Abstract
The autotransporters are a large and diverse family of bacterial secreted and outer membrane proteins, which are present in many Gram-negative bacterial pathogens and play a role in numerous environmental and virulence-associated interactions. As part of a larger systematic study on the autotransporters of Burkholderia pseudomallei, the causative agent of the severe tropical disease melioidosis, we have constructed an insertion mutant in the bpss1439 gene encoding an unstudied predicted trimeric autotransporter adhesin. The bpss1439 mutant demonstrated a significant reduction in biofilm formation at 48 hours in comparison to its parent 10276 wild-type strain. This phenotype was complemented to wild-type levels by the introduction of a full-length copy of the bpss1439 gene in trans. Examination of the wild-type and bpss1439 mutant strains under biofilm-inducing conditions by microscopy after 48 hours confirmed that the bpss1439 mutant produced less biofilm compared to wild-type. Additionally, it was observed that this phenotype was due to low levels of bacterial adhesion to the abiotic surface as well as reduced microcolony formation. In a murine melioidosis model, the bpss1439 mutant strain demonstrated a moderate attenuation for virulence compared to the wild-type strain. This attenuation was abrogated by in trans complementation, suggesting that bpss1439 plays a subtle role in the pathogenesis of B. pseudomallei. Taken together, these studies indicate that BPSS1439 is a novel predicted autotransporter involved in biofilm formation of B. pseudomallei; hence, this factor was named BbfA, Burkholderia biofilm factor A.
Introduction
Burkholderia pseudomallei is a motile, Gram-negative bacillus, which is principally an environmental saprophyte of tropical and sub-tropical soils and water. It is the causative agent of melioidosis, a febrile illness with disease states ranging from acute pneumonia or septicaemia to chronic or localised abscess formation. The disease is acquired by subcutaneous inoculation, inhalation and ingestion. Melioidosis has become an increasingly important disease in endemic areas such as South East Asia and Northern Australia causing a significant number of deaths despite antibiotic treatment. B. pseudomallei has a large genome encoding many virulence factors, including capsule, lipopolysaccharide (LPS), flagella, quorum sensing (QS) systems and several protein secretion systems, including type III and type VI pathways [reviewed in [1], [2]].
B. pseudomallei grows in microcolonies in vitro and in vivo for which the production of exopolysaccharride have been implicated [3]. It has been postulated that biofilm formation may allow bacterial cells to evade the host immune system and persist within patients causing chronic or relapsing disease. Melioidosis is refractory to antibiotic therapy and B. pseudomallei biofilms have been shown to have very high levels of resistance to antibiotics which are effective against planktonic cells [4], [5]. The quantity of biofilm produced by B. pseudomallei is strain dependent [4], [6], [7] and affected by the growth conditions. Interestingly, the addition of glucose (2–50 mM) enhances biofilm formation [6]; the authors proposed that this may in part explain why chronic and relapsing infections are more common in diabetic patients with poor glycemic control [8].
The relative effects of a variety of mutants on the formation of biofilms have been tested. An acapsular wcbB mutant demonstrated wild-type levels of biofilm formation [4] while a second acapsular wzm mutant had reduced biofilm formation in minimal media but not in LB [9]. This may indicate that under certain conditions, capsule is important for biofilm production; however, the wzm mutant also demonstrated susceptibility to desiccation and oxidative stress [9] which may indirectly affect biofilm formation. An LPS O-antigen wbiI mutant also displayed wild-type levels of biofilm which concurs with the observation that the level of biofilm production by a strain does not correlate with the presence of O-antigen observed by silver staining [4].
An aflagellate fliC mutant demonstrated reduced biofilm formation [4]. Additionally, a pkk (polyphosphate kinase) mutant was found to be aflagellate and also demonstrated reduced biofilm formation; however, this mutant also displayed reduced sensitivity to oxidative stress [10]. To overcome the loss of flagella-based motility, centrifugation of these two mutants onto an abiotic surface was performed, but biofilm formation was still significantly reduced. Microscopy demonstrated that no microcolony formation occurred for either strain while the pkk mutant also lacked exopolysaccharide production [10]. These data indicate that flagella play a role in microcolony formation. However, this aflagellate phenotype can also be compensated by overexpression of other factors which promote microcolony formation. A cpdA mutant (encoding a c-di-GMP phosphodiesterase), with increased intracellular GMP levels, lacks flagella but produced increased levels of exopoolysaccharide, thus, more autoagglutination and biofilm formation [11].
It is unclear from the multiple phenotypes of the cpdA mutant whether c-di-GMP levels directly or indirectly affect biofilm. Intracellular levels of c-di-GMP affect the QS system [11]. B. pseudomallei has three QS synthases and five QS regulators [2]. A bspI mutant, which cannot produce the major stationary phase QS molecule C8HL, displayed reduced biofilm formation, as does a mutant in its regulator bspR. When the secondary producer of C8HL (bspI3) was inactivated, it also demonstrated reduced biofilm formation although comparatively less than the bspI mutant [12]. Biofilm levels could be restored to wild-type levels by the addition of exogenous C8HL [12]; these data indicated that one or more factors required for biofilm formation is/are expressed under stationary phase conditions and that this expression may be either directly or indirectly influenced by the QS regulator(s). Another regulatory factor that has been identified to potentially play a role in biofilm formation is the alternative sigma factor σE [13]. Proteomic analysis of protein expression by a B. pseudomallei rpoE mutant relative to its parent strain did not identify any proteins with known or putative roles in biofilm production [14].
To date, only one study has focused on genes directly involved in biofilm formation as the mutations discussed above have pleiotropic effects. A random mutagenesis study identified two strains which produced very low levels of biofilm; the first mutant, M6, had a transposon insertion within a gene encoding a putative polysaccharide biosynthesis protein, and the second, M10, had an insertion in gene encoding a putative sugar transferase [7]. These mutants demonstrated wild-type virulence in the BALB/c melioidosis model [7] and, although demonstrating some reduction in antibiotic diffusion and viability, still show biofilm-like levels of antibiotic resistance [7], [15]. However, these unexpected results are likely to be caused by the mutagenesis system used in this study; a subsequent paper by the same authors' identified a non-specific reduction in biofilm production due to the transposon used [16]. Therefore, factors directly involved in biofilm formation, and the role of biofilm in virulence, remain to be elucidated.
The autotransporters (ATs) are a large and diverse family of bacterial secreted and outer membrane proteins, which are present in many Gram-negative bacterial pathogens and play roles in numerous environmental and virulence-associated interactions including persistence, adhesion, serum resistance and biofilm formation. ATs utilise the Type V secretion mechanism, one of seven recognised secretion pathways in Gram-negative bacteria. A typical AT will have a 20–400 kDa passenger domain that contains the effector functions and a 10–30 kDa β domain facilitating translocation across the outer membrane [17]. ATs can be further divided into classical ATs and trimeric autotransported adhesins (TAAs). TAAs are so named because each protein trimerises to form a single 12-stranded β barrel from their three 4-stranded β barrel domains. The functional passenger domain of a typical AT acts predominantly as an adhesin [17], [18]. The B. pseudomallei K96243 genome contains eleven predicted ATs, based on sequence similarity to known ATs [19], of which nine are predicted to be TAAs [20]. Only two TAA adhesins have been characterised; mutants in boaA and boaB are impaired in epithelial adhesion while expression of recombinant BoaA and BoaB in a heterologous E. coli host increased the adhesive phenotype of the bacterial cells [21]. As part of a larger systematic study on the autotransporters of B. pseudomallei, we have constructed an insertion mutant in the bpss1439 gene encoding an unstudied predicted TAA. This mutant was found to be affected in its ability to form biofilm, and demonstrated a moderate attenuation for virulence compared to the wild-type strain in the acute murine melioidosis model, suggesting that production of biofilm may play a role in virulence of B. pseudomallei.
Materials and Methods
Ethics Statement
All investigations involving animals were carried out according to the requirements of the Animal (Scientific Procedures) Act 1986. Ethical approval was granted by our local (Defence Science and Technology Laboratory, University of Leicester) ethical review process according to the requirements of the Animal (Scientific Procedures) Act 1986. Predetermined humane end points were employed where possible and animals were culled via cervical dislocation according to schedule 1 of the Animal (Scientific Procedures) Act 1986.
Bacterial strains, plasmids, media and growth conditions
All bacterial strains and plasmids reported in these studies are listed in Table 1%. The strains were routinely grown in Luria-Bertani (LB) broth with shaking or on 1.5 w/v agar at 37°C. Cultures were supplemented with antibiotics and/or 10 mM isopropyl-β-D-thiogalactopyranoside (IPTG) as required; kanamycin was used at a final concentration of 500 µg/ml and tetracycline at 25 µg/ml.
Table 1. Strains, plasmids and cell lines used in this study.
| Strain, plasmid or cell line | Characteristics | Reference or source |
| B. pseudomallei: | ||
| 10276 | Clinical isolate (wild-type) | Dr T. Pitt (Public Health Laboratory Service, London, UK) |
| 10276 (pME) | 10276 harbouring the pME6032 plasmid | This study |
| bpss1439 (bbfA) mutant | 10276 harbouring a pKNOCK-Kan single cross-over insertion in bpss1439 (bbfA) | This study |
| bpss1439 (bbfA) mutant (pME) | 10276 harbouring a pKNOCK-Kan single cross-over insertion in bpss1439 (bbfA) and the pME6032 plasmid | This study |
| bpss1439 (bbfA) mutant (pME-1439) | 10276 harbouring a pKNOCK-Kan single cross-over insertion in bpss1439 (bbfA) and the pME6032 plasmid containing a full-length copy of bpss1439 | This study |
| E. coli: | ||
| S17-1 λpir | Laboratory strain (λpir hsdR pro thi; chromosomal integrated RP4-2 Tc::Mu Km::Tn7). | [36] |
| Plasmids: | ||
| pKNOCK-Kan | 2.2 kb, KanR, λpir-dependent vector (oriR6K) with RP4 oriT | [23] |
| pKNOCK-Kan-bpss1439 | pKNOCK-Kan containing a 623 bp internal fragment of bpss1439 (bbfA) | This study |
| pME (pME6032) | 9.8 kb, TetR, pVS1 derived shuttle vector with IPTG inducible ptac promoter | [24] |
| pME-1439 | pME6032 containing the full-length (4593 bp) bpss1439 (bbfA) gene, including 146 bp upstream | This study |
| Eukaryotic cell lines: | ||
| J774.2 | Murine macrophage-like cells | ECACC 85011428 |
| A549 | Human lung airway epithelial cells | ECACC 86012804 |
Recombinant DNA techniques
Plasmid DNA isolation, PCR fragment purification and gel extraction were performed using Bioline kits as per manufacturer's instructions. Genomic DNA was isolated from B. pseudomallei as previously described [22]. Restriction endonucleases and DNA modifying enzymes (New England Biolabs) were used as per manufacturer's instructions. PCR amplifications were performed using Advantage Taq (Clontech) or Taq DNA polymerase (New England Biolabs) in accordance with manufacturer's instructions. DNA sequencing was carried out at the Protein and Nucleic Acid Laboratory of the University of Leicester.
The bpss1439 single cross-over insertion mutant was generated using pKNOCK-Kan [23] as described previously. Briefly, an internal fragment of the bpss1439 gene was amplified by PCR from B. pseudomallei 10276 using primers EF (5′ TCCGAGGGATCCAACGGCAAGCGCGGCGGC) and M5R (5′ CCCGTCTCTAGAGAAGTGGAAAGCGAGGTCAG). The PCR product containing the internal fragment was digested with BamHI and XbaI alongside the pKNOCK-Kan plasmid and ligated to form pKNOCK-Kan-bpss1439. This recombinant plasmid was introduced into B. pseudomallei 10276 via conjugation from E. coli S17-1λpir [23] and insertion inactivation of bpss1439 was confirmed by PCR and sequencing.
To allow for in trans complementation, the full-length bpss1439 gene was amplified by PCR from B. pseudomallei 10276 using primers FC (5′ ATCTCTAGACCATGATGAACAAGATCTATAAAACC) and RC (5′ ATCGGATCCTTCATGTGCTTGAGCCAGTC). The PCR product containing the bpss1439 gene was digested with NcoI and BamHI and ligated into similarly digested pME6032 [24] under the control of a lac promoter to form pME6032-bpss1439 (pME-1439).
Biofilm assays
Biofilm formation on an abiotic polystyrene surface was studied using 96-well trays (Nunc) as described previously [10]. Briefly, overnight cultures of B. pseudomallei strains were diluted 1∶10 in LB and incubated at 37°C under static conditions for 48 h. Biofilms were washed in distilled water, stained with 1% w/v crystal violet for 5 minutes, washed and then solubilised in 96% v/v ethanol. The degree of biofilm formation stained by the crystal violet was determined by the optical density at 595 nm. Eight experimental replicates were assessed in triplicate experiments.
Microscopic analysis of biofilms
Biofilms were allowed to form on 20 mm glass coverslips (Menzel Gläser) within 24 well trays (Nunc) as described above and fixed overnight in 4% w/v paraformaldehyde or 2.5% v/v glutaldehyde for examination by light microscopy or scanning electron microscopy (SEM) respectively. For light microscopy, fixed biofilms grown on coverslips were visualised with a Peroidic Acid Scheiff stain (Sigma) and examined using a Nikon Diaphot 300 inverted microscope with a ×100 oil immersion lens. For SEM, fixed biofilm grown on coverslips were dehydrated, sputter coated with gold and examined using a Hitachi S3000H SEM (Leicester Imaging Technologies, University of Leicester). At least ten fields of view were assessed in duplicate experiments.
Phenotypic testing
The bpss1439 mutant and its parent wild-type 10276 strain were assessed for various phenotypes which may impact of biofilm formation using published methods. Phenotypes examined were: polysaccharide capsule using mAb staining [25], LPS using silver stain [4], flagella and pili using motility plates [10], oxidative stress using peroxide sensitivity [13], osmotic stress using NaCl sensitivity [13], desiccation using drying sensitivity [9] and exopolysaccharide production using Congo Red binding [11]. Also in vitro attachment and net intracellular survival assays were performed using immortalised A549 human airway epithelial cells [11] and J774.2 murine macrophage-like cells [13].
Assessment of virulence in mice
Three independent animal trials to assess the virulence of the bpss1439 mutant were performed as follows.
Groups of six BALB/c mice (6–8 weeks old) were challenged by the intra-peritoneal route with either the wild-type or bpss1439 mutant at 3.5×105 CFU or 3.07×105 CFU. Mice were monitored for signs of disease over a 35 day period and humanely culled when pre-defined end-points were reached; a log-rank (Mantel-Cox) test was used to evaluate the statistical significance of time to death between the wild-type and bpss1439 mutant.
Six groups of five BALB/c mice (6–8 weeks old) were separately challenged by the intra-peritoneal route with the bpss1439 mutant at doses rising in ten-fold increments from 8.8×102 to 8.8×107 CFU. The wild-type 10276 parent strain was tested at a single dose (3×106 CFU) to confirm consistent results with the previously determined MLD of 1.6×104 CFU from 2004 and 2009 (data not shown). Mice were monitored for signs of disease over a 35 day period and humanely culled when pre-defined end-points were reached. The medium lethal dose for the bpss1439 mutant was determined using the Reed and Meunch calculation based on cumulative infections.
Groups of three BALB/c mice (6–8 weeks old) were challenged by the intra-peritoneal route with an equal ratio of wild-type harbouring the pME6032 vector and the bpss1439 mutant harbouring either the pME6032 vector or the pME-1439 complementation vector (4×105 CFU). Mice were monitored for signs of disease over the 24 hour experimental period and then humanely culled. Livers and spleens were harvested and plated for bacterial recovery. Colonies were patched onto kanamycin and tetracycline agar plates and the percentage of in vivo growth of the bpss1439 mutant versus the wild-type was determined by subtracting the number of tetR, kanR (bpss1439 mutant harbouring pME6032 or pME-1439) colonies from the number of tetR, kanS (wild-type harbouring pME6032) colonies. Statistical significance was assessed using Student's T test.
Results and Discussion
Bioinformatic analysis of BPSS1439, an unstudied predicted TAA
The B. pseudomallei K96243 genome encodes nine predicted TAAs; the bpss1439 gene is located on chromosome two and has been predicted to be located in an operon with two downstream genes, bpss1442 and bpss1443 (C. Ong, personal communication; [26]) (Figure 1). Both of these downstream genes are annotated as encoding hypothetical proteins which lack homology to known proteins in available databases. As a result of revision of the K96243 genome annotation, there are no longer genes numbered bpss1440 or bpss1441. The closest orthologue to bpss1439 is the upstream gene bpss1434;, which is predicted to encode another unstudied TAA bpss1434 is a significantly larger gene of 7899 bp (versus the 4581 bp bpss1439). These genes share 85% identity with most of the variation occurring in the 5′ end of the gene which encodes the functional N-terminal region of the predicted TAA (data not shown) suggestive that these genes encode TAAs with divergent functions. The high degree of sequence similarity between bpss1439 and bpss1434 has resulted in the mistaken earlier observation that orthologues of both these genes are present in B. thailandensis E264 [27], which in fact only has a full-length orthologue for bpss1434 and a truncated pseudogene (639 bp) in place of bpss1439. Interestingly, the bpss1439 orthologue also occurs as a pseudogene in B. mallei ATCC 23344 (BMAA0810; 459 bp). Furthermore, truncation of bpss1439 also occurs within some of the sequenced B. pseudomallei strains (14, 112, 91, NCTCC13177, BCC215, 7894, DM98, B7210, BPC006, 1106b, 668); these pseudogenes not only vary in length but in whether they are 5′ or 3′ truncations. Other strains have a full-length copy of the bpss1439 gene (MSHR346, K96243, 1106a, 1710a, 1710b, MSHR305, 1026b) with small size differences due to variable numbers of repeat regions (1488 bp to 1616 bp). Agarose gel electrophoration of the PCR amplification of the bpss1439 gene from the 10276 strain used in this study indicated that a full-length copy was present on the genome (data not shown).
Figure 1. The arrangement of the bpss1439 operon.

The bpss1439 gene is located in an operon with two downstream genes, bpss1442 and bpss1443; both which encode hypothetical proteins of no significant database hits. The closest orthologue to bpss1439 is the upstream gene (bpss1434) encoding another unstudied predicted TAA.
The bpss1439 gene encodes for a 1530 aa predicted TAA which contains an extended signal peptide (pfam13018) with a predicted cleavage site at 24 aa, and the characteristic C-terminal domains annotated as HIM (pfam05662; short motif found in invasins and haemoagglutinins) and YadA-like (pfam03895; beta barrel region) [28]. The remainder of the protein largely consists of low-complexity repeat regions which form the neck structure responsible for ensuring the adhesin domains are accessible to their host target. A single cross-over insertion mutation disrupting the bpss1439 gene was constructed using the sucide vector pKNOCK-Kan in which a 623 bp internal fragment of bpss1439 was cloned. The resulting plasmid was recombined into bpss1439; sequencing of an amplicon of the mutated region on the chromosome confirmed the disruption of the gene at a point which would produce a transcript encoding a severely truncated 207 aa protein lacking both a portion of the passenger domain and the C-terminal domain responsible for mediating outer member translocation (data not shown).
The bpss1439 mutant displayed reduced biofilm formation compared to the wild-type strain
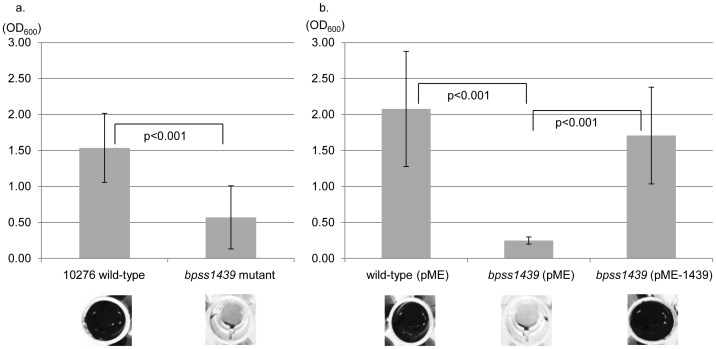
The reported functions of TAAs includes roles in both autoagglutination and biofilm formation [29]. To assess if the unstudied predicted TAA encoded by bpss1439 has a role in either of these phenotypes, a comparative analysis of the bpss1439 mutant and its parent wild-type 10276 strain was performed. The strains were incubated for 48 hours in LB at 37°C under static conditions and monitored for altered autoagglutination by the measurement of the optical density of the growth media; the bpss1439 mutant and wild-type strains demonstrated the same level of autoagglutination (data not shown). However, when these cultures were assessed for their ability to form biofilms, the bpss1439 mutant demonstrated a significant (p<0.001) reduction in biofilm formation at 48 hours in LB at 37°C compared to the wild-type strain (Figure 2a°). This same phenotypic profile was found to occur at temperatures of 27C and 30°C, as well as in minimal media and LB enriched with 50 mM glucose (data not shown).
Figure 2. The bpss1439 mutant displayed reduced biofilm formation which was complemented by in trans bpss1439 expression.
a. The wild-type and bpss1439 mutant were grown in LB under static conditions at 37°C for 48 hours. Biofilms were stained with 1% w/v crystal violet, solubilised and quantified using an optimal density reading at 595 nm. Results are plotted with standard deviation error bars from triplicate experiments each consisting of eight experimental replicates; the p value was calculated using a paired Student's T test. b. The trans-complemented bpss1439 mutant (pME-1439), as well as the wild-type and bpss1439 pME strain, were also assessed for biofilm production as described previously.
To confirm that the observed reduction in biofilm formation is due to the disruption of bpss1439, rather than downstream genes in the operon (Figure 1), the bpss1439 mutant was complemented in trans. A full-length copy of the bpss1439; gene was cloned into the inducible pME6032 vector the resultant plasmid (pME-1439) was transformed into the bpss1439 mutant by electroporation. Additionally, B. pseudomallei wild-type and the bpss1439 strain harbouring empty pME6032 (pME) vector were constructed as controls to account for any potential effects of the plasmid per se on biofilm formation. All strains were tested and found to display wild-type levels of in vitro growth under the conditions used for the biofilm assays (Figure S1). Biofilm assays were repeated as described previously and the expected levels of biofilm were observed for the wild-type (pME) and bpss1439 (pME) strains. However, biofilm production by the bpss1439 (pME-1439) strain was restored to wild-type levels in the presence of inducer (Figure 2b). Therefore, it can be concluded that bpss1439 is a novel factor involved in biofilm formation of B. pseudomallei. We have therefore named this gene bbfA, Burkholderia biofilm factor A.
The bbfA mutant demonstrated reduced adhesion and microcolony formation
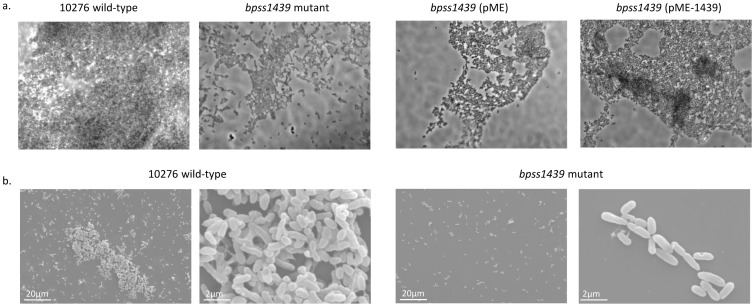
Microscopy was used to confirm and examine the defect in biofilm production by the bbfA mutant. The wild-type and bbfA mutant, along with the control strains harbouring pME or pME-1439, were grown on glass coverslips under static conditions in LB at 37°C for 48 hours. Biofilms were stained for exopolysaccharide using the periodic acid-Schiff protocol. When grown under biofilm-inducing conditions, the bbfA mutant demonstrated reduced adhesion as seen by lower total bacterial numbers, as well as reduced microcolony formation as noted by the lack of clumping or autoagglutination of bacterial cells in comparison with the wild-type (Figure 3a). As previously found with the crystal violet biofilm assay, the wild-type (pME) and the complemented bbfA (pME-1439) strains demonstrated wild-type levels of biofilm production with extensive microcolony formation while the bbfA mutant and bbfA (pME) strains displayed reduced adhesion and microcolony formation (Figure 3a).
Figure 3. The bbfA mutant demonstrated reduced adhesion and microcolony formation.
A. The wild-type, bbfA mutant and trans-complemented bbfA mutant (pME-1439), along with the control wild-type and bbfA mutant strains harbouring pME, were grown in LB under static conditions at 37°C for 48 hours. Biofilms were stained for exopolysaccharide using the periodic acid-Schiff protocol and examined by light microscopy. B. The wild-type and bbfA mutant biofilms were also fixed with 2.5% v/v glutaldehyde and examined by scanning electron microscopy.
Biofilm formation of the wild-type and bbfA strains was further examined by SEM (Figure 3b). These images were congruent with the periodic acid-Schiff staining with the bbfA mutant demonstrating reduced adhesion and microcolony production in comparison to the wild-type. Additionally, evidence of biofilm maturation in terms of the depth of the wild-type biofilm formation is clearly lacking in the bbfA mutant samples grown in biofilm-inducing conditions. Therefore, bbfA appears to play a role in the initial adhesion of B. pseudomallei to an abiotic surface based on reduced bacterial numbers at the 48 hour time-point of biofilm formation.
The bbfA mutant did not demonstrate any of the additional phenotypes reported for other biofilm reduced mutant strains
All previously characterised B. pseudomallei mutants with reduced ability to form biofilms also showed pleiotropic effects on multiple other phenotypes. Therefore, to further understand whether the BbfA protein acts directly in biofilm formation, the following phenotypes were examined: polysaccharide capsule (monoclonal antibody staining), LPS (silver stain), flagella and pili (swimming, swarming and twitching motility plates), oxidative stress (peroxide sensitivity), osmotic stress (NaCl sensitivity), dessication (viability post-drying) and exopolysaccharide production (Congo red binding). Additionally, the bbfA mutant was tested for net intracellular replication in both epithelial A549 and macrophage J774.2 cell lines. The bbfA mutant was found to display wild-type characteristics for all of these phenotypes (Table 2; Figure S2). These data suggest the adhesion phenotype of BbfA-mediated biofilm formation described earlier is directly responsible for the reduced production of biofilm in the bbfA mutant.
Table 2. Comparison of the bbfA mutant with other reported biofilm reduced strains.
| Mutant | Annotation | Source | Other phenotypes | Cell lines |
| wzn | ABC transporter | [9] | acapsular, ↓ oxidative survival, desiccation | |
| fliC | Flagellin | [4] | aflagellate | |
| cpdA | c-di-GMP phosphodiesterase | [11] | aflagellate, ↑ EPS†, autoagglutination | ↓ A549 & THP1 invasion |
| pkk | polyphosphate kinase | [10] | aflagellate, ↓ oxidative survival | |
| rpoE | Alternative sigma factor | [13] | ↓ oxidative & osmotic survival | ↓ J774 invasion |
| bspI | QS synthase 1 | [12] | no QS 1 | |
| bspI3 | QS synthase 3 | [12] | no QS 3 | |
| bspR | QS regulator 1 | [12] | no QS 1 | |
| bbfA | TAA | This study | wt A549 & J774 invasion |
EPS = exopolysaccharide.
The bbfA mutant exhibited an attenuation for virulence in the BALB/c murine melioidosis model compared to the parent strain
To assess the role of biofilm formation in the pathogenesis of B. pseudomallei, the bbfA mutant was tested for virulence in a murine melioidosis model. Groups of six BALB/c mice were challenged via the intra-peritoneal route and median time to death was determined by the Mantel-Cox log-rank test at 35 days post-infection (Figure 4). Compared to mice infected with the parent 10276 wild-type, the mice challenged with the bbfA mutant demonstrated a significant delay in time to death, with a mean survival time of 3.5 days versus 2 days for the wild-type (p = 0.03). However, this delay in time to death could, in part, be a product of the marginally lower inoculum (3.07×105 CFU for bbfA vs. 3.5×105 CFU for the wild-type); therefore, subsequent animal studies were performed. Six groups of five BALB/c mice were challenged via the intra-peritoneal route and the median lethal dose (mice were humanely culled when pre-defined end-points were reached) was determined by the using the Reed and Meunch calculation based on cumulative lethal dose. Compared to the parent 10276 wild-type, the bbfA mutant demonstrated a 13-fold increase in medium lethal dose (2.09×105 vs. 1.6×104 CFU). To confirm that this attenuation is directly linked to BbfA, in trans complementation of the bbfA mutant was performed using a competitive in vivo growth assay. An equal ratio of the wild-type harbouring empty pME6032 vector and the bbfA mutant harbouring either pME6032 or the pME-1439 complementation vector was inoculated into groups of three BALB/c mice via the intra-peritoneal route. Mice were humanely culled at 24 hours post-infection and livers and spleens were harvested and plated for bacterial recovery. Colonies were patched to determine the percentage of in vivo growth by the bbfA mutant versus the wild-type strain by subtracting the number of tetR, kanR (bbfA mutant harbouring pME6032 or pME-1439) colonies from the number of tetR, kanS (wild-type harbouring pME6032) colonies. The bbfA mutant harbouring empty pME6032 vector demonstrated reduced in vivo growth compared to the wild-type harbouring pME6032. However, this attenuation was abrogated by the complemented bbfA mutant strain that displayed a comparable level of in vivo survival to the wild-type harbouring pME6032, both within the spleen (0.33%±0.58% vs. 42.3%±12.3%, p = 0.004) and the liver (0%±0% vs. 42.5%±20.6%, p = 0.023). Bacteria isolated from the liver and spleen of control mice infected with only the complemented bbfA mutant strain demonstrated between 0% to 4% loss of the pME-1439 plasmid over the 24 hour experimental period. These data suggest that bbfA-mediated biofilm formation may play a subtle role in the pathogenesis of melioidosis. Future studies could focus on whether biofilm production relates to chronic disease rather than the acute infection seen in the BALB/c melioidosis model [6], or when mice are challenged at a mucosal surface rather than by a parenteral route. Alternatively, studies could examine differences between strains; it has been previously observed that B. pseudomallei strains demonstrate varying levels of biofilm production, while B. mallei and B. thailandensis strains produce relatively low levels of biofilm [4], [6], [7]. Therefore, it is of interest to note that the bbfA gene occurs as a pseudogene in some B. pseudomallei strains, as well as B. thailandensis and B. mallei, and this may explain the variable biofilm levels seen.
Figure 4. Mice infected with the bbfA mutant demonstrated a delay to death in comparison to those challenged with the parental strain.
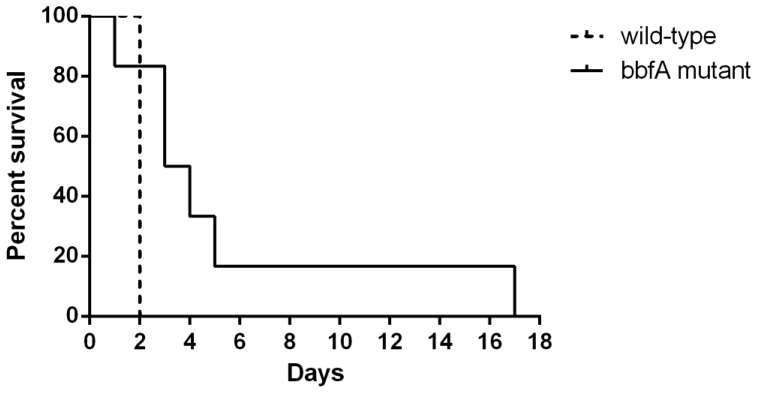
Groups of six BALB/c mice were challenged via the intra-peritoneal route and the median time to death was determined by the Mantel-Cox log-rank test at 35 days post-infection. Mice inoculated with the bbfA mutant had a mean survival time of 3.5 days versus 2 days for those dosed with the wild-type (p = 0.03).
Biofilm formation has been demonstrated to be of importance in dynamic environments where there is a flow of fluid [30]. In agreement with this observation, it has been proposed that the ability of B. pseudomallei to form biofilms is important to its colonisation of bore wells where it has been implicated in melioidosis outbreaks [31]. While a role for biofilm formation for persistence in the environment is often referenced [6], [13], [32], it is important to remember that inoculation events, which are essential for pathogenesis, occur at the environmental-host interface. Furthermore, it is tempting to speculate, that similar to the related pathogens Burkholderia cenocepacia [33] and Pseudomonas aeruginosa [34], autotransporter-mediated biofilm formation of B. pseudomallei may be involved in persistent infection of airways and lungs. Unfortunately, little similarity (38% amino acid identity overall) is seen with between BbfA and the B. cenocepacia biofilm factor (BCAM0223); the functional domains of TAAs demonstrate significant sequence divergence [35]. Yet an indication for a potential role of biofilm production in the virulence of B. pseudomallei is implicated by the identification of bbfA as one of the subset of genes within the B. pseudomallei core genome which is undergoing positive selection. This subset of genes, which demonstrate an above-background rate of functional variation, is enriched for genes with proposed or experimentally validated roles in virulence [26]. Additionally, BbfA was recognised in convalescent sera from melioidosis patients indicating that it is expressed in vivo [20]. One could propose that biofilm is important for the unique lifestyle of B. pseudomallei in which it transitions between survival, and persistence, in both the environment and various hosts.
Supporting Information
The bbfA mutant, and the complemented strains, demonstrate wild-type levels of in vitro growth. a. The wild-type and bbfA mutant were grown in LB at 37°C for 48 hours; in vitro growth was quantified using optimal density readings at 600 nm. Average values from biological triplicates are plotted. b. The trans-complemented bbfA mutant (pME-1439), as well as the wild-type and bbfA pME strain, were also assessed for in vitro growth under biofilm production conditions. There is a slightly lower final (48 h) OD600 reading for the wild-type strain with pME; this strain demonstrates slightly higher biofilm formation which may affect the OD reading at this time point.
(TIF)
Phenotypic analysis of the bbfA mutant. The bbfA mutant and its parental wild-type 10276 strain were assessed for various phenotypes which may impact of biofilm formation using published methods. The following phenotypes were examined: a. polysaccharide capsule (monoclonal antibody staining), b. LPS (silver stain), c. flagella and pili (swimming, swarming and twitching motility plates), d. exopolysaccharide production (Congo red binding) and e. intracellular replication in both epithelial A549 and macrophage J774.2 cell lines. The bbfA mutant was found to display wild-type characteristics for all of these phenotypes.
(TIF)
Acknowledgments
We are grateful to the staff at the Leicester Imaging Technologies Electron Microscopy suite, University of Leicester for their assistance in the preparation and imaging of the biofilm samples for SEM.
Funding Statement
This research was funded by the Wellcome Trust Research Grant #085972 (http://www.wellcome.ac.uk/Funding/Biomedical-science/index.htm). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Galyov EE, Brett PJ, DeShazer D (2010) Molecular insights into Burkholderia pseudomallei and Burkholderia mallei pathogenesis. Annual review of microbiology 64: 495–517. [DOI] [PubMed] [Google Scholar]
- 2. Lazar Adler NR, Govan B, Cullinane M, Harper M, Adler B, et al. (2009) The molecular and cellular basis of pathogenesis in melioidosis: how does Burkholderia pseudomallei cause disease? FEMS Microbiology Reviews 33: 1079–1099. [DOI] [PubMed] [Google Scholar]
- 3. Vorachit M, Lam K, Jayanetra P, Costerton JW (1995) Electron microscopy study of the mode of growth of Pseudomonas pseudomallei in vitro and in vivo . Journal of Tropical Medicine & Hygiene 98: 379–391. [PubMed] [Google Scholar]
- 4. Sawasdidoln C, Taweechaisupapong S, Sermswan RW, Tattawasart U, Tungpradabkul S, et al. (2010) Growing Burkholderia pseudomallei in biofilm stimulating conditions significantly induces antimicrobial resistance. PLoS One 5: e9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vorachit M, Lam K, Jayanetra P, Costerton JW (1993) Resistance of Pseudomonas pseudomallei growing as a biofilm on silastic discs to ceftazidime and co-trimoxazole. Antimicrobial agents and Chemotheraphy 37: 2000–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramli NS, Eng Guan C, Nathan S, Vadivelu J (2012) The effect of environmental conditions on biofilm formation of Burkholderia pseudomallei clinical isolates. PLoS One 7: e44104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taweechaisupapong S, Kaewpa C, Arunyanart C, Kanla P, Homchampa P, et al. (2005) Virulence of Burkholderia pseudomallei does not correlate with biofilm formation. Microbial Pathogenesis 39: 77–85. [DOI] [PubMed] [Google Scholar]
- 8. Chanchamroen S, Kewcharoenwong C, Susaengrat W, Ato M, Lertmemongkolchai G (2009) Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infection & Immunity 77: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuen CW, Ong EB, Mohamad S, Manaf UA, Najimudin N (2012) Construction and characterization of a Burkholderia pseudomallei wzm deletion mutant. Journal of microbiology and biotechnology 22: 1336–1342. [DOI] [PubMed] [Google Scholar]
- 10. Tunpiboonsak S, Mongkolrob R, Kitudomsub K, Thanwatanaying P, Kiettipirodom W, et al. (2010) Role of a Burkholderia pseudomallei polyphosphate kinase in an oxidative stress response, motilities, and biofilm formation. Journal of microbiology 48: 63–70. [DOI] [PubMed] [Google Scholar]
- 11. Lee HS, Gu F, Ching SM, Lam Y, Chua KL (2010) CdpA is a Burkholderia pseudomallei cyclic di-GMP phosphodiesterase involved in autoaggregation, flagellum synthesis, motility, biofilm formation, cell invasion, and cytotoxicity. Infection Immunity 78: 1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gamage AM, Shui G, Wenk MR, Chua KL (2011) N-Octanoylhomoserine lactone signalling mediated by the BpsI-BpsR quorum sensing system plays a major role in biofilm formation of Burkholderia pseudomallei . Microbiology 157: 1176–1186. [DOI] [PubMed] [Google Scholar]
- 13. Korbsrisate S, Vanaporn M, Kerdsuk P, Kespichayawattana W, Vattanaviboon P, et al. (2005) The Burkholderia pseudomallei RpoE (AlgU) operon is involved in environmental stress tolerance and biofilm formation. FEMS Microbiology Letters 252: 243–249. [DOI] [PubMed] [Google Scholar]
- 14. Thongboonkerd V, Vanaporn M, Songtawee N, Kanlaya R, Sinchaikul S, et al. (2007) Altered proteome in Burkholderia pseudomallei rpoE operon knockout mutant: insights into mechanisms of rpoE operon in stress tolerance, survival, and virulence. Journal of Proteome Research 6: 1334–1341. [DOI] [PubMed] [Google Scholar]
- 15. Pibalpakdee P, Wongratanacheewin S, Taweechaisupapong S, Niumsup PR (2012) Diffusion and activity of antibiotics against Burkholderia pseudomallei biofilms. Internation journal of antimicrobial agents 39: 356–359. [DOI] [PubMed] [Google Scholar]
- 16. Songsri J, Proungvitaya T, Wongratanacheewin S, Homchampa P (2012) Tn5-OT182 should not be used to identify genes involved in biofilm formation in Burkholderia pseudomallei . The Southeast Asian journal of tropical medicine and public health 43: 124–128. [PubMed] [Google Scholar]
- 17. Dautin N, Bernstein H (2007) Protein secretion in gram-negative bacteria via the autotransporter pathway. Annual review of microbiology 61: 89–112. [DOI] [PubMed] [Google Scholar]
- 18. Cotter SE, Surana NK, St Geme JWr (2005) Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends in microbiology 13: 199–205. [DOI] [PubMed] [Google Scholar]
- 19. Lazar Adler NR, Stevens JM, Stevens MP, Galyov EE (2011) Autotransporters and Their Role in the Virulence of Burkholderia pseudomallei and Burkholderia mallei . Frontiers in Microbiology 2: 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tiyawisutsri R, Holden MT, Tumapa S, Rengpipat S, Clarke SR, et al. (2007) Burkholderia Hep_Hag autotransporter (BuHA) proteins elicit a strong antibody response during experimental glanders but not human melioidosis. BMC Microbiology 7. [DOI] [PMC free article] [PubMed]
- 21.Balder R, Lipski S, Lazarus JJ, Grose W, Wooten RM, et al. (2010) Identification of Burkholderia mallei and Burkholderia pseudomallei adhesins for human respiratory epithelial cells. BMC Microbiology 10: doi:10.1186/1471-2180-1110-1250. [DOI] [PMC free article] [PubMed]
- 22. Mack K, Titball RW (1996) Transformation of Burkholderia pseudomallei by electroporation. Analytical Biochemistry 242: 73–76. [DOI] [PubMed] [Google Scholar]
- 23. Alexeyev M (1999) The pKNOCK series of broad-host-range mobilizable suicide vectors for gene knockout and targeted DNA insertion into the chromosome of gram-negative bacteria. Biotechniques 26: 824–826. [DOI] [PubMed] [Google Scholar]
- 24. Heeb S, Itoh Y, Nishijyo T, Schnider U, Keel C, et al. (2000) Small, stable shuttle vectors based on the minimal pVS1 replicon for use in gram-negative, plant-associated bacteria. Molecular Plant-Microbe Interactions 13: 232–237. [DOI] [PubMed] [Google Scholar]
- 25. Atkins T, Prior R, Mack K, Russell P, Nelson M, et al. (2002) Characterisation of an acapsular mutant of Burkholderia pseudomallei identified by signature tagged mutagenesis. Journal of Medicial Microbiology 51: 539–547. [DOI] [PubMed] [Google Scholar]
- 26. Nandi T, Ong C, Singh AP, Boddey J, Atkins T, et al. (2010) A genomic survey of positive selection in Burkholderia pseudomallei provides insights into the evolution of accidental virulence. PLoS Pathogens 6: e1000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazar Adler NR, Stevens JM, Stevens MP, Galyov EE, 15. FMdfEJ (2011) Autotransporters and Their Role in the Virulence of Burkholderia pseudomallei and Burkholderia mallei. Frontiers in microbiology 2. [DOI] [PMC free article] [PubMed]
- 28. Marchler-Bauer A, Zheng C, Chitsaz F, Derbyshire MK, Geer LY, et al. (2013) CDD: conserved domains and protein three-dimensional structure. Nucleic Acids Research 41: D384–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henderson IR, Nataro JP (2001) Virulence functions of autotransporter proteins. Infection Immunity 69: 1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Müller NF, Kaiser PO, Linke D, Schwarz H, Riess T, et al. (2011) Trimeric autotransporter adhesin-dependent adherence of Bartonella henselae, Bartonella quintana, and Yersinia enterocolitica to matrix components and endothelial cells under static and dynamic flow conditions. Infection Immunity 79: 2544–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Currie BJ, Mayo M, Anstey NM, Donohoe P, Haase A, et al. (2001) A cluster of melioidosis cases from an endemic region is clonal and is linked to the water supply using molecular typing of Burkholderia pseudomallei isolates. Americian Journal of Tropical Medicine and Hygiene 65: 177–179. [DOI] [PubMed] [Google Scholar]
- 32. Kamjumphol W, Chareonsudjai S, Chareonsudjai P, Wongratanaceewin S, Taweechaisupapong S (2013) Environmental factors affecting Burkholderia pseudomallei biofilm formation. Southeast Asian Journal of Tropical Medicine and Public Health 44: 72–81. [PubMed] [Google Scholar]
- 33. Mil-Homens D, Fialho AM (2012) A BCAM0223 mutant of Burkholderia cenocepacia is deficient in hemagglutination, serum resistance, adhesion to epithelial cells and virulence. PLoS One 7: e41747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilhelm S, Gdynia A, Tielen P, Rosenau F, Jaeger KE (2007) The autotransporter esterase EstA of Pseudomonas aeruginosa is required for rhamnolipid production, cell motility, and biofilm formation. Journal of Bacteriology 189: 6695–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Henderson IR, Navarro-Garcia F, Desvaux M, Fernandez RC, Ala′Aldeen D (2004) Type V Protein Secretion Pathway: the Autotransporter Story. Microbiology and Molecular Biology Reviews 68: 692–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Simon R, Priefer U, Puhler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio-Technology 1: 784–791. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The bbfA mutant, and the complemented strains, demonstrate wild-type levels of in vitro growth. a. The wild-type and bbfA mutant were grown in LB at 37°C for 48 hours; in vitro growth was quantified using optimal density readings at 600 nm. Average values from biological triplicates are plotted. b. The trans-complemented bbfA mutant (pME-1439), as well as the wild-type and bbfA pME strain, were also assessed for in vitro growth under biofilm production conditions. There is a slightly lower final (48 h) OD600 reading for the wild-type strain with pME; this strain demonstrates slightly higher biofilm formation which may affect the OD reading at this time point.
(TIF)
Phenotypic analysis of the bbfA mutant. The bbfA mutant and its parental wild-type 10276 strain were assessed for various phenotypes which may impact of biofilm formation using published methods. The following phenotypes were examined: a. polysaccharide capsule (monoclonal antibody staining), b. LPS (silver stain), c. flagella and pili (swimming, swarming and twitching motility plates), d. exopolysaccharide production (Congo red binding) and e. intracellular replication in both epithelial A549 and macrophage J774.2 cell lines. The bbfA mutant was found to display wild-type characteristics for all of these phenotypes.
(TIF)