Abstract
As interest in the study of antioxidant intake from foods and other agricultural products increases, methods for performing radical scavenging activity assays based on the electron paramagnetic resonance spectroscopic method, in which there is no interference from the sample color and turbidity, are required. In this study, we have developed a rapid and simple electron paramagnetic resonance based assay to evaluate the alkylperoxyl radical scavenging activity of several antioxidants. The alkylperoxyl radical species was generated by the photolysis of azo-radical initiator 2,2'-azobis(isobutyronitrile), in which the radical generation rate and period were controlled by the illumination light. The relative alkylperoxyl radical scavenging activity was obtained by a simple formula of competing reaction of antioxidant and spin trap toward the oxygen radical. The scavenging activities toward alkylperoxyl radical and alkoxy radical species were evaluated in six antioxidants. Although quercetin showed the highest activity toward both radicals, the order of the relative activities in the other antioxidants was different mutually between the alkylperoxyl radical and the alkoxyl radical. This alkylperoxyl radical scavenging activity assay based on electron paramagnetic resonance spectroscopy is useful for evaluation of colored and turbid food samples.
Keywords: alkylperoxyl radical, radical scavenging activity, EPR spin trapping, AIBN
Introduction
Previously, we reported the electron paramagnetic resonance based oxygen radical absorbance capacity (ORAC-EPR) assay by employing the electron paramagnetic resonance (EPR) spin trap method, which evaluates the radical scavenging activity (RSA) of antioxidants toward the oxygen radical produced from the azo-radical initiator 2,2'-azobis(2-amidinopropane) dihydrochloride (AAPH).(1) This was developed as a rapid ORAC assay with simpler data analysis in comparison to the original fluorescence-based ORAC (ORAC-FL) assay developed by Ou et al.(2) It was originally thought that the oxygen radical produced by AAPH in the original ORAC-FL assay was an alkylperoxyl radical (ROO•). However, the experiments have showed the oxygen radical to be alkoxyl radical (RO•) in nature.(3,4) Accordingly, the objective of this study was to develop a novel assay to evaluate the RSA of antioxidants toward ROO• by adopting the framework of the ORAC-EPR.
ROO•, one of the reactive oxygen radicals generated in organisms, also functions as good oxidizing agents.(5) ROO• is reproduced in the chain reaction processes as well as damages biological molecules in organisms.(6) Such propagation of consequent damage to biological molecules continues until reproduced ROO• is scavenged. It has been thought that ROO• of hydrophilic proteins damages other biological molecules in organisms.(7) Since the production of ROO• is associated with the propagation of serious damage to biological molecules in organisms, hydrophilic antioxidants are thought to scavenge ROO• frequently.(8) The ROO• scavenging activity of hydrophilic antioxidants is an important area of reactive oxygen species scavenging, which should be further evaluated.
Because of increasing interest in the reduction of oxidative stress by the intake of antioxidants through a proper daily diet,(9,10) an RSA evaluation method for samples extracted from fruits, vegetables, and other edible natural products is desired. Many samples extracted from these sources are colored owing to the presence of natural pigments such as anthocyanidins or carotenoids. EPR spectroscopy, the method employed in this study, is a magnetic resonance spectroscopy, which is not affected by sample color owing to utilizing microwave. The assay to evaluate the RSA of colored samples would be advantageous in comparison to other spectrophotometric methods.
Materials and Methods
Materials
AAPH, acetonitrile, 2,2'-azobis(isobutyronitrile) (AIBN), caffeic acid, glutathione, n-propyl gallate (PG), quercetin, trolox, uric acid and sodium phosphate were purchased from Wako Pure Chem. Ind. Ltd. (Osaka, Japan). The spin trap CYPMPO [5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline-N-oxide] was obtained from Radical Research Inc. (Tokyo, Japan).
Instrumentation
A UV light illuminator (RUVF-203SR, Radical Research Inc., Tokyo, Japan) was employed to generate oxygen radicals from the azo-radical initiator. The light source was a 200-W Xe arc lamp (San-ei Electronics, Osaka, Japan). The illuminator was equipped with a quartz fiber-optic guide and its far end was mechanically fitted to the hole in front of the EPR cavity. The illuminator was equipped with a computer-controllable mechanical shutter, which controlled the illumination time. The illumination period was determined so that a sufficient amount of the oxygen radical adduct would be obtained. Light from the Xe arc lamp light was attenuated to minimize the photochemical decomposition of the antioxidants. For EPR measurements, a JEOL RRX-1XS X-band ESR spectrometer equipped with 100 kHz field modulation (Tokyo, Japan) and the WIN-RAD operation software (Radical Research Inc.) was employed. The spectrometer settings were as follows: field modulation width, 0.1 mT; microwave power, 6 mW; field scan width/rate, ± 7.5 mT/2 min; time constant, 0.1 s. A disposable borosilicate flat sample cell (Radical Research Inc.), which was loaded with the sample liquid, was placed inside the EPR cavity and the EPR signal was recorded. The magnetic field of the observed spectrum was corrected by the spectrum of the same radical observed in the other EPR spectrometer. The hyperfine coupling constants [HFCC, A(H), A(N), A(P)] were estimated by fitting the Gaussian type function to the observed spectrum using the least square method. HFCC is represented in militesra in this paper.
ROO• scavenging activity assay
AIBN and quercetin were dissolved in acetonitrile. The other antioxidants (caffeic acid, glutathione, PG, trolox and uric acid) and CYPMPO were dissolved in sodium phosphate buffer (100 mM, pH 7.4). The assay mixture was prepared by mixing the constituent solutions. The typical concentrations of the constituents in the assay mixture were as follows: AIBN, 10 mM; CYPMPO, 10 mM; antioxidant, 0.1 mM; acetonitrile, 20% (w/w); 100 mM sodium phosphate buffer, 80% (w/w). After loading into the flat sample cell, the liquid sample was illuminated for 180 s inside the cavity. The EPR signal was recorded immediately after termination of the illumination.
RO• scavenging activity assay
Quercetin was dissolved in acetonitrile, and the other chemicals were dissolved in sodium phosphate buffer (100 mM, pH 7.4). The assay mixture was prepared by mixing the constituent solutions. The concentrations of the constituents in the assay mixture were as follows: AAPH, 20 mM; CYPMPO, 10 mM; antioxidant, 0.1 mM in 100 mM sodium phosphate buffer. Twenty percent (w/w) of the solvent was replaced by acetonitrile in RSA assay of quercetin and the blank for quercetin. The peak height of the EPR spectrum did not show a statistically significant change as compared to the blank prior to solvent replacement (p = 0.36, two-sided t test, n = 5). Once loaded into the flat sample cell, the liquid sample was illuminated for 60 s inside the cavity. The EPR signal was recorded immediately after termination of the illumination.
Data analysis
The ROO• and RO• scavenging activity was evaluated by comparison of the rate constants in this assay. The reactions of ROO• (or RO•), spin trap CYPMPO (C), and antioxidant (A) were assumed to be as follows:
| ROO• + C → S, rate constant: kC | (1) |
| ROO• + A → X, rate constant: kA | (2) |
S, and X represent the spin adduct and product generated by the reaction, respectively. It was assumed that no other reactions between the components occurred and that only the spin adduct gave the EPR signal. The rate equations are given as follows, respectively.
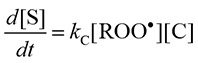 |
(3) |
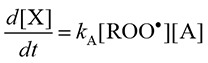 |
(4) |
The ratio of [X] and [S] is derived as follows:
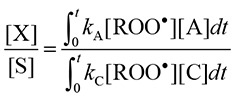 |
(5) |
[X] can be replaced by [S]B – [S], where [S]B and [S] represent the concentration of the spin adduct in the presence of the spin trap alone (the blank) and in the combined presence of the antioxidant and spin trap, respectively. In addition, since the EPR peak heights are proportional to the concentration of the spin adduct, ([S]B – [S])/[S] on the left side of Equation 5 is replaced by (IB – I)/I, where IB and I denote the peak height in the presence of the spin trap alone and in the combined presence of the antioxidant and spin trap, respectively. The amounts of C and A consumed are assumed to be negligible in the reaction and [C] and [A] are nearly equal to [C]0 and [A]0, respectively, in which [ ]0 denotes the initial concentration. Finally the rate constant of the antioxidant normalized by spin trap CYPMPO is given as follows:
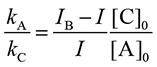 |
(6) |
The height of the 5th peak from the lower-magnetic-field side was employed for the analysis as IB and I.
Results
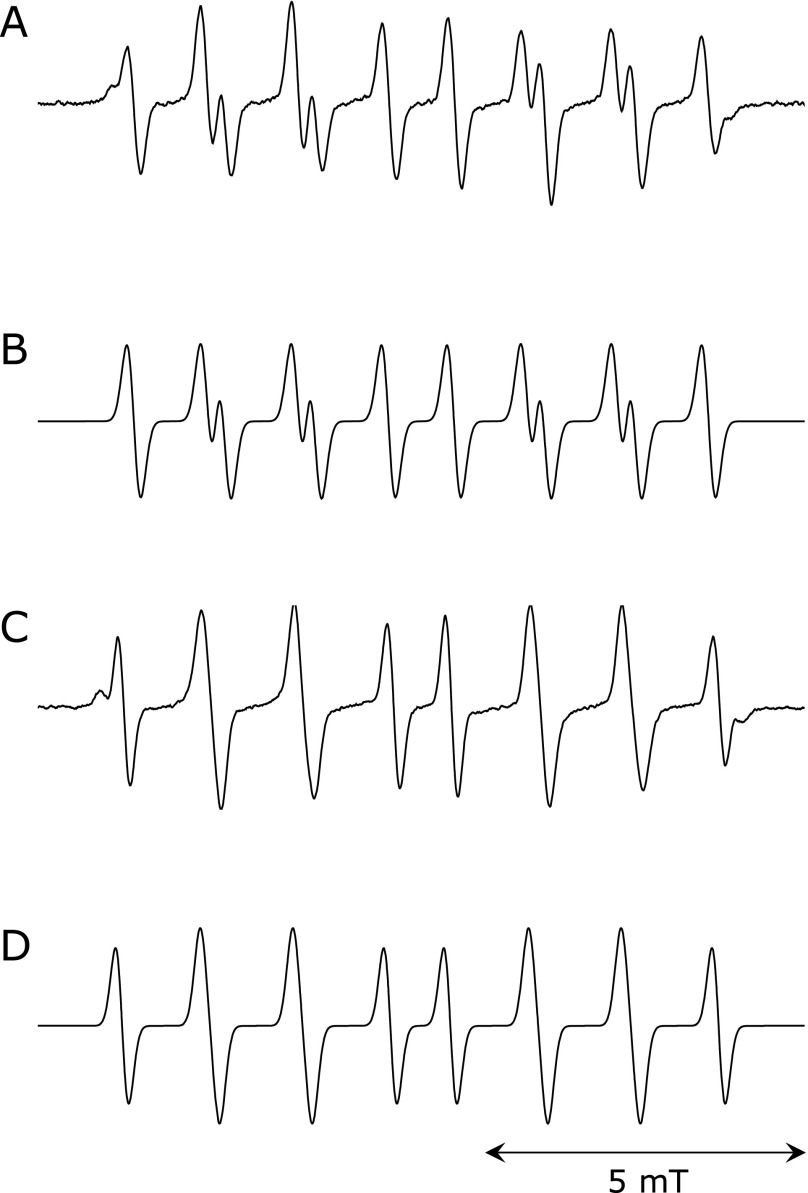
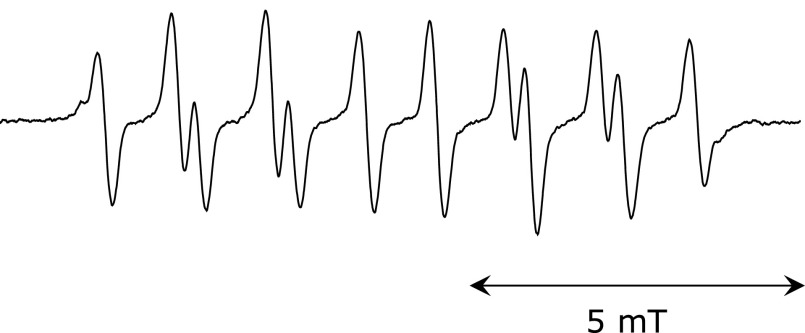
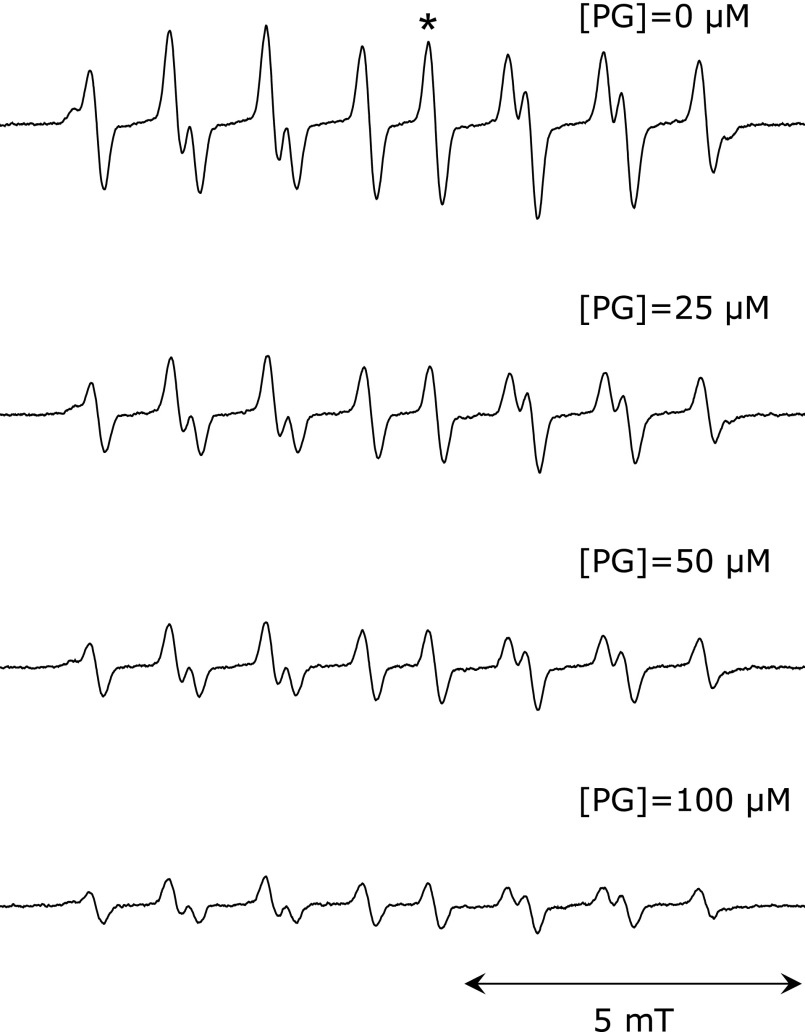
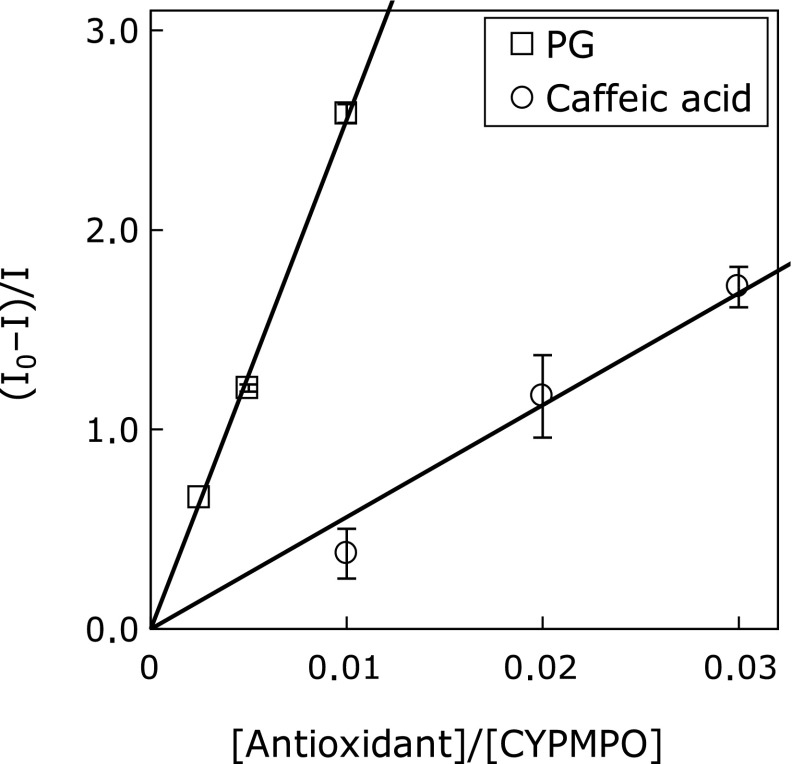
The EPR spectra of the spin adduct of CYPMPO when coexisting AIBN was photolyzed is shown in Fig. 1A, and its simulated spectrum is shown in Fig. 1B. The EPR spectrum pattern was same as the reported spectrum generated by the photolysis of AIBN.(4) The HFCCs were estimated as follows: A(H) = 1.11, A(N) = 1.33, A(P) = 4.68. This spectrum was assigned to ROO• adduct of CYPMPO [R is -C(CH3)2-CN]. The EPR spectrum of radical adduct of CYPMPO by the photolysis of AAPH and its simulation spectrum are shown in Fig. 1C and D, respectively. The relative differences of the estimated HFCCs [A(H) = 1.21, A(N) = 1.35, A(P) = 4.78] were less than 3% compared to the previously reported values.(4) This spectrum was assigned to RO• adduct of CYPMPO [R is -C(CH3)2-C(NH2)NH2+]. The same spectrum pattern was also obtained in experiments where an AIBN solution cooled in an ice bath during illumination was mixed with CYPMPO solution after termination of illumination (Fig. 2). The estimated hyperfine coupling constants were as follows: A(H) = 1.03, A(N) = 1.30, A(P) = 4.62. The EPR spectra of the ROO• adduct in the presence of various concentrations of PG are shown in Fig. 3. As the concentration of PG increased, the peak height decreased. The value of (IB – I)/I was plotted as a function of [Antioxidant]/[CYPMPO] in Fig. 4 in which the antioxidant was caffeic acid, and PG. The theoretical relationship is represented as a line passing through the origin by the equation, (IB – I)/I = kA/kC [Antioxidant]/[CYPMPO], derived from Equation 6. The results were well fitted to the theoretical line. The relative RSAs of the antioxidants toward ROO• and RO• are summarized in Table 1. The orders of the relative RSAs were different between ROO• and RO• but quercetin which was the top in both two radicals.
Fig. 1.
Observed EPR spectra of radical adduct of CYPMPO and their simulated spectra. A) An observed EPR spectrum of CYPMPO AIBN-derived radical. A 80 µl solution containing 10 mM AIBN, 10 mM CYPMPO, 80% (w/w) of 100 mM phosphate buffer, and 20% (w/w) of acetonitrile was illuminated with UV light for 180 s. B) The EPR spectrum simulating the observed spectrum shown in Fig. 1A. The hyperfine coupling constants (mT) used for the simulation were as follows: A(H) = 1.07, A(N) = 1.32, A(P) = 4.66. C) An observed EPR spectrum of CYPMPO AAPH-derived radical. A 80 µl solution containing 20 mM AAPH, 10 mM CYPMPO, and 100 mM phosphate buffer was illuminated with UV light for 60 s. D) The EPR spectrum simulating the observed spectrum shown in Fig. 1C. The hyperfine coupling constants (mT) used for the simulation were as follows: A(H) = 1.21, A(N) = 1.35, A(P) = 4.78.
Fig. 2.
EPR spectrum of spin adduct of CYPMPO trapping the radical generated from AIBN after termination of illumination. A 72 µl solution containing 11 mM AIBN, 67% (w/w) of 100 mM phosphate buffer, and 33% (w/w) of acetonitrile was illuminated by UV light for 30 s on an ice bath, followed by addition of an 8 µl solution of 100 mM CYPMPO after termination of illumination.
Fig. 3.
EPR spectra of AIBN-derived alkylperoxyl radical adduct of CYPMPO in the presence of several concentrations of PG. The height of the peak marked with an asterisk (*) was used for the analysis. A 80 µl solution containing 1 mM AIBN, 10 mM CYPMPO, PG, 95% (w/w) of 100 mM phosphate buffer, and 5% (w/w) of acetonitrile was illuminated with UV light for 60 s.
Fig. 4.
Plot of (IB – I)/I against [Antioxidant]/[CYPMPO] and the regression line. PG and caffeic acid were chosen as antioxidants. The value of (IB – I)/I is the average of three measurements. The error bar represents the standard deviation. The coefficients of determinations of the regression line were 0.997 and 0.959 in PG and caffeic acid, respectively.
Table 1.
Summary of ROO• and RO• scavenging activity of antioxidants evaluated in this study
| Antioxidant | ROO• scavenging activity |
RO• scavenging activity |
||
|---|---|---|---|---|
| Order | kA/kC† | Order | kA/kC‡ | |
| Caffeic acid | 6 | 79 ± 6.6 (8.4) | 3 | 550 ± 40 (7.3) |
| Glutathione | 4 | 120 ± 16 (13) | 6 | 160 ± 27 (17) |
| PG | 3 | 210 ± 15 (7.1) | 2 | 820 ± 160 (20) |
| Quercetin | 1 | 330 ± 46 (14) | 1 | 1200 ± 130 (11) |
| Trolox | 2 | 230 ± 21 (9.1) | 5 | 260 ± 54 (21) |
| Uric acid | 5 | 87 ± 13 (15) | 4 | 280 ± 31 (11) |
†Mean ± SD (Relative SD, %), n = 5.
‡Mean ± SD (Relative SD, %), n = 4 or 5.
Discussion
By employing the framework of the ORAC-EPR, a method for evaluating the ROO• scavenging activity of the hydrophilic antioxidants was developed. Some merits, including the moderate concentration of the azo-radical initiator (10 mM) required, the controllable generation of ROO• by adjusting the intensity and the period of the illumination light, and the simple data analysis, were inherited from the original ORAC-EPR method.(1) Minimal photodamage of the antioxidants and sufficient generation of ROO• could be accomplished by optimizing the power of the illumination source and the illumination period. Previously, Rodrigues et al.(11) developed an alkylperoxy RSA assay of lipophilic antioxidants by employing the thermolysis of AIBN (175 mM, 41°C), similar to the ORAC-FL assay. The assay period in this study (180 s) was much shorter than that in the above-mentioned thermolysis assay (120 min). Since the framework employed in this study is similar to that of the ORAC-EPR assay, the RSA assay toward two oxygen radicals (RO• and ROO•) could be implemented by changing the radical generation reagent and the illumination period. Additionally, the chemical decomposition, or photolysis of tert-butyl hydroperoxide has been utilized to generate ROO• in hydrophilic solution.(12,13) It has also been reported that oxygen radicals other than ROO• were generated simultaneously by the chemical decomposition, and photolysis of tert-butyl hydroperoxide.(13,14) In contrast, AIBN which was utilized in this study was decomposed into alkyl radical and nitrogen by the photolysis. This alkyl radical species rapidly reacts with dissolved oxygen molecule to generate ROO• (second-order rate constant: ca. 109 M−1s−1).(3) The EPR spectra of the spin adduct assigned to other radicals were not observed in the photolysis of AIBN. Some radicals which are not assumed in this assay are generated from antioxidants by the UV light illumination, or in radical scavenging reactions possibly. When the spin trap and the spin adduct are consumed by some extra radicals, the evaluation of the RSA is affected directly. The linear relationship of (IB – I)/I and [Antioxidant]/[CYPMPO] and the EPR spectrum pattern can be useful to find extra radicals. If the linearity is insufficient, or other spectrum pattern is superimposed on the EPR spectrum of the spin adduct, the spin adduct, or spin trap may react with extra radicals. The experimental condition should be modified to inhibit the extra radical generation in the case.
Although the absolute RSAs toward ROO• and RO• can not be compared by kA/kC obtained in this study, the relative activity can be compared by kA/kC. The relative RSA toward both ROO• and RO• was the highest in quercetin among the six antioxidants. Both ROO• and RO• cause hydrogen abstraction reaction.(15) This common reactivity of these radicals and rich hydroxyl groups of quercetin may be responsible for these relatively high activities. Trolox, ranked 5th in relative RSA order toward RO•, could be ranked second in relative RSA order toward ROO• among the six antioxidants. This difference is reasonable considering that trolox is an analog of α-tocopherol, which is an inhibitor of lipid peroxidation.(16) No common order among the relative activities was evident in any of the antioxidants except for quercetin. The difference in the reactivity of the oxygen radical species (ROO• and RO•) with antioxidants contributed to the difference of the relative RSAs of the antioxidant toward ROO• and RO•. The corresponding oxygen radical should be generated in the oxygen RSA assay of antioxidants. It was thought that the other factors (the difference of alkyl group, the presence of acetonitrile, and so on) also contributed to the difference of the relative RSAs.
The estimated HFCCs of the AIBN-derived peroxyl radical adduct in this study was larger than the previously reported values [A(H) = 0.84, A(N) = 1.24, A(P) = 4.39].(4) Although the previous reported HFCCs were measured in benzene, we obtained the HFCCs in the mixed solvent of phosphate buffer and acetonitrile. It was reported that the increased value of HFCC was observed when the nitroxide radical was contained in polar solvent.(17) The larger values of our HFCCs were thought to due to be the difference of the solvent.
Assay solutions including the antioxidant and AIBN were illuminated by UV light to generate ROO• in this study. If the antioxidant is sensitive to UV light, photochemical reactions will result from UV light exposure. We propose a potential solution to resolve this shortcoming. It was found that radicals were also generated after termination of the illumination, provided the AIBN solution was sufficiently cooled during illumination (Fig. 2). Engel(18) has reported the isomerization of azoalkanes in which two alkyl groups bonded to the azo group adopt the stable trans-configuration. Absorption of UV light promotes transformation to the unstable cis-isomer, which then decomposes to two alkyl radicals and a nitrogen molecule. AIBN also decomposes rapidly via isomerization of the stable trans-isomer to the unstable cis-isomer by UV absorption. It is thought that cooling decreased the decomposition rate. The remaining cis-isomer was allowed to generate the radicals after termination of illumination. If this radical generation method is adopted in our assay, UV-induced photochemical damage of antioxidants will not be caused by addition of illuminated AIBN solution to antioxidant solution. Further investigation of optimal experimental parameters, including cooling temperature, UV illumination period, and AIBN concentration, is needed.
In addition to agricultural and natural products, antioxidants can be also taken through processed foods in which antioxidants are enriched. Addition of antioxidants derived from natural sources to raw materials of processed foods is common during the manufacturing process. Processing such as heating might result in deterioration of the antioxidant RSA. The decrease in the RSA of processed foods found in each processing step is a concern as well as the RSA in the final products. If the processing conditions are optimized to prevent decrease of the RSA by evaluating the consequent change in RSA of the processed foods, the rapid RSA assay described here will be useful. Processed foods also contain natural pigments derived from materials or added coloring agents. Though several spectrophotometric methods that determine RSA have been developed, these methods show possible interference by sample pigmentation.(19,20) In the 1,1-diphenyl-2-picrylhydrazyl (DPPH) RSA assay, in which the same sample was evaluated by using both EPR and UV-visible spectrophotometry, it was suggested that absorption by the decomposition products of the antioxidants accounts for the difference in the outcomes of the two techniques.(21) If necessary, turbid samples, which are not extracted, can be measured by the EPR based assay reported herein. In conclusion, this RSA assay employing EPR spectroscopy is highly useful for the determination of ROO• scavenging activities of colored or turbid food samples.
Acknowledgments
This work was supported by a grant from Sapporo Biocluster “Bio-S”, the Knowledge Cluster Initiative of the Ministry of Education, Sports, Science, and Technology (MEXT).
Abbreviations
- AAPH
2,2'-azobis(2-amidinopropane) dihydrochloride
- AIBN
2,2'-azobis(isobutyronitrile)
- CYPMPO
5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline-N-oxide
- EPR
electron paramagnetic resonance
- HFCC
hyperfine coupling constant
- ORAC-EPR
electron paramagnetic resonance based oxygen radical absorbance capacty
- PG
n-propyl gallate
- ROO•
alkylperoxyl radical
- RO•
alkoxyl radical
- RSA
radical scavenging activity
- UV
ultraviolet
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Kohri S, Fujii H, Oowada S, et al. An oxygen radical absorbance capacity-like assay that directly quantifies the antioxidant's scavenging capacity against AAPH-derived free radicals. Anal Biochem. 2009;386:167–171. doi: 10.1016/j.ab.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 2.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 3.Krainev AG, Bigelow DJ. Comparison of 2,2'-azobis(2-amidinopropane) hydrochloride (AAPH) and 2,2'-azobis(2,4-dimethylvaleronitrile) (AMVN) as free radical initiators: a spin-trapping study. J Chem Soc Perkin Trans. 1996;2:747–754. [Google Scholar]
- 4.Sueishi Y, Yoshioka D, Oowada S, et al. Is the oxygen radical absorbance capacity (ORAC) method a peroxyl-radical scavenging assay? Z Phys Chem. 2010;224:921–928. [Google Scholar]
- 5.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Archiv Biochem Biophys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 6.Choe E, Min DB. Chemistry and reactions of reactive oxygen species in foods. J Food Sci. 2005;70:R142–R159. doi: 10.1080/10408390500455474. [DOI] [PubMed] [Google Scholar]
- 7.Gebicki JM, Du J, Collins J, Tweeddale H. Peroxidation of proteins and lipids in suspensions of liposomes, in blood serum, and in mouse myeloma cells. Acta Biochim Pol. 2000;47:901–911. [PubMed] [Google Scholar]
- 8.Nauser T, Koppenol WH, Gebicki JM. The kinetics of oxidation of GSH by protein radicals. Biochem J. 2005;392:693–701. doi: 10.1042/BJ20050539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lordan S, Ross RP, Stanton C. Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar Drugs. 2011;9:1056–1100. doi: 10.3390/md9061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traka MH, Mithen RF. Plant science and human nutrition: challenges in assessing health-promoting properties of phytochemicals. Plant Cell. 2011;23:2483–2497. doi: 10.1105/tpc.111.087916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodrigues E, Mariutti LR, Chisté RC, Mercadante AZ. Development of a novel micro-assay for evaluation of peroxyl radical scavenger capacity: application to carotenoids and structure-activity relationship. Food Chem. 2012;135:2103–2111. doi: 10.1016/j.foodchem.2012.06.074. [DOI] [PubMed] [Google Scholar]
- 12.Kalyanaraman B, Mottley C, Mason RP. A direct electron spin resonance and spin-trapping investigation of peroxyl free radical formation by hematin/hydroperoxide systems. J Biol Chem. 1983;258:3855–3858. [PubMed] [Google Scholar]
- 13.Davies MJ, Slater TF. Studies on the photolytic breakdown of hydroperoxides and peroxidized fatty acids by using electron spin resonance spectroscopy. Spin trapping of alkoxyl and peroxyl radicals in organic solvents. Biochem J. 1986;240:789–795. doi: 10.1042/bj2400789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies MJ. Detection of peroxyl and alkoxyl radicals produced by reaction of hydroperoxides with heme-proteins by electron spin resonance spectroscopy. Biochim Biophys Acta. 1988;964:28–35. doi: 10.1016/0304-4165(88)90063-3. [DOI] [PubMed] [Google Scholar]
- 15.Marnett LJ. Peroxyl free radicals: potential mediators of tumor initiation and promotion. Carcinogenesis. 1987;8:1365–1373. doi: 10.1093/carcin/8.10.1365. [DOI] [PubMed] [Google Scholar]
- 16.Davies MJ, Forni LG, Willson RL. Vitamin E analogue Trolox C. E.s.r. and pulse-radiolysis studies of free-radical reactions. Biochem J. 1988;255:513–522. [PMC free article] [PubMed] [Google Scholar]
- 17.Mukai K, Nishiguchi H, Ishizu K, Deguchi Y, Takaki H, et al. Solvent effects in the electron spin resonance spectra of some phenoxyl, nitroxide, and anilino radicals. Bull Chem Soc Jpn. 1967;40:2731–2739. [Google Scholar]
- 18.Engel PS. Mechanism of the thermal and photochemical decomposition of azoalkanes. Chem Rev. 1980;80:99–150. [Google Scholar]
- 19.Huang D, Ou B, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 20.Karadag A, Ozcelik B, Saner S. Review of methods to determine antioxidant capacities. Food Anal Methods. 2009;2:41–60. [Google Scholar]
- 21.Sanna D, Delogu G, Mulas M, Schirra M, Fadda A. Determination of free radical scavenging activity of plant extracts through DPPH assay: An EPR and UV-Vis study. Food Anal Methods. 2012;5:759–766. [Google Scholar]