Abstract
Purpose
To examine the associations of age-related macular degeneration (AMD) with incident coronary heart disease (CHD) and stroke in the Cardiovascular Health Study.
Design
Population-based prospective cohort study.
Participants
A total of 1786 white and African-American participants free of CHD or 2228 participants free of stroke, aged 69 to 97 years.
Methods
AMD was evaluated from photographs taken in 1997 and 1998.
Main Outcome Measures
Incident CHD and stroke ascertained using standardized methods.
Results
Of the 1786 persons free of CHD, 303 developed incident CHD over 7 years. Participants with early AMD (n = 277) had a higher cumulative incidence of CHD than participants without early AMD (25.8% vs. 18.9%, P = 0.001). By adjusting for age, gender, race, systolic and diastolic blood pressure, hypertension status, fasting glucose, triglyceride, low-density lipoprotein cholesterol, cigarette smoking, pack years of smoking, and C-reactive protein, the presence of early AMD was associated with an increased risk of incident CHD (hazard ratio 1.57; 95% confidence interval, 1.17–2.22). Late AMD (n = 25) was not associated with incident CHD (hazard ratio 0.78; 95% confidence interval, 0.25–2.48). Among 2228 persons at risk, 198 developed incident stroke; neither early nor late AMD was associated with incident stroke.
Conclusions
This study suggests persons with early AMD have a higher risk of CHD but not stroke in a population aged 69 to 97 years. This provides further support that AMD is associated with underlying systemic vascular disease.
Age-related macular degeneration (AMD) is a leading cause of irreversible vision loss for individuals aged more than 40 years in Western society.1 Untreated patients with neovascular AMD lose 3 lines of visual acuity within 1 year.2 Although the cause of AMD remains poorly understood,3 increasing evidence suggests that it may share similar risk factors and common pathogenic mechanisms with cardiovascular disease (CVD).4,5 Both AMD and CVD are linked with various cardiovascular risk factors (e.g., smoking, hypertension), inflammatory markers (e.g., C-reactive protein [CRP]),6,7 and common genetic variants (e.g., apolipoprotein E gene and complement factor H).8–12 However, cross-sectional studies have not found a consistent association of AMD with various subclinical (e.g., cerebral white matter lesions detected by magnetic resonance imaging), clinical CVD (e.g., stroke history), or risk (e.g., plasma triglycerides) factors.13–17
There are limited data that have investigated prospectively whether AMD is an independent predictor of subsequent CVD.18,19 In the Atherosclerosis Risk In the Communities Study (ARIC), which prospectively examined middle-aged persons (51–72 years), the presence of early-stage AMD was associated with a higher adjusted risk for stroke (hazard ratio [HR] 1.85; 95% confidence interval [CI], 1.19–2.87)18 but not incident coronary heart disease (CHD) (HR 1.08; 95% CI, 0.82–1.42).19 Also in the ARIC study, late AMD was associated with a 3-fold greater risk of incident CHD (HR 3.10; 95% CI, 1.15–8.31).19 AMD has also been linked with decreased survival, an association partly reflecting underlying CVD in persons with AMD.20 However, no association of AMD status and overall or CVD specific mortality has been found in other studies.21 The purpose of the current study is to examine the association of AMD with the incidence of CHD and stroke events in a population age ranged 69 to 97 years, while controlling for common risk factors.
Materials and Methods
Study Population
The Cardiovascular Health Study (CHS) is a population-based cohort study of CVD in adults 65 years of age and older.22 The study sample and conduct have been described in detail.23 In brief, recruitment of the original cohort of 5201 persons took place at 4 field centers in the United States from 1989 to 1990. An additional 687 eligible African-Americans were recruited from Forsyth County, Sacramento County, and Allegheny County from 1992 to 1993. The total number of participants enrolled in the CHS was 5888, consisting of 4925 whites, 924 African-Americans, and 39 from other ethnic groups. Differences between those recruited and those not recruited have been presented.23
The current study population is derived from participants who returned for the clinic examination in 1997 and 1998 and had a retinal photograph taken approximately 9 years after the baseline examination. Of the 4249 persons (95.5% of baseline survivors) who were contacted at this examination, we initially excluded 29 participants (0.7%) whose race was neither white nor African-American, 1852 participants (43.6%) who did not have retinal photography or who had an ungradable photograph with AMD signs, 140 persons who had prevalent stroke, or 582 persons who had prevalent CHD, leaving 1786 persons (42.0% of the original 4249) or 2228 persons (52.4%) who were considered eligible for the analysis of incident CHD or stroke. Comparisons of persons with and without gradable photographs have been reported.24 In general, persons who did not have retinal photography or who had ungradable photographs were older and more likely to be male and African-American; to have hypertension and diabetes; to have a higher systolic and diastolic blood pressure, fasting glucose, and plasma total cholesterol levels; and to be current cigarette smokers.
Institutional review boards at each study site approved the study. Informed consent was obtained from all participants, and the study was conducted in accordance with the Declaration of Helsinki.
Retinal Photography and Age-Related Macular Degeneration Grading
The retinal photography procedure and the assessment of AMD in the CHS have been reported.13,25 After 5 minutes of dark adaptation, 1 randomly selected eye of each participant was photographed using a 45-degree nonmydriatic camera, 1 photograph centered at the optic disc and 1 photograph centered at the macula field.
Trained investigators, masked to subject information, graded the photographs using a modification of the Wisconsin AMD grading system at the Fundus Photographs Reading Center, Wisconsin. Soft drusen were defined as those having a diameter larger than 63 μm. Retinal pigment epithelial (RPE) depigmentation and increased retinal pigment associated with AMD (the presence of granules or clumps of gray or black pigment in or beneath the retina) were defined as present, absent, or questionable. Early AMD was defined as the presence of soft drusen alone, RPE depigmentation alone, or a combination of soft drusen with increased retinal pigment or RPE depigmentation in the absence of late AMD. Late AMD was defined as the presence of signs of exudative AMD or pure geographic atrophy.
The intergrader agreement ranged from 66% to 73% of eyes for 4 drusen characteristics (size, type, area, and confluence). There was even higher intergrader reproducibility for other maculopathy characteristics (kappa of ≥ 0.88). The intragrader agreement ranged from 62.5% for drusen type to 100% for geographic atrophy.26
Incident Cardiovascular Disease
Ascertainment of new CHD and stroke events in the CHS has been described.27–29 Participants, family members, or other previously identified informants reported new cardiovascular events during semiannual contacts by telephone or at a clinic visit. Medical records were obtained to confirm the diagnosis, and events were adjudicated by a committee.27,28 The 2 cardiovascular outcomes of interest in this study were incident CHD, which includes fatal and nonfatal myocardial infarction, fatal CHD and other CHD deaths (e.g., sudden cardiac death), and incident stroke, which includes fatal and nonfatal stroke. Adjudicated CHD and stroke events occurring between the date of retinal photograph in 1997–1998 and June 30, 2004, were available for analysis, providing a maximum of 7 years of follow-up for the present study. The implementation of multiple quality control procedures for the ascertainment of CHD and stroke events, especially the adjudication process, produced consistent results of outcome classification in the CHS.27
Definition of Other Variables
Participants underwent a standardized assessment of cardiovascular risk factors, including examiner-administered questionnaires, electrocardiography, carotid ultrasonography, echocardiography, and blood chemistry profiles, described in detail elsewhere.24,30 Prevalent CHD and stroke were assessed and classified by an adjudication process involving medical history, physical examination, and laboratory criteria.30 Standardized procedures were pre-formed for blood sample collection and processing to determine fasting glucose, lipids, and CRP levels.31 Resting blood pressure was measured according to a standardized protocol, and the mean of the first and the second measurements was used for analyses.32 Participants were classified as hypertensive if systolic blood pressure was ≥140 mmHg, diastolic blood pressure was ≥90 mmHg, or by the combination of self-reported high blood pressure diagnosis and use of antihypertensive medications. Education, hypertension and diabetes history, cigarette smoking (current, ever, never), alcohol consumption, and medication use were ascertained from standardized questionnaires. Diabetes mellitus was defined as a fasting glucose level ≥126 mg/dl (≥7.0 mmol/l), use of insulin or oral diabetes medication, or a self-reported history of physician-diagnosed diabetes. Height and weight were measured with participants wearing light cloth and without shoes. Body mass index was calculated as kilograms/meters squared.
All variables defined were based on the 1997–1998 clinic examination when retinal photography was taken, except data on some blood chemistries including CRP, high-density lipoprotein (HDL) cholesterol, and total triglyceride, which were obtained from the 1992–1993 examination, and fasting glucose, diabetes medications, and standing height, which were taken from the 1996–1997 examination.
Statistical Analysis
Student t test and chi-square test were used to compare the means and proportions of baseline characteristics by AMD status (none, early, late), incident CHD, and incident stroke status (absent, present). We preformed linear and logistic regression to compute age-, gender-, and ethnicity-adjusted P values for comparison of means or proportions.
Cox proportional hazards models were used to estimate the 7-year cumulative incidence of CHD and stroke among individuals with and without early AMD, adjusting for age, gender, and ethnicity. We also used Cox proportional hazards models to estimate the HR and corresponding 95% CIs for incident CHD and incident stroke by AMD status, adjusting initially for age, gender, and ethnicity, and then further for systolic blood pressure, diastolic blood pressure, hypertension, fasting glucose, diabetes status, low-density lipoprotein (LDL) cholesterol and HDL cholesterol, triglyceride, pack years of cigarette smoking, smoking status, and CRP. We calculated the 7-year cumulative incidence of CHD and stroke (defined as 1 − [100 × Kaplan–Meier CHD {or stroke}-free survival at 7 years]) in individuals with and without AMD. Survival time was based on time from retinal photography to incident CHD and stroke, death, or censoring at June 30, 2004.
We also conducted stratified analysis to examine potential interaction with ethnicity, gender, hypertension, and cigarette smoking status. All statistics analyses were performed using STATA version 10 (Stata Corp., College Station, TX).
Results
Among the 1786 participants free of CHD, 277 had early AMD and 25 had late AMD. After 7 years of follow-up (average follow-up, 6 years), there were 303 persons who developed an incident CHD event. Among 2228 persons free of stroke, there were 198 incident stroke events over a 7-year period.
There were 1715 participants free of both CHD and stroke, consisting of 1450 whites and 265 African-Americans. The prevalence of AMD was 17.0% (292 persons). There were 268 (15.6%) early AMD cases and 24 (1.4%) late AMD cases.
Table 1 presents participant characteristics according to AMD status and whether participants developed an incident CHD and stroke event. Persons with early and late AMD were significantly older compared with persons without AMD. Persons with early AMD were more likely to be white and had lower plasma total triglyceride and lower plasma LDL cholesterol compared with persons without AMD. Participants who developed incident CHD were significantly older, more likely to be male, and more likely to have hypertension; had higher mean systolic blood pressure but lower diastolic blood pressure; were more likely to have diabetes; and had higher glucose levels, lower plasma HDL cholesterol level, and higher plasma triglyceride level than participants who did not develop an incident CHD. Participants with an incident stroke were also significantly older and more likely to have hypertension, and had higher systolic blood pressure than participants without a stroke event.
Table 1.
Participant Characteristics at Baseline, by Age-Related Macular Degeneration, Incident Coronary Heart Disease Status, and Incident Stroke Status
| AMD
|
Incident CHD
|
Incident Stroke
|
|||||
|---|---|---|---|---|---|---|---|
| Absent (n = 1423) | Early (n = 268) | Late (n = 24) | No (n = 1483) | Yes (n = 303) | No (n = 2030) | Yes (n = 198) | |
| Age, y | 77.8 | 79.8a | 82.1a | 78.0 | 79.4a | 78.3 | 79.7a |
| Male, % | 34.6 | 33.6 | 37.5 | 32.0 | 45.5a | 39.3 | 34.8 |
| African-American, % | 16.9 | 9.0a | 4.2 | 16.8 | 12.9 | 14.9 | 15.2 |
| High school graduate, % | 48.7 | 51.2 | 59.1 | 49.0 | 51.6 | 48.5 | 49.5 |
| Hypertension, % | 57.2 | 55.5 | 75.0 | 55.5 | 66.6a | 57.2 | 66.8a |
| Systolic blood pressure, mmHg | 132.1 | 130.7 | 133.9 | 131.4 | 134.6a | 131.0 | 136.7a |
| Diastolic blood pressure, mmHg | 67.1 | 67.5 | 66.4 | 67.4 | 65.9a | 66.4 | 68.0 |
| Diabetes, % | 16.1 | 14.6 | 14.3 | 14.9 | 21.0a | 17.2 | 20.1 |
| Glucose, mg/dl | 97.5 | 97.7 | 97.6 | 96.4 | 102.3a | 98.4 | 98.6 |
| Body mass index, kg/m2 | 27.1 | 26.6 | 28.5 | 26.9 | 27.4 | 27.0 | 26.9 |
| Total cholesterol, mg/dl | 205.6 | 202.9 | 198.0 | 205.7 | 203.4 | 202.3 | 206.5 |
| HDL cholesterol, mg/dl | 54.7 | 56.4 | 60.3 | 55.4 | 53.7a | 53.7 | 54.0 |
| LDL cholesterol, mg/l | 129.0 | 124.2a | 115.7 | 128.1 | 128.8 | 128.6 | 127.6 |
| Total triglyceride, mg/dl | 126.2 | 109.5a | 110.6 | 120.9 | 133.8a | 125.6 | 125.2 |
| Cigarette smoking, ever, % | 5.9 | 8.2 | 11.7 | 6.3 | 5.8 | 5.6 | 8.1 |
| Alcohol use, ever, % | 1.0 | 0.9 | 0.6 | 1.0 | 0.8 | 1.1 | 1.0 |
AMD = age-related macular degeneration; CHD = coronary heart disease; HDL = high-density lipoprotein; LDL= low-density lipoprotein.
P<0.05; represents differences in means or proportions, adjusted for age, gender, and ethnicity (except for mean age, male, and African-American ethnicity, which were not adjusted for age, gender, and ethnicity, respectively).
Table 2 shows the cumulative incidence of CHD and stroke associated with the presence of early or late AMD signs. Participants who had signs of early-stage AMD had a significantly higher cumulative incidence of CHD than participants without early AMD (25.76% vs. 18.88%, P = 0.001). There was a significantly higher cumulative incidence of CHD for persons with specific early AMD signs (soft drusen, hyperpigmentation, and RPE de-pigmentation) than for persons without these signs. In contrast, there was no difference of cumulative incidence of stroke between persons with early or late AMD and those without these signs.
Table 2.
Cumulative Incidence of Coronary Heart Disease and Stroke, by Presence of Early and Late Age-related Macular Degeneration Signs
| No. at Risk | CHD
|
No. at Risk | Stroke
|
|||||
|---|---|---|---|---|---|---|---|---|
| No. of Events | Incidence % | Pa | No. of Events | Incidence % | Pa | |||
| Early AMD | ||||||||
| Absent | 1484 | 234 | 18.88 | 0.001 | 1849 | 158 | 9.89 | 0.21 |
| Present | 277 | 64 | 25.76 | 350 | 36 | 12.24 | ||
| Soft drusen | ||||||||
| Absent | 1500 | 240 | 19.09 | 0.005 | 1870 | 162 | 10.00 | 0.42 |
| Present | 261 | 58 | 25.00 | 329 | 32 | 11.76 | ||
| Hyperpigmentation | ||||||||
| Absent | 1656 | 266 | 18.02 | <0.001 | 2064 | 182 | 10.19 | 0.81 |
| Present | 105 | 32 | 46.78 | 135 | 12 | 11.28 | ||
| RPE depigmentation | ||||||||
| Absent | 1717 | 285 | 19.68 | 0.006 | 2149 | 187 | 10.09 | 0.13 |
| Present | 44 | 13 | 33.38 | 50 | 7 | 17.76 | ||
| Late AMD | ||||||||
| Absent | 1465 | 156 | 18.88 | 0.56 | 1849 | 158 | 9.89 | 0.22 |
| Present | 25 | 4 | 21.14 | 29 | 4 | 13.79 | ||
AMD = age-related macular degeneration; CHD = coronary heart disease; RPE = retinal pigment epithelial.
%: Cumulative incidence of CHD/stroke, defined as 100 − (1-Kaplan–Meier cumulative survival at 7 years follow-up).
P value based on log-rank test.
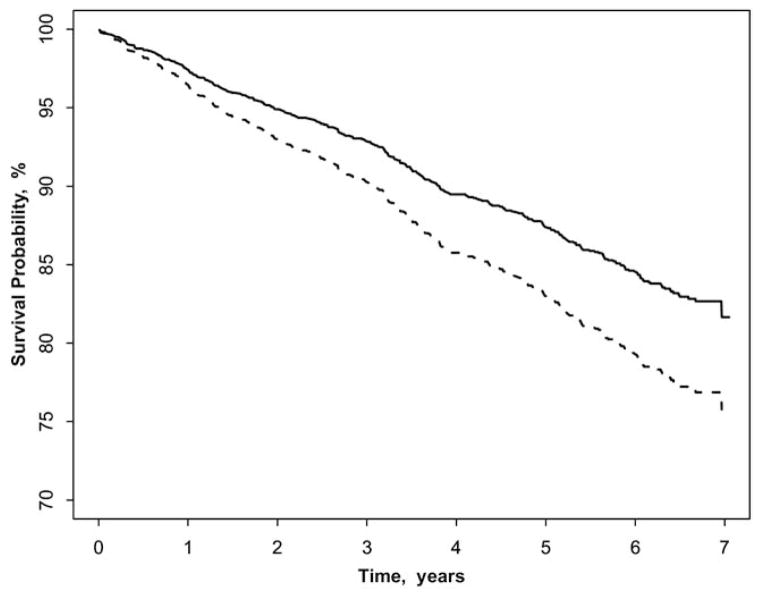
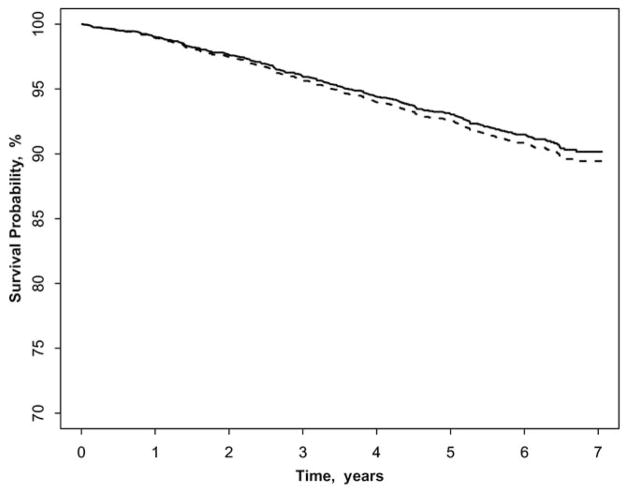
Figure 1 shows that participants with early AMD had a significantly higher incidence of CHD than those without the signs, after adjusting for age, gender, and ethnicity. In contrast, persons with early AMD did not have a higher incidence of stroke compared with those without early AMD (Fig 2). The associations of early and late AMD signs with incident CHD and stroke are presented in Table 3. After controlling for age, gender, and ethnicity, early AMD signs were associated with incident CHD (HR 1.38; 95% CI, 1.04–1.84). Further adjustment for potential confounders of systolic and diastolic blood pressure, hypertension, fasting glucose, diabetes, triglyceride, pack-years of smoking, smoking status, LDL cholesterol, and CRP did not alter this relationship (HR 1.57; 95% CI, 1.17–2.12). Some specific early AMD signs were also related to incident CHD (marginally for soft drusen and RPE depigmentation). Neither early nor late AMD was associated with incident stroke events.
Figure 1.
Coronary heart disease-free survival in participants with and without early age-related macular degeneration (AMD), adjusted for age, gender, and ethnicity. Solid line represents early AMD absent; dash line represents early AMD present.
Figure 2.
Stroke-free survival in participants with and without early age-related macular degeneration (AMD), adjusted for age, gender, and ethnicity. Solid line represents early AMD absent; dash line represents early AMD present.
Table 3.
Hazard Ratios of Incident Coronary Heart Disease and Stroke, by Early and Late Age-related Macular Degeneration Signs
| CHD
|
Stroke
|
|||
|---|---|---|---|---|
| Age-Gender-Race HR (95% CI)a | Multivariable HR (95% CI)b | Age-Gender-Race HR (95% CI)a | Multivariable HR (95% CI)b | |
| Early AMD | 1.38 (1.04–1.84) | 1.57 (1.17–2.12) | 1.08 (0.75–1.56) | 1.05 (0.69–1.58) |
| Soft drusen | 1.29 (0.96–1.73) | 1.46 (1.07–1.99) | 1.00 (0.68–1.47) | 0.99 (0.64–1.52) |
| Hyperpigmentation | 1.69 (1.16–2.46) | 1.68 (1.12–2.54) | 0.88 (0.49–1.59) | 0.71 (0.34–1.46) |
| RPE depigmentation | 1.72 (0.97–3.02) | 1.90 (1.06–3.39) | 1.46 (0.68–3.13) | 0.95 (0.35–2.60) |
| Late AMD | 0.96 (0.39–2.34) | 0.78 (0.25–2.48) | 1.36 (0.50–3.70) | 1.43 (0.45–4.54) |
AMD = age-related macular degeneration; CHD = coronary heart disease; RPE = retinal pigment epithelial; HR = hazard ratio; CI = confidence interval.
Hazard ratio (95% CI) of CHD/stroke, adjusted for age, gender, and ethnicity.
Hazard ratio (95% CI) of CHD/stroke, adjusted for age, gender, ethnicity, systolic and diastolic blood pressure, hypertension, fasting glucose, diabetes, triglyceride, pack-years of smoking, current smoking status, low-density lipoprotein cholesterol, and C-reactive protein.
In the stratified analysis, associations of AMD with CHD were somewhat stronger in younger than older people. Among persons aged 69 to 78 years, the adjusted HR for CHD in association with early AMD was 1.80 (95% CI, 1.16–2.79), whereas in persons 79 years and older, the HR was 1.45 (95% CI, 0.97–2.16, age group/ early AMD cross-product interaction term, P = 0.07). The association was also slightly stronger in whites compared with African-Americans, in men compared with women, in persons without hypertension compared with persons with hypertension, and in persons who were never/past cigarette smokers compared with current smokers (data not shown). These later differences were not statistically significant (P>0.10 for all cross-product interaction terms).
Discussion
In the current study, we examined prospectively the relationship of AMD to 7-year incident CHD and stroke in a population aged 69 to 97 years. Our study demonstrates that older persons with early AMD signs are at higher risk of CHD events than persons without these signs. This association was independent of age, gender, race, systolic and diastolic blood pressure, hypertension, fasting glucose, diabetes, triglyceride, pack-years of smoking, current smoking status, LDL cholesterol, and CRP. Late AMD, however, was not associated with incident CHD. Furthermore, neither early nor late AMD was associated with incident stroke in our study population.
Although many previous studies have examined cross-sectional associations of AMD and CVD, few of them found a consistent link between AMD and CVD.16,33 In the same CHS cohort, older participants with early AMD signs were more likely to have subclinical cerebral lesions on magnetic resonance imaging.13 The lack of association with incident clinical stroke was therefore unexpected.
There are few studies that have prospectively examined AMD with incident CVD. These are summarized in Table 4. A recent retrospective cohort study using hospitalized CVD database found contradictory results, showing significantly lower rates of CVD in persons with neovascular AMD than controls (adjusted rate ratio 0.58; 95% CI, 0.48–0.72 for myocardial infarction; 0.56, 95% CI, 0.45–0.70 for cerebrovascular accidents).34 The lack of adjustment for important confounders (e.g., smoking and race) in the analyses and potential selection bias, however, make it difficult to interpret.
Table 4.
Age-related Macular Degeneration and Incident Cardiovascular Disease
| No. | Age Range (yrs) | Follow-up (yrs) | AMD Status | HR of CHD (95% CI)a | HR of Stroke (95% CI)a | |
|---|---|---|---|---|---|---|
| US Medicare data35 | 1,445,677 | ≥65 | 2 | Late | 1.19 (1.16–1.22) | — |
| US Medicare data43 | 1,303,186 | ≥65 | 2 | Late | — | 1.21 (1.18–1.23) |
| ARIC Study18,19 | 10,000+ | 51–72 | 10 | Early | 1.08 (0.82–1.42) | 1.85 (1.19–2.87) |
| Late | 3.10 (1.15–8.31) | — | ||||
| Blue Mountains Eye Study20 | 3654 | ≥49 | 11 | Early | 2.32 (1.03–5.19)b | — |
| Late | 5.57 (1.35–22.99)b,c | 10.2 (2.39–43.6)b,c | ||||
| Beaver Dam Eye Study21 | 4926 | 43–84 | 14 | Early or late | 0.95 (0.87–1.07) | 0.97 (0.75–1.23) |
| Cardiovascular Health Study | 1786 | 69–97 | 7 | Early | 1.57 (1.17–2.22) | 1.05 (0.69–1.58) |
| Late | 0.78 (0.25–2.48) | 1.43 (0.45–4.54) |
AMD = age-related macular degeneration; CHD = coronary heart disease; ARIC = Atherosclerosis In Community Study; HR = hazard ratio; CI = confidence interval.
Multivariable hazard ratios of CHD or stroke (CHD or stroke mortality for the Blue Mountains Eye Study and Beaver Dam Eye Study).
Only in persons aged < 75 yrs.
Age and gender adjusted only.
We previously reported in the ARIC study that persons with early AMD have a higher incidence of stroke than those without early AMD (HR 1.85; 95% CI, 1.19–2.87),18 although a similar association was not found for incident CHD in this study.19 Late AMD was found to be linked to a 3-fold higher risk of incident CHD (HR 3.10; 95% CI, 1.15–8.31).19 Our present study extends these observations and provides further evidence that these early AMD signs predict subsequent development of CHD events in an older population, which was also consistent with the recent finding of an older population sampled from the US Medicare beneficiaries (HR of CHD 1.19; 95% CI, 1.16–1.22).35 Although both early and late AMD are largely related to same risk factors and processes, there are some subtle differences. Drusen and RPE changes result from slow degenerative processes associated with age and may be related to local choroidal vascular ischemia caused by the same risk factors that induce atherosclerosis.4 Late “exudative” forms of AMD have in addition an increase in vascular endothelial growth factor activity that leads to choroidal neovascularization.4 In the Visual Impairment Project conducted in Australia, early AMD was associated with age, cigarette smoking for more than 40 years, and a history of ever having taken angiotensin-converting enzyme inhibitors and lipid-lowering medications.36 The magnitude of all these risk factors was slightly higher for late AMD, except that having received lipid-lowering medications was not significant.36 We note both CHS and ARIC had similar grading and definition of AMD, as well as definitions of incident CHD and stroke (both included fatal and nonfatal events and were based on active patient recall, tracing of hospitalization and death records, and adjudication by central committee of experts). It is therefore likely that the contrasting findings between the ARIC study and the CHS may be due to differences in study population and characteristics, in length of follow-up time, and the different vascular risk factor profiles such as age, smoking, hypertension, and other risk factors, which are strongly associated with both early and late AMD. In addition, our study had more than 90% power to detect an association between early AMD and incident stroke with an HR of 1.8 based on the ARIC study showing this strength of association. Thus, taken in totality, the differences in association between early and late AMD with CVD may reflect slightly different underlying pathophysiology and risk factors.
We note a slightly stronger association of AMD with CVD in younger than older people. In the Blue Mountains Eye Study, the reported association of AMD with cardiovascular mortality was seen only in persons younger than 75 years.20 However, in the Beaver Dam Eye Study, AMD severity was not associated with CHD or stroke mortality in the 14-year follow-up, even in younger members of the cohort.21 Further research is required to determine if younger persons with AMD are at higher risk of CVD.
The association of AMD and CHD may be explained by common and broad underlying pathogenic mechanisms shared with both conditions. For example, atherosclerosis, a traditional risk factor of CHD, may represent a pathogenic process implicated in the AMD development, based on its effects on the choroid capillaries and extracellular drusen (lipids) deposits.37 Inflammatory mechanisms seem to be another plausible biological basis that may involve both coronary and retinal circulation.38 Genetic factors have also been postulated to contribute to the pathogenesis of both AMD and CHD. Of note, a genetic epidemiologic study recently reported that CHF is a connecting gene linking 1 disease (CHD) to another (AMD).39,40 The exact molecular pathway linking AMD and CHD, however, remains unclear.
Strengths of the CHS include a prospective observation from a community-sampled population, the use of photographic methods to evaluate AMD, validated ascertainment of incident CHD and stroke events, and detailed information on a variety of potential confounders. Our study also had several important limitations. First, retinal photography was performed approximately 10 years after the baseline study, and a significant proportion of photographs were ungradable because of media opacity or poor pupil dilation in this older population. Biases related to nonattendance for photography, ungradable photographs, and selective mortality may lead to an underestimation of the true risks. For example, the lack of association of early or late AMD signs and incident stroke events may be related to higher mortality among participants with AMD at baseline who did not attend the follow-up examinations. Second, there is increased grading variability, and AMD is less likely to be detected using a 45-degree fundus photograph of 1 eye taken by a nonmydriatic camera.41 In addition, some people with AMD would be missed because of the possibility of the involved eye not being photographed, although AMD is often symmetric between eyes. Nevertheless, if AMD was associated with CHD, nondifferential misclassification of AMD (AMD cases misclassified as absent) would result in a bias toward the null; and thus the true association between AMD and incident CHD may be stronger than that observed here. Third, we cannot totally exclude residual confounding given some factors (e.g., CRP, LDL, and HDL cholesterol) were measured 5 years before retinal photography. This may partly explain the unexpected observation of LDL cholesterol level being significantly lower in participants with early AMD in Table 1. Although this could also be due to lipid-lowering medication use in participants with early AMD, this information was not available in this study. The limitations of the study also include a decreased power of detecting an association of late AMD with incident CVD, given the relatively few cases of late AMD in the present study.
In conclusion, in this older cohort, we found that early AMD signs were associated with incident CHD events, although not incident stroke. Our findings suggest that AMD may be associated with underlying systemic atherosclerotic vascular disease and may have broader implications of cardiovascular safety for the many patients with AMD who are treated with long-term antivascular endothelial growth factor therapy.42
Acknowledgments
The research reported in this article was supported by the National Heart, Lung, and Blood Institute (contracts N01-HC-85079 to N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, and U01 HL080295], with additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided by the National Heart, Lung, and Blood Institute, National Institute of Health (grant number R21-HL077166) and the Sylvia and Charles Viertel Clinical Investigator Award (TYW). A full list of principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm. The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Presented at: The Macula Society Meeting, May 2007, London, England.
Financial Disclosure(s): The author(s) have no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Wong TY, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–26. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Holz FG, Pauleikhoff D, Klein R, Bird AC. Pathogenesis of lesions in late age-related macular disease. Am J Ophthalmol. 2004;137:504–10. doi: 10.1016/j.ajo.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 4.Snow KK, Seddon JM. Do age-related macular degeneration and cardiovascular disease share common antecedents? Ophthalmic Epidemiol. 1999;6:125–43. doi: 10.1076/opep.6.2.125.1558. [DOI] [PubMed] [Google Scholar]
- 5.Wong TY, Mitchell P. The eye in hypertension. Lancet. 2007;369:425–35. doi: 10.1016/S0140-6736(07)60198-6. [DOI] [PubMed] [Google Scholar]
- 6.Seddon JM, Gensler G, Milton RC, et al. Association between C-reactive protein and age-related macular degeneration. JAMA. 2004;291:704–10. doi: 10.1001/jama.291.6.704. [DOI] [PubMed] [Google Scholar]
- 7.Cao JJ, Arnold AM, Manolio TA, et al. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: the Cardiovascular Health Study. Circulation. 2007;116:32–8. doi: 10.1161/CIRCULATIONAHA.106.645606. [DOI] [PubMed] [Google Scholar]
- 8.Lindsberg PJ, Ohman J, Lehto T, et al. Complement activation in the central nervous system following blood-brain barrier damage in man. Ann Neurol. 1996;40:587–96. doi: 10.1002/ana.410400408. [DOI] [PubMed] [Google Scholar]
- 9.Edwards AO, Ritter R, III, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 10.Klaver CC, Kliffen M, van Duijn CM, et al. Genetic association of apolipoprotein E with age-related macular degeneration. Am J Hum Genet. 1998;63:200–6. doi: 10.1086/301901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song Y, Stampfer MJ, Liu S. Meta-analysis: apolipoprotein E genotypes and risk for coronary heart disease. Ann Intern Med. 2004;141:137–47. doi: 10.7326/0003-4819-141-2-200407200-00013. [DOI] [PubMed] [Google Scholar]
- 12.McCarron MO, Delong D, Alberts MJ. APOE genotype as a risk factor for ischemic cerebrovascular disease: a meta-analysis. Neurology. 1999;53:1308–11. doi: 10.1212/wnl.53.6.1308. [DOI] [PubMed] [Google Scholar]
- 13.Klein R, Klein BE, Marino EK, et al. Early age-related maculopathy in the Cardiovascular Health Study. Ophthalmology. 2003;110:25–33. doi: 10.1016/s0161-6420(02)01565-8. [DOI] [PubMed] [Google Scholar]
- 14.Klein R, Klein BE, Jensen SC, et al. Age-related maculopathy in a multiracial United States population: the National Health and Nutrition Examination Survey III. Ophthalmology. 1999;106:1056–65. doi: 10.1016/S0161-6420(99)90255-5. [DOI] [PubMed] [Google Scholar]
- 15.Hyman L, Schachat AP, He Q, Leske MC Age-Related Macular Degeneration Risk Factors Study Group. Hypertension, cardiovascular disease, and age-related macular degeneration. Arch Ophthalmol. 2000;118:351–8. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Knudtson MD, et al. Subclinical atherosclerotic cardiovascular disease and early age-related macular degeneration in a multiracial cohort: the Multiethnic Study of Atherosclerosis. Arch Ophthalmol. 2007;125:534–43. doi: 10.1001/archopht.125.4.534. [DOI] [PubMed] [Google Scholar]
- 17.Smith W, Mitchell P, Leeder SR, Wang JJ. Plasma fibrinogen levels, other cardiovascular risk factors, and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 1998;116:583–7. doi: 10.1001/archopht.116.5.583. [DOI] [PubMed] [Google Scholar]
- 18.Wong TY, Klein R, Sun C, et al. Atherosclerosis Risk in Communities Study. Age-related macular degeneration and risk for stroke. Ann Intern Med. 2006;145:98–106. doi: 10.7326/0003-4819-145-2-200607180-00007. [DOI] [PubMed] [Google Scholar]
- 19.Wong TY, Tikellis G, Sun C, et al. Age-related macular degeneration and risk of coronary heart disease: the Atherosclerosis Risk in Communities Study. Ophthalmology. 2007;114:86–91. doi: 10.1016/j.ophtha.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 20.Tan JS, Wang JJ, Liew G, et al. Age-related macular degeneration and mortality from cardiovascular disease or stroke. Br J Ophthalmol. 2008;92:509–12. doi: 10.1136/bjo.2007.131706. [DOI] [PubMed] [Google Scholar]
- 21.Knudtson MD, Klein BE, Klein R. Age-related eye disease, visual impairment, and survival: the Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:243–9. doi: 10.1001/archopht.124.2.243. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 23.Tell GS, Fried LP, Hermanson B, et al. Recruitment of adults 65 years and older as participants in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:358–66. doi: 10.1016/1047-2797(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 24.Wong TY, Klein R, Sharrett AR, et al. The prevalence and risk factors of retinal microvascular abnormalities in older persons: the Cardiovascular Health Study. Ophthalmology. 2003;110:658–66. doi: 10.1016/S0161-6420(02)01931-0. [DOI] [PubMed] [Google Scholar]
- 25.Sun C, Tikellis G, Klein R, et al. Depressive symptoms and age-related macular degeneration in older people: the Cardiovascular Health Study. Ophthalmic Epidemiol. 2007;14:127–33. doi: 10.1080/09286580601186742. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Davis MD, Magli YL, et al. The Wisconsin Age-related Maculopathy Grading System. Ophthalmology. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 27.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events: the Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 28.Price TR, Psaty B, O’Leary D, et al. Assessment of cerebrovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1993;3:504–7. doi: 10.1016/1047-2797(93)90105-d. [DOI] [PubMed] [Google Scholar]
- 29.Wong TY, Kamineni A, Klein R, et al. Quantitative retinal venular caliber and risk of cardiovascular disease in older persons: the Cardiovascular Health Study. Arch Intern Med. 2006;166:2388–94. doi: 10.1001/archinte.166.21.2388. [DOI] [PubMed] [Google Scholar]
- 30.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 31.Robbins J, Wahl P, Savage P, et al. Hematological and biochemical laboratory values in older Cardiovascular Health Study participants. J Am Geriatr Soc. 1995;43:855–9. doi: 10.1111/j.1532-5415.1995.tb05526.x. [DOI] [PubMed] [Google Scholar]
- 32.Tell GS, Rutan GH, Kronmal RA, et al. Cardiovascular Health Study (CHS) Collaborative Research Group. Correlates of blood pressure in community-dwelling older adults: the Cardiovascular Health Study. Hypertension. 1994;23:59–67. doi: 10.1161/01.hyp.23.1.59. [DOI] [PubMed] [Google Scholar]
- 33.Klein R, Deng Y, Klein BE, et al. Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: Women’s Health Initiative Sight Exam ancillary study. Am J Ophthalmol. 2007;143:473–83. doi: 10.1016/j.ajo.2006.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen-Khoa BA, Goehring EL, Jr, Werther W, et al. Hospitalized cardiovascular diseases in neovascular age-related macular degeneration. Arch Ophthalmol. 2008;126:1280–6. doi: 10.1001/archopht.126.9.1280. [DOI] [PubMed] [Google Scholar]
- 35.Duan Y, Mo J, Klein R, et al. Age-related macular degeneration is associated with incident myocardial infarction among elderly Americans. Ophthalmology. 2007;114:732–7. doi: 10.1016/j.ophtha.2006.07.045. [DOI] [PubMed] [Google Scholar]
- 36.McCarty CA, Mukesh BN, Fu CL, et al. Risk factors for age-related maculopathy: the Visual Impairment Project. Arch Ophthalmol. 2001;119:1455–62. doi: 10.1001/archopht.119.10.1455. [DOI] [PubMed] [Google Scholar]
- 37.Friedman E. The role of the atherosclerotic process in the pathogenesis of age-related macular degeneration. Am J Ophthalmol. 2000;130:658–63. doi: 10.1016/s0002-9394(00)00643-7. [DOI] [PubMed] [Google Scholar]
- 38.Donoso LA, Kim D, Frost A, et al. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51:137–52. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topol EJ, Smith J, Plow EF, Wang QK. Genetic susceptibility to myocardial infarction and coronary artery disease. Hum Mol Genet. 2006;15(suppl):R117–23. doi: 10.1093/hmg/ddl183. [DOI] [PubMed] [Google Scholar]
- 40.Kardys I, Klaver CC, Despriet DD, et al. A common polymorphism in the complement factor H gene is associated with increased risk of myocardial infarction: the Rotterdam Study. J Am Coll Cardiol. 2006;47:1568–75. doi: 10.1016/j.jacc.2005.11.076. [DOI] [PubMed] [Google Scholar]
- 41.Klein R, Meuer SM, Moss SE, Klein BE. Detection of drusen and early signs of age-related maculopathy using a nonmy-driatic camera and a standard fundus camera. Ophthalmology. 1992;11:1686–92. doi: 10.1016/s0161-6420(92)31745-2. [DOI] [PubMed] [Google Scholar]
- 42.Wong TY, Liew G, Mitchell P. Clinical update: new treatments for age-related macular degeneration. Lancet. 2007;370:204–6. doi: 10.1016/S0140-6736(07)61104-0. [DOI] [PubMed] [Google Scholar]