Abstract
Clathrin assembly proteins AP180 and CALM regulate the assembly of clathrin-coated vesicles (CCVs), which mediate diverse intracellular trafficking processes, including synaptic vesicle (SV) recycling at the synapse. Although studies using several invertebrate model systems have indicated a role for AP180 in SV recycling, less is known about AP180’s or CALM’s function in the synapse of mammalian neurons. In this study, we examined synapses of rat hippocampal neurons in which the level of AP180 or CALM had been reduced by RNA interference (RNAi). Using light microscopy, we visualized synaptic puncta in these AP180- or CALM-reduced neurons by co-expressing Synaptophysin::EGFP (Syp::EGFP). We found that neurons with reduced AP180 or reduced CALM had smaller Syp::EGFP-illuminated puncta. Using electron microscopy, we further examined the ultrastructure of the AP180- or CALM-reduced presynaptic terminals. We found that SVs became variably enlarged in both the AP180-reduced and CALM-reduced presynaptic terminals. Lower AP180 and CALM also reduced the density of SVs and the size of SV clusters. Our findings demonstrate that in the presynaptic terminals of hippocampal neurons, AP180 and CALM have a similar role in regulating synaptic vesicles. This overlapping activity may be necessary for high-precision and high-efficacy SV formation during endocytosis.
Keywords: AP180, CALM, Hippocampal synapse, Synaptic vesicle
Introduction
Efficient and precisely regulated synaptic vesicle (SV) recycling is a crucial process in assuring the quantity and quality of SVs in the synapse of the neuron, a prerequisite for the proper formation and adaptive plasticity of the presynaptic terminal. It is now well accepted that endocytosis mediated by clathrin-coated vesicles (CCVs) plays a key role in SV recycling (Morgan et al. 2002; Dittman and Ryan 2009; Royle and Lagnado 2010). The structural components of a CCV, in the simplest sense, include the clathrin protein, the underlying plasma membrane, and the adaptor protein complex that tethers the clathrin to the membrane (Brodsky et al. 2001; McMahon and Boucrot 2011). In addition, an abundance of regulatory proteins that control various aspects of CCVs or CCV-mediated endocytosis has been discovered (Lafer 2002; Robinson 2004; Brett and Traub 2006; Schmid and McMahon 2007; Ungewickell and Hinrichsen 2007; McMahon and Boucrot 2011).
The clathrin assembly protein AP180, as its name implies, is involved in the assembly of CCVs (Morris et al. 1993; Zhou et al. 1993). Biochemical studies have revealed that AP180 makes the assembly of CCVs more efficient and restricts them to a uniform size (Ahle and Ungewickell 1986; Ye and Lafer 1995). Disruptions of AP180 in several invertebrate models have also shown impaired SV recycling (Zhang et al. 1998; Morgan et al. 1999; Nonet et al. 1999), indicating the in vivo role of AP180 in SV recycling at invertebrate synapses. However, the function and physiological significance of AP180 in the synapses of vertebrate or mammalian neurons is incompletely understood.
CALM (clathrin assembly lymphoid myeloid protein) is a clathrin assembly protein that has a domain structure similar to AP180 (Dreyling et al. 1996; Tebar et al. 1999). Functionally, CALM—albeit in non-neuronal cells—also bears a resemblance to AP180, as a reduction of CALM results in clathrin-coated structures of irregular size and shape (Meyerholz et al. 2005). In a previous study, we compared the subcellular locations of AP180 and CALM in hippocampal neurons (Yao et al. 2005). We found that while CALM is more broadly distributed than AP180 in neurons, these two proteins coexist and intermingle with SVs in a similar manner within the presynaptic terminal (Yao et al. 2003, 2005; Petralia and Yao 2007).
To learn about the contributions of AP180 and CALM to the presynaptic terminal of the hippocampal neurons, we used cultured hippocampal neurons as a model system and RNAi-mediated reduction of AP180 and CALM. This was achieved by the transfection of a vector-based short-hairpin RNA (shRNA), AP180shRNA or CALMshRNA, together either with Synaptophysin::EGFP (Syp::EGFP) to visualize the transfected synaptic terminals by light microscopy or with EGFP::mHRP to examine the ultrastructure of the presynaptic terminals by electron microscopy.
Materials and Methods
Animals
All animal procedures were approved by the NIA Animal Care and Use Committee and complied with the NIH Guide for Care and Use of Laboratory Animals. Timed pregnant female Sprague–Dawley rats were used as the source of embryonic brains to establish hippocampal cultures.
DNA Constructs
The design and generation of the pSuper-AP180shRNA (shRNA2157) and pSuper-CALMshRNA (shRNA1649) have been described in detail in previous studies (Bushlin et al. 2008; Harel et al. 2008). The specificity and efficacy of the AP180shRNA and CALMshRNA in reducing AP180 or CALM, respectively, and the corresponding control shRNA, have also been characterized (Bushlin et al. 2008; Harel et al. 2008; Wu et al. 2009; Schwartz et al. 2010). The additional pSuper-AP180shRNA (shRNA373) and pSuper-CALMshRNA (shRNA365 and shRNA594) are described in Supplementary Information. Syp::EGFP was a kind gift of Dr. Jane Sullivan (University of Washington). EGFP::mHRP was a kind gift of Dr. Hollis T. Cline (The Scripps Research Institute); its construction and usage has been described (Li et al. 2010).
Hippocampal Neuron Culture, Transfection, Immunolabeling, and FM Dye Uptake
Cultures of hippocampal neurons were prepared from embryonic day-18 rat brains as described previously (Banker and Cowan 1977; Mattson et al. 1988; Kaech and Banker 2006; Bushlin et al. 2008). In brief, hippocampal tissues were dissected and incubated for 15 min in a trypsin solution (2 mg/ml). Dissociated neurons were then plated on coverslips coated with poly-d-lysine at a density of 150–200 cells/mm2. The neurons were grown in Neuro-basal medium/B27 (Invitrogen) supplemented with 0.5 mM glutamine. Neurons were transfected using a calcium phosphate-based kit (Invitrogen) and analyzed 4–5 days later. For light microscopy studies, neurons were co-transfected with Syp::EGFP and a testing shRNA construct. For electron microscopy studies, neurons were co-transfected with EGFP::mHRP and a testing shRNA.
Immunolabeling was carried out as described (Bushlin et al. 2008). Neurons were fixed with 4 % paraformaldehyde and 4 % sucrose for 15 min, washed, and permeabilized in 0.2 % Triton X-100 for 5 min, and blocked in 10 % BSA. Neurons were then incubated with either Synapsin 1 antibody (1:2,500; Chemicon International, #AB1543P) or Bassoon antibody (1:100; Stressgen, #VAM-PS003) overnight at 4 °C. After washing multiple times in PBS, neurons were incubated in secondary antibody (Alexa Fluor 568 goat anti-rabbit for Synapsin 1; Alexa Fluor 568 goat anti-mouse for Bassoon).
For FM dye uptake, neurons were incubated in a buffer containing fixable FM 4–64 (5 µM) and 45 mM KCl at 37 °C for 2 min. Neurons were washed for 2 min with Ca2+-free saline twice, followed by a 2 min wash with 1 mM ADVASEP (Kay et al. 1999) in Ca2+-free saline. ADVASEP, a sulfobutylated derivative of β-cyclodextrin, has a high affinity for FM dye and, therefore, is effective in removing nonspecific FM dye labeling (Kay et al. 1999). Following additional washes in Ca2+-free saline, neurons were fixed with 4 % paraformaldehyde and mounted for imaging by fluorescence microscopy.
Fluorescence Microscopy and Image Analysis
The fixed neurons were examined using a 40X or a 63X objective on a Zeiss LSM510 or LSM710 laser scanning confocal microscope. All images were acquired at a 1,024 × 1,024 pixel resolution, and each image was an average of four scans at the same position. The confocal acquisition settings were kept as consistent as possible among the experiments throughout the course of the study. The brightness and contrast of the images were minimally adjusted (in Adobe Photoshop 8.0) for those images presented. No additional digital image processing was performed.
At least 4 independent cultures were used for each experiment. Two to three coverslips (at least 10 microscopic fields per coverslip) from each culture were used for analysis. Synaptic puncta number (number per 10 µm) and size (µm2) were measured using a customized Plugin in ImageJ that provided measurements with identical threshold settings.
Electron Microscopy and Data Analysis
Four to five days after co-transfections with EGFP::mHRP and a testing shRNA, neurons were processed for electron microscopy as described (Petralia and Wenthold 1999; Petralia et al. 2010; Mitchell et al. 2012). Briefly, neurons on coverslips were fixed in 4 % paraformaldehyde +0.1 % glutaraldehyde in PBS at room temperature for 30 min. Following washes, they were treated with sodium borohydride (0.5 mg/ml), washed, and processed using the tyramide signal amplification method (TSA; PerkinElmer, Waltham, MA). This included treatment with the TSA working solution for 5 min, washing, treatment with 0.5 % blocking medium for 5 min, then 1/100 streptavidin-horseradish peroxidase for 30 min, washing, and then incubation in diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA) for 5 min, followed by washing in PBS and then 0.1 M cacodylate buffer. Then, coverslips were postfixed in 1 % osmium tetroxide in cacodylate buffer for 30 min and dehydrated in an ethanol series (including 10 min in 1 % uranyl acetate in 50 % ethanol) and then in propylene oxide and embedded in epon. The glass coverslip was removed with hydrofluoric acid, and thin sections were stained with 0.03 % lead citrate for 3 min and examined in a JEOL JEM-1010 electron microscope.
The experiment was repeated 4 times (4 independent cultures). At least 100 randomly selected fields from each group in each experiment were photographed. A total of 1,096 micrographs were examined.
Data Analysis and Statistics
Statistical significance was determined by the two-tailed Student’s t-test. The Mann–Whitney U test was used when the assumptions were not met. All data are expressed as mean ± SEM. Statistical significance was assumed when p<0.05. In figures, *p<0.05, **p<0.01, and ***p<0.001.
Results
Light Microscopy Reveals Smaller Presynaptic Puncta in AP180- and CALM-Knocked Down Neurons
The design, construction, and characterization of the AP180shRNA and CALMshRNA, as well as their usage in neurons, have been described in detail in several previous studies (Bushlin et al. 2008; Harel et al. 2008; Wu et al. 2009; Schwartz et al. 2010). The AP180shRNA reduced AP180 protein level by 95 % (95 ± 3 %) in cultured hippocampal neurons (Bushlin et al. 2008). The CALMs-hRNA reduced CALM protein level by 70 % (70 ± 5 %) in these neurons (Bushlin et al. 2008; Harel et al. 2008).
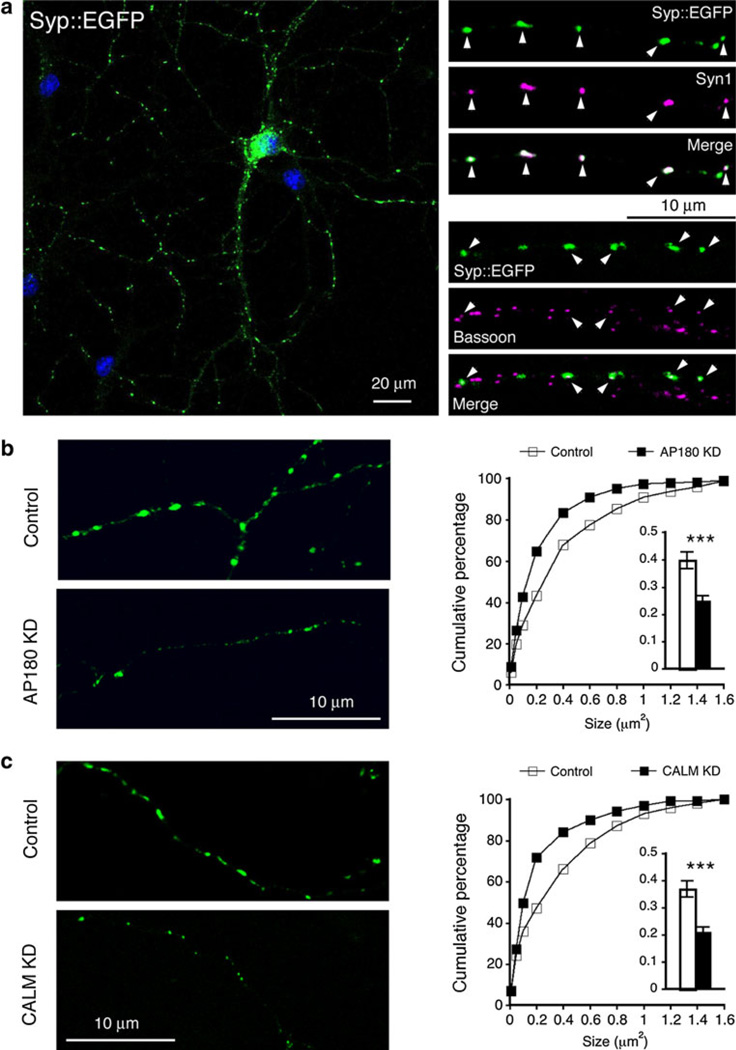
The Syp::EGFP or similar constructs have been used for marking presynaptic terminals—defined at the level of light microscopy as presynaptic puncta (for examples see: Staras et al. 2010; Williams et al. 2011). Figure 1a (left) shows an example of the typical punctate expression pattern of Syp::EGFP by cultured hippocampal neurons. Most of these Syp::EGFP-illuminated puncta contained the endogenous synaptic vesicle protein Synapsin1 (Syn1) and co-localized with the endogenous presynaptic active zone protein Bassoon (white arrowheads in Fig. 1a, right).
Fig. 1.
AP180- and CALM-knocked down (KD) neurons have smaller presynaptic puncta. Neurons were transfected on 16 div (days in vitro) and examined 4 days later. a left, confocal image of a hippocampal neuron expressing Syp::EGFP; right, the Syp::EGFP-positive puncta (green) contain the endogenous SV protein Synapsin1 (Syn1; magenta) and co-localize with endogenous active zone protein Bassoon (magenta). b Neurons were co-transfected with Syp::EGFP and empty shRNA vector (Control) or AP180shRNA (AP180 KD). Additional examples are shown in supplemental Figure S1. Results using a different AP180shRNA are shown in supplemental Figure S2. Cumulative frequency plot of puncta size and the average size (insert) shows smaller puncta in AP180 KD-neurons. c Synaptic puncta of CALM KD-neurons are also smaller. Additional examples are shown in supplemental Figure S3, and results using a different CALMshRNA are shown in supplemental Figure S4. For each experiment, at least 200 axonal segments (exemplified in b and c) from 8 to 10 coverslips obtained from 4 to 5 cultures were analyzed. Data represent mean ± SEM. ***p<0.001 (Color figure online)
We co-transfected the hippocampal neurons with the Syp::EGFP and a control vector or an shRNA construct (AP180 KD for AP180 knocked down). We conducted the experiments using neurons at two culture ages: (1) neurons were transfected at 6 div (days in vitro) and analyzed 4 days later (6 div + 4 ds), and (2) neurons were transfected at 16 div and analyzed 4 days later (16 div + 4 ds).
The size of Syp::EGFP puncta appeared visibly smaller in both young (Fig. S1A) and older AP180 KD-neurons (Fig. 1b, left; also additional examples in Fig. S1C). Quantitative assessments of the average size and cumulative distribution of the puncta were concordant with the visual indication: presynaptic puncta in AP180 KD-neurons were significantly smaller than those in control neurons (Fig. S1B for young neurons: 0.2 ± 0.01 µm2 in AP180 KD-neurons versus 0.26 ± 0.01 µm2 in control neurons, p<0.01; Fig. 1b right and insert histogram for older neurons: 0.4 ± 0.03 µm2 in control neurons versus 0.25 ± 0.02 µm2 in AP180 KD-neurons, p<0.001; see also Table 1).
Table 1.
Reduced SV cluster size revealed by electron microscopy corresponds with reduced puncta size seen with light microscopy
| AP180 KD/control ratio | CALM KD/control ratio | |
|---|---|---|
| Syp::EGFP puncta size (light microscopy) | 0.625 | 0.568 |
| SV cluster size (electron microscopy) | 0.428 | 0.384 |
The ratio for Syp::EGFP puncta size was calculated by dividing the average puncta size of either AP180 KD or CALM KD by the puncta size of control vector-transfected neurons (16 div + 4 ds). The ratio for SV cluster size was calculated by dividing the average SV cluster size of AP180 KD or CALM KD by the SV cluster size of control vector-transfected neurons (also 16 div + 4 ds)
The number of Syp::EGFP puncta, on the other hand, did not appear to be significantly affected by AP180 KD in either age group (Fig. S1D; for young neurons: 3.2 ± 0.2 per 10 µm in control neurons versus 2.6 ± 0.2 per 10 µm in AP180 KD-neurons, p = 0.103; for older neurons: 4.4 ± 0.3 per 10 µm in control neurons versus 5.1 ± 0.2 per 10 µm in AP180 KD-neurons, p = 0.07).
We also examined the effect of a different AP180shRNA (shRNA373). Figure S2A shows the efficacy of the shRNA373 in reducing AP180 expression in hippocampal neurons. Figure S2B shows that neurons expressing the shRNA373 similarly displayed smaller presynaptic puncta. Thus, reduction of AP180 seems to affect the size, but not the number, of Syp::EGFP presynaptic puncta.
Knocking down CALM also produced noticeably smaller Syp::EGFP puncta in both young (Fig. S3A) and older neurons (Fig. 1c, left; additional examples are shown in Fig. S3C). Quantitative assessments showed a significant reduction in the average size of puncta (Fig. S3B for young neurons: 0.29 ± 0.02 µm2 in control versus 0.2 ± 0.01 µm2 in CALM KD, p<0.001; Fig. 1c, right and insert histogram for older neurons: 0.37 ± 0.03 µm2 in control vs. 0.21 ± 0.02 µm2 in CALM KD, p<0.001; also Table 1), and a left shift in cumulative distribution (Fig. S3B and Fig. 1c).
In addition, the knockdown of CALM in the young neurons induced a small but statistically significant decrease in puncta number (Fig. S3D; 2.8 ± 0.1 per 10 µm in control neurons vs. 2.3 ± 0.1 per 10 µm in CALM KD-neurons; p<0.05). In older neurons, however, there was no difference between the control and CALM KD-neurons (Fig. S3D; 3.8 ± 0.4 per 10 µm in control neurons vs. 3.7 ± 0.4 per 10 µm in CALM KD-neurons).
We tested an additional CALMshRNA construct (shRNA365). Figure S4A shows the efficacy of the shRNA365 in reducing the expression of CALM in hippocampal neurons. Figure S4B shows similarly smaller Syp::EGFP puncta in neurons expressing the shRNA365.
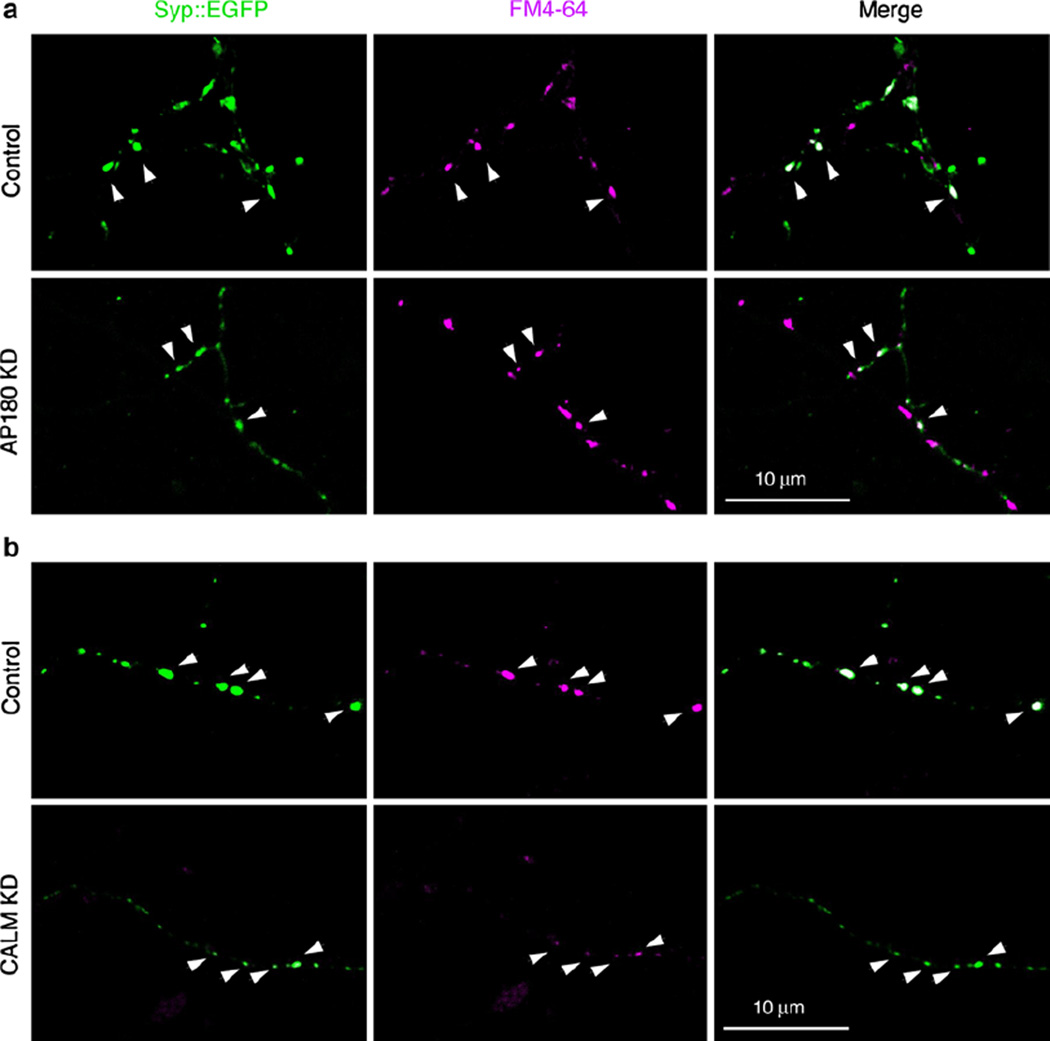
Using the fluorescent styryl FM dye (Kay et al. 1999), we evaluated the endocytosis ability of the Syp::EGFP presynaptic puncta that are from either the AP180 KD- or the CALM KD-neurons. Following a brief incubation with fixable FM4-64 dye in 45 mM KCl, both transfected and nontransfected Syp::EGFP-positive synapses internalized the FM dye (Fig. 2). The synaptic puncta from the AP180 KD-neurons, although considerably smaller, did take up the FM dye (denoted by arrowheads in Fig. 2a). Likewise, the smaller synaptic puncta from the CALM KD-neurons were also capable of internalizing the FM dye (denoted by arrowheads in Fig. 2b). While the FM uptake experiment conducted at a single time point does not reveal endocytosis kinetics or synaptic vesicle exocytosis, the results imply that the endocytosis continues to take place in the Syp::EGFP presynaptic puncta of both AP180 KD- and CALM KD-neurons. Furthermore, the co-localization of the activity-dependent endocytic marker FM4-64 with the Syp::EGFP puncta suggests that the Syp::EGFP protein molecules localized at their expected sites, the synaptic vesicles in the presynaptic terminals.
Fig. 2.
Endocytosis of FM dye by AP180- and CALM KD-neurons. Live neurons expressing Syp::EGFP (green) and AP180shRNA (a; AP180 KD) or CALMshRNA (b; CALM KD) were incubated with fixable FM 4–64 (magenta) and then fixed. Although the Syp::EGFP puncta are considerably smaller in AP180- and CALM KD-neurons, these smaller puncta internalize the FM dye (white arrowheads). The experiment was repeated three times (Color figure online)
Electron Microscopy Reveals Enlarged SVs and Decreased SV Density and SV Cluster Size in AP180- and CALM-Knocked Down Neurons
Intrigued by the light microscopic observations of the Syp::EGFP puncta, we next examined the ultrastructure of the presynaptic terminals. We took advantage of a recently developed construct EGFP::mHRP (mHRP, membrane-targeted horseradish peroxidase) that allows one to locate transfected neurons and identify their synaptic terminals using electron microscopy (Li et al. 2010). We focused on one of the two AP180shRNAs (shRNA2157) and one of the two CALMshRNAs (shRNA1649). We co-transfected neurons with the EGFP::mHRP and the control vector, or the AP180shRNA, or the CALMshRNA (*16 div), and processed the neurons 4–5 days post-transfection (16 div + 4 ds).
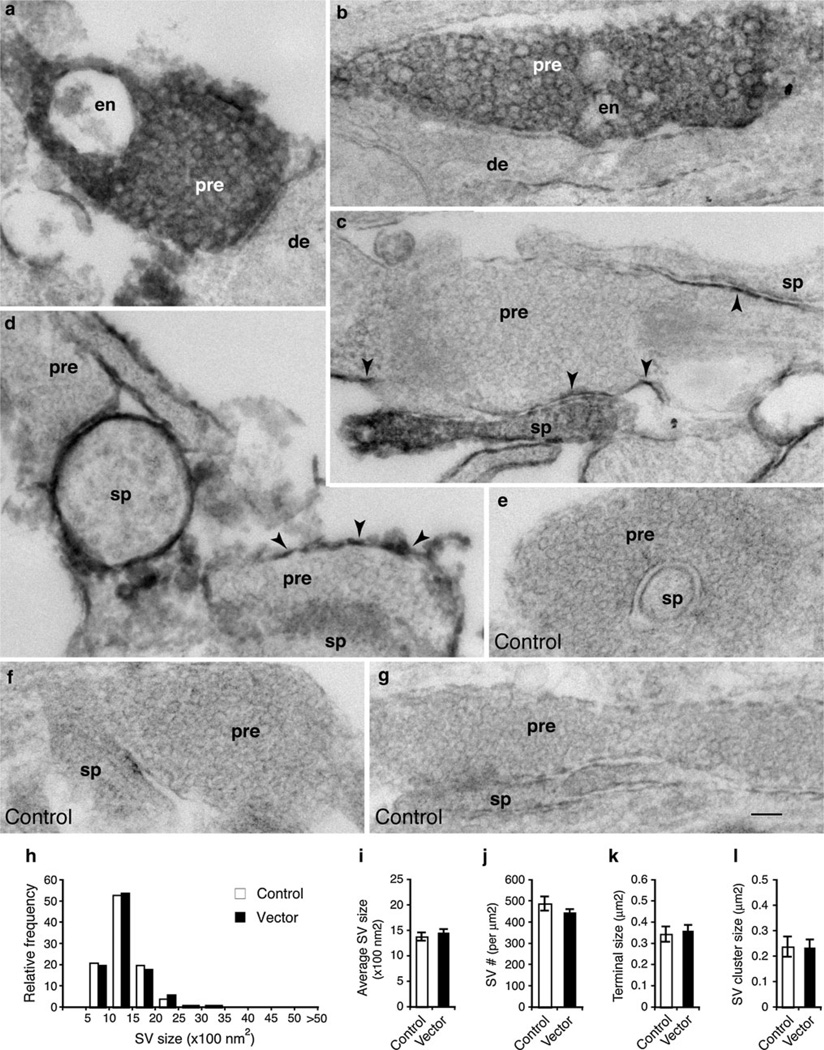
While the expression of the EGFP::mHRP was restricted to the plasma membrane (Li et al. 2010), we found DAB reaction products catalyzed by the mHRP, in some cases, spread out into the cytoplasm of the EGFP::mHRP-expressing neurons. Figure 3a, b depict examples of the EGFP::mHRP-expressing presynaptic terminals (white letters) filled with DAB reaction products. Nevertheless, the DAB products, although dark, did not seem to preclude the visualization of SVs. Figure 3c, d are examples of the EGFP::mHRP-expressing presynaptic terminals in which DAB labeling was mostly confined to the plasma membranes (black arrowheads). Comparing the DAB-filled with the DAB-outlined presynaptic terminals, we did not observe any obvious differences in the ultrastructural characteristics (from 4 cultures). Therefore, we pooled the data from both types of the presynaptic terminals. Furthermore, because our initial analysis did not show detectable differences between the presynaptic terminals that oppose the transfected (thus DAB labeled) or non-transfected postsynaptic terminals, we analyzed all the clearly identifiable transfected presynaptic terminals, irrespective of the type or the presence of postsynaptic contacts.
Fig. 3.
Presynaptic terminals of cultured hippocampal neurons revealed by electron microscopy. Following co-transfection with empty shRNA vector and EGFP–mHRP, neurons were processed for DAB detection and then examined by electron microscopy. Some presynaptic terminals (pre) of transfected neurons are filled with dark DAB reaction products (denoted by white letters in a and b), while other presynaptic terminals have DAB reaction products only on the plasma membrane or a part of the membrane (denoted by black arrowheads in c and d). Ultrastructural characteristics are not visibly different between the DAB-filled or DAB-outlined presynaptic terminals. Densely packed synaptic vesicles are homogenous in size and shape. Endosomes (en) are seen in some presynaptic terminals. There is also no difference between the presynaptic terminals that oppose transfected (DAB labeled) or nontransfected (no DAB labeling) postsynaptic dendrites (de) or spines (sp). Synapses formed from nontransfected presynaptic terminals onto nontransfected post-synaptic terminals (from the same culture) serve as controls (e–g). Scale bar in g is 100 nm, and it applies to all micrographs. h and i show size distribution and average size of SVs. Data are pooled from DAB-filled and DAB-outlined presynaptic terminals. Total of 306 potential EM micrographs from two cultures were analyzed (control, n = 1,160 synaptic vesicles; vector, n = 2,511 synaptic vesicles). j The density of SVs was calculated by counting the number of synaptic vesicles in randomly selected areas and normalizing to per lm2. k The size of presynaptic terminals. l The size of SV clusters. Data represent mean ± SEM
The nontransfected synapses from the same culture were used as the control for each experiment (Fig. S5). While the morphology and size of the presynaptic terminals of cultured hippocampal neurons varied considerably, SVs in these terminals exhibited remarkable homogeneity in the size, shape, and density (Fig. 3e–g). Transfection with the control vector did not change the ultrastructural features of SVs (compare Fig. 3a–d to e–g, and Fig. S5). The size of SVs, assessed by either size distribution or average size, was almost identical between the nontransfected control and the control vector transfected (Fig. 3h, i). We also compared the density of the SVs. We quantified SV density in randomly selected terminals where SVs were unambiguously visible (Fig. 3j). Our measurements showed that the density of SVs was similar between the nontransfected and the vector-transfected groups (Fig. 3j). Additionally, the size of presynaptic terminals (Fig. 3k) and SV clusters (Fig. 3l) was also similar between the two groups.
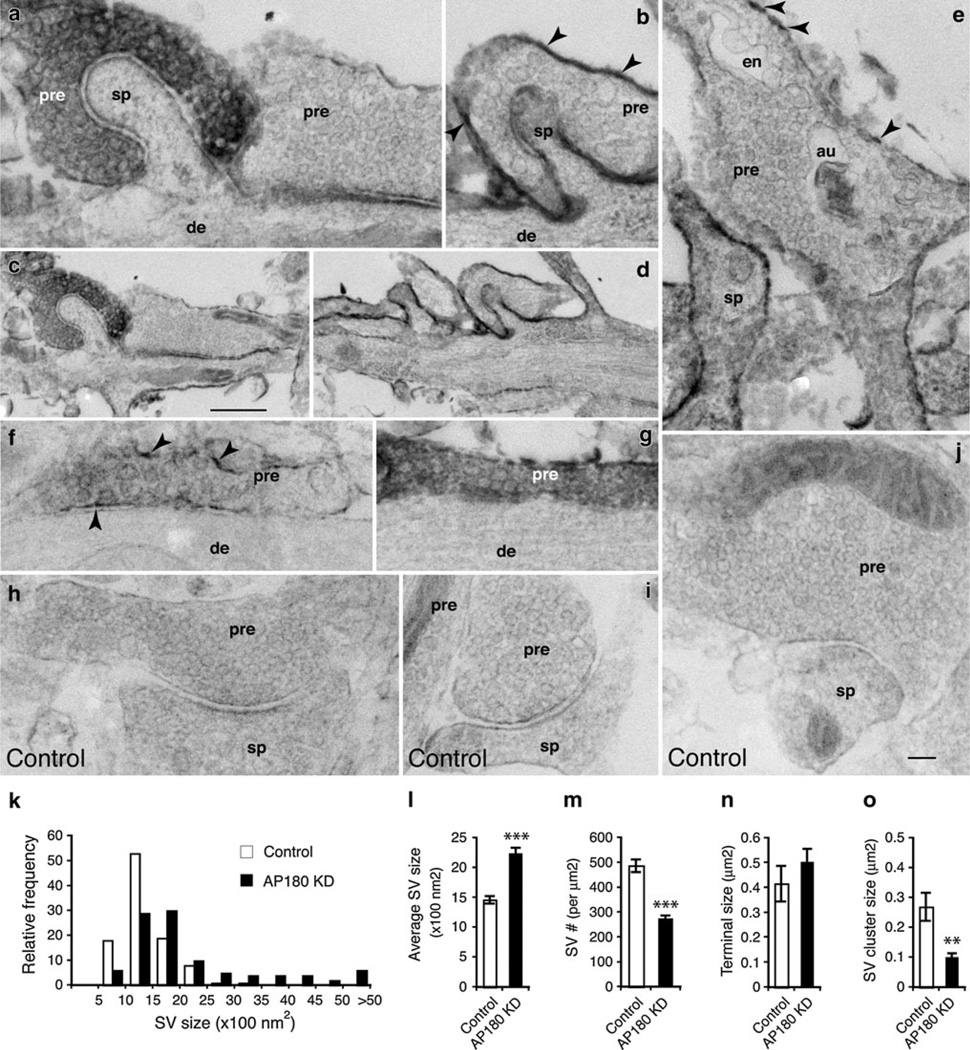
We next examined the neurons that had been co-transfected with the EGFP::mHRP and the AP180shRNA (AP180 KD). The examples in Fig. 4 showed that SVs in the presynaptic terminals of the AP180 KD-neurons appeared larger than those in the control neurons (compare Fig. 4a–g to h–j). Control SVs averaged 1,461 ± 63 nm2 in area, with a typically narrow range of 1,000–1,500 nm2 (n = 1,366; Fig. 4k, l). In contrast, the AP180 KD-SVs averaged 2,235 ± 98 nm2, with a broad range of 1,000–5,000 or greater (n = 3,160; Fig. 4k, l). In addition to their increased size, the AP180 KD-SVs were less densely packed: more empty spaces were observed in some terminals (for example, Fig. 4b, f). Quantitative assessments agreed with the visual impression: the density of SVs in the AP180 KD-neurons was significantly lower than in the control (Fig. 4m; 274 ± 13 per µm2 in AP180 KD vs. 487 ± 26 per µm2 in control; p<0.001). The presynaptic terminals of AP180 KD-neurons also seemed larger as exemplified in Fig. S6, but the difference was not statistically significant (Fig. 4n; 0.502 ± 0.054 µm2 in AP180 KD vs. 0.415 ± 0.071 µm2 in control; p>0.05). When comparing the size of SV clusters, however, we found that the size of SV clusters in AP180 KD-presynaptic terminal was only a half of that in control (Fig. 4o; 0.1 ± 0.012 µm2 in AP180 KD vs. 0.269 ± 0. 047 µm2 in control; p<0.01; and Fig. S6; see also Table 1).
Fig. 4.
Synaptic vesicles of AP180 KD-neurons. (Notice that all micrographs presented in this figure are *20 % smaller than those in Figs. 3 and 5). Neurons were transfected with AP180shRNA together with EGFP–mHRP. c is a lower magnification of a, and d is a lower magnification of b. Presynaptic terminals (pre) of transfected neurons are either filled with dark DAB reaction products (white letters in a and g) or have the labeling on the plasma membranes only (black arrowheads in b, e, and f). Synapses formed between nontransfected presynaptic terminals and nontransfected postsynaptic terminals (from the same culture) serve as controls (h–j). Synaptic vesicles become noticeably larger and less densely packed in the AP180 KD-presynaptic terminals than in the control. These changes are seen in the presynaptic terminals irrespective of opposing transfected or nontransfected postsynaptic spines (sp) or dendrites (de). en, endosome; au, autophagosome. Scale bar in c is 500 nm and it applies to d. Scale bar in j is 100 nm and it applies to a, b, e–j. k Size distribution of synaptic vesicles from control and AP180 KD-neurons. Data are pooled from DAB-filled and DAB-outlined presynaptic terminals. Total of 254 potential EM micrographs from two cultures were analyzed (control, n = 1,366 synaptic vesicles; AP180 KD, n = 3,160 synaptic vesicles). i shows that the average size of synaptic vesicles in the AP180 KD-neurons is significantly increased. m shows significantly reduced synaptic vesicle density in the AP180 KD-neurons, as assessed by counting the number of synaptic vesicles per unit area (normalized to lm2). n shows the size of presynaptic terminals. o shows significantly reduced SV cluster size. ***p<0.001; **p<0.01. Data represent mean ± SEM. See also Figure S6
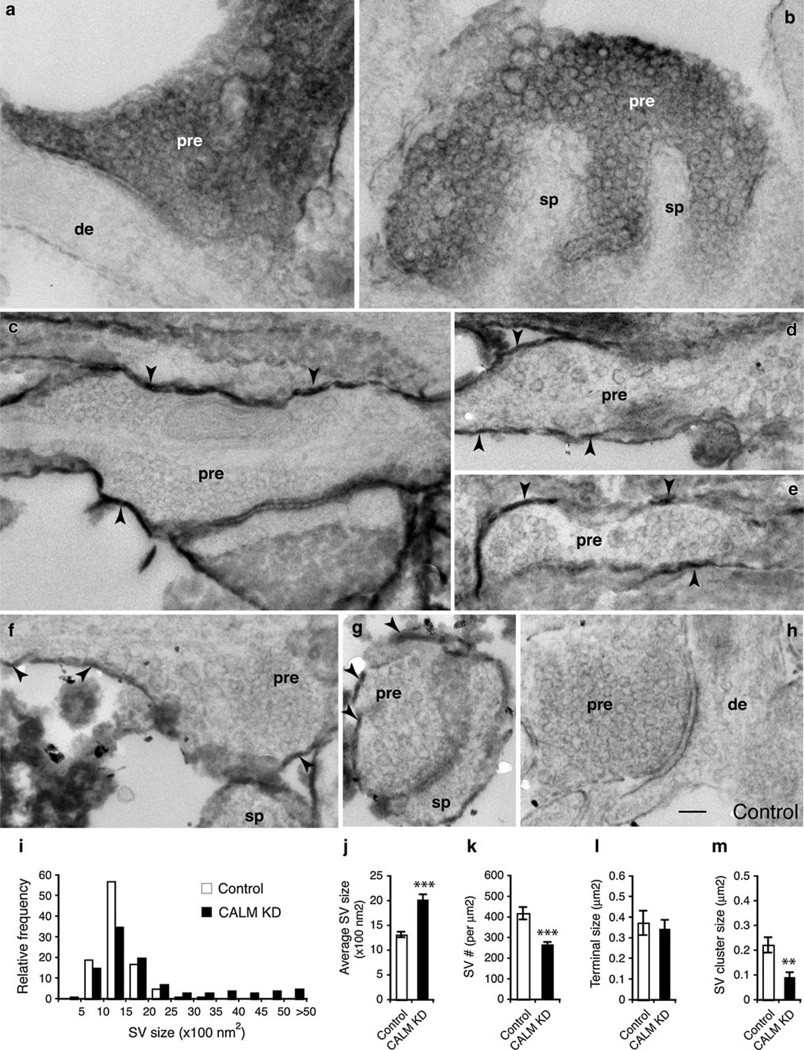
The CALM KD-neurons displayed similar presynaptic changes. Figure 5a, b are examples of the presynaptic terminals from the CALM KD-neurons; although darkened by diffused DAB product, large sized vesicles—particularly at the peripheral of the SV cluster—were clearly noticeable. Additional CALM KD-presynaptic terminals, whose SVs became considerably variable, are illustrated by examples in Fig. 5c–g. On average, the size of control SVs was 1,317 ± 58 nm2 in area, with a narrow range of 1,000–1,500 nm2 (n = 1,010; Fig. 5i, j). However, the size of CALM KD-SVs was 2,021 ± 110 nm2, with a broad range of 1,000–5,000 or greater (n = 3,212; Fig. 5i, j). Also, the density of SVs in the CALM KD-neurons was lower than in the control (Fig. 5k; 267 ± 13 per µm2 in CALM KD vs. 419 ± 30 per µm2 in control; p<0.001). While the size of presynaptic terminals was unaffected by CALM KD (Fig. 5l), SV clusters became significantly smaller (Fig. 5m; 0.09 ± 0.021 µm2 in CALM KD vs. 0.222 ± 0.031 µm2 in control; p<0.01; also Table 1).
Fig. 5.
Synaptic vesicles of CALM KD-neurons. Neurons were transfected with CALMshRNA together with EGFP–mHRP. Transfected neurons have dark DAB reaction products filling their presynaptic terminals (pre) in some cases (white letters in a and b), or the labeling is only on the presynaptic membranes in other cases (black arrowheads in c–g). de, dendrite; sp, spines. Nontransfected presynaptic terminals opposing nontransfected postsynaptic terminals from the same culture are used as the controls (h). Synaptic vesicles from the CALM KD-neurons are distinctly variable in size and shape. Scale bar in h is 100 nm and it applies to all micrographs. i Histogram shows broader size distribution of synaptic vesicles from the CALM KD-neurons. Total of 274 potential EM micrographs from two cultures were analyzed (control, n = 1,010 synaptic vesicles; CALM KD, n = 3,212 synaptic vesicles). j The average size of synaptic vesicles of the CALM KD-neurons is significantly increased. k shows reduced synaptic vesicle density in the CALM KD-neurons. l shows unchanged presynaptic terminal size. m shows significantly reduced SV cluster size. ***p<0.001; **p<0.01. Data represent mean ± SEM
Taken together, these electron microscopic observations suggest that the reduction of AP180 and CALM leads to similar morphological changes in the presynaptic terminals of hippocampal neurons: enlarged SVs and decreased SV density and SV cluster size.
Discussion
This study combines two techniques to explore the cell-autonomous effect of AP180- or CALM-knockdown on the presynaptic terminals of hippocampal neurons: Light microscopy of the reporter Syp::EGFP was used to provide a coarse view of the presynaptic puncta, and electron microscopy was used to provide a closer view of the presynaptic terminals and SVs. With these two techniques, we provide evidence that the clathrin assembly proteins AP180 and CALM are both involved in regulating synaptic vesicles.
Our results expand existing knowledge about AP180 and CALM in two ways. First, for AP180, it has been known that AP180 has a specific role in keeping CCVs uniform in size during endocytosis (Ahle and Ungewickell 1986; Ye and Lafer 1995). Given that CCVs are essential for the formation of SVs during exocytosis–endocytosis cycles (Morgan et al. 2002; Dittman and Ryan 2009; Royle and Lagnado 2010), it is not surprising that defective AP180 results in abnormally sized SVs in several invertebrate models (Zhang et al. 1998; Morgan et al. 1999; Nonet et al. 1999). From studies of these invertebrate models, it has been inferred that AP180 must also have the same activity in the mammalian synapses. Second, for CALM, because CALM and AP180 are similar in their domain structure (Dreyling et al. 1996; Ford et al. 2001; Mao et al. 2001), it has been thought that CALM and AP180 possibly have similar functions. While it is true that CALM’s activity is reminiscent of AP180’s activity in affecting the size of clathrin-coated vesicles in non-neuronal cells (Meyerholz et al. 2005), what role CALM actually plays in the synapse of neurons had not been explicitly examined.
Now, we have demonstrated that AP180 indeed regulates SVs in the mammalian synapses. We have also found that, at least in the presynaptic terminals of the hippocampal neurons, CALM’s activity seems similar to AP180’s. Based on their well-characterized activity in the formation of CCVs (Meyerholz et al. 2005), we suggest that AP180 and CALM possibly work together or in parallel along the clathrin-mediated pathway for SV formation. Given that the size and shape and number of SVs are all essential for normal neurotransmitter release, it may be necessary for neurons to use multiple assembly proteins to ascertain whether SVs are precisely constructed following each cycle of exocytosis and endocytosis.
The smaller Syp::EGFP-marked synaptic puncta revealed by light microscopy could reflect smaller presynaptic terminals or fewer SVs within the presynaptic terminals. Our electron microscopic measurements did not show any significant changes in the size of the presynaptic terminals. Nonetheless, the low SV density, in particular smaller SV clusters, revealed by electron microscopy (Figs. 3–5; Table 1; and Fig. S6) correlates well with the smaller Syp::EGFP puncta (also Table 1).
While this work was under review, a study by Koo et al. was published that reported their investigation into AP180 and CALM in synaptic endocytosis (Koo et al. 2011). Their observation of morphologically normal synaptic terminals in AP180shRNA- and CALMshRNA-expressing neurons is similar to ours. Moreover, their finding of enlarged SVs in the synapses of AP180shRNA-expressing neurons is fully consistent with ours (Koo et al. 2011). One discrepancy, however, between our finding and that of Koo et al. is for the CALM KD-neurons. While we found significantly enlarged SVs in the CALM KD-neurons (Fig. 5), Koo et al. observed no CALM KD-induced change in the size of SVs (Koo et al. 2011). One possible explanation for this discrepancy is the age of cultured neurons. We performed the shRNA transfection on 16 div neurons, whereas Koo et al. transfected neurons at 6–8 div, a much younger culture age (Koo et al. 2011). Cultured hippocampal neurons that are younger than 10 div, although they already possess SVs, undergo mostly spontaneous neurotransmitter release; only when neurons become more mature (older than 10 div), do they switch to evoked neurotransmitter release (Andreae et al. 2012). It is conceivable that the requirement for SV recycling changes according to the mode of neurotransmitter release. CALM’s role in regulating SV recycling might be negligible in the SV recycling machinery in young neurons.
Supplementary Material
Acknowledgments
We thank the anonymous reviewers for helpful suggestions. We also thank Dr. Jane Sullivan for Synaptophysin::EGFP and Dr. Hollis T. Cline for the EGFP::mHRP construct. This work was supported by the Intramural Research Programs of the NIA/NIH and NIDCD/NIH.
Footnotes
Electronic supplementary material The online version of this article (doi: 10.1007/s12017-012-8194-x) contains supplementary material, which is available to authorized users.
Contributor Information
Ronald S. Petralia, Advanced Imaging Core, NIDCD/NIH, Bethesda, MD 20892, USA
Ya-Xian Wang, Advanced Imaging Core, NIDCD/NIH, Bethesda, MD 20892, USA.
Fred E. Indig, Confocal Imaging Facility, Laboratory of Clinical Investigation, NIA/NIH, Baltimore, MD 21224, USA
Ittai Bushlin, Laboratory of Neurosciences, NIA/NIH Biomedical Research Center, 251 Bayview Boulevard, Baltimore, MD 21224, USA.
Fangbai Wu, Laboratory of Neurosciences, NIA/NIH Biomedical Research Center, 251 Bayview Boulevard, Baltimore, MD 21224, USA.
Mark P. Mattson, Laboratory of Neurosciences, NIA/NIH Biomedical Research Center, 251 Bayview Boulevard, Baltimore, MD 21224, USA
Pamela J. Yao, Email: yaopa@grc.nia.nih.gov, Laboratory of Neurosciences, NIA/NIH Biomedical Research Center, 251 Bayview Boulevard, Baltimore, MD 21224, USA.
References
- Ahle S, Ungewickell E. Purification and properties of a new clathrin assembly protein. EMBO Journal. 1986;5:3143–3149. doi: 10.1002/j.1460-2075.1986.tb04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreae LC, Fredj NB, Burrone J. Independent vesicle pools underlie different modes of release during neuronal development. Journal of Neuroscience. 2012;32:1867–1874. doi: 10.1523/JNEUROSCI.5181-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker G, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Research. 1977;126:397–425. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Brett TJ, Traub LM. Molecular structures of coat and coat-associated proteins: Function follows form. Current Opinion in Cell Biology. 2006;18:395–406. doi: 10.1016/j.ceb.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: Formation and function of clathrin-coated vesicles. Annual Reviews of Cell and Developmental Biology. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- Bushlin I, Petralia RS, Wu F, Harel A, Mughal MR, Mattson MP, et al. Clathrin assembly protein AP180 and CALM differentially control axogenesis and dendrite outgrowth in embryonic hippocampal neurons. Journal of Neuroscience. 2008;28:10257–10271. doi: 10.1523/JNEUROSCI.2471-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annual Reviews of Cell and Developmental Biology. 2009;25:133–160. doi: 10.1146/annurev.cellbio.042308.113302. [DOI] [PubMed] [Google Scholar]
- Dreyling MH, Martinez-Climent JA, Zheng M, Mao J, Rowley JD, Bohlander SK. The t(10;11)(p13;q14) in the U937 cell line results in the fusion of the AF10 gene and CALM, encoding a new member of the AP-3 clathrin assembly protein family. Proceedings of the National Academy of Sciences. 1996;93:4804–4809. doi: 10.1073/pnas.93.10.4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MG, Pearse BM, Higgins MK, Vallis Y, Owen DJ, Gibson A, et al. Simultaneous binding of PtdIns(4,5)P2 and clathrin by AP180 in the nucleation of clathrin lattices on membranes. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- Harel A, Wu F, Mattson MP, Morris CM, Yao PJ. Evidence for CALM in directing VAMP2 trafficking. Traffic. 2008;9:417–429. doi: 10.1111/j.1600-0854.2007.00694.x. [DOI] [PubMed] [Google Scholar]
- Kaech S, Banker G. Culturing hippocampal neurons. Nature Protocols. 2006;1:2406–2415. doi: 10.1038/nprot.2006.356. [DOI] [PubMed] [Google Scholar]
- Kay AR, Alfonso A, Alford S, Cline HT, Holgado AM, Sakmann B, et al. Imaging synaptic activity in intact brain and slices with FM1-43 in, Celegans, lamprey, and rat. Neuron. 1999;24:809–817. doi: 10.1016/s0896-6273(00)81029-6. [DOI] [PubMed] [Google Scholar]
- Koo SJ, Markovic S, Puchkov D, Mahrenholz CC, Beceren-Braun F, Maritzen T, et al. SNARE motif-mediated sorting of synaptobrevin by the endocytic adaptors clathrin assembly lymphoid myeloid leukemia (CALM) and AP180 at synapses. Proceedings of the National Academy of Sciences. 2011;108:13540–13545. doi: 10.1073/pnas.1107067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafer EM. Clathrin-protein interactions. Traffic. 2002;3:513–520. doi: 10.1034/j.1600-0854.2002.30801.x. [DOI] [PubMed] [Google Scholar]
- Li J, Wang Y, Chiu SL, Cline HT. Membrane targeted horseradish peroxidase as a marker for correlative fluorescence and electron microscopy studies. Front Neural Circuits. 2010;26:4–6. doi: 10.3389/neuro.04.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Chen J, Maynard JA, Zhang B, Quiocho FA. A novel all helix fold of the AP180 amino-terminal domain for phosphoinositide binding and clathrin assembly in synaptic vesicle endocytosis. Cell. 2001;104:433–440. doi: 10.1016/s0092-8674(01)00230-6. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Dou P, Kater SB. Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. Journal of Neuroscience. 1988;8:2087–2100. doi: 10.1523/JNEUROSCI.08-06-02087.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nature Reviews Molecular Cell Biololgy. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Meyerholz A, Hinrichsen L, Groos S, Esk PC, Brandes G, Ungewickell EJ. Effect of clathrin assembly lymphoid myeloid leukemia protein depletion on clathrin coat formation. Traffic. 2005;6:1225–1234. doi: 10.1111/j.1600-0854.2005.00355.x. [DOI] [PubMed] [Google Scholar]
- Mitchell N, Petralia RS, Yao PJ, Currier DG, Wang YX, Kim A, et al. Sonic hedgehog regulates presynaptic terminal size, ultrastructure and function in hippocampal neurons. Journal of Cell Science. 2012 doi: 10.1242/jcs.105080. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Zhao X, Womack M, Prasad K, Augustine GJ, Lafer EM. A role for the clathrin assembly domain of AP180 in synaptic vesicle endocytosis. Journal of Neuroscience. 1999;19:10201–10212. doi: 10.1523/JNEUROSCI.19-23-10201.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Augustine GJ, Lafer EM. Synaptic vesicle endocytosis: the races, places, and molecular faces. NeuroMolecular Medicine. 2002;2:101–114. doi: 10.1385/NMM:2:2:101. [DOI] [PubMed] [Google Scholar]
- Morris SA, Schroder S, Plessmann U, Weber K, Ungewickell E. Clathrin assembly protein AP180: Primary structure, domain organization and identification of a clathrin binding site. EMBO Journal. 1993;12:667–675. doi: 10.1002/j.1460-2075.1993.tb05700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet ML, Holgado AM, Brewer F, Serpe CJ, Norbeck BA, Holleran J, et al. UNC-11, a Caenorhabditis elegans AP180 homologue, regulates the size and protein composition of synaptic vesicles. Molecular Biology of the Cell. 1999;10:2343–2360. doi: 10.1091/mbc.10.7.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wenthold RJ. Immunocytochemistry of NMDA receptors. Methods Molecular Biololgy. 1999;128:73–92. doi: 10.1385/1-59259-683-5:73. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Yao PJ. AP180 and CALM in the developing hippocampus: Expression at the nascent synapse and localization to trafficking organelles. Journal of Comparative Neurology. 2007;504:314–327. doi: 10.1002/cne.21454. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Hua F, Yi Z, Zhou A, Ge L, et al. Organization of NMDA receptors at extrasynaptic locations. Neuroscience. 2010;167:68–87. doi: 10.1016/j.neuroscience.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS. Adaptable adaptors for coated vesicles. Trends in Cell Biology. 2004;14:167–174. doi: 10.1016/j.tcb.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Royle SJ, Lagnado L. Clathrin-mediated endocytosis at the synaptic terminal: Bridging the gap between physiology and molecules. Traffic. 2010;11:1489–1497. doi: 10.1111/j.1600-0854.2010.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448:883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- Schwartz CM, Cheng A, Mughal MR, Mattson MP, Yao PJ. Clathrin assembly proteins AP180 and CALM in the embryonic rat brain. Journal of Comparative Neurology. 2010;518:3803–3818. doi: 10.1002/cne.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staras K, Branco T, Burden JJ, Pozo K, Darcy K, Marra V, et al. A vesicle superpool spans multiple presynaptic terminals in hippocampal neurons. Neuron. 2010;66:37–44. doi: 10.1016/j.neuron.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tebar F, Bohlander SK, Sorkin A. Clathrin assembly lymphoid myeloid leukemia (CALM) protein: Localization in endocytic-coated pits, interactions with clathrin, and the impact of overexpression on clathrin-mediated traffic. Molecular Biology of the Cell. 1999;10:2687–2702. doi: 10.1091/mbc.10.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell EJ, Hinrichsen L. Endocytosis: Clathrin-mediated membrane budding. Current Opinion in Cell Biology. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Williams ME, Wilke SA, Daggett A, Davis E, Otto S, Ravi D, et al. Cadherin-9 regulates synapse-specific differentiation in the developing hippocampus. Neuron. 2011;71:640–655. doi: 10.1016/j.neuron.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F, Matsuoka Y, Mattson MP, Yao PJ. The clathrin assembly protein AP180 regulates the generation of amyloid-beta peptide. Biochemical and Biophysical Research Communications. 2009;385:247–250. doi: 10.1016/j.bbrc.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PJ, Zhang P, Mattson MP, Furukawa K. Heterogeneity of endocytic proteins: Distribution of clathrin adaptor proteins in neurons and glia. Neuroscience. 2003;121:25–37. doi: 10.1016/s0306-4522(03)00431-7. [DOI] [PubMed] [Google Scholar]
- Yao PJ, Petralia RS, Bushlin I, Wang Y, Furukawa K. Synaptic distribution of the endocytic accessory proteins AP180 and CALM. Journal of Comparative Neurology. 2005;481:58–69. doi: 10.1002/cne.20362. [DOI] [PubMed] [Google Scholar]
- Ye W, Lafer EM. Bacterially expressed F1-20/AP-3 assembles clathrin into cages with a narrow size distribution: Implications for the regulation of quantal size during neurotransmission. Journal of Neuroscience. 1995;41:15–26. doi: 10.1002/jnr.490410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- Zhou S, Tannery NH, Yang J, Puszkin S, Lafer EM. The synapse-specific phosphoprotein F1-20 is identical to the clathrin assembly protein AP-3. Journal of Biological Chemistry. 1993;268:12655–12662. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.