Abstract
National data indicate that patients treated with buprenorphine for opiate use disorders are more likely to be White, highly educated, and to have greater incomes than those receiving methadone, but patterns of buprenorphine dissemination across demographic areas have not been documented in major metropolitan areas where poverty, minority populations and injection heroin use are concentrated. Rates of buprenorphine and methadone treatment are compared among areas of New York City defined by their income and ethnic/racial composition.
Residential social areas (hereinafter called social areas) were defined as aggregations of ZIP codes with similar race/ethnicity and income characteristics, and were formed based on clustering techniques. Treatment rates were obtained for each New York City ZIP code: buprenorphine treatment rates were based on the annual number of buprenorphine prescriptions written, and the methadone treatment rate on the number of methadone clinic visits for persons in each ZIP code. Treatment rates were correlated univariately with ethnicity and income characteristics of ZIP codes. Social area treatment rates were compared using individual ANOVA models for each rate.
Buprenorphine and methadone treatment rates were significantly correlated with the ethnicity and income characteristics of ZIP codes, and treatment rates differed significantly across the social areas. Buprenorphine treatment rates were highest in the social area with the highest income and lowest percentage of Black and Hispanic residents. Conversely, the methadone treatment rate was highest in the social area with the highest percentage of low income and Hispanic residents.
The uneven dissemination of 0pioid maintenance treatment in New York City may be reflective of the limited public health impact of buprenorphine in ethnic minority and low income areas. Specific policy and educational interventions to providers are needed to promote the use of buprenorphine for opiate use disorders in diverse populations.
INTRODUCTION
Opiate agonist therapies for opiate dependence lower HIV incidence and arrest rates among injection drug users1,2,3, so enhancing access to these treatments is a major public health priority. Buprenorphine, a partial opiate receptor agonist approved by the U.S. FDA in 2002 for treatment of opiate dependence, offers important advantages over methadone maintenance. Buprenorphine is less lethal in overdose than methadone4. The most commonly prescribed formulation of buprenorphine, in which it is combined with the opiate antagonist naloxone, produces opiate withdrawal when injected, limiting its abuse potential5. Because of these lower risks, buprenorphine can be prescribed by office-based generalist physicians, potentially increasing treatment access and reducing stigma in comparison to methadone6, which is restricted to federally regulated methadone clinics.
Few physicians offer buprenorphine treatment despite its advantages7. While policies such as state Medicaid coverage of buprenorphine are related to physician adoption8,9, and while Federal and regional initiatives have sought to increase the number of prescribers, the number remains inadequate to care for opiate dependent people10 particularly among low income patients. For example, based on a comparison of the Federal register of buprenorphine certified prescribers published by the U.S. Substance Abuse and Mental Health Services Administration with the New York City Medicaid provider network provided by the New York State Medicaid office as of 2012 only 94 of the 899 buprenorphine certified prescribers in the five boroughs of New York City (10.4%) accept Medicaid payments. In contrast, Medicaid pays for a large percentage of methadone patients in New York City11. The way that opiate dependent people find their way to treatment also differs by medication: buprenorphine providers are listed on an internet site and rely on patients’ initiative and access to web-based resources, while methadone clinics get referrals from a wide range of agencies serving low income people, including public health clinics, social welfare offices and the criminal justice system12,13,14.
As might be predicted based on these differences in provider characteristics and treatment referral pathways, national data regarding the demographic characteristics of patients treated with buprenorphine indicate racial/ethnic and socioeconomic disparities in its use. National surveys of buprenorphine and methadone patients revealed that buprenorphine patients were more likely to be White (92% vs. 53% of methadone patients), employed (56% vs 29%), to have some college education (56% vs 19%), and that 75% were prescription opioid dependent rather than heroin dependent15.
Since buprenorphine has been disproportionately taken up by prescription opioid dependent patients in rural and suburban areas, these national data may not represent usage in major US cities. Little is known about racial, ethnic, and socio-economic patterns of buprenorphine treatment in major metropolitan areas, where impoverished ethnic minorities are residentially concentrated, and where the prevalence of heroin injection–and associated risks of infection and overdose–is high. The geographic concentration of poverty in American cities and its relationship to enduring racial/ethnic segregation are well documented phenomena16,17, leading to increased risk of interrelated health problems such as substance abuse, HIV, Hepatitis C and diabetes18. The disparate geographic dissemination of corresponding treatments therefore has important implications for public health.
This is particularly relevant to New York City, the largest city in the U.S., with residentially concentrated poverty, the greatest number of opioid abusers (of heroin or illicit opioids) with estimates ranging from 92,000 to 200,000, and only 21,600 of these initiating treatment in 200619, 20 (McNeely 2012). New York City also has the highest AIDS case rate of any U.S. city21,22. Additionally, the New York City Department of Health took initiative as early as 2003 to promote buprenorphine in its public hospitals as an HIV prevention measure, and New York State Medicaid covers buprenorphine prescriptions. Based on the size of its opiate dependent population, the impact of HIV on this population, and the early efforts of local officials to make buprenorphine accessible to low income patients, New York City offers an important study of buprenorphine uptake in urban, low income, ethnic minority areas.
Existing New York City data was used to examine the correlation of residential area-level measures of poverty, race, and ethnicity with rates of buprenorphine and methadone treatment use . The hypotheses tested is that methadone treatment rates are highest in the residential areas with the lowest incomes and highest percentages of black and Hispanic residents, while buprenorphine treatment rates are highest in the residential areas with the highest incomes and lowest percentages of black and Hispanic residents.
METHOD
Data
The study conducts secondary analyses of data collected for administrative purposes. Buprenorphine use is determined from data collected by the Federal Drug Enforcement Agency (DEA) on the number of outpatient buprenorphine prescriptions written in 2007 by postal ZIP code of the patient treated supplied to the authors by the New York State Bureau of Narcotics Enforcement. Although independent verification of the completeness of this data is not available, it is the most complete publicly available data set on buprenorphine prescriptions because retail pharmacies are Federally mandated to report the age, address and date of service for each buprenorphine prescription recipient to the DEA. Data on methadone use was obtained from the New York State Office of Alcoholism and Substance Abuse Services (OASAS) and are the number of all patients in a postal Zip code receiving methadone maintenance in methadone clinics in New York City in 2007. Estimates of missing data in this set are unavailable, but missing data are expected to be rare since all New York City methadone maintenance clinics are regulated by the New York State Office of Alcohol and Substance Abuse Services, and this data set is linked to reimbursements and used for budgeting purposes. To describe Zip codes, data on the proportions of the population who are living at less than two times below the poverty level, are Black non-Hispanic, and are Hispanic were obtained from 2000 US Census ZIP Code Tabulation area reports. Postal ZIP codes were matched into census ZIP code equivalents23 to align them with census data. After exclusion of codes denoting water areas and non-residential areas, the sample consisted of 181 New York City census ZIP codes.
Measures
Annual buprenorphine and methadone treatment rates were calculated for persons living in each ZIP code who had received treatment within the year. To be noted is that persons living in these zip codes, as is likely in NYC, could have traveled to other zip codes for their treatments. The annual total number of buprenorphine prescriptions (n=27,571) written to patients residing in each ZIP code was divided by 12 to approximate an unduplicated number of persons receiving prescriptions. To obtain a rate this number was divided by the number of persons residing in the ZIP code. The particular divisor used to unduplicate the count does not impact the analysis conducted. The methadone treatment rate was defined as the annual total number of people in a Zip code enrolled for any period of time in a methadone clinic (n=39,231), divided by the number of persons residing in the ZIP code. The buprenorphine and methadone treatment rates are in different units and hence not directly comparable. The study focus, however, is to separately assess the dissemination pattern of each medication, buprenorphine and methadone, among NYC residential areas. To accomplish this, separate comparisons of each usage rate were made among aggregations of ZIP codes that were homogeneous with regard to income, race/ethnicity (social areas defined below).
Social areas
Social areas are residential areas in which persons have similar living standards, ethnic backgrounds, and life-style24,25,26,27. For this study, ZIP codes were aggregated into social areas based on similarities in ZIP code racial/ethnic and income characteristics. Social area analysis is an established approach used in public health research to examine effects of residential characteristics on health and behavior, and to examine inequalities in health, when residential information is available for each subject but not other demographic information28,29,30,31.
Social areas were formed based on the three census variables that capture income and race/ethnicity using Ward’s minimum-variance method in the SAS 9.2 Cluster procedure32. Starting with a pre-specified number of social areas, the procedure clusters Zip codes to minimize within-cluster variance over the three variables. A heuristic procedure was used to select the optimal number of clusters for ANOVA analyses of treatment rates. The results of clustering based on increasing numbers of social areas were reviewed. The final choice of the number of social areas was based on: (1) having enough clusters to minimize within-cluster variance and (2) having few enough clusters to have a sufficient number of Zip codes (the unit of analysis) to maximize power in the analysis of variance. Five social areas were selected.
Given that only publicly available aggregate (zip code level) data without personal identifiers were used in this study, the study was exempted from informed consent procedures by the Human Subject Review Boards of New York University and of Nathan Kline Institute for Psychiatric Research.
Statistical analyses
Two levels of ecological analyses were conducted between treatment rates and neighborhood characteristics. First, ZIP code level treatment rates were related to income and racial/ethnic proportions of a ZIP code. Each rate was correlated univariately with race/ethnicity and income using Pearson’s Product-Moment Correlation Coefficient. Significance is reported at the 1% level. While ANOVAs (or regression models) on zip codes might have been used for this analysis, multiple interaction terms would have been required in the models and their effects are notably difficult to interpret. Second, ANOVA models were use to compare treatment rates among social areas defined by the three census variables. A one-way analysis of variance was used to compare mean treatment rates of each (the average of rates across the ZIP codes in a social area) among the five social areas. Rates between areas were contrasted using the Tukey Studentized range test for pairwise comparisons corrected for multiplicity, which controls the family wise error rate at the 5% level. No variables were controlled for in the analysis of variance since the social area itself encapsulates race, ethnicity and income. No ZIP code level data were available on insurance status which could have impacted use; however in the U.S. income level and ethnicity are correlated with percent persons with insurance coverage33,34. All study analyses were conducted in SAS 9.235.
RESULTS
Zip code level analyses
Across ZIP codes, the buprenorphine treatment rate was significantly (p<.01) negatively correlated with percent of residents who are in poverty, Black non-Hispanic, and Hispanic while methadone rates were significantly (p<.01) positively correlated with these variables (Table 1). Methadone rates were substantially (R>.55) correlated with percent poverty and percent Hispanic. Buprenorphine rates were weakly correlated with all census variables (R<−.29).
Table 1.
Corellation coefficients (R and p values) for associations between treatment rates and proportion of residents who are impoverished, Black non-Hispanic, and Hispanic in New York City ZIP codes
| Proportion of Zip Code Area with Demographic Characteristic | |||
|---|---|---|---|
| Treatment | % Below 2X Poverty | Black, non-Hispanic | Hispanic |
| Methadone | 0.57 (<.0001) | 0.24 (.001) | 0.55 (<.0001) |
| Buprenorphine | −0.26 (.0005) | −0.29 (<.001) | −0.18 (.01) |
Correlation coefficients are univariate, unadjusted estimates. See text for definition of methadone and buprenorphine treatment rates.
Social area analyses
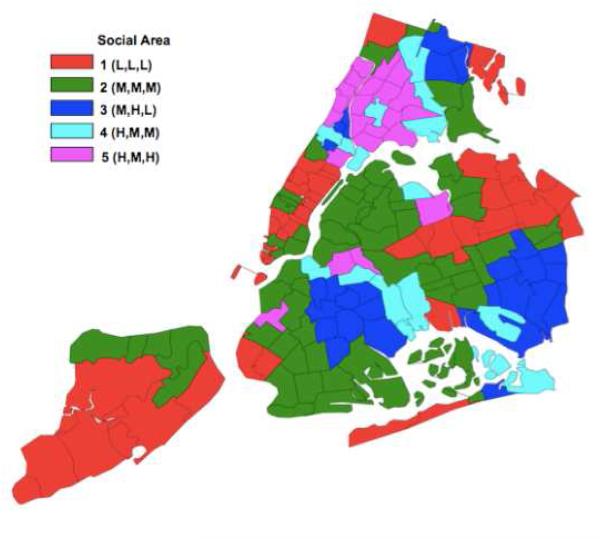
In order to better describe the social areas in terms of the census variables (percent of a characteristic), each characteristic was assigned a value of high (H), medium (M) or low (L) based on cutpoints obtained from a one dimensional clustering of the variable values across all zip codes into three tertiles. The tertile cut points for percent below two times poverty level are 33% and 51%; for percent Black non-Hispanic are 10% and 46% and for percent Hispanic are 22% and 47%. Fifty five ZIP codes were aggregated into social area 1 described as low poverty, low percent Black non-Hispanic, low percent Hispanic and denoted by L,L,L; an aggregation of 62 ZIP codes (social area 2) was characterized as moderate income, moderately non-Hispanic Black and moderately Hispanic (M,M,M ); 27 ZIP codes (social area 3) as moderate income, highly Black non-Hispanic and low percent Hispanic (M,H,L ); 15 ZIP codes (social area 4) as high poverty, moderate percent Black non-Hispanic, moderate percent Hispanic (H,M,M ), and 22 ZIP codes (social area 5) as high poverty, moderate percent non-Hispanic Black, high percent Hispanic (H,M,H). The clusters were mainly but not necessarily geographically contiguous (Figure 1).
Figure 1. Map or New York City Social Areas.
Social area notations in parentheses indicate proportions of area residents who are impoverished, Black non-Hispanic, and Hispanic, respectively. L,M, and H denote low, medium, and high proportions, respectively. See text for definitions of social areas and treatment rates.
The social area L,L,L had the highest rate of buprenorphine users, and this rate significantly differed from rates of all other social areas (p<.05 for all contrasts, Table 3).
Table 3.
Mean annual number of buprenorphine and methadone treatments per 10,000 residents (treatment rates), by social area
| Social Area | # of buprenorphine prescriptions filled (SE) |
# of persons receiving methadone (SE) |
|---|---|---|
| 1 (L,L,L) | 5.1 (4.9) 1 | 20.6 (20.4) 3 |
| 2 (M,M,M) | 2.9 (2.4) 2 | 37.1 (34.7) 3 |
| 3 (M,H,L) | 1.2 (1.4) 2 | 45.4 (30.2) 4 |
| 4 (H,M,M) | 1.3 (1.1) 2 | 75.6 (69.6) 4,5 |
| 5 (H,M,H) | 2.8 (3.4) 2 | 114.2 (71.1) 6 |
Social area notations in parentheses indicate relative proportions of area residents who are <2X poverty level, Black non-Hispnic, and Hispanic, resplectively. L, M, and H denote low, medium, and high tertiles of variables within social areas, respectively. SE denotes standard error. See text for definitions of social areas and treatment rates.
Significantly greater than the buprenoorphine treatment rates for social areas 2, 3, 4 and 5.
Significantly less than the buprenorphne treatment rate for social area 1.
Significantly less than the methadone treatment rates for social areas 4 and 5.
Significantly less than the methadone treatment rates for social area 5.
Significantly greater than the the methadone treatment rate for area 1.
Significantly greater than the methadone treatment rates for social areas 1, 2,3,4.
Social area treatent rates contrasted using the Tukey studentized range test for pairwise comparisons corrected for multiplicity, controlling family wise error rate at the p<.05 level.
Methadone treatment rates were the highest in social area 5 (H, M, H). Methadone treatment rates monotonically increased from social area 1 to 5 from a low of 21 persons per 10,000 in the highest income area to a high of 114 persons per 10,000 in the poorest area. The methadone rate for the high poverty area that was predominately Hispanic differed significantly from the rates of all other social areas. The high poverty area with moderately high proportions of both Blacks and Hispanics (H,M,M) had significantly higher rates of methadone use than the low poverty, predominately White area (L,L,L) and the moderate income area with moderate proportions of Blacks and Hispanics (M,M,M).
DISCUSSION
Since demographic data on individual buprenorphine users is not routinely collected, information relevant to the population level distribution of buprenorphine treatment, relative to methadone treatment, has not been available at the regional level. This paper is unique in its comparison of buprenorphine with methadone usage patterns by the demographics of the neighborhoods of users, providing insight into dissemination patterns. It was found that buprenorphine treatment is unevenly distributed among New York City social areas and is concentrated in areas with the highest incomes and highest percentage of White residents. ZIP code level findings of a negative correlation of buprenorphine treatment rates, and a positive correlation of methadone treatment rates with poverty and Black or Hispanic ethnicity were confirmed by social area level multivariate analysis which shows that methadone has an inverse geographic distribution to that of buprenorphine.
The fifth social area (H,M,H), a high poverty, predominantly Hispanic social area that had an intermediate buprenorphine treatment rate and a high methadone treatment rate, is an exception. This area includes zip codes in the South Bronx served by Montefiore, a large hospital that offered buprenorphine to its primarily Medicaid insured and uninsured patients starting shortly after buprenorphine’s FDA approval (New York City Department of Health Buprenorphine Task Force, personal communication, 2005). The influence of this hospital’s early buprenorphine initiative is informative to dissemination analysis and may explain the intermediate buprenorphine treatment rate in this low income, ethnic minority social area. The social area of highest buprenorphine use included affluent ZIP codes of Staten Island, lower and midtown Manhattan, Queens bordering suburban Long Island, and one area of the North Bronx bordering suburban Westchester County. Impoverished neighborhoods of East New York and Harlem were in the social area with the lowest buprenorphine use. Stark geographic differences in buprenorphine and methadone treatment across New York City social areas are particularly concerning given the existence of local policies intended to support equitable dissemination of buprenorphine treatment. New York State was one of the first states to offer Medicaid coverage of buprenorphine-related office visits and buprenorphine prescriptions. The New York City Department of Health declared buprenorphine dissemination among low income patients a public health priority in 2003, one year after buprenorphine’s FDA approval36; it launched an outreach campaign to public clinics which distributed promotional materials on buprenorphine, and offered buprenorphine certification training for public clinic physicians. Although the impact of these efforts on buprenorphine dissemination in public clinics has not been systematically evaluated, because few other major U.S. cities promote buprenorphine for publicly insured patients, and not all states’ Medicaid programs cover buprenorphine prescriptions8, differences in New York City by social area may be smaller than those in other cities.
The low number of Medicaid-accepting buprenorphine prescribers relative to the potential demand for buprenorphine suggests a problem of access to buprenorphine prescribers among low income people. In contrast, buprenorphine usage in high income, largely White areas is most likely linked to providers who accept private insurance coverage. Furthermore the dissemination of buprenorphine as a viable treatment may be promoted by social networks of neighborhood residents who share treatment information; such networks have been shown to reinforce socio-economic and ethnic/racial differences in treatment37. Availability of providers in a ZIP code could potentially impact the dissemination of the two medications. However, the widespread availability of mass transit in New York City would strongly mitigate the effect of provider location on treatment provision . A shortage of public sector physicians who prescribe buprenorphine has been identified as a major barrier to dissemination among low income patients in a national study, and most buprenorphine prescribers are in private practice or are addiction specialists9. Interviews with buprenorphine prescribers reveal that private practice physicians charge substantial fees for buprenorphine treatment, while there are few professional or economic incentives for public sector physicians to offer buprenorphine treatment (Hansen H, unpublished data).
Other factors that may contribute to the low rates of buprenorphine treatment in low income, ethnic minority neighborhoods could be linked to professional and institutional treatment biases. These factors include: 1) Some addiction specialists express the view that buprenorphine is more clinically appropriate for employed, “stable” prescription opiate abusers than “unstable” heroin injectors38, 39. 2) The manufacturer’s marketing of buprenorphine relies on internet-based publicity displaying employed, White prescription opiate abusing patients40, which may reflect that buprenorphine was approved for treatment of opiate dependence on the heels of a major U.S. epidemic of White, middle class prescription opiate abuse41. 3) A major mechanism for referral of patients to buprenorphine certified physicians is an online network sponsored by the federal Substance Abuse and Mental Health Services Administration42; low income patients may not have access to online services.
Research and interventions to identify and redress the institutional and professional barriers to buprenorphine provision are needed. Effective dissemination of buprenorphine requires targeted efforts to promote buprenorphine prescribing among public sector physicians, particularly those in primary care who come into contact with large numbers of patients. Studies of buprenorphine dissemination through the NIDA Clinical Trials Network, and of the Physician Clinical Support System, indicate that linking buprenorphine experienced providers with inexperienced providers is effective in promoting buprenorphine prescription by widening circles of physicians43. Physicians are also more likely to prescribe buprenorphine when they believe their patient population has a need for treatment for opioid dependence, and when they perceive the treatment as relatively easy to administer44. These perceptions could be addressed in interventions that promote buprenorphine accessibility in public clinics.
Unlike buprenorphine treatment, methadone maintenance is targeted to low income patients. Methadone clinics have long been funded by city and state governments, methadone is considered one of New York City’s major HIV prevention initiatives, and is routinely offered to opiate dependent recipients of public assistance13. There is, however, little clinical evidence of any advantage of methadone over buprenorphine for treatment of low-income patients. A clinical trial comparing buprenorphine with methadone treatment of low income opiate dependent arrestees initiating treatment prior to prison release, found no difference between patients in the two groups with regard to relapse, re-arrest or re-incarceration after release; in fact, treatment continuation rates were higher among those on buprenorphine45. Other studies suggest that patient preferences for, and outcomes on, buprenorphine are influenced by disease severity and comorbidity46,47; these factors should also be examined in future investigations of geographic and demographic patterns in buprenorphine dissemination.
This study has several limitations. The ethnic and socioeconomic backgrounds of buprenorphine and methadone users cannot be directly inferred from the characteristics of the area of their residence due to the risk of ecological fallacy. The ecological fallacy occurs when population level characteristic are ascribed to individuals in the area who do not have these characteristics, e.g. by assuming that all persons in a predominantly Black area are Black. Refraining from making inferences about how individual characteristics influence treatment, avoids the ecological fallacy, but does not allow for extrapolating from geographic distribution of treatment use to the characteristics of individual treatment users. Second, unmeasured neighborhood characteristics which influence methadone and buprenorphine treatment rates–such as disease prevalence, incidence, and severity–may be heterogeneously distributed across neighborhoods and may confound the relationships observed. Third, the estimate of the unduplicated count of buprenorphine users does not reflect the possible mixed frequency of prescription fillings. In any event, direct comparisons are not made between buprenorphine and methadone treatment rates within a social area or ZIP code due to differences in their units of measurement. Fourth, the U.S. Census demographic data used was collected seven years before the buprenorphine treatment data. Shifts in neighborhood population characteristics may have occurred in that time. Finally, New York City findings may not be generalizable to other U.S. cities.
Implications for Behavioral Health
The unequal distribution of the new, lower risk treatment technology reported here is predicted by social epidemiological theory: new technologies disproportionately benefit high income consumers with the means to access them. Therefore treatment innovations can widen disparities unless concerted public efforts counteract social processes underlying disparities48,49. In addition, analyses that take into consideration the interaction of geographic patterns of health and health care with local cultural and institutional practices are crucial for developing appropriate and context-dependent interventions50. For example, access to a full range of convenient, safe treatments for opiate dependence in low income and ethnic minority neighborhoods is a particular public health imperative because the most severe consequences of untreated opiate dependence–HIV infection, overdose and incarceration–are concentrated there. Physicians serving low income patients already face the challenge of growing caseloads and declining reimbursements due to reduced governmental support for the public health sector. In New York State, providers saw $204.9 million in cuts Medicaid for mental health services between 2009-201251, and $46 million in cuts to the Office of Alcoholism and Substance Abuse in 2011 alone52. Specific physician incentives to increase the number of buprenorphine prescribers in the public sector - those who serve the largest number of patients at risk for opiate related infection, overdose and incarceration - should be considered as a preventive health investment. Follow up studies of the physician-level, institutional and policy factors related to the adoption of buprenorphine should provide information to refine treatment dissemination interventions and achieve the public health gains heralded with the introduction of buprenorphine treatment.
Table 2.
Demographic Characteristics of social areas
| Mean % if characteristic across zip codes in social area (SD) | |||||
|---|---|---|---|---|---|
| Social Area |
Social Area Label |
# (%) of ZIP Codes in Area |
% Below 2X Poverty Level |
Black, non-Hispanic |
Hispanic |
| 1 | (L,L,L) | 55 (30.4) | 17.2 (5.7) | 3.1 (2.9) | 9.0 (4.2) |
| 2 | (M,M,M) | 62 (34.2) | 37.9 (12.1) | 11.5 (10.5) | 23.6 (11.3) |
| 3 | (M,H,L) | 27 (14.9) | 39.2 (14.3) | 75.6 (12.6) | 11.6 (5.8) |
| 4 | (H,M,M) | 15 (8.3) | 57.1 (12.3) | 43.8 (10.8) | 37.1 (11.4) |
| 5 | (H,M,H) | 22 (12.2) | 61.9 (6.4) | 21.9 (10.9) | 66.3 (7.4) |
L, M, and H denote low, medium, or high tertiles variables within social area.
SD denotes standard deviation.
See text for definition of social areas.
Footnotes
Conflict of Interest Statement None of the contributing authors have conflicts of interest with regard to ties to manufacturers or promoters of treatments for opiate dependence. This study was supported by the Substance Abuse and Mental Health Services Administration/American Psychiatric Association Minority Fellowship, the New York State Office of Mental Health’s Center of Excellence in Culturally Competent Mental Health, and the Robert Wood Johnson Health and Society Scholars Fellowship Program.
REFERENCES
- 1.Lawrinson P, Ali R, Buavirat A, et al. Key findings from the WHO collaborative study on substitution therapy for opioid dependence and HIV/AIDS. Addiction. 2008;103(9):1493–4. doi: 10.1111/j.1360-0443.2008.02249.x. [DOI] [PubMed] [Google Scholar]
- 2.Sees KL, Delucchi KL, Masson C, et al. Methadone maintenance vs 180-day psychosocially enriched detoxification for treatment of opioid dependence: a randomized controlled trial. JAMA. 2000;283(10):1303–10. doi: 10.1001/jama.283.10.1303. [DOI] [PubMed] [Google Scholar]
- 3.Appel PW, Joseph H, Kott A, et al. Selected in-treatment outcomes of long-term methadone maintenance treatment patients in New York State. Mt. Sinai Journal of Medicine. 2001;68(1):55–61. [PubMed] [Google Scholar]
- 4.Bell JR, Butler B, Lawrence A, et al. Comparing overdose mortality associated with methadone and buprenorphine. Drug and Alcohol Dependence. 2009;104(1-2):73–7. doi: 10.1016/j.drugalcdep.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Food and Drug Administration [Accessed March 28, 2013];FDA Talk Paper: Subutex and Suboxone approved to treat opiate dependence. Avaliable online at: http://www.fda.gov/bbs/topics/ANSWERS/2002/ANS01165.html.
- 6.Merrill JO. Policy progress for physician treatment of opiate addiction. Journal of General Internal Medicine. 2002;17(5):361–8. doi: 10.1046/j.1525-1497.2002.10628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roman PM, Abraham AJ, Knudsen HK. Using medication-assisted treatment for substance use disorders: Evidence of barriers and facilitators of implementation. Addictive Behavior. 2011;36(6):584–9. doi: 10.1016/j.addbeh.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducharme LJ, Abraham AJ. State policy influence on the early diffusion of buprenorphine in community treatment programs. Substance Abuse Treatment Prevention and Policy. 2008;3:17. doi: 10.1186/1747-597X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallack SS, Thomas CP, Martin TC, et al. Substance Abuse Treatment Organizations as Mediators of Social Policy: Slowing the Adoption of a Congressionally Approved Medication. Journal of Behavioral Health Services & Research. 2010;37(1):64–78. doi: 10.1007/s11414-008-9132-4. [DOI] [PubMed] [Google Scholar]
- 10.Roman PM, Abraham AJ, Rothrauff TC, et al. A longitudinal study of organizational formation, innovation adoption, and dissemination activities within the National Drug Abuse Treatment Clinical Trials Network. Journal of Substance Abuse Treatment. 2010;38(Suppl 1):S44–S52. doi: 10.1016/j.jsat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne-Miller A. The Praeger International Collection on Addictions. Greenwood Publishers; Westport, CT: 2009. [Google Scholar]
- 12.Benoit E, Young R, Magura S, et al. The impact of welfare reform on methadone treatment: policy lessons from service providers in New York City. Substance Use & Misuse. 2004;39(13-14):2355–90. doi: 10.1081/ja-200034643. [DOI] [PubMed] [Google Scholar]
- 13.Morgenstern J, Hogue A, Dauber S, et al. A practical clinical trial of coordinated care management to treat substance use disorders among public assistance beneficiaries. J Consult Clin Psychol. 2009;77(2):257–69. doi: 10.1037/a0014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunn A, Zeller N, Dickman S, et al. Methadone and buprenorphine prescribing and referral practices in US prison systems: results from a nationwide survey. Drug and Alcohol Dependence. 2011;113(2-3):252. doi: 10.1016/j.drugalcdep.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanton A, McLeod C, Luckey B, et al. [Accessed March 28, 2013];SAMHSA/CSAT Evaluation of the Buprenorpine Waiver Program. Available online at http://buprenorphine.samhsa.gov/Overview_Evaluation_Waiver_Program.pdf.
- 16.Massey DS, Denton NA. American Apartheid: Segregation and the Making of the American Underclass. Harvard University Press; Cambridge, MA: 1993. [Google Scholar]
- 17.Fullilove MT. Rootshock: How Tearing Up City Neighborhoods Hurts America, and What We Can Do About It. Random House; New York: 2005. [Google Scholar]
- 18.Singer M. Introduction to Syndemics: A Critical Systems Approach to Public and Community Health. John Wiley & Sons; San Francisco: 2009. [Google Scholar]
- 19.Frank B. An overview of heroin trends in New York City: Past, present and future. The Mount Sinai Journal of Medicine. 2000;67(5-6):340–6. [PubMed] [Google Scholar]
- 20.McNeely N, Gourevitch MN, Paone D, et al. Estimating the prevalence of illicit opioid use in New York City using multiple data sources. BMC Public Health. 2012;12:443. doi: 10.1186/1471-2458-12-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NYC Department of Health and Mental Hygiene [Accessed March 28, 2013];HIV/AIDS Information. Available online at http://www.nyc.gov/html/doh/html/ah/ah.shtml.
- 22.Courtwright DT. The prepared mind: Marie Nyswander, methadone maintenance, and the metabolic theory of addiction. Addiction. 1997;92(3):257–65. [PubMed] [Google Scholar]
- 23.Grubesic TH, Matisziw TC. On the use of ZIP codes and ZIP code tabulation areas (ZCTAs) for the spatial analysis of epidemiological data. International Journal of Health Geography. 2006;5:58. doi: 10.1186/1476-072X-5-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith MP, Olatunde O, White C. Inequalities in disability-free life expectancy by area deprivation: England, 2001-04 and 2005-08. Health Statistics Quarterly. 2010;48:36–57. doi: 10.1057/hsq.2010.20. [DOI] [PubMed] [Google Scholar]
- 25.Araujo EM, Costa Mda C, Oliveira NF, et al. Spatial distribution of mortality by homicide and social inequalities according to race/skin color in an intra-urban Brazilian space. Brazilian Review of Epidemiology (Rev Bras Epidemiol) 2010;13(4):549–60. doi: 10.1590/s1415-790x2010000400001. [DOI] [PubMed] [Google Scholar]
- 26.Chau KL. Ecological analysis of health care utilization for China’s rural population: association with a rural county’s socioeconomic characteristics. BMC Public Health. 2010;10:664. doi: 10.1186/1471-2458-10-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bocquier A, Cortaredona S, Nauleau S, et al. Prevalence of treated diabetes: Geographical variations at the small-area level and their association with area-level characteristics. A multilevel analysis in Southeastern France. Diabetes Metab. 2011;37(1):39–46. doi: 10.1016/j.diabet.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Das-Munshi J, Becares L, Dewey ME, et al. Understanding the effect of ethnic density on mental health: multi-level investigation of survey data from England. British Medical Journal. 2010;341:c5367. doi: 10.1136/bmj.c5367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottfredson DC, McNeil RJ, Gottfredson GD. Social area influences on delinquency: A multilevel analysis. Journal of Research in Crime and Delinquency. 1991;28(2):197–226. [Google Scholar]
- 30.Shevky E, Bell W. Social area analysis: theory, illustrative application and computational procedures. Stanford University Press; Stanford, CA: 1995. [Google Scholar]
- 31.Scott-Samuel A. Social area analysis in community medicine. British Journal of Preventive and Social Medicine. 1977;31:199–204. doi: 10.1136/jech.31.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heye C, Leuthold H. [Accessed March 28, 2013];Theory-based social area analysis: An approach considering the conditions of a post-industrial society. 14th European Colloquium on Theoretical and Quantitative Geography. 2005 Available online at http://www.sotomo.ch/media/publis/ch_hl_2005_socialarea.pdf. [Google Scholar]
- 33.Adams PR, Martinez ME, Vickerie JL. Summary health statistics for the U.S. population: national health interview survey, 2009. Vital Health Statistics. 2009;10(248):1–115. [PubMed] [Google Scholar]
- 34.Liao Y, Bang D, Cosgrove S, et al. Surveillance of health status in minority communities – racial and ethnic approaches to community health across the U.S. (REACH U.S.) risk factor survey, United States 2009. MMWR Surveillance Summary. 2011;60(60):1–44. [PubMed] [Google Scholar]
- 35.SAS Institute, Inc . SAS/STAT® 9.2 User’s Guide. SAS Institute Inc.; Cary, NC: 2008. [Google Scholar]
- 36.Taylor C. [Accessed March 28, 2013];New option to wean off heroin. New York City Newsday. Available online at http://www.mapinc.org/newscsdp/v05/n1099/a05.html.
- 37.Duhan D, Johnson S, Wilcox J, et al. Influences on consumer use of word-of- mouth recommendation sources. Journal of the Academy of Marketing Science. 2007;25(4):283–95. [Google Scholar]
- 38.Casadonte P. Community treatment programs take up buprenorphine. Science & Practice Perspectives. 2004;2(2):24–6. doi: 10.1151/spp042224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore BA, Fiellin DA, Barry DT, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. Journal of General Internal Medicine. 2007;22(4):527–30. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Alliance of Advocates for Buprenorphine Treatment [Accessed March 28, 2013];National Alliance of Advocates for Buprenorphine Treatment (NAABT) Available online at www.naabt.org.
- 41.Van Zee A. The promotion and marketing of OxyContin: Commercial triumph, public health tragedy. Journal of the American Public Health Association. 2009;99(2):221–7. doi: 10.2105/AJPH.2007.131714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Substance Abuse and Mental Health Services Administration (SAMHSA) [Accessed March 28, 2013];Buprenorphine Physician and Treatment Program Locator. Available online at http://buprenorphine.samhsa.gov/bwns_locator/aboutphysician.htm.
- 43.Egan JE, Casadonte P, Gartenmann T, et al. The Physician Clinical Support System-Buprenorphine (PCSS-B): a novel project to expand/improve buprenorphine treatment. Journal of General Internal Medicine. 2010;25(9):936–41. doi: 10.1007/s11606-010-1377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barry DT, Irwin KS, Jones ES, et al. Integrating buprenorphine treatment into office-based practice: a qualitative study. Journal of General Internal Medicine. 2009;24(2):218–25. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magura S, Lee JD, Hershberger J, et al. Buprenorphine and methadone maintenance in jail and post-release: a randomized clinical trial. Drug and Alcohol Dependence. 2009;99(1-3):222–30. doi: 10.1016/j.drugalcdep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pinto H, Maskrey V, Swift L, et al. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. Journal of Substance Abuse Treatment. 2010;39(4):340–52. doi: 10.1016/j.jsat.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan LE, Moore BA, O’Connor PG, et al. The association between cocaine use and treatment outcomes in patients receiving office-based buprenorphine/naloxone for the treatment of opioid dependence. American Journal of Addictions. 2010;19(1):53–8. doi: 10.1111/j.1521-0391.2009.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Link B. Epidemiological sociology and the social shaping of population health. Journal of Health and Social Behavior. 2008;49:367–84. doi: 10.1177/002214650804900401. [DOI] [PubMed] [Google Scholar]
- 49.Rubin MS, Colen CG, Link G. Examination of inequalities in HIV/AIDS mortality in the United States from a fundamental cause perspective. American Journal of Public Health. 2009;100(6):1053–9. doi: 10.2105/AJPH.2009.170241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cutchin MP. The need for the “New Health Geography” in epidemiological studies of environment and health. Health Place. 2007;13(3):725–42. doi: 10.1016/j.healthplace.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Honberg R, Kimball A, Diehl S, et al. [Accessed March 28, 2013];State mental health cuts: the continuing crisis. National Alliance for the Mentally Ill (NAMI) Available online at http://www.nami.org/ContentManagement/ContentDisplay.cfm?Content FileID=147763.
- 52.New York Association of Alcoholism & Substance Abuse Providers (NYAASAP) [Accessed March 28, 2013];Federal funding for addiction-related programming critical, especially in the face of budget cuts. Available online at www.asapnys.org/PDF/DCPacket/2011StrongFundingNeededFacingState BudgetCuts.pdf.