purchased from Gibco-BRL 15710-015). Sterilize by filtration.
0.1× MMR, 6% (w/v) Ficoll, 10 μg/mL gentamycin. Sterilize by filtration.
0.1× MMR, 10 μg/mL gentamycin.
Linearized plasmid (100 ng/μL in water): Any enzyme can be used for linearization of plasmid. Digest DNA using standard conditions, and purify by phenol/chloroform extraction and ethanol precipitation. There is no need to gel-purify the plasmid.
Restriction enzyme: Dilute in water before adding to the transplantation reaction. Although any enzyme can be used, do not use an enzyme that digests within regulatory or protein coding regions of the construct (see Note 1).
Agarose-coated injection dishes: 1.0% agarose in 0.1× MMR is poured into 60-mm Petri dishes. Before the agarose solidifies, a template is laid onto it. After the agarose has solidified, the templates are removed and the dishes are wrapped in para film and stored at 4°C until use. As a template we routinely use a 35-mm × 35-mm square, small weighing boat, which holds about 400 X. laevis eggs.
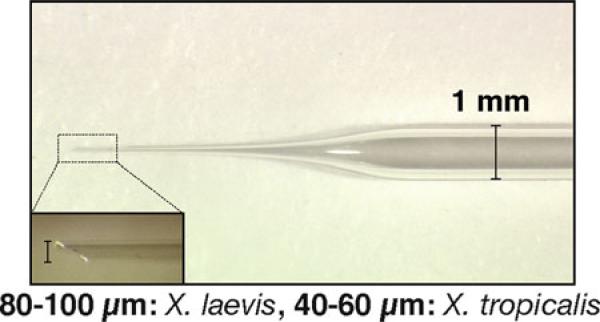
Transplantation needles: 30-μL Drummond MICROCAPS ® (Cat. No. 1-000-0300) are pulled to produce large needles with long, gently sloping tips (Fig. 2). We use a Flaming/Brown Micropipet Puller Model P-87 (Sutter Instruments Co.) for pulling needles using a condition, p = 50, v = 100, and t = 5. Needles are clipped with a forceps to produce a beveled tip of 80–100 μm diameter, using the ocular micrometer of a dissecting microscope for measurement (Fig. 2).
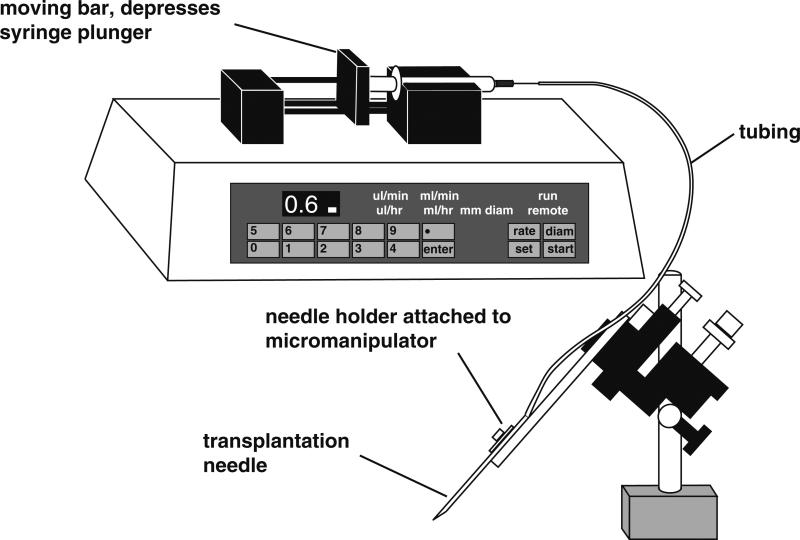
Transplantation apparatus: Most commercial injection apparatus used for RNA and DNA injections which are based on air pressure are not suitable for nuclear transplantation, due to the difference in needle tip size. Therefore we use an oil-filled injection system, Harvard apparatus 22 syringe pump (NP 55-2222) with two 2.5 mL Hamilton Gas Tight Syringes and plastic tubing (ID = 0.7 mm, OD = 2.4 mm, Tygon ® tubing, R3603) (Fig. 3). Two people can transplant nuclei at the same time. We use Mineral Oil (Sigma M-8410) in the system. The infusion pump allows us to control flow rate easily. We set the speed of flow at 0.6 μL/min (0.2 μL/min for X. tropicalis, see below).
Fig. 2.
Transplantation needle has a gently sloping tip and is clipped with forceps to produce a beveled, 80–100 μm wide tip for Xenopus laevis and a 40–60 μm wide tip for Xenopus tropicalis.
Fig. 3.
We use an oil-filled injection system for the nuclear transplantations. The syringe and tubing are filled with mineral oil and the infusion pump depresses the syringe plunger, resulting in a constant, desirable flow rate (0.6 μL/min for X. laevis; 0.2 μL/min for X. tropicalis).
2.4. Nuclear Transplantation Reagents and Equipment (X. tropicalis Protocol)
2% l-Cysteine, in 0.1× MMR, pH.8.0 (titrate with NaOH, Sigma, C-7352). Make up freshly.
0.1× MMR.
Sperm Injection Buffer modified for X. tropicalis (MOH) (3) : 10 mM KPO4, pH 7.2, 125 mM Potassium gluconate, 5 mM NaCl, 0.5 mM MgCl2, 250 mM Sucrose, 0.25 mM Spermidine, 0.125 mM Spermine.
0.1× MMR, 6% (w/v) Ficoll (Sigma, F-4375). Sterilize by filtration.
0.01× MMR, 6% (w/v) Ficoll (Sigma, F-4375). Sterilize by filtration.
0.01× MMR.
Linearized plasmid (100 ng/μL in water) as described in Subheading 2.3, item 8.
Agarose-coated injection dishes as described in Subheading 2.3, item 10.
Transplantation needles as described in Subheading 2.3, item 11. Needles are clipped with a forceps to produce a beveled tip of 40–60 μm for X. tropicalis, using the ocular micrometer of a dissecting microscope for measurement (Fig. 2).
Transplantation apparatus: Harvard apparatus 22 syringe pump (NP 55-2222) with two 0.1 mL Hamilton Gas Tight Syringes and Tygon tubing. The speed of flow is set at 0.2 μL/min for X. tropicalis.
Agarose-coated 24 well dishes: 1.0% agarose in 0.1× MMR is poured into 24 well dishes.
Agarose-coated 90 mm Petri dish. We use agarose-coated dishes to culture X. tropicalis embryos until they hatch at around stage 28, because their vitellin membrane is quite sticky.
3. Methods
3.1. Transgenesis Method for Xenopus laevis
3.1.1. High-Speed Extract Preparation
This protocol is an adaptation of Murray (4). Briefly, a crude cytostatic factor (CSF) arrested egg extract (cytoplasm arrested in meiotic metaphase) is prepared.
Calcium is then added to allow the extract to progress into inter-phase, and a highspeed spin is performed to obtain a purer cytoplasmic fraction. Cytochalasin is omitted from the protocol, since carryover of cytochalasin into the final extract used for sperm incubations interferes with normal development of transplant embryos. Use of high-speed rather than crude cytoplasmic extracts is advantageous, because high-speed extracts promote swelling of added sperm nuclei (and some chromatin decondensation), but do not promote DNA replication. Replication of sperm DNA incubated in these extracts occurs after transplantation of the nucleus into the egg rather than in the extract. High-speed extract can be stored frozen in small aliquots (at –80°C) and thawed before use.
Prime 8–12 female adult X. laevis about 3–5 days prior to HCG injection by injecting 50 U of PMSG into the dorsal lymph sac. The evening before the extract preparation begins, inject each frog with 500 U HCG and place two frogs/container into 2 L 1× MMR. Since one frog with lysing or activating eggs can compromise the whole extract preparation, we prefer to separate the frogs into pairs for the ovulation. The frogs are then placed at 15–18°C overnight (12–14 h). On the next morning, the egg quality from each container is screened before mixing all the eggs and starting the extract preparation. All the eggs released in a container with mottled, lysing, or dying eggs are left out of the extract preparation.
All solutions should be prepared before beginning the extract preparation, since the procedure should be carried through all steps promptly once it is initiated; optimally, the high-speed spin should begin within 45–60 min of dejellying the eggs. Gently, manually expel eggs from each frog into large beakers containing 1× MMR, and collect unbroken eggs with even pigmentation. Good eggs can also be collected from the 1× MMR in the frog buckets. Total volume of eggs should be 100 mL or greater before dejellying.
Remove as much MMR as possible from the eggs. Dejelly eggs in 2% cysteine in XB salts (no HEPES/sucrose). Add a small amount at a time, swirl eggs, and partially replace with fresh cysteine several times during dejellying. Remove broken eggs with a pipet during dejellying. Dejellying should be initiated separately for different batches of eggs, and batches that show breakage or egg activation are discarded. The rest of the eggs can then be combined.
Wash eggs in XB (with HEPES/sucrose). We use about 35 mL for each wash, and do four washes.
Wash eggs in CSF-XB with protease inhibitors. We do two 25 mL washes.
Using a wide-bore Pasteur pipet, transfer eggs into Beckman ultraclear tubes. For these volumes, we typically use 14 × 95 mm tubes (Beckman, 344060). If multiple tubes will be used, try to transfer an equal volume of eggs per tube. Allow the eggs to settle and remove as much CSF-XB as possible.
Spin for about 60 s at 1,000 rpm (150 g) in a Beckman SW 40 Ti Rotor (or similar rotor) in an ultracentrifuge. Remove the excess CSF-XB and then balance the tubes.
Spin the tubes for 10 min at 16,000× (10,000 rpm) at 2°C in a Beckman SW 40 Ti Rotor (or similar rotor) in an ultracentrifuge to crush the eggs. The eggs should be separated into three layers: lipid (top), cytoplasm (center), and yolk (bottom). Collect the cytoplasmic layer from each tube with an 18-gage needle by inserting the needle at the base of the cytoplasmic layer and withdrawing slowly. Transfer cytoplasm to a fresh Beckman tube on ice. If large volumes of darkly pigmented eggs are used, the cytoplasmic layer may be grayish rather than golden at this step. After a second spin to clarify this extract, it should be golden.
Add protease inhibitors to the isolated cytoplasm (do not add cytochalasin); recentrifuge the cytoplasm in Beckman tubes for an additional 10 min at 16,000× to clarify, again using a swinging bucket rotor. Collect the clarified cytoplasm as before. Expect to obtain 0.75–1 mL cytoplasm/batch of eggs collected from one frog.
Add 1/20 vol of the ATP-regenerating system (energy mix). Transfer the clarified cytoplasm into TL100.3 thick-wall polycarbonate tubes (Beckman, 349622). Tubes hold about 3 mL each and should be at least half full.
Add CaCl2 to each tube to a final concentration of 0.4 mM; this inactivates CSF and pushes the extract into interphase. Incubate at room temperature for 15 min and then balance for the high-speed spin.
Spin tubes in a Beckman tabletop ultracentrifuge in a TL100.3 rotor (gold top; fixed angle) at 70,000 rpm for 1.5 h at 4°C.
The cytoplasm will fractionate into four layers, top to bottom: lipid, cytosol, membranes/mitochondria, and glycogen/ribosomes. Remove the cytosolic layer from each tube (about 1 mL if 2–3 mL were loaded into the tube) by inserting a syringe into the top of the tube through the lipid layer. Transfer this fraction to fresh TL-100 tubes, and spin again at 70,000 rpm for 20 min at 4°C.
Aliquot the high-speed cytosol supernatant into 10 μL aliquots in 0.5-mL Eppendorf tubes. Quick-freeze aliquots in liquid nitrogen, and store at –80°C until use. We typically obtain 1–2 mL of high-speed cytosol from preparations of this scale. Sperm nuclei should be incubated in an aliquot of extract and stained with Hoechst as described below in Subheading 3.1.2, step 16 to determine whether extract is effective. If active, interphase extract should cause nuclei to swell visibly (thicken and lengthen) within 10 min of addition at room temperature.
3.1.2. Sperm Nuclei Preparation (Xenopus laevis)
We follow the standard protocol of Murray (4), but omit the protease inhibitors leupeptin and phenylmethylsulfonyl fluoride from all steps to avoid transfer into the final mixture, which is diluted for egg injections. We always obtain better sperm nuclei when males are injected with hormones.
Prime one or two males about 3–5 days prior to HCG injection with 50 U of PMSG.
Inject the male or males 12–15 h before the preparation with 500 U of HCG.
- Dissect and isolate the testes from the male:
- Anaesthetize a male by immersion in 0.1% Tricaine Methanesulfonate/0.1% sodium bicarbonate for at least 20 min (immersion of the animal in ice water for 20 min may also be used), and pith it.
- Cut through the ventral body wall and musculature, and lift the yellow fat bodies to isolate the two testes, which are attached to the base of the fat bodies, one on each side of the midline.
- Remove the testes with dissecting scissors, and roll them on a dry paper towel to remove the blood, blood vessels and fat body.
- Wash the testes briefly in a 60-mm Petri dish containing cold 1× MMR, removing any attached pieces of fat body or debris with a forceps. Take care not to puncture the testes, as this releases the sperm.
- Rinse the testes in cold 1× NPB.
Move the cleaned testes to a dry 60-mm Petri dish, and macerate the testes well (until clumps are no longer visible to the naked eye) with a pair of clean forceps.
Add 2 mL cold 1× NPB and mix well by pipetting the solution up and down with a 10 mL plastic pipet.
Squirt the sperm suspension through four thicknesses of cheesecloth placed into a funnel, and collect the solution in a 14-mL tube (Falcon 2059).
Rinse the dish with an additional 3 mL of cold 1× NPB, and force this through the cheesecloth into the 14-mL tube.
Add 5 mL of cold 1× NPB and squeeze the cheesecloth by hand, wearing gloves, to get any remaining liquid through the funnel into the 14-mL tube.
Pellet the sperm by centrifugation at 3,000 rpm for 10 min at 4°C (we use a Sorvall HB-4 or similar swinging bucket rotor with the appropriate adapters). During spin, allow 1 mL of 1× NPB to equilibrate to room temperature. Note that the pellet of sperm should be primarily white, but it may contain a central core of redness, due to pelleted erythrocytes. The erythrocytes are heavier than the sperm, so they pellet first.
Decant the supernatant and resuspend the sperm in 9 mL 1× NPB using a 10-mL plastic pipet and repellet by centrifugation at 3,000 rpm for 10 min at 4°C. During this spin, dissolve 1 mg of Lysolecithin in 100 μL of water (10 mg/mL) at room temperature.
Decant the supernatant and resuspend the pellet with a 1 mL blue tip in the 1 mL 1× NPB that has equilibrated at room temperature.
Add 50 μL of 10 mg/mL lysolecithin. Mix gently and incubate for 5 min at room temperature.
Add 10 mL of cold 3% BSA/1× NPB to the suspension to stop the reaction, and centrifuge at 3,000 rpm for 10 min at 4°C. Note that after this spin, the pellet should be softer, and more spread out, and it should not contain any redness, as the plasma membrane of the erythrocytes should have been disrupted, thus releasing the hemoglobin into the supernant.
Decant the supernatant and resuspend the pellet in 5 mL cold 0.3% BSA/1× NPB. Mix well by pipetting with 5-mL plastic pipet and centrifuge at 3,000 rpm for 10 min at 4°C.
Take supernatant carefully and resuspend the pellet in 500 μL of sperm storage buffer, and transfer suspension into a 1.5-mL Eppendorf tube.
Count the number of sperm nuclei using a hemacytometer: Cut off the end of a yellow tip with a razor blade and mix the sperm nuclei well by pipetting. Dilute 1 μL of the sperm nuclei with 100 μL of SDB, and add 1 μL of 1:100 diluted Hoechst stock to visualize the sperm nuclei under a fluorescence microscope. For a 1:100 dilution of our sperm stock, we typically obtain counts of 100–200 (×104 nuclei/mL) in 1-mm × 1-mm × 0.1-mm square of an improved Neubauer hemacytometer. At this concentration, the undiluted stock contains 1–2 × 105 nuclei/μL. If your sperm stock is substantially less concentrated (i.e., a count of <50 for a 1:100 dilution), let the sperm settle for a few hours or overnight, and remove some of supernatant. We leave fresh nuclei overnight at 4°C to allow the penetration of glycerol for best cryopreservation of the sperm. Then next day the sperm is aliquoted (20 μL/aliquot) and fast frozen in liquid nitrogen. The frozen aliquots are then stored at –80°C.
3.1.3. Transgenesis by Sperm Nuclear Transplantation into Unfertilized Eggs from Xenopus laevis
Prime two females 3–5 days before HCG injection with 50 U of PMSG.
Inject the females with 500 U of HCG, 12–15 h before egg collection.
Allow an aliquot of SDB to equilibrate to room temperature, and make up 2% Cysteine in 1× MMR, pH 8.0.
Turn on the switch of the Harvard Apparatus and start the infusion pump to get the flow to stabilize before injection.
Set up a reaction using a clipped yellow tip: mix 4 μL sperm stock (~4–8 × 105 nuclei) and 1–2 μL linearized plasmid (100 ng/μL), and incubate for 5 min.
Dilute 0.5 μL of a restriction enzyme in 4.5 μL of water, and mix 1 μL of the diluted enzyme with 18 μL of SDB, 2 μL of 100 mM MgCl2, and 2 μL of high-speed egg extract.
Add the mixture to the sperm/DNA and mix well by gentle pipetting (using a clipped yellow tip). Incubate for 15 min at room temperature.
During the reaction, collect eggs in a beaker by squeezing frogs and dejelly them in 2% cysteine/1× MMR, pH 8.0. This usually takes about 10 min, so by the time the eggs are ready, the reaction is nearly complete. Squeeze eggs directly into a dry beaker and add cysteine immediately to keep egg quality. One may need to dejelly eggs from different females separately, as dejellying times vary or poor quality eggs make the solution dirty (see Note 2).
Wash the dejellied eggs with 1× MMR at least three times, and transfer the eggs to injection dishes containing 0.4% MMR/6% Ficoll using a wide-bore Pasteur pipet. We generally fill the square space with eggs so that no gap is left between the eggs. After about 5 min in 0.4× MMR, 6% Ficoll, the eggs will pierce easily. Transplantation should be performed at around 16°C. To achieve this we place the injection dish on the plastic box half-filled with ice and place the box and eggs under the injection microscope.
After the incubation with extracts, mix the sperm nuclei gently by pipetting with a clipped yellow tip. Then transfer 5 μL of the reaction into 150 μL of SDB that has equilibrated at room temperature.
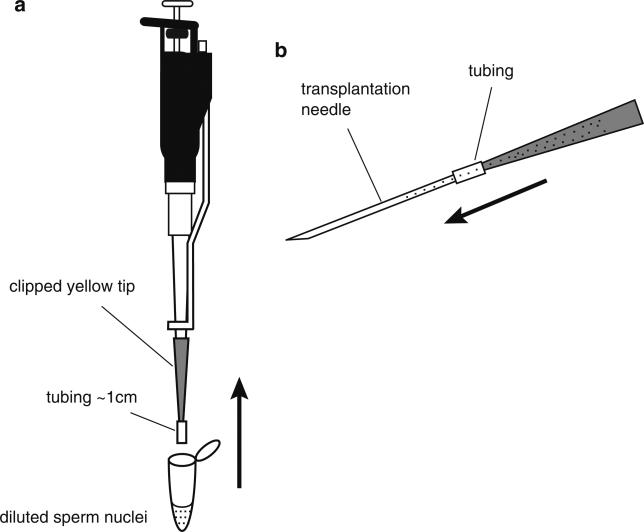
Mix well but avoid making bubbles, using a clipped yellow tip with a piece of plastic tube attached (Fig. 4a). Fill the clipped yellow tip with the diluted sperm suspension, carefully detach the clipped yellow tip, keeping the tip horizontal and back fill a transplantation needle by attaching it to the tube (Fig. 4b). You can keep the yellow tip with the remaining nuclei, by placing it horizontally, in case you need to load another needle. Keep decondensed sperm nuclei at room temperature and transplant them within an hour, but preferably within 30 min.
Attach the needle to the tube filled with mineral oil that is connected to the syringe on the Harvard Apparatus.
Check the flow and start injecting. Keep the needle inside each egg for approximately 0.5 s, and move the needle fairy rapidly from egg to egg piercing the plasma membrane of each egg with single, sharp motion. We usually transplant for about 15–20 min. If needle is blocked by debris during transplantation, change needle or try to fix by pinching the tube or cutting the tip of needle using forceps.
After injection, incubate embryos at 16°C.
When the embryos reach the 4-cell stage (about 3–4 h after injection at 16°C), gently transfer normally dividing embryos to 10-cm Petri dish containing 0.1× MMR, 6%Ficoll, 10 μg/mL gentamycin using a wide-bore Pasteur pipet (see Note 3).
The next day, when embryos are around stage 12, transfer healthy embryos to a new 90-mm Petri dish containing 0.1× MMR, 10 μg/mL gentamycin without Ficoll. Because of the large needle tip used for transplantations, embryos often develop large blebs at the site of injection. These blebs occur when cells are forced out of the hole left in the vitelline membrane at the injection site, but they generally do not affect development. The blebs usually fall off at the neurula or tailbud stages.
Incubate embryos until they reach the stage that you need to analyze at 14–22°C. Approximately 10–30% of embryos proceeded to post-gastrula show stable expression of transgenes (see Note 4).
Fig. 4.
Method for back filling nuclei into a transplantation needle. (a) The diluted reaction mix is drawn into a clipped yellow tip containing about 1 cm of Tygon tubing. (b) The yellow tip containing the Tygon tubing and dilute sperm nuclei is carefully detached from the pipetman and connected to the back of the needle using the tubing. The needle is gently loaded with the dilute sperm nuclear reaction by gravity. This is done by slowly increasing the angle of the yellow tip/tubing/needle so that the mixture flows gently into the needle. Once the needle is completely filled, the needle is detached from the yellow tip and the needle is ready to connect to the infusion pump. The remaining sperm mixture can be set aside horizontally and used to reload another needle, if two people are injecting simultaneously or if a needle is accidentally damaged or blocked.
3.2. Transgenesis Method for Xenopus tropicalis
X. tropicalis has a diploid genome and shorter generation time than X. laevis, but retains many of the advantages of X. laevis (3, 5). The basic protocol for transgenesis in X. tropicalis is very similar to the procedure used for X. laevis.
3.2.1. High-Speed Extract Preparation
We use a high-speed egg extract from X. laevis (see Subheading 3.1.1) in the protocol for X. tropicalis.
3.2.2. Sperm Nuclei Preparation (X. tropicalis)
Prime two males the day before the sperm nuclei preparation with 10 U of PMSG.
Inject the males 3 h before the nuclei preparation with 50 U of HCG.
Dissect and isolate the testes from the male as described in Subheading 3.1.2. Take care not to puncture the testes, as this releases the sperm. This is particularly important for X. tropicalis.
Move the cleaned testes to a dry 60-mm Petri dish, and macerate the testes well (until clumps are no longer visible to the naked eye) with a pair of clean forceps.
Add 2 mL cold 1× NPB and mix well by pipetting the solution up and down with a 10 mL plastic pipet.
Squirt the sperm suspension through four thicknesses of cheesecloth placed into a funnel, and collect the solution in a 14-mL tube (Falcon 2059).
Rinse the dish with an additional 3 mL of cold 1× NPB, and force this through the cheesecloth into the 14-mL tube.
Add 5 mL of cold 1× NPB and squeeze the cheesecloth by hand, wearing gloves, to get any remaining liquid through the funnel into the 14-mL tube.
Pellet the sperm by centrifugation at 3,000 rpm for 10 min at 4°C (Sorvall HB-4 or similar swinging bucket rotor with the appropriate adapters). During spin, allow 1 mL of 1× NPB to equilibrate to room temperature.
Decant the supernatant and resuspend the sperm in 9 mL 1× NPB using a 10-mL plastic pipet and repellet by centrifugation at 3,000 rpm for 10 min at 4°C. During this spin, dissolve 1 mg of Lysolecithin in 100 μL of water (10 mg/mL) at room temperature.
Decant the supernatant and resuspend the pellet with a 1 mL blue tip in the 1 mL 1× NPB that has equilibrated at room temperature.
Dilute 20 μL of 10 mg/mL lysolecithin with 980 μL of water (0.2 mg/mL) and add 50 μL of that diluted lysolecithin to the sperm. Mix gently and incubate for 5 min at room temperature.
Add 10 mL of cold 3% BSA/1× NPB to the suspension to stop the reaction, and centrifuge at 3,000 rpm for 10 min at 4°C.
Decant the supernatant and resuspend the pellet in 5 mL cold 0.3% BSA/1× NPB. Mix well by pipetting with 5-mL plastic pipet and centrifuge at 3,000 rpm for 10 min at 4°C.
Take supernatant carefully and resuspend the pellet in 100 μL of sperm storage buffer, and transfer suspension into a 1.5-mL Eppendorf tube.
Count of the sperm nuclei: For a 1:100 dilution of our sperm stock, we typically obtain counts of ~80 (×104 nuclei/mL) in 1-mm × 1-mm × 0.1-mm square of an improved Neubauer hemacytometer. At this concentration, the undiluted stock contains 8 × 104 nuclei/μL, and we use 10 μL of that for a reaction. If the sperm stock is substantially less concentrated, let the sperm settle for a few hours or overnight, and remove some of supernatant. If the count is still low, you can use up to 30 μL of the sperm nuclei per reaction. Sperm nuclei can be frozen in aliquots at –80°C.
3.2.3. Nuclear Transplantations (Xenopus tropicalis)
Prime two females the day before the nuclear transplanations with 15 U of PMSG. Inject the females 3–4 h before the nuclear transplantation with 75 U of HCG and leave frogs at 22–24°C. We use two females for two rounds of the nuclear transplantation a day. If more rounds of transgenesis are needed in the same day, inject another two females 1 or 2 h after the first pair. This will allow for four rounds of transgenics, each around 1 h apart.
Allow an aliquot of MOH buffer to equilibrate to room temperature, and make up 2% Cysteine in 1× MMR, pH 8.0.
Turn on the switch of the Harvard Apparatus and start the infusion pump.
Set up a reaction: Take 1–2 μL of linearized DNA into a 1.5-mL tube. Using a clipped yellow tip, mix sperm nuclei stock and take 10 μL of sperm nuclei (~8 × 105 nuclei) to mix with the DNA.
Incubate the mixture for 5 min at room temperature.
Add 2 μL of egg extract to the sperm/DNA and mix well by gentle pipetting using a clipped yellow tip. Incubate for 15 min at room temperature.
During the reaction, collect eggs in a beaker by squeezing frogs and dejelly them in 2% Cysteine/0.1× MMR, pH 8.0.
Wash the dejellied eggs with 0.1× MMR three times and transfer the eggs to injection dishes containing 0.1% MMR/6% Ficoll. We generally fill the half of square space with eggs, because we need to inject eggs within 30 min.
After 15 min incubation, dilute all the reaction mix with 130 μL of MOH that has equilibrated at room temperature. Mix well and back fill a needle using a clipped yellow tip with a piece of tube. If you use more than 10 μL of the sperm (up to 30 μL), amount of MOH buffer should be reduced. The concentration of nuclei must be considerably higher with X. tropicalis, since the volume injected into each egg is about 1/5 the amount used for X. laevis.
Attach the needle to the injection system and inject eggs checking the flow regularly. Transplantation is performed at room temperature (22–24°C).
Incubate embryos at 22–24°C.
When embryos reach at the 4-cell stage, collect normally dividing embryos and transfer them to agarose-coated 24 well dishes (1–2 embryo/well) containing 0.01× MMR/6% Ficoll. Embryos reach the 4-cell stage approximately 1.5 h after injection at 22–24°C, therefore it may be necessary for one person to sort the embryos while others continue later rounds of injections (i.e., third and fourth rounds of injections). Culture embryos overnight at 22°C.
The next day transfer healthy embryos to an agarose-coated 90 mm Petri dish containing 0.01× MMR, and culture embryos at 22°C.
Footnotes
Generally the same restriction enzyme used to linearize the transgene is used in the transgenic reaction before the nuclear transplantation. However, a different enzyme, which was not used to linearize the transgene, can still be used for the reaction, as long as it does not cut within the transgene. We usually use enzymes, NotI, SalI, or SfiI purchased from Roche. Asp700 (XmnI) and SwaI can also be used. Asp700 digests once within Ampicilin resistant gene. We often use SwaI when the construct is generated by Tol2 kit (6) or pTransgenesis (7), which digests once within Tol2 transposon element. SwaI sites are generally quite rare in constructs. Some calibration may be required to determine the optimal amount of enzyme to add to each reaction, as too much enzyme may adversely affect the development of embryos derived from nuclear transplantations. To co-integrate two different constructs into the genome, both DNAs can be digested with either the same enzyme or with different enzymes, without loss of co-integration efficiency. Although it is possible to omit restriction enzyme from the transgenic reaction, this usually results in a lower efficiency of transgenesis. This is desirable for some applications such as establishment of transgenic lines, as embryos generated from reactions that do not include restriction enzyme tend to develop to postmetamorphic and adult stages with fewer anomalies.
Egg quality is one of the factors that can affect transgenesis efficiency. Needles for nuclear transplantation are much larger than those used for RNA injections, in order to not damage swollen nuclei. Therefore eggs need to be of high quality to recover after injection. Eggs used for nuclear transplantation should, in particular, have a firm cortex and maintain a regular round shape.
One can determine whether the sperm dilution and the flow rate used for injections were appropriate by watching the first cleavage of the transplanted eggs. If few of the eggs received a nucleus, the frequency of cleavage will be low; one-third of our transplantations typically result in normally cleaving embryos. Eggs that were injected with more than one nucleus will divide at the time of first cleavage abnormally into three or four (or more) cells. Many of these embryos will develop to blastula stages, but most fail during gastrulation; in some, a region of the embryo will fail to cellularize and die. Eggs injected with multiple nuclei that do gastrulate usually do so abnormally; typically, blastopore closure is incomplete, resulting in embryos that form two wings of somites and neural tissue on each side of the exposed yolky tissue lying in the center of the trunk. This type of gastrulation failure is also common to stressed or unhealthy embryos (particularly embryos derived from “soft” eggs).
One of the main factors affecting the efficiency of transgenesis is the amount of chromosomal damage sustained by sperm nuclei prior to transplantation. Chromosomal damage can occur during sperm nuclear preparation and is also deliberately induced by addition of restriction enzyme to the sperm, extract, and plasmid reaction mixture used for transplantations. Nuclei with very little chromosomal damage will promote a much higher frequency of normal embryonic development to late stages. However, nuclei that have sustained more chromosomal damage give rise to transgenic embryos at higher frequencies. Thus a balance must be achieved between attaining normal development of embryos vs. promoting high frequency transgenesis. We generally use a procedure that results in a lower (10–30%) efficiency of transgenesis, but with improved overall survival of the embryos.
References
- 1.Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122(10):3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- 2.Hartley KO, Nutt SL, Amaya E. Targeted gene expression in transgenic Xenopus using the binary Gal4-UAS system. Proc Natl Acad Sci U S A. 2002;99(3):1377–1382. doi: 10.1073/pnas.022646899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Offield MF, Hirsch N, Grainger RM. The development of Xenopus tropicalis transgenic lines and their use in studying lens developmental timing in living embryos. Development. 2000;127(9):1789–1797. doi: 10.1242/dev.127.9.1789. [DOI] [PubMed] [Google Scholar]
- 4.Murray AW. Cell cycle extracts. Methods Cell Biol. 1991;36:581–605. [PubMed] [Google Scholar]
- 5.Amaya E, Offield MF, Grainger RM. Frog genetics: Xenopus tropicalis jumps into the future. Trends Genet. 1998;14(7):253–255. doi: 10.1016/s0168-9525(98)01506-6. [DOI] [PubMed] [Google Scholar]
- 6.Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien CB. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236(11):3088–3099. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- 7.Love NR, Thuret R, Chen Y, Ishibashi S, Sabherwal N, Paredes R, Alves-Silva J, Dorey K, Noble AM, Guille MJ, Sasai Y, Papalopulu N, Amaya E. pTransgenesis: a cross-species, modular transgenesis resource. Development. 2011;138(24):5451–5458. doi: 10.1242/dev.066498. [DOI] [PMC free article] [PubMed] [Google Scholar]