Abstract
B-cell mediated humoral responses are triggered in many human diseases including autoimmune, cancer, neurologic, and infectious diseases. However, the full exploitation of the information contained within a patient's antibody repertoire, for diagnosis, monitoring and even disease prediction has been limited due to the poor diagnostic performance of many immunoassay formats. We have developed Luciferase immunoprecipitation systems (LIPS) that harnesses light emitting proteins to generate high definition antibody profiles optimal for both diagnostics and biomarker discovery. Here we describe the results and implications from a range of LIPS antibody profiling studies performed in our laboratory. These include highly sensitive diagnostics for domestic and global pathogens, insights into infection-related diseases, discovery of new biomarkers for human diseases, subcategorization of symptoms and identification of pathogenic autoantibodies against self-proteins. These investigations highlight the types of humoral response profiles associated with different diseases, provide new information related to disease pathogenesis, and provide a framework for incorporating LIPS antibody profiling into global health initiatives and disease monitoring.
Keywords: antibody profiling, autoimmune, autoantibodies, biomarker, chronic disease, infectious disease, infectious disease, Luciferase immunoprecipitation systems (LIPS)
Introduction
The ability to detect a disease early is often a crucial factor in successful treatment because overall disease burden is low and associated tissue destruction is limited. While antibodies detected against specific antigenic targets have long been utilized as important clinical biomarkers for many diseases, a generalized disease surveillance technology based on antibody profiling is currently not available. In the case of infectious agents, the detection of antibodies is often critical for diagnosis and monitoring, and understanding vaccine responses. In autoimmune diseases, autoantibodies directed against self proteins are highly useful for diagnosis and even for disease prediction [1]. Autoantibodies are also present as biomarkers in many other human diseases including cancer, neurological and degenerative diseases, where the primary pathogenesis is not ostensibly related to autoimmunity. Unfortunately, most existing antibody detection technologies lack the sensitivity, specificity, multiplex capacity and robustness needed to fully exploit patient antibody profiling. Luciferase immunoprecipitation systems (LIPS) is a relatively new and highly informative antibody profiling technology for biomarker discovery and disease diagnostics. The goal of this short review is to illustrate the many advantages and new information that can be discovered by LIPS. These studies also lay the foundation for using LIPS as part of generalized disease surveillance technology.
Why is LIPS a powerful antibody profiling technology?
The detection of antibodies usually involves solid phase assays such as Western blotting, ELISA and protein arrays. For these assay formats, target antigens are directly immobilized to plates or membranes, which often prevents the proper presentation of many of the epitopes needed to detect antibodies with high sensitivity and specificity. Since the antigens used for solid phase ELISA and protein arrays are usually produced in bacteria or with bacterial extracts, there are often high backgrounds despite using blocking agents. Even after optimization for background noise, these solid phase assays have a narrow dynamic range of detection and sub-optimal detection of conformational epitopes. As an alternative, we developed the liquid phase LIPS assays, which employ Renilla luciferase (Ruc)-tagged antigens to detect antibodies to protein targets [2]. In these LIPS assays, chimeric genes encoding pathogen antigens fused to Renilla luciferase are expressed in mammalian cells, and crude extracts are prepared and used in immunoprecipitation assays to yield quantitative antibody profiles. LIPS, like other liquid phase assays is the preferred method for serological diagnosis of many autoimmune diseases because of its high sensitivity in detecting autoantibodies directed against both conformational and linear epitopes [3]. Despite the unique approach of using light-emitting proteins to measure antibody titers, the assay's general format is not patentable. Nevertheless, a key benefit of LIPS is its highly scalable format allowing facile and fast screening of panels of antigens. A detailed protocol and corresponding video describing the technical aspects of LIPS can be found on the internet [4]. Since protein targets are genetically fused to luciferase, there is no need to purify antigens. Furthermore, radioactive tracers, which are required for radiobinding liquid phase assays, are not needed, thereby eliminating the need for repeated labeling and associated disposal considerations. The major time-consuming step for producing antigens for LIPS analysis involves the cloning required to generate the Ruc-antigen fusion constructs [2]. Following expression in mammalian cells, the extracts are harvested and can be stored stably at −80° C until needed. These antigens are then used in the standard LIPS format without the need for assay optimization [4]. Generally, most bona fide antigenic targets used in the LIPS assay show high sensitivity, specificity and wide dynamic range of detection. These many advantages support LIPS as an ideal platform for profiling large panel of antigens for antibodies to infectious agents and autoantibodies to self-proteins.
Infectious disease diagnostics and antigen discovery by LIPS
Infectious agents represent major environmental factors that can cause human illness and disease. In an attempt to create a universal platform for comprehensively detecting antibodies against a large panel of human infectious agents, we have developed many different diagnostic tests for various filarial/helminthic [5-7], fungal [8], bacterial [9], and viral pathogens [8,10-14] LIPS assays for these diverse infectious agents often have higher sensitivity, specificity, and a larger dynamic range over existing standard assays. For example, LIPS tests were more effective than ELISAs for the diagnosis of filarial and helminthic infections including Loa loa [6], Strongyloides [7], and Onchocerciasis [5]. Not only did these LIPS tests diagnostically outperform existing ELISAs, but additional modifications of the LIPS format, which decrease incubation time, show promise for point-of-care testing [5,6]. The wide dynamic range of antibody detection for many of these LIPS tests is advantageous for monitoring response to treatment. LIPS profiling of antibodies generated against multiple hepatitis C virus (HCV) proteins has lead to the identification of biomarkers for differentiating long-term responders for HCV treatment in HCV-HIV-coinfected individuals [12]. In these studies the combined antibody titers to three HCV proteins, core, ENV1 and NS4, had potential for identification of long term responders from relapsers at 40 weeks following treatment with interferon-α and ribavirin for HCV infection. Although both long term responders and relapsers showed similar low levels of HCV RNA at the end of treatment, only the long-term responders exhibited significant decreases in anti-HCV antibody titers from pretreatment conditions. These results highlight the novel clinical information that can be gained from antibody LIPS profiling and its ability to guide clinical decision making.
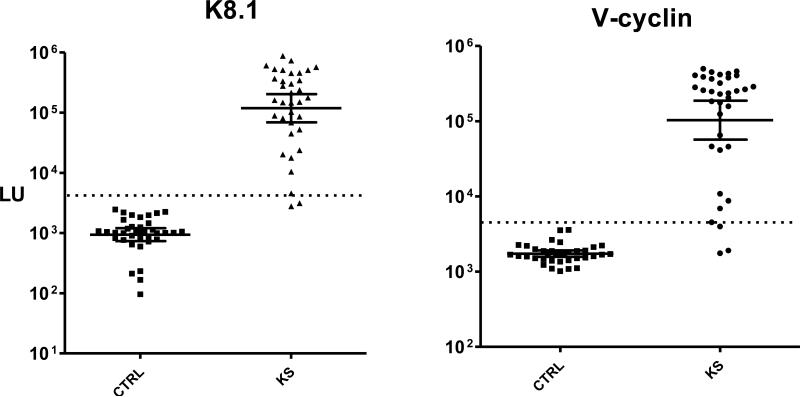
The ability to quickly generate and screen panels of proteins by LIPS is remarkably effective for identifying novel antigens. For LIPS testing, only a small amount of serum (1 microliter per test or less) is needed. Upon testing twenty different proteins from the Kaposi Sarcoma-associated herpes virus (KSHV/HHV-8) proteome, v-cyclin was identified as a new, informative antigen for diagnosis of KSHV infection in Kaposi Sarcoma (KS) patients [13]. Figure 1 shows LIPS detection of antibodies against the established K8.1 KSHV antigen and v-cyclin in KS patients. Of note, previous Western blot studies failed to detect antibodies to v-cyclin [15]. The ability to use v-cyclin for serological testing as an additional antibody marker is quite useful in light of the difficulty in diagnosing KSHV infection. Synthetic proteins combining elements from several protein domains or different bacterial or viral strains can also be expressed as recombinant Ruc fusions and serve as antigenic target. Such a synthetic antigen, which we called VOVO, formed the basis for a LIPS test for Lyme disease [9]. This new test can detect antibody titers spanning over 10,000-fold and demonstrates great promise for monitoring response to antibiotic treatment and diagnostic evaluation of Borrelia burgdorferi.
Figure 1. Anti-K8.1 and anti-v-cyclin antibodies in uninfected controls and KS patients.
Shown are antibody titer results from LIPS screening of 36 uninfected normal controls (CTRL) and 35 KS patients. Each symbol represents the average antibody titer from duplicate testing from an individual patient or control. The geometric mean antibody titer and 95% CI for anti-K8.1 lytic antibodies anti-v-cyclin latent antibody in light units (LU) are plotted on the Y-axis using a log10 scale.
While profiling single antigens from a particular infectious agent is the classical approach for diagnosis, the capacity to simultaneously measure antibody responses to large ensembles of proteins provides a route to high-content evaluation of individual patient responses. We recently constructed LIPS arrays encompassing the whole proteome of HIV and other infectious agents[16]. Using such arrays, highly robust titers of approximately 300-400 times the control samples can be found in the HIV-infected compared to uninfected controls [16]. LIPS arrays have also been used to study antibody responses to 25% of the proteins from the Epstein-Barr virus (EBV) proteome [16]. Antibodies to EBV were detected to over 50% of the antigens tested and more impressive titers were obtained compared to conventional protein arrays. Antibody titers generated by LIPS arrays are comparable to those generated by single antigen testing using LIPS indicating no loss of sensitivity using the array format. Because LIPS arrays show impressive titer differences compared to solid phase arrays and require little or no optimization, they comprise a new platform for antigen discovery. Future adaptations, including high efficiency cloning of the needed Ruc-antigen fusions, will make profiling even larger proteomes possible.
One of the most powerful applications of the LIPS technology is for detecting host humoral responses to novel infectious agents as evidence for in vivo expression of a pathogen. Based on the large number of newly described potential human viruses discovered from metagenomics studies, serological tests are needed to determine if these agents are infectious and whether they can cause disease. As an alternative to ELISAs, LIPS offers low backgrounds and the ability to generate diagnostically useful serodeterminations without any true positive and true negative control samples. Recently, human humoral responses were detected by LIPS to a new Astrovirus [Burbelo, P.D., Ching, K.H., Esper, F., Iadarola, M.J., Delwart, E., Lipkin, W. I., Kapoor, A, Unpublished Data]. This finding suggests that LIPS will likely provide a highly informative tool for investigation of novel agents to determine their pathogenic potential.
Infectious disease monitoring and disease stratification by LIPS
A full understanding of antibody responses to most infectious agents as it relates to disease severity and symptomatology has not been fully explored. In many cases, a single infectious agent can cause a broad range of symptoms, from mild to fatal illnesses. To examine biomarkers of infection-associated clinical symptoms, LIPS antibody profiles were determined from patients with different conditions or symptom presentations caused by the same infectious agent (Table I). Infection by the HTLV-I retrovirus provides a proof of concept. HTLV-1 infection can result in three clinical conditions: asymptomatic infection, HTLV-I –related T cell lymphoma (ATLL) and a HTLV-I associated demyelinating neurological disease, HAM/TSP. From evaluating antibody titers to different HTLV-I proteins in these three conditions, antibody titers to the HTLV-I envelope in HAM/TSP patients were observed to be much higher than in asymptomatic infected patients or patients with HTLV-I-associated lymphoma [14]. In contrast, antibody titers to the HTLV-I Gag protein were essentially identical between the three groups. These results suggest that anti-envelope antibodies are a biomarker for HTLV-I associated neurological disease and that immune responses to the envelope may be involved in the pathogenesis of HAM/TSP.
Table I.
Stratifying Different Diseases Caused by the same Infectious Agent
| Infectious Agent | LIPS antibody Profile in Disease State | Reference |
|---|---|---|
| HTLV-I | High anti-ENV antibodies in HAM/TSP vs. lower anti-ENV antibody titers in ATLL and asymptomatic-HTLV-infected. | [14] |
| KSHV | High anti-lytic antibodies in MCD vs. lower titer in KS. Low anti-latent antibodies in MCD vs. higher titer in KS. | [10] |
| EBV | High anti-lytic antibodies in CAEBV vs. low titer in asymptomatic EBV-infected. Low anti-EBNA1 antibodies in CAEBV vs. higher titer in asymptomatic EBV-infected. | [18] |
In another study, antibody responses to the herpes virus, KSHV, were studied in asymptomatic individuals and patients with Kaposi sarcoma (KS) or two lymphoproliferative diseases, Multicentric Castleman's disease (MCD) and primary effusion lymphoma (PEL). In some patients, MCD can occur by itself (MCD+/KS−) or together with KS (MCD+/KS+). LIPS profiling of antibodies against K8.1, a KSHV lytic antigen, revealed the lowest titers in KS, intermediate in MCD+/KS+, and highest in the MCD+/KS− and PEL samples (Table I) [17]. In contrast, antibodies against two latent antigens, v-cyclin and LANA, were over 14-fold higher in the KS samples compared to the MCD samples (Table I). The combined sum of anti-v-cyclin and anti-LANA antibody titers discriminated KS from MCD+/KS+ with 93% sensitivity and 83% specificity [17]. These results support the possibility that the higher anti-latent antibody responses in KS as compared to MCD may include greater expression of KSHV latent antigens in KS or blunting of specific antibodies in MCD. Here again, the wide dynamic range of antibody titers detected by LIPS enabled the separation of these different disease groups.
High level of infection by another herpes virus, EBV, causes a rare lymphoproliferative condition called chronic active EBV disease (CAEBV). From LIPS analysis, a unique anti-EBV antibody profile was detected in CAEBV patients compared to healthy controls [18]. While nearly all healthy controls have been exposed to EBV and have antibodies to certain EBV proteins, CAEBV patients showed markedly higher antibody titers to a number of lytic antigens, including the capsid proteins p23 and p18 and a DNA processivity factor (BMRF1) (Table I). In contrast, low levels of antibody responses to the latent EBNA1 antigen were detected in CAEBV patients compared to controls (Table I). This differential LIPS antibody profile allowed the CAEBV patients to be distinguished and is consistent with high levels of EBV replication in CAEBV patients. Together these studies with EBV-, KSHV- and HTLV-I-associated diseases suggest that detailed analysis of antibody titers against other infectious agents associated with different disease states or symptoms might yield insights into infection-related diseases and subcategorization of symptoms. Lastly, the ability to profile multiple antigens in a single format such as LIPS represents a powerful tool in infectious disease monitoring and diagnosis.
Detection of autoantibodies by LIPS in autoimmune diseases
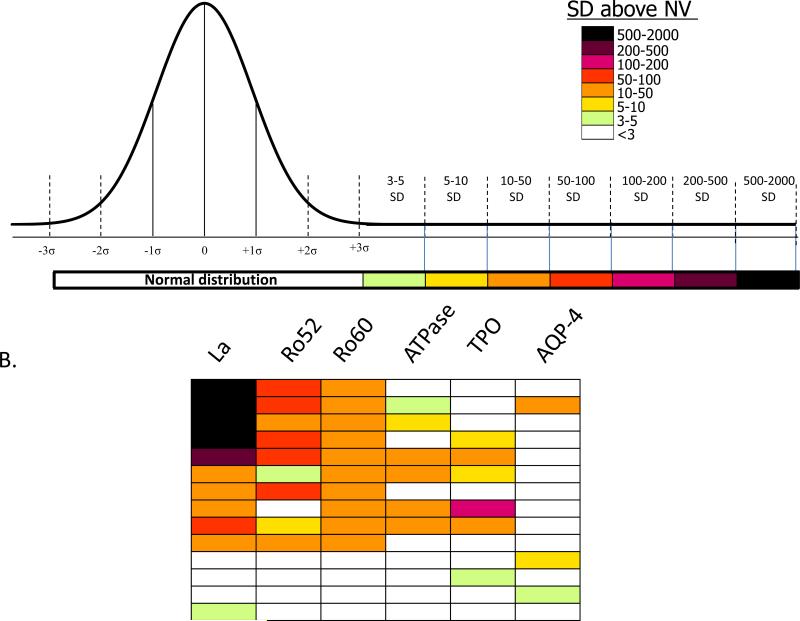
Human autoantibodies represent a rich source of biomarkers and can provide novel insights into disease and associated symptoms. However, the exact titers and spectrum of autoantibodies against most proteins are presently not known in healthy subjects and the identification of novel autoantibodies associated with a particular disease is an active area of research. LIPS offers a unique perspective on the range of antibodies that can be observed for a given antigen. Important antibody biomarker responses that range from above the control mean plus 3 standard deviations (SD) to over 2000 standard deviations greater than control subjects (Figure 2A) can be detected by LIPS in many different autoimmune diseases including Sjögren's syndrome (SjS) [19-21], Myasthenia gravis [22], Stiff person syndrome [23], and Type I diabetes [24,25]. Due to the large number of antigens that can be tested by LIPS and the wide dynamic range of each assay, a heatmap is often used to visualize the data. A representative heatmap of the types of antibody responses that can be detected by LIPS in SjS patients, an autoimmune disease characterized by immune attack against salivary and lacrimal glands, is shown in Figure 2B. Of note, the robust autoantibody titer values detected by LIPS make it possible for rapid point of care testing [20] and even for using saliva instead of serum in SjS testing [26]. LIPS antibody profiling also yields insights into gastrointestinal, neurological and other symptoms present in some patients with SjS. Testing for autoantibodies against a number of extraglandular targets, revealed autoantibodies to thyroid and stomach proteins suggesting that these organs are also susceptible to autoimmune attack in certain subgroups of SjS patients [21]. Interestingly, upon correlating antibody profiles with clinical data, autoantibodies to the AQP-4 water channel were identified as a specific marker of neuropathy in SjS [21]. In conclusion, by evaluating a large repertoire of autoantibodies in SjS, and likely other autoimmune diseases, LIPS antibody profiles yield important insights into symptoms research and disease stratification that is useful for treatment, disease monitoring and understanding pathogenesis of autoimmune disorders.
Figure 2. Autoantibody biomarker detection by LIPS.
(A) Autoantibodies detected by LIPS often show a wide range of titers. Autoantibody titers are determined in a control normal volunteer (NV) group as a reference scale. Antibody titer values for each antigen-antibody measurement greater than the control mean plus 3 SD were color-coded to signify the relative number of standard deviations above these cut-off values. In some autoimmune diseases antibody responses ranging from the mean plus 3 standard deviations (SD) to over 2000 standard deviations greater than control subjects can be detected. (B) A heatmap of autoantibody responses in Sjögren's syndrome (SjS). The heatmap shows the heterogeneity of responses and antibody titers to the different antigens in selected SjS patients, whereby each row in the heatmap represents one patient. Of note, approximately 15% of the SjS cohort examined by LIPS did not show statistical response to any of the autoantigens tested [21].
In type I diabetes (T1D), the detection of autoantibodies is not only useful for diagnosis but also can be used for predicting T1D in certain high risk children [1]. Due to the highly conformational epitopes associated with T1D autoantigens, radioactive liquid phase assays, which generally have higher sensitivity and specificity than solid phase assays, are the preferred method for detecting autoantibodies to IA2, GAD65 and other T1D autoantigens [3]. LIPS offers a non-radioactive alternative for detecting these T1D-associated autoantibodies with high diagnostic performance [24,25]. Although not yet explored in T1D, the ability to employ antigen panels and the large dynamic range of antibody detection with LIPS may provide additional information for disease prediction and stratification. Lastly, LIPS provides an ideal format to investigate, in parallel, the role infectious agents play, if any, in triggering or modulating T1D or other autoimmune diseases.
Discovery of antibody biomarkers by LIPS for complex human diseases
In addition to infectious and autoimmune diseases, profiling autoantibody biomarkers offers a potentially inexpensive tool for the diagnosis of cancer, neurologic and other acute and chronic diseases. However, to date, many autoantibody studies using solid phase formats show sub-optimal sensitivity, specificity and robustness required for these tests to have any practical clinical value. As an alternative, LIPS offers a promising tool for detecting autoantibodies in a host of diseases including cancer [27], neurologic conditions [28] and even in acute illnesses [29]. In ARDS and sepsis, two conditions known to have widespread immune activation and cytokine storms, LIPS antibody profiling detected a wide spectrum of autoantigenic targets. For example, a subset of patients was found to have autoantibodies against several major organ sites including the KCNRG lung protein, the gastric ATPase, several neural antigens and even several cytokines [29]. Interestingly, closely spaced longitudinal sampling disclosed that these autoantibody responses appear very rapidly, often occurring within 72 hours following admittance to the intensive care unit and in some cases remain elevated through the end of the study. While understanding the full-spectrum of autoantibodies generated during ARDS and other diseases is a difficult task, these autoantibody profiles may provide new biomarkers for early detection and yield insight into pathogenesis. Clearly, LIPS has many features that would be optimal for large scale autoantibody analysis. Future studies screening large panels of antigens by LIPS offers the possibility of identifying many new autoantigen biomarkers that ultimately could be incorporated into a generalized disease surveillance technology for a wide spectrum of different diseases.
Discovery of pathogenic autoantibodies by LIPS
LIPS also provides the opportunity to discover pathogenic autoantibodies directed at other receptors, channels, cytokines, and extracellular ligands that interfere with normal cellular function. For example in Myasthenia Gravis, LIPS has been used to study pathogenic autoantibodies, directed against the muscle endplate derived nicotinic acetylcholine receptor, which causes muscle weakness and tissue destruction [22]. Despite the membrane-spanning properties of the multi-subunit nicotinic acetylcholine receptor, luciferase fusion proteins derived from the single alpha1 subunit of the nicotinic acetylcholine receptor were used to study autoantibodies in patients with Myasthenia Gravis. One fusion protein containing the single alpha1 subunit of the nicotinic acetylcholine in LIPS showed 32% sensitivity compared with the native multi-subunit receptor used in a radiobinding assay with 63% sensitivity [22]. These results highlight the ability of LIPS to detect autoantibodies directed at specific individual subunits of a given receptor and suggest that incorporation of additional neurotransmitter receptors subunits in the scalable format will likely increase the diagnostic sensitivity.
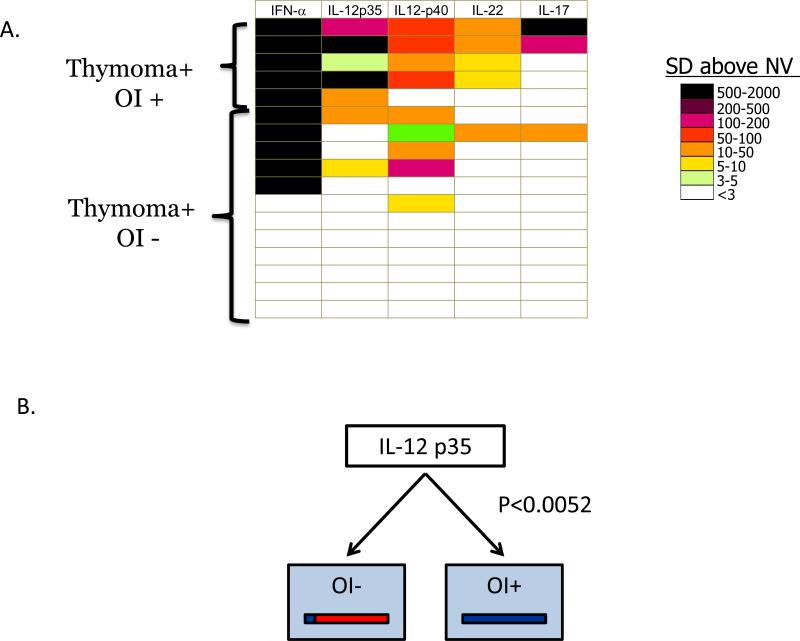
Extracellular ligands involved in cellular communication can also be targets of pathogenic autoantibodies. One group of molecules that has not been thoroughly explored as autoantigen targets are cytokines. In certain patients, autoantibodies targeting different cytokines may alter and even block immune responses to certain infectious agents resulting in various types of immunodeficiency and consequently opportunistic infection [30]. To explore this issue, we have created a large panel of over 40 cytokines as a tool to study pathogenic autoantibodies associated with immunodeficiency [31]. From screening this cytokine panel in thymoma patients with and without opportunistic infections, a diverse spectrum of anti-cytokine autoantibodies were identified in a subset of these patients. As previously observed [32,33], high titer autoantibodies to interferons were often found in thymoma patients. Overall, high titer autoantibodies were detected in different thymoma patients against 17 of the 40 cytokines in the panel. Additional functional assays such as bioassays for signaling showed that many of these high titer anti-cytokine autoantibodies detected by LIPS blocked normal cytokine function [31]. A heatmap showing the autoantibody profile against a selected number of these cytokines in these thymoma patients is shown in Figure 3A. The high quality LIPS anti-cytokine autoantibody data is also suitable for unbiased machine-based data analysis. For example, a decision tree algorithm with the LIPS autoantibody data showed that anti-IL12 p35 antibodies were the most informative and were able to differentiate most thymoma patients with opportunistic infection from those without (Figure 3B). Together these findings suggest that LIPS provides a rapid tool to screen for autoantibodies against large numbers of candidate cytokines, which then can be further analyzed in detail using functional assays to prove their pathogenic potential. Lastly, understanding the full extent of anti-cytokine and other pathogenic autoantibodies represents a rich translational area for studying human disease.
Figure 3. Anti-cytokine autoantibodies detected by LIPS in thymoma patients with opportunistic infection correlate with immunodeficiency.
(A) Heatmap analysis of anti-cytokine autoantibody profiles in thymoma patients. Anti-cytokine autoantibody titers to 5 informative cytokines are shown for each of five thymoma patients with and twelve thymoma patients without opportunistic infection (OI). Each row in the heatmap represents one patient from a total of 17 thymoma patients analyzed. The titer values greater than the mean of the 30 normal volunteers plus 3 standard deviations were color-coded from green to black to signify the relative number of standard deviations above these reference values. (B) Anti-IL-12 p35 autoantibodies correlate with OI. A decision tree algorithm using rapid miner (www.rapidminer.com) identified IL-12 p35 as the most informative anti-cytokine response for distinguishing thymoma patient with and without OI.
Expert Commentary
It is likely that antibody biomarkers can be discovered by LIPS for diagnosis, monitoring and prediction of many human diseases. In particular, the highly quantitative antibody profiles generated by LIPS are ideal for developing diagnostics for domestic and global pathogens and provide novel insights into infection-related disease. The development of LIPS arrays offer the opportunity to simultaneously screen partial or whole proteomes of infectious agents, as well as potentially hundreds of antigens from different human infectious agents. Similarly, the detection of autoantibodies by LIPS represents a new and highly useful tool for studying diverse autoimmune conditions and for gaining insight into diagnosis, prediction, and subcategorization of symptoms and even for the identification of pathogenic autoantibodies. Ultimately a collection of antigenic targets from infectious disease and autoantigenic targets could be incorporated into a comprehensive disease surveillance technology, which could be employed in routine medical assessments throughout a patient's lifetime as a means of obtaining highly informative and potentially predictive information on a wide spectrum of diseases.
Five-year view
It is expected that in the next 5 years, antibody profiles generated by LIPS will continue to provide important information related to antigen discovery, disease diagnosis, and understanding of human disease pathogenesis. LIPS antibody profiling of both autoantigens and infectious agents will also allow the exploration of many human disease states, such as autism and chronic fatigue, in which etiology of the disease is not known.
Acknowledgments
This work was supported by the intramural research program of the National Institute of Dental and Craniofacial Research, NIH. The authors thank the many patients who volunteered for these studies. We are also indebted to our many intramural and extramural collaborators.
Footnotes
Financial & competing interest disclosure
Two of the authors (P.D.B. and M.J.I.) have patent applications submitted using LIPS. The authors have no relevant affiliation or other financial involvement with any organization. No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•of interest
••of considerable interest
- 1•.Notkins AL. New predictors of disease. Molecules called predictive autoantibodies appear in the blood years before people show symptoms of various disorders. Tests that detected these molecules could warn of the need to take preventive action. Scientific American. 2007;296(3):72–79. [Provides an overview of how autoantibodies can be used as disease predictors.] [PubMed] [Google Scholar]
- 2.Burbelo PD, Ching KH, Bush ER, Han BL, Iadarola MJ. Antibody-profiling technologies for studying humoral responses to infectious agents. Expert review of vaccines. 2010;9(6):567–578. doi: 10.1586/erv.10.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu E, Eisenbarth GS. Accepting clocks that tell time poorly: fluid-phase versus standard ELISA autoantibody assays. Clin Immunol. 2007;125(2):120–126. doi: 10.1016/j.clim.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Burbelo PD, Ching KH, Klimavicz CM, Iadarola MJ. Antibody profiling by Luciferase Immunoprecipitation Systems (LIPS). J Vis Exp. 2009;(32) doi: 10.3791/1549. [Contains a detailed protocol and video describing the technical aspects of performing LIPS.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burbelo PD, Leahy HP, Iadarola MJ, Nutman TB. A four-antigen mixture for rapid assessment of Onchocerca volvulus infection. PLoS Negl Trop Dis. 2009;3(5):e438. doi: 10.1371/journal.pntd.0000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burbelo PD, Ramanathan R, Klion AD, Iadarola MJ, Nutman TB. Rapid, novel, specific, high-throughput assay for diagnosis of Loa loa infection. Journal of clinical microbiology. 2008;46(7):2298–2304. doi: 10.1128/JCM.00490-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. The Journal of infectious diseases. 2008;198(3):444–451. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burbelo PD, Ching KH, Mattson TL, Light JS, Bishop LR, Kovacs JA. Rapid antibody quantification and generation of whole proteome antibody response profiles using LIPS (luciferase immunoprecipitation systems). Biochem Biophys Res Commun. 2007;352(4):889–895. doi: 10.1016/j.bbrc.2006.11.140. [DOI] [PubMed] [Google Scholar]
- 9•.Burbelo PD, Issa AT, Ching KH, Cohen JI, Iadarola MJ, Marques A. Rapid, simple, quantitative, and highly sensitive antibody detection for lyme disease. Clin Vaccine Immunol. 2010;17(6):904–909. doi: 10.1128/CVI.00476-09. [Describes the use of a synthetic antigen containing immunodominant epitopes for the diagnosis of Lyme disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbelo PD, Hoshino Y, Leahy H, et al. Serological diagnosis of human herpes simplex virus type 1 and 2 infections by luciferase immunoprecipitation system assay. Clin Vaccine Immunol. 2009;16(3):366–371. doi: 10.1128/CVI.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burbelo PD, Issa AT, Ching KH, et al. Highly quantitative serological detection of anti-cytomegalovirus (CMV) antibodies. Virol J. 2009;6:45. doi: 10.1186/1743-422X-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbelo PD, Kovacs JA, Ching KH, et al. Proteome-wide anti-hepatitis C virus (HCV) and anti-HIV antibody profiling for predicting and monitoring the response to HCV therapy in HIV-coinfected patients. The Journal of infectious diseases. 2010;202(6):894–898. doi: 10.1086/655780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burbelo PD, Leahy HP, Groot S, et al. Four-antigen mixture containing v-cyclin for serological screening of human herpesvirus 8 infection. Clin Vaccine Immunol. 2009;16(5):621–627. doi: 10.1128/CVI.00474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14••.Burbelo PD, Meoli E, Leahy HP, et al. Anti-HTLV antibody profiling reveals an antibody signature for HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Retrovirology. 2008;5:96. doi: 10.1186/1742-4690-5-96. [Demonstrates that detection of anti-Envelope but not anti-GAG antibodies against HTLV-I by LIPS is a biomarker of HAM/TSP.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katano H, Iwasaki T, Baba N, et al. Identification of antigenic proteins encoded by human herpesvirus 8 and seroprevalence in the general population and among patients with and without Kaposi's sarcoma. Journal of virology. 2000;74(8):3478–3485. doi: 10.1128/jvi.74.8.3478-3485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Burbelo PD, Bren KE, Ching KH, et al. LIPS arrays for simultaneous detection of antibodies against partial and whole proteomes of HCV, HIV and EBV. Molecular BioSystems. 2011 doi: 10.1039/c0mb00342e. [Describes a LIPS array format for simultaneous testing of multiple antigens from partial and whole proteomes of small viruses.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Burbelo PD. Distinct Profiles of Antibodies to Kaposi's Sarcoma-Associated Herpesvirus Antigens in Patients with Kaposi Sarcoma, Multicentric Castlemen's Disease, and Primary Effusion Lymphoma. The Journal of infectious diseases. 2010;198(3):444–451. doi: 10.1086/652869. al e. [Describes how antibodies against lytic and latent antigens of KSHV can discriminate between different KSHV-associated diseases.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen JI, Jaffe ES, Dale J, Pittaluga S, Heslop H, Rooney C, Gottschalk S, Rao K, Marques A, Burbelo PD, Turk S-P, Fulton R, Wayne A, Little R, Cairo MS, El-Mallawany NK, Fowler D, Sportes C, Bishop M, Wilson, Straus SE. Treatment of Chronic Active Epstein-Barr Virus Disease: A 28 Year Experience in the United States. Blood. 2011 doi: 10.1182/blood-2010-11-316745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burbelo PD, Ching KH, Han BL, Bush ER, Reeves WH, Iadarola MJ. Extraordinary antigenicity of the human Ro52 autoantigen. American journal of translational research. 2010;2(2):145–155. [PMC free article] [PubMed] [Google Scholar]
- 20.Burbelo PD, Ching KH, Issa AT, et al. Rapid serological detection of autoantibodies associated with Sjogren's syndrome. Journal of translational medicine. 2009;7:83. doi: 10.1186/1479-5876-7-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burbelo PD, Leahy HP, Issa AT, et al. Sensitive and robust luminescent profiling of anti-La and other autoantibodies in Sjogren's syndrome. Autoimmunity. 2009;42(6):515–524. doi: 10.1080/08916930902911738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ching KH, Burbelo PD, Kimball RM, Clawson LL, Corse AM, Iadarola MJ. Recombinant expression of the AChR-alpha1 subunit for the detection of conformation-dependent epitopes in Myasthenia Gravis. Neuromuscul Disord. 2011 doi: 10.1016/j.nmd.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burbelo PD, Groot S, Dalakas MC, Iadarola MJ. High definition profiling of autoantibodies to glutamic acid decarboxylases GAD65/GAD67 in stiff-person syndrome. Biochem Biophys Res Commun. 2008;366(1):1–7. doi: 10.1016/j.bbrc.2007.11.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burbelo PD, Hirai H, Issa AT, et al. Comparison of radioimmunoprecipitation with luciferase immunoprecipitation for autoantibodies to GAD65 and IA-2beta. Diabetes care. 2010;33(4):754–756. doi: 10.2337/dc09-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Burbelo PD, Hirai H, Leahy H, et al. A new luminescence assay for autoantibodies to mammalian cell-prepared insulinoma-associated protein 2. Diabetes care. 2008;31(9):1824–1826. doi: 10.2337/dc08-0286. [Documents LIPS as an alternative to RBA testing for autoantibodies to type I diabetes autoantigen.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ching KH, Burbelo PD, Gonzalez-Begne M, et al. Salivary anti-Ro60 and anti-Ro52 Antibody Profiles to Diagnose Sjogren's Syndrome. Journal of dental research. 2011 doi: 10.1177/0022034510390811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burbelo PD, Goldman R, Mattson TL. A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins. BMC Biotechnol. 2005;5:22. doi: 10.1186/1472-6750-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ching KH, Burbelo PD, Carlson PJ, Drevets WC, Iadarola MJ. High levels of Anti-GAD65 and Anti-Ro52 autoantibodies in a patient with major depressive disorder showing psychomotor disturbance. Journal of neuroimmunology. 2010;222(1-2):87–89. doi: 10.1016/j.jneuroim.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29••.Burbelo PD, Seam N, Groot S, et al. Rapid induction of autoantibodies during ARDS and septic shock. Journal of translational medicine. 2010;8:97. doi: 10.1186/1479-5876-8-97. [Documents by LIPS the non-autoimmune induction of humoral responses to self proteins during acute inflammatory conditions.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Browne SK, Holland SM. Anticytokine autoantibodies in infectious diseases: pathogenesis and mechanisms. The Lancet infectious diseases. 2010;10(12):875–885. doi: 10.1016/S1473-3099(10)70196-1. [DOI] [PubMed] [Google Scholar]
- 31••.Burbelo PD, Browne SK, Sampaio EP, et al. Anti-cytokine autoantibodies are associated with opportunistic infection in patients with thymic neoplasia. Blood. 2010;116(23):4848–4858. doi: 10.1182/blood-2010-05-286161. [Important publication describing the robust detection of autoantibodies against cytokines by LIPS and their association with opportunistic infection.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meager A, Vincent A, Newsom-Davis J, Willcox N. Spontaneous neutralising antibodies to interferon--alpha and interleukin-12 in thymoma-associated autoimmune disease. Lancet. 1997;350(9091):1596–1597. doi: 10.1016/s0140-6736(05)64012-3. [DOI] [PubMed] [Google Scholar]
- 33.Meager A, Wadhwa M, Dilger P, et al. Anti-cytokine autoantibodies in autoimmunity: preponderance of neutralizing autoantibodies against interferon-alpha, interferon-omega and interleukin-12 in patients with thymoma and/or myasthenia gravis. Clinical and experimental immunology. 2003;132(1):128–136. doi: 10.1046/j.1365-2249.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]