Abstract
Adverse early nutrition leads to metabolic aberrations in adulthood. Molecular and cellular mechanisms responsible are emerging; specific nutritional causes remain unclarified. We investigated gestational dietary intake and its influences on metabolism in offspring. Three groups of pregnant Sprague-Dawley rats were fed either AIN93G standard diet as control, isocaloric high fat sucrose diet or calorie restriction diet (50% of control) until delivery. All dams were fed control diet ad libitum during lactation. Offsprings’ metabolic parameters were assessed at three weeks. Visceral fat and plasma triglycerides of high fat sucrose diet offspring were significantly higher than those of control diet and calorie restriction diet offspring. Plasma leptin level was higher in high fat sucrose diet than control offspring. Conversely, plasma adiponectin was lower in high fat sucrose diet and calorie restriction diet offspring compared to controls. Significant inductions of hepatic mRNA expression of stearoyl-CoA desaturase1 and Δ-5 desaturase genes, were observed in high fat sucrose diet and calorie restriction diet offspring. Gestational high sugar and fat intake even without over energy intake would be more detrimental to metabolisms of offspring compared to calorie restriction.
Keywords: visceral fat, triglyceride, SCD1 gene, high fat sucrose diet, intrauterine growth retardation
Introduction
During the intrauterine and early neonatal period, environmental factors including nutrition may have an influence on the development of the fetus. Adult metabolic disease may arise in-utero due to sub-optimal intra-uterine conditions. This has been demonstrated by epidemiological studies as well as research on laboratory animals.(1,2) Maternal undernutrition and overnutrition during this crucial period have been implicated for disease risk in offspring during adulthood.(3,4) Sub-optimal fetal nutrition brings about adaptations that permanently impair the physiology and metabolism of the offspring, hence predisposing them to metabolic, endocrine and cardiovascular disorders during adulthood.
The molecular and cellular mechanisms of fetal responses to nutritional environment leading to metabolic disorders later in life are largely unknown. However, several studies on the causes of insufficient intrauterine nutrition and later development of metabolic abnormalities have emerged. Lack of proper development of fetal organs, causes altered morphology and function. For instance, poor β-cell development affects insulin production and reduced number of glomeruli, which initiates hypertension later in life.(5,6) Hormonal programming leading to alterations in the fetal hypothalamic-pituitary-adrenal (HPA) axis has also been implicated.(7) Lastly, through the “thrifty phenotype theory”, it has also been postulated that an undernourished fetus may establish a “thrifty” way of handling food, which later becomes inappropriate in conditions of plenty, after birth. If post-natal nutrient availability is greater than prenatally “predicted”, then eventually adult insulin resistance will result.(8)
Conversely, altered function of adult offspring following maternal overnutrition, has been proposed by “predictive adaptive response (PAR) hypothesis” as resulting from mismatch between pre and post-natal environments.(9) Gestational high fat diet predisposes offspring to obesogenic phenotype(10) and has the potential to program gluconeogenic capacity of the offspring by epigenetic alterations and consequently lead to hyperglycemia and insulin insensitivity in later life.(11)
Hyperphagia has been implicated for fetal programming and is considered a key contributing factor in adult pathophysiology. Hyperleptinaemia and hyperinsulinaemia, especially in intrauterine growth retardation (IUGR) animals are critical in the development of hyperphagia, obesity and hypertension.(12) Nevertheless, the specific way in which metabolic programming happens and how genes interact with nutritional exposure to affect metabolic disorders is currently not very clear and remains largely unexplored. Although some studies have explored epigenetic regulation as a possible inducer of nutritional programming leading to disease in adulthood,(13–17) we sought a novel responsible gene for metabolic abberations in offspring, occasioned by an adverse diet. In this study, we found stearoyl Co-A desaturase 1 (SCD1) gene can be a novel candidate gene, that is gene is up-regulated in the CR as well as in the high fat and sucrose (HFS) diet offspring.
Recently, low glycemic diet or low carbohydrate diet has widely been applied to normal young women for reducing weight as well as patients with obesity, insulin resistance, diabetes mellitus or metabolic diseases. These diets can reveal lower postprandial blood glucose levels and be beneficial for control of blood glucose and insulin levels. However, low carbohydrate diet, as a result, consists of high fat and/or high protein diet. Effect of the difference of carbohydrate/fat/protein ratio in diet during gestational period on metabolic aberrations of offspring has not been clarified.
In the present study, we investigated the effect of isocaloric administration of HFS diet, which is widely utilized as a western type of diet with low carbohydrate ratio, during gestational period on metabolic aberrations of offspring in comparison with calorie restriction (CR) diet or control diet. HFS diet was assessed side by side with a 50% calorie restricted diet as models of adverse nutrition based on under nutrition on one side and over nutrition without caloric overload on the other side, to compare the differences in metabolic abberations appropriated by both diets. We demonstrate that exposure to isocaloric administration of HFS diet during gestational period can trigger metabolic abnormalities in offspring more than calorie restriction diet. To the best of our knowledge, this is the first study to examine side by side, the effect of high fat sucrose gestational diet and a calorie restriction diet on the offspring and to suggest the role of the SCD1 gene in giving rise to metabolic abberations after adverse gestational nutrition.
Materials and Methods
Animals
Sixteen 10-week-old virgin female Sprague-Dawley (SD) rats (Charles River) weighing 230–250 g, were mated by introducing a male rat to the standard rat cages where the female rats were individually housed. Confirmation of mating was by sighting of a plug in the morning after the dark cycle period. All the rats were maintained in the same room with a constant temperature and a 12 h light; 12 h dark cycle. Before confirmation of mating, the dams were allowed free access to standard rodent diet and water. After confirmation of mating, the dams were assigned to either of three experimental diets, for the entire gestational period. The dams delivered spontaneously after a period of about 21 days. They were fed a standard American Institute of Nutrition AIN93G diet ad libitum, during lactation. At 3 weeks, prior to weaning, male offsprings were anesthetized and sacrificed by exsanguination. Body length was taken and organs were removed immediately, frozen in liquid nitrogen and then stored at –80°C. All animal procedures were approved by the institutional Animal Care and Oversight Committee and carried out in accordance with the guidelines and principles for the care and use of animals at the University of Tokushima.
Diet and experimental design
The dams were randomly assigned to 3 dietary groups during the gestational period: (i) high fat and sucrose (HFS) diet (5 dams) (HFS Diet; 20% calories from sucrose, 15% calories from corn starch, 5% calories from fibre, 25% calories from protein, 30% calories from fat, 5% calories from vitamin and mineral mixture, as shown in Table 1), (ii) control diet AIN93G (5 dams) 10% calories from sucrose, 53% calories from corn starch, 5% calories from fibre, 20% calories from protein, 7% calories from fat, 5% calories from vitamin and mineral mixture) and (iii) calorie restriction (CR) diet which was 50% of control diet (6 dams). For the calorie restriction diet, the total dietary intake of the control group from the previous day was computed and 50% of this amount was fed to the CR group. The dams in the HFS diet group and the control group were allowed free access to food and water throughout the 3 week-gestation period.
Table 1.
Nutrient composition of experimental diets (% in total energy)
| High fat sucrose diet (HFS) (%) | Control diet (AIN-93G) (%) | |
|---|---|---|
| Carbohydrates | 39.9 | 67.9 |
| Corn starch | 14.9 | 52.9 |
| Sucrose | 20.0 | 10.0 |
| Fibre | 5.0 | 5.0 |
| Protein | 25.4 | 20.3 |
| Casein | 25.0 | 20.0 |
| L-cystein | 0.38 | 0.3 |
| Fat | 30.0 | 7.0 |
| Soybean oil | 2.0 | 7.0 |
| Beef tallow | 14.0 | 0 |
| Lard | 14.0 | 0 |
| Vitamins and minerals | 4.8 | 4.7 |
| AIN93G-Mineral mix | 3.5 | 3.5 |
| AIN93G-Vitamin mix | 1.0 | 1.0 |
| Cholinebitartrate | 0.25 | 0.25 |
| tert-butylhydroquinone | 0.006 | 0.0014 |
| Total energy (kcal/g) | 4.81 | 3.95 |
AIN-93G: American Institute of Nutrition recommended growth diet for young, pregnant and lactating rodents. Isocaloric high fat and sucrose diet (HFS); Protein:Fat:Carbohydrate = 25:30:40, control diet; Protein:Fat:Carbohydrate = 20:7:68.
Determination of body weight and plasma glucose
At birth, each littermate from all groups fed with experimental diets, was weighed and an average birth weight assigned to each of the three groups. Some female offspring were culled but the gender preference was only so as to allow for adequate feeding during lactation. After 21 days of lactation, the male offspring were weighed and blood glucose taken, after which they were forced to fast overnight (20:00–09:00). Prior to euthanization the following day, glucose tolerance tests of 2 g/kg glucose load were performed on the fasted animals. Blood samples were drawn from the tip of the tail at 0, 30, 60, 90 and 120 min after administration of the glucose load. Blood glucose concentrations were measured with a glucometer (Accu-check Active; Roche Diagnostics, Mannheim, Germany).
Blood chemistry analysis
After exsanguination of the animals at 3 weeks, the blood was centrifuged to obtain the plasma sample. Plasma insulin was measured using an ELISA kit (Morinaga, Yokohama, Japan). Total cholesterol, nonesterified fatty acid (NEFA) and plasma triglyceride (TG) concentrations were measured using specific assay kits (Wako Pure Chem. Ind. Ltd., Osaka, Japan), respectively. Plasma adiponectin and leptin were measured by ELISA (Morinaga).
RNA preparation and quantitative RT-PCR
Organs including the liver, pancreas, visceral fat and soleus muscle were excised, weighted and stored at –80°C until analysis. The mRNA levels of hepatic lipid homeostasis genes were determined by reverse transcription of total RNA followed by PCR analysis. Total RNA was extracted from liver sample using Trizol Reagent (Invitrogen, Carlsbad, CA). First-strand cDNA were reverse-transcribed at 42°C for 60 min from 5 µg of the extracted total RNA, with M-MLV reverse transcriptase (RT) (Invitrogen) and Oligo dT primer. Real time PCR was performed using the primers shown in Table 2 and SYBR premix Ex Taq (Takara BIO Inc., Shiga, Japan), in a lightCycler real-time PCR system (Roche Diagnostics), according to the manufacturer instructions as previously described.(18) The relative amount of mRNA was calculated with β-actin mRNA as the invariant control. The ratio for the data from the CT group was set arbitrarily at 1.
Table 2.
Sequence of oligonucleotide primers for quantitative RT-PCR analysis
| Gene name | Size (bp) | Accession No. | Primer sequence |
|---|---|---|---|
| ACC | 192 | NT_033778 | F:5'-GGAAAACGGTCAGGTACGGA-3' |
| R:5'-TTCGGACGTTGTATGCACCA-3' | |||
| G6PDH | 128 | NC_013047 | F:5'-TCACCTTGTGCTGTCCGTTT-3' |
| R:5'-CGATCGGGCTCACACAAGTA-3' | |||
| L-PK | 233 | NC_005101 | F:5'-CCACCAGGCACAGAAACACT-3' |
| R:5'-TGAGCTTGGCAGAGGATGAC-3' | |||
| GK | 144 | NM_001124249 | F:5'-CCAGTGTCAAAATGCTGCCC-3' |
| R:5'-CCTTCCATCCCCTCTCCTCA-3' | |||
| SCD-1 | 373 | NM_139192 | F:5'-CTAGGTGGGTGGCTGTTGAG-3' |
| R:5'-ATGTAACTGCGGGAAGGGTG-3' | |||
| D5D | 108 | NC_005100 | F:5'-CCACTACGCTGGTCAGGATG-3' |
| R:5'-GCGCTTGCATTCTGGGTCTA-3' | |||
| FAS | 168 | NC_005100 | F:5'-CTAGGCGGGGAACTGCTTTC-3' |
| R:5'-TCTGGATCTGTGGAGTGGCT-3' | |||
| ChREBP | 420 | NC_005111 | F:5'-GGCGAAGGGAATTCAGGACA-3' |
| R:5'-GGGCCACCCGTTTAGCTATC-3' | |||
| PPARα | 164 | NC_005106.3 | F:5'-CAAACCATCCTAGTGTGCCCT-3' |
| R:5'-CCACAGAGGGCAGCATTTCT-3' | |||
| PGC1α | 102 | NC_005113.3 | F:5'-ACTCTTGAGGGCTTACAACCT-3' |
| R:5'-AGCACTGGGCTTATTTGTGGA-3' | |||
| CPT1 | 308 | NC_005106 | F:5'-CCTCACCACTCCAATCCCAC-3' |
| R:5'-CGTCCCGAAATCGAGTCTGA-3' | |||
| ACS | 173 | NC_003076 | F:5'-GCCCCAATCTGGAAATGGCA-3' |
| R:5'-CTTACAGCGAGGAGCTTCGT-3' | |||
| FGF21 | 119 | NC_005100.3 | F:5'-TGCAGGCCTCAGGATCAAAG-3' |
| R:5'-GGCTGGACCTGAATCTGGAG-3' | |||
| ACOX | 246 | NC_002754 | F:5'-GGGCAGTATACAAAGGGGCA-3' |
| R:5'-CCCCTACATACCAACCGCTC-3' | |||
| HSL | 250 | NC_005100 | F:5'-GGGCTGCTAAGCTCAGGAAA-3' |
| R:5'-TCGGTTAACCACAGCTTCCC-3' | |||
| β-Actin | 108 | NC_005111 | F:5'-AGAAGGGACACCGTAGAGGG-3' |
| R:5'-GCGGGAATACGACTGCAAAC-3' |
F, forward; R, reverse; ACC, acetyl CoA carboxylase; G6PDH, glucose-6-phosphate dehydrogenase; LPK, liver pyruvate kinase; GK, glucokinase; SCD1, stearoyl-CoA desaturase 1; D5D, Δ5 desaturase; FAS, tumor necrosis factor receptor superfamily member 6 (TNFRSF6); ChREBP, carbohydrate responsive element binding protein; PPARα, peroxisome proliferator activated receptor alpha; PGC1α, peroxisome proliferator activated receptor gamma co-activator alpha; CPT1, carnitine palmitoyltransferase 1; ACS, acetyl-CoA synhtetate; FGF21, fibroblast growth factor 21; ACOX, acyl-CoA oxidase; HSL, hormone sensitive lipase; β-actin.
Statistical analysis
Statistical analysis was performed using GraphPad Prism version 5.0d (San Diego, CA). Significant effects of diet were identified by 1-way ANOVA followed by Tukey’s multiple comparison test. Data was presented as means ± SEM and significance was assigned when p<0.05.
Results
Food intake, body and organ weight changes in dams and offspring
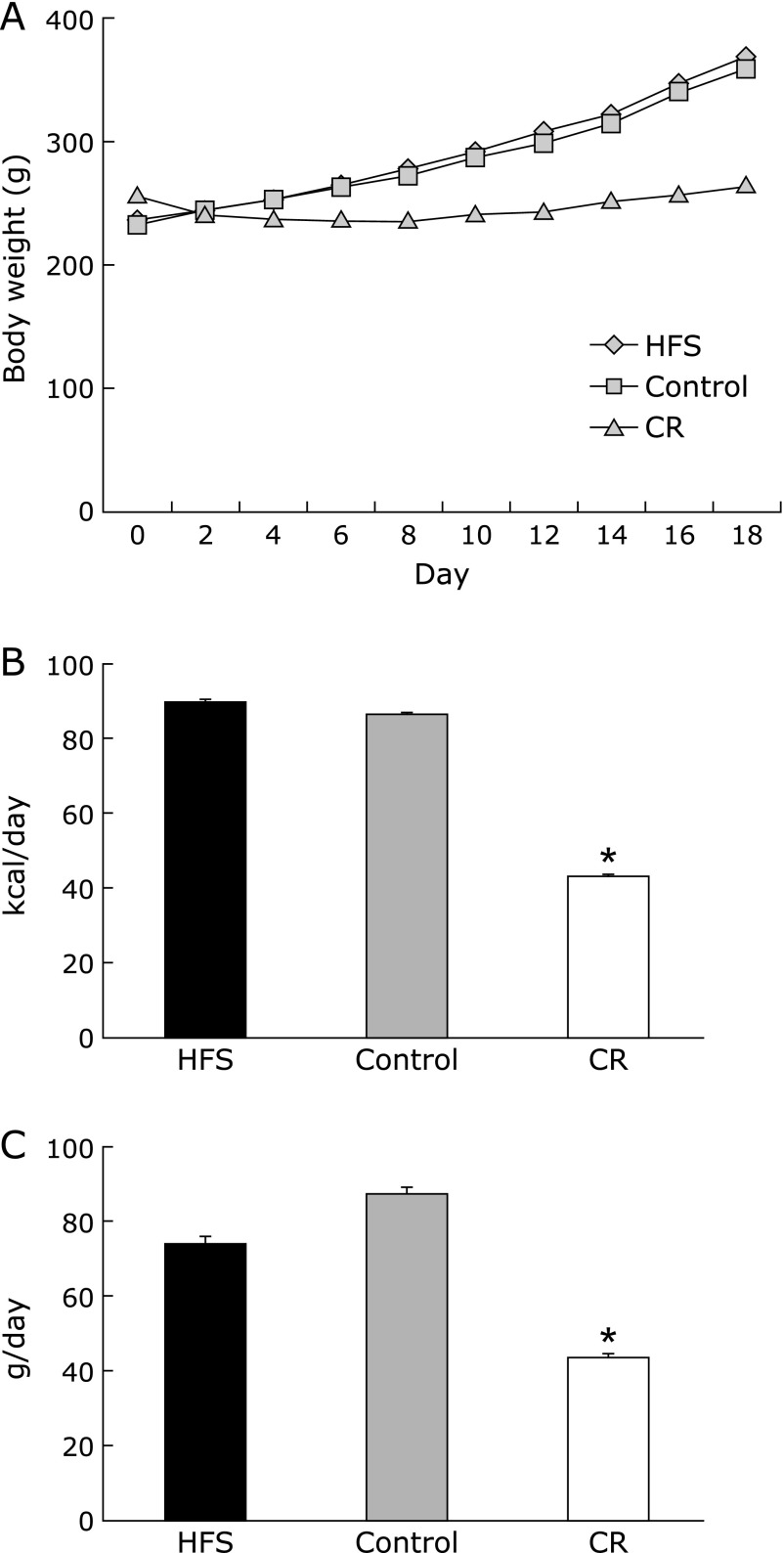
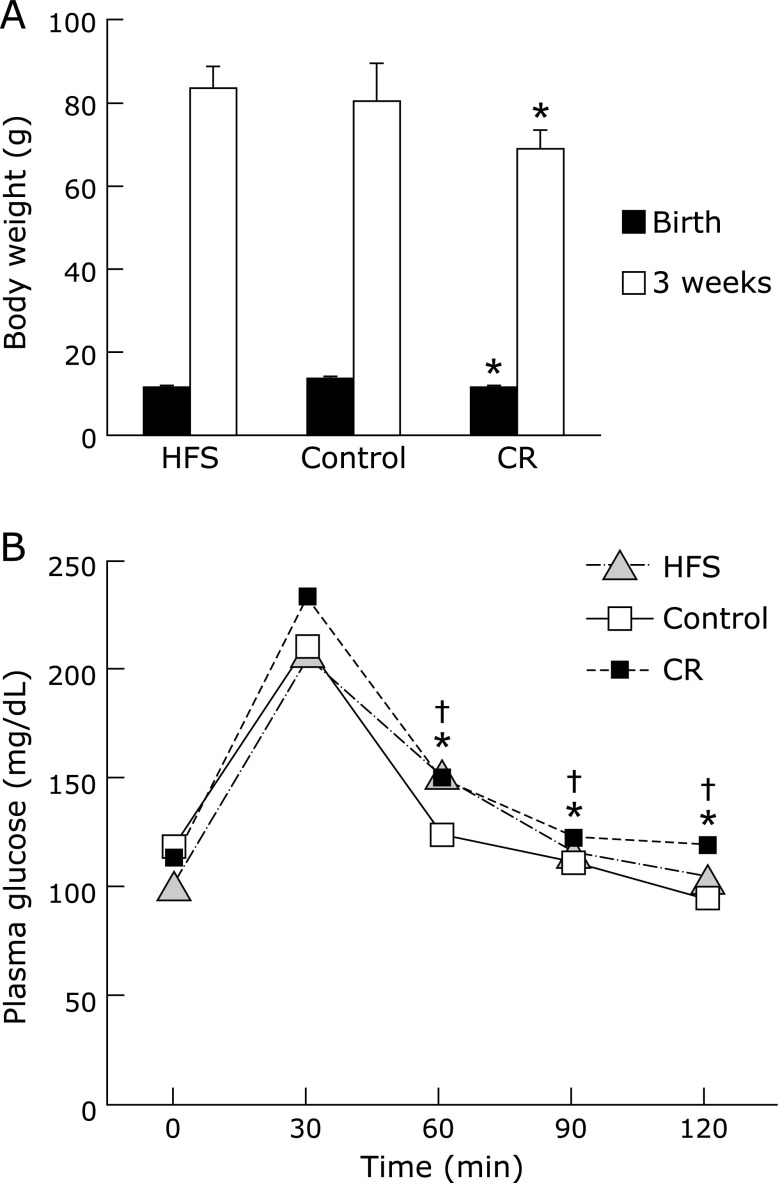
The daily food intake and energy intake of CR diet group was just a half of control diet group (Fig. 1B and C). Conversely, the food intake of HFS diet group was slight but not significantly lower than that of control diet group (Fig. 1C). Resulting from the decrease in food intake in HFS diet group, the calculated daily energy intake of HFS diet group was same as that of control group (Fig. 1B). Therefore, this condition was defined as isocaloric administration of HFS diet (isocaloric HFS diet). The change in body weight among the dams was significantly lower in CR group compared to control group (Fig. 1A). Gestational calorie restriction caused intra-uterine growth retardation (IUGR) in offspring of the CR diet group and their body weight at 3 weeks remained significantly lower as opposed to the isocaloric HFS diet and control diet groups (Table 3 and Fig. 2A).
Fig. 1.
Changes in body weight, daily energy intake and food intake of dams during dietary intervention. (A) Body weight was measured every other day. (B) Daily energy intake was calculated from recorded daily food intake. (C) Daily food intake was recorded during dietary intervention from mating to delivery. HFS, isocaloric high fat and sucrose diet; control, control AIN93G diet; CR, calorie restriction diet (50% of control diet). Data are mean ± SEM. *p<0.05 vs control.
Table 3.
Birth data of offspring
| Variable | HFS | Control | CR |
|---|---|---|---|
| Birth weight (g) | 5.85 ± 0.01 | 6.83 ± 0.24 | 5.12 ± 0.17* |
| Average No. of pups | 14 | 12 | 14 |
| Male:famale ratio | 1:01 | 1:01 | 1:01 |
Fig. 2.
Body weight changes and oral glucose tolerance test in offspring. (A) Body weight of offspring was measured at birth and at 3 week-old. Data are mean ± SEM *p<0.05 vs CT group. (B) Oral glucose tolerance test in offspring was performed as described in Materials and Methods. After fasting for 13 h, the rats were given an oral dose of glucose 2 g/kg body weight. Blood samples were taken at 0, 30, 60, 90 and 120 min after glucose administration. Data are mean ± SEM, n = 6–10 rats/group, *p<0.01 vs CT group for CR at 60, 90 and 120 min and †p<0.05 vs CT group for HFS at 60, 90 and 120 min.
Hyperglycemia and insulin resistance
Random blood glucose value in isocaloric HFS diet group was significantly lower than those of other groups (Table 4). Despite this, the results of the oral glucose tolerance test indicated that plasma glucose levels in the isocaloric HFS diet and CR diet groups were significantly higher than control group 60, 90 and 120 min after oral glucose administration (Fig. 2B). the random plasma insulin levels showed no significant difference between the groups (Table 4).
Table 4.
Body weight, organ weight, blood chemistry of the offspring at 3 weeks after gestational diet
| Variable | HFS | Control | CR |
|---|---|---|---|
| Body Weight (g) | 41.9 ± 2.5 | 40.2 ± 4.6 | 34.5 ± 2.3** |
| Visceral fat (g/kg body weight) | 11.6 ± 0.98** | 7.6 ± 0.99 | 6.3 ± 0.91** |
| Epididymal WAT (g/kg body weight) | 2.6 ± 0.24** | 1.8 ± 0.3 | 1.4 ± 0.4** |
| Mesenteric WAT (g/kg body weight) | 5.8 ± 0.7** | 4.5 ± 1.5 | 3.9 ± 0.5 |
| Retroperitoneal WAT (g/kg body weight) | 3.2 ± 1.1** | 1.3 ± 0.3 | 1.0 ± 0.3** |
| Soleus (g/kg body weight) | 0.6 ± 0.1 | 0.7 ± 0.2 | 0.7 ± 0.1 |
| Liver (g) | 1.36 ± 0.13 | 1.27 ± 0.18 | 1.0 ± 0.09** |
| Pancreas (g) | 0.14 ± 0.02 | 0.13 ± 0.03 | 0.11 ± 0.02** |
| Glucose (mg/dl) | 103 ± 7.6 ** | 113 ± 7.2 | 119 ± 5.04 |
| Insulin (ng/mL) | 0.49 ± 0.09 | 0.44 ± 0.09 | 0.47 ± 0.11 |
| Total Cholesterol (mg/dl) | 162 ± 7.7 | 173 ± 9.2 | 217 ± 17* |
| Triglyceride (mg/dl) | 117 ± 19* | 67.9 ± 12.3 | 42.8 ± 7.3* |
| NEFA (mEq/L) | 0.55 ± 0.03 | 0.55 ± 0.03 | 0.66 ± 0.05 |
| Leptin (ng/ml) | 2.02 ± 0.15 | 1.6 ± 0.28 | 1.2 ± 0.11* |
| Adiponectin (ng/ml) | 53.3 ± 4.9 | 65.2 ± 5.4 | 54.0 ± 9.0 |
Values are means ± SEM, n = 51. HFS, high fat and sucrose diet; CT, control diet; CR, calorie restriction diet. *p<0.05, **p<0.001 vs CT Group.
Visceral fat accumulation and plasma lipids
Visceral fat accumulation, as well as plasma triglycerides levels, in the offspring from isocaloric HFS diet group were significantly higher than those of control diet and CR diet group. Gestational isocaloric HFS diet induced visceral obesity and hypertriglyceridemia in offspring. Such metabolic aberrations were however not observed in the CR diet group, which displayed significantly lower visceral fat and plasma triglycerides levels than the control diet group. Nevertheless, the CR diet group exhibited significant elevation of total cholesterol, relative to the CT group (Table 4).
Plasma adipocytokine levels
Plasma leptin level of offspring was significantly lower in the CR diet group than control diet group, but that in the isocalroric HFS diet group exhibited no significant change (Table 4). Conversely, plasma adiponectin levels were no significant difference between the groups (Table 4).
Hepatic mRNA expression of lipogenesis-related genes
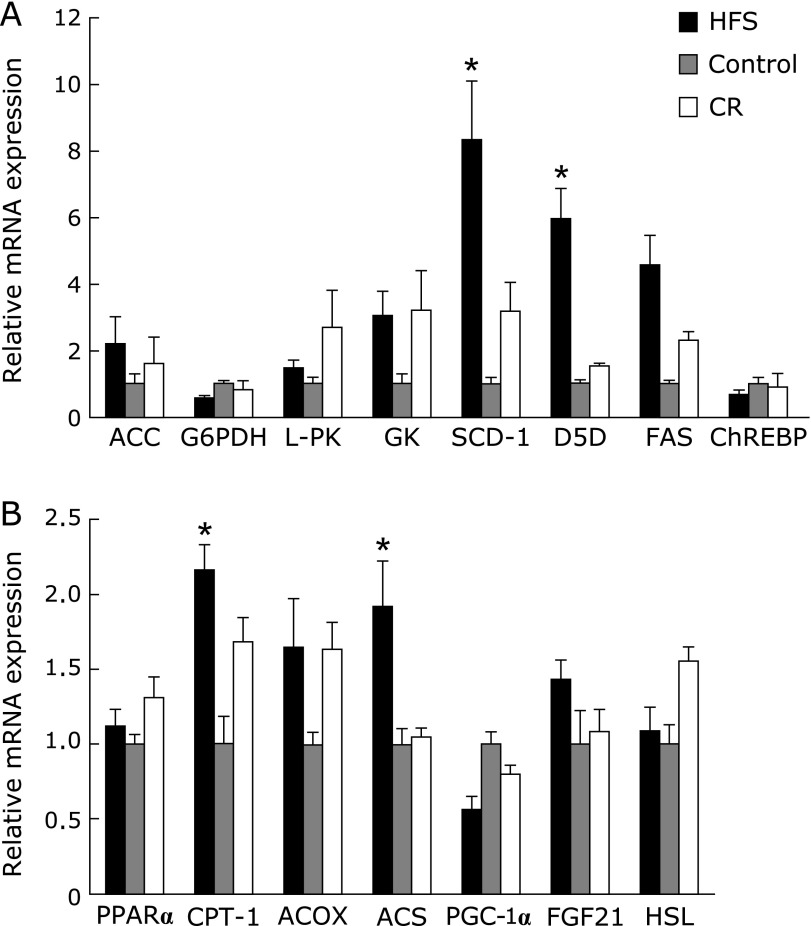
To explore the cellular and molecular mechanisms of metabolic abnormalities caused by isocaloric HFS or CR diets, the expressions of lipogenesis genes in the liver were investigated. ACC, L-PK, GK and FAS genes tended to increase in both the CR diet and isocaloric HFS diet groups, but we observed no statistically difference between the groups (Fig. 3A). The expressions of SCD-1 gene (p<0.05 for CR and isocaloric HFS diet vs control diet) and D5D gene (p<0.05 for isocaloric HFS diet) were significantly increased in the liver of offspring in the isocaloric HFS diet group. These results suggest that increase in the expression of SCD-1 and D5D genes which has been implicated in increasing fatty acids and triglycerides in the liver plays a key role in metabolic abnormalities in offspring.
Fig. 3.
Messenger RNA expression of lipogenesis-related and lipolysis (A) or lipid oxidation-related (B) genes in the liver of offspring. Messenger RNA expression levels were determined by quantitative RT-PCR analysis with appropriate specific primer sets. Data represent means ± SEM, n = 6–10 rats/group *p<0.05 vs CT group.
Hepatic mRNA expression of lipolysis and fatty acid oxidation genes
The expression of genes that regulate lipolysis and fatty acid oxidation in the liver was also investigated. The hepatic expressions of CPT-1 gene and ACS gene in the offspring in isocaloric HFS diet group were also significantly increased. Taken together with increases in serum triglycerides level, lipogenic gene expression and lipolytic gene expression, both lipogenesis and lipolysis could be stimulated, but induction rate of lipogenesis could be relatively larger than that of lipolysis, resulted in elevation of serum triglycerides level of the offspring from the dam with gestational isocaloric HFS diet.
Discussion
In the present study, we investigated the effect of isocaloric administration of HFS diet during gestational period on metabolism of offspring rats with comparison to calorie restriction diet. To the best of our knowledge, this is the first finding that in-utero exposure to HFS diet without over calorie intake can trigger hypertriglyceridemia and visceral obesity in offspring through increase in the expression of lipogenic genes such as SCD-1 and D5D. Generally, HFS diet offspring can develop obesity and fatty liver primarily due to increased total energy intake. HFS diet offspring also showed impaired glucose tolerance, elevated serum triglycerides (TGs) and cholesterol as previously reported.(19) In addition, Chen et al.(20) reported that in-utero exposure to both carbohydrates and fats led to fetal programming that caused hyperinsulinemia, insulin insensitivity and other adverse metabolic effects in non-obese offspring. These results suggest that the proportion of dietary macronutrient in gestational period can influence the onset of obesity and metabolic complications in offspring. This is independent of the total energy intake as also previously reported.(21)
Gestational calorie restriction caused IUGR in offspring as previous reports,(22–24) however, no rapid catch-up growth in the CR diet group was observed after a period of lactation during which dams were allowed ad libitum access to the control diet. Consistent with previous reports, this study also demonstrated not only reduced body weight but also organ weights in the offspring of the CR diet group relative to the control diet group.(23,25,26) Under the present condition, much more time may be needed to show catch-up growth in the CR diet group.
An observation of significant importance made by this study was the possible higher capacity for gluconeogenesis and/or insulin resistance as evidenced by demonstration of higher plasma glucose values in the CR offspring more than in the isocaloric administration of HFS diet offspring. This is in congruence with reported increased hepatic glucose production and increased glucose disposal in IUGR offspring which were more glucose intolerant compared to controls.(27) In our study, both offspring from CR diet and isocaloric administration of HFS diet group demonstrated significant hyperglycemia after glucose tolerance test compared to control diet group. We observed also that the pancreatic weight of the CR diet group was significantly reduced compared to the other diet groups. CR diet can suppress insulin/IGF-1 signal in offspring, resulting in suppression of development of the pancreas.(28) The decrease in pancreatic weight may have triggered insufficient pancreatic β-cell mass, and cannot sufficiently respond to plasma glucose level as concerned in various studies with intrauterine undernutrition.(5,29,30)
However, we did not observe the reduction of pancreatic weight in the isocaloric administration of HFS diet group. In this case, the problem may be addressed in the difference of dietary composition between CR and HFS diet. It has been well-known that high fat diet can induce apoptosis of pancreatic β-cell in adult rats by changing lipid profile in β-cell membrane. This is called as lipotoxicity.(31) Therefore, lipotoxicity dependent metabolic complications may be programmed in offspring. These different mechanisms between the isocaloric administration of HFS diet group and the CR diet group may explain the difference in the phenotype.
Another difference in phenotype was serum leptin level. Serum leptin was slightly increased in the isocaloric administration of HFS diet offspring, while it decreased in the offspring of CR diet group. Generally, serum leptin level is positively associated with fat mass.(32) In our study, fat mass was highest in the isocaloric administration HFS diet group and lowest in the CR diet group. Therefore, the leptin level reflected fat mass. Conversely, adiponectin levels tended to decrease in the offspring of both isocaloric administration HFS and the CR diet groups. This result agreed with a study that was conducted on the effects of late gestational high-fat diet on body weight, metabolic regulation and adipokine expression in offspring.(33) It is also notable that reduced adiponectin has been identified as an independent risk factor for developing metabolic syndrome.(34) These adipokines also reflect metabolic abnormalities in the offspring of the isocaloric administration HFS diet group as well as the CR diet group.
Given the results from gene expression analysis, SCD-1 gene expression may prove to be a key factor for nutritional programming of the metabolic syndrome, because its expression was highly and significantly elevated, especially in the offspring from isocaloric administration HFS diet offspring. SCD-1 gene a key metabolic gene is responsible for de novo lipogenesis which increases fatty acids and triglycerides in the liver. It has been previously identified as an obesity gene responsible for hepatic steatosis and factors of the metabolic syndrome such as leptin and insulin resistance. SCD-1 has a critical role in the up-regulation of liver lipogenesis in response to high carbohydrate feeding, especially for diets high in sugars.(35) In this study, the isocaloric administration HFS diet was composed of 20% sucrose and this may induce the highly significant expression of the SCD-1 gene. Deficiency of the gene in SCD-1 knockout (LKO) mice has also been found to protect mice from carbohydrate-induced adiposity and hepatic steatosis.(36,37)
Isocaloric administration of HFS diet also significantly increased hepatic D5D gene expression in the offspring. D5D can introduce a double bond at the 5th position from the carboxyl group of long-chain fatty acid. This reaction is required for the synthesis of polyunsaturated fatty acids (PUFAs), especially arachidonic acid and eicosapentaenoic acid.(38) Generally, D5D activity can be controlled by feedback regulation with SREBP-1c and PPARα. Deficient of essential fatty acid increases D5D activity, but supplementation of PUFAs inhibits. Indeed, decrease in D5D activity was observed in obese patients.(39,40) Relationship between decrease in D5D activity and obesity or metabolic syndrome has been arguable. However, the latest study reported by Lopez-Vicario et al.(41) demonstrated that increase in D5D mRNA and protein expression together with increase in content of n-6 PUFAs was observed in the liver from mice with high-fat diet-induced obesity and non-alcoholic steatohepatitis. They also demonstrated that supplementation of n-3 PUFAs or treatment with a combined inhibitor of D5D and D6D, CP-24879, significantly reduced intracellular lipid accumulation, n-6 PUFAs and inflammation in hepatocytes. Thus, increase in hepatic D5D expression can increase inflammatory n-6 PUFAs and lipid accumulation in the liver. Our present study suggests that gestational isocaloric HFS diet may cause metabolic complications in the liver of offspring due to increase in D5D expression compared to other diet group.
Another possibility is low carbohydrate composition. There are some difference in phenotypes between the isocaloric administration HFS diet group and the CR diet group as described above. Nevertheless, similar phenotypes were also observed as oral glucose tolerance test, serum adiponectin level, pattern in the expression of lipogenesis-related and lipolysis-related genes. Lower carbohydrate contents causes suppression of postprandial glucose increase. This is believed to be beneficial for prevention of diabetes and metabolic syndrome by avoiding hyperinsulinemia and insulin resistance. However, lower carbohydrate diet during gestational period may be harmful to fetus according to thrifty phenotype theory. HFS diet contains 59% of carbohydrate of control diet. In addition, food intake of HFS diet group was 80% of control diet. Therefore, calculated net carbohydrate intake in the isocaloric administration of HFS diet was almost same as that in CR diet. Lower carbohydrate intake results in lower postprandial increase in blood glucose level, resulting in lower postprandial insulin secretion and suppression of IGF-1 signaling pathway.(42) Although the exact mechanism still remains unclear, lower postprandial increase in blood glucose level somehow may be memorized, i.e., epigenetic mechanism, and may be involved in the induction of the SCD1 gene and occasioned metabolic abnormalities observed in the isocaloric administration of HFS diet and CR diet group.
In conclusion, our study shows that in-utero exposure to high fat high sucrose diet imposes on the progeny the propensity to metabolic dysfunction, more adversely than a calorie restriction diet. This conclusion is supported by highly significant visceral fat accumulation, elevation of serum triglycerides level, and induction of hepatic lipogenic gene expressions, all of which are key factors for metabolic complications. The mechanisms by which high sucrose fat diet imposes more adverse metabolic consequences than calorie restriction may be attributable to induction of the SCD-1 gene, a lipogenesis-related gene, responsible for increasing fatty acids and triglycerides in the liver. However, possible strategies in the future point towards exploration of DNA methylation and histone acetylation appropriated by both diets on the SCD-1 gene and how these appropriations relate or differ. Calorie restriction diet during pregnancy and lactation is detrimental to offspring health, but even more detrimental is high fat high sugar diet. Conclusively, based on this evidence, there should be measures in medical and public health practice seeking to enhance good fetal and infant nutrition through proper maternal nourishment.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research (B) 22300237 and (B) 21300277 from the Ministry of Education, Culture, Sports, Science and Technology in Japan (to Y.T. and E.T.).
Conflict of Interest
No potential conflicts of interest were disclosed.
Contribution statement
VW, YT, and ET were principal investigators and responsible for the study. VW, HO, YT, KA, HO, HY, and ET conceived and designed the study, and participated in the analysis and interpretation of the data. VW drafted the manuscript, and all authors revised it critically for intellectual content. All authors approved the version to be published.
References
- 1.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 2.Ozanne SE, Constância M. Mechanisms of disease: the developmental origins of disease and the role of the epigenotype. Nat Clin Pract Endocrinol Metab. 2007;3:539–546. doi: 10.1038/ncpendmet0531. [DOI] [PubMed] [Google Scholar]
- 3.Buckley AJ, Jaquiery AL, Harding JE. Nutritional programming of adult disease. Cell Tissue Res. 2005;322:73–79. doi: 10.1007/s00441-005-1095-7. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 5.Inoue T, Kido Y, Asahara S, et al. Effect of intrauterine undernutrition during late gestation on pancreatic β cell mass. Biomed Res. 2009;30:325–330. doi: 10.2220/biomedres.30.325. [DOI] [PubMed] [Google Scholar]
- 6.Brenner BM, Chertow GM. Congenital oligonephropathy: an inborn cause of adult hypertension and progressive renal injury? Curr Opin Nephrol Hypertens. 1993;2:691–695. [PubMed] [Google Scholar]
- 7.Webster JI, Sternberg EM. Role of the hypothalamic-pituitary-adrenal axis, glucocorticoids and glucocorticoid receptors in toxic sequelae of exposure to bacterial and viral products. J Endocrinol. 2004;181:207–221. doi: 10.1677/joe.0.1810207. [DOI] [PubMed] [Google Scholar]
- 8.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 9.Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Q, Suzuki M. Parental obesity and overweight affect the body-fat accumulation in the offspring: the possible effect of a high-fat diet through epigenetic inheritance. Obes Rev. 2006;7:201–208. doi: 10.1111/j.1467-789X.2006.00232.x. [DOI] [PubMed] [Google Scholar]
- 11.Strakovsky RS, Zhang X, Zhou D, Pan YX. Gestational high fat diet programs hepatic phosphoenolpyruvate carboxykinase gene expression and histone modification in neonatal offspring rats. J Physiol. 2011;589:2707–2717. doi: 10.1113/jphysiol.2010.203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- 13.Stevens A, Begum G, Cook A, et al. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology. 2010;151:3652–3664. doi: 10.1210/en.2010-0094. [DOI] [PubMed] [Google Scholar]
- 14.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 15.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–1073. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lillycrop KA, Phillips ES, Torrens C, Hanson MA, Jackson AA, Burdge GC. Feeding pregnant rats a protein-restricted diet persistently alters the methylation of specific cytosines in the hepatic PPARα promoter of the offspring. Br J Nutr. 2008;100:278–282. doi: 10.1017/S0007114507894438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinclair KD, Allegrucci C, Singh R, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A. 2007;104:19351–19356. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amo K, Arai H, Uebanso T, et al. Effects of xylitol on metabolic parameters and visceral fat accumulation. J Clin Biochem Nutr. 2011;49:1–7. doi: 10.3164/jcbn.10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu JH, Sun HS, Wang Y, Zheng WQ, Shi ZY, Wang QJ. The effects of a fat- and sugar-enriched diet and chronic stress on nonalcoholic fatty liver disease in male Wistar rats. Dig Dis Sci. 2010;55:2227–2236. doi: 10.1007/s10620-009-1019-6. [DOI] [PubMed] [Google Scholar]
- 20.Chen CY, Crott J, Liu Z, Smith DE. Fructose and saturated fats predispose hyperinsulinemia in lean male rat offspring. Eur J Nutr. 2010;49:337–343. doi: 10.1007/s00394-009-0091-1. [DOI] [PubMed] [Google Scholar]
- 21.Lomba A, Milagro FI, García-Díaz DF, Marti A, Campión J, Martínez JA. Obesity induced by a pair-fed high fat sucrose diet: methylation and expression pattern of genes related to energy homeostasis. Lipids in Health and Disease. 2010;9:60. doi: 10.1186/1476-511X-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim K, Armitage JA, Stefanidis A, Oldfield BJ, Black MJ. IUGR in the absence of postnatal “catch-up” growth leads to improved whole body insulin sensitivity in rat offspring. Paediatr Res. 2011;70:339–344. doi: 10.1203/PDR.0b013e31822a65a3. [DOI] [PubMed] [Google Scholar]
- 23.Tosh DN, Fu Q, Callaway CW, et al. Epigenetics of programmed obesity: alteration in IUGR rat hepatic IGF1 mRNA expression and histone structure in rapid vs. delayed postnatal catch-up growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1023–G1029. doi: 10.1152/ajpgi.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bieswal F, Ahn MT, Reusens B, et al. The importance of catch-up growth after early malnutrition for the programming of obesity in male rats. Obesity (Silver Spring) 2006;14:1330–1343. doi: 10.1038/oby.2006.151. [DOI] [PubMed] [Google Scholar]
- 25.Desai M, Gayle D, Babu J, Ross MG. The timing of nutrient restriction during rat pregnancy/lactation alters metabolic syndrome phenotype. Am J Obstet Gynecol. 2007;196:555.e551–e557. doi: 10.1016/j.ajog.2006.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 27.Garg M, Thamotharan M, Rogers L, Bassilian S, Lee WN, Devaskar SU. Glucose metabolic adaptations in the intrauterine growth-restricted adult female rat offspring. Am J Pysiol Endocrinol Metab. 2006;290:E1218–E1226. doi: 10.1152/ajpendo.00474.2005. [DOI] [PubMed] [Google Scholar]
- 28.Hofman PL, Cutfield WS, Robinson EM, et al. Insulin resistance in short children with intrauterine growth retardation. J Clin Endocrinol Metab. 1997;82:402–406. doi: 10.1210/jcem.82.2.3752. [DOI] [PubMed] [Google Scholar]
- 29.Garofano A, Czernichow P, Bréant B. In utero undernutrition impairs rat β-cell development. Diabetologia. 1997;40:1231–1234. doi: 10.1007/s001250050812. [DOI] [PubMed] [Google Scholar]
- 30.Matveyenko AV, Singh I, Shin BC, Georgia S, Devaskar SU. Differential effects of prenatal and postnatal nutritional environment on β-cell mass development and turnover in male and female rats. Endocrinology. 2010;151:5647–5656. doi: 10.1210/en.2010-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golson ML, Ackermann Misfeldt A, Kopsombut UG, Petersen CP, Gannon M. High fat diet regulation of β-cell proliferation and β-cell mass. The Open Endocrinol J. 2010;4:66–77. doi: 10.2174/1874216501004010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.You JS, Park JY, Zhao X, Jeong JS, Choi MJ, Chang KJ. Relationship among serum taurine, serum adipokines, and body composition during 8-week human body weight control program. Adv Exp Med Biol. 2013;776:113–120. doi: 10.1007/978-1-4614-6093-0_12. [DOI] [PubMed] [Google Scholar]
- 33.Khalyfa A, Carreras A, Hakim F, Cunningham JM, Wang Y, Gozal D.Effects of late gestational high-fat diet on body weight, metabolic regulation and adipokine expression in offspring Int J Obes (Lond) 2013. DOI: 10.1038/ijo.2013.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renaldi O, Pramono B, Sinorita H, Purnomo LB, Asdie RH, Asdie AH. Hypoadiponectinemia: a risk factor for metabolic syndrome. Acta Med Indones. 2009;41:20–24. [PubMed] [Google Scholar]
- 35.Miyazaki M, Dobrzyn A, Man WC, et al. Stearoyl-CoA desaturase 1 gene expression is necessary for fructose-mediated induction of lipogenic gene expression by sterol regulatory element-binding protein-1c-dependent and -independent mechanisms. J Biol Chem. 2004;279:25164–25171. doi: 10.1074/jbc.M402781200. [DOI] [PubMed] [Google Scholar]
- 36.Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 37.Ntambi JM, Miyazaki M, Stoehr JP, et al. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc Natl Acad Sci U S A. 2002;99:11482–11486. doi: 10.1073/pnas.132384699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakamura MT, Nara TY. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Ann Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 39.Do HJ, Chung HK, Moon J, Shin MJ. Relationship between the estimates of desaturase activities and cardiometabolic phenotypes in Koreans. J Clin Biochem Nutr. 2011;49:131–135. doi: 10.3164/jcbn.10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saito E, Okada T, Abe Y, et al. Abdominal adiposity is associated with fatty acid desaturase activity in boys: implications for C-reactive protein and insulin resistance. Prostaglandins Leukot Essent Fatty Acids. 2013;88:307–311. doi: 10.1016/j.plefa.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 41.López-Vicario C, González-Périz A, Rius B, et al. Molecular interplay between Δ5/Δ6 desaturases and long-chain fatty acids in the pathogenesis of non-alcholic steatohepatitis Gut 2013. DOI: 10.1136/gutjnl-2012-303179 [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki M, Flowers MT, Sampath H, et al. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metabolism. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]