Abstract
While free-living protists are usually subjected to hyposmotic environments, parasitic protists are also in contact with hyperosmotic habitats. Recent work in one of these parasites, Trypanosoma cruzi, has revealed that its contractile vacuole complex, which usually collects and expels excess water as a mechanism of regulatory volume decrease after hyposmotic stress, has also a role in cell shrinking when the cells are submitted to hyperosmotic stress. Trypanosomes also have an acidic calcium store rich in polyphosphate (polyP), named the acidocalcisome, which is involved in their response to osmotic stress. Here, we review newly emerging insights on the role of acidocalcisomes and the contractile vacuole complex in the cellular response to hyposmotic and hyperosmotic stresses. We also review the current state of knowledge on the composition of these organelles and their other roles in calcium homeostasis and protein trafficking.
1. Introduction
Volume regulation is a homeostatic mechanism present in all cells. With exception of those having a cell wall, most cells respond to osmotic changes by swelling or shrinking through the activation of a number of transporters and metabolic pathways that return the cells to their original volume, and gene expression changes that adapt the cells to the new environmental conditions. These physiological adaptations to osmotic stress have been studied extensively in a wide variety of vertebrate cell types.
Upon exposure to a reduction in external osmolarity, cells initially swell but soon regain nearly normal volume by a process that has been termed the Regulatory Volume Decrease (RVD), which is accompanied by the efflux of various inorganic ions (such as Na+ and K+) and organic osmolytes, including glycerophosphorylcholine, sorbitol, inositol, betaine, and amino acids. During the response to hyposmotic stress, all of these can be released to the extracellular medium to various degrees in different cell types (Rohloff and Docampo, 2008). By far the most functionally significant efflux, in terms of total contribution to RVD, seems to involve the amino acids, particularly the β-amino acid taurine. Efflux is hypothesized to occur through a non-specific, volume-sensitive organic osmolyte anion channel (VSOAC) that can mediate the efflux of both organic osmolytes and inorganic ions (Lang et al., 1998b). The molecular candidates for this VSOAC channel are numerous, although it is unlikely that only a single channel is responsible for all observations across multiple cell types (Furst et al., 2002). It should also be kept in mind that, in many of the best-characterized vertebrate systems, the contribution of inorganic ion efflux to the RVD far exceeds that of organic osmolytes (Lang et al., 1998b).
On the other hand, upon exposure to an elevation in external osmolarity, cells shrink and then regain normal volume by a process called Regulatory Volume Increase (RVI). However, this RVI response seems not to be a general process; a number of cell types from mammals have been shown not to regain its original volume after hyperosmotic stress, at least within an hour (see (O'Neill, 1999) for a review). Following these initial changes gene expression changes also occur in a variety of cells, which are necessary for their adaptation to the new osmotic conditions (Alfieri and Petronini, 2007; Causton et al., 2001; Gasch et al., 2000).
In contrast to vertebrate cells most protists live in environments of low osmolarity and their mechanism of volume regulation appears to differ. While protists with a rigid cell wall resist swelling in these environments, several protists devoid of cell wall possess a contractile vacuole complex (CVC), which accumulates and expels excess water. Some protists also need to deal with high osmolarity conditions under certain circumstances, and a role for the CVC under these conditions has also been demonstrated (Li et al., 2011).
Recent work in several protists has revealed a link between the CVC and the organelles named the acidocalcisomes (Docampo et al., 2011). Acidocalcisomes are acidic calcium stores rich in polyphosphate (polyP), a polymer of few to hundreds of phosphate units, whose function in osmoregulation has been better studied in trypanosomatid parasites (Docampo and Moreno, 2011). These parasites alternate between an insect vector and a mammalian host, where the parasites are exposed not only to low but also to high osmolarities.
This review provides an overview of the cellular and molecular events underlying the role of acidocalcisomes and the CVC in volume homeostasis in Trypanosoma cruzi, the etiological agent of Chagas disease, with additional references to similarities and differences with other protists.
2. Acidocalcisomes in Protists
2.1. History
In 1992 a research article on calcium homeostasis in Dictyostelium discoideum (Rooney and Gross, 1992) reported the presence of a Ca2+-ATPase in organelles called the acidosomes, which were thought to be part of the contractile vacuole apparatus of this slime mold. The name acidosome was used because they are acidic as indicated by their sensitivity to nigericin (a K+/H+ ionophore). Nigericin-sensitive calcium compartments had also been described in Leishmania donovani (Philosoph and Zilberstein, 1989), as well as in Trypanosoma brucei (Ruben et al., 1991). We therefore tested, in permeabilized cells, whether it was possible to detect a Ca2+-ATPase activity in the calcium-containing acidic compartment of T. brucei. We demonstrated the presence of proton uptake sensitive to vacuolar ATPase (V-H+-ATPase) inhibitors, Ca2+ uptake sensitive to vanadate (Ca2+-ATPase), and the presence of organelles in these parasites that stained with Acridine Orange and were responsible for the responses to inhibitors and ionophores. We named these organelles the acidocalcisomes to indicate that they are acidic and contain calcium (Vercesi et al., 1994). Further work in T. cruzi, now using intact cells loaded with Fura-2, a calcium dye indicator, allowed the physiological characterization of these organelles (Docampo et al., 1995).
An important aspect of this early work was the identification of acidocalcisomes at the ultrastructural level. The best candidates were the polyP granules. These had been described very early in trypanosomes when they were known as volutin granules (Swellengrebel, 1908). Ormerod’s work in the 1950s characterized them very well from a morphological point of view (Ormerod, 1958). Although they were known as polyP granules nobody had ever documented in trypanosomes that they actually contained polyP. However, work by Vickerman and Tetley (Vickerman and Tetley, 1977), and later by Dvorak et al. (Dvorak et al., 1988) and LeFurgey et al. (LeFurgey et al., 1990), using X-ray microanalysis, had described the presence of large amounts of calcium and phosphorus in these granules. By doing incubations of intact trypanosomes, with and without nigericin, and using quick freezing, ultracryomicrotomy, and electron probe microanalysis, the problem was solved: the granules that contained calcium increased their K+ concentration after nigericin treatment indicating that they were acidic and that polyP granules and acidocalcisomes were the same entity (Scott et al., 1997).
The nature of the abundant phosphorus compounds present in acidocalcisomes was still a mystery, but the use of 31P-NMR led to the identification of very large amounts of pyrophosphate (PPi) in T. cruzi, which is preferentially localized in the acidocalcisomes (Urbina et al., 1999). This was followed by the identification of short and long chain polyP in cells and isolated acidocalcisomes of different trypanosomatids using 31P-NMR (Moreno et al., 2002; Moreno et al., 2000) and biochemical techniques (Ruiz et al., 2001b).
While this work was going on, the gene of the first pump described in acidocalcisomes, the Ca2+-ATPase, was cloned and the protein co-localized with the vacuolar H+-ATPase (Lu et al., 1998), and was later also cloned and characterized in T. brucei (Luo et al., 2004). The presence of a V-H+-ATPase made the acidocalcisomes look very similar to the plant vacuole that was known to contain both a V-H+-ATPase and a V-H+-pyrophosphatase. This, together with the finding of large amount of PPi in trypanosomes suggested that perhaps acidocalcisomes also had a V-H+-PPase. In fact, a PPi-driven proton uptake was found in permeabilized cells, and the enzyme was localized to acidocalcisomes using antibodies against the V-H+-PPase from plants (Scott et al., 1998). The discovery of this enzyme, which at the time was known to be present only in bacteria and plants, was also important because it was the marker needed to purify the organelle, a process that was developed in T. cruzi (Scott et al., 1998), and later used to isolate acidocalcisomes from T. brucei (Rodrigues et al., 1999a) and L. donovani (Rodrigues et al., 1999b). The gene encoding for this enzyme in T. cruzi was then cloned and functionally expressed in yeast (Hill et al., 2000), and was also studied in T. brucei (Lemercier et al., 2002).
The recent years of acidocalcisome research have been very exciting. The isolation method for these organelles was improved (Salto et al., 2008; Scott and Docampo, 2000; Yagisawa et al., 2009); acidocalcisomes were isolated and characterized in other trypanosomatids (Mendoza et al., 2002; Miranda et al., 2004a; Miranda et al., 2004b; Miranda et al., 2004c; Moraes Moreira et al., 2005; Soares Medeiros et al., 2005), Apicomplexan parasites (Marchesini et al., 2000; Moreno and Zhong, 1996; Ruiz et al., 2004b; Soares Medeiros et al., 2011), Chlamydomonas reinhardtii (Ruiz et al., 2001a), D. discoideum (Marchesini et al., 2002), the bacteria Agrobacterium tumefaciens (Seufferheld et al., 2003) and Rhodospirillum rubrum (Seufferheld et al., 2004), human platelets (Ruiz et al., 2004a), mast cells (Moreno-Sanchez et al., 2012), insect (Motta et al., 2009; Ramos et al., 2011), chicken (Ramos et al., 2010b), and sea urchin (Ramos et al., 2010a) eggs; their chemical composition investigated (Ferella et al., 2008; Salto et al., 2008); a number of pumps, channels and exchangers in their membranes were biochemically characterized and their genes cloned, and expressed (Fang et al., 2007; Huang, 2013; Montalvetti et al., 2004; Rohloff et al., 2004); their biogenesis was studied (Besteiro et al., 2008; de Jesus et al., 2010; Huang et al., 2011; Madeira da Silva and Beverley, 2010); and the investigation of their functional roles was started (Docampo et al., 2011). Some of these studies will be the subject of this review.
2.2. Structure and composition
Acidocalcisomes of protists are in general spherical, and can be detected with dyes that accumulate in acidic vesicles like Acridine Orange (Docampo et al., 1995) or LysoSensor (Seufferheld et al., 2003), or with techniques that detect polyP, such as staining with DAPI (Ruiz et al., 2004a) or with antibodies against the polymer (Ramos et al., 2010a). Their number varies in different species and could reach hundreds in some cells. By standard electron microscopy they appear as empty vacuoles or vacuoles containing a thin layer of dense material or an inclusion that sticks to the inner face of the membrane (Fig. 1). The electron dense material inside acidocalcisomes is better preserved with the use of cryomethods (Scott et al., 1997) where the organelles seem completely filled by an electron dense material. Protist acidocalcisomes are usually about 0.2 µm diameter and distributed at random (Docampo et al., 2005). Leishmania amazonensis (∼0.6 µm) (Rodrigues et al., 2002) and Tetrahymena pyriformis (up to 2–3 µm) (Rosenberg, 1966; Rosenberg and Munk, 1969) possess very large acidocalcisomes.
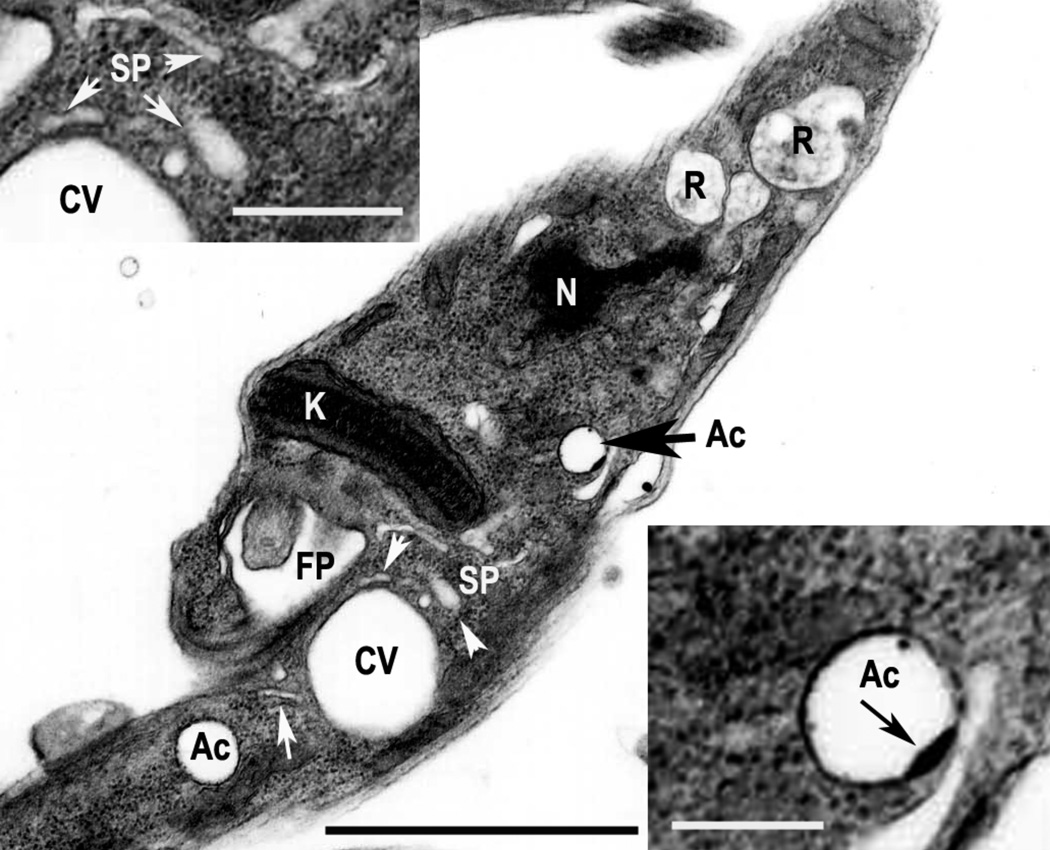
Figure 1. Acidocalcisomes of T. cruzi.
Epimastigotes were observed by transmission electron microscopy. Notations are flagellar pocket (FP), acidocalcisome (Ac), contractile vacuole (CV), spongiome (SP), nucleus (N) and reservosome (R). Insets show the spongiome (left) and one acidocalcisome (right) at higher magnification. Note the electron-dense inclusion (black arrow) in the membrane of the acidocalcisome, which also has an electron dense periphery. White arrows show the tubules of the spongiome. Bar = 2.5 µm (main picture), and 0.2 µm (inset).
Besides polyP, acidocalcisomes of protists also contain orthophosphate (Pi), and PPi. These phosphorus compounds are in close association to cations (sodium, potassium, magnesium, calcium, zinc and iron) and basic amino acids (Docampo et al., 2005; Rohloff et al., 2003). Trypanosomatids are very rich in very short chain polyP such as polyP3, polyP4, and polyP5 (Moreno et al., 2000). Taking into account its total concentration and the relative volume of acidocalcisomes in some of these cells (about 1–2% of the total cell volume), the intraorganellar concentration would be in the molar range (∼ 3 M) (Docampo et al., 2005).
The membrane of the acidocalcisome possesses pumps, antiporters, and channels. Ca2+-ATPases were found in several protists, and the genes encoding some of them were cloned and expressed in yeast to demonstrate their function (Lu et al., 1998; Luo et al., 2004; Luo et al., 2001). The proteins are closely related to the family of plasma membrane calcium ATPases (PMCA). In T. brucei, knockdown of the acidocalcisome Ca2+-ATPase by RNAi results in reduced levels of mobilizable calcium from these stores and impaired growth (Luo et al., 2004). Ablation of the acidocalcisome Ca2+-ATPase from T. gondii also affects growth, and the cells have a serious defect in invasion and virulence (Luo et al., 2001).
Two proton pumps were found in acidocalcisomes of protists. One is the vacuolartype H+-ATPase, a macromolecular complex of 14 subunits (Bowman et al., 2009; Lu et al., 1998; Marchesini et al., 2002; Rodrigues et al., 2000; Ruiz et al., 2001a; Yagisawa et al., 2009), and the other is the V-H+-PPase, a single subunit protein that uses PPi instead of ATP to transport protons (Drozdowicz et al., 2003; Rodrigues et al., 1999a; Ruiz et al., 2001a; Scott et al., 1998; Yagisawa et al., 2009). Only the gene for the T. cruzi V-H+-PPase could be functionally expressed in yeast (Hill et al., 2000). In addition, the N-terminal region of the T. cruzi V-H+-PPase can enhance the functional expression of other V-H+-PPases in yeast (Drake et al., 2010).
There is biochemical evidence for the presence of Na+/H+ and Ca2+/H+ antiporters in acidocalcisomes of some trypanosomatids (Rodrigues et al., 1999b; Vercesi and Docampo, 1996; Vercesi et al., 1997; Vercesi et al., 2000) and T. gondii (Rohloff et al., 2011), and molecular evidence of a Ca2+/H+ antiporter in acidocalcisomes of Neurospora crassa (Bowman et al., 2009). An homolog to a zinc transporter was detected in T. cruzi acidocalcisomes (Ferella et al., 2008). A water channel or aquaporin was also found in acidocalcisomes of T. cruzi (Rohloff et al., 2004). In contrast to the aquaporins of T. brucei, this protein is unable to transport glycerol when expressed in Xenopus oocytes (Montalvetti et al., 2004).
Recently, an inositol 1,4,5-trisphosphate receptor was found in the acidocalcisomes of T. brucei, which is the long sought channel for Ca2+ release from these organelles (Huang, 2013). Finally, a vacuolar transporter chaperone complex (VTC complex) was also detected in the acidocalcisome membrane of trypanosomatids (Fang et al., 2007), T. gondii (Rooney et al., 2011) and the red alga Cyanidioschyzon merolae (Yagisawa et al., 2009). The complex is apparently formed by two proteins, VTC1 (in trypanosomes) or VTC2 (in T. gondii) and VTC4, of which VTC4 is the catalytic subunit that synthesizes and translocates polyP into the acidocalcisomes (Hothorn et al., 2009).
An exopolyphosphatase (Rodrigues et al., 2002), a soluble inorganic pyrophosphatase (Lemercier et al., 2004), and a metacaspase (Lee et al., 2007) have also been localized to acidocalcisomes by immunofluorescence analyses. An acid phosphatase activity was also detected using cytochemical methods (Gomes et al., 2006). A protein with significant sequence identity to proteins of peptidase family M13 (CMP249C), a prenylated Rab receptor (CMJ260C), an ABC transporter (CMS401C), and an o-methyltransferase (CMT369C) were found in acidocalcisomes of C. merolae, using specific antibodies or expression of HA-tagged proteins (Yagisawa et al., 2009). The enzymes and transporters identified in acidocalcisomes of protists are listed in Table 1.
Table 1.
Proteins localized to the acidocalcisomes of different protists
| Protein name | Name of Gene* |
Protist | Reference |
|---|---|---|---|
| Ca2+-ATPase | Tca1 | T. cruzi | (Lu et al., 1998) |
| TbPMC1/TbPMC2 | T. brucei | (Luo et al., 2004) | |
| TgA1 | T. gondii | (Luo et al., 2005) | |
| DdpatA | D. discoideum | (Marchesini et al., 2002) | |
| H+-V-ATPase | T. cruzi | (Lu et al., 1998) | |
| T. brucei | (Vercesi et al., 1994) | ||
| D. discoideum | (Marchesini et al., 2002) | ||
| C. reinhardtii | (Ruiz et al., 2001a) | ||
| T. gondii | (Rohloff et al., 2011) | ||
| N. crassa | (Bowman et al., 2009) | ||
| C. merolae | (Yagisawa et al., 2009) | ||
| H+-V-PPase | TcPPase | T. cruzi | (Hill et al., 2000; Scott et al., 1998) |
| TbVP1 | T. brucei | (Lemercier et al., 2002; Rodrigues et al., 1999a) | |
| L. donovani | (Rodrigues et al., 1999b; Sahin etal., 2008) | ||
| T. gondii | (Drozdowicz et al., 2003; Rodrigues et al., 2000) | ||
| C. merolae | (Yagisawa et al., 2009) | ||
| C. reinhardtii | (Ruiz et al., 2001a) | ||
| Ca2+/H+ exchanger | L. donovani | (Vercesi et al., 2000) | |
| T. brucei | (Vercesi and Docampo, 1996; Vercesi et al., 1997) | ||
| T. gondii | (Rohloff et al., 2011) | ||
| Cax | N. crassa | (Bowman et al., 2009) | |
| Na+/H+ exchanger | L. donovani | (Vercesi et al., 1994) | |
| T. brucei | (Vercesi and Docampo, 1996; Vercesi et al., 1997) | ||
| T. gondii | (Rohloff et al., 2011) | ||
| Aquaporin | TcAQP1 | T. cruzi | (Montalvetti et al., 2004; Rohloff et al., 2004) |
| InsP3R | TbIP3R | T. brucei | (Huang, 2013) |
| VTC proteins | TbVTC1 | T. brucei | (Fang et al., 2007) |
| TgVTC2 | T. gondii | (Rooney et al., 2011) | |
| PPX | TcPPX | T. cruzi | (Ruiz et al., 2001b) |
| LdPPX | L. donovani | (Rodrigues et al., 2002) | |
| V-PPase | TbVSP | T. brucei | (Lemercier et al., 2004) |
| Metacaspase | LdMCP | L. donovani | (Lee et al., 2007) |
| Acid phosphatase | T. rangeli | (Gomes et al., 2006) | |
| Zinc transporter | EAN89594 | T. cruzi | (Ferella et al., 2008) |
| Peptidase (M13) | CMP249C | C. merolae | (Yagisawa et al., 2009) |
| ABC transporter | CMS401C | C. merolae | (Yagisawa et al., 2009) |
| Rab receptor protein | CMJ260C | C. merolae | (Yagisawa et al., 2009) |
| o-Methyltransferase | CMT369C | C. merolae | (Yagisawa et al., 2009) |
When a gene name is indicated it means that it has been cloned, expressed and the protein product localized to acidocalcisomes.
2.3. Evolutionary distribution
When the characteristic features of acidocalcisomes were revealed, it became apparent that they were morphologically and chemically similar to the “granules” described more than 100 years ago as “metachromatic granules”, because they had the ability to turn purple basic blue dyes (Babes, 1895). These were also called “volutin granules” because they were initially found in Spirillum volutans (Meyer, 1904). Volutin granules were renamed polyP granules after Wiame found that the number of granules in yeast correlated with the amount of polyP (Wiame, 1947). Volutin or polyphosphate granules were found in a number of eukaryotic microbes using the “Meyer test”, based on their methachromasy, including coccidia (Kunze, 1907), trypanosomes (Swellengrebel, 1908), and Sarcosporidia (Erdnmann, 1910).
Early reports (Friedberg and Avigad, 1968; Jensen, 1968) suggested the presence of a membrane surrounding the bacterial granule, but since this contradicted current thought that bacteria lack an endomembrane system, for many years they were assumed to lack an internal structure or limiting membrane (Shively, 1974; Shively et al., 1988). However, the presence of a membrane in acidocalcisomes of eukaryotes suggested that this was probably not the case. The finding of enzymes and transporters in the surrounding membrane of these organelles was fundamental in understanding their potential function and origin, and these studies started after their description in trypanosomatid and Apicomplexan parasites (Docampo et al., 2005).
Work in Agrobacterium tumefaciens (Seufferheld et al., 2003) and Rhodospirillum rubrum (Seufferheld et al., 2004) demonstrated that acidocalcisomes in bacteria are also membrane-bounded. Evidence for the presence of a limiting membrane included: [1] its detection by electron microscopy of intact bacteria and subcellular fractions; [2] the staining of the organelles by dyes that accumulate in acidic compartments, such as LysoSensor, and cycloprodigiosin; and [3] the detection in the acidocalcisome membranes of a vacuolar proton pyrophosphatase (V-H+-PPase), which contains several transmembrane domains, by immunofluorescence and immunoelectron microscopy and by subcellular fractionation. The more recent discovery of acidocalcisome-like organelles in human platelets (Ruiz et al., 2004a) and mast cells (Moreno-Sanchez et al., 2012) established that acidocalcisomes are the only membrane-bounded organelle present from bacteria to humans (Docampo et al., 2005) and suggested that the this is an organelle that either evolved before bacterial and eukaryotic lineages diverged, or appeared independently by convergent evolution (Docampo et al., 2010; Seufferheld et al., 2011).
2.4. Biogenesis
Acidocalcisomes of eukaryotes are considered lysosome-related organelles (LROs). These are a group of organelles with similarities to lysosomes, as for example melanosomes, lytic granules, major histocompatibiliy complex (MHC) class II compartments, platelet dense granules, basophil granules, and neutrophil azurophil granules (Dell'Angelica et al., 2000). Human platelet dense granules (Ruiz et al., 2004a) and mast cell granules (Moreno-Sanchez et al., 2012) are considered acidocalcisomes because they are acidic calcium stores rich in polyP.
One of the main differences with lysosomes is that acidocalcisomes do not accumulate endocytic tracers, such as transferrin (Scott et al., 1997), horseradish peroxidase (Coppens et al., 1993) or FM4-64 (Mullin et al., 2001). However, a common origin cannot be ruled out because a L. major mutant deficient in sphingolipid synthesis was found to be defective in biogenesis of both multivesicular bodies (or late endosomes) and acidocalcisomes (Zhang et al., 2005c).
Adaptor protein (AP) complexes are important mediators for vesicular transport of membrane proteins between cellular compartments, such as Golgi complex, endosomes, lysosomes and plasma membrane (Boehm and Bonifacino, 2002). Five main basic AP complexes have been described: AP-1 to AP-5 (Hirst et al., 2013). Each of these complexes is composed of two large, one medium and one small subunits or adaptins. AP-3 is involved in sorting of proteins to lysosomes and lysosome-related organelles from the Golgi (Peden et al., 2004) or from endosomes (Theos et al., 2005).
To study whether biogenesis of acidocalcisomes in T. brucei is linked to the expression of AP-3 function we investigated the effects of ablation of its large β and δ subunits (Tbβ3 and Tbδ) by RNAi (Huang et al., 2011). In contrast to the results reported in L. major, where knockout of the δ subunit of AP-3 did not affect growth in culture or acidocalcisome biogenesis (Besteiro et al., 2008) knockdown of the β3 or δ subunits of the AP-3 complex affected growth in vitro, and led to a decrease in the number of acidocalcisomes in both procyclic (PCF) and bloodstream form (BSF) trypanosomes (Huang et al., 2011). These phenotypic changes were revealed by immmunofluorescence and electron microscopy assays, and by the decrease in their acidic calcium, PPi, and polyP content (Huang et al., 2011). However, as occurs with T. brucei mutants, L. major promastigotes mutants deficient in functional acidocalcisomes, were also less virulent in vivo (Besteiro et al., 2008).
Although the mechanism for the phenotypic differences between L. major and T. brucei in vitro is unknown, a possible explanation is the formation of partial adaptor complexes in L. major constituted by just two subunits, a phenomenon that has been described for mouse models deficient in the AP-3 δ or β3 chain (Peden et al., 2002). Our results using C-terminal tagged β3 and δ subunits of AP-3 suggest similar predominant endosomal localization in T. brucei procyclic form trypanosomes. However, we also observed partial co-localization with markers of the trans-Golgi network (TGN) and with acidocalcisomes, suggesting that, as it was indicated for other LROs, acidocalcisome integral membrane proteins can follow a pathway from the TGN to endosomes rather than from the TGN to the plasma membrane and then to endosomes (Huang et al., 2011). The traffic of proteins from the Golgi complex to acidocalcisomes is also supported by results with L. donovani. Dominant negative ADP-ribosylation factor like-1 (ARL-1) L. donovani had no VP1 in their acidocalcisomes (Sahin et al., 2008).
An important role of target of rapamycin (TOR) kinase 3 (TOR3 kinase) in the biogenesis of acidocalcisomes of T. brucei (de Jesus et al., 2010) and L. major (Madeira da Silva and Beverley, 2010) has also been described. However, while knockdown of TOR3 in T brucei led to increases in polyP and PPi levels, larger acidocalcisomes, and more sensitivity to growth under hyperosmotic conditions (de Jesus et al., 2010), TOR3 knockout mutant promastigotes of L. major had smaller acidocalcisomes with apparently less polyP, and the parasites were less responsive to hyposmotic stress (Madeira da Silva and Beverley, 2010). The results suggest that TOR3 could have different roles in acidocalcisome biogenesis of each parasite.
2.5. Functional roles
Storage of phosphorus compounds (Pi, PPi and polyP) and cations (calcium, magnesium, sodium, potassium, zinc and iron) is one of the main roles of acidocalcisomes from different protists. This storage in an intracellular compartment reduces the osmotic effect of large pools of these compounds in the cytosol.
PolyP has roles in development, sporulation and predation (Zhang et al., 2005a; Zhang et al., 2005b), in stress adaptation (Castro et al., 1999; Castro et al., 1995; Pick and Weiss, 1991; Pick et al., 1991; Weiss et al., 1991) and in osmoregulation in different protists (Li et al., 2011; Ruiz et al., 2001b). Several protist parasites are less virulent when they contain lower amounts of polyP in their acidocalcisomes (Lemercier et al., 2004; Luo et al., 2005). The recent discovery that polyP has critical roles in blood clotting (Smith et al., 2006) and inflammation (Muller et al., 2009) suggests that polyP present in microorganisms could be involved in their pathogenicity.
The discovery of an inositol 1,4,5-trisphosphate receptor (IP3R) in acidocalcisomes of T. brucei (Huang, 2013) indicates that these organelles have a significant role in Ca2+ signaling. The IP3R is the primary target responsible for the initiation of intracellular Ca2+ signaling in most eukaryotic cells. Ca2+ release via IP3Rs stimulates activities critical for life. In this regard, it has been reported that acidocalcisome Ca2+ has a role in host cell invasion. Depletion of acidocalcisome Ca2+ by pretreatment of invasive stages of T. cruzi with ionomycin plus nigericin or ionomycin plus NH4Cl, inhibited invasion of host cells (Fernandes et al., 2006; Neira et al., 2002). In T gondii, knockout of TgA1, the enzyme necessary for pumping Ca2+ into the organelle results in de-regulation of cytosolic calcium, altered microneme secretion, and decreased virulence (Luo et al., 2004).
Acidocalcisomes also appear to have a role in regulation of intracellular pH. RNAi experiments in T. brucei to reduce the acidocalcisome V-H+-PPase activity resulted in their inability to recover their normal pH when they were exposed to an external basic pH >7.4, and the same cells recovered from intracellular acidification at a slower rate and to a more acidic final intracellular pH (Lemercier et al., 2002).
Acidocalcisomes have also an important role in osmoregulation. There is rapid hydrolysis or synthesis of acidocalcisome polyP during hypo- or hyperosmotic stress, respectively, in T. cruzi (Li et al., 2011; Ruiz et al., 2001b), as well as changes in sodium and chloride content in acidocalcisomes of L. major in response to acute hyposmotic stress (LeFurgey et al., 2001). Cyclic AMP levels increase when epimastigotes of T. cruzi are subjected to hyposmotic stress (Rohloff et al., 2004). Acidocalcisomes, which have an aquaporin (TcAQP1), traffic towards the contractile vacuole after hyposmotic stress, as revealed by direct observation of cells expressing TcAQP1 tagged with green fluorescent protein (GFP). This traffic is stimulated by cAMP analogs or phosphodiesterase (PDE) inhibitors and is inhibited by adenylyl cyclase inhibitors, and results in fusion of acidocalcisomes with the contractile vacuole and translocation of TcAQP1 (Rohloff et al., 2004). It has been proposed that the stimulus of cell swelling causes a spike in intracellular cAMP through an as yet unidentified adenylyl cyclase, resulting in fusion of acidocalcisomes with the contractile vacuole and translocation of aquaporin. This process helps the elimination of water by the contractile vacuole and is terminated by the action of the PDE (Rohloff and Docampo, 2008; Schoijet et al., 2011).
3. The Contractile Vacuole
3.3. History
The first description of a contractile vacuole complex is attributed to Lazzaro Spallanzani (Spallanzani, 1776), who noted a pulsatile star-shaped organelle in a free-swimming organism, presumably a Paramecium, and he postulated that it was involved in respiration. Numerous descriptions of this organelle in a wide variety of amoeba, algae, flagellates, and ciliates, followed since then. Their role in osmoregulation was defined by Kitching, using a variety of fresh-water and marine protists, in a series of articles published since 1934 (Kitching, 1934). The CVC is also present in single cell stages of some multicellular fungi and some cells of freshwater sponges but is absent from other multicellular species.
The first description of a contractile vacuole complex in trypanosomes, including T. cruzi, was from Clark (Clark, 1959). Linder and Staehelin (Linder and Staehelin, 1979) provided a model for fluid secretion involving an exocytic mechanism for the CVC of the trypanosomatid Leptomonas collosoma, and besides a few morphological descriptions (i.e., Leishmania amazonensis (Molyneux et al., 1975), Bodo sp. (Attias et al., 1996), the presence and functions of this organelle in trypanosomatids were mostly ignored until we began our studies on osmoregulation in these parasites (Rohloff et al., 2004).
3.2. Structure and composition
Several electron microscopic studies determined that the structure of the contractile vacuole is bipartite, consisting of a central vacuole or bladder and a surrounding loose network of tubules and vesicles named the spongiome (Allen and Naitoh, 2002). The morphological characteristics of the CVC of a number of protists have been carefully reviewed in a previous issue of this series (Allen and Naitoh, 2002), and we will limit our discussion to recent results on their structure and composition.
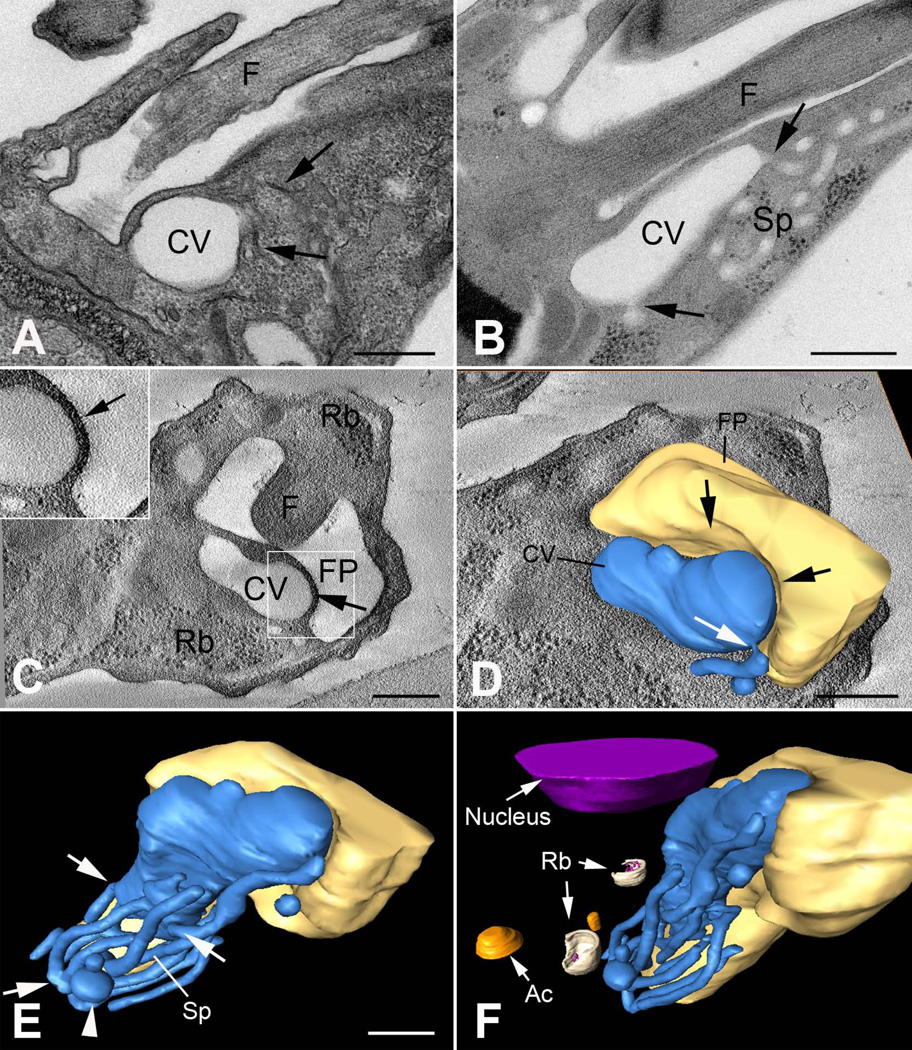
The three-dimensional architecture of the CVC of epimastigotes of T. cruzi was recently described (Girard-Dias et al., 2012). Using high-pressure freezing and freeze substitution the ultrastructure of T. cruzi was analyzed by serial electron tomography. A well-conserved CVC, containing a turgid central vacuole and spongiome tubules forming an interconnected network was observed (Fig. 2).
Figure 2. The CVC in T. cruzi epimastigotes.
(A) Thin section of chemically fixed epimastigote showing the CVC. Note the collapsed aspect of the spongiome (arrows). (B) Thin section of a high pressure-freeze substituted epimastigote in the G2 phase of the cell cycle showing the CV and the interconnected tubules (arrows) that form the spongiome (Sp). (C) Virtual section showing the CV docked to the flagellar pocket (FP) and the electron dense region between both structures (arrow and inset). (D) Virtual section and 3D model of the CVC and flagellar pocket (FP) where a deformation in the FP was observed (black arrows) and a tubule of the spongiome was connected to the central vacuole (white arrow). (E) 3D model of the CVC and FP showing the interconnected spongiome connected to the CV (arrows) and concentrated in the anterior region of the CV. Some vesicles were also connected to the spongiome (arrowhead). (F) 3D model showing the organization of the CVC and surrounding structures, such as ribosomes (Rb), acidocalcisomes (Ac) and nucleus. Scale bars = 200 nm. Reproduced with kind permission from ©Springer Science+Business Media, from from Fig. 5 of (Girard-Dias et al., 2012).
A number of proteins have been localized in the CVC of a variety of protists (Table 2). As occurs with acidocalcisomes, two proton pumps, a vacuolar proton ATPase (V-H+-ATPase, (Fok et al., 1993; Heuser et al., 1993; Nishihara et al., 2008; Rooney and Gross, 1992; Ruiz et al., 2001a; Ulrich et al., 2011) and a vacuolar H+ pyrophosphatase (V-H+-PPase or VP1, (Rohloff et al., 2004; Ruiz et al., 2001a; Ulrich et al., 2011) have been localized to the contractile vacuole of different protists. However, the pH of the CVC does not appear to be very acidic (for example, one study (Stock et al., 2002) calculated the pH of the CV in P. multimicronucleatum to be 6.4) and it has been proposed that the proton gradient is more likely a factor in the mechanism of fluid accumulation rather than in acidifying the organelle (Heuser et al., 1993).
Table 2.
Proteins localized to the contractile vacuole of different protists
| Protein name | CVC localization (name) | Protist | Reference |
|---|---|---|---|
| Membrane Transporters/Channels/Receptors | |||
| V-H+-ATPase | bladder /spongiome | D. discoideum | (Fok et al., 1993; Heuser et al., 1993; Rooney et al., 1994) |
| bladder | C. reinhardtii | (Ruiz et al., 2001a) | |
| bladder | T. cruzi | (Ulrich et al., 2011) | |
| bladder | A. proteus | (Nishihara et al., 2008) | |
| H+-PPase | bladder (VP1) | C. reinhardtii | (Ruiz et al., 2001a) |
| T. cruzi | (Rohloff et al., 2004) | ||
| Ca2+-ATPase | bladder (PAT1) | D. discoideum | (Marchesini et al., 2002; Moniakis et al., 1999) |
| IP3 receptor | bladder/spongiome | ||
| (CRC-II-1b) | P. tetraurelia | (Ladenburger et al., 2006) | |
| Calmodulin | bladder (CaM) | D. discoideum | (Zhu and Clarke, 1992; Zhu et al., 1993) |
| bladder (CaM) | P. multimicronucleatum | (Fok et al., 2008) | |
| spongiome (CaM) | T. cruzi | (Ulrich et al., 2011) | |
| Copine A | bladder (CpnA) | D. discoideum | (Damer et al., 2007) |
| P2X receptor | bladder (P2X) | D. discoideum | (Fountain et al., 2007; Ludlow et al., 2009; Sivaramakrishnan and Fountain, 2012a, b) |
| Ammonium transporter | bladder (Amtb) | D. discoideum | (Kirsten et al., 2008) |
| Rh50 (CO2 transporter) | bladder (RH50) | D. discoideum | (Benghezal et al., 2001) |
| Polyamine transporter | bladder (POT1.1) | T. cruzi | (Hasne et al., 2010) |
| Phosphate transporter | bladder (PHO1) | T. cruzi | (Ulrich et al., 2011) |
| Aquaporin | bladder (AQP1) | T. cruzi | (Rohloff et al., 2004) |
| bladder (AQP1) | L. major | (Figarella et al., 2007) | |
| bladder (AQP) | A. proteus | (Nishihara et al., 2008) | |
| bladder (CreMIP1) | C. reinhardtii | (Komsic-Buchmann et al., 2012) | |
| Trafficking Proteins | |||
| Rab8a | bladder /spongiome | D. discoideum | (Essid et al., 2012) |
| Rab11a | bladder /spongiome | D. discoideum | (Harris et al., 2001) |
| bladder | T. cruzi | (Ulrich et al., 2011) | |
| Rab11c | bladder/spongiome | D. discoideum | (Du et al., 2008) |
| Rab14 | spongiome (RabD) | D. discoideum | (Bush et al., 1994; Harris and Cardelli, 2002) |
| Rab 32 | bladder | T. cruzi | (Ulrich et al., 2011) |
| Disgorgin | bladder /spongiome | D. discoideum | (Du et al., 2008) |
| Drainin | bladder | D. discoideum | (Becker et al., 1999) |
| LvsA | spongiome | D. discoideum | (Gerald et al., 2002) |
| Clathrin | bladder | D. discoideum | (Heuser, 2006; Stavrou and O'Halloran, 2006) |
| AP180 | bladder | D. discoideum | (Wen et al., 2009) |
| bladder | T. cruzi | (Ulrich et al., 2011) | |
| AP2 | bladder | D. discoideum | (Wen et al., 2009) |
| Epsin | bladder | D. discoideum | (Wen et al., 2009) |
| SNARE2.1 | spongiome | T. cruzi | (Ulrich et al., 2011) |
| SNARE2.2 | spongiome | T. cruzi | (Ulrich et al., 2011) |
| Vamp7B | bladder | D. discoideum | (Wen et al., 2009) |
| SNAP-25 | bladder | P. tetraurelia | (Schilde et al., 2008) |
| Sec8 | bladder | D. discoideum | (Essid et al., 2012) |
| Sec15 | bladder | D. discoideum | (Essid et al., 2012) |
| MEGAPs | spongiome | D. discoideum | (Heath and Insall, 2008a, b) |
| Myosin 1c | bladder | D. discoideum | (Zhu and Clarke, 1992) |
| Myosin V | bladder/spongiome | (MyoJ) D. discoideum | (Jung et al., 2009) |
| Golvesin | bladder /spongiome | D. discoideum | (Gerisch et al., 2004; Schneider et al., 2000) |
| Dajumin | bladder | D. discoideum | (Gabriel et al., 1999) |
| Phosphoprotein | spongiome | C. luciliae thermophila | (Baqui et al., 2000) |
| Soluble proteins and enzymes | |||
| Alkaline phosphatase | lumen | D. discoideum | (Zhu and Clarke, 1992) |
| DdCAD-1 | lumen | D. discoideum | (Sesaki et al., 1997; Siu et al., 2011; Sriskanthadevan et al., 2009; Sriskanthadevan et al., 2011) |
| Discoidin 1 | lumen | D. discoideum | |
| Carbonic anhydrase | lumen | D. discoideum | (Ruiz et al., 2001a) |
| Phosphodiesterase C | spongiome | T. cruzi | (Schoijet et al., 2011) |
| Protein kinase | lumen | D. discoideum | (Betapudi and Egelhoff, 2009; Betapudi et al., 2005) |
| Metacaspase | bladder | D. discoideum | (Saheb et al., 2012) |
The presence of several proteins related to Ca2+ signaling underscores the role of the CVC in this process (see below): a Ca2+-ATPase (PAT1) in D. discoideum (Marchesini et al., 2002; Moniakis et al., 1999), an IP3R in Paramecium tetraurelia (Ladenburger et al., 2006), the Ca2+ binding proteins calmodulin (CaM) (Zhu and Clarke, 1992; Zhu et al., 1993) and copine A (Damer et al., 2007) in D. discoideum, and also CaM in P. multimicronucleatum (Fok et al., 2008) and T. cruzi (Ulrich et al., 2011), and the P2X receptors in D. discoideum (Fountain et al., 2007; Ludlow et al., 2009; Sivaramakrishnan and Fountain, 2012a, b). These receptors are Ca2+ permeable ligand-gated ion channels activated by ATP. Other transporters, such as an ammonium (NH4+) transporter (AmtB, (Kirsten et al., 2008)), a homologous to the Rh protein in red blood cells (RH50, (Benghezal et al., 2001)) that could act as CO2 transporter (Kustu and Inwood, 2006), in D. discoideum, and polyamine (TcPOT1, (Hasne et al., 2010)) and phosphate transporters (TcPHO1, (Ulrich et al., 2011)), in T. cruzi, have also been localized to the CVC.
Although a water channel was postulated to be involved in water accumulation by the CVC (Allen and Naitoh, 2002) only recently an aquaporin was discovered, first in the organelle of T. cruzi (Montalvetti et al., 2004; Rohloff et al., 2004), and later in those of L. major (Figarella et al., 2007), A. proteus (Nishihara et al., 2008), and C. reinhardtii (Komsic-Buchmann et al., 2012).
A number of proteins involved in trafficking and vacuolar fusion have also been identified. Rab8a (Du et al., 2008), 11a (Harris et al., 2001), 11c (Du et al., 2008), and 14 (Harris and Cardelli, 2002) have been localized to the CVC of D. discoideum, and Rab11 and 32 to the CVC of T. cruzi (Ulrich et al., 2011). Disgorgin was identified as a Rab8a GTPase activating protein (GAP) and functions in the CV cycle (Du et al., 2008). Drainin is a Rab-GAP like protein (although apparently inactive) that regulates CVC discharge (Becker et al., 1999; Du et al., 2008).
The Dictyostelium homologue to one of the Chediak-Higashi syndrome (CHS) proteins or large vacuole sphere A (LvsA) labels the CVC bladder when it reaches its maximal diameter and remains associated throughout the discharge phase until it concentrates in a patch at the plasma membrane (Gerald et al., 2002). Clathrin also contributes to CVC function and has been detected in the bladder of the complex of D. discoideum (Heuser, 2006; Stavrou and O'Halloran, 2006). Clathrin-coated vesicles on CVC bladders contain adaptor proteins AP180, AP-2 and epsin, and the SNARE, Vamp7B (Wen et al., 2009). AP180 and a VMAP7 homologue, and two SNAREs have also been found in the bladder and the spongiome of the CVC of T. cruzi, respectively (Ulrich et al., 2011). A homolog to SNAP-25, another SNARE, was also detected in the CVC of P. tetraurelia (Schilde et al., 2008). Sec15 and Sec8, two components of the exocyst complex, have also been localized to the CVC of D. discoideum especially during their discharge (Essid et al., 2012).
Another group of proteins are related to the cytoskeleton and involved in membrane tubulation and motility. The MEGAPs (mental retardation GTPase-activating proteins) are GAPs that localize to the tubules of the CVC of D. discoideum and transiently to the bladder when it is distended (Heath and Insall, 2008a, b). In addition, two myosins, a type I myosin (myosin 1c, (Zhu and Clarke, 1992)) and type V myosin (MyoJ, (Jung et al., 2009)) have been localized to the CVC of D. discoideum. MyoJ is required for the normal steady state distribution of membranes in the actin-rich cortex and to drive the actin-based cortical motility of the membrane tubules that arise from collapsed bladder membranes after water discharge (Jung et al., 2009). In addition to this role of myosin in CVC motility it has also been described that tubules and vesicles of the spongiome move bidirectionally between the cortex and the microtubule-organizing center (MTOC) via plus and minus end-directed mitrotubule motors (Jung et al., 2009).
Two proteins, golvesin and dajumin, have been used as markers of different compartments in D. discoideum. Golvesin is a protein that localizes in endosomes and the contractile vacuole, but localizes in the Golgi apparatus when its C-terminal region is blocked with GFP (Gerisch et al., 2004; Schneider et al., 2000). Dajumin-GFP is the only integral membrane protein to unequivocally identify constituents of the CVC (Gabriel et al., 1999). However, little is known about the function of these proteins. Another very large phosphorylated protein of unidentified function has been found in the CVC of Crithidia luciliae thermophila (Baqui et al., 2000)
Several proteins with enzymatic activity localize to the CVC of different protists: an alkaline phosphatase activity in D. discoideum (Nolta and Steck, 1994; Zhu and Clarke, 1992) and T. cruzi (Rohloff et al., 2004) has been used as marker for subcellular fractionation studies; a phosphodiesterase C, which is involved in the termination of cyclic AMP stimulation after hyposmotic stress was found in T. cruzi (Schoijet et al., 2011); an acetazolamide-sensitive carbonic anhydrase activity was detected in D. discoideum and could be important for the filling of the bladder (Marchesini et al., 2002). An unconventional protein kinase (protein kinase alpha) that contains an N-terminal von Willebrand factor A (vWFA)-like motif (vWFA kinase) and is able to autophosphorylate and bind to calmodulin is enriched in membranes of the CVC and Golgi-like structures of D. discoideum (Betapudi and Egelhoff, 2009; Betapudi et al., 2005). A metacaspase has recently been localized to the CVC of D. discoideum, when overexpressed (Saheb et al., 2012).
Two soluble proteins, DdCAD-1 (Sesaki et al., 1997; Siu et al., 2011; Sriskanthadevan et al., 2009; Sriskanthadevan et al., 2011) and discoidin 1 (Sriskanthadevan et al., 2009) are transported to the cell surface previous residence in the lumen of the CVC. Polyphosphate (polyP) has also been detected by DAPI staining in the lumen of the CVC of C. reinhardtii (Ruiz et al., 2001a), and D. discoideum (Marchesini et al., 2002), while orthophosphate (Pi) was enriched in subcellular fractions of T. cruzi containing the CVC (Rohloff et al., 2004), suggesting hydrolysis of polyP during fractionation. This argues against the idea that the bladders are empty inside and that they contain only water or a dilute electrolyte and favors an early hypothesis suggesting that contractile vacuoles might be filled with an expandable hydrocolloid that accumulates and retains water (Heywood, 1978).
3.3. Biogenesis
Ultrastructural studies in D. discoideum have suggested that the CVC is a post-Golgi compartment (Gabriel et al., 1999; Heuser et al., 1993). Biochemical and molecular data also support the Golgi-related origin of many of its constituents. For example, it has been demonstrated in D. discoideum that overexpressed golvesin, can be detected using monoclonal antibodies and is present in vesicles of various sizes including the CVC (Schneider et al., 2000). When the protein is CVC and that the C-terminal region of the protein is important for this translocation.
There is evidence that the transport of proteins from the Golgi apparatus to the CVC is through budding of clathrin-coated vesicles from the trans-Golgi network, as occurs in mammalian cells (Boehm and Bonifacino, 2001). The function of clathrin and its adaptors in the biogenesis of the contractile vacuole can be inferred from studies showing mislocalization of contractile vacuole components in AP-1 and AP-2 knockouts (Lefkir et al., 2003; Sosa et al., 2012). Studies in D. discoideum in which the µ1 adaptin of AP-1 was knocked out revealed that the contractile vacuole proteins Rh50 and dajumin-GFP were mislocalized to punctate structures inside the cell (Lefkir et al., 2003; Sosa et al., 2012) while the contractile vacuole was completely absent (Lefkir et al., 2003).
By simultaneously imaging fluorescently tagged clathrin and AP-2, Macro et al. (Macro et al., 2012) recently described that the CVC marker dajumin-GFP is trafficked via the plasma membrane and identified it as a cargo that is internalized by clathrindependent, AP-2 independent mechanisms. The finding that clathrin mediated endocytosis is required for internalization of CVC proteins from the cell membrane explains the CV biogenesis defect in D. discoideum cells lacking clathrin. Osmoregulation phenotypes of varying severity are observed in both clathrin light chain (clc-) (Wang et al., 2003) and heavy chain (chcA-) knockouts (O'Halloran and Anderson, 1992), as well as in knockouts of AP180 (Stavrou and O'Halloran, 2006), the α, β1/2, or µ2 subunits of AP-2 (Sosa et al., 2012; Wen et al., 2009), and the µ1 subunit of AP1 (Lefkir et al., 2003). Clathrin-mediated endocytosis has therefore a role in the biogenesis and/or maintenance of the contractile vacuole by functioning in retrieval of proteins from the cell surface indicating that the plasma membrane is another potential source of membrane for the contractile vacuole (Macro et al., 2012).
3.4. Role in osmoregulation
The CVC accumulates excess water from the cell resulting in swelling of the bladder, which eventually enters in contact with the plasma membrane expelling this excess water from the cell. This is a homeostatic process in organisms possessing a CVC, and it has a periodicity that varies from species to species and changes after modifications in the osmotic environmental conditions. The CVC of T. cruzi, for example, contracts every min and a half (Clark, 1959).
Several questions need to be considered: How water is accumulated? Which is the ionic composition of the CV? Which is the origin of the membrane that forms the swollen bladders and how the vacuole bladder contracts? How the bladder enters in contact with the plasma membrane?
How water is accumulated was unknown until the presence of an aquaporin or water channel was described in the CVC of different protists (Figarella et al., 2007; Komsic-Buchmann et al., 2012; Montalvetti et al., 2004; Nishihara et al., 2008). A water channel had been previously postulated to be involved in the CVC function (Allen and Naitoh, 2002) and calculations of water permeability in the CVC of Amoeba proteus suggested that their membrane was equipped with water channels (Nishihara et al., 2004). Experiments in T. cruzi clearly established a role for the CVC-located aquaporin, TcAQP1, in the cellular response to both hyposmotic and hyperosmotic stresses (Li et al., 2011). Knockdown of the expression of TcAQP1 reduced the regulatory volume decrease (RVD) after hyposmotic stress and the shrinking of the cells after hyperosmotic stress (Li et al., 2011). When TcAQP1 is knocked down and the cells are submitted to hyposmotic stress, water entry into the CVC is diminished and the cells have reduced capacity to pump water into the medium and recover their original volume (shrink) (Li et al., 2011). Conversely, when these cells are submitted to hyperosmotic stress the contractile vacuole is deficient in water accumulation and the cells cannot shrink by eliminating water into the medium as the wild type cells do (Li et al., 2011). Therefore TcAQP1 is important for cell shrinking during volume recovery after hyposmotic stress and during the initial phase of the response to hyperosmotic stress. These studies were also the first to reveal a role for the CVC under hyperosmotic stress.
The question regarding the ionic composition of the CVC is more difficult to answer. Early micropuncture studies in C. chaos and A. proteus showed that the CVC is hyposmolar respective to the cytosol (Riddick, 1968; Schmidt-Nielsen and Schrauger, 1963) but Heuser et al. (Heuser et al., 1993) argued that the cell cannot afford to expend valuable osmolytes to mitigate this osmotic gradient. They speculated that expendable metabolic byproducts, such as ammonia (NH3) and bicarbonate (HCO3−), might be important sources for generating an osmotic gradient across the contractile vacuole membrane and that the vacuolar H+-ATPase would provide the electrochemical gradient needed for water accumulation. NH3 is membrane permeable and would be retained by transformation into ammonium (NH4+) with the protons provided by the H+-V-ATPase (or the H+-V-PPase, where available). An anion channel would transport HCO3−. Some evidence for this pathway was found in D. discoideum (Marchesini et al., 2002). Incubation of amoebas in the presence of the anion exchanger inhibitor H2DIDS (Cabantchik and Greger, 1992) or the V-H+-ATPase inhibitor bafilomycin A1 (Bowman et al., 1988) produced a dose-dependent prolongation of their CV contraction cycle, measured as the interval between two contractile vacuole discharges in the same cell (Marchesini et al., 2002). In addition, the carbonic anhydrase inhibitor acetazolamide prolonged the contraction cycle of the CV (Marchesini et al., 2002). Furthermore, carbonic anhydrase activity was present in CV fractions and most of the acetazolamidesensitive carbonic anhydrase activity co-localized with the contractile vacuole markers (Marchesini et al., 2002). Interestingly, evidence for an ammonium (NH4+) transporter (AmtB) (Kirsten et al., 2008) and a potential CO2 transporter (RH50) (Benghezal et al., 2001) in the CVC of D. discoideum has also been provided. Although the orientation of these transporters is not known their presence suggest that NH4+ and CO2 could return to the cytosol once the water is eliminated by the CV discharge. A model is shown in Fig. 3.
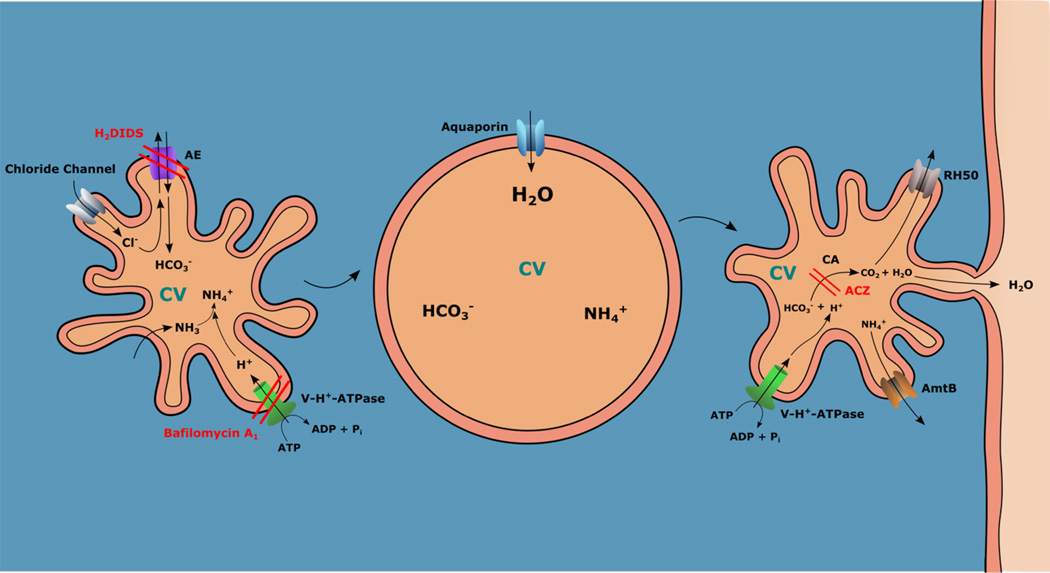
Figure 3. Model proposed for filling of the CVC in protists.
Ammonia (NH3) is transported to the CVC where it combines with H+ pumped in by the action of the vacuolar H+-ATPase (which is inhibited by bafilomycin A1) to generate ammonium (NH4+), which is charged and retained. An anion exchanger (AE, which is inhibited by H2DIDS) transports HCO3− in exchange of Cl−, which enters the vacuole through a chloride channel. NH4+ and HCO3− are osmolytes that attract water, which enters through an aquaporin (AQP) and swells the CV. The CV contacts the plasma membrane (kiss and run) through a porosome, releasing water to the medium. A carbonic anhydrase (which is inhibited by acetazolamide, ACZ) generates CO2 from HCO3− and H+ (pumped in by the V-H+-ATPase). CO2 is released into the cytosol through the protein RH50, while ammonium is released into the cytosol through an ammonium transporter (AmtB).
An alternative, although not excluding, possibility was considered for T. cruzi, where it was postulated that fusion of acidocalcisomes with the CVC and concomitant hydrolysis of polyP would lead to an increase in phosphate and cations in the bladder, which would result in water accumulation (Rohloff and Docampo, 2008). After water elimination, cations and phosphate would return to the cytosol. The presence of a phosphate transporter in the CVC (Ulrich et al., 2011) and cation exchangers that could be transferred from the acidocalcisome membranes together with aquaporin upon their fusion (Rohloff et al., 2004) would favor this hypothesis. Since similar associations of acidocalcisomes with the CVC of D. discoideum (Marchesini et al., 2002) and C. reinhardtii (Ruiz et al., 2001a) have been described, these possibilities are not necessarily mutually exclusive. See Fig. 4 for a model.
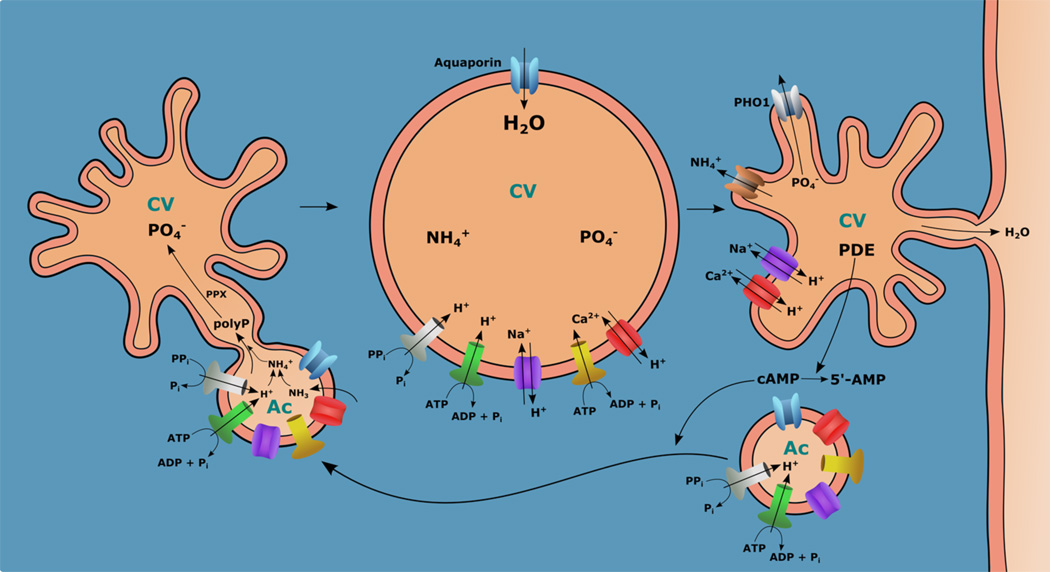
Figure 4. Alternative model for filling of the CVC.
Stimulation of an adenylyl cyclase results in a microtubule-dependent movement and fusion of acidocalcisomes with the CVC with transfer of membrane and matrix content. NH3 enters the spongiome and combined with H+ pumped in by the V-H+-ATPase or the H+-PPase forms NH4+ and increases the pH of the vacuole stimulating an exopolyphosphatase (PPX) to cleave polyP. Ammonium, Pi, and cations transferred by the different pumps and exchangers translocated from acidocalcisomes, are the osmolytes that attract water that enters through the aquaporin and swells the vacuole. When the vacuole enters in contact with the plasma membrane porosomes, Pi is transported back to the cytosol by the phosphate transporter (PHO1), while cations are also transported back to the cytosol by inversion of the exchangers. NH4+ is transported to the cytosol through the ammonium transporter.
The CV bladder does not burst during diastole and to increase the surface area during expansion, the membrane is provided by the tubules and vesicles that form the spongiome. It has been proposed (Clarke et al., 2002; Gerisch et al., 2002) that the contraction phase is not due to muscle-like contraction since no F-actin or myosin are present in the bladder (Heuser et al., 1993) but to an asymmetry in the phospholipids of the network. Filling the bladders would place a strain on the membrane, which has a tendency to tubulate, and this tendency would be responsible for driving the emptying process.
Finally, the mechanism that the CV bladder uses to make contact with the plasma membrane appears to differ in different protists. In Paramecium the CVC is highly differentiated and the intracellular position of the CVC (2 per cell) is fixed and a permanent surface indentation of the plasma membrane called the CV pore is recognizable (Allen and Naitoh, 2002). The situation is significantly different with the CVC of D. discoideum; the form of which is always changing and its position is not fixed in the cell. However, there is evidence that the CV discharge occurs by “kiss and run” exocytosis, where the discharging entity retains its identity, so its membrane does not need to be recycled via a compensatory endocytic event, except under non-physiological circumstances (Heuser, 2006). In this regard, the GTPase Rab8a interacts with the exocyst complex in tethering of the CV to the plasma membrane, and its fusion and attachment, thereby regulating the “kiss and run” exocytosis (Essid et al., 2012). This hypothesis is compatible with the presence of porosomes, which are supramolecular cup-shaped lipoprotein structures at the cell membrane, where membrane-bound secretory vesicles transiently dock and fuse to release intravesicular contents to the outside during secretion (Jena, 2013). Finally, the CV of trypanosomes is attached to the side of the flagellar pocket by a specialized electron-dense region that has been termed the adhesion plaque, first described in L. collosoma (Linder and Staehelin, 1979) and more recently found in T. cruzi (Girard-Dias et al., 2012) (Fig. 2).
3.5. Role in calcium homeostasis
Contractile vacuoles are considered acidic calcium stores (Patel and Docampo, 2010) and they have been proposed to be involved in Ca2+ secretion and signaling.
In D. discoideum expression of the CVC Ca2+-ATPase PAT1 is upregulated, in a calcineurin-dependent manner, when the cells are grown in calcium-rich medium (Moniakis et al., 1999). Conditions that impair CVC function reduce the rate of Ca2+ secretion and antisense patA RNA or calcineurin antagonists affect the growth of cells in high Ca2+ medium (Moniakis et al., 1999). The results suggest a role of the CVC in Ca2+ sequestration and excretion pathways, especially under conditions of high extracellular Ca2+. In agreement with these results, isolated CVs from D. discoideum have been shown to take up Ca2+ (Malchow et al., 2006). It has also been proposed that the CVC P2X receptors of D. discoideum are Ca2+ release channels (Ludlow et al., 2009) that are stimulated by changes in luminal ATP, which would be translocated into the CVC through and ATP-specific transporter (Sivaramakrishnan and Fountain, 2012b).
Ca2+ release from the CVC was observed in P. tetraurelia when the InsP3R located in this organelle was stimulated by uncaging InsP3 (Ladenburger et al., 2006), suggesting a role of the CVC in Ca2+ signaling. Peptides corresponding to a homolog of the InsP3R were also found in the proteomic analysis of the CVC of T. cruzi but the role of this channel in T. cruzi is not yet known (Ulrich et al., 2011).
3.6. Role in protein trafficking
There is evidence that the CVC of different protists could act as a trafficking hub, receiving and delivering proteins to the plasma membrane. Although it has been indicated that normally there is no much mixing or ‘scrambling’ of contractile vacuole and plasma membranes (Heuser, 2006), transfer of membrane proteins from the CVC to the plasma membrane has been observed in several instances. For example, V-H+-ATPase and CaM translocate to the plasma membrane of D. discoideum when cells are starved during stationary phase (Heuser et al., 1993). The Ca2+-ATPase PAT1 moves to the plasma membrane when D. discoideum is incubated at high Ca2+ concentrations (Moniakis et al., 1999). The CVC polyamine transporter of T. cruzi (TcPOT1) is transferred to the plasma membrane when the incubation medium is deficient in polyamines (Hasne et al., 2010). The adhesive protein DdCAD-1 is also targeted to the cell surface via the CVC in D. discoideum (Sesaki et al., 1997) by an unconventional protein-trafficking pathway, being imported to the CV after forming vesicular structures in its lumen (Sriskanthadevan et al., 2009). In addition, it is remarkable that Rab11, a protein that localizes in recycling endosomes in most cells, localizes to the CVC of T. cruzi (Ulrich et al., 2011) and D. discoideum (Harris et al., 2001), suggesting that this compartment could share the function of recycling endosomes that usually send proteins back to the plasma membrane. The studies described above (Biogenesis) showing that the CVC marker dajumin-GFP is trafficked via the cell membrane and is internalized by a clathrin-dependent mechanism suggest that clathrin-mediated endocytosis may also function as a back-up mechanism in case of transfer of proteins from the contractile vacuole to the plasma membrane (Macro et al., 2012).
4. Volume control in T. cruzi
4.4. Need for osmoregulation
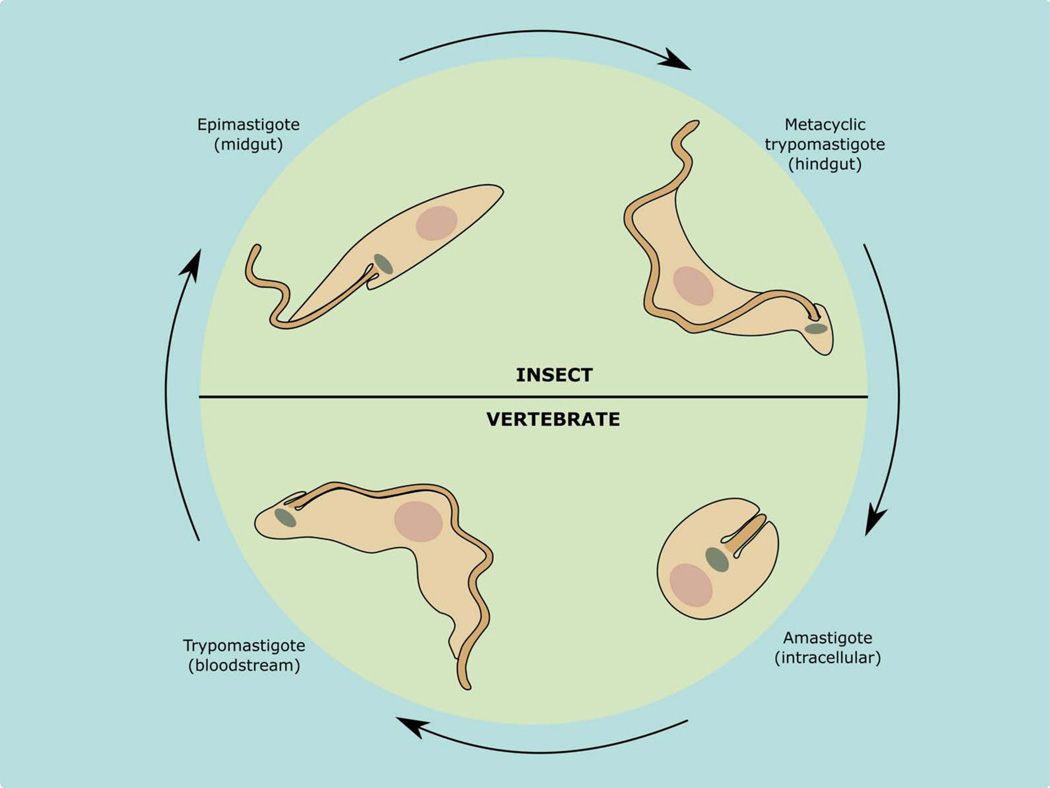
The life cycle of T. cruzi involves four major developmental stages that alternate between an insect vector and a mammalian host. The parasite enters the mammalian host when the insect vector defecates in the vicinity of the bite, and the natural infective stage, the metacyclic trypomastigote, is carried into the wound by scratching, and then penetrates and infects nearby cells. Once inside the host cells, metacyclic trypomastigotes differentiate into amastigotes. These replicative forms multiply in the cytoplasm and, after several rounds of replication, differentiate back into trypomastigotes, which gain access into the bloodstream and eventually invade new cells, thus perpetuating the infection. When the insect bites an infected mammal, the trypomastigotes carried over with the blood meal differentiate into epimastigotes, which are a free replicative form living in the insect intestine. In the rectum, where the insect’s urine is discharged, the epimastigotes differentiate to metacyclic trypomastigotes by a process termed metacyclogenesis, and these forms are able to start a new round of infection (Fig. 5).
Figure 5. Life cycle of T. cruzi.
Two main forms are present in the insect vector: epimastigotes and metacyclic trypomastigotes; and two main forms in the vertebrate host: intracellular amastigotes and bloodstream trypomastigotes.
During its developmental cycle in the mammalian and insect hosts, T. cruzi faces critical environmental challenges and one that is especially dramatic is osmolarity. Each time the trypomastigote stage passes through the kidney of its mammalian host it must be able to resist 1,300–1,400 mOsm/Kg and return to isosmotic conditions of 300 mOsm/kg (Lang, 2007). Some organs that are infected by T. cruzi (liver, spleen, lymphoid tissues) have also higher osmolarity than serum (330 vs 300 mOsm/kg) (Go et al., 2004). In the insect vector, the parasite is found in the epimastigote form and in this environment the osmolarity also increases dramatically from the feces to the urine of the vector, and reaches values of up to 1,000 mOsm/kg in the yellow rectal content (Kollien et al., 2001). In addition to the drastic changes in osmolarity to which it is exposed, as all cells (Lang, 2007), T. cruzi need to regulate its volume continuously. Recent work has revealed that this parasite has very unique mechanisms to deal with these challenges. The parasite acidocalcisomes and the CVC appear to have a central role in the adaptation to osmotic changes.
4.2. Response to hyposmotic stress
A regulatory volume decrease mechanism is present in amastigotes, epimastigotes and trypomastigotes of T. cruzi (Rohloff et al., 2003). This process is rapid and essentially complete in all T. cruzi stages by 5 min. An amino acid efflux mechanism accounts for approximately 50% of the regulatory volume decrease. A number of uncharged or acidic amino acids are mobilized during hyposmotic stress in all three stages and there is a marked absence of mobilization of cationic amino acids. Glu, Gly, Pro, and Ala account for nearly 90% of the total amino acids mobilized (Rohloff et al., 2003). These results suggest that amino acid efflux in T. cruzi occurs through anion channels or transporters with properties similar to those previously described in other cells (Lang et al., 1998a; Lang et al., 1998b; Vieira et al., 1996). Although acidocalcisomes have large amounts of amino acids, nearly 90% of the amino acid pool of the acidocalcisome consists of Arg and Lys, minor components of the amino acids released extracellularly during regulatory volume decrease (Rohloff et al., 2003).
A rise in intracellular Ca2+ occurs upon hyposmotic stress which is completely dependent on extracellular Ca2+ and, although it plays a role in modulating the early phase of amino acid efflux, is not a key determinant of the final outcome of the regulatory volume decrease (Rohloff et al., 2003). Na+, phosphate and inositol are not released extracellularly, while K+ efflux in epimastigotes could account for only about 7% of the regulatory volume decrease (Rohloff and Docampo, 2008).
Taken together, these results showed that an osmolyte efflux mechanism alone does not entirely account for the regulatory volume decrease in T. cruzi, and that the function of the CVC is necessary for a complete RVD. It was found that cyclic AMP levels increase when T. cruzi epimastigotes are subjected to hyposmotic stress and that modulators of cyclic AMP levels and microtubule function affect trafficking of TcAQP1 from the acidocalcisomes to the contractile vacuole (Rohloff et al., 2004). The results suggested that either a mechanosensitive adenylyl cyclase is activated or a mechanosensitive channel leads to the influx of ions, such as Ca2+, and activation of an adenylyl cyclase upon hyposmotic stress. A model was proposed (Rohloff et al., 2004) in which the stimulus of cell swelling causes a spike in intracellular cyclic AMP through an as yet unidentified adenyl cyclase, resulting in a microtubule-dependent fusion of acidocalcisomes with the contractile vacuole and translocation of an aquaporin. A simultaneous rise in ammonia (Rohloff and Docampo, 2006), and its sequestration in acidocalcisomes as NH4+, would activate an acidocalcisome exopolyphosphatase, which cleaves polyphosphate (Ruiz et al., 2001b), releasing inorganic phosphate residues and also the various polyphosphate-chelated osmolytes, such as basic amino acids and calcium. The resulting osmotic gradient sequesters water, through the aid of TcAQP1, which is subsequently ejected into the flagellar pocket. A cyclic AMP phosphodiesterase (PDE) would terminate the signaling pathway by hydrolyzing cAMP to 5’-AMP (Rohloff et al., 2004) (Fig. 4).
Since this model was proposed, evidence was presented for the localization of a PDE C in the CVC (spongiome) of the parasites (Schoijet et al., 2011). Inhibition of this enzyme by newly developed compounds caused inhibition of the RVD and cell killing validating this process as a target for chemotherapy (King-Keller et al., 2010). It was also found that a class III phosphatidylinositol 3-kinase (TcPI3K), related to the yeast vacuolar protein sorting 34, Vps34p, was also important for RVD (Schoijet et al., 2008). Overexpression of TcPI3K was shown to affect the RVD (Schoijet et al., 2008). In addition, several proteins important for vacuolar fusion (SNAREs) were shown to localize to the CVC (Table 2) and a putative phosphate transporter was found in the bladder of the CVC (Ulrich et al., 2011). This transporter could be involved in recycling of phosphate produced by the hydrolysis of poly P during RVD (Fig. 4).
4.3. Response to hyperosmotic stress
Hyperosmotic stress in most mammalian cells causes cell shrinkage due to osmotic efflux of water leading to increases in intracellular ionic strength (Alfieri and Petronini, 2007). This rapid reduction in cell volume is corrected by the regulatory volume increase (RVI), which is mediated by ion transport systems, including the Na+-K+-Cl− co-transporter, the Na+/H+ exchanger and the Cl−/HCO3− exchanger (Lang et al., 1998a; McManus et al., 1995). The increase of intracellular ions and the accompanying influx of water cause RVI. Cells counteract the additional increase in ionic strength produced by the further uptake of inorganic ions by substituting them by either the synthesis or uptake and cellular accumulation of compatible osmolytes, such as neutral amino acids or their derivatives, polyols such as sorbitol and myo-inositol, and methylamines such as betaine (Alfieri and Petronini, 2007). Compatible osmolytes replace the inorganic ions without impairing normal biochemical functions such as protein synthesis (Alfieri and Petronini, 2007). Compatible osmolytes also protect the cells from apoptosis and modulate their adaptive responses (Alfieri et al., 2002; Kitamura et al., 1997).
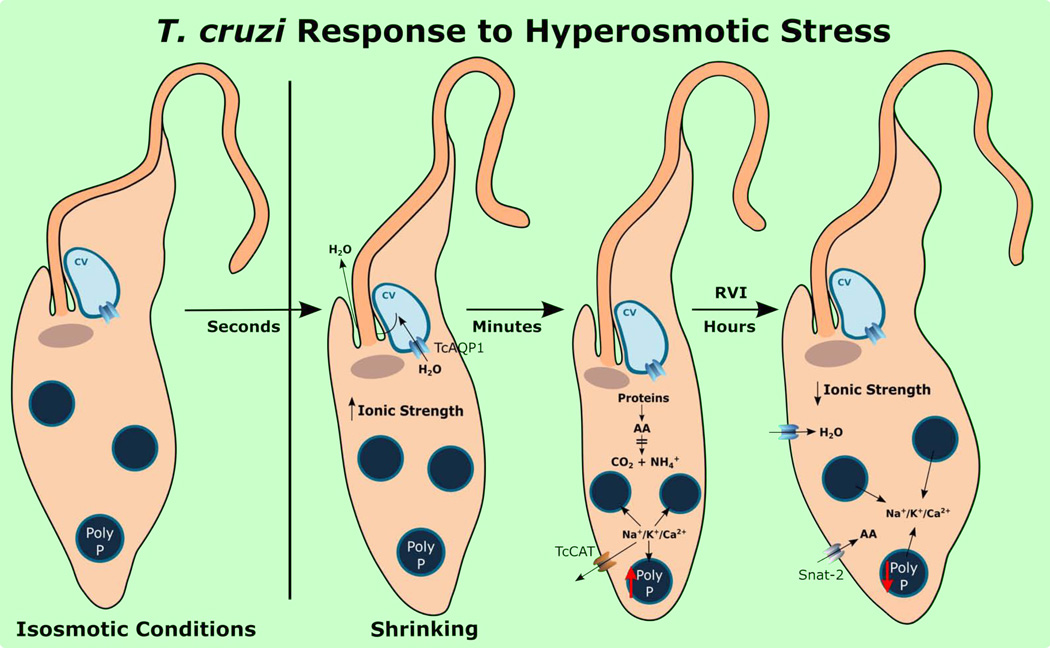
In contrast to what happens with mammalian cells, when epimastigotes of T. cruzi are subjected to hyperosmotic stress they shrink within a few seconds, but do not significantly regain their normal volume, suggesting that there is no immediate inorganic ion and water uptake (Li et al., 2011). There is an initial great increase in the size of the contractile vacuole suggesting that water efflux is mediated through the CVC (Li et al., 2011). In spite of the initial increase in intracellular ionic strength they adapt well to these conditions, being virtually indistinguishable in terms of motility from control cells maintained in isosmotic buffer (Li et al., 2011). However, within minutes of hyperosmotic stress there is a decrease in ammonium production and accumulation of amino acids, which then stabilize at concentrations higher than those under isosmotic conditions (Li et al., 2011). Protein content also decreases within three hours suggesting protein degradation to increase the amino acid pool. PolyP synthesis is stimulated within minutes of hyperosmotic stress and this stimulation results in a 3- and 2.35-fold increase in long-chain polyP content at 3 and 6 hours, respectively, after hyperosmotic stress (Li et al., 2011). Taken together, these results are in agreement with a model in which reduced amino acid catabolism and increased protein degradation result in amino acid accumulation (Li et al., 2011). These amino acids are the compatible osmolytes that replace the inorganic ions (Na+, K+) that are sequestered together with newly formed polyP in the acidocalcisomes, thus reducing the cytosolic ionic strength increased after water elimination, and preventing cell damage (Li et al., 2011) (Fig. 6).
Figure 6. Model showing changes in T. cruzi submitted to hyperosmotic stress.
When transferred to hyperosmotic conditions cells rapidly shrink in a process facilitated by water transport to the CVC through an aquaporin (AQP). Within minutes acidocalcisome polyP synthesis increases trapping cations (K+, Na+, Ca2+) inside the organelles and restoring the cytosol ionic strength. Cations can also be transported outside through a cation channel (TcCAT). Protein degradation together with inhibition of amino acid catabolism results in cytosolic amino acids increase. Amino acids are the organic osmolytes replacing cytosolic cations. Within hours acidocalcisome polyP is hydrolyzed returning cations to the cytosol, and water entry restores the cell volume (regulatory volume decrease). Amino acid transport at this stage is facilitated by the induced expression of a sodium dependent (Snat-2) amino acid transporter.
Treatment of the epimastigotes with low concentrations of HgCl2, a known inhibitor of T. cruzi aquaporin 1 (TcAQP1), or knockdown of TcAQP1 expression reduces the intensity of shrinking after hyperosmotic stress while overexpression of TcAQP1 increased shrinking, suggesting that the CVC mediates water efflux during hyperosmotic challenge (Li et al., 2011). Shrinking is also probably due to cation elimination through a cation channel (TcCAT) that is translocated to the plasma membrane of epimastigotes submitted to hyperosmotic stress (Jimenez and Docampo, 2012). Inhibitors of TcCAT (BaCl2, 4-aminopyridine) inhibit shrinking of trypomastigotes under hyperosmotic stress (Jimenez and Docampo, 2012). Early synthesis of polyP and sequestration of inorganic ions in acidocalcisomes of epimastigotes and the simultaneous increase in compatible osmolytes prevents the deleterious effects of a cellular increase in ionic strength.
A second phase of recovery after hyperosmotic stress of epimastigotes is characterized by induction of amino acid transporters (Li et al., 2011). The higher expression of genes encoding for amino acid transporters suggest that amino acids are the compatible osmolytes needed to replace the inorganic ions sequestered by the stimulated synthesis of polyP in the acidocalcisomes (Li et al., 2011). Interestingly, some of these amino acid transporters have similarity to the sodium-dependent neutral amino acid transporter-2 (SNAT2) known as System A, which increases upon exposure of mammalian cells to hyperosmotic stress (Alfieri et al., 2002).
The response of epimastigotes to hyperosmotic stress is therefore different from that observed in mammalian cells or yeasts. An aquaporin and the contractile vacuole are involved in water efflux leading to cell shrinkage, and there is no early regulatory volume increase. The results suggest that the increase in ionic strength is counteracted by the early synthesis of polyP and sequestration of inorganic ions in acidocalcisomes. Amino acids are the compatible osmolytes that replace the inorganic ions sequestered in acidocalcisomes, and they initially accumulate by a reduction in their catabolism, and later on by protein degradation and by uptake through induced amino acid transporters. A model for the changes occurring in T. cruzi during hyperosmotic stress is shown in Fig. 6.
5. Conclusions and Open Questions
T. cruzi is exposed to environments of different osmolarities and has developed novel mechanisms to deal with these changes. A contractile vacuole complex (CVC) is important not only to expel water as a mechanism of regulatory volume decrease (RVD) but also to help in shrinking the cells when submitted to hyperosmotic stress. The CVC of T. cruzi also appears to have a role as a trafficking hub, and could be important for Ca2+ signaling. T. cruzi possesses acidic Ca2+ stores rich in polyP, which are termed the acidocalcisomes, and which participate in both the response to hyposmotic and hyperosmotic stresses. Roles for polyP in the generation of inorganic osmolytes during hyposmotic stress and in sequestering inorganic osmolytes to prevent the increase in the cytosolic ionic strength of the cells under hyperosmotic stress have been proposed in T. cruzi. Furthermore, a water channel or aquaporin is important not only for the filling of the CVC during hyposmotic stress but also under hyperosmotic stress to facilitate shrinking of the cells. Reduced levels of polyP are associated to decreased ability to respond to osmotic stress and decreased pathogenicity in vivo. A microtubule- and cAMP-dependent signaling pathway is stimulated by hyposmotic stress and results in the transfer of the aquaporin from acidocalcisomes to the CVC. A PI3K is also involved in the response to hyposmotic stress. Acidocalcisomes alkalinize due to ammonia accumulation and also increase their volume in response to hyposmotic stress. A T. cruzi PDE C was localized to the CVC and demonstrated to be essential for volume regulation and survival of the parasite providing a novel target for chemotherapy.
Acknowledgments
We thank Wendell Girard Diaz and Kildare Miranda for the use of Figure 2, and Christina Moore for the drawings of Figures 3–6. Work in our laboratory was supported in part by grants from the U.S. National Institute of Allergy and Infectious Diseases (AI068647 to RD and AI101167 to VJ), and a pre-doctoral fellowship from the American Heart Association Southeast Affiliate (to NL).
References
- Alfieri RR, Cavazzoni A, Petronini PG, Bonelli MA, Caccamo AE, Borghetti AF, Wheeler KP. Compatible osmolytes modulate the response of porcine endothelial cells to hypertonicity and protect them from apoptosis. J Physiol. 2002;540:499–508. doi: 10.1113/jphysiol.2001.013395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri RR, Petronini PG. Hyperosmotic stress response: comparison with other cellular stresses. Pflugers Arch. 2007;454:173–185. doi: 10.1007/s00424-006-0195-x. [DOI] [PubMed] [Google Scholar]
- Allen RD, Naitoh Y. Osmoregulation and contractile vacuoles of protozoa. Int Rev Cytol. 2002;215:351–394. doi: 10.1016/s0074-7696(02)15015-7. [DOI] [PubMed] [Google Scholar]
- Attias M, Vommaro RC, de Souza W. Computer aided three-dimensional reconstruction of the free-living protozoan Bodo sp. (Kinetoplastida:Bodonidae) Cell Struct Funct. 1996;21:297–306. doi: 10.1247/csf.21.297. [DOI] [PubMed] [Google Scholar]
- Babes V. Beoachtungen über die metachromatischen körperchen, sporenbildung, verzwiegung, kolben- und kapsel-bildung pathogener bakterien. Zentrabl. Bakteriol. arasitenkd. Infektionskr. 1895;20:412–420. [Google Scholar]
- Baqui MM, De Moraes N, Milder RV, Pudles J. A giant phosphoprotein localized at the spongiome region of Crithidia luciliae thermophila . J Eukaryot Microbiol. 2000;47:532–537. doi: 10.1111/j.1550-7408.2000.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Becker M, Matzner M, Gerisch G. Drainin required for membrane fusion of the contractile vacuole in Dictyostelium is the prototype of a protein family also represented in man. Embo J. 1999;18:3305–3316. doi: 10.1093/emboj/18.12.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benghezal M, Gotthardt D, Cornillon S, Cosson P. Localization of the Rh50-like protein to the contractile vacuole in Dictyostelium . Immunogenetics. 2001;52:284–288. doi: 10.1007/s002510000279. [DOI] [PubMed] [Google Scholar]
- Besteiro S, Tonn D, Tetley L, Coombs GH, Mottram JC. The AP3 adaptor is involved in the transport of membrane proteins to acidocalcisomes of Leishmania . J Cell Sci. 2008;121:561–570. doi: 10.1242/jcs.022574. [DOI] [PubMed] [Google Scholar]
- Betapudi V, Egelhoff TT. Roles of an unconventional protein kinase and myosin II in amoeba osmotic shock responses. Traffic. 2009;10:1773–1784. doi: 10.1111/j.1600-0854.2009.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betapudi V, Mason C, Licate L, Egelhoff TT. Identification and characterization of a novel alpha-kinase with a von Willebrand factor A-like motif localized to the contractile vacuole and Golgi complex in Dictyostelium discoideum . Mol Biol Cell. 2005;16:2248–2262. doi: 10.1091/mbc.E04-07-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS. Adaptins: the final recount. Mol Biol Cell. 2001;12:2907–2920. doi: 10.1091/mbc.12.10.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm M, Bonifacino JS. Genetic analyses of adaptin function from yeast to mammals. Gene. 2002;286:175–186. doi: 10.1016/s0378-1119(02)00422-5. [DOI] [PubMed] [Google Scholar]
- Bowman BJ, Draskovic M, Freitag M, Bowman EJ. Structure and distribution of organelles and cellular location of calcium transporters in Neurospora crassa . Eukaryot Cell. 2009;8:1845–1855. doi: 10.1128/EC.00174-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush J, Nolta K, Rodriguez-Paris J, Kaufmann N, O'Halloran T, Ruscetti T, Temesvari L, Steck T, Cardelli J. A Rab4-like GTPase in Dictyostelium discoideum colocalizes with V-H+-ATPases in reticular membranes of the contractile vacuole complex and in lysosomes. J Cell Sci. 1994;107:2801–2812. doi: 10.1242/jcs.107.10.2801. [DOI] [PubMed] [Google Scholar]
- Cabantchik ZI, Greger R. Chemical probes for anion transporters of mammalian cell membranes. Am J Physiol. 1992;262:C803–C827. doi: 10.1152/ajpcell.1992.262.4.C803. [DOI] [PubMed] [Google Scholar]
- Castro CD, Koretsky AP, Domach MM. NMR-Observed phosphate trafficking and polyphosphate dynamics in wild-type and vph1-1 mutant Saccharomyces cerevisae in response to stresses. Biotechnol Prog. 1999;15:65–73. doi: 10.1021/bp9800743. [DOI] [PubMed] [Google Scholar]
- Castro CD, Meehan AJ, Koretsky AP, Domach MM. In situ 31P nuclear magnetic resonance for observation of polyphosphate and catabolite responses of chemostat-cultivated Saccharomyces cerevisiae after alkalinization. Appl Environ Microbiol. 1995;61:4448–4453. doi: 10.1128/aem.61.12.4448-4453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causton HC, Ren B, Koh SS, Harbison CT, Kanin E, Jennings EG, Lee TI, True HL, Lander ES, Young RA. Remodeling of yeast genome expression in response to environmental changes. Mol Biol Cell. 2001;12:323–337. doi: 10.1091/mbc.12.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark TB. Comparative morphology of four genera of trypanosomatidae. J. Protozool. 1959;6:227–232. [Google Scholar]
- Clarke M, Kohler J, Arana Q, Liu T, Heuser J, Gerisch G. Dynamics of the vacuolar H+-ATPase in the contractile vacuole complex and the endosomal pathway of Dictyostelium cells. J Cell Sci. 2002;115:2893–2905. doi: 10.1242/jcs.115.14.2893. [DOI] [PubMed] [Google Scholar]
- Coppens I, Baudhuin P, Opperdoes FR, Courtoy PJ. Role of acidic compartments in Trypanosoma brucei, with special reference to low-density lipoprotein processing. Mol Biochem Parasitol. 1993;58:223–232. doi: 10.1016/0166-6851(93)90044-x. [DOI] [PubMed] [Google Scholar]
- Damer CK, Bayeva M, Kim PS, Ho LK, Eberhardt ES, Socec CI, Lee JS, Bruce EA, Goldman-Yassen AE, Naliboff LC. Copine A is required for cytokinesis, contractile vacuole function, and development in Dictyostelium . Eukaryot Cell. 2007;6:430–442. doi: 10.1128/EC.00322-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus TC, Tonelli RR, Nardelli SC, da Silva Augusto L, Motta MC, Girard-Dias W, Miranda K, Ulrich P, Jimenez V, Barquilla A, Navarro M, Docampo R, Schenkman S. Target of rapamycin (TOR)-like 1 kinase is involved in the control of polyphosphate levels and acidocalcisome maintenance in Trypanosoma brucei . J Biol Chem. 2010;285:24131–24140. doi: 10.1074/jbc.M110.120212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. Faseb J. 2000;14:1265–1278. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SN. Acidocalcisomes - conserved from bacteria to man. Nat Rev Microbiol. 2005;3:251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- Docampo R, Jimenez V, King-Keller S, Li ZH, Moreno SN. The role of acidocalcisomes in the stress response of Trypanosoma cruzi . Adv Parasitol. 2011;75:307–324. doi: 10.1016/B978-0-12-385863-4.00014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Moreno SN. Acidocalcisomes. Cell Calcium. 2011;50:113–119. doi: 10.1016/j.ceca.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Scott DA, Vercesi AE, Moreno SN. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi . Biochem J. 1995;310(Pt 3):1005–1012. doi: 10.1042/bj3101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R, Ulrich P, Moreno SN. Evolution of acidocalcisomes and their role in polyphosphate storage and osmoregulation in eukaryotic microbes. Philos Trans R Soc Lond B Biol Sci. 2010;365:775–784. doi: 10.1098/rstb.2009.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake R, Serrano A, Perez-Castineira JR. N-terminal chimaeras with signal sequences enhance the functional expression and alter the subcellular localization of heterologous membrane-bound inorganic pyrophosphatases in yeast. Biochem J. 2010;426:147–157. doi: 10.1042/BJ20091491. [DOI] [PubMed] [Google Scholar]
- Drozdowicz YM, Shaw M, Nishi M, Striepen B, Liwinski HA, Roos DS, Rea PA. Isolation and characterization of TgVP1, a type I vacuolar H+-translocating pyrophosphatase from Toxoplasma gondii. The dynamics of its subcellular localization and the cellular effects of a diphosphonate inhibitor. J Biol Chem. 2003;278:1075–1085. doi: 10.1074/jbc.M209436200. [DOI] [PubMed] [Google Scholar]
- Du F, Edwards K, Shen Z, Sun B, De Lozanne A, Briggs S, Firtel RA. Regulation of contractile vacuole formation and activity in Dictyostelium . Embo J. 2008;27:2064–2076. doi: 10.1038/emboj.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak JA, Engel JC, Leapman RD, Swyt CR, Pella PA. Trypanosoma cruzi: elemental composition heterogeneity of cloned stocks. Mol Biochem Parasitol. 1988;31:19–26. doi: 10.1016/0166-6851(88)90141-7. [DOI] [PubMed] [Google Scholar]
- Erdnmann R. Kern und metachromatische körper bei sarkosporidien. Arch. Protistenk. 1910;20:239–243. [Google Scholar]
- Essid M, Gopaldass N, Yoshida K, Merrifield C, Soldati T. Rab8a regulates the exocyst-mediated kiss-and-run discharge of the Dictyostelium contractile vacuole. Mol Biol Cell. 2012;23:1267–1282. doi: 10.1091/mbc.E11-06-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Rohloff P, Miranda K, Docampo R. Ablation of a small transmembrane protein of Trypanosoma brucei (TbVTC1) involved in the synthesis of polyphosphate alters acidocalcisome biogenesis and function, and leads to a cytokinesis defect. Biochem J. 2007;407:161–170. doi: 10.1042/BJ20070612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferella M, Nilsson D, Darban H, Rodrigues C, Bontempi EJ, Docampo R, Andersson B. Proteomics in Trypanosoma cruzi-localization of novel proteins to various organelles. Proteomics. 2008;8:2735–2749. doi: 10.1002/pmic.200700940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes AB, Neira I, Ferreira AT, Mortara RA. Cell invasion by Trypanosoma cruzi amastigotes of distinct infectivities: studies on signaling pathways. Parasitol Res. 2006;100:59–68. doi: 10.1007/s00436-006-0236-6. [DOI] [PubMed] [Google Scholar]
- Figarella K, Uzcategui NL, Zhou Y, LeFurgey A, Ouellette M, Bhattacharjee H, Mukhopadhyay R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: possible role in volume regulation and osmotaxis. Mol Microbiol. 2007;65:1006–1017. doi: 10.1111/j.1365-2958.2007.05845.x. [DOI] [PubMed] [Google Scholar]
- Fok AK, Aihara MS, Ishida M, Allen RD. Calmodulin localization and its effects on endocytic and phagocytic membrane trafficking in Paramecium multimicronucleatum . J Eukaryot Microbiol. 2008;55:481–491. doi: 10.1111/j.1550-7408.2008.00347.x. [DOI] [PubMed] [Google Scholar]
- Fok AK, Clarke M, Ma L, Allen RD. Vacuolar H+-ATPase of Dictyostelium discoideum. A monoclonal antibody study. J Cell Sci. 1993;106:1103–1113. doi: 10.1242/jcs.106.4.1103. [DOI] [PubMed] [Google Scholar]
- Fountain SJ, Parkinson K, Young MT, Cao L, Thompson CR, North RA. An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum . Nature. 2007;448:200–203. doi: 10.1038/nature05926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg I, Avigad G. Structures containing polyphosphate in Micrococcus lysodeikticus . J Bacteriol. 1968;96:544–553. doi: 10.1128/jb.96.2.544-553.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furst J, Gschwentner M, Ritter M, Botta G, Jakab M, Mayer M, Garavaglia L, Bazzini C, Rodighiero S, Meyer G, Eichmuller S, Woll E, Paulmichl M. Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. Pflugers Arch. 2002;444:1–25. doi: 10.1007/s00424-002-0805-1. [DOI] [PubMed] [Google Scholar]
- Gabriel D, Hacker U, Kohler J, Muller-Taubenberger A, Schwartz JM, Westphal M, Gerisch G. The contractile vacuole network of Dictyostelium as a distinct organelle: its dynamics visualized by a GFP marker protein. J Cell Sci. 1999;112:3995–4005. doi: 10.1242/jcs.112.22.3995. [DOI] [PubMed] [Google Scholar]
- Gasch AP, Spellman PT, Kao CM, Carmel-Harel O, Eisen MB, Storz G, Botstein D, Brown PO. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerald NJ, Siano M, De Lozanne A. The Dictyostelium LvsA protein is localized on the contractile vacuole and is required for osmoregulation. Traffic. 2002;3:50–60. doi: 10.1034/j.1600-0854.2002.30107.x. [DOI] [PubMed] [Google Scholar]
- Gerisch G, Benjak A, Kohler J, Weber I, Schneider N. GFP-golvesin constructs to study Golgi tubulation and post-Golgi vesicle dynamics in phagocytosis. Eur J Cell Biol. 2004;83:297–303. doi: 10.1078/0171-9335-00393. [DOI] [PubMed] [Google Scholar]
- Gerisch G, Heuser J, Clarke M. Tubular-vesicular transformation in the contractile vacuole system of Dictyostelium . Cell Biol Int. 2002;26:845–852. doi: 10.1006/cbir.2002.0938. [DOI] [PubMed] [Google Scholar]
- Girard-Dias W, Alcantara CL, Cunha ESN, de Souza W, Miranda K. On the ultrastructural organization of Trypanosoma cruzi using cryopreparation methods and electron tomography. Histochem Cell Biol. 2012;138:821–831. doi: 10.1007/s00418-012-1002-8. [DOI] [PubMed] [Google Scholar]
- Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci U S A. 2004;101:10673–10678. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes SA, Fonseca de Souza AL, Silva BA, Kiffer-Moreira T, Santos-Mallet JR, Santos AL, Meyer-Fernandes JR. Trypanosoma rangeli: Differential expression of cell surface polypeptides and ecto-phosphatase activity in short and long epimastigote forms. Exp Parasitol. 2006;112:253–262. doi: 10.1016/j.exppara.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Harris E, Cardelli J. RabD, a Dictyostelium Rab14-related GTPase, regulates phagocytosis and homotypic phagosome and lysosome fusion. J Cell Sci. 2002;115:3703–3713. doi: 10.1242/jcs.00050. [DOI] [PubMed] [Google Scholar]
- Harris E, Yoshida K, Cardelli J, Bush J. Rab11-like GTPase associates with and regulates the structure and function of the contractile vacuole system in Dictyostelium . J Cell Sci. 2001;114:3035–3045. doi: 10.1242/jcs.114.16.3035. [DOI] [PubMed] [Google Scholar]
- Hasne MP, Coppens I, Soysa R, Ullman B. A high-affinity putrescine-cadaverine transporter from Trypanosoma cruzi . Mol Microbiol. 2010;76:78–91. doi: 10.1111/j.1365-2958.2010.07081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RJ, Insall RH. Dictyostelium MEGAPs: F-BAR domain proteins that regulate motility and membrane tubulation in contractile vacuoles. J Cell Sci. 2008a;121:1054–1064. doi: 10.1242/jcs.021113. [DOI] [PubMed] [Google Scholar]
- Heath RJ, Insall RH. F-BAR domains: multifunctional regulators of membrane curvature. J Cell Sci. 2008b;121:1951–1954. doi: 10.1242/jcs.023895. [DOI] [PubMed] [Google Scholar]
- Heuser J. Evidence for recycling of contractile vacuole membrane during osmoregulation in Dictyostelium amoebae--a tribute to Gunther Gerisch. Eur J Cell Biol. 2006;85:859–871. doi: 10.1016/j.ejcb.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Heuser J, Zhu Q, Clarke M. Proton pumps populate the contractile vacuoles of Dictyostelium amoebae. J Cell Biol. 1993;121:1311–1327. doi: 10.1083/jcb.121.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood P. Osmoregulation in the alga Vacuolaria virescens. Structure of the contractile vacuole and the nature of its association with the Golgi apparatus. J Cell Sci. 1978;31:213–224. doi: 10.1242/jcs.31.1.213. [DOI] [PubMed] [Google Scholar]
- Hill JE, Scott DA, Luo S, Docampo R. Cloning and functional expression of a gene encoding a vacuolar-type proton-translocating pyrophosphatase from Trypanosoma cruzi . Biochem J. 2000;351:281–288. doi: 10.1042/0264-6021:3510281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Irving C, Borner GH. Adaptor protein complexes AP-4 and AP-5: new players in endosomal trafficking and progressive spastic paraplegia. Traffic. 2013;14:153–164. doi: 10.1111/tra.12028. [DOI] [PubMed] [Google Scholar]
- Hothorn M, Neumann H, Lenherr ED, Wehner M, Rybin V, Hassa PO, Uttenweiler A, Reinhardt M, Schmidt A, Seiler J, Ladurner AG, Herrmann C, Scheffzek K, Mayer A. Catalytic core of a membrane-associated eukaryotic polyphosphate polymerase. Science. 2009;324:513–516. doi: 10.1126/science.1168120. [DOI] [PubMed] [Google Scholar]
- Huang G, Bartlett PD, Thomas AP, Moreno SNJ, Docampo R. Acidocalcisomes of Trypanosoma brucei have an inositol 1,4,5-trisphosphate receptor that is required for growth and infectivity. Proc. Natl. Acad. Sci USA. 2013 doi: 10.1073/pnas.1216955110. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Fang J, Sant'Anna C, Li ZH, Wellems DL, Rohloff P, Docampo R. Adaptor protein-3 (AP-3) complex mediates the biogenesis of acidocalcisomes and is essential for growth and virulence of Trypanosoma brucei . J Biol Chem. 2011;286:36619–36630. doi: 10.1074/jbc.M111.284661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena BP. Porosome: the secretory NanoMachine in cells. Methods Mol Biol. 2013;931:345–365. doi: 10.1007/978-1-62703-056-4_17. [DOI] [PubMed] [Google Scholar]
- Jensen TE. Electron microscopy of polyphosphate bodies in a blue-green alga, Nostoc pruniforme. Arch, Mikrobiol. 1968;62:144–152. [Google Scholar]
- Jimenez V, Docampo R. Molecular and electrophysiological characterization of a novel cation channel of Trypanosoma cruzi . PLoS Pathog. 2012;8:e1002750. doi: 10.1371/journal.ppat.1002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G, Titus MA, Hammer JA., 3rd The Dictyostelium type V myosin MyoJ is responsible for the cortical association and motility of contractile vacuole membranes. J Cell Biol. 2009;186:555–570. doi: 10.1083/jcb.200810147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Keller S, Li M, Smith A, Zheng S, Kaur G, Yang X, Wang B, Docampo R. Chemical validation of phosphodiesterase C as a chemotherapeutic target in Trypanosoma cruzi, the etiological agent of Chagas' disease. Antimicrob Agents Chemother. 2010;54:3738–3745. doi: 10.1128/AAC.00313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsten JH, Xiong Y, Davis CT, Singleton CK. Subcellular localization of ammonium transporters in Dictyostelium discoideum . BMC Cell Biol. 2008;9:71. doi: 10.1186/1471-2121-9-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H, Yamauchi A, Nakanishi T, Takamitsu Y, Sugiura T, Akagi A, Moriyama T, Horio M, Imai E. Effects of inhibition of myo-inositol transport on MDCK cells under hypertonic environment. Am J Physiol. 1997;272:F267–F272. doi: 10.1152/ajprenal.1997.272.2.F267. [DOI] [PubMed] [Google Scholar]
- Kitching J. The physiology of contractive vacuoles: I. Osmotic relations. J. Exp. Biol. 1934;11:364–381. [Google Scholar]
- Kollien AH, Grospietsch T, Kleffmann T, Zerbst-Boroffka I, Schaub GA. Ionic composition of the rectal contents and excreta of the reduviid bug Triatoma infestans . J Insect Physiol. 2001;47:739–747. doi: 10.1016/s0022-1910(00)00170-0. [DOI] [PubMed] [Google Scholar]
- Komsic-Buchmann K, Stephan LM, Becker B. The SEC6 protein is required for contractile vacuole function in Chlamydomonas reinhardtii . J Cell Sci. 2012;125:2885–2895. doi: 10.1242/jcs.099184. [DOI] [PubMed] [Google Scholar]
- Kunze W. Uber orcheobius herpobdellae Schuberg et Kunze. Arch. Protistenk. 1907;9:382–390. [Google Scholar]
- Kustu S, Inwood W. Biological gas channels for NH3 and CO2: evidence that Rh (Rhesus) proteins are CO2 channels. Transfus Clin Biol. 2006;13:103–110. doi: 10.1016/j.tracli.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Ladenburger EM, Korn I, Kasielke N, Wassmer T, Plattner H. An Ins(1,4,5)P3 receptor in Paramecium is associated with the osmoregulatory system. J Cell Sci. 2006;119:3705–3717. doi: 10.1242/jcs.03075. [DOI] [PubMed] [Google Scholar]
- Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613S–623S. doi: 10.1080/07315724.2007.10719667. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998a;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Volkl H. The diversity of volume regulatory mechanisms. Cell Physiol Biochem. 1998b;8:1–45. doi: 10.1159/000016269. [DOI] [PubMed] [Google Scholar]
- Lee N, Gannavaram S, Selvapandiyan A, Debrabant A. Characterization of metacaspases with trypsin-like activity and their putative role in programmed cell death in the protozoan parasite Leishmania . Eukaryot Cell. 2007;6:1745–1757. doi: 10.1128/EC.00123-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkir Y, de Chassey B, Dubois A, Bogdanovic A, Brady RJ, Destaing O, Bruckert F, O'Halloran TJ, Cosson P, Letourneur F. The AP-1 clathrin-adaptor is required for lysosomal enzymes sorting and biogenesis of the contractile vacuole complex in Dictyostelium cells. Mol Biol Cell. 2003;14:1835–1851. doi: 10.1091/mbc.E02-10-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFurgey A, Ingram P, Blum JJ. Elemental composition of polyphosphate-containing vacuoles and cytoplasm of Leishmania major . Mol Biochem Parasitol. 1990;40:77–86. doi: 10.1016/0166-6851(90)90081-v. [DOI] [PubMed] [Google Scholar]
- LeFurgey A, Ingram P, Blum JJ. Compartmental responses to acute osmotic stress in Leishmania major result in rapid loss of Na+ and Cl. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:385–394. doi: 10.1016/s1095-6433(00)00319-6. [DOI] [PubMed] [Google Scholar]
- Lemercier G, Dutoya S, Luo S, Ruiz FA, Rodrigues CO, Baltz T, Docampo R, Bakalara N. A vacuolar-type H+-pyrophosphatase governs maintenance of functional acidocalcisomes and growth of the insect and mammalian forms of Trypanosoma brucei . J Biol Chem. 2002;277:37369–37376. doi: 10.1074/jbc.M204744200. [DOI] [PubMed] [Google Scholar]
- Lemercier G, Espiau B, Ruiz FA, Vieira M, Luo S, Baltz T, Docampo R, Bakalara N. A pyrophosphatase regulating polyphosphate metabolism in acidocalcisomes is essential for Trypanosoma brucei virulence in mice. J Biol Chem. 2004;279:3420–3425. doi: 10.1074/jbc.M309974200. [DOI] [PubMed] [Google Scholar]
- Li ZH, Alvarez VE, De Gaudenzi JG, Sant'Anna C, Frasch AC, Cazzulo JJ, Docampo R. Hyperosmotic stress induces aquaporin-dependent cell shrinkage, polyphosphate synthesis, amino acid accumulation, and global gene expression changes in Trypanosoma cruzi . J Biol Chem. 2011;286:43959–43971. doi: 10.1074/jbc.M111.311530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder JC, Staehelin LA. A novel model for fluid secretion by the trypanosomatid contractile vacuole apparatus. J Cell Biol. 1979;83:371–382. doi: 10.1083/jcb.83.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HG, Zhong L, de Souza W, Benchimol M, Moreno S, Docampo R. Ca2+ content and expression of an acidocalcisomal calcium pump are elevated in intracellular forms of Trypanosoma cruzi . Mol Cell Biol. 1998;18:2309–2323. doi: 10.1128/mcb.18.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow MJ, Durai L, Ennion SJ. Functional characterization of intracellular Dictyostelium discoideum P2X receptors. J Biol Chem. 2009;284:35227–35239. doi: 10.1074/jbc.M109.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo S, Rohloff P, Cox J, Uyemura SA, Docampo R. Trypanosoma brucei plasma membrane-type Ca(2+)-ATPase 1 (TbPMC1) and 2 (TbPMC2) genes encode functional Ca2+-ATPases localized to the acidocalcisomes and plasma membrane, and essential for Ca2+ homeostasis and growth. J Biol Chem. 2004;279:14427–14439. doi: 10.1074/jbc.M309978200. [DOI] [PubMed] [Google Scholar]
- Luo S, Ruiz FA, Moreno SN. The acidocalcisome Ca2+-ATPase (TgA1) of Toxoplasma gondii is required for polyphosphate storage, intracellular calcium homeostasis and virulence. Mol Microbiol. 2005;55:1034–1045. doi: 10.1111/j.1365-2958.2004.04464.x. [DOI] [PubMed] [Google Scholar]
- Luo S, Vieira M, Graves J, Zhong L, Moreno SN. A plasma membrane-type Ca2+-ATPase co-localizes with a vacuolar H+-pyrophosphatase to acidocalcisomes of Toxoplasma gondii . Embo J. 2001;20:55–64. doi: 10.1093/emboj/20.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macro L, Jaiswal JK, Simon SM. Dynamics of clathrin-mediated endocytosis and its requirement for organelle biogenesis in Dictyostelium . J Cell Sci. 2012 doi: 10.1242/jcs.108837. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira da Silva L, Beverley SM. Expansion of the target of rapamycin (TOR) kinase family and function in Leishmania shows that TOR3 is required for acidocalcisome biogenesis and animal infectivity. Proc Natl Acad Sci U S A. 2010;107:11965–11970. doi: 10.1073/pnas.1004599107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow D, Lusche DF, Schlatterer C, De Lozanne A, Muller-Taubenberger A. The contractile vacuole in Ca2+-regulation in Dictyostelium: its essential function for cAMP-induced Ca2+-influx. BMC Dev Biol. 2006;6:31. doi: 10.1186/1471-213X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesini N, Luo S, Rodrigues CO, Moreno SN, Docampo R. Acidocalcisomes and a vacuolar H+-pyrophosphatase in malaria parasites. Biochem J 347 Pt. 2000;1:243–253. [PMC free article] [PubMed] [Google Scholar]
- Marchesini N, Ruiz FA, Vieira M, Docampo R. Acidocalcisomes are functionally linked to the contractile vacuole of Dictyostelium discoideum . J Biol Chem. 2002;277:8146–8153. doi: 10.1074/jbc.M111130200. [DOI] [PubMed] [Google Scholar]
- McManus ML, Churchwell KB, Strange K. Regulation of cell volume in health and disease. N Engl J Med. 1995;333:1260–1266. doi: 10.1056/NEJM199511093331906. [DOI] [PubMed] [Google Scholar]
- Mendoza M, Mijares A, Rojas H, Rodriguez JP, Urbina JA, DiPolo R. Physiological and morphological evidences for the presence acidocalcisomes in Trypanosoma evansi: single cell fluorescence and 31P NMR studies. Mol Biochem Parasitol. 2002;125:23–33. doi: 10.1016/s0166-6851(02)00166-4. [DOI] [PubMed] [Google Scholar]
- Meyer A. Orientierende untersuchungen ueber verbreitung. Morphologie, und chemie des volutins. Bot. Zeit. 1904;62:113–152. [Google Scholar]
- Miranda K, Docampo R, Grillo O, de Souza W. Acidocalcisomes of trypanosomatids have species-specific elemental composition. Protist. 2004a;155:395–405. doi: 10.1078/1434461042650361. [DOI] [PubMed] [Google Scholar]
- Miranda K, Docampo R, Grillo O, Franzen A, Attias M, Vercesi A, Plattner H, Hentschel J, de Souza W. Dynamics of polymorphism of acidocalcisomes in Leishmania parasites. Histochem Cell Biol. 2004b;121:407–418. doi: 10.1007/s00418-004-0646-4. [DOI] [PubMed] [Google Scholar]
- Miranda K, Rodrigues CO, Hentchel J, Vercesi A, Plattner H, de Souza W, Docampo R. Acidocalcisomes of Phytomonas francai possess distinct morphological characteristics and contain iron. Microsc Microanal. 2004c;10:647–655. doi: 10.1017/S1431927604040887. [DOI] [PubMed] [Google Scholar]
- Molyneux DH, Killick-Kendrick R, Ashford RW. Leishmania in phlebotomid sandflies. III. The ultrastructure of Leishmania mexicana amazonensis in the midgut and pharynx of Lutzomyia longipalpis . Proc R Soc Lond B Biol Sci. 1975;190:341–357. doi: 10.1098/rspb.1975.0098. [DOI] [PubMed] [Google Scholar]
- Moniakis J, Coukell MB, Janiec A. Involvement of the Ca2+-ATPase PAT1 and the contractile vacuole in calcium regulation in Dictyostelium discoideum . J Cell Sci. 1999;112:405–414. doi: 10.1242/jcs.112.3.405. [DOI] [PubMed] [Google Scholar]
- Montalvetti A, Rohloff P, Docampo R. A functional aquaporin co-localizes with the vacuolar proton pyrophosphatase to acidocalcisomes and the contractile vacuole complex of Trypanosoma cruzi . J Biol Chem. 2004;279:38673–38682. doi: 10.1074/jbc.M406304200. [DOI] [PubMed] [Google Scholar]
- Moraes Moreira BL, Soares Medeiros LC, Miranda K, de Souza W, Hentschel J, Plattner H, Barrabin H. Kinetics of pyrophosphate-driven proton uptake by acidocalcisomes of Leptomonas wallacei . Biochem Biophys Res Commun. 2005;334:1206–1213. doi: 10.1016/j.bbrc.2005.06.205. [DOI] [PubMed] [Google Scholar]
- Moreno B, Rodrigues CO, Bailey BN, Urbina JA, Moreno SN, Docampo R, Oldfield E. Magic-angle spinning 31P NMR spectroscopy of condensed phosphates in parasitic protozoa: visualizing the invisible. FEBS Lett. 2002;523:207–212. doi: 10.1016/s0014-5793(02)02977-0. [DOI] [PubMed] [Google Scholar]
- Moreno B, Urbina JA, Oldfield E, Bailey BN, Rodrigues CO, Docampo R. 31P NMR spectroscopy of Trypanosoma brucei, Trypanosoma cruzi, and Leishmania major. Evidence for high levels of condensed inorganic phosphates. J Biol Chem. 2000;275:28356–28362. doi: 10.1074/jbc.M003893200. [DOI] [PubMed] [Google Scholar]
- Moreno SN, Zhong L. Acidocalcisomes in Toxoplasma gondii tachyzoites. Biochem J. 1996;313:655–659. doi: 10.1042/bj3130655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Sanchez D, Hernandez-Ruiz L, Ruiz FA, Docampo R. Polyphosphate is a novel pro-inflammatory regulator of mast cells and is located in acidocalcisomes. J Biol Chem. 2012;287:28435–28444. doi: 10.1074/jbc.M112.385823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta LS, Ramos IB, Gomes FM, de Souza W, Champagne DE, Santiago MF, Docampo R, Miranda K, Machado EA. Proton-pyrophosphatase and polyphosphate in acidocalcisome-like vesicles from oocytes and eggs of Periplaneta americana . Insect Biochem Mol Biol. 2009;39:198–206. doi: 10.1016/j.ibmb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Muller F, Mutch NJ, Schenk WA, Smith SA, Esterl L, Spronk HM, Schmidbauer S, Gahl WA, Morrissey JH, Renne T. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell. 2009;139:1143–1156. doi: 10.1016/j.cell.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullin KA, Foth BJ, Ilgoutz SC, Callaghan JM, Zawadzki JL, McFadden GI, McConville MJ. Regulated degradation of an endoplasmic reticulum membrane protein in a tubular lysosome in Leishmania mexicana . Mol Biol Cell. 2001;12:2364–2377. doi: 10.1091/mbc.12.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neira I, Ferreira AT, Yoshida N. Activation of distinct signal transduction pathways in Trypanosoma cruzi isolates with differential capacity to invade host cells. Int J Parasitol. 2002;32:405–414. doi: 10.1016/s0020-7519(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Nishihara E, Shimmen T, Sonobe S. Functional characterization of contractile vacuole isolated from Amoeba proteus . Cell Struct Funct. 2004;29:85–90. doi: 10.1247/csf.29.85. [DOI] [PubMed] [Google Scholar]
- Nishihara E, Yokota E, Tazaki A, Orii H, Katsuhara M, Kataoka K, Igarashi H, Moriyama Y, Shimmen T, Sonobe S. Presence of aquaporin and V-ATPase on the contractile vacuole of Amoeba proteus . Biol Cell. 2008;100:179–188. doi: 10.1042/BC20070091. [DOI] [PubMed] [Google Scholar]
- Nolta KV, Steck TL. Isolation and initial characterization of the bipartite contractile vacuole complex from Dictyostelium discoideum . J Biol Chem. 1994;269:2225–2233. [PubMed] [Google Scholar]
- O'Halloran TJ, Anderson RG. Clathrin heavy chain is required for pinocytosis, the presence of large vacuoles, and development in Dictyostelium . J Cell Biol. 1992;118:1371–1377. doi: 10.1083/jcb.118.6.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill WC. Physiological significance of volume-regulatory transporters. Am J Physiol. 1999;276:C995–C1011. doi: 10.1152/ajpcell.1999.276.5.C995. [DOI] [PubMed] [Google Scholar]
- Ormerod WE. A comparative study of cytoplasmic inclusions (volutin granules) in different species of trypanosomes. J Gen Microbiol. 1958;19:271–288. doi: 10.1099/00221287-19-2-271. [DOI] [PubMed] [Google Scholar]
- Patel S, Docampo R. Acidic calcium stores open for business: expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol. 2004;164:1065–1076. doi: 10.1083/jcb.200311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden AA, Rudge RE, Lui WW, Robinson MS. Assembly and function of AP-3 complexes in cells expressing mutant subunits. J Cell Biol. 2002;156:327–336. doi: 10.1083/jcb.200107140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philosoph H, Zilberstein D. Regulation of intracellular calcium in promastigotes of the human protozoan parasite Leishmania donovani . J Biol Chem. 1989;264:10420–10424. [PubMed] [Google Scholar]
- Pick U, Weiss M. Polyphosphate Hydrolysis within acidic vacuoles in response to amine-induced alkaline stress in the halotolerant alga Dunaliella salina . Plant Physiol. 1991;97:1234–1240. doi: 10.1104/pp.97.3.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick U, Zeelon O, Weiss M. Amine accumulation in acidic vacuoles protects the halotolerant alga Dunaliella salina against alkaline stress. Plant Physiol. 1991;97:1226–1233. doi: 10.1104/pp.97.3.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos I, Gomes F, Koeller CM, Saito K, Heise N, Masuda H, Docampo R, de Souza W, Machado EA, Miranda K. Acidocalcisomes as calcium- and polyphosphate-storage compartments during embryogenesis of the insect Rhodnius prolixus Stahl. PLoS One. 2011;6:e27276. doi: 10.1371/journal.pone.0027276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos IB, Miranda K, Pace DA, Verbist KC, Lin FY, Zhang Y, Oldfield E, Machado EA, De Souza W, Docampo R. Calcium- and polyphosphate-containing acidic granules of sea urchin eggs are similar to acidocalcisomes, but are not the targets for NAADP. Biochem J. 2010a;429:485–495. doi: 10.1042/BJ20091956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos IB, Miranda K, Ulrich P, Ingram P, LeFurgey A, Machado EA, de Souza W, Docampo R. Calcium- and polyphosphate-containing acidocalcisomes in chicken egg yolk. Biol Cell. 2010b;102:421–434. doi: 10.1042/BC20100011. [DOI] [PubMed] [Google Scholar]
- Riddick DH. Contractile vacuole in the amoeba Pelomyxa carolinensis . Am J Physiol. 1968;215:736–740. doi: 10.1152/ajplegacy.1968.215.3.736. [DOI] [PubMed] [Google Scholar]
- Rodrigues CO, Ruiz FA, Vieira M, Hill JE, Docampo R. An acidocalcisomal exopolyphosphatase from Leishmania major with high affinity for short chain polyphosphate. J Biol Chem. 2002;277:50899–50906. doi: 10.1074/jbc.M208940200. [DOI] [PubMed] [Google Scholar]
- Rodrigues CO, Scott DA, Bailey BN, De Souza W, Benchimol M, Moreno B, Urbina JA, Oldfield E, Moreno SN. Vacuolar proton pyrophosphatase activity and pyrophosphate (PPi) in Toxoplasma gondii as possible chemotherapeutic targets. Biochem J 349 Pt. 2000;3:737–745. doi: 10.1042/bj3490737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues CO, Scott DA, Docampo R. Characterization of a vacuolar pyrophosphatase in Trypanosoma brucei and its localization to acidocalcisomes. Mol Cell Biol. 1999a;19:7712–7723. doi: 10.1128/mcb.19.11.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues CO, Scott DA, Docampo R. Presence of a vacuolar H+-pyrophosphatase in promastigotes of Leishmania donovani and its localization to a different compartment from the vacuolar H+-ATPase. Biochem J. 1999b;340:759–766. [PMC free article] [PubMed] [Google Scholar]
- Rohloff P, Docampo R. Ammonium production during hypo-osmotic stress leads to alkalinization of acidocalcisomes and cytosolic acidification in Trypanosoma cruzi . Mol Biochem Parasitol. 2006;150:249–255. doi: 10.1016/j.molbiopara.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Rohloff P, Docampo R. A contractile vacuole complex is involved in osmoregulation in Trypanosoma cruzi . Exp Parasitol. 2008;118:17–24. doi: 10.1016/j.exppara.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohloff P, Miranda K, Rodrigues JC, Fang J, Galizzi M, Plattner H, Hentschel J, Moreno SN. Calcium uptake and proton transport by acidocalcisomes of Toxoplasma gondii . PLoS One. 2011;6:e18390. doi: 10.1371/journal.pone.0018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohloff P, Montalvetti A, Docampo R. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi . J Biol Chem. 2004;279:52270–52281. doi: 10.1074/jbc.M410372200. [DOI] [PubMed] [Google Scholar]
- Rohloff P, Rodrigues CO, Docampo R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol Biochem Parasitol. 2003;126:219–230. doi: 10.1016/s0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- Rooney EK, Gross JD. ATP-driven Ca2+/H+ antiport in acid vesicles from Dictyostelium . Proc Natl Acad Sci U S A. 1992;89:8025–8029. doi: 10.1073/pnas.89.17.8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney EK, Gross JD, Satre M. Characterisation of an intracellular Ca2+ pump in Dictyostelium . Cell Calcium. 1994;16:509–522. doi: 10.1016/0143-4160(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Rooney PJ, Ayong L, Tobin CM, Moreno SN, Knoll LJ. TgVTC2 is involved in polyphosphate accumulation in Toxoplasma gondii. Mol Biochem Parasitol. 2011;176:121–126. doi: 10.1016/j.molbiopara.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H. The isolation and identification of “volutin” granules from Tetrahymena . Exp Cell Res. 1966;41:397–410. doi: 10.1016/s0014-4827(66)80147-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg H, Munk N. Transport phenomena associated with the deposition and disappearance of pyrophosphate granules in Tetrahymena pyriformis . Biochim Biophys Acta. 1969;184:191–197. doi: 10.1016/0304-4165(69)90114-7. [DOI] [PubMed] [Google Scholar]
- Ruben L, Hutchinson A, Moehlman J. Calcium homeostasis in Trypanosoma brucei. Identification of a pH-sensitive non-mitochondrial calcium pool. J Biol Chem. 1991;266:24351–24358. [PubMed] [Google Scholar]
- Ruiz FA, Lea CR, Oldfield E, Docampo R. Human platelet dense granules contain polyphosphate and are similar to acidocalcisomes of bacteria and unicellular eukaryotes. J Biol Chem. 2004a;279:44250–44257. doi: 10.1074/jbc.M406261200. [DOI] [PubMed] [Google Scholar]
- Ruiz FA, Luo S, Moreno SN, Docampo R. Polyphosphate content and fine structure of acidocalcisomes of Plasmodium falciparum . Microsc Microanal. 2004b;10:563–567. doi: 10.1017/S1431927604040875. [DOI] [PubMed] [Google Scholar]
- Ruiz FA, Marchesini N, Seufferheld M, Govindjee Docampo R. The polyphosphate bodies of Chlamydomonas reinhardtii possess a proton-pumping pyrophosphatase and are similar to acidocalcisomes. J Biol Chem. 2001a;276:46196–46203. doi: 10.1074/jbc.M105268200. [DOI] [PubMed] [Google Scholar]
- Ruiz FA, Rodrigues CO, Docampo R. Rapid changes in polyphosphate content within acidocalcisomes in response to cell growth, differentiation, and environmental stress in Trypanosoma cruzi . J Biol Chem. 2001b;276:26114–26121. doi: 10.1074/jbc.M102402200. [DOI] [PubMed] [Google Scholar]
- Saheb E, Trzyna W, Bush J. An Acanthamoeba castellanii metacaspase associates with the contractile vacuole and functions in osmoregulation. Exp Parasitol. 2012 doi: 10.1016/j.exppara.2012.12.001. in press. [DOI] [PubMed] [Google Scholar]
- Sahin A, Espiau B, Tetaud E, Cuvillier A, Lartigue L, Ambit A, Robinson DR, Merlin G. The Leishmania ARL-1 and Golgi traffic. PLoS One. 2008;3:e1620. doi: 10.1371/journal.pone.0001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salto ML, Kuhlenschmidt T, Kuhlenschmidt M, de Lederkremer RM, Docampo R. Phospholipid and glycolipid composition of acidocalcisomes of Trypanosoma cruzi . Mol Biochem Parasitol. 2008;158:120–130. doi: 10.1016/j.molbiopara.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilde C, Lutter K, Kissmehl R, Plattner H. Molecular identification of a SNAP-25-like SNARE protein in Paramecium . Eukaryot Cell. 2008;7:1387–1402. doi: 10.1128/EC.00012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Nielsen B, Schrauger CR. Amoeba proteus: studying the contractile vacuole by micropuncture. Science. 1963;139:606–607. doi: 10.1126/science.139.3555.606. [DOI] [PubMed] [Google Scholar]
- Schneider N, Schwartz JM, Kohler J, Becker M, Schwarz H, Gerisch G. Golvesin-GFP fusions as distinct markers for Golgi and post-Golgi vesicles in Dictyostelium cells. Biol Cell. 2000;92:495–511. doi: 10.1016/s0248-4900(00)01102-3. [DOI] [PubMed] [Google Scholar]
- Schoijet AC, Miranda K, Girard-Dias W, de Souza W, Flawia MM, Torres HN, Docampo R, Alonso GD. A Trypanosoma cruzi phosphatidylinositol 3-kinase (TcVps34) is involved in osmoregulation and receptor-mediated endocytosis. J Biol Chem. 2008;283:31541–31550. doi: 10.1074/jbc.M801367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoijet AC, Miranda K, Medeiros LC, de Souza W, Flawia MM, Torres HN, Pignataro OP, Docampo R, Alonso GD. Defining the role of a FYVE domain in the localization and activity of a cAMP phosphodiesterase implicated in osmoregulation in Trypanosoma cruzi . Mol Microbiol. 2011;79:50–62. doi: 10.1111/j.1365-2958.2010.07429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, de Souza W, Benchimol M, Zhong L, Lu HG, Moreno SN, Docampo R. Presence of a plant-like proton-pumping pyrophosphatase in acidocalcisomes of Trypanosoma cruzi . J Biol Chem. 1998;273:22151–22158. doi: 10.1074/jbc.273.34.22151. [DOI] [PubMed] [Google Scholar]
- Scott DA, Docampo R. Characterization of isolated acidocalcisomes of Trypanosoma cruzi . J Biol Chem. 2000;275:24215–24221. doi: 10.1074/jbc.M002454200. [DOI] [PubMed] [Google Scholar]
- Scott DA, Docampo R, Dvorak JA, Shi S, Leapman RD. In situ compositional analysis of acidocalcisomes in Trypanosoma cruzi . J Biol Chem. 1997;272:28020–28029. doi: 10.1074/jbc.272.44.28020. [DOI] [PubMed] [Google Scholar]
- Sesaki H, Wong EF, Siu CH. The cell adhesion molecule DdCAD-1 in Dictyostelium is targeted to the cell surface by a nonclassical transport pathway involving contractile vacuoles. J Cell Biol. 1997;138:939–951. doi: 10.1083/jcb.138.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufferheld M, Lea CR, Vieira M, Oldfield E, Docampo R. The H+-pyrophosphatase of Rhodospirillum rubrum is predominantly located in polyphosphate-rich acidocalcisomes. J Biol Chem. 2004;279:51193–51202. doi: 10.1074/jbc.M406099200. [DOI] [PubMed] [Google Scholar]
- Seufferheld M, Vieira MC, Ruiz FA, Rodrigues CO, Moreno SN, Docampo R. Identification of organelles in bacteria similar to acidocalcisomes of unicellular eukaryotes. J Biol Chem. 2003;278:29971–29978. doi: 10.1074/jbc.M304548200. [DOI] [PubMed] [Google Scholar]
- Seufferheld MJ, Kim KM, Whitfield J, Valerio A, Caetano-Anolles G. Evolution of vacuolar proton pyrophosphatase domains and volutin granules: clues into the early evolutionary origin of the acidocalcisome. Biol Direct. 2011;6:50. doi: 10.1186/1745-6150-6-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shively JM. Inclusion bodies of prokaryotes. Annu Rev Microbiol. 1974;28:167–187. doi: 10.1146/annurev.mi.28.100174.001123. [DOI] [PubMed] [Google Scholar]
- Shively JM, Bryant DA, Fuller RC, Konopka AE, Stevens SE, Jr, Strohl WR. Functional inclusions in prokaryotic cells. Int Rev Cytol. 1988;113:35–100. doi: 10.1016/s0074-7696(08)60846-3. [DOI] [PubMed] [Google Scholar]
- Siu CH, Sriskanthadevan S, Wang J, Hou L, Chen G, Xu X, Thomson A, Yang C. Regulation of spatiotemporal expression of cell-cell adhesion molecules during development of Dictyostelium discoideum . Dev Growth Differ. 2011;53:518–527. doi: 10.1111/j.1440-169X.2011.01267.x. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan V, Fountain SJ. Intracellular P2X receptors as novel calcium release channels and modulators of osmoregulation in Dictyostelium: A comparison of two common laboratory strains. Channels (Austin) 2012a;7 doi: 10.4161/chan.22737. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan V, Fountain SJ. A mechanism of intracellular P2X receptor activation. J Biol Chem. 2012b;287:28315–28326. doi: 10.1074/jbc.M112.372565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SA, Mutch NJ, Baskar D, Rohloff P, Docampo R, Morrissey JH. Polyphosphate modulates blood coagulation and fibrinolysis. Proc Natl Acad Sci U S A. 2006;103:903–908. doi: 10.1073/pnas.0507195103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares Medeiros LC, Gomes F, Maciel LR, Seabra SH, Docampo R, Moreno S, Plattner H, Hentschel J, Kawazoe U, Barrabin H, de Souza W, Damatta RA, Miranda K. Volutin granules of Eimeria parasites are acidic compartments and have physiological and structural characteristics similar to acidocalcisomes. J Eukaryot Microbiol. 2011;58:416–423. doi: 10.1111/j.1550-7408.2011.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares Medeiros LC, Moreira BL, Miranda K, de Souza W, Plattner H, Hentschel J, Barrabin H. A proton pumping pyrophosphatase in acidocalcisomes of Herpetomonas sp. Mol Biochem Parasitol. 2005;140:175–182. doi: 10.1016/j.molbiopara.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Sosa RT, Weber MM, Wen Y, O'Halloran TJ. A single beta adaptin contributes to AP1 and AP2 complexes and clathrin function in Dictyostelium . Traffic. 2012;13:305–316. doi: 10.1111/j.1600-0854.2011.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallanzani L. Opuscoli di fisica animale e vegetabile. Modena: Societa Tipografica; 1776. [Google Scholar]
- Sriskanthadevan S, Lee T, Lin Z, Yang D, Siu CH. Cell adhesion molecule DdCAD-1 is imported into contractile vacuoles by membrane invagination in a Ca2+-and conformation-dependent manner. J Biol Chem. 2009;284:36377–36386. doi: 10.1074/jbc.M109.057257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriskanthadevan S, Zhu Y, Manoharan K, Yang C, Siu CH. The cell adhesion molecule DdCAD-1 regulates morphogenesis through differential spatiotemporal expression in Dictyostelium discoideum . Development. 2011;138:2487–2497. doi: 10.1242/dev.060129. [DOI] [PubMed] [Google Scholar]
- Stavrou I, O'Halloran TJ. The monomeric clathrin assembly protein, AP180, regulates contractile vacuole size in Dictyostelium discoideum . Mol Biol Cell. 2006;17:5381–5389. doi: 10.1091/mbc.E06-06-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock C, Gronlien HK, Allen RD. The ionic composition of the contractile vacuole fluid of Paramecium mirrors ion transport across the plasma membrane. Eur J Cell Biol. 2002;81:505–515. doi: 10.1078/0171-9335-00272. [DOI] [PubMed] [Google Scholar]
- Swellengrebel NH. La volutine chez les trypanosomes. C. R. Soc. Biol Paris. 1908:64. [Google Scholar]
- Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, Bonifacino JS, Marks MS, Raposo G. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich PN, Jimenez V, Park M, Martins VP, Atwood J, Moles K, 3rd, Collins D, Rohloff P, Tarleton R, Moreno SN, Orlando R, Docampo R. Identification of contractile vacuole proteins in Trypanosoma cruzi . PLoS One. 2011;6:e18013. doi: 10.1371/journal.pone.0018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina JA, Moreno B, Vierkotter S, Oldfield E, Payares G, Sanoja C, Bailey BN, Yan W, Scott DA, Moreno SN, Docampo R. Trypanosoma cruzi contains major pyrophosphate stores, and its growth in vitro and in vivo is blocked by pyrophosphate analogs. J Biol Chem. 1999;274:33609–33615. doi: 10.1074/jbc.274.47.33609. [DOI] [PubMed] [Google Scholar]
- Vercesi AE, Docampo R. Sodium-proton exchange stimulates Ca2+ release from acidocalcisomes of Trypanosoma brucei . Biochem J. 1996;315(Pt 1):265–270. doi: 10.1042/bj3150265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi AE, Grijalba MT, Docampo R. Inhibition of Ca2+ release from Trypanosoma brucei acidocalcisomes by 3,5-dibutyl-4-hydroxytoluene: role of the Na+/H+ exchanger. Biochem J. 1997;328:479–482. doi: 10.1042/bj3280479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi AE, Moreno SN, Docampo R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei . Biochem J. 1994;304:227–233. doi: 10.1042/bj3040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercesi AE, Rodrigues CO, Catisti R, Docampo R. Presence of a Na+/H+ exchanger in acidocalcisomes of Leishmania donovani and their alkalization by anti-leishmanial drugs. FEBS Lett. 2000;473:203–206. doi: 10.1016/s0014-5793(00)01531-3. [DOI] [PubMed] [Google Scholar]
- Vickerman K, Tetley L. Recent ultrastructural studies on trypanosomes. Ann Soc Belg Med Trop. 1977;57:441–457. [PubMed] [Google Scholar]
- Vieira LL, Lafuente E, Gamarro F, Cabantchik Z. An amino acid channel activated by hypotonically induced swelling of Leishmania major promastigotes. Biochem J. 1996;319(Pt 3):691–697. doi: 10.1042/bj3190691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Virta VC, Riddelle-Spencer K, O'Halloran TJ. Compromise of clathrin function and membrane association by clathrin light chain deletion. Traffic. 2003;4:891–901. doi: 10.1046/j.1600-0854.2003.00144.x. [DOI] [PubMed] [Google Scholar]
- Weiss M, Bental M, Pick U. Hydrolysis of polyphosphates and permeability changes in response to osmotic shocks in cells of the halotolerant alga Dunaliella . Plant Physiol. 1991;97:1241–1248. doi: 10.1104/pp.97.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Stavrou I, Bersuker K, Brady RJ, De Lozanne A, O'Halloran TJ. AP180-mediated trafficking of Vamp7B limits homotypic fusion of Dictyostelium contractile vacuoles. Mol Biol Cell. 2009;20:4278–4288. doi: 10.1091/mbc.E09-03-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiame JH. Etude d’une substance polyphosphorée, basophile et m´tachromatique chez les levures. Biochim. Biophys. Acta. 1947;1:234–255. [Google Scholar]
- Yagisawa F, Nishida K, Yoshida M, Ohnuma M, Shimada T, Fujiwara T, Yoshida Y, Misumi O, Kuroiwa H, Kuroiwa T. Identification of novel proteins in isolated polyphosphate vacuoles in the primitive red alga Cyanidioschyzon merolae. Plant J. 2009;60:882–893. doi: 10.1111/j.1365-313X.2009.04008.x. [DOI] [PubMed] [Google Scholar]
- Zhang H, Gomez-Garcia MR, Brown MR, Kornberg A. Inorganic polyphosphate in Dictyostelium discoideum: influence on development, sporulation, and predation. Proc Natl Acad Sci U S A. 2005a;102:2731–2735. doi: 10.1073/pnas.0500023102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Rao NN, Shiba T, Kornberg A. Inorganic polyphosphate in the social life of Myxococcus xanthus: motility, development, and predation. Proc Natl Acad Sci U S A. 2005b;102:13416–13420. doi: 10.1073/pnas.0506520102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Hsu FF, Scott DA, Docampo R, Turk J, Beverley SM. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Mol Microbiol. 2005c;55:1566–1578. doi: 10.1111/j.1365-2958.2005.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Clarke M. Association of calmodulin and an unconventional myosin with the contractile vacuole complex of Dictyostelium discoideum. J Cell Biol. 1992;118:347–358. doi: 10.1083/jcb.118.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q, Liu T, Clarke M. Calmodulin and the contractile vacuole complex in mitotic cells of Dictyostelium discoideum. J Cell Sci. 1993;104(Pt 4):1119–1127. doi: 10.1242/jcs.104.4.1119. [DOI] [PubMed] [Google Scholar]