Abstract
Purpose
Nilotinib is a second-generation oral tyrosine kinase inhibitor (TKI) with superior efficacy compared with imatinib mesylate in the treatment of chronic phase chronic myelogenous leukemia (CML). Calcium carbonate is commonly used as a source of calcium supplementation or as antacid to ameliorate the gastrointestinal side effects associated with nilotinib, which could have unknown effects on nilotinib absorption. The purpose of this study was to provide information on the effect of calcium carbonate on the PK of nilotinib in healthy volunteers.
Methods
Healthy subjects were enrolled in a 2-period, open-label, single-institution, randomized, cross-over, fixed-schedule study. In one period, each subject received 400 mg of nilotinib p.o. In the other period, 4000 mg calcium carbonate (4 X Tums Ultra 1000®) were administered p.o. 15 minutes prior to the nilonitib dose. Plasma samples were collected at specified timepoints, concentrations of nilotinib were quantitated by LC-MS, and data were analyzed non-compartmentally.
Results
Eleven subjects were evaluable. Calcium supplementation did not significantly affect nilotinib pharmacokinetic parameters including area under the plasma concentration versus time curve (AUC) (18.4 μg/mL•h alone versus 16.9 μg/mL•h with calcium carbonate, P=0.83; 80% power); maximum plasma concentration (Cmax) (0.670 μg/mL alone versus 6.18 μg/mL with calcium carbonate, P=0.97); or half-life (18.9 h alone versus 17.2 h with calcium carbonate, P=0.18).
Conclusions
Our results indicate that the use of calcium carbonate does not significantly affect nilotinib pharmacokinetics.
Keywords: nilotinib, calcium, CML, interaction
Introduction
Nilotinib (Tasigna®) is a second-generation oral tyrosine kinase inhibitor (TKI) with greater binding affinity to the Bcr-Abl fusion protein that is characteristic of chronic myelogenous leukemia (CML). Its superior efficacy in chronic-phase CML was recently demonstrated[1,2]. It is currently approved by the FDA as a first- or second-line therapy in patients with chronic-phase CML. Its application is currently being investigated further in other malignancies, such as gastrointestinal stromal tumors (GIST) and metastastic melanoma, with some degree of success[3,4]. There is increasing evidence that TKIs have exposure response relationships, with a lower exposure resulting in suboptimal therapeutic efficacy[5,6,7], and it is likely that nilotinib also has an exposure response relationship. Similar to other oral TKIs, nilotinib can cause gastrointestinal symptoms, such as nausea, for which patients self-administer various over-the-counter remedies, including calcium carbonate[8]. In addition, nearly a quarter of the population over age 20 has been reported to use calcium supplements, a number that is expected to continue to rise in parallel with the number of older adults at risk for osteoporosis[9,10]. Calcium carbonate has the potential to alter the pharmacokinetics (PK) of nilotinib when taken concomitantly as it can: 1) increase the pH of the stomach content; 2) delay gastric emptying; and 3) supplies calcium ions capable of forming non-absorbable complexes with drugs (e.g. as occurs with tetracycline antibiotics). Consequently, the absorption of nilotinib may be adversely affected by calcium salts taken for either gastric upset or calcium supplementation, resulting in sub-therapeutic concentrations of nilotinib and potentially leading to selection of resistant subclones and ultimately relapse.
We conducted a prospective open-label randomized cross-over study to obtain clinically relevant information on the effect of calcium carbonate on the PK of nilotinib in healthy volunteers.
Methods
Subjects
We conducted a pharmacokinetic study approved by the University of Pittsburgh Institutional Review Board in 12 healthy subjects (6 men, 6 women; ≥ 18 years of age; body mass index < 31 kg/m2 after they provided informed consent. Exclusion criteria were: abnormal bone marrow function; renal dysfunction (proteinuria, estimated creatinine clearance < 60 mL/min/1.73 m2); impaired hepatic function (liver enzymes or bilirubin > the normal upper limit); QTcF > 450 msec on screening ECG (using the QTcF formula); electrolyte abnormality (e.g., hypokalemia, hypomagnesemia, hypophosphatemia, hyperkalemia, hypocalcemia, hyponatremia); pregnancy or breast-feeding; use of any medications (including over-the-counter products, herbal products or mineral supplements) within two weeks of start of the study; or use of an investigational new drug within 28 days of start of the study. Daily multivitamin preparations or oral contraceptives (for women) were allowed.
Study design
The study had a 2-period, open-label, randomized, cross-over, fixed-sequence design and had 80% power to detect a 39% difference in nilotinib AUC with a 5% type I error, assuming a within-subject variability of 30%[11]. In one phase, each subject received 400 mg of nilotinib (Tasigna®, Novartis Pharmaceuticals Corp, East Hanover, NJ) p.o. with 200 mL of water, while in the other phase, 4000 mg calcium carbonate (4 X Tums Ultra 1000®) were administered p.o. 15 minutes prior to the nilotinib dose. The two nilotinib doses were separated by a wash-out period of at least 14 days.
On treatment days, subjects were fasted from midnight the night before dosing until 1 hour after dosing (approximately 10:00 AM), at which time they received a light breakfast. At approximately 11:00 AM, subjects were given a light snack, and after approximately 1:00 PM, subjects could eat per normal schedule. Water was allowed ad libitum. While participating in the study, subjects could not consume any alcoholic or caffeine-containing beverages for 24 h prior to the screening visit or 24 h prior to the day of dosing through the end of PK sampling. Also, subjects could not consume any grapefruit, grapefruit juice, or star fruit for 48 h prior to dosing, and through the end of PK sampling.
Pharmacokinetic sampling and bioanalysis
Venous blood samples (N=13 per subject, 6 mL each) were drawn from an indwelling catheter into heparinized Vacutainer tubes before and at 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, and 72 h after nilotinib administration. Blood samples were centrifuged at 4 °C, 3000 x g for 10 min, and the resulting plasma was aspirated and stored at −20 °C or below until analyzed.
Plasma samples were analyzed for nilotinib using an FDA validated LC-MS method with stable-isotope internal standard that was previously developed and validated in our laboratory over a range of 5–5000 ng/mL[12].
Pharmacokinetic data analysis
The nilotinib plasma pharmacokinetic parameters were determined by standard non-compartmental methods, with PK Solutions 2.0 (Summit Research Services, Montrose, CO; www.summitPK.com). The maximum concentration (Cmax) and time to reach the maximum concentration (tmax) were determined by visual inspection of the plasma concentration versus time curves. The nilotinib elimination rate constant (ke) was obtained using non-linear least-square regression of the terminal concentration versus time data. The nilotinib area under the concentration versus time curve (AUC) was calculated by the trapezoidal rule with extrapolation to infinity (AUC0–∞). The percentage of AUC0–∞ extrapolated beyond the last sample time (Clast), indicating the fraction of the AUC0–∞ that is not based on plasma determinations, was calculated. Ideally, the percentage extrapolated is <20%.
Statistical analysis
Whether or not calcium carbonate had a significant effect on the pharmacokinetics of nilotinib was determined with SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). Pharmacokinetic parameters between day 1 and day 15 were compared using the two-tailed exact Wilcoxon signed rank test. Data were considered significantly different when p < 0.05. The presence of a sequence effect was assessed by the Wilcoxon rank-sum test (R version 2.14.1).
We also performed an analysis of bioequivalence by calculating the 90% confidence intervals of the nilotinib AUC ratio and the Cmax ratio, based on log-transformed data. Equivalence limits were defined as 80–125% [13].
Results
A total of twenty subjects were enrolled, six patients failed screening mostly secondary to abnormal laboratory values. A total of fourteen patients received nilotinib at least once, two patients withdrew consent after the first dose, both experienced grade 1 headache and decided not to proceed but were included in the safety data. Eleven complete data sets were obtained. For 1 subject, the samples from the second visit were not available for analysis. All other subjects tolerated the administrations of nilotinib and calcium carbonate well. The following adverse events were noted: three events of grade 1 headache (3/14, 21%), two events of grade 1 nausea that responded to antiemetics (2/14, 14%), and one patient experienced a grade 1 acneiform rash that was self-limiting (1/14, 7%). All these events were considered possibly related to nilotinib.
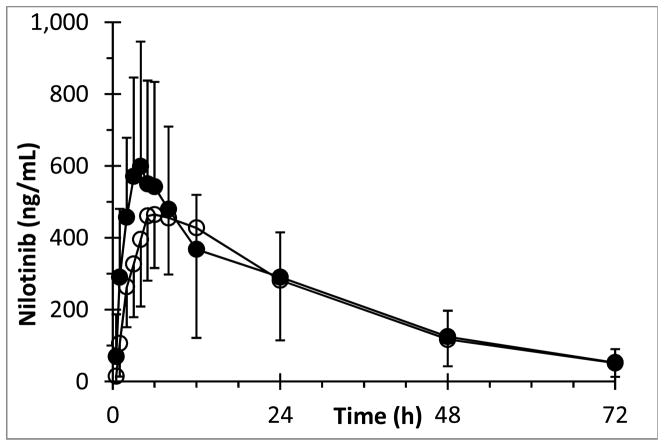
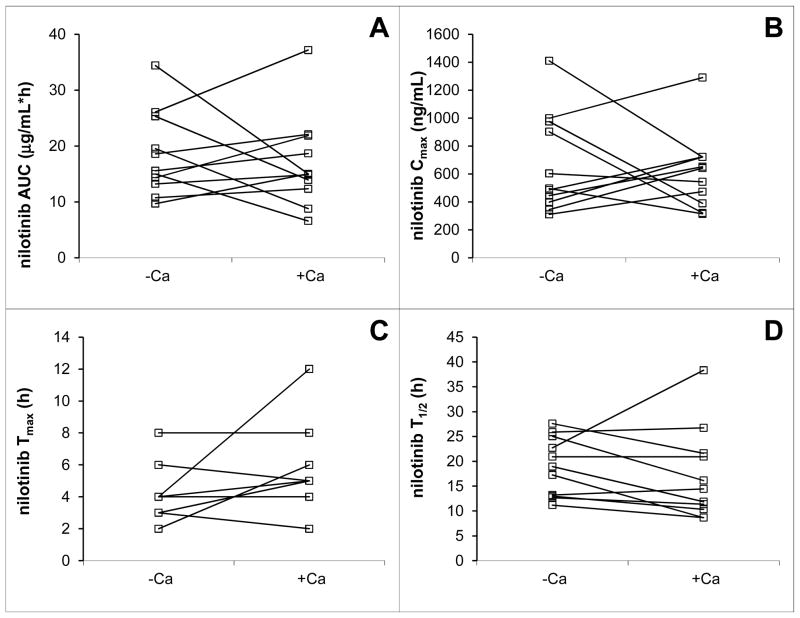
Average concentration versus time curves of nilotinib in the presence and absence of calcium carbonate, respectively, are shown in Fig 1. The pharmacokinetic parameter estimates for nilotinib are shown in Table 1. Statistically, there was no significant difference in nilotinib plasma AUC after dosing of nilotinib alone when compared with the AUC after co-administration of calcium carbonate with nilotinib (P = 0.83). There was no sequence effect in the nilotinib AUC, i.e. the pharmacokinetics were not different between the first and second administration of nilotinib (P = 0.21). The average percentage of the nilotinib AUC extrapolated beyond Clast was 7.7%, with a maximum of 26%, allowing us to interpret our data with confidence. Visual inspection of the average nilotinib plasma concentration versus time curves suggests a slight decrease in Cmax and increase in Tmax in the context of calcium supplementation. However, this observation did not reach statistical significance, nor did we find a significant effect of calcium carbonate on any of the other nilotinib pharmacokinetic parameters (Table 1 and Fig 2).
Fig 1.
Mean (±standard deviation) concentration versus time profile of nilotinib after p.o. administration of 400 mg nilotinib without calcium carbonate (●) and with calcium carbonate (○) in healthy volunteers.
Table 1.
Pharmacokinetic parameter estimates for nilotinib after p.o. administration of nilotinib alone and with co-administration of calcium carbonate (N = 11)
| Parameter | Unit | Nilotinib | Nilotinib + Ca | p-value |
|---|---|---|---|---|
| AUC0-inf | (μg/mL•h) | 18.4 (7.5) | 16.9 (8.2) | 0.831 |
| Cmax | (μg/mL) | 0.670 (0.351) | 0.618 (0.273) | 0.966 |
| tmax | (h) | 4.3 (1.7) | 5.6 (2.5) | 0.117 |
| t1/2 | (h) | 18.9 (5.9) | 17.2 (9.1) | 0.182 |
| Vss/F | (L) | 714 (275) | 739 (330) | 0.898 |
| Cl/F | (L/h) | 24.9 (9.2) | 28.7 (13.9) | 0.765 |
The data are expressed as mean (standard deviation).
The percentage of the AUC0–∞ extrapolated beyond Clast was <26% (average 7.7%).
Fig 2.
Intra-individual changes of nilotinib AUC (A), Cmax (B), Tmax (C), and half-life (D) when p.o. nilotinib was co-administered with calcium carbonate to healthy volunteers.
The 90% confidence intervals of the log-transformed nilotinib AUC ratio (mean 0.89, 90% confidence interval 0.63–1.28) and Cmax ratio (mean 0.96, 90% confidence interval 0.63–1.45) did not fall within the limits (0.80–1.25) set for bioequivalence [13].
Discussion
This healthy volunteer study demonstrates that the use of calcium carbonate was not associated with a significant change in nilotinib pharmacokinetics.
The results are similar to results obtained when nilotinib was co-administered with a Mg2+-Al3+-based antacid [14], but are in contrast to data demonstrating that proton pump inhibitor use is associated with a 27% decrease in nilotinib Cmax and a 34% decrease in nilotinib AUC[15]. The reported decrease in exposure after proton pump inhibitor use may be due to limited dissolution of the nilotinib base in an environment with elevated pH. Based on our results, it appears that a transient elevation in gastric pH, such as occurs with calcium carbonate, has less impact on nilotinib absorption than the more prolonged suppression of gastric acid production associated with proton pump inhibitor use. Moreover, complexation with calcium does not appear to limit nilotinib absorption.
This study was not powered for bioequivalence, however, the 90% confidence interval of the log-transformed ratios of nilotinib AUC and Cmax did not fall within the bioequivalence limits, so we may not conclude that the two modes of administration are equivalent. However, any effect that could occur would likely be small and not clinically relevant.
As cancer continues to transform into a chronic disease treated with oral agents, the potential polypharmacy resulting from concomitant therapy with prescription or over-the-counter medications poses new challenges. The mechanism of action of novel anticancer oral therapies such as TKIs is generally not curative but rather suppressive of tumor growth and is largely exposure dependent. Therefore, drug-drug interactions are likely to significantly impact the outcomes of therapy. Systematic evaluations of such interactions are critical to ensure that failures of cancer therapeutics are not inadvertently induced by seemingly harmless combinations. Conversely, drug-drug interactions could also lead to increased exposure potentially altering the delicate balance between efficacy and toxicity in cancer therapy.
In conclusion, our results show that calcium carbonate use either as an antacid or for calcium supplementation is not associated with significant alterations in nilotinib pharmacokinetics.
What is already known about this subject
Gastric upset is a common side effect of nilotinib therapy, and calcium carbonate is frequently used concomitantly, either as antacid or as calcium supplementation. With the increasing number of oral agents in cancer therapy, oral drug-drug interactions are becoming more relevant. Nilotinib has already been shown to be absorbed to a much lesser extent when co-administered with proton-pump inhibitors. Because exposure to subtherapeutic concentrations of anticancer drugs such as nilotinib may result in selection of resistant clones and ultimately relapse, we studied the effect of a calcium carbonate supplement (Tums Ultra 1000®) on nilotinib pharmacokinetics.
What this study adds
Calcium carbonate may be coadministered with nilotinib without significantly affecting the pharmacokinetics of nilotinib and potentially impacting efficacy.
Acknowledgments
We thank the nursing staff of the University of Pittsburgh Clinical Translational Research Center for their invaluable assistance, and the University of Pittsburgh Cancer Institute Merrill Egorin Writing Group for constructive suggestions regarding the manuscript. This work was supported by Novartis Pharmaceuticals Corporation (East Hanover, NJ), and NIH/NCRR/CTSA Grant UL1 RR024153. This project used the UPCI Clinical Pharmacology Analytical Facility (CPAF) and was supported in part by award P30CA047904.
References
- 1.Saglio G, Kim DW, Issaragrisil S, le CP, Etienne G, Lobo C, Pasquini R, Clark RE, Hochhaus A, Hughes TP, Gallagher N, Hoenekopp A, Dong M, Haque A, Larson RA, Kantarjian HM. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, Hochhaus A, Saglio G, De SC, Flinn IW, Stenke L, Goh YT, Rosti G, Nakamae H, Gallagher NJ, Hoenekopp A, Blakesley RE, Larson RA, Hughes TP. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12:841–851. doi: 10.1016/S1470-2045(11)70201-7. [DOI] [PubMed] [Google Scholar]
- 3.Cho JH, Kim KM, Kwon M, Kim JH, Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs. 2011 doi: 10.1007/s10637-011-9763-9. [DOI] [PubMed] [Google Scholar]
- 4.Sawaki A, Nishida T, Doi T, Yamada Y, Komatsu Y, Kanda T, Kakeji Y, Onozawa Y, Yamasaki M, Ohtsu A. Phase 2 study of nilotinib as third-line therapy for patients with gastrointestinal stromal tumor. Cancer. 2011 doi: 10.1002/cncr.26120. [DOI] [PubMed] [Google Scholar]
- 5.Ravaud A, Bello CL. Exposure-response relationships in patients with metastatic renal cell carcinoma receiving sunitinib: maintaining optimum efficacy in clinical practice. Anticancer Drugs. 2011;22:377–383. doi: 10.1097/CAD.0b013e3283442039. [DOI] [PubMed] [Google Scholar]
- 6.Larson RA, Druker BJ, Guilhot F, O’Brien SG, Riviere GJ, Krahnke T, Gathmann I, Wang Y. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 7.Demetri GD, Wang Y, Wehrle E, Blanke C, Joensuu H, von Mehren M. Correlation of imatinib plasma levels with clinical benefit in patients (Pts) with unresectable/metastatic gastrointestinal stromal tumors (GIST). ASCO Gastrointestinal Cancers Symposium.2008. [Google Scholar]
- 8.Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A, Griffin JD, Hoelzer D, Albitar M, Dugan M, Cortes J, Alland L, Ottmann OG. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 9.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160:339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 10.Straub DA. Calcium supplementation in clinical practice: a review of forms, doses, and indications. Nutr Clin Pract. 2007;22:286–296. doi: 10.1177/0115426507022003286. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MH, Williams G, Johnson JR, Duan J, Gobburu J, Rahman A, Benson K, Leighton J, Kim SK, Wood R, Rothmann M, Chen G, KMU, Staten AM, Pazdur R. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin Cancer Res. 2002;8:935–942. [PubMed] [Google Scholar]
- 12.Parise RA, Egorin MJ, Christner SM, Shah DD, Zhou W, Beumer JH. A high-performance liquid chromatography-mass spectrometry assay for quantitation of the tyrosine kinase inhibitor nilotinib in human plasma and serum. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1894–1900. doi: 10.1016/j.jchromb.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Campos DR, Vieira NR, Bernasconi G, Barros FA, Meurer EC, Marchioretto MA, Coelho EC, Calafatti SA, Sommer C, Couto JM, Buranello S, Silva AR, Amarante AR, Abib E, Junior JP. Bioequivalence of two enteric coated formulations of pantoprazole in healthy volunteers under fasting and fed conditions. Arzneimittelforschung. 2007;57:309–314. doi: 10.1055/s-0031-1296624. [DOI] [PubMed] [Google Scholar]
- 14.Sparano BA, Egorin MJ, Parise RA, Walters J, Komazec KA, Redner RL, Beumer JH. Effect of antacid on imatinib absorption. Cancer Chemother Pharmacol. 2009;63:525–528. doi: 10.1007/s00280-008-0778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin OQ, Gallagher N, Fischer D, Demirhan E, Zhou W, Golor G, Schran H. Effect of the proton pump inhibitor esomeprazole on the oral absorption and pharmacokinetics of nilotinib. J Clin Pharmacol. 2010;50:960–967. doi: 10.1177/0091270009346061. [DOI] [PubMed] [Google Scholar]