Abstract
Objective
Dysregulated angiotensin II (Ang II) signaling induces local vascular interleukin-6 (IL-6) secretion, producing leukocyte infiltration and life-threatening aortic dissections. Precise mechanism(s) by which IL-6 signaling induces leukocyte recruitment remain(s) unknown. T-helper 17lymphocytes (Th17) have been implicated in vascular pathology, but their role in the development of aortic dissections is poorly understood. Here, we tested the relationship of IL-6-STAT3 signaling with Th17-induced inflammation in the formation of Ang II-induced dissections in C57BL/6 mice.
Methods and Results
Ang II infusion induced aortic dissections and CD4+-interleukin 17A (IL-17A)-expressing, Th17 cell accumulation in C57BL/6 mice. A blunted local Th17 activation, macrophage recruitment, and reduced incidence of aortic dissections were seen in IL-6−/− mice. To determine pathological roles of Th17 lymphocytes, we treated Ang II infused mice with IL-17A neutralizing antibody (IL17A NAb), or infused Ang II in genetically deficientIL-17A mice, and found decreased aortic chemokine MCP-1 production and macrophage recruitment, leading to a reduction in aortic dissections. This effect was independent of blood pressure in IL17ANAb experiment. Application of a cell-permeable STAT3 inhibitor to downregulate the IL-6 pathway decreased aortic dilation and Th17 cell recruitment. We also observed increased aortic Th17 infiltration and IL-17 mRNA expression in patients with thoracic aortic dissections. Lastly, we found that Ang II mediated aortic dissections occurred independent of blood pressure changes.
Conclusions
Our results indicate that the IL-6-STAT3 signaling pathway converges on Th17 recruitment and IL-17A signaling upstream of macrophage recruitment, mediating aortic dissections.
Keywords: Angiotensin II, Aortic dissection, IL-6, Th17, Vascular inflammation
Angiotensin II (Ang II) is the major effector peptide of the renin angiotensin system, and its signaling via the type 1 Ang II receptor induces vascular contractility, hypertrophy and extracellular remodeling.1 More recently, Ang II has been shown to induce inflammation, a process mediated by monocyte/macrophage cell recruitment into the adventitial and medial layers of large arteries. Importantly, both human and experimental animal studies have suggested a role for Ang II in the development of aortic dissections.2
Vascular inflammation is a stereotypic process producing recruitment of activated leukocytes, monocytes/macrophages and lymphocytes, into all layers of the vascular wall.3, 4 Circulating leukocytes are recruited from the circulation into the vessel wall either through the intimal (“inside-out”) or adventitial (“outside-in”) surfaces, through a coordinated process of demargination, tissue infiltration, and local cellular activation.5, 6 Of these, monocyte/macrophages mediate the final pathological consequences of vascular inflammation. Ang II-stimulated monocytes are major generators of ROS stress, producers of matrix metalloproteases, and secretors of additional cytokines in the vessel wall.7–9 These effects result in extracellular matrix degradation, enhanced reactivity to inflammatory agents, endothelial dysfunction, and vascular dissection.10 The mechanisms that Ang II-induced cytokines play in this process of local vascular inflammation are not well understood.
IL-6 is the most highly upregulated cytokine in Ang II-stimulated vessels yet identified,11, 12 and it has been identified as an independent biomarker of vascular atherosclerotic risk and of aneurysmal rupture.13, 14 IL-6 is a member of a superfamily of cardioactive cytokines whose members include cardiotropin, IL-11, and -12, and G-CSF that bind to unique alpha receptors and whose actions are mediated through a common gp130 signal transducer converging on the signal transducer and activator of transcription (STAT)-3.15, 16 Cellular targets of IL-6 signaling include vascular smooth muscle cells, endothelial cells, and monocyte/macrophage populations. Recently, we demonstrated that IL-6 plays a major pathogenic role in aortic dissections induced by Ang II because its deficiency significantly blocked aortic dissections, monocyte infiltration, reactive oxygen species (ROS) formation and chemotactic cytokine amplification.11 Although our findings suggest that IL-6 is necessary for macrophage activation in the early stages of vascular inflammation leading to aortic dissection, IL-6 lacks chemotactic activity and therefore, its effects on monocyte recruitment have not been fully explained.
Earlier studies have linked vascular effects of Ang II as mediated by lymphocyte populations.2, 17 Not only are T and B lymphocytes found in Ang II-induced vascular diseases,18 but the effect of Ang II on hypertension, vasomotor dysfunction, oxidative stress, arteriolar thrombosis, and atherosclerosis is prevented with total T-lymphocyte deficiency in mice.19, 20 These studies suggest that T lymphocytes may play a central role in vascular pathogenesis. More recently, the CD4+ T helper subset characterized by IL-17A secretion (Th17), a subset distinct from the polarized Th1, Th2, and Treg populations, has been implicated in Ang II-induced atherosclerosis and vascular dysfunction in a hyperlipidemic background.21–26 The role of IL-17A/Th17 activation and the mechanism for their accumulation in the development and progression of aortic dissections is not known.
In this study, we explored the relationship of IL-6 signaling on formation of the Th17 cell population, and the pathogenic role of IL-17Ain aortic dilation and dissections induced by Ang II. Our approach utilized Ang II challenge of normolipidemic mouse models11 with IL-6 and IL-17A deficiencies to assess the rate of aortic dissection. To establish clinical relevance, we also examined Th17 recruitment in human ascending aortic dissections. Our results suggest that a major consequence of vascular IL-6 signaling is the STAT3-dependent formation and recruitment of Th17 lymphocytes. Th17, in turn, plays an important role in monocyte recruitment and aortic dissections.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Supplement.
RESULTS
IL-6 deficiency reduced aortic dissections and Th17 recruitment induced by Ang II
We have previously reported that chronic subcutaneous infusion of Ang II (2,500 ng/kg/min) induced aortic dissections (defined as intramural hematoma in the suprarenal aorta17) in 35–50 % of aged mice.27 In this study, aortic hematomas/dissections were demonstrated with aortic ultrasonography and tissue histochemistry. In WT mice in the C57BL/6 background, histochemical analysis of cross sections in the suprarenal abdominal aorta consistently showed adventitial thickening and blood-filled false lumens located in the tunica adventitia in the Ang II-treated mice (Supplementary Figure 1). Approximately40 % of Ang II-infused mice developed areas of focal hemorrhages, visualized as false lumens indicating aortic dissection. All mice that developed dissections maintained an aortic size 50 % greater than control aortas.
We reproduced our earlier studies of Ang II infusions conducted in IL-6−/− mice in the C57BL/6 background, where we observed a significantly reduced early incidence of aortic dissections after 7 d (31 % in WT, n=16, vs. 0 % in IL-6−/−, n=12, respectively, 7 d, p<0.05). This reduction in the incidence of dissections was not accounted for by changes in the systolic blood pressure of IL-6−/− mice.28 Here, Ang II induced a pressor effect of 30 mmHg after 7 d in WT mice (from mean 102 ± 3 mmHg to a mean of 132 ± 6 mmHg, n=10, p=0.002, Supplementary Figure 2) which was not statistically different from the pressor response in IL-6−/− mice of the same background (mean 103 ± 4 mmHg to 119 ± 7 mmHg, n=10, p= 0.048).
Earlier studies have shown that Ang II induces Th17 recruitment in hyperlipidemic vascular tissues, a cell type mediating hypertension, endothelial dysfunction and atherosclerosis in the ApoE−/− background.23, 24, 29 To establish whether Ang II induces Th17 cell recruitment into the aortic wall in normolipidemic mice, we measured the abundance of IL-17A mRNA in the aorta and found it was increased 4-fold relative to sham-infused mice (Figure 1A, p<0.01). IL-17A-positive immunostaining was found in both the medial and adventitial layers (Figure 1A, p<0.01).
Figure 1.

Ang II promoted Th17 cell accumulation in aortic tissues. Age matched WT mice were treated with sham or Ang II for 14 d. (A) Sham and Ang II-treated WT mice were examined for aortic Th17 recruitment. Left panel: IL-17A expression was analyzed using Q-RT- PCR. Circles: sham- treated mice. Squares: Ang II-treated mice. Right panel: Aortic sections were stained for IL-17A-expressing cells. Cell numbers were quantified microscopically and expressed as cells/visual field under 200X magnification. **, p<0.01. Bottom panel, IHC for IL-17A expression in sham and Ang II infused aortas. IL-17A immunostaining is increased in the adventitial-medial border (adventitial border is indicated by arrows). (B) Flow cytometric analysis of aortic CD4 and IL-17A-positive Th17 cells was performed and amount of double-positive cells was measured. *, p<0.05. (C) Flow cytometric analysis of aortic ROR-γT-expressing cells with CD4+ gating was performed. CD4+ ROR-γT+ cells were quantified. White bars: sham-treated animals. Black bars: animals treated with Ang II for 14 d. n=4 in each group. **, p<0.01.
To confirm that Ang II induced the aortic accumulation of the Th17 cell population, we conducted flow cytometric analysis of the dissociated aortae staining for both CD4 and IL-17A. Ang II induced a significant 5-fold recruitment of the CD4+IL17+ Th17 cell population (Figure 1B, p<0.01). The retinoic-acid receptor related orphan receptor (ROR)γ-T, directs Th17 cell differentiation.30 To further confirm recruitment of Th17 cells, we quantified CD4+RORγT+ cells by flow cytometry. A similar 5-fold increase of RORγT+ cells (Figure 1C, p<0.05) was also observed in response to Ang II infusion.
We next tested whether local macrophage and T lymphocyte recruitment were affected in the IL-6−/− background. Flow cytometric staining of aortic CD11b+ macrophages indicated that Ang II-induced macrophage recruitment was abolished in IL-6−/− mice (Figure 2A). We also tested if IL-6 deficiency affected expression of IL-17A. A 5-fold increase in aortic wall IL-17A mRNA was produced by Ang II in the IL-6+/+ genotype, while IL-17A transcript level was decreased in the IL-6−/− background (Figure 2B; 5-fold vs. 2-fold, Ang II-treated WT vs. Ang II-treated IL-6−/−, p<0.01). We also observed by flow cytometry that aortic CD4+ IL-17A+ cells were decreased with IL-6 deficiency (Figure 2C, 7.3 % vs. 5.8 %, Ang II-treated WT vs. IL-6−/−). These data indicate that IL-6 deficiency reduces Ang II-induced aortic IL-17A expression and accumulation of Th17 lymphocytes.
Figure 2. IL-6 deficiency reduced Ang II-induced macrophage and Th17 recruitment.
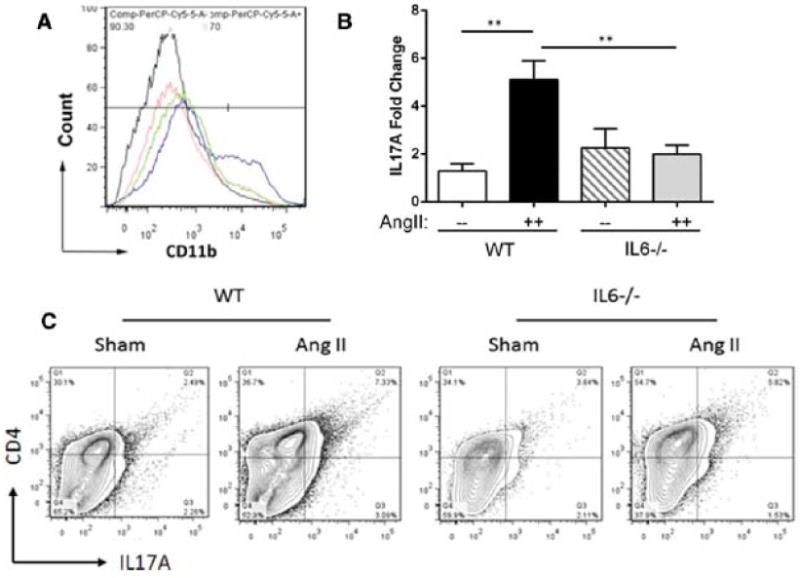
Age matched WT and IL-6−/− mice were treated with Ang II or saline (sham) for 14 d. (A) Flow cytometric analysis of CD11b-positive macrophages was performed using disassociated aortic cells and the number of CD11b-positive cells was measured. Black curve: Sham-treated WT. Blue curve: Ang II-treated WT. Red curve: Sham-treated IL-6−/−. Green curve: Ang II-treated IL-6−/−. n=4 in each group. (B) IL-17A expression was analyzed using Q-RT-PCR. White bar: Sham-treated WT. Black bar: Ang II-treated WT. Cross bars: Sham-treated IL-6−/−. Grey bar: Ang II-treated IL-6−/−. n= 3–5 in each group. *, p<0.05.**, p<0.01.(C) Flow cytometric analysis of CD4-positive and IL-17A-positive cells was performed and number of double-positive cells was measured. Representative panels corresponding to each group are shown (n=6). IL-6−/− showed abated Th17 recruitment to the aorta.
IL-17A neutralization reduced aortic inflammation and dissections induced by Ang II
Since IL-6 plays a pivotal role in Th17 differentiation, we hypothesized that the reduced inflammatory phenotype observed in Ang II-treated IL-6−/− mice was due, at least in part, to decreased Th17 activation. To test the pathogenic role of IL-17A, we infused Ang II in mice treated with an IL-17A neutralizing antibody (NAb) or an isotype control antibody (ICAb). First, we confirmed by ELISA that the IL-17A NAb reduced IL-17A secretion from aortic explants in tissue culture (Figure 3A). We found that a two-week IL-17A NAb treatment significantly reduced Ang II-induced aortic dissections (Figure 3B; 33 % in Ang II plus ICAb, n=12, vs. 0 % in Ang II plus IL-17A NAb, n=13; p<0.01). Also, IL-17A neutralization reduced aortic dilation (Figure 3B, p<0.05 at d 12) and reduced aortic adventitial thickening. IL-17A NAb also abolished Ang II-induced aortic Th17 recruitment (Figure 3C, 12 % in ICAb treatment vs. 4 % with IL-17A NAb treatment, p<0.05). Neutralization of IL-17A also reduced the aortic macrophage population (Figures 3D and 3E), indicating that IL-17A plays a role upstream of macrophage recruitment in Ang II-induced inflammation.
Figure 3. IL-17A neutralization ablated Ang II-induced aortic inflammation and dissection.
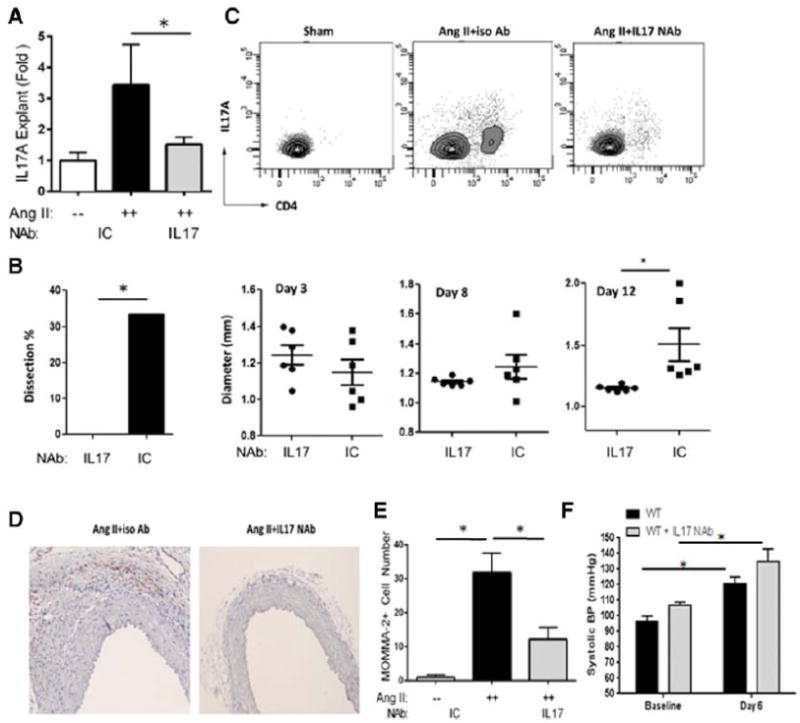
Mice were treated with Ang II and IL-17A NAb or ICAb for 14 d.(A) IL-17A secretion was quantified in aortic explants. White bars: Sham. Black bars: Ang II and ICAb-treated. Grey bars: Ang II and IL-17A NAb-treated. n=4 in each group. *, p<0.05. (B) During Ang II treatment, in vivo imaging of aortas was performed with ultrasonography and maximum diameter of suprarenal aortas was measured. At 14 d, percentage of aortic dissection featured by presence of intramural hematomas was recorded (left panel). Grey bar: animals treated with Ang II and IL-17A NAb, n=13. Black bars: animals treated with Ang II and ICAb, n=12. Right panel, aortic diameter was quantified at d 3, 8 and 12 for each treatment group. Circles: Ang II and IL-17A NAb-treated mice. Squares: Ang II and ICAb-treated mice.*, p<0.05. (C) Flow cytometric analysis of aortic CD4 and IL-17A-positive Th17 cells was performed and number of double-positive cells was measured. n=5 in each group. (D) Aortic sections were immunostained for macrophages using MOMA-2 antibodies. Representative images of each treatment group from 3 different experiments are shown; both images magnified at 200X. (E) Quantification of aortic macrophages for each treatment condition. MOMA-2+ cells were quantified microscopically as cells/visual field at 200x magnification. *, p<0.05. (F) Systolic blood pressure measurements, recorded with tail-cuff plethysmography, were not different between Ang II and IL-17A NAb-treated mice at baseline or at 6 d of Ang II infusion. n=5 mice per group. *, p<0.05.
IL17 has been implicated in the Ang II-induced pressor response because IL-17A deficiency blunts the increase in blood pressure from Ang II infusion.29 To determine whether IL17A neutralization produced a similar confounding pressor effect, we measured systolic blood pressures. We observed that at both baseline and after Ang II-infusion, pressor effects were indistinguishable in untreated WT mice vs. the IL17A NAb-treated mice. The untreated WT mice mean systolic blood pressures changed from 96 ± 4 mmHg to 121 ± 4 mmHg after Ang II treatment, whereas the mean systolic blood pressure of the IL17A NAb-treated mice changed from 106 ± 2 mmHg to 135 ± 8 mmHg (Figure 3F). The pairwise differences in baseline and Ang II induced blood pressures were not significant by antibody treatment. These data indicate that the IL17 NAb reduction in aortic dissections was independent of the pressor response.
IL-17A deficiency blunted inflammatory responses and aortic dissections
We next utilized IL-17A-deficient mice to test the role of Th17 lymphocytes in Ang II-induced vascular inflammation and aortic dissection. We found that, compared with WT mice, age-matched IL-17A−/− mice had a significantly lower incidence of Ang II-induced early (7 d) and late (14 d) aortic dissections (Figure 4A; 41 and 50 % in C57BL/6 vs. 0 and 8 % IL-17A−/− mice, respectively, n=12, p<0.05). In addition, IL-17A−/− mice developed aortic dissections later (12 d), suggesting that IL-17A plays an early pathogenic role in the formation of aortic dissection and dilatation (Figure 4A, diameters of suprarenal aorta in C57BL/6 and IL-17A−/− were 1.3 mm vs. 1.0 mm at 6 d, p<0.05). We confirmed the absence of Th17 cells in IL-17A−/− mice (Figure 4B, 12 % in C57BL/6 vs. 2 % in IL-17A−/−), suggesting abnormal Th17 homing in these mice may account for protection against aortic aneurysms.
Figure 4.

IL-17A deficiency blunted inflammatory response and aortic dissections. Age matched WT and IL-17A−/− mice were treated with Sham or Ang II for 14 d. During Ang II treatment, in vivo imaging of aortas was performed with ultrasonography and diameters of aortas were measured (A). Percentage of aortic dissection featured by presence of intramural hematomas was recorded (left panel). White bars: animals treated with Ang II for 7 d. Black bars: animals treated with Ang II for 14 d. n=12 in each group. Right panel: aortic diameters were recorded at 6 and 12 d for each treatment group. Circles: sham-treated mice, n=5 mice respectively for WT and IL-17A−/−. Squares: Ang II-treated mice, n=6 for WT mice and n=9 for IL-17A−/− mice. *, p<0.05; ns, no significance. (B) Flow cytometric analysis of aortic CD4-positive and IL-17A-positive cells was performed and number of double-positive cells was measured. n=4 in each group. (C) MCP-1 was measured in aortic explant culture medium. White bars: sham-treated WT; Black bars Ang II-treated WT; Grey bars: Ang II-treated IL-17A−/−. n=5–6 in each group. *, p<0.05. (D) Flow cytometric analysis of CD11b-positive macrophages in dissociated aortic cells was performed. A representative measurement is shown. Grey curves: Sham-treated WT at 7 d. Red curves: Ang II-treated WT at 7 d. Blue curves: Ang II-treated IL-17−/− at 7d. n=2 in each group. (E) Systolic blood pressure was measured via a non-invasive tail-cuff method in conscious WT (> 4 months old) and IL-17−/− mice (> 8 months old) at baseline and at d 7 of Ang II infusion, as indicated. *, p<0.05.
We further examined cytokine secretion from aortic tissue in response to Ang II treatment. Multi-plex cytokine/chemokine measurements in aortic explant tissue culture media demonstrated that Ang II enhanced expression of MCP-1, which was decreased in the IL-17A−/− background (Figure 4C). In addition, aortic macrophage recruitment in response to Ang II was decreased in IL-17A−/− mice (Figure 4D; 21 % in WT vs. 7 % in IL-17A−/−). To determine the pressor response of the IL-17A−/− mice, systolic blood pressure was monitored. Here we observed that IL-17A−/− mice had reduced resting blood pressure compared to WT controls (92 ± 2 mmHgvs.101 ± 2 mmHg, p = 0.015), and demonstrated a weak pressor response after Ang II infusion (104 ± 5vs.120 ± 4mmHg, p=ns); the mean systolic pressure of the Ang II infused IL-17A−/− mice was lower than the mean pressure of Ang II infused WT controls (p = 0.04, Figure 4E).
IL-6-STAT3 signaling mediated Ang II-induced Th17 lymphocyte formation
IL-6 signaling via the gp130 transducer activates intracellular signaling mediated by the STAT3 or NF-IL6 pathway.16 Our previous work has shown that STAT3 is activated in aortic monocytes in a manner that is absolutely dependent on IL-6.27 To test the role of IL-6-STAT3 signaling, we synthesized a peptide derivative of the STAT3 second helix, a domain that binds specifically with STAT3 but not STAT1,31 fused to penetratin32, that we and others have shown is a potent cell-permeant inhibitor of STAT3 action (Supplementary Figure 4).31, 33 We found that the subcutaneous infusion of penetratin-STAT3 inhibitory peptide (pSTAT3ip) significantly reduced Ang II-induced aortic SOCS3 mRNA expression (Figure 5A) as well as the suprarenal aortic dilation in WT mice (1.38 ± 0.2 mm in Ang II- treated vs. 1.19 ± 0.05 mm in Ang II + pSTAT3ip treated, p <0.05, Figure 5B). The effect of pSTAT3ip on formation of Th17 lymphocytes was measured in splenic lymphocytes. Here, we observed that Ang II induced a dramatic formation of Th17 cells, where 22% of the splenic lymphocytes were CD4+IL17+ Th17 lymphocytes, and this number was significantly reduced to 13% in the presence of the pSTAT3ip (p<0.05, Figure 5C). Together, these data indicate that STAT3 is a critical intracellular signal for Ang II-induced Th17 formation.
Figure 5. STAT3 signaling mediated aortic dilation and Th17 formation.
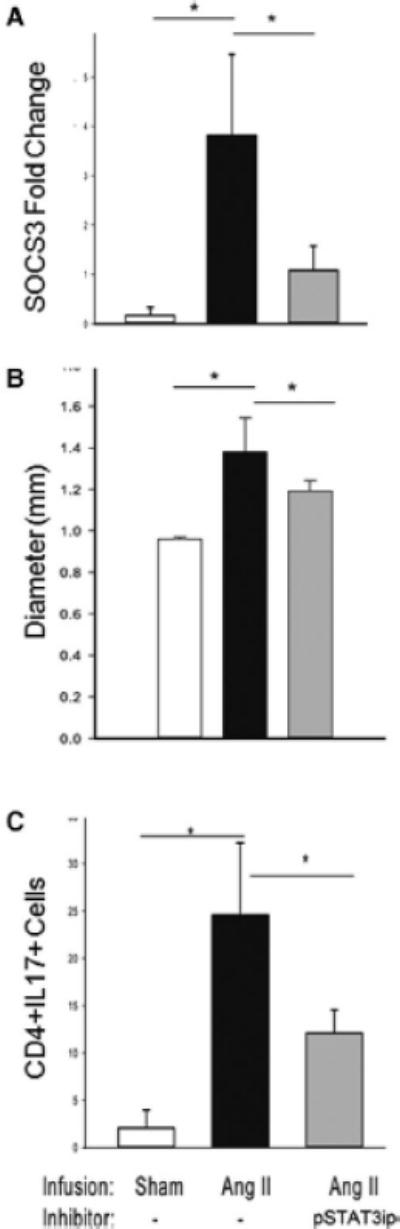
WT mice were infused for 7 d with PBS (sham, n=3), Ang II (n=5) or Ang II + pSTAT3ip (n=6). Both Ang II and pSTAT3ip were delivered subcutaneously by osmotic mini-pumps. (A) Expression of aortic SOSC3 mRNA was measured by Q-RT-PCR. *, p<0.05. (B) Aortic ultrasonography was used to monitor the full diameter of the suprarenal segment of the aorta. *, p<0.05. (C) Quantification of Th17 splenic cell population was performed by flow cytometry (n=3 in each group). *, p<0.05.
Th17 lymphocyte recruitment in patients with thoracic aortic aneurysms
Previous work has shown that macrophages and T lymphocytes are present in human aortic aneurysms.3 To determine whether aortic Th17 recruitment is increased in humans with thoracic aortic aneurysm and dissection (TAAD), we quantified IL-17A expression using IHC in thoracic aortic samples from patients with TGF-β receptor mutation (TGFBR2 R460C). We observed IL-17A immunostaining predominantly at the media-adventitia border (Figure 6A–F). Rarely, IL-17Aimmunostainingwas observed in the medialor intimal layers. Compared to controls, ascending aortic samples from patients with Type A dissections caused by TGFBR2 mutation showed significant enhancement in IL-17A-expressing cell recruitment (4 ± 2 cells/field vs. 82 ± 23 cells/field, control vs. TAAD, respectively, p<0.01, Figure 6G). To confirm local accumulation of Th17 cells, total RNA was extracted from the same samples, and subjected to Q-RT-PCR for hIL-17 mRNA. We observed a 2.8-fold increase in hIL-17 mRNA in TAAD samples relative to control (p<0.05, Figure 6H). These results extend the pathophysiological relevance of our observations that IL-17A-expressing Th17 cells are recruited into the aortic wall and mediate aortic dissections in a mouse model by suggesting that Th17 cells may also be important contributors to human aortic aneurysms and dissections.
Figure 6. IL-17-positive cell accumulation was observed in patients with thoracic aortic aneurysms and dissections (TAAD).
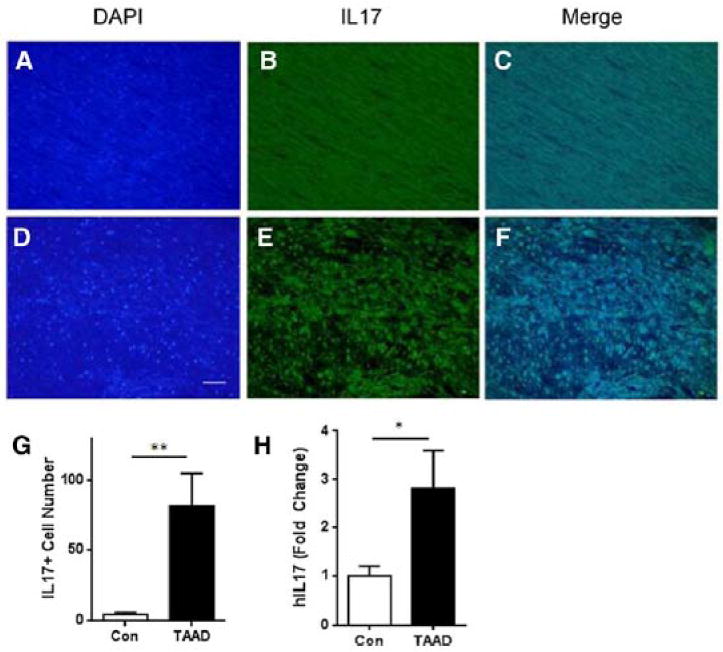
In thoracic aortic samples from patients with TGF-β receptor mutation (TGFβR2 R460C), IL-17 was detected by immunofluorescence microscopy. Positive staining is shown in green and counterstaining with DAPI in blue is shown in blue. Representative images of aortic sections from control patients (A–C) and patients with TGFβR2 mutations (D–F)are shown. (G) Quantification of IL-17-positive cells in human aortic samples. IL-17-positive cells per visual filed were counted under a microscope at 200x magnification. White bar: control patients. Black bar: patients with TGFβR2 mutations and Type A dissection. n=3 in each group. **, p<0.01. (H) Q-RT-PCR analysis for hIL-17 mRNA normalized to GAPDH. Fold change of hIL-17 mRNA in TAAD patients relative to control patients is presented.
Ang II-induced aortic dissections independent of its vasopressor effects
Other labs have demonstrated that the acceleration of atherosclerosis and abdominal aortic aneurysms in Ang II infused mice is independent of Ang II mediated increase in blood pressure.2, 14, 34 To extend these observations to Ang II-induced aortic dissections, we examined the effect of Ang II on dissections in mice made normotensive using the vasodilator hydralazine. WT mice were treated with hydralazine (Supplementary Materials) before and throughout infusion with Ang II. Under these conditions, the hydralazine-treated mice did not exhibit an Ang II-induced pressor response (mean of 101 ± 3mmHg to a mean of 96 ± 5 mmHg, p= ns, Figure 7). However 33% of the normotensive hydralazine-treated mice developed aortic dissections, which was similar to 45% incidence of dissection in mice that were made hypertensive with Ang II (p= ns, Figure 7B). These data indicate that Ang II-induced aortic dissections are independent of the systolic pressor response.
Figure 7. Ang II induction of dissections is independent of systolic blood pressure.
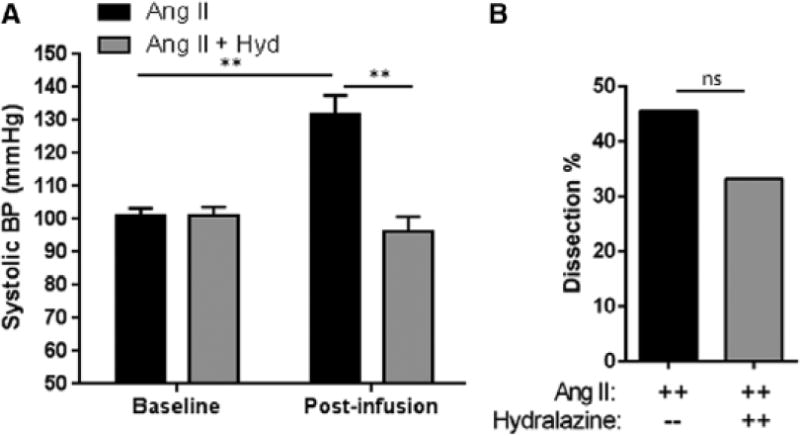
WT mice were infused with Ang II for 7 days in the absence or presence of hydralazine. (A) Baseline and post-Ang II infusion systolic blood pressures were measured using the tail cuff method. Black bars: Ang II-treated WT. Grey bars: Ang II and hydralazine-treated WT. n= 9–11 mice per group. **, p<0.01. (B) The percent of dissections in each group was determined at the end of the study. ns, no significance.
DISCUSSION
Ang II is a potent inducer of vascular inflammation, IL-6 production and monocyte recruitment and activation. In this study, we have found that IL-6 signaling converges on the recruitment of Th17 cells, a cell type necessary for the development of Ang II-induced aortic inflammation and dissections via its involvement in macrophage recruitment to the aortic wall. This work extends our previous study which identified IL-6-STAT3 signaling in monocyte activation to macrophages, by providing a unifying mechanistic pathway where monocyte/macrophage recruitment into the aortic wall is coordinated by CD4+IL-17A+Th17 lymphocytes. To our knowledge, this is the first application of a genetic deletion of IL-17A in normolipidemic C57BL/6 mice to study the role of IL17A in aortic dissection and dilation induced by Ang II.
Lacking direct chemotactic activity, the role of IL-6 in mediating inflammation has been elusive. Previously, we demonstrated that IL-6 signaling in Ang II-stimulated vascular disease was mediated by macrophage activation, a process involving phospho-Tyr STAT3 formation, loss of F4/80 cell surface staining, and induction of matrix-modifying MMPs.12, 27 Interpreted together, these data indicate that IL-6 is locally produced in sufficient concentrations to induce intracellular signaling, which directly leads to monocyte to macrophage differentiation. Our results here are surprising since they suggest that a second major target of aortic IL-6 secretion is the naïve Th0 lymphocyte population, which is stimulated towards Th17 differentiation. Our findings that significant induction of Th17 lymphocyte is in the spleen of Ang II-infused animals suggest that Ang II signaling significantly alters the lymphocyte population systemically. We interpret our data to mean that IL-6 is a potent regulator of aortic IL-17A production: it mediates the differentiation of Th cells into IL-17 producing Th17 cells found in the aorta by stimulating STAT3 activity.
Our data suggest that IL-17A expression is largely dependent on the IL-6 signaling. However, a small induction of IL17 remains in aortas of IL-6−/− mice (Figure 2B). Although many studies have shown IL-6 is required for T cell lineage development into Th17 cells, some innate immune cells are not dependent on IL-6 induction of RORγt and IL-17. For example, subsets of γδT cells and invariant NKT (iNKT) cells that do not undergo T cell receptor selection constitutively express RORγT and can preferentially develop into IL-17 producing cells.35 Similarly, stimulation of γδT cells with IL-1β or IL-23 promotes IL-17 secretion and does not require IL-6.36 Furthermore, analysis of human abdominal aortic aneurysmal tissue has indicated the presence of an oligoclonal population of γδT cells; these data suggest γδT cell are involved in aortic inflammation.37 One possible explanation of our findings is that Ang II infusion also stimulates innate γδT and iNKT cells to produce IL-17A even in absence of IL-6. This IL-6 independent induction of IL-17A production would lead to monocyte chemotaxis and may account for the delayed inflammation and dissection observed in IL-6 deficient mice.
Several recent studies have employed hyperlipidemic ApoE−/− mice to study the effect of total T lymphocytes38 or Th17 cells26 deficiency on aneurysm formation. Although these studies indicated that total T cell or Th17 deficiency was not sufficient to attenuate Ang II-induced aneurysm formation in ApoE−/− mice after 28 days of Ang II infusion, our results clearly suggest that defects in Th17 development protects against early development of aortic dissection and dilation by reducing vascular leukocyte infiltration and cytokine/chemokine expression. In this setting reduced leukocyte infiltration may result in fewer medial breaks, an initial precursor lesion that is followed by aortic dissection and later, aneurysm formation (Supplementary Figure 1).17
Th17 cells have been implicated in the pathogenesis of autoimmune and inflammatory diseases,39 and more recently in cardiovascular disease.40 Increased circulating Th17 cells and Th17 cell infiltration into the aorta are found in Ang II-induced hypertension, and IL-17A deficiency blunts these responses and prevents hypertension.29 Our data indicate that Th17 cells are enriched in the medial-adventitial border of suprarenal aortas (Figure 1A, bottom panel), indicating their accumulation at the principal site of dissection in this model. Others have shown that Th17 cells as well as IL-17 expression in atherosclerosis are increased, and blockade of IL-17A reduced aortic macrophage infiltration, cytokine secretion, and atherosclerotic plaque formation.24, 38 Our data are consistent with these observations, where we observe reduced MCP-1 expression in IL-17A deficient mice. Interestingly, other studies have shown that IL-6 expression is itself induced by IL-17A and reduced by blockade of IL-17A signaling,24 suggesting an auto-amplification loop where the proinflammatory effects of IL-6 are enhanced by activated Th17 cells. These studies highlight an important proinflammatory role for T cells, especially the Th17 subset, in vascular inflammation and dissection.
Our flow cytometry data suggest that CD4+ T cells are a primary source of IL-17A in aortic tissue, but there is a component of IL-17A that comes from CD4-negative cell types (Figure 1B). Although Th17 cells are classically the main producers of IL-17A,41 other cells such as γδT cells,42 lymphoid tissue inducer (LTi)- like cells,43 and iNKT cells44 have also been shown to produce IL-17A. It is possible that Ang II stimulates IL-17A production in these cell types, in addition to CD4+ cells. Further work will be required to determine cell-specific contribution to IL-17A production and formation of aortic dissection.
Monocyte/macrophage recruitment and differentiation play key pathogenic roles in Ang II-induced aortic aneurysms.45 Discussed above, IL-17Acontributes to inflammatory processes by promoting monocyte chemotaxis, adhesion and migration. Indeed, reduced monocyte recruitment is seen upon neutralization of IL-17A (Figure 3E). It has been recently reported that IL-17 induces monocyte migration partially through MCP-1 induction, consistent with our results of reduced MCP-1 expression in IL-17A−/− mice (Figure 4).46, 47 IL-17A treatment of aorta from atherosclerotic mice promoted aortic CXCL1 expression and monocyte adhesion.24 Together, these results highlight an important role of Th17/IL-17A in the pathogenesis of aortic inflammation by promoting cytokine production and monocyte recruitment.
Besides vascular inflammation, hypertension is another well-established pro-pathogenic factor for many cardiovascular diseases, including aortic dissections. Moreover, we and others have previously shown that2500 ng/kg/min dose of Ang II administered subcutaneously by osmotic mini pump results in hypertension.27 Many labs have also demonstrated that infusion of a lower dose of Ang II (1,000ng/kg/min) than used in our studies promotes abdominal aortic aneurysms independent of hypertension in hypercholesterolemic mice.14, 48 In this study, we examined the pressor effects of Ang II on IL-6 knockout mice. Interestingly, although Lee et al. reported that IL-6 mediates the pressor effect of Ang II,28 we observed a pressor effect in IL-6−/− that was not statistically different from that of WT mice (Supplementary Figure 2). These differences may be due to strain effects or differences in Ang II dosing. Nevertheless, the finding that Ang II induced similar pressor responses suggests that the permissive role of IL-6 in aortic dissections is largely independent of a blunted pressor effect.
Our study expands our understanding of the inflammatory process in aortic dissections by identifyingTh17cellsas a central coordinator of aortic inflammation. Since IL-17A acts on vascular tissue and induces the production of proinflammatory cytokines and chemokines23, 24, 49 and ROS,25 we hypothesized that IL-17A-expressing Th17 cells may mediate recruitment of leukocytes to sites of inflammation in the aorta. Vascular cells, including endothelial cells,49 smooth muscle cells,25 and monocytes22 express IL-17RA, the major component of the receptor complex for IL-17A and IL-17F.50 Recent in vitro and in vivo studies suggest an important role of IL-17A in mediating monocyte chemotaxis in different systems.22, 47 Rheumatoid arthritis patients treated with IL-17A antibody showed inhibited monocyte chemotaxis.46 Ldlr−/− mice with IL-17R signaling disruption in bone marrow-derived cells resulted in a reduced IL-6 production and attenuated atherosclerosis.21 ApoE−/− mice with IL-17RA deficiency demonstrated decreased production of proinflammatory cytokines/chemokine and reduced recruitment of macrophages, T cells, and neutrophils.22 Consistent with these findings, our results indicate that abnormalities in Th17 activation caused by IL-17A deficiency or IL-6 deficiency lead to a reduction in macrophage recruitment and cytokine/chemokine expression in the wall of the aorta.
Previous work has shown that IL-17A-deficient mice have a blunted long-term pressor response to Ang II infusion.29 In our studies, we surprisingly found IL17 NAb did not affect resting or pressor responses to Ang II, however, the IL-17A−/− mice had a reduced resting systolic blood pressure and a blunted Ang II pressor response. These divergent observations on the pressor responses may suggest that there may be developmental role of IL17A on vascular tone in IL17−/− mice that is not apparent with short-term IL17 neutralization. Moreover, the divergent observations on the pressor responses between IL17A−/− and IL-6−/− background may suggest that there may be compensation for IL-6 deficiency via other IL-6 superfamily of cytokines (IL-11, LIF, oncostatin-M, cardiotropin, and others) that sustain IL-17 cells and the pressor response.
The TGFBR2 R460C mutation is an activating receptor mutation, producing both tonic TGFβ pathway stimulation51 and lymphocytic inflammation.3 This tonic TGFβ signaling is shared by the well-established Marfan FBN1 mutation, a mutation that releases latent TGFβ from the extracellular matrix. Currently, there is evidence that TGFβ and Ang II cross-talk plays an important role in vascular pathology. For example, the pathology induced by TGFβ signaling in Marfan disease is significantly attenuated by Ang II antagonism.52 We therefore suspect that pathology in patients with TGFBR2 R460C mutation may be, in part, the consequence of Ang II-IL6 signaling. Our findings for Th17 cell accumulation in these patients suggest that investigation of the Ang II-IL6-Th17 pathway may be further warranted.
In summary, our data suggest that the Th17-IL-17 axis, which is regulated by IL-6-STAT3 signaling, is an important effector arm of Ang II mediated vascular inflammation. It functions upstream of monocyte/macrophage activation and is independent of Ang II pressor response. Lastly, the data indicates that the Th17-IL-17 pathway has direct correlates in human aortic aneurysms and dissections.
Supplementary Material
SIGNFICANCE.
Angiotensin II is a potent activator of inflammatory signaling in vascular tissue, leading to aortic dissections. Previous work has shown that interleukin -6 (IL-6) is an important effector arm for Ang II induced monocyte/macrophage recruitment and vascular disease. We show here that IL-6 acts as a key signaling pathway inducing the formation and recruitment of T helper (Th)-17 using mice genetically deficient in IL17 and administration of neutralizing antibody to IL17. Moreover, induction of the Th17 axis requires the signal transducer and activator of transcription (STAT)-3 because administration of a cell-permeant inhibitor of STAT3 blocks Th17 formation and aortic dissections. Correlations of Th17 activity are identified in human ascending aortic dissections. These data suggest that one of the actions of IL-6 signaling is to modulate adaptive immunity through specific Th subsets. These findings have broad implications for the diagnosis and treatment of aortic dissections.
Acknowledgments
The authors acknowledge Dr. Heidi Spratt for professional statistical analysis of data, theUTMB Histopathology and Flow Cytometry Core Facility at UTMB, Dr. Stefan Serabyn for synthesizing Ang II and pSTAT3ip, and Dr. Ken Fujise for providing us access to the CODA blood pressure apparatus.
SOUCES OF FUNDING
This work was supported by the National Institutes of Health (P50 HL083794 to ARB and DMM, HL70925 to ARB, DK079053 to RGT) and the Ted Nash Long Life Foundation to ARB.
Abbreviations
- Ang
angiotensin
- IL
interleukin
- MCP
monocytic chemotactic factor
- NAb
neutralizing antibody
- pSTAT3ip
phospho-STAT3 inhibitory peptide
- STAT
signal transducers and activators of transcription
- Th
T helper lymphocyte
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
References
- 1.Schluter KD, Wenzel S. Angiotensin ii: A hormone involved in and contributing to pro-hypertrophic cardiac networks and target of anti-hypertrophic cross-talks. Pharmacology & therapeutics. 2008;119:311–325. doi: 10.1016/j.pharmthera.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Daugherty A, Manning MW, Cassis LA. Angiotensin ii promotes atherosclerotic lesions and aneurysms in apolipoprotein e-deficient mice. Journal of Clinical Investigation. 2001;105:1605–1612. doi: 10.1172/JCI7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He R, Guo DC, Estrera AL, Safi HJ, Huynh TT, Yin Z, Cao SN, Lin J, Kurian T, Buja LM, Geng YJ, Milewicz DM. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. The Journal of thoracic and cardiovascular surgery. 2006;131:671–678. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Daugherty A, Cassis LA. Mechanisms of abdominal aortic aneurysm formation. Curr Atheroscler Rep. 2002;4:222–227. doi: 10.1007/s11883-002-0023-5. [DOI] [PubMed] [Google Scholar]
- 5.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovascular Research. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasier AR. The nuclear factor-kb interleukin-6 signalling pathway mediating vascular inflammation. Cardiovascular Research. 2010;86:211–218. doi: 10.1093/cvr/cvq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopalan S, Meng XP, Ramasamy S, Harrison DG, Galis ZS. Reactive oxygen species produced by macrophage-derived foam cells regulate the activity of vascular matrix metalloproteinases in vitro. Implications for atherosclerotic plaque stability. Journal of Clinical Investigation. 1996;98:2572–2579. doi: 10.1172/JCI119076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oya K, Sakamoto N, Ohashi T, Sato M. Combined stimulation with cyclic stretching and hypoxia increases production of matrix metalloproteinase-9 and cytokines by macrophages. Biochem Biophys Res Commun. 2011;412:678–682. doi: 10.1016/j.bbrc.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–552. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pearce WH, Shively VP. Abdominal aortic aneurysm as a complex multifactorial disease: Interactions of polymorphisms of inflammatory genes, features of autoimmunity, and current status of mmps. Ann N Y Acad Sci. 2006;1085:117–132. doi: 10.1196/annals.1383.025. [DOI] [PubMed] [Google Scholar]
- 11.Tieu BC, Ju X, Lee C, Sun H, Lejeune W, Recinos IA, Brasier AR, Tilton RG. Aortic adventitial fibroblasts participate in angiotensin-induced vascular wall inflammation and remodeling. Journal of Vascular Research. 2011;48:261–272. doi: 10.1159/000320358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Recinos A, 3rd, LeJeune WS, Sun H, Lee CY, Tieu BC, Lu M, Hou T, Boldogh I, Tilton RG, Brasier AR. Angiotensin ii induces il-6 expression and the jak-stat3 pathway in aortic adventitia of ldl receptor-deficient mice. Atherosclerosis. 2007;194:125–133. doi: 10.1016/j.atherosclerosis.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuett H, Luchtefeld M, Grothusen C, Grote K, Schieffer B. How much is too much? Interleukin-6 and its signalling in atherosclerosis. Thrombosis and haemostasis. 2009;102:215–222. doi: 10.1160/TH09-05-0297. [DOI] [PubMed] [Google Scholar]
- 14.Norgren L, Swartbol P. Biological responses to endovascular treatment of abdominal aortic aneurysms. Journal of endovascular surgery : the official journal of the International Society for Endovascular Surgery. 1997;4:169–173. doi: 10.1177/152660289700400208. [DOI] [PubMed] [Google Scholar]
- 15.Mihara M, Hashizume M, Yoshida H, Suzuki M, Shiina M. Il-6/il-6 receptor system and its role in physiological and pathological conditions. Clin Sci (Lond) 2012;122:143–159. doi: 10.1042/CS20110340. [DOI] [PubMed] [Google Scholar]
- 16.Hou J, Tieu B, Ray S, Recinos A, III, Cui R, Tilton R, Brasier AR. Roles of il-6-gp130 signaling in vascular inflammation. Current Cardiology Reviews. 2008;4:179–192. doi: 10.2174/157340308785160570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saraff K, Babamusta F, Cassis LA, Daugherty A. Aortic dissection precedes formation of aneurysms and atherosclerosis in angiotensin ii-infused, apolipoprotein e-deficient mice. Arteriosclerosis, Thrombosis & Vascular Biology. 2003;23:1621–1626. doi: 10.1161/01.ATV.0000085631.76095.64. [DOI] [PubMed] [Google Scholar]
- 18.Ocana E, Bohorquez JC, Perez-Requena J, Brieva JA, Rodriguez C. Characterisation of t and b lymphocytes infiltrating abdominal aortic aneurysms. Atherosclerosis. 2003;170:39–48. doi: 10.1016/s0021-9150(03)00282-x. [DOI] [PubMed] [Google Scholar]
- 19.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the t cell in the genesis of angiotensin ii induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senchenkova EY, Russell J, Kurmaeva E, Ostanin D, Granger DN. Role of t lymphocytes in angiotensin ii-mediated microvascular thrombosis. Hypertension. 2011;58:959–965. doi: 10.1161/HYPERTENSIONAHA.111.173856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Es T, van Puijvelde GH, Ramos OH, Segers FM, Joosten LA, van den Berg WB, Michon IM, de Vos P, van Berkel TJ, Kuiper J. Attenuated atherosclerosis upon il-17r signaling disruption in ldlr deficient mice. Biochem Biophys Res Commun. 2009;388:261–265. doi: 10.1016/j.bbrc.2009.07.152. [DOI] [PubMed] [Google Scholar]
- 22.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The il-17a/il-17ra axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–687. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Böckler D, Katus HA, Dengler TJ. Inhibition of il-17a attenuates atherosclerotic lesion development in apoe-deficient mice. The Journal of Immunology. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 24.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17a results in reduced atherosclerosis in apolipoprotein e-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pietrowski E, Bender B, Huppert J, White R, Luhmann HJ, Kuhlmann CR. Pro-inflammatory effects of interleukin-17a on vascular smooth muscle cells involve nad(p)h- oxidase derived reactive oxygen species. J Vasc Res. 2011;48:52–58. doi: 10.1159/000317400. [DOI] [PubMed] [Google Scholar]
- 26.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tieu B, Lee C, Sun H, LeJeune W, Ju X, Spratt H, Guo D, Milewicz DM, Recinos A, Tilton R, Brasier AR. Adventitial il-6-mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection. Journal of Clinical Investigation. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee DL, Sturgis LC, Labazi H, Osborne JB, Jr, Fleming C, Pollock JS, Manhiani M, Imig JD, Brands MW. Angiotensin ii hypertension is attenuated in interleukin-6 knockout mice. AJP – Heart and Circulatory Physiology. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 29.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG. Interleukin 17 promotes angiotensin ii-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. doi: 10.1161/HYPERTENSIONAHA.109.145094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–29. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 31.Timofeeva OA, Gaponenko V, Lockett SJ, Tarasov SG, Jiang S, Michejda CJ, Perantoni AO, Tarasova NI. Rationally designed inhibitors identify stat3 n-domain as a promising anticancer drug target. ACS Chem Biol. 2007;2:799–809. doi: 10.1021/cb700186x. [DOI] [PubMed] [Google Scholar]
- 32.Dom G, Shaw-Jackson C, Matis C, Bouffioux O, Picard JJ, Prochiantz A, Mingeot-Leclerc MP, Brasseur R, Rezsohazy R. Cellular uptake of anetennapedia penetratin peptides is a two-step process in which phase transfer precedes a tryptophan-dependent translocation. Nucleic Acids Research. 2003;31:556–561. doi: 10.1093/nar/gkg160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ray S, Ju X, Sun H, Finnerty CC, Herndon DN, Brasier AR. Il-6 trans-signaling-stat3 pathway mediates ecm and cellular proliferation in fibroblasts from hypertrophic scar. Journal of Investigative Dermatology. 2013 doi: 10.1038/jid.2012.499. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassis LA, Gupte M, Thayer S, Zhang X, Charnigo R, Howatt DA, Rateri DL, Daugherty A. Ang ii infusion promotes abdominal aortic aneurysms independent of increased blood pressure in hypercholesterolemic mice. American journal of physiology. Heart and circulatory physiology. 2009;296:H1660–1665. doi: 10.1152/ajpheart.00028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michel M-L, Mendes-da-Cruz D, Keller AC, Lochner M, Schneider E, Dy M, Eberl G, Leite-de-Moraes MC. Critical role of ror-γt in a new thymic pathway leading to il-17-producing invariant nkt cell differentiation. Proceedings of the National Academy of Sciences. 2008;105:19845–19850. doi: 10.1073/pnas.0806472105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and il-23 induce innate il-17 production from gammadelta t cells, amplifying th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Platsoucas CD, Lu S, Nwaneshiudu I, Solomides C, Agelan A, Ntaoula N, Purev E, Li LP, Kratsios P, Mylonas E, Jung WJ, Evans K, Roberts S, Lu Y, Layvi R, Lin WL, Zhang X, Gaughan J, Monos DS, Oleszak EL, White JV. Abdominal aortic aneurysm is a specific antigen-driven t cell disease. Ann N Y Acad Sci. 2006;1085:224–235. doi: 10.1196/annals.1383.019. [DOI] [PubMed] [Google Scholar]
- 38.Uchida HA, Kristo F, Rateri DL, Lu H, Charnigo R, Cassis LA, Daugherty A. Total lymphocyte deficiency attenuates angii-induced atherosclerosis in males but not abdominal aortic aneurysms in apoe deficient mice. Atherosclerosis. 2010;211:399–403. doi: 10.1016/j.atherosclerosis.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng X, Yu X, Ding YJ, Fu QQ, Xie JJ, Tang TT, Yao R, Chen Y, Liao YH. The th17/treg imbalance in patients with acute coronary syndrome. Clinical immunology. 2008;127:89–97. doi: 10.1016/j.clim.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Eid RE, Rao DA, Zhou J, Lo SF, Ranjbaran H, Gallo A, Sokol SI, Pfau S, Pober JS, Tellides G. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating t cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–1432. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cua DJ, Tato CM. Innate il-17-producing cells: The sentinels of the immune system. Nature reviews Immunology. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 42.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, il-17-producing gamma delta t cells. Journal of Immunology. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of il-17 and il-22. Journal of Experimental Medicine. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-De-Moraes MC. Identification of an il-17-producing nk1.1(neg) inkt cell population involved in airway neutrophilia. Journal of Experimental Medicine. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tieu BC, Lee C, Sun H, Lejeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial il-6/mcp1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shahrara S, Pickens SR, Dorfleutner A, Pope RM. Il-17 induces monocyte migration in rheumatoid arthritis. J Immunol. 2009;182:3884–3891. doi: 10.4049/jimmunol.0802246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shahrara S, Pickens SR, Mandelin AM, Karpus WJ, Huang Q, Kolls JK, Pope RM. Il-17–mediated monocyte migration occurs partially through cc chemokine ligand 2/monocyte chemoattractant protein-1 induction. The Journal of Immunology. 2010;184:4479–4487. doi: 10.4049/jimmunol.0901942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rutherford JD, Pfeffer MA, Moye LA, Davis BR, Flaker GC, Kowey PR, Lamas GA, Miller HS, Packer M, Rouleau JL. Effects of captopril on ischemic events after myocardial infarction. Results of the survival and ventricular enlargement trial. Save investigators. Circulation (Dallas TX) 1994;90:1731–1788. doi: 10.1161/01.cir.90.4.1731. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Crother TR, Arditi M. Emerging role of il-17 in atherosclerosis. Journal of innate immunity. 2010;2:325–333. doi: 10.1159/000314626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaffen SL. Structure and signalling in the il-17 receptor family. Nature reviews Immunology. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inamoto S, Kwartler CS, Lafont AL, Liang YY, Fadulu VT, Duraisamy S, Willing M, Estrera A, Safi H, Hannibal MC, Carey J, Wiktorowicz J, Tan FK, Feng X-H, Pannu H, Milewicz DM. Tgfbr2 mutations alter smooth muscle cell phenotype and predispose to thoracic aortic aneurysms and dissections. Cardiovascular Research. 2010;88:520–529. doi: 10.1093/cvr/cvq230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an at1 antagonist, prevents aortic aneurysm in a mouse model of marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


