Abstract
Huanglongbing (HLB) presumably caused by Candidatus Liberibacter asiaticus (CLas) threatens the commercial U.S. citrus crop of an annual value of $3 billion. The earliest shift in metabolite profiles of leaves from greenhouse-grown sweet orange trees infected with Clas, and of healthy leaves, was characterized by HPLC-MS concurrently with PCR testing for the presence of Clas bacteria and observation of disease symptoms. Twenty, 8-month-old ‘Valencia’ and ‘Hamlin’ trees were grafted with budwood from PCR-positive HLB source trees. Five graft-inoculated trees of each variety and three control trees were sampled biweekly and analyzed by HPLC-MS and PCR. Thirteen weeks after inoculation, Clas was detected in newly growing flushes in 33% and 55% of the inoculated ‘Hamlin’ and ‘Valencia’ trees, respectively. Inoculated trees remained asymptomatic in the first 20 weeks, but developed symptoms 30 weeks after grafting. No significant differences in the leaf metabolite profiles were detected in Clas-infected trees 23 weeks after inoculation. However, 27 weeks after inoculation, differences in metabolite profiles between control leaves and those of Clas-infected trees were evident. Affected compounds were identified with authentic standards or structurally classified by their UV and mass spectra. Included among these compounds are flavonoid glycosides, polymethoxylated flavones, and hydroxycinnamates. Four structurally related hydroxycinnamate compounds increased more than 10-fold in leaves from ‘Hamlin’ and ‘Valencia’ sweet orange trees in response to Clas infection. Possible roles of these hydroxycinnamates as plant defense compounds against the Clas infection are discussed.
Introduction
Citrus greening disease, also known as huanglongbing (HLB), is the most destructive citrus disease worldwide [1]. It is believed to be caused by the bacterium Candidatus Liberibacter asiaticus (Clas), which is transmitted by the Asian citrus psyllid (Diaphorina citri, ACP) [1]. Recently, HLB was discovered in the largest citrus growing regions in the USA and Brazil [2]. HLB symptoms are displayed on different parts of the plant; the leaves are characterized by a yellow blotchy mottle, or asymmetrical chlorosis, and the fruits are under-developed, lopsided, and green colored with aborted or stained seeds [3]. As the disease severity increases, both yield and fruit quality decrease. Yield reduction due to fruit drop can reach 30 to 100% [1].
Clas bacterium is not the only type of Candidatus Liberibacter that causes a destructive disease in commercial crops. In fact, the phloem-limited bacterium (Candidatus Liberibacter solanacearum), which is transmitted by the potato psyllids Bactericera cockerelli Sulc. (Hemiptera: Triozidae), can negatively affect a range of vegetable hosts [4]. In potatoes, Candidatus Liberibacter solanacearum is associated with zebra chips (ZC) disease. The ZC symptoms include purple or yellow discoloration, leaf burn, aerial tubers, and early death [5].Tubers from infected plants showed brown discoloration after slicing and very dark blotches, stripes, or streaks upon frying [5].
The incubation period for HLB within citrus trees ranges from a few months to one or more years [1]. At about 3 months after inoculation, Clas was detected in 70% of inoculated sweet orange and grapefruit seedlings [6], and severe asymmetrical yellowing of leaves was clearly observed 5–6 months after grafting. In a similar study [7], Clas bacterium was detected in 60% of the ‘Valencia’ trees 1 month after inoculation, and typical HLB symptoms (chlorosis of leaves) were observed 6–8 months after inoculation. Quantification of the bacterium using qPCR showed that the Clas bacterium was present in different parts of the infected plant; however, it was unevenly distributed [8]. Although the previous studies provided useful information about the etiology of the HLB disease, transmission, symptom development, and Clas detection, they did not provide any information about the effect of Clas on the health and the biochemical change in fruits and leaves of infected plants.
Studies of the effects of HLB on the orange juice encompassed investigations into the effects of HLB disease on the taste and phytochemical compositions of the fruits. An increase in the concentrations of limonoids, a number of alkaloids, hydroxycinnamates (HCAs), phenylalanine, and citrate have been observed in ‘Valencia’ orange juice samples obtained from Clas-infected trees [9,10].
Early studies of the effects of HLB on the phytochemical profiles of citrus leaves targeted starch [11] and gentisic acid [12]. More recent studies have characterized part of the metabolome of leaves from Clas-infected and healthy citrus trees using high performance liquid chromatography-mass spectrometry (HPLC-MS) [13,14], capillary electrophoresis with photodiode array detection [15], and gas chromatography-mass spectrometry (GC-MS) [16]. However, these studies involved investigations of leaves obtained from commercial groves for which it was not possible to determine the time of initial infection, and for which multiple inoculations by psyllids likely occurred. Moreover, all of these studies focused on the metabolic difference between highly symptomatic and healthy leaves; hence, little has been achieved in characterizing potential early-occurring phytochemical markers in newly-infected pre-symptomatic leaves. The objective of our study was to evaluate biochemical changes that occur in the leaves of Clas-infected sweet orange trees over time.
Materials and Methods
Tree inoculation with Candidatus Liberibacter asiaticus (Clas) and sampling procedures
‘Valencia’ and ‘Hamlin’ (Citrus sinensis (L.) Osbeck) plants used in this study were grafted on Volkamer lemon (Citrus limonia Osbeck ‘Volkameriana’). Twenty plants (8–10 months old, 0.5‑1 m tall) from each variety were scion grafted with four pieces of budwood from PCR-positive HLB source trees. The grafts were 10 cm apart and the lowest graft was at about 15 cm above ground. Inoculated plants were kept in a USDA-APHIS approved secure greenhouse with the temperature controlled at 28–32°C. Plants were regularly watered twice a week and fertilized once a week using 20-10-20 fertilizer (Allentown, PA). For each variety, eight plants were grafted with disease-free budwood and were used as controls. Five inoculated trees of each variety and three control trees were sampled at 3, 23, 27, 29, 35, and 38 weeks after inoculation. The sampling plan was based on our previous observations in early studies [6,17]. From each tree, two leaves were randomly (regardless to symptoms) harvested from the same shoot, bagged, immediately placed on ice for transportation, frozen, and stored at -80°C.
PCR analyses and leaf symptoms
The second leaf from each sampled tree was used for PCR analysis to check for the presence of the Clas bacterium as described by Tatineni et al. [8]. Briefly, 250 mg of midrib leaf tissue were extracted in 2.5 ml extraction buffer (100 mM Tris-HCL pH 8.0; 50 mM EDTA; 500 mM NaCl; 10 mM dithiothreitol). A volume of 1500 µl was transferred to a 2.5 ml Eppendorf tube, and 100 µl 20% (w/v) SDS was added, and incubated at 65°C for 30 minutes. A 500 µl aliquot of 5 M potassium acetate (pH = 5.2) was added, mixed thoroughly, and incubated on ice for 20 minutes. DNA was recovered by centrifugation and precipitation with isopropanol and kept at -20°C overnight. Resuspended DNA was analyzed by conventional PCR using 0.2 μg of DNA template, 0.2 μM each primer (HLB-65 and HLB-66) [8], 0.25 mM dNTPs, 1× buffer (FBII; Takara Bio), and 0.125 μl (5 U/μl) of SpeedSTAR HS DNA polymerase. The amplification conditions were as follows: 94°C for 2 minutes; followed by 35 cycles at 94°C for 20 seconds, 54°C for 20 seconds, and 72°C for 60 seconds; and final extension at 72°C for 5 minutes. PCR reactions were analyzed in 1.0% agarose gel. DNA bands were visualized by ethidium bromide staining. All reactions were done in triplicate with positive, healthy, and water controls. Control and Clas-inoculated trees were photographed before sampling, and sampled leaves were also photographed before storage to document any leaf symptoms (chlorosis, blotchy-mottled, or leaf yellowing).
Sample preparation and HPLC-MS analyses of leaf metabolites
Methanol, dimethylsulfoxide, and formic acid were purchased from Sigma (St. Louis, MO). Diosmin, 6,8-di-C-glucosyl apigenin, 2′′-xylosylvitexin, 8-C-glucosyldiosmetin, luteolin-7-O-rutinoside, hesperidin, isosakuranetin rutinoside, sinensetin, nobelitin, 3,5,6,7,8,3′,4′-heptamethoxyflavone, tangeretin, feruloylputrescine, and 5-hydroxy-6,7,4′-trimethoxyflavone were obtained as authentic standards from the USDA (Fort Pierce, FL).
One leaf from each sampled tree was removed from storage and a weighed portion (approximately 0.5 g) was ground with a mortar and pestle to a fine powder under liquid nitrogen. A 10-ml aliquot of methanol and dimethylsulfoxide (1:1) was added, and the sample was shaken overnight at 200 rpm in a cold chamber at 0°C using Innova 2100 platform shaker from New Brunswick Science (Edison, NJ). The samples were equilibrated to room temperature and filtered using 0.45 μm Titan 2 HPLC filters from Thermo Fisher Scientific (Pittsburg, PA). A 0.9-ml aliquot of the filtrate was spiked with 0.1 ml of 2500 ppm of 5-hydroxy-6,7,4′-trimethoxyflavone as an internal standard.
Metabolite profiles were analyzed by HPLC-MS, using Varian ProStar 210 pumps and a ProStar 410 autosampler controlled by Star software (ver. 6.41). Compound separations were achieved with a Waters Xbridge C8 column (4.6 × 150 mm i.d.). The mobile phase composition and the flow rate are given in Table 1. MS peak detection was achieved with a Leco APCI (atmospheric pressure chemical ionization) source coupled with a LECO Unique HT MS analyzer (St. Joseph, MI). MS parameters were as follows: positive atmospheric pressure ionization (+ APCI), interface temperature 99°C, nebulizer pressure 300 kPa, desolvation temperature 350°C, desolvation gas flow 2.0 L min-1, nozzle voltage 100 V, skimmer voltage 52 V, and quad RF voltage 165 V. Data processing was done with Leco ChromaTOF (ver. 4.0). Peak identifications were achieved by comparing retention times and mass spectra of sample peaks with those of authentic standards.
Table 1. Mobile phase composition and flow rate.
| Time (min) | % A (0.5% formic acid, H2O) | % B (acetonitrile) | Flow (ml·min-1) |
|---|---|---|---|
| 0 | 86 | 14 | 0.30 |
| 16 | 72 | 28 | 0.30 |
| 21 | 62 | 38 | 0.30 |
| 28 | 50 | 50 | 0.30 |
| 43 | 45 | 65 | 0.50 |
| 48 | 30 | 70 | 0.75 |
| 53 | 30 | 70 | 0.75 |
| 58 | 86 | 14 | 0.75 |
| 63 | 86 | 14 | 0.75 |
| 64 | 86 | 14 | 0.30 |
| 70 | 86 | 14 | 0.30 |
Data processing and statistical analysis
Data was manually aligned using retention time and mass values. Data from infected and non-infected trees were normalized by dividing the area of each peak by the area of the internal standard (5-hydroxy-6,7,4′-trimethoxyflavone). Normalized data was analyzed using principal component analysis (PCA) using Unscrambler® X (www.camo.com). The mean of the normalized values (relative amount) of each compound in the Clas-inoculated trees was also compared to the mean of the control trees at each sampling date using two tailed t-test with unequal replication using Microsoft Excel, and significant differences were reported at 95% confidence level. Data from Clas-inoculated trees that were PCR-negative for the Clas bacterium throughout the entire duration of the experiment were not included (one ‘Valencia’ and two ‘Hamlin’) in the statistical analyses, because those trees did not acquire the disease. In addition, analysis of covariance (ANCOVA) was carried on the whole data set for each cultivar, using time as a covariate, to study the effect of Clas infection on the metabolites of sweet orange over time.
Results and Discussion
PCR analyses and leaf symptoms
Our preliminary PCR analysis showed that Clas bacterium was detected in 3/9 and 5/9 of the inoculated ‘Hamlin’ and ‘Valencia’ plant, respectively, 13 weeks after inoculation. At 33 weeks after inoculation, 5/5 of the tested ‘Valencia’ and 4/5 of the tested ‘Hamlin’ plants were PCR positive (Table 2). Leaves from certain inoculated trees were PCR positive 13 weeks after inoculation, while leaves from the same trees but at different locations were PCR negative 21 and 29 weeks after inoculation, and then these trees were PCR positive 45 weeks after inoculation. These observations in both sweet orange varieties indicated that the bacterium was not evenly distributed in the inoculated trees. In addition, while some of the inoculated trees were PCR positive 13 weeks after inoculation, others did not show any PCR positive results before 45 weeks.
Table 2. PCR results for inoculated ‘Hamlin’ and ‘Valencia’ trees.
| Weeks after inoculation | ‘Hamlin’ | ‘Valencia’ |
|---|---|---|
| 3 | 0/5 a | 0/5 |
| 23 | 2/7 | 2/5 |
| 25 | 3/5 | 3/5 |
| 27 | 2/5 | 1/5 |
| 29 | 0/5 | 1/5 |
| 31 | 4/5 | 3/5 |
| 33 | 4/5 | 5/5 |
| 35 | 4/5 | 3/4 |
| 38 | 4/5 | 3/5 |
aNumber of PCR positive plants over number of sampled plants at each sampling week.
Leaves of the graft-inoculated trees remained asymptomatic in the first 19 weeks (Figure 1A-B and Figure 2A-B). Leaf symptoms characteristic of HLB started to develop in shoots of grafted plants at 19 weeks and were pronounced at 29 weeks after inoculation (Figure 1C-F and Figure 2 C-F). As with the uneven detection of the Clas bacterium throughout the plants (Table 2), HLB leaf symptoms were also unevenly distributed throughout different portions of individual plants; some newly developed leaves on certain trees were highly symptomatic, while others were asymptomatic. These observations are consistent with those of Batool et al. [3] where HLB-symptoms occurred on individual shoots or branches, while other portions of the tree remained asymptomatic.
Figure 1. Progression of huanglongbing (HLB)-related symptoms in ‘Candidatus Liberibacter asiaticus’-inoculated ‘Valencia’ sweet orange seedlings.
A) leaves from control plants 19 weeks after inoculation; B) leaves from HLB-grafted plants 19 weeks after inoculation; C) leaves from control plants 29 weeks after inoculation; D) leaves from HLB-grafted plants 29 weeks after inoculation; E) leaves from control plants 35 weeks after inoculation; F) leaves from HLB-grafted trees 35 weeks after inoculation.
Figure 2. Progression of huanglongbing (HLB)-related symptoms in ‘Candidatus Liberibacter asiaticus’-inoculated ‘Hamlin’ sweet orange seedlings.
A) leaves from control plants 19 weeks after inoculation; B) leaves from CLas-grafted plants 19 weeks after inoculation; C) leaves from control plants 29 weeks after inoculation; D) leaves from CLas-grafted plants 29 weeks after inoculation; E) leaves from control plants 35 weeks after inoculation; F) leaves from CLas-grafted trees 35 weeks after inoculation.
In this study, observations of leaf symptoms (Figure 1 and Figure 2), together with the PCR analyses, indicate that the Clas bacterium in inoculated citrus trees are detected before the development of leaf symptoms, and that the probability of detecting Clas is higher in the symptomatic leaves (31−37 weeks after inoculation). These findings are in close agreement with those reported in previous studies [6,8]. No Clas bacterium was detected by qPCR in inoculated sweet orange and grapefruit 2 months after grafting, while 70% of the inoculated trees were PCR positive after 3 months [6]. Although Clas bacterium has been found in different parts of infected citrus plants, qPCR showed that it was unevenly distributed [8]. In agreement with our study, no disease-related symptoms were observed at 3 months after inoculation; however, by 5−6 months, slight yellowing was observed [6]. In another study, Clas bacterium was detected in 60% of the ‘Valencia’ trees one month after inoculation, and typical HLB symptoms (chlorosis of leaves) were observed 6−8 months after inoculation [7]. The results of this study together with the results of the previous studies [6,7,8] suggest that the observed HLB related symptoms and the PCR results are reproducible. However, the level of Clas titer in the inoculation source, greenhouse temperature, grafting time, number of grafts per tree, applied nutrition, watering schedule, and the type of rootstock may result in slight variation between different studies.
Although the lack of visual leaf symptoms, prior to the detection of the Clas bacteria in our study, suggests that the presence of the bacteria in the leaves may be necessary for the development of the visual symptoms, this hypothesis remains inconclusive. It is still possible that the bacterium could block the phloem of lower stems, thus producing chlorosis in the upper leaves [8], which may explain in part the inconsistent correlation between symptoms and PCR results.
Metabolite profile analysis
The effects of Clas graft-inoculation on disease symptom development in the ‘Hamlin’ and ‘Valencia’ trees were further explored by analyzing the changes in the leaf metabolite profiles, comprised mainly of phenolic flavonoids, hydroxycinnamates, and alkaloids (Table 3). The identification of a number of these compounds was achieved by observing co-elutions and matching MS and UV spectra with authentic standards. In addition, the UV spectra of these compounds were further compared to earlier published spectra [18]. For a number of the remaining compounds, structural classifications were made by inferences from their UV and mass spectra. Previous studies have shown that flavone-C- and -O-glycosides, primarily those of diosmetin (5,7,3′-trihydroxy-4′-methoxyflavone), apigenin (5,7,4′-trimethoxyflavone), and luteolin (5,7,3′,4′-tetrahydroxyflavone) are particularly abundant in leaves of many common citrus cultivars, including sweet orange [14,19,20]. Flavanone-O-glycosides are also abundant in citrus leaves, and compounds 20-22 in Table 3 are indicative of such compounds. Latter flavonoids in sweet orange occur as rutinosides [19] and are identified by sets of ions ([M+H]+, [M+H-(rhamnose-H2O)]+, [M+H-(rutinose-H2O)]+) readily detected in the positive APC-mass spectra of these compounds. Based on the above criteria and overlaps with standards, compounds 16, 19, 20, and 22 were identified as luteolin-7-O- rutinoside, diosmin, hesperidin, and isosakuranetin rutinoside, respectively. In contrast, flavone-C-glycosides typically exhibit protonated molecular ions [M+H]+, and ion fragments corresponding to [M+H-nH2O]+ where n = 1–4. Several of the metabolites in Table 3 exhibited such patterns, including 8, 12, and 17, and were identified as 6, 8-di-C-glucosylapigenin, 2′′-O-xylosylvitexin, and 8-C-glucosyldiosmetin, respectively. Several of the remaining unknown compounds listed in Table 3 also exhibited similar fragment ion patterns; but, due to an absence of standards, they remained unidentified. Also prevalent in the leaf chromatograms were compounds that exhibited UV and mass spectra of polymethoxylated flavones (PMFs) [20], and a number of these are listed in Table 3. Several other remaining compounds in Table 3 also exhibited UV spectra similar to the PMFs but had molecular weights 14 atomic mass units lower than the identified PMFs. These compounds may represent several of the 5-desmethyl PMFs previously reported in sweet orange tissues [20].
Table 3. APC mass-spectra in positive ion detection mode “m/z” and absorbance maximum (nm) for detected compounds.
| Peak no. | RT (min) | Compound | “m/z” (+) | λmax (nm) |
|---|---|---|---|---|
| 1 | 5.2 | Unknown | 313.8 | |
| 2 | 5.3 | Unknown | 268 | |
| 3 | 5.4 | Unknown | 151 | |
| 4 | 10.2 | Feruloylputrescinea | 265/177 | 292/317 |
| 5 | 10.5 | HCAb | 386/195/177 | 325/230 |
| 6 | 12.5 | HCAb | 386/195/177 | 325/231 |
| 7 | 14.1 | HCAb | 386/195/177 | 328/234 |
| 8 | 14.4 | 6,8-di-C-Glucosylapigenina | 595/577/559/541 | 271/335 |
| 9 | 16.1 | HCAb | 386/195/177 | 328/234 |
| 10 | 18.5 | Unknown | 595 | |
| 11 | 19.4 | Unknown | 573 | |
| 12 | 19.5 | 2′′-O-xylosylvitexina | 565/433/415/397 | 268/337 |
| 13 | 20.2 | Unknown | 565 | |
| 14 | 20.3 | Unknown | 595 | |
| 15 | 20.5 | Flavanone-O-rutinosideb | 611/465/303 | |
| 16 | 21.2 | Luteolin-7-O-rutinosidea | 595/595/449/287 | 255/266sh/348 |
| 17 | 22.1 | 8-C-glucosyldiosmetina | 463/445/427 | 254/266/348 |
| 18 | 24.4 | Flavone-O-rutinosideb | 609/463/301 | |
| 19 | 25.8 | Diosmina | 609/463/301 | 253/267/347 |
| 20 | 26.3 | Hesperidina, PCA | 611/465/449/303 | 283/331 |
| 21 | 29.1 | Flavanone-O-rutinosideb | 611/465/303 | |
| 22 | 32.2 | Isosakuranetin rutinosidea | 595/449/287 | 283/331 |
| 23 | 33.5 | Unknown | 728 | |
| 24 | 33.5 | Unknown | 359 | |
| 25 | 34.1 | Unknown | 713 | |
| 26 | 34.4 | Unknown | 359 | |
| 27 | 35.2 | Unknown | 359 | |
| 28 | 35.3 | Unknown | 331 | |
| 29 | 36.1 | Isosinensetina | 373 | 249/270/342 |
| 30 | 37.1 | DesmethylPMFb | 389 | |
| 31 | 37.6 | Sinensetina, PCA | 373 | 243/264/333 |
| 32 | 38.4 | DesmethylPMFb | 345 | |
| 33 | 39.4 | Nobiletina | 403 | 249/270/334 |
| 34 | 40.1 | Tetramethyl-O-Scutellareina | 343 | 265/322 |
| 35 | 40.2 | Unknown | 375 | |
| 36 | 40.5 | Heptamethoxyflavonea | 433 | 254/270sh/341 |
| 37 | 41.3 | DesmethylPMFb | 359 | |
| 38 | 41.5 | Tangeretinb | 373 | 271/321 |
| 39 | 42.3 | DesmethylPMFb | 359 | |
| 40 | 43.2 | 5-Desmethylnobiletina | 389 |
aIdentified by matching their retention time, mass spectra, and UV spectra with known standard. The UV spectra were further compared to earlier published spectra [18].
bTentatively identified using +APCI-MS fragmentation patterns.
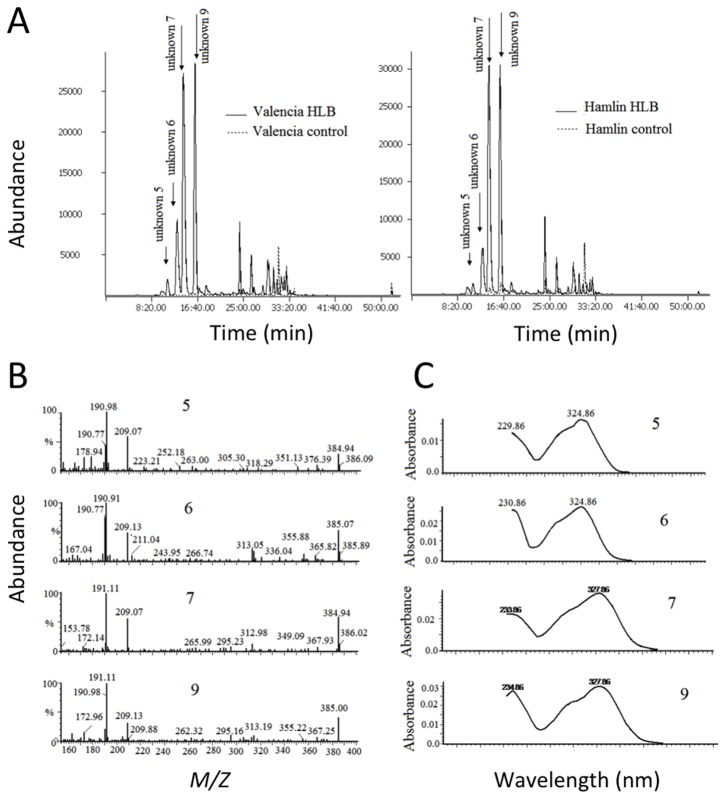
Also present in the leaf metabolite profiles of the ‘Hamlin’ and ‘Valencia’ seedlings were compounds with spectroscopic properties indicative of a set of structurally-related HCAs. Compounds 5–7 and 9 (Figure 3A) each exhibited a mass spectra and a UV spectra (Figure 3B-C) and fluorescence emission spectra (data not shown) similar to feruloyl-derivatives of galactaric and glucaric (aldaric) acids reported in sweet orange peels [21,22], rye leaves [23], and cowpeas [24]. In cowpeas, six isomeric forms of trans-feruloylaldaric acid have been identified. Compounds 5–7 and 9 exhibited fragment ions at 177 m/z and 195 m/z m/z (Table 3) with positive electrospray ionization (ESI) and atmospheric pressure chemical ionization (APCI), while with negative ESI, fragment ions at 191, 209, and 385 m/z were additionally observed (Figure 3B). The 177 and 195 m/z ions are indicative of fragment ions generated from ferulic acid (FA) [FA-H2O+H] + and [FA+H]+ ions, respectively [21]. The 191 m/z ion fragments observed with negative ESI likely originated from [galactaric acid (GalA)-H2O-H]- or [glucaric (GluA) acid-H2O-H]- moieties, the 209 m/z from [GalA-H]- or [GluA-H]-, and the 385 m/z ions from the ionized molecular ions [FA GalA-H]- or [FA GluA-H]- [24]. Ferulic acid-containing alkaloid feruloylputrescine, compound 4, was also detected with positive ESI and APCI [25,26,27].
Figure 3. Typical APCI-HPLC-MS chromatograms of ‘Hamlin’ and ‘Valencia’ leaf extracts and mass-spectra and UV spectra of unknown 5, 6, 7, and 9.
A) selective ion recording chromatograms of “m/z” specie of 177 in the positive ion mode; B) negative electrospray ionization mass spectra ; C) UV spectra of unknown 5, 6, 7, and 9.
Effect of Clas infection on sweet orange leaf metabolites
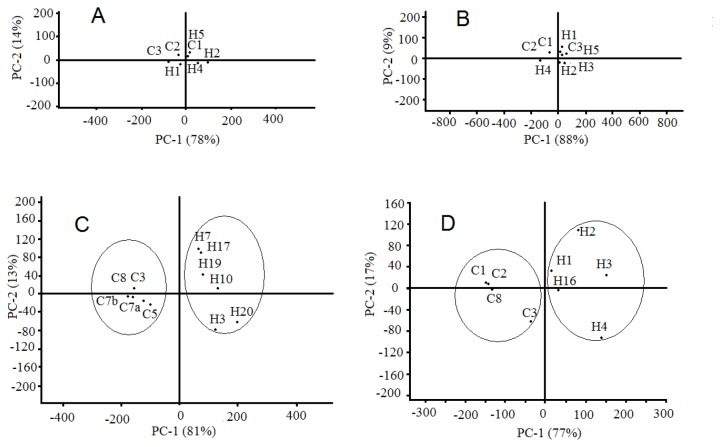
Principal component analysis (PCA) and the t-test were carried out on each set of leaf samples to compare the metabolite profiles of the control to those from Clas-inoculated trees and to identify compounds whose relative amounts were significantly affected by the progression of HLB disease. The PCA and t-test are the most common statistical analysis approach used in untargeted large-scale plant metabolomics studies [28,29]. No group clustering was observed in the score plots from PCA of ‘Valencia’ leaf metabolites during the first 23 weeks (data not shown). However, at 27 weeks, clustering into two groups (HLB and control) began to form (data not shown), and the clustering was well-defined by 38 weeks (Figure 4C). Loading values from PCA for the first or second principal components (Table 4) of compound 6, 7, 9, and 20, were greatest, suggesting that they were responsible for the group clustering.
Figure 4. Principal component analysis of HPLC-MS leaf metabolites from ‘Valencia’ and ‘Hamlin’ trees.
A) score plot of leaf metabolites from ‘Valencia’ trees 3 weeks after inoculation; B) score plot of leaf metabolites from ‘Hamlin’ trees 3 weeks after inoculation; C) score plot of leaf metabolites from ‘Valencia’ trees 38 weeks after inoculation; D) score plot of leaf metabolites from ‘Hamlin’ trees 38 weeks after inoculation.
Table 4. Loadings from PCA for metabolites in leaves from ‘Valencia’ or ‘Hamlin’ at sampling dates when group clustering was observeda.
| Compound |
Loadings |
|||||
|---|---|---|---|---|---|---|
|
|
Time after inoculation (week) |
|||||
| 27 | 29 | 35 | 35 | 38 | ||
| ‘Valencia’ | ‘Hamlin’ | |||||
| PC-1 | 7 | 0.24 | 0.15 | 0.35 | ||
| 9 | 0.27 | |||||
| 19 | 0.19 | |||||
| 20 | 0.82 | 0.91 | 0.89 | 0.93 | 0.81 | |
| 31 | 0.28 | 0.21 | 0.21 | |||
| 33 | -0.15 | -0.25 | ||||
| 34 | 0.25 | |||||
| 37 | 0.22 | |||||
| 38 | -0.10 | |||||
| PC-2 | 6 | 0.26 | 0.23 | 0.24 | ||
| 7 | 0.54 | 0.67 | 0.74 | 0.67 | ||
| 9 | 0.42 | 0.58 | 0.47 | 0.49 | ||
| 20 | 0.54 | 0.21 | 0.29 | -0.24 | -0.47 | |
| 33 | 0.48 | |||||
| 38 | -0.22 | |||||
| 40 | 0.25 | |||||
aCompounds relevant to both varieties were bolded.
Similar score plots from PCA of ‘Hamlin’ leaves provided evidence of clustering into two groups (HLB and control) 35 weeks after grafting (data not shown) and the clustering was well-defined at 38 weeks (Figure 4D). Cluster regions were arbitrarily drawn in Figure 4C-D to help visualization. As in the changes that occurred in ‘Valencia’ leaves, compounds 6, 7, 9, 18–20, 31, and 37 were the main compounds responsible for group clustering in ‘Hamlin’ leaves.
Comparison of means of metabolite normalized area (relative amounts) from HLB-inoculated versus control ‘Valencia’ leaves revealed no significant increase in any metabolite at 3 and 23 weeks after inoculation (Table 5). However, comparison of means of metabolite relative amounts between weeks 27 and 35 revealed that at one or more sampling dates 13 metabolites were five times higher in the HLB-affected leaves than in the control leaves, and four compounds were five-fold higher in the HLB-affected leaves at two sampling dates. These compounds are highlighted on Table 5. In particular, the relative amount of compound 13 was 58.7 and 46.3 times higher in the HLB-affected leaves in weeks 29 and 35, respectively; the relative amount of compound 6 was 13.0 and 25.8 times higher in weeks 27 and 29, respectively, and compound 7 was 17.2 and 11.1 times higher in weeks 27 and 29, respectively.
Table 5. The relative amounts of metabolites from leaves of CLas-inoculated ‘Valencia’ trees with respect to control ‘Valencia’ trees.
| Peak no. | Compound | Week 3 | Week 23 | Week 27 | Week 29 | Week 35 | Week 38 | Time effect (p-value)a | Group effect (p-value)a |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | 0.7 | 0.9 | 9.8 | 1.5 | 1.4 | 22.6 | 0.3940 | 0.0380 |
| 2 | Unknown | 0.9 | 1.5 | 1.5 | 1.2 | 1.0 | 1.7 | 0.0060 | 0.2010 |
| 3 | Unknown | 1.0 | 0.7 | 3.6 | 1.3 | 1.2 | 2.3 | 0.3780 | 0.0290 |
| 4 | Feruloylputrescine | 1.6 | 1.0 | 2.0 | 2.0 | 3.3 | 12.2 | 0.0820 | 0.0056 |
| 5 | HCA | 1.6 | 1.3 | 10.5 | 6.4 | 3.2 | 127.7 | 0.1280 | 0.0003 |
| 6 | HCAPCA | 1.5 | 3.3 | 13.0 | 25.8 | 291.2 | 158.3 | 0.0056 | <0.0001 |
| 7 | HCAPCA | 2.3 | 2.2 | 17.2 | 11.1 | 105.6 | 48.8 | 0.0007 | <0.0001 |
| 8 | 6,8-di-C-Glucosylapigenin | 1.3 | 0.7 | 4.7 | 2.4 | 9.9 | 4.5 | 0.2000 | <0.0001 |
| 9 | HCAPCA | 1.3 | 2.0 | 6.8 | 9.3 | 45.8 | 24.1 | 0.0064 | <0.0001 |
| 10 | Unknown | 1.3 | 2.2 | NA | 6.5 | 114.8 | 31.0 | 0.0206 | 0.0078 |
| 11 | Unknown | 0.9 | 2.7 | 12.3 | 31.8 | 4.0 | 273.6 | 0.0087 | 0.0003 |
| 12 | 2′′-O-xylosylvitexin | 0.9 | 1.0 | 7.2 | 202.4 | 81.9 | 86.6 | 0.0381 | 0.0147 |
| 13 | Unknown | 1.2 | 12.5 | 3.2 | 58.7 | 46.3 | 26.2 | 0.0104 | 0.0008 |
| 14 | Unknown | 1.4 | 1.0 | NA | NA | 10.2 | 4.5 | 0.0513 | 0.0009 |
| 15 | Flavanone-O-rutinoside | 0.6 | 1.4 | 15.4 | 12.7 | 10.3 | 0.3 | 0.0200 | 0.7039 |
| 16 | Luteolin-7-O-rutinoside | 1.4 | 2.8 | 10.9 | 2.2 | 1.5 | 1.3 | 0.0413 | 0.0383 |
| 17 | 8-C-glucosyldiosmetin | 1.4 | 14.8 | 11.0 | 3.6 | 6.5 | 20.0 | 0.6120 | 0.0002 |
| 18 | Flavone-O-rutinoside | 1.1 | 1.7 | 2.3 | 1.6 | 5.3 | 2.0 | 0.2540 | 0.0058 |
| 19 | Diosmin | 1.2 | 1.3 | 4.3 | 1.6 | 2.2 | 2.4 | 0.9260 | 0.0020 |
| 20 | HesperidinPCA | 1.2 | 1.6 | 4.1 | 2.2 | 1.8 | 7.5 | 0.1860 | 0.0006 |
| 21 | Flavanone-O-rutinoside | 1.4 | 3.6 | 5.0 | 0.4 | 2.3 | 10.7 | 0.4840 | 0.1179 |
| 22 | Isosakuranetin rutinoside | 1.2 | 1.8 | 5.5 | 2.9 | 3.4 | 8.2 | 0.2003 | < 0.0001 |
| 23 | Unknown | 1.0 | 1.0 | 3.9 | 2.9 | 1.8 | 16.6 | 0.1849 | 0.0024 |
| 24 | Unknown | 0.8 | 1.0 | 5.4 | 4.9 | 2.0 | 6.4 | 0.8840 | 0.0003 |
| 25 | Unknown | 0.9 | 0.8 | 3.4 | 5.7 | 2.8 | 227.2 | 0.0001 | 0.0095 |
| 26 | Unknown | 0.8 | 1.2 | 4.6 | 4.0 | 2.6 | 1.9 | 0.0520 | 0.0041 |
| 27 | Unknown | 0.7 | 0.6 | 3.2 | 4.2 | 3.5 | 1.3 | 0.1740 | 0.1055 |
| 28 | Unknown | 0.8 | 1.2 | 4.9 | 7.7 | 2.1 | 3.1 | 0.3660 | 0.0002 |
| 29 | Isosinensetin | 0.7 | 0.4 | 0.8 | 1.1 | 1.5 | 0.2 | 0.4450 | 0.0029b |
| 30 | DesmethylPMF | 0.7 | 0.7 | 1.8 | 3.4 | 4.2 | 0.8 | 0.6540 | 0.5315 |
| 31 | SinensetinPCA | 0.8 | 1.2 | 4.8 | 4.7 | 2.4 | 10.3 | 0.0001 | 0.0037 |
| 32 | DesmethylPMF | 0.7 | 1.4 | 4.8 | 2.9 | 2.9 | 19.3 | 0.0019 | 0.0030 |
| 33 | Nobiletin | 0.8 | 0.6 | 1.5 | 2.2 | 1.9 | 0.6 | 0.2510 | 0.4680 |
| 34 | Tetramethyl-O-ScutellareinPCA | 0.9 | 1.1 | 6.0 | 4.3 | 2.0 | 2.0 | 0.0223 | 0.0082 |
| 35 | Unknown | 0.7 | 0.6 | 2.1 | 2.6 | 3.4 | 0.7 | 0.4580 | 0.7399 |
| 36 | Heptamethoxyflavone | 0.7 | 0.9 | 3.1 | 2.6 | 2.7 | 0.8 | 0.1890 | 0.2531 |
| 37 | DesmethylPMFPCA | 0.9 | 1.5 | 5.7 | 2.9 | 2.4 | 14.3 | 0.0136 | 0.0003 |
| 38 | TangeretinPCA | 0.9 | 0.7 | 2.1 | 2.8 | 2.2 | 1.0 | 0.2340 | 0.1070 |
| 39 | DesmethylPMF | 0.8 | 0.5 | 1.0 | 1.2 | 1.4 | 0.3 | 0.2018 | 0.0117b |
| 40 | 5-Desmethylnobiletin | 0.8 | 0.6 | 1.4 | 2.6 | 2.3 | 0.7 | 0.2590 | 0.8380 |
Bolded ratio means that the averages of these compounds in CLas-inoculated trees (n = 5) were significantly different (p-value < 0.05) from the controls (n = 3).
a p-value for group effect and time effect for the whole model (all sampling dates) using ANCOVA analysis.
bThe average of the relative amount of these compounds in all sampling dates was significantly lower in CLas-inoculated plants. P-values (in the same column) lower than 0.05 without any superscript are higher in CLas-inoculated plants.
PCA Compound with loading > 0.2 from principal component analysis, hence, accounts for group clustering.
NA means that the level of the compound was below the detection limit.
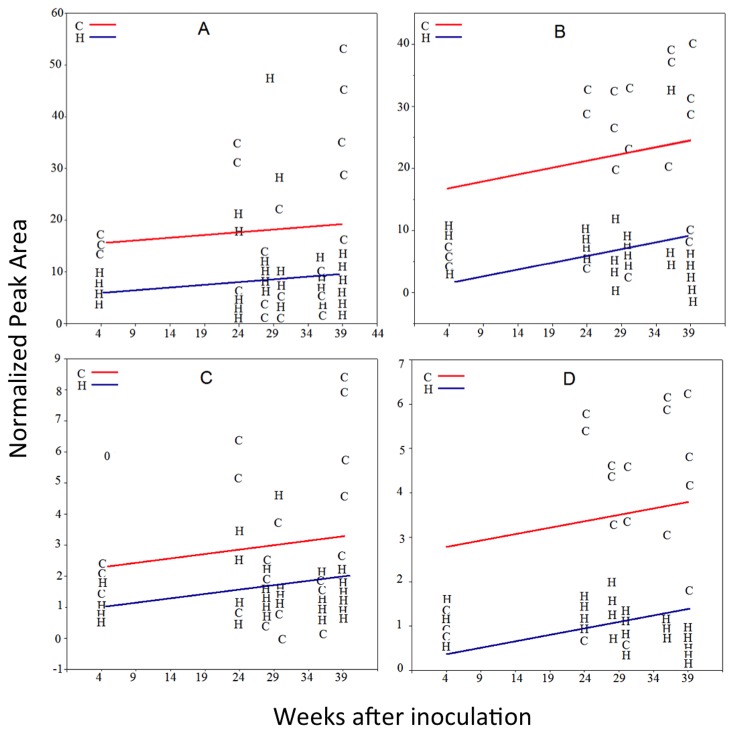
In ‘Hamlin’ trees, compounds 5, 16, 22, 27, and 32 occurred at significantly higher levels at week 3 in leaves of Clas-inoculated trees than in control leaves (Table 6). Because the ratios of these compounds in Clas-inoculated trees were slightly higher than those from the control and none of these compounds was significantly higher at 23 weeks after inoculation, these differences are not likely to be caused by Clas infection. Slight variation in leaf metabolite levels could result from differences in leaf maturity and leaf age. At week 23, there was no significant difference in any leaf metabolite, and only a slight increase (20%) in the relative amount of compound 2 in leaves from Clas-inoculated ‘Hamlin’ trees was observed 2 weeks later. At week 38, where the leaf symptoms were clearly evident, dramatic increases in the numbers of compounds with significantly different means between the HLB-affected and control leaves occurred. The number of compounds that was induced in the HLB-affected ‘Hamlin’ leaves was less than those induced in the HLB-affected ‘Valencia’ leaves, yet those compounds observed in the HLB-affected ‘Hamlin’ leaves matched the compounds found at earlier dates in ‘Valencia’ trees. In agreement with the later development of visual leaf symptom in the Clas-infected trees, changes in metabolite profiles appeared later in ‘Hamlin’ leaves than those in ‘Valencia’. Table 6 highlights the metabolites that were higher in leaves from Clas-infected ‘Hamlin’ plants than in leaves from controls. The flavone glycoside (17) was 32 times more concentrated at week 35, and the putative HCAs (6 and 7) were more than 10 times in leaves from HLB-infected ‘Hamlin’ trees 38 weeks after inoculation. A number of the PMFs and PMF-derivatives, 29, 36, and 39 occurred at significantly lower levels in leaves of the HLB-affected trees at week 27, and compounds 29, 33, 36 , and 39 occurred at significantly lower levels in leaves of HLB-affected trees at week 38 (Table 6). The abundance of compounds 29 and 39 against time in control and Clas-inoculated trees is shown in Figure 5. Decreased levels of PMFs were similarly reported in field-grown HLB-symptomatic ‘Valencia’ and ‘Midsweet’ orange leaves [14].
Table 6. The relative amounts of metabolites from CLas-inoculated ‘Hamlin’ trees with respect to control ‘Hamlin’ trees.
| Peak no. | Compound | Week 3 | Week 23 | Week 27 | Week 29 | Week 35 | Week 38 | Time effect (p-value)a | Group effect (p-value)a |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Unknown | 1.2 | 0.6 | 9.2 | 1.5 | 41.5 | 1.0 | 0.9890 | 0.0900 |
| 2 | Unknown | 1.0 | 0.8 | 1.2 | 2.1 | 1.0 | 1.6 | 0.0050 | 0.7300 |
| 3 | Unknown | 1.3 | 0.7 | 1.3 | 1.4 | 2.1 | 1.2 | 0.0149 | 0.1673 |
| 4 | Feruloylputrescinea | 2.0 | 1.1 | 16.4 | 7.9 | 41.1 | 1.7 | 0.0210 | 0.0137 |
| 5 | HCA | 8.7 | 1.1 | 0.9 | NA | 1.0 | 5.2 | 0.0187 | 0.0059 |
| 6 | HCAPCA | 4.5 | 11.4 | 14.0 | 7.0 | 17.0 | 33.0 | 0.0128 | 0.0006 |
| 7 | HCAPCA | 4.1 | 43.3 | 4.3 | 4.8 | 88.9 | 12.0 | 0.0007 | 0.0001 |
| 8 | 6,8-di-C-Glucosylapigenina | 2.9 | 1.9 | 2.0 | 1.5 | 13.5 | 1.8 | 0.4298 | 0.0007 |
| 9 | HCAPCA | 2.6 | 14.5 | 2.8 | 3.2 | 39.3 | 7.2 | 0.0183 | 0.0001 |
| 10 | Unknown | 1.8 | 1.3 | 0.3 | 3.6 | 52.0 | 6.0 | 0.0907 | 0.0132 |
| 11 | Unknown | 2.0 | 8.1 | 36.2 | 4.2 | 146.8 | 6.9 | 0.0180 | 0.0005 |
| 12 | 2′′-O-xylosylvitexin | 2.5 | 2.0 | NA | 4.5 | 355.3 | 23.6 | 0.0660 | 0.0194 |
| 13 | Unknown | 1.9 | 2.4 | 1.1 | 4.3 | 62.1 | 9.6 | 0.0936 | 0.0123 |
| 14 | Unknown | 1.6 | 1.4 | NA | 2.0 | 17.3 | 5.4 | 0.4280 | 0.0084 |
| 15 | Flavanone-O-rutinoside | 3.7 | 15.6 | 0.3 | 0.2 | 1.3 | 1.0 | 0.0180 | 0.1520 |
| 16 | Luteolin-7-O-rutinoside | 3.2 | 1.6 | 0.4 | 1.4 | 3.6 | 1.9 | 0.0952 | 0.0327 |
| 17 | 8-C-glucosyldiosmetin | 2.5 | 1.5 | 4.4 | 2.4 | 32.0 | 2.6 | 0.8600 | 0.0017 |
| 18 | Flavone-O-rutinoside | 1.5 | 3.0 | 1.3 | 1.3 | 2.4 | 2.9 | 0.0605 | 0.0047 |
| 19 | Diosmin | 1.7 | 0.9 | 1.2 | 1.5 | 3.7 | 1.9 | 0.5138 | 0.0035 |
| 20 | HesperidinPCA | 1.8 | 1.1 | 5.8 | 2.0 | 14.1 | 2.0 | 0.5210 | 0.0008 |
| 21 | Flavanone-O-rutinoside | 1.8 | 0.9 | NA | 2.2 | 11.9 | 2.3 | 0.7970 | 0.0018 |
| 22 | Isosakuranetin rutinoside | 1.7 | 0.1 | NA | 1.4 | 13.9 | 2.0 | 0.4800 | 0.7350 |
| 23 | Unknown | 1.1 | 1.6 | 4.7 | 1.3 | 7.8 | 2.1 | 0.0030 | 0.0010 |
| 24 | Unknown | 1.4 | 1.5 | 2.6 | 1.9 | 2.2 | 1.1 | 0.0932 | 0.0001 |
| 25 | Unknown | 0.9 | 2.4 | 130.0 | 2.3 | 225.1 | 2.4 | 0.0010 | 0.0039 |
| 26 | Unknown | 1.2 | 0.7 | 1.8 | 1.6 | 2.9 | 0.7 | 0.0724 | 0.2864 |
| 27 | Unknown | 2.1 | 0.8 | 0.7 | 1.7 | 1.4 | 0.5 | 0.6949 | 0.9887 |
| 28 | Unknown | 1.6 | 0.8 | 1.3 | 2.9 | 3.2 | 1.8 | 0.2536 | 0.0006 |
| 29 | Isosinensetin | 1.2 | 0.5 | 0.2 | 0.3 | 0.3 | 0.2 | 0.0951 | 0.0010b |
| 30 | DesmethylPMF | 1.5 | 0.8 | 1.1 | 0.5 | 0.6 | 0.8 | 0.1669 | 0.4856 |
| 31 | SinensetinPCA | 1.2 | 2.1 | 3.3 | 1.7 | 4.2 | 1.1 | 0.0010 | 0.0027 |
| 32 | DesmethylPMF | 1.5 | 1.3 | 2.6 | 1.9 | 15.6 | 1.7 | 0.0003 | 0.0003 |
| 33 | Nobiletin | 1.2 | 0.7 | 0.8 | 0.4 | 0.4 | 0.4 | 0.9670 | 0.0030b |
| 34 | Tetramethyl-O-ScutellareinPCA | 1.1 | 1.2 | 1.6 | 2.1 | 11.6 | 1.4 | 0.0003 | 0.0023 |
| 35 | Unknown | 1.3 | 0.7 | 2.0 | 0.6 | 0.7 | 0.7 | 0.6110 | 0.9910 |
| 36 | Heptamethoxyflavone | 1.4 | 0.7 | 0.4 | 0.6 | 0.2 | 0.2 | 0.9960 | 0.0014b |
| 37 | DesmethylPMFPCA | 1.1 | 1.8 | 3.7 | 2.1 | 6.0 | 1.5 | 0.0043 | 0.0010 |
| 38 | TangeretinPCA | 1.1 | 0.7 | 1.9 | 0.5 | 1.5 | 0.5 | 0.5310 | 0.0013b |
| 39 | DesmethylPMF | 1.1 | 0.5 | 0.3 | 0.5 | 0.4 | 0.2 | 0.1100 | 0.0001b |
| 40 | 5-Desmethylnobletin | 1.1 | 0.6 | 1.0 | 0.4 | 0.3 | 0.4 | 0.1530 | 0.0260 |
Bolded ratio means that the averages of these compounds in CLas-inoculated trees (n = 5) were significantly different (p-value < 0.05) from the controls (n = 3).
a p-value for group effect and time effect for the whole model (all sampling dates) using ANCOVA analysis.
bThe average of the relative amount of these compounds in all sampling dates was significantly lower in CLas-inoculated plants. P-values (in the same column) lower than 0.05 without any superscript are higher in CLas-inoculated plants.
PCA Compound with loading > 0.2 from principal component analysis, hence, accounts for group clustering.
NA means that the level of the compound was below the detection limit.
Figure 5. Relative amounts versus time (week) of different citrus leaf metabolites from controls and CLas-inoculated trees.
A) unknown 29 in ‘Valencia’ leaves; B) unknown 29 in ‘Hamlin’ leaves; C) unknown 39 in ‘Valencia’ leaves; D) unknown 39 in ‘Hamlin’ leaves.
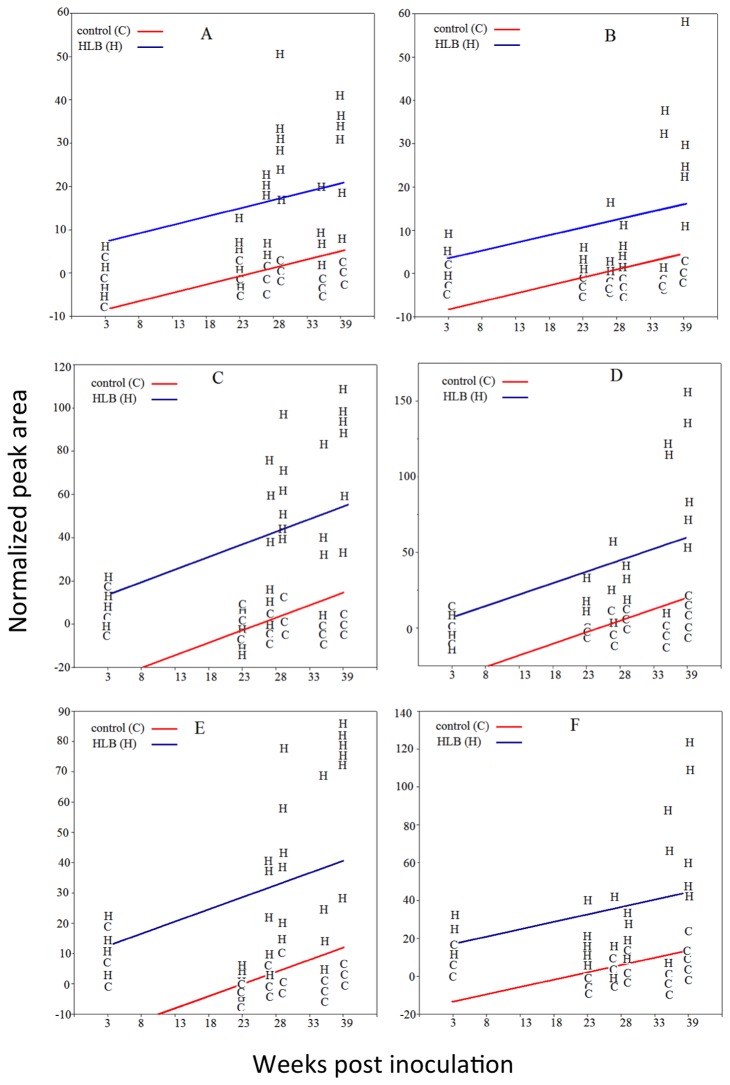
The t-test results support the findings made by PCA, particularly pertaining to the putative HCAs, 6, 7, and 9. As shown in Figure 3A and Figure 6, the levels of these compounds were higher in leaves from Clas-infected ‘Valencia’ and ‘Hamlin’ trees, and their levels increased with time and symptoms progression. These elevated levels of HCAs in the HLB-affected trees are consistent with findings reported earlier with mature field-grown ‘Valencia’ and ‘Midsweet’ orange trees [14]. At week 3, the levels of these putative HCAs in leaves from HLB-infected trees were equal to those from controls. However, the levels of these compounds were higher in leaves from Clas-infected trees at 27, 29, 35, and 38 weeks after inoculation.
ANCOVA analysis (columns 8 and 9 in Table 5 and Table 6) showed that most of the detected leaf metabolites were significantly affected by Clas infection. The use of ANCOVA analysis was necessary to account for the effect of the covariant (time) and to reduce the error that was caused by the none-even distribution of plant response. ANCOVA analysis showed that time effect was significant in the model, especially for those metabolites that were significantly affected by Clas infection. The ANCOVA analysis showed that 28 compounds were significantly higher in leaves from Clas-inoculated ‘Valencia’ plants, 2 metabolites (29 and 39) were lower, and 10 were not affected. Similar effects were also observed in ‘Hamlin’ leaf metabolites. Twenty-seven metabolites were higher in leaves from Clas-inoculated ‘Hamlin’ trees, five metabolites were lower (29, 33, 36, 38, and 39), and eight metabolites were not affected.
The results from the ANCOVA analysis were consistent with t-test and PCA results. For example, the t-test showed that ratios of the relative abundance of metabolite 29 in leaves from Clas-inoculated plants were always less than 1 at one or more sampling dates (Table 5 and Table 6). The ANCOVA analysis of the whole data set for ‘Hamlin’ and ‘Valencia’ leaves also showed that compound 29 was significantly lower in Clas-inoculated plants (Table 5 and Table 6 and Figure 5). Metabolites 6, 7, and 9 are another example. The t-test showed that the mean of these metabolites in Clas-inoculated trees was significantly higher than those in the controls at one or more sampling dates (Table 5 and Table 6). In agreement with the t-test results, the ANCOVA analysis showed that these compounds were also higher in Clas-infected plants (Table 5 and Table 6 and Figure 6). In addition, the ANCOVA analysis confirmed the PCA results. For example, the PCA analysis showed that unknowns 6, 7, and 9 were responsible for group clustering and the ANCOVA analysis showed that these metabolites were significantly higher in Clas-inoculated trees (p-value < 0.001).
Figure 6. Relative amounts versus time (week) of different citrus leaf metabolites from controls and CLas-inoculated trees.
A) unknown 6 in ‘Valencia’ leaves; B) unknown 6 in ‘Hamlin’ leaves; C) unknown 7 in ‘Valencia’ leaves; D) unknown 7 in ‘Hamlin’ leaves; E) unknown 9 in ‘Valencia’ leaves; F) unknown 9 in ‘Hamlin’ leaves.
In agreement with our results, change in secondary metabolites has been observed in many plants after pathogen infection. A significant increase in concentrations of phenolic compounds was observed in xylem tissues of grapevines 2 months after inoculation with Xylella fastidiosa [30]. The increase in phenolic compounds suggested that the cell wall of the infected plant becomes thicker and more resistant to degradation by Xylella fastidiosa [30]. Higher levels of several amino acids, phenolic compounds, reducing sugars, and defense enzymes were also observed in ‘Candidatus Liberibacter solanacearum’-infected potato tubers [4]. Tubers that were infected 5 weeks before harvest showed higher levels than those infected one week before harvest. No correlation between elevated compounds and ‘Candidatus Liberibacter solanacearum’ titer was observed except for phenolics. However, there was a strong association between biochemical response and symptom severity [4].
Our results showed that hydroxycinnamate compounds were induced in leaves from ‘Hamlin’ and ‘Valencia’ sweet orange trees in response to Clas infection. In fact, accumulation of phenylpropanoids (hydroxycinnamic acid derivatives) was also observed in tomato plant infected with the bacterial pathogen Pseudomonas syringae pv. Tomato [31,32]. In addition, an accumulation of p-coumaric, caffeic, and ferulic acids mainly forming esters with glucose has been observed in cucumber infected with prunus necrotic ringspot virus or melon infected with melon necrotic spot virus [33]. In agreement with the induction of the phenylpropanoids observed, both viral infections studied induced the expression of cinnamic acid 4-hydroxylase (C4H) and phyenylalanine ammonia-lyase (PAL) genes. Because an increase of p-coumaric, caffeic, and ferulic acids was also observed in leaves from cucumber treated with powdery mildew [34], Bellés et al. [33] suggested that accumulation of HCAs is involved in plant defense against different biotic stresses.
The involvement of feruloyl HCAs as components of plant responses against microbial attacks has been previously studied [31,35]. The molecular actions of HCAs in plant defenses are not well-defined, but it has been proposed that they can be incorporated into the plant cell wall to strengthen it against microbial attack, can act as direct antimicrobial agents, or may act as signal molecules [36]. HCAs are produced by the activation of the phenylpropanoid biosynthetic pathway which is induced in response to biotic and abiotic stress [31]. The first step in the phenylpropanoid pathway is the conversion of phenylalanine to trans-cinnamic acid (phenylpropenoic acid) by phenylalanine ammonia-lyase (PAL) [31]. In the second step, cinnamic acid is being converted to 4-hydroxycinnamic acid by cinnamate 4-hydroxylase (C4H) [31]. The hydroxycinnamic acids (p-coumaric and ferulic) serve as a precursor for many phenylpropanoid derivatives with antimicrobial function [31].
In conclusion, this study showed that sweet orange leaf metabolites were significantly affected by Clas infection. The earliest shift in metabolite profiles of leaves from Clas-infected trees was noticed 27 weeks after inoculation. Most of the detected metabolites increased with time, while few decreased. The abundance of some metabolites like HCAs increased more than 10-fold in leaves from Clas-infected trees, whereas the abundance of some metabolites like isosinensetin and desmethylPMF decreased more than 3-fold. The increase in HCAs and other metabolite levels in the leaves of Clas-infected plants may be potentially linked to sweet orange’s response to the Clas infection. Further studies are needed to examine the effects of Clas infection on both resistant and susceptible cultivars to test whether the induced metabolites are related with plant resistance.
Acknowledgments
We want to thank Ms. Veronica Cook for technical assistance and assistance with manuscript preparation, and we gratefully acknowledge Dr. Nabil Killiny for critical reading of the manuscript and helpful discussions.
Funding Statement
This research was funded by the USDA CSREES NIFA project number 2009-34446-20228. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gottwald TR (2010) Current epidemiological understanding of citrus huanglongbing. In: VanAlfen NK, Bruening G, Leach JE. Annu Rev Phytopathol 48: 119–139. [DOI] [PubMed] [Google Scholar]
- 2. Bové JM (2006) Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J Plant Pathol 88: 7–37. [Google Scholar]
- 3. Batool A, Iftikhar Y, Mughal SM, Khan MM, Jaskani MJ et al. (2007) Citrus greening disease – A major cause of citrus decline in the world: A review. J Hort_Sci 34: 159–166. [Google Scholar]
- 4. Rashed A, Wallis CM, Paetzold L, Workneh F, Rush CM (2013) Zebra chip disease and potato biochemistry: Tuber physiological changes in response to ‘Candidatus Liberibacter solanacearum’ infection over time. Phytopathology 103: 419–426. doi: 10.1094/PHYTO-09-12-0244-R. PubMed: 23425237. [DOI] [PubMed] [Google Scholar]
- 5. Lin H, Gudmestad NC (2013) Aspects of pathogen genomics, diversity, epidemiology, vector dynamics, and disease management for a newly emerged disease of potato: Zebra chip. Phytopathology 103: 524–537. doi: 10.1094/PHYTO-09-12-0238-RVW. PubMed: 23268582. [DOI] [PubMed] [Google Scholar]
- 6. Folimonova SY, Achor DS (2010) Early events of citrus greening (huanglongbing) disease development at the ultrastructural level. Phytopathology 100: 949–958. doi: 10.1094/PHYTO-100-9-0949. PubMed: 20701493. [DOI] [PubMed] [Google Scholar]
- 7. Coletta-Filho HD, Carlos EF, Alves KCS, Pereira MAR, Boscariol-Camargo RL et al. (2010) In planta multiplication and graft transmission of ‘Candidatus Liberibacter asiaticus’ revealed by real-time PCR. Eur J Plant Pathol 126: 53–60. doi: 10.1007/s10658-009-9523-2. [DOI] [Google Scholar]
- 8. Tatineni S, Sagaram US, Gowda S, Robertson CJ, Dawson WO et al. (2008) In planta distribution of ‘Candidatus Liberibacter asiaticus’ as revealed by polymerase chain reaction (PCR) and real-time PCR. Phytopathology 98: 592–599. doi: 10.1094/PHYTO-98-5-0592. PubMed: 18943228. [DOI] [PubMed] [Google Scholar]
- 9. Baldwin E, Plotto A, Manthey J, McCollum G, Bai J et al. (2010) Effect of Liberibacter infection (huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: Chemical and physical analyses. J Agr Food Chem 58: 1247–1262. PubMed: 20030384. [DOI] [PubMed] [Google Scholar]
- 10. Slisz AM, Breksa AP III, Mishchuk DO, McCollum G, Slupsky CM (2012) Metabolomic analysis of citrus infection by ‘Candidatus Liberibacter’ reveals insight into pathogenicity. J Proteome Res 11: 4223–4230. doi: 10.1021/pr300350x. PubMed: 22698301. [DOI] [PubMed] [Google Scholar]
- 11. Takushi T, Toyozato T, Kawano S, Taba S, Taba K et al. (2007) Scratch method for simple, rapid diagnosis of citrus huanglongbing using iodine to detect high accumulation of starch in the citrus leaves. Jpn. J Phytopathol 73: 3–8. doi: 10.3186/jjphytopath.73.3. [DOI] [Google Scholar]
- 12. Hooker ME, Lee RF, Civerolo EL, Wang SY (1993) Reliability of gentisic acid, a fluorescent marker, for diagnosis of citrus greening disease. Plant Dis 77: 174–180. doi: 10.1094/PD-77-0174. [DOI] [Google Scholar]
- 13. Cevallos-Cevallos J, Etxeberria E, Rouseff R, Reyes-De-Corcuera J (2008) Metabolite profiling of healthy and huanglongbing-infected citrus leaves. Proc Fla State Hort Soc 121 pp. 85–89. [Google Scholar]
- 14. Manthey J (2008) Differences in secondary metabolites in leaves from orange (Citrus sinensis L.) trees affected with greening disease (Huanglongbing). Proc Fla State Hort Soc 12: 285–288. [Google Scholar]
- 15. Cevallos-Cevallos JM, Rouseff R, Reyes-De-Corcuera JI (2009) Untargeted metabolite analysis of healthy and huanglongbing-infected orange leaves by CE-DAD. Electrophoresis 30: 1240–1247. doi: 10.1002/elps.200800594. PubMed: 19283697. [DOI] [PubMed] [Google Scholar]
- 16. Cevallos-Cevallos JM, García-Torres R, Etxeberria E, Reyes-De-Corcuera JI (2011) GC‑MS analysis of headspace and liquid extracts for metabolomic differentiation of citrus huanglongbing and zinc deficiency in leaves of ‘Valencia’ sweet orange from commercial groves. Phytochem Anal 22: 236–246. doi: 10.1002/pca.1271. PubMed: 21046688. [DOI] [PubMed] [Google Scholar]
- 17. Cevallos-Cevallos JM, Futch DB, Shilts T, Folimonova SY, Reyes-De-Corcuera JI (2012) GC-MS metabolomic differentiation of selected citrus varieties with different sensitivity to citrus huanglongbing. Plant Physiol Biochem 53: 69–76. doi: 10.1016/j.plaphy.2012.01.010. PubMed: 22326359. [DOI] [PubMed] [Google Scholar]
- 18. Mabry TJ, Markham KR, Thomas MB (1970) The systematic identification of flavonoids. New York: Springer-Verlag. 354 pp. [Google Scholar]
- 19. Kanes K, Tisserat B, Berhow M, Vandercook C (1993) Phenolic composition of various tissues of rutaceae species. Phytochemistry 32: 967–974. doi: 10.1016/0031-9422(93)85237-L. [DOI] [Google Scholar]
- 20. Manthey JA, Grohmann K, Berhow MA, Tisserat B (2000) Changes in citrus leaf flavonoid concentrations resulting from blight-induced zinc-deficiency. Plant Physiol Biochem 38: 333–343. doi: 10.1016/S0981-9428(00)00748-8. [DOI] [Google Scholar]
- 21. Risch B, Herrmann K, Wray V, Grotjahn L (1987) 2’-(e)-o-para-coumaroylgalactaric acid and 2’-(e)-o-feruloylgalactaric acid in citrus. Phytochemistry 26: 509–510. doi: 10.1016/S0031-9422(00)81444-2. [DOI] [Google Scholar]
- 22. Risch B, Herrmann K, Wray V (1988) (E)-0-p-coumaroyl-, (E)-0-feruloyl-derivatives of glucaric acid in citrus. Phytochemistry 27: 3327–3329. doi: 10.1016/0031-9422(88)80059-1. [DOI] [Google Scholar]
- 23. Strack D, Engela U, Weissenböcka G, Grotjahnb L, Wray V (1986) Ferulicacidesters of sugarcarboxylicacids from primary leaves of rye. Phytochemistry 25: 2605-2608. doi: 10.1016/S0031-9422(00)84518-5. [DOI] [Google Scholar]
- 24. Duenas M, Fernandez D, Hernandez T, Estrella I, Munoz R (2005) Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J Sci Food Agric 85: 297–304. doi: 10.1002/jsfa.1924. [DOI] [Google Scholar]
- 25. Wheaton TA, Stewart I (1965a) Quantitative analysis of phenolic amines using ion-exchange chromatography. Anal Biochem 12: 585–592. doi: 10.1016/0003-2697(65)90226-5. PubMed: 5323674. [DOI] [PubMed] [Google Scholar]
- 26. Wheaton TA, Stewart I (1965b) Feruloylputrescine: Isolation and identification from citrus leaves and fruit. Nature 206: 620–621. doi: 10.1038/206620a0. PubMed: 5832837. [DOI] [PubMed] [Google Scholar]
- 27. Manthey JA, Grohmann K (2001) Phenols in citrus peel byproducts: Concentrations of hydroxycinnamates and polymethoxylated flavones in citrus peel molasses. J Agr Food Chem 49: 3268–3273. [DOI] [PubMed] [Google Scholar]
- 28. De Vos RCH, Moco S, Lommen A, Keurentjes JJB, Bino RJ et al. (2007) Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat Protoc 2: 778–791. doi: 10.1038/nprot.2007.95. PubMed: 17446877. [DOI] [PubMed] [Google Scholar]
- 29. Dettmer K, Aronov PA, Hammock BD (2007) Mass spectrometry-based metabolomics. Mass Spectrom Rev 26: 51–78. doi: 10.1002/mas.20108. PubMed: 16921475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wallis CM, Chen J (2012) Grapevine phenolic compounds in xylem sap and tissues are significantly altered during infection by Xylella fastidiosa . Phytopathology 102: 816–826. doi: 10.1094/PHYTO-04-12-0074-R. PubMed: 22671027. [DOI] [PubMed] [Google Scholar]
- 31. López-Gresa MP, Torres C, Campos L, Lison P, Rodrigo I et al. (2011) Identification of defence metabolites in tomato plants infected by the bacterial pathogen Pseudomonas syringae . Environ Exp Bot 74: 216–228. doi: 10.1016/j.envexpbot.2011.06.003. [DOI] [Google Scholar]
- 32. López-Gresa MP, Maltese F, Bellés JM, Conejero V, Kim HK et al. (2010) Metabolic response of tomato leaves upon different plant-pathogen interactions. Phytochem Anal 21: 89–94. doi: 10.1002/pca.1179. PubMed: 19866456. [DOI] [PubMed] [Google Scholar]
- 33. Bellés JM, Lopez-Gresa MP, Fayos J, Pallas V, Rodrigo I et al. (2008) Induction of cinnamate 4-hydroxylase and phenylpropanoids in virus-infected cucumber and melon plants. Plant Sci 174: 524–533. doi: 10.1016/j.plantsci.2008.02.008. [DOI] [Google Scholar]
- 34. Daayf M, Ongena M, Boulanger R, El Hadrami I, Be´langer RR (2000) Induction of phenolic compounds in two cultivars of cucumber by treatment of healthy and powdery mildew-infected plants with extracts of Reynoutria sachalinensis . J Chem Ecol 26: 1579–1593. doi: 10.1023/A:1005578510954. [DOI] [Google Scholar]
- 35. Pearce G, Marchand PA, Griswold J, Lewis NG, Ryan CA (1998) Accumulation of feruloyltyramine and p-Coumaroyltyramine in tomato leaves in response to wounding. Phytochemistry 47: 659–664. doi: 10.1016/S0031-9422(97)00620-1. [DOI] [Google Scholar]
- 36. von Roepenack-Lahaye E, Newman MA, Schornack S, Hammond-Kosack KE, Lahaye T et al. (2003) p-Coumaroylnoradrenaline, a novel plant metabolite implicated in tomato defense against pathogens. J Biol Chem 278: 43373–43383. doi: 10.1074/jbc.M305084200. PubMed: 12900412. [DOI] [PubMed] [Google Scholar]