Abstract
Reactive oxygen species (ROS) mediate cell-signaling processes in response to various ligands and play important roles in the pathogenesis of cardiovascular diseases. The present study reports that interleukin-22 (IL-22) elicits signal transduction in vascular smooth muscle cells (SMCs) through a ROS-dependent mechanism. We find that pulmonary artery SMCs express IL-22 receptor alpha 1 and that IL-22 activates STAT3 through this receptor. IL-22-induced signaling is found to be mediated by NADPH oxidase, as indicated by the observations that the inhibition and siRNA knock-down of this enzyme inhibit IL-22 signaling. IL-22 triggers the oxidative modifications of proteins through protein carbonylation and protein glutathionylation. Mass spectrometry identified some proteins that are carbonylated in response to IL-22 stimulation, including α-enolase, heat shock cognate 71 kDa protein, mitochondrial 60 kDa heat shock protein, and cytoplasmic 2 actin and determined that α-tubulin is glutathionylated. Protein glutathionylation and STAT3 phosphorylation are enhanced by the siRNA knock-down of glutaredoxin, while IL-22-mediated STAT3 phosphorylation is suppressed by knocking down thioredoxin interacting protein, an inhibitor of thioredoxin. IL-22 is also found to promote the growth of SMCs via NADPH oxidase. In rats, pulmonary hypertension is found to be associated with increased smooth muscle IL-22 expression. These results show that IL-22 promotes the growth of pulmonary vascular SMCs via a signaling mechanism that involves NADPH oxidase-dependent oxidation.
Keywords: Interleukin-22, Reactive oxygen species, Redox Signaling, Vascular smooth muscle
Introduction
The growth of smooth muscle cells (SMCs) plays an important role in the development of pulmonary vascular remodeling, which contributes to the pathogenesis and complications of pulmonary hypertension [1-3]. Understanding complex cell signaling mechanisms for SMC growth should contribute to the development of therapeutic strategies to prevent and/or treat pulmonary hypertension as well as other vascular diseases. Factors that have been reported to mediate the growth of pulmonary vascular SMCs include endothelin-1, serotonin, and growth factors such as platelet-derived growth factor (PDGF) [4]. Recently, inflammatory cytokines have been proposed to play important roles as well [5].
Accumulating evidence suggests the importance of reactive oxygen species (ROS) in the mechanism of cell signaling for SMC growth induced by various factors [6,7]. NADPH oxidase plays a central role in producing ROS in response to ligand/receptor interactions during cell signaling [8,9]. ROS, in turn, promote oxidative modifications such as thiol oxidation [6,10] and protein carbonylation [11-13]. The precise mechanism of ROS signaling is, however, incompletely understood, and this interferes with developing effective therapeutic strategies that target ROS. For example, while endothelin-1, serotonin and PDGF have all been shown to produce ROS in SMCs (9,14,15), it is unclear if ROS indeed are the converging point for these distinct signaling stimuli. Other factors such as cytokines may also produce ROS and may contribute to the development of pulmonary vascular remodeling. Thus, the identification of new factors that promote SMC growth via ROS should provide important information to further understand the role of ROS in the pathogenesis of vascular remodeling.
Interleukin-22 (IL-22) is a relatively new member of the IL-10 family of cytokines, which is usually produced by Th17 cells [16,17]. Recently, airway SMCs have been shown to elicit IL-22 signaling [18,19]. The relationships between IL-22 and vascular SMCs, however, have not been reported. The present study demonstrates that IL-22 can elicit cell signaling in pulmonary vascular SMCs and that its mechanism involves NADPH oxidase and oxidative protein modifications.
Methods
Cell culture
Human pulmonary artery SMCs from ScienCell Research Laboratories (Carlsbad, CA, USA) were cultured in accordance with the manufacturers’ instructions in 5% CO2 at 37°C. Cells in passages 3-7 were used. Cells were growth-arrested for 2 days in media containing 0.01% fetal bovine serum (FBS) for proliferation assays or serum-starved overnight for signaling studies. Cells were treated with recombinant human IL-22 (PeproTech, Inc., Rocky Hill, NJ, USA) or human recombinant PDGF-BB (Invitrogen, Carlsbad, CA, USA). For siRNA knockdown, cells were transfected with siRNA Transfection Reagent and gene silencing siRNAs along with control with scrambled sequence from Santa Cruz Biotechnology (Dallas, TX, USA). Cells were used for experiments 2 days after transfection. Cell growth was assessed using a tetrazolium salt-based XTT assay (Trevigen, Inc., Gaithersburg, MD, USA).
Animal studies
Male Sprague Dawley rats (250 – 300 g) were subjected to chronic hypoxia for 2 weeks in a chamber regulated by an OxyCycler Oxygen Profile Controller (BioSpherix, Redfield, NY, USA) that was set to maintain 10% O2 with an influx of N2 [20]. Ventilation was adjusted to remove CO2, so that its level did not exceed 5,000 ppm. Normoxia controls were subjected to ambient 21% O2 in another chamber. Animals were fed normal rat chaw. The Georgetown University Animal Care and Use Committee approved all animal experiments, and the investigation conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Four micron sections of formalin-fixed tissues were taken, and immunohistochemical (IHC) staining of IL-22 and IL-22 receptor alpha 1 (IL-22Ra1) was performed using antibodies purchased from Santa Cruz Biotechnology.
Immunoblotting
Cell lysates were prepared and immunoblotting was performed as previously described [11] using antibodies for phospho-STAT3 (Tyr705) (Cell Signaling Technology, Danvers, MA, USA), cyclin D2, and glutathione (GSH) (Santa Cruz). Electrophoresis through an SDS-polyacrylamide gel was followed by electroblotting onto a nitrocellulose membrane. The Enhanced Chemiluminescence System (Amersham Biosciences, Piscataway, NJ, USA) was used for detection. Carbonyl content was monitored in 2,4-dinitrophenylhydrazine (DNPH)-derivatized proteins using an OxyBlot Protein Oxidation Detection Kit (Millipore, Billerica, MA, USA).
Statistical analysis
Means ± SEM were calculated and then comparisons between two groups were analyzed using a two-tailed Student's t test, while comparisons between three or more groups were analyzed using ANOVA with a Student-Newman-Keuls post-hoc test. P < 0.05 was considered to be significant.
Results
Pulmonary vascular SMCs express a functional IL-22 receptor
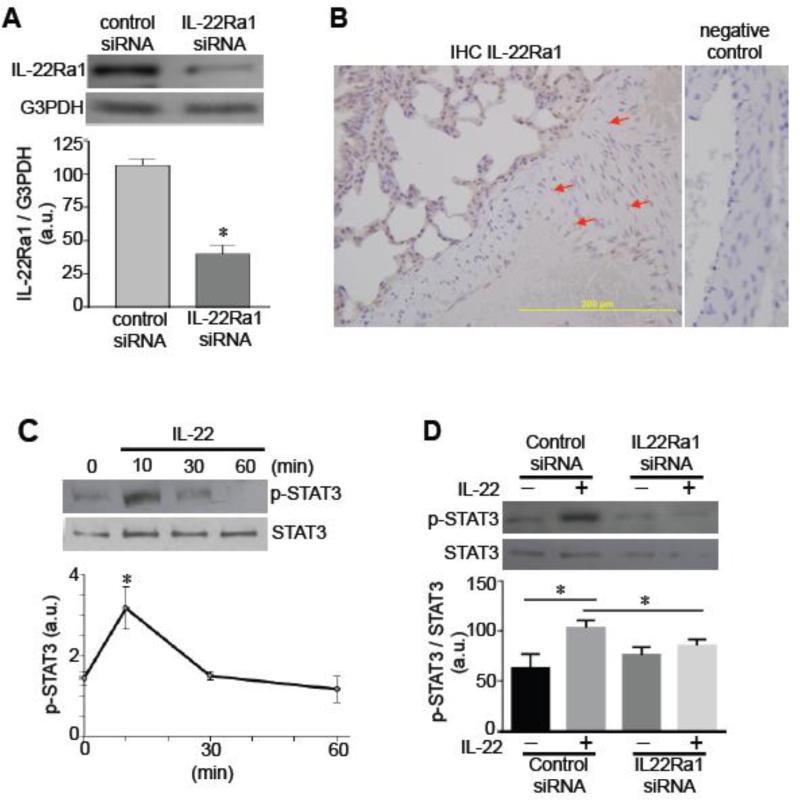
In cultured human pulmonary artery SMCs, the expression of IL-22Ra1 protein (an IL-22 receptor) was detected by immunoblotting (Fig. 1A). This IL-22Ra1 band was reduced by knocking down IL-22Ra1 in the cells by siRNA (Fig. 1A). IHC studies of the lung showed that the pulmonary artery medial smooth muscle layer of rats with pulmonary hypertension expresses IL-22Ra1 (Fig. 1B). To test whether the IL-22 receptor is functional, human pulmonary artery SMCs were treated with human recombinant IL-22. IL-22 promoted the phosphorylation of STAT3 at 10 min (Fig. 1C) that was blocked by knocking down IL-22Ra1 (Fig. 1D). To our knowledge, this is the first demonstration that vascular SMCs express a functional IL-22 receptor.
Fig. 1. Expression of functional IL-22 receptor in pulmonary artery SMCs.
[A] Human pulmonary artery SMCs were transfected with control siRNA or IL-22Ra1 siRNA. Cell lysates were subjected to immunoblotting with the IL-22Ra1 antibody. The glyceraldehyde-3-phosphate dehydrogenase (G3PDH) expression was monitored as a control. The bar graph represents means ± SEM (n = 6) of the ratio of IL-22Ra1 to G3PDH as expressed in arbitrary units (a.u.). The symbol * denotes values significantly different from control at P<0.05. [B] Rat lungs were immersed in buffered 10% paraformaldehyde, and embedded in paraffin for IHC with the IL-22Ra1 antibody. Brown stains in the pulmonary artery medial layer are indicated by the red arrows. [C] Human pulmonary artery SMCs were treated with IL-22 (10 ng/ml) for durations indicated. Cell lysates were prepared and subjected to immunoblotting for phosphorylated STAT3 (p-STAT3). The line graph represents means ± SEM (n = 3). * denotes values significantly different from control at P<0.05. [D] Human pulmonary artery SMCs were transfected with control siRNA or IL-22Ra1 siRNA, then treated with IL-22 for 10 min to monitor phosphorylated STAT3 by immunoblotting. The bar graph represents means ± SEM (n = 4). * denotes values significantly different from each other at P<0.05.
IL-22 promotes oxidative protein modifications
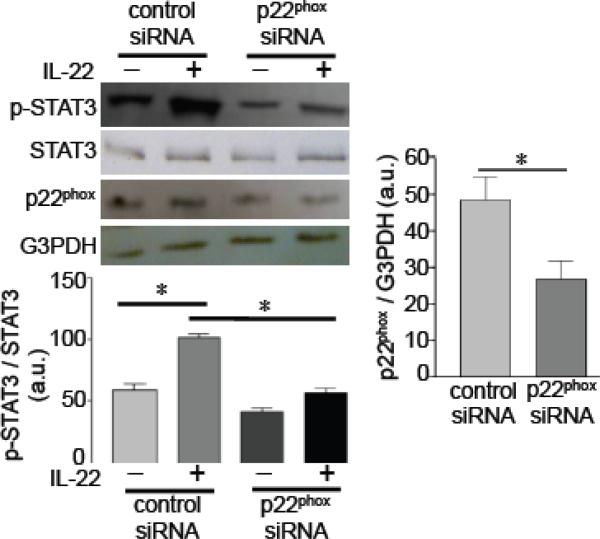
For the mechanism of IL-22-induced STAT3 phosphorylation, the role of NADPH oxidase was examined. The IL-22-mediated activation of STAT3 did not occur when the NADPH oxidase subunit, p22phox, was knocked down by siRNA (Fig. 2).
Fig. 2. Role of NADPH oxidase in IL-22 signaling.
Human pulmonary artery SMCs were transfected with control siRNA or p22phox siRNA, then treated with IL-22 (10 ng/ml) for 10 min to monitor phosphorylated STAT3 by immunoblotting. * denotes values significantly different from each other at P<0.05 (n = 5).
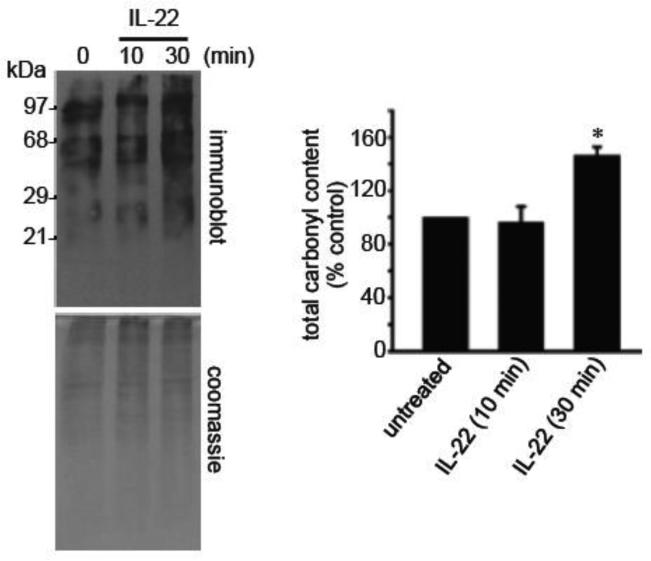
We next tested whether NADPH oxidase-generated ROS promotes the oxidative modification of proteins, specifically carbonylation and glutathionylation. A number of proteins were found to be carbonylated in response to IL-22 at 10 and 30 min as determined by derivatizing the carbonyl groups with DNPH (11,21) followed by immunoblotting (Fig. 3). Total protein carbonyl levels were significantly increased after 30 min of IL-22 stimulation. Two-dimensional gel electrophoresis identified 12 protein spots, which were carbonylated by IL-22 (Supplemental Figure). Mass spectrometry identified these proteins to be α-enolase (Spots 1 and 2), heat shock cognate 71 kDa protein (Spots 3 - 5), mitochondrial 60 kDa heat shock protein (Spots 6 - 8), and cytoplasmic 2 actin (Spots 9 - 12). Among them, heat shock cognate 71 kDa protein and cytoplasmic 2 actin were found to be carbonylated after 10 min of IL-22 treatment.
Fig. 3. IL-22 promotes protein carbonylation.
Human pulmonary artery SMCS were treated with IL-22 (10 ng/ml) to monitor carbonylated proteins using the OxyBlot kit. * denotes values significantly different from untreated control at P<0.05.
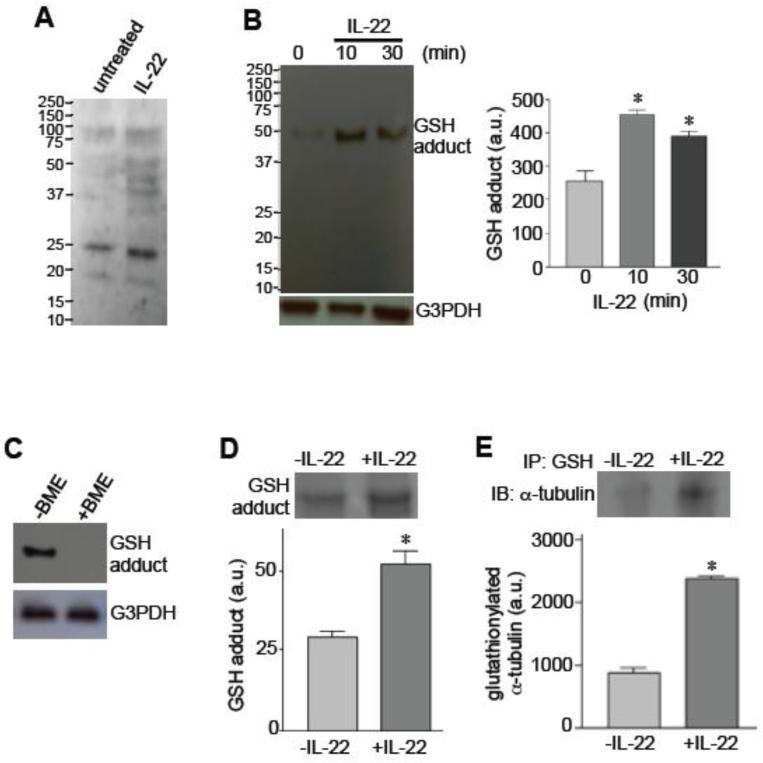
IL-22 increased the total GSH adduct formation in various proteins (protein glutathionylation) after 10 min of stimulation. While the GSH antibody from Millipore (Billerica, MA, USA) exhibited multiple proteins (Fig. 4A), we detected one clear band using the antibody from Santa Cruz Biotechnology. The glutathionylation of this ~50kDa band increased in response to IL-22 stimulation (Fig. 4B). These results were obtained using non-reducing gels to preserve GSH-protein interactions, and the 50kDa band was not observed in the presence of a reducing agent, β-mercaptothanol (BME), as shown in Fig. 4C.
Fig. 4. IL-22 promotes protein glutathionylation.
[A] Human pulmonary artery SMCS were treated with IL-22 (10 ng/ml) for 10 min, and the levels of GSH-protein adducts were monitored by non-reducing SDS-PAGE and immunoblotting using a GSH antibody from Millipore. [B] Human pulmonary artery SMCS were treated with IL-22 (10 ng/ml), and the levels of GSH-protein adducts were monitored by non-reducing SDS-PAGE and immunoblotting using a GSH antibody from Santa Cruz Biotechnology. [C] Immunoblotting for detecting GSH-protein adducts with the GSH antibody from Santa Cruz was performed without or with BME in SDS-PAGE. [D] Lysates from cells treated without or with IL-22 for 10 min were immunoprecipitated with the GSH antibody from Santa Cruz, followed by non-reducing SDS-PAGE and Coomassie blue staining. [E] Lysates from cells treated without or with IL-22 for 10 min were immunoprecipitated (IP) with the GSH antibody from Santa Cruz, followed by non-reducing SDS-PAGE and immunoblotting (IB) with the α-tubulin antibody to monitor the levels of glutathionylated α-tubulin. * denotes values significantly different from untreated control at P<0.05 (n = 3).
To identify the protein that was glutathionylated, cell lysate proteins were immunoprecipitated with the GSH antibody and subjected to SDS-PAGE. Consistent with the immunoblotting results, the Coomassie blue staining of the gels showed a 50 kDa band that increased in response to the IL-22 treatment of pulmonary artery SMCs (Fig. 4D). Mass spectrometry identified that the 50 kDa protein glutathionylated in response to IL-22 stimulation is α-tubulin. This finding was confirmed by performing immunoprecipitation/immunoblotting experiments with GSH and α-tubulin antibodies (Fig. 4E).
Role of glutaredoxin (GRX) and thioredoxin in IL-22 signaling
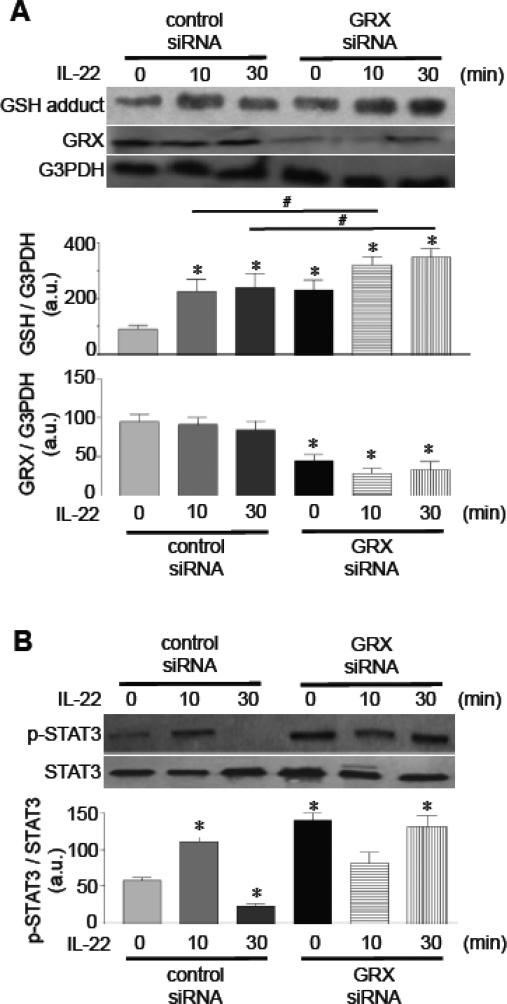
Glutathionylated proteins can be deglutathionylated by GRX or thioredoxin, while GRX is a specific enzyme that catalyzes the deglutathionylation reaction [22-25]. Consistently, the siRNA knock-down of GRX enhanced basal and IL-22-induced glutathionylation levels (Fig. 5A). The siRNA knock-down of GRX enhanced STAT3 phosphorylation (Fig. 5B), directly linking glutathionylation to STAT3 activation.
Fig. 5. Effects of GRX knock-down on IL-22 signaling.
Cells were transfected with control siRNA or GRX siRNA, then treated with IL-22 (10 ng/ml) to monitor [A] GSH protein adducts and [B] phosphorylated STAT3. Bar graphs represent means ± SEM (n = 3 - 7). * denotes values significantly different from untreated control at P<0.05. # denotes values significantly different from each other.
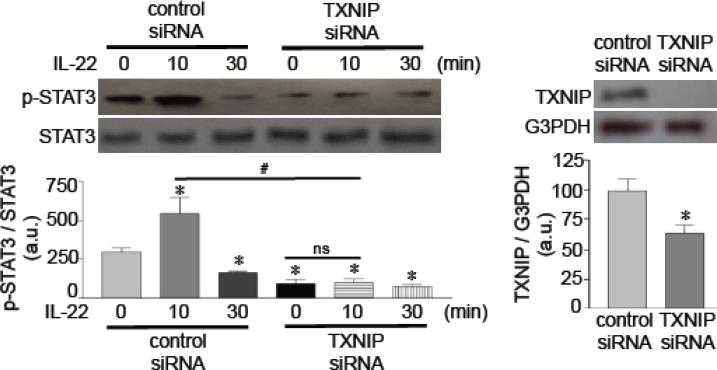
We tested the role of thioredoxin in the regulation of IL-22 signaling. Thioredoxin is inhibited by an endogenous regulator, thioredoxin interacting protein (TXNIP) [26]. Thus, the siRNA knock-down of TXNIP is expected to increase the activity of thioredoxin. We found that TXNIP knock-down inhibited IL-22-induced STAT3 phosphorylation (Fig. 6), providing causal evidence for the role of redox regulation in IL-22 signaling.
Fig. 6. Effects of TXNIP knock-down on IL-22 signaling.
Cells were transfected with control siRNA or TXNIP siRNA, then treated with IL-22 (10 ng/ml) to monitor phosphorylated STAT3. Bar graphs represent means ± SEM (n = 3). * denotes values significantly different from control at P<0.05. # denotes values significantly different from each other. The symbol ns denotes not significantly different from each other.
IL-22 is a growth factor of vascular SMCs
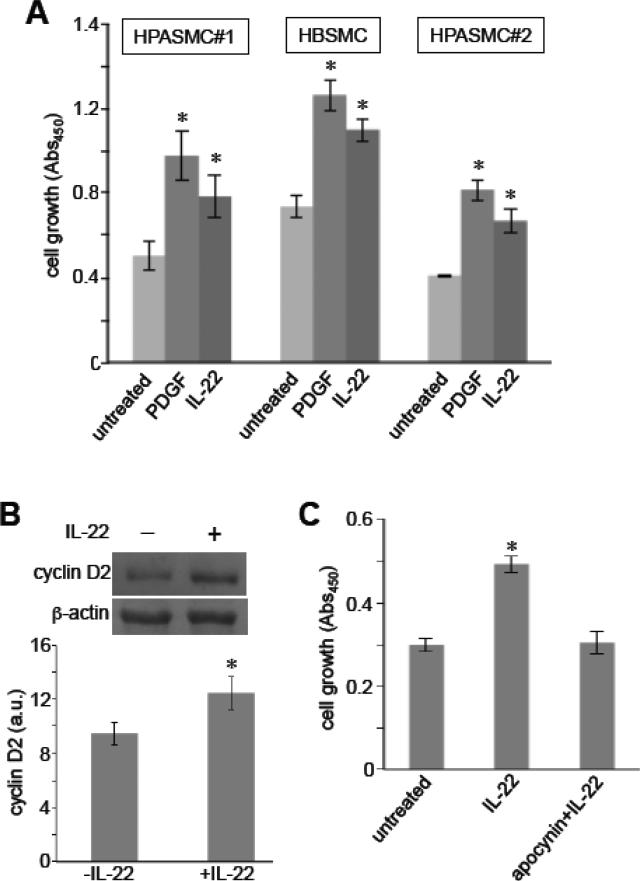
To determine the function of IL-22 in pulmonary artery SMCs, we tested whether IL-22 promotes pulmonary artery SMC growth, which is an important pathogenic mechanism for pulmonary vascular remodeling. We found that IL-22 promoted the proliferation of human pulmonary artery SMCs (Fig. 7A). While this and other experiments were performed using human pulmonary artery SMCs from ScienCell (HPASMC#1), similar results were obtained in human pulmonary artery SMCs from another supplier (Cell Applications, San Diego, CA; HPASMC#2). In addition to lung vascular SMCs, IL-22 also promoted the cell growth of airway SMCs (human bronchial SMCs; HBSMCs) [19] in a comparable fashion. In all these cells, the ability of 10 ng/ml human recombinant IL-22 to promote SMC growth was slightly weaker but comparable to the abilities of PDGF (10 ng/ml). In human pulmonary artery SMCs, IL-22 also promoted the gene expression of a cell cycle regulator, cyclin D2, which promotes cell proliferation (Fig. 7B). The inhibition of NADPH oxidase activity by apocynin significantly attenuated the IL-22-induced proliferation of pulmonary artery SMCs (Fig. 7C), suggesting the role of oxidant signaling in IL-22-induced cell growth.
Fig. 7. IL-22 promotes SMC growth.
[A] Human pulmonary artery SMCs (HPASMC) and human bronchial SMCs (HBSMC) were treated with 10 ng/ml PDGF or IL-22. Cell growth was analyzed using XTT assay (n = 6). HPASMC#1 were purchased from ScienCell, and HPASMC#2 were from Cell Applications. [B] Human pulmonary artery SMCs from ScienCell were treated with IL-22 for 2 days, and cyclin D2 protein expression was analyzed by immunoblotting (n = 3). [C] Cells were treated with apocynin (50 M), then treated with IL-22. Cell growth was monitored by XTT assay. * denotes values significantly different from untreated control at P<0.05.
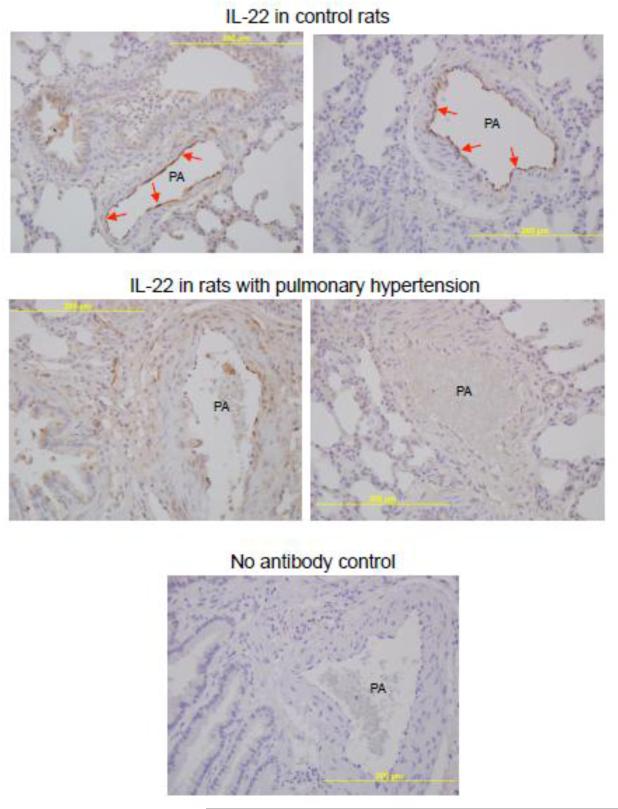
In rats, IHC analyses showed that pulmonary arteries of control normal rats have IL-22 protein highly expressed in the endothelium (Fig. 8). The promotion of pulmonary hypertension and pulmonary vascular remodeling by subjecting rats to chronic hypoxia for 2 weeks resulted in decreased IL-22 expression in the endothelium and some expression being detected in the smooth muscle media layer (Fig. 8). Brown positive staining for the IL-22 protein expression was not observed in control experiments without the use of the primary antibody. This increase in smooth muscle IL-22 was observed as early as 4 days (data not shown).
Fig. 8. IL-22 protein expression in the pulmonary artery (PA) of rats with pulmonary hypertension.
Rats were exposed to normoxia (control) or hypoxia (10% O2) for two weeks to induce pulmonary hypertension. Lungs were harvested, immersed in buffered 10% paraformaldehyde, and embedded in paraffin for IHC with the IL-22 antibody. Brown staining shows the IL-22 protein expression. Experiments were repeated at least three times. Two representative images from two different animals are shown for each condition. Red arrows indicate the strong IL-22 expression in the intimal endothelial layer in control rats.
Discussion
The present study demonstrates that IL-22 can promote cell growth signaling in vascular SMCs and that IL-22 activates ROS signaling. Specifically, we demonstrate the occurrence of IL-22 signaling in pulmonary artery SMCs, linking this cytokine to the pathogenesis of pulmonary hypertension and suggesting the possibility that IL-22/IL-22 receptor may be a therapeutic target for this disease.
In vivo animal experiments performed in the present study intriguingly showed that IL-22 is highly expressed in the pulmonary vascular endothelium in normal rats and that the promotion of pulmonary hypertension by the chronic exposure to hypoxia resulted in decreased endothelial IL-22 and the detection of some levels of IL-22 in the medial smooth muscle layer. These observations could suggest that the release of endothelial IL-22 may affect pulmonary vascular SMCs in a paracrine fashion. Indeed, pulmonary artery SMCs were found to express the functional IL-22 receptor and IL-22 can promote the growth of pulmonary artery SMCs. Thus, we propose that IL-22 may be a mediator of pulmonary vascular remodeling.
The present study also demonstrates that IL-22 can trigger oxidant signaling. Oxidants have been well recognized as cell signaling mediators, and many ligands have been shown to promote ROS production [6,7]. However, to date, the promotion of oxidant signaling by IL-22 has not been reported. Our data show that IL-22-mediated signaling events can be suppressed by inhibiting NADPH oxidase and that IL-22 can promote cellular oxidative protein modifications including protein carbonylation and glutathionylation.
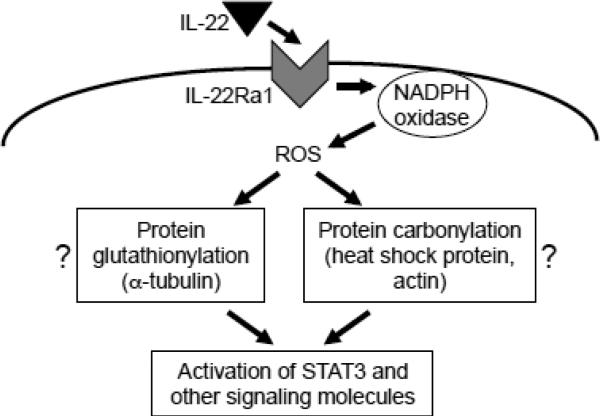
We previously reported that protein carbonylation mediates cell signaling in response to receptor activation by ligands such as endothelin-1 and PDGF [11-13,27]. However, IL-22 signaling may not utilize protein carbonylation for STAT3 activation, since the phosphorylation of STAT3 occurs 10 min after the IL-22 stimulation, while most proteins are not carbonylated until 30 min. We did identify that heat shock cognate 71 kDa protein and cytoplasmic 2 actin are carbonylated after 10 min of IL-22 treatment and thus oxidative modifications of these proteins via carbonylation may play a role in IL-22 signaling. We also found that IL-22 can elicit protein glutathionylation 10 min after the stimulation. Moreover, knocking-down GRX enhanced glutathionylation and STAT3 phosphorylation, and knocking-down TXNIP, a thioredoxin inhibitor, suppressed the IL-22-mediated events, suggesting that protein glutathionylation may mediate IL-22 signaling for STAT3 activation. We identified that α-tubulin is one protein that is glutathionylated by IL-22. It is possible that the modification of this structural protein may result in transmitting signals to protein kinase cascades, leading to STAT3 phosphorylation. Fig. 9 depicts these proposed signaling pathways. Further studies are needed to clarify this mechanism.
Fig. 9. A proposed pathway for IL-22-induced oxidant signaling.
IL-22 binds to IL-22Ra1 receptor and activates NADPH oxidase, which produces ROS. ROS, in turn, promote oxidative modifications of some proteins through glutathionylation and carbonylation. We propose that these oxidative modifications activate STAT3 and other signaling molecules.
In summary, the present study demonstrates that IL-22, which is rather a new cytokine without well-defined biologic roles, is a mitogen of the SMCs of multiple types (vascular and airway). Since the growth of SMCs is critical for the pathogenesis of various diseases including pulmonary hypertension, asthma, arteriosclerosis, and systemic hypertension, the results of the present study may have identified an important therapeutic target. Further work should include the functional elucidation of this cytokine in various diseases that involve SMC growth. The mechanisms of how the IL-22 receptor activates NADPH oxidase and how oxidative modifications mediate signaling for cell growth are also fruitful directions for future investigations.
Supplementary Material
Highlights.
- IL-22-mediated cell growth signaling in lung vascular smooth muscle cells is NADPH oxidase-dependent.
- IL-22 promotes oxidative protein modifications including carbonylation and glutathionylation.
- Protein glutathionylation and STAT3 phosphorylation is enhanced by knocking-down glutaredoxin.
- IL-22-mediated STAT3 phosphorylation is inhibited by knocking-down thioredoxin interacting protein.
- Endothelial IL-22 may be released to target pulmonary vascular smooth muscle for promoting vascular remodeling.
Acknowledgments
This work was supported in part by grants from National Institutes of Health (R01HL72844 and R01HL97514) and the American Heart Association Mid-Atlantic Affiliate (to YJS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakao S, Tatsumi K, Voelkel NF. Am. J. Respir. Cell Mol. Biol. 2010;43:629–634. doi: 10.1165/rcmb.2009-0389TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeffery TK, Morrell NW. Prog. Cardiovasc. Dis. 2002;45:173–202. doi: 10.1053/pcad.2002.130041. [DOI] [PubMed] [Google Scholar]
- 3.Stenmark KR, Fagan KA, Frid MG. Circ. Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 4.Wong CM, Bansal G, Pavlickova L, Marcocci L, Suzuki YJ. Antioxid. Redox Signal. 2013;18:1789–1796. doi: 10.1089/ars.2012.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hassoun PM, Mouthon L, Barberà JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. J. Am. Coll. Cardiol. 2009;54:S10–S19. doi: 10.1016/j.jacc.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Rhee SG, Bae YS, Lee SR, Kwon J. Sci STKE.2000. 2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki YJ, Forman HJ, Sevanian A. Free Radic. Biol. Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 8.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, Black SM. Circ. Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 9.Lee SL, Wang WW, Fanburg BL. Free Radic. Biol. Med. 1998;24:855–858. doi: 10.1016/s0891-5849(97)00359-6. [DOI] [PubMed] [Google Scholar]
- 10.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Wong CM, Cheema AK, Zhang L, Suzuki YJ. Circ. Res. 2008;102:310–318. doi: 10.1161/CIRCRESAHA.107.159814. [DOI] [PubMed] [Google Scholar]
- 12.Wong CM, Marcocci L, Liu L, Suzuki YJ. Antioxid. Redox Signal. 2010;12:393–404. doi: 10.1089/ars.2009.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong CM, Bansal G, Marcocci L, Suzuki YJ. Redox Rep. 2012;17:90–94. doi: 10.1179/1351000212Y.0000000007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 15.Wedgwood S, Dettman RW, Black SM. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L1058–L1067. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- 16.Eyerich S, Eyerich K, Cavani A, Schmidt-Weber C. Trends Immunol. 2010;31:354–361. doi: 10.1016/j.it.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Zenewicz LA, Flavell RA. Int. Immunol. 2011;23:159–163. doi: 10.1093/intimm/dxr001. [DOI] [PubMed] [Google Scholar]
- 18.Chang Y, Al-Alwan L, Risse PA, Roussel L, Rousseau S, Halayko AJ, Martin JG, Hamid Q, Eidelman DH. J Allergy Clin Immunol. 2011;127:1046–1053. doi: 10.1016/j.jaci.2010.12.1117. [DOI] [PubMed] [Google Scholar]
- 19.Chang Y, Al-Alwan L, Risse PA, Halayko AJ, Martin JG, Baglole CJ, Eidelman DH, Hamid Q. FASEB J. 2012;26:5152–5160. doi: 10.1096/fj.12-208033. [DOI] [PubMed] [Google Scholar]
- 20.Park AM, Wong CM, Jelinkova L, Liu L, Nagase H, Suzuki YJ. Hypertension. 2010;56:1145–1151. doi: 10.1161/HYPERTENSIONAHA.110.160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine RL, Williams JA, Stadtman ER, Shacter E. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- 22.Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, Rossi R. Antioxid. Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 23.Peltoniemi MJ, Karala AR, Jurvansuu JK, Kinnula VL, Ruddock LW. J. Biol. Chem. 2006;281:33107–33114. doi: 10.1074/jbc.M605602200. [DOI] [PubMed] [Google Scholar]
- 24.Lillig CH, Berndt C, Holmgren A. Biochim. Biophys. Acta. 2008;1780:1304–1317. doi: 10.1016/j.bbagen.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Pastore A, Piemonte F. Eur. J. Pharm. Sci. 2012;46:279–292. doi: 10.1016/j.ejps.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Spindel ON, World C, Berk BC. Thioredoxin interacting protein: redox dependent and independent regulatory mechanisms. Antioxid Redox Signal. 2012;16:587–596. doi: 10.1089/ars.2011.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong CM, Preston IR, Hill NS, Suzuki YJ. Iron chelation inhibits the development of pulmonary vascular remodeling. Free Radic Biol Med. 2012;53:1738–1747. doi: 10.1016/j.freeradbiomed.2012.08.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.