Abstract
Introduction
Transesophageal echocardiography (TEE) is used to evaluate for left atrial appendage (LAA) thrombi prior to restoration of sinus rhythm in atrial fibrillation (AF). We examined the relationship of atrial fibrosis quantified using late gadolinium enhancement MRI (LGE-MRI) with TEE findings.
Methods and Results
We included 178 patients with AF, undergoing TEE and LGE-MRI prior to ablation or cardioversion. LGE-MRI and subsequent image processing was used to quantify atrial fibrosis based on signal intensity analysis. The mean CHADS2 score was 1.24±1.08 and CHA2DS2-VASc was 2.08±1.33. The LAA was classified as normal, spontaneous echo contrast (SEC) present or thrombus present. LAA thrombus was found in 12 patients (6.7%) while SEC was identified in 19 patients (10.7%). Patients with thrombus had higher atrial fibrosis compared to patients without thrombus (26.9±17.4% vs 16.7±10.5%; p<0.01). Atrial fibrosis was also higher in patients with SEC (23.3±13.7%) compared to those without SEC (16.7±10.8%; p=0.01). Patients with high atrial fibrosis (>20%) were more likely to have a LAA thrombus (Odds Ratio 4.6; p=0.02) and SEC (Odds ratio 2.6; p=0.06). Multivariate logistic regression showed high fibrosis (Odds Ratio 3.6; p<0.01) and CHADS2≥2 (Odds Ratio 3.5; p<0.01) were significant predictors of TEE abnormalities (LAA thrombus or SEC). The area under the curve for the model including high fibrosis, AF type and CHADS2≥2 or CHA2DS2-VASc≥2 was 0.73 compared to 0.63 and 0.65 for CHADS2 and CHA2DS2-VASc alone.
Conclusions
Atrial fibrosis is independently associated with appendage thrombus and spontaneous contrast. It provides additional risk stratification not captured by clinical parameters.
Keywords: Atrial fibrillation, transesophageal echocardiography, magnetic resonance imaging, appendage thrombus, stroke, atrial fibrillation, catheter ablation
Introduction
Atrial fibrillation (AF) is associated with increased mortality1 and a 5-fold higher risk of stroke2. Embolic strokes associated with AF cause more severe deficits and debilitation compared to ischemic strokes unrelated to the arrhythmia.3 One of the early decision points in managing AF patients is the assessment of thromboembolic risk. Several risk stratification tools, including the CHADS2 score, are in use and rely on clinical parameters and comorbidities. The decision whether to recommend oral anticoagulation therapy is made depending on the number of risk factors identified.
AF is associated with tissue as well as overall atrial chamber structural remodeling that has been demonstrated in both animal models and humans.4 This remodeling process is integral to the pathophysiology of the arrhythmia and constitutes the substrate required for its maintenance.5 We have demonstrated that atrial tissue remodeling and fibrosis can be noninvasively assessed and quantified using advanced cardiac imaging with Late-Gadolinium Enhancement MRI (LGE-MRI)6, 7. Thromboembolism risk stratification tools currently do not incorporate any measure of the atrial remodeling seen in AF patients.
Transesophageal echocardiography (TEE) is a commonly used imaging modality in AF patients. It is often performed to evaluate for atrial thrombi prior to interventions aimed at restoration of sinus rhythm, such as direct current cardioversion, antiarrhythmic drug therapy and catheter ablation. TEE is especially performed when duration of arrhythmia and adequate therapeutic anticoagulation cannot be properly ascertained.
In this study, we sought to evaluate the relationship of LA fibrosis quantified using LGE-MRI and the presence of TEE abnormalities. We hypothesized that advanced atrial fibrosis is associated with a higher risk of TEE abnormalities including LA appendage thrombi and spontaneous echocardiographic contrast (SEC). We also examined the predictive ability of a model that includes atrial fibrosis in addition to the usual clinical risk factors (CHADS2 and CHA2DS2-VASc scores).
Methods
Study Population
One hundred and seventy-eight patients were retrospectively selected from the University of Utah atrial fibrillation database. Selected patients had to have both LGE-MRI of the heart as well as transesophageal echocardiography between April 2009 and September 2010. The institutional review board approved the atrial fibrillation program database protocol. Patient related data were de-identified and protected in compliance with Health Insurance Portability and Accountability Act (HIPAA) regulations. Baseline patient demographics, medical conditions and comorbidities, pertinent lab results especially international normalized ratios (INRs), drug treatments, echocardiographic and LGE-MRI data pertaining to chamber size and function as well as significant valvular abnormalities were collected and analyzed for the purpose of the study.
Late-Gadolinium Enhancement MRI Acquisition
LGE-MRI was obtained in all patients for decision-making and management of atrial fibrillation. The details of MRI acquisition techniques have been previously described6, 7. Briefly, all scans were performed on a 3 Tesla Verio clinical scanner (Siemens Healthcare, Erlangen, Germany). High-resolution LGE images of the left atrium were acquired about 15 minutes following contrast agent injection (0.1mmol/kg, Multihance [Bracco Diagnostics Inc., Princeton, NJ, USA]) using a 3-dimensional (3D) inversion recovery prepared, respiration-navigated, electrocardiogram (ECG)-gated, gradient echo pulse sequence. Typical acquisition paramaters were: free breathing using respiratory navigation, a transverse imaging volume with voxel size=1.25 × 1.25 × 2.55mm (reconstructed to 0.625 × 0.625 × 1.25mm), repetition time/echo time (TR/TE) = 3.1/1.4 milliseconds, flip angle = 14 degrees, inversion time (TI) = 280–330 milliseconds, and GRAPPA with a reduction factor of 2. Inversion pulse was applied every heart beat and fat saturation was applied immediately before data acquisition. Data acquisition was limited to 15% of the cardiac cycle and was performed during the diastolic phase of the atrial cycle. The TE of the scan (1.4 milliseconds) was chosen such that fat and water were almost out of phase and the signal intensity of partial volume fat-tissue voxels was reduced allowing improved delineation of the atrial wall boundaries. The TI value for the LGE-MRI scan was identified using a TI scout scan. Typical scan time for the LGE-MRI study was 4–9 minutes depending of subject respiration.
Quantification of atrial tissue fibrosis
Left atrial wall volumes were manually segmented by expert observers from the LGE-MRI images, using the Corview image processing software (MARREK Inc., Salt Lake City, UT). The protocol for segmentation proceeded as follows. First, the endocardial border of the LA was defined, including an extent of pulmonary vein (PV) sleeves, by manually tracing the LA-PV blood pool in each slice of the LGE-MRI volume. Next, the endocardial segmentation was morphologically dilated and then manually adjusted to create an assessment of the boundary of the epicardial LA surface. Finally, the endocardial segmentation was subtracted from the epicardial segmentation to define a wall segmentation, which was manually edited to exclude the mitral valve and PVs. Thus, the resulting LA wall segmentation included the 3D extent of both the LA wall and the antral regions of the PVs.
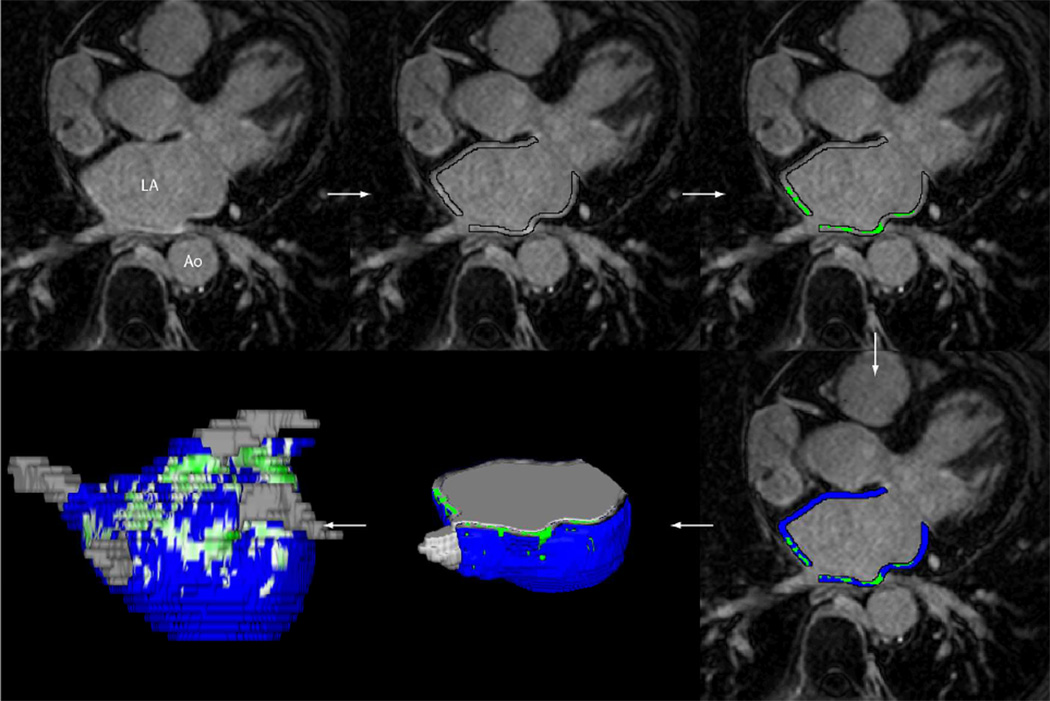
To delineate regions of fibrosis in LGE-MRI images, we defined enhancement through an intensity threshold that was determined by expert inspection. To assist this process, initial visualization used a volume-rendering tool in Corview that allowed the operator to visualize the distribution of enhancement in 3D. A custom transfer function allowed the operator to define gradations of enhancements, while suppressing blood and normal tissue with a transfer function. The threshold was defined for each patient at two standard deviations above the mean enhancement. Figure 1 illustrates the steps described above. Quantification of atrial tissue fibrosis was reported as a percentage of the left atrial wall volume.
Figure 1.
Example of MRI image processing is shown for a patient with a quantified fibrosis of 13.0%. The steps from MRI image acquisition to quantification of fibrosis are illustrated.
Processing of the MRI scans was completed in a blinded independent fashion relative to the trans-esophageal echocardiograms.
Interobserver Variability
Three blinded observers were asked to quantify fibrosis on randomly selected subjects from this study. The correlation coefficients between observers ranged from 0.82 to 0.97 indicating significant reproducibility and advanced expertise in our laboratory in acquisition, segmentation and processing of LGE-MRI images.
Transesophageal echocardiographic evaluation
Transesophageal echocardiograms were obtained after the MRI scan with an average time of one week between the two studies. All studies were conducted under a standardized protocol that includes local hypopharyngeal anesthesia and monitored sedation with midazolam and fentanyl. Transesophageal image acquisition was performed using commercially available, multiplane phased array imaging probes and cardiac ultrasound systems (Sequoia C512: Siemens Medical Solutions, Mountain View, California; Philips iE33: Philips Medical Systems, Bothell, WA). Cine loops were stored and images were available for offline review and evaluation for the presence of appendage thrombus and left atrial spontaneous echocardiographic contrast. All scans were performed in the presence of two cardiovascular disease physicians with extensive echocardiography experience. Spontaneous echocardiographic contrast was defined as smoke-like material with a characteristic swirling motion that persisted throughout the cardiac cycle and was seen with optimized gain settings. Thrombus was defined as an echo-dense mass lesion that was present in multiple imaging planes and clearly distinguishable from trabeculation and atrial endocardium.8 Intravenous injection of perflutren lipid microspheres (Definity: Lantheus Medical Imaging, North Billerica, MA) was selectively used to clarify whether ambiguous lesions represented spontaneous contrast versus thrombus.
Statistical Analysis
Statistical analysis was performed using Stata version 12 (StataCorp, College Station, TX, USA). Continuous variables are reported as means and standard deviations; categorical variables are reported as percentages of the cohort. Student’s t-test was used to compare continuous variables and Fisher’s exact test (two-sided) was used to compare categorical variables. Logistic regression was used to determine the odds of a positive TEE finding. Since we only found 12 patients with thrombus and 19 patients with SEC, we combined the two findings (28 patients with LAA thrombus, SEC or both) in order to perform multivariate regression analysis. Receiver operating characteristics (ROC) analysis was performed to assess the performance of the prediction models using the CHADS2 score alone and in combination with atrial fibrosis and AF type. A p value of less than 0.05 was considered significant for all results.
Results
Patient characteristics are summarized in Table 1. The mean patient age was 66±11 years; 29% were women. The prevalence of persistent atrial fibrillation in this cohort was 68% or 119 patients. Sixty-three patients (35.4%) had a CHADS2 score ≥ 2 and 117 patients (65.7%) had a CHADS2-VASc ≥2. Thirty-seven patients (20.8%) had coronary artery disease, 29.3% had greater than mild mitral valve regurgitation. The average LV ejection fraction was 58% with 13 patients (7.6%) having an ejection fraction less than 40%.
Table 1.
Baseline Patient characteristics compared between high (≥20%) and low (<20%) fibrosis groups
| Overall n=178 |
LA fibrosis <20% N=120 |
LA fibrosis ≥ 20% N=58 |
P value | |
|---|---|---|---|---|
| Age | 66±11 | 65±11 | 67±11 | 0.22 |
| Gender (%Female) | 29 | 23% | 41% | 0.01 |
| Persistent AF (%) | 68.0 | 66.9% | 69.0% | 0.79 |
| HTN (%) | 65.3 | 69.5% | 56.9% | 0.10 |
| DM (%) | 13.6 | 14.4% | 12.1% | 0.67 |
| CAD (%) | 20.6 | 20.7% | 12.6% | 0.19 |
| CHF (%) | 11.4 | 11.7% | 10.3% | 0.78 |
| TIA/CVA (%) | 5.6 | 2.5% | 3.4% | 0.73 |
| LVEF(%) | 58±10 | 58±11 | 58±10 | 0.98 |
| Mitral valve regurgitation (>1+) | 29.3 | 27.4% | 33.3% | 0.42 |
| CHADS2 | 1.24±1.08 | 1.22±1.06 | 1.21±1.14 | 0.96 |
| CHADS2≥2 [n (%)] | 63 (35.4%) | 43 (35.8%) | 20 (34.5%) | 0.85 |
| CHA2DS2-VASc | 2.08±1.33 | 1.98±1.35 | 2.28±1.26 | 0.17 |
| CHA2DS2-VASc ≥2 [n (%)] | 117 (65.7%) | 72 (60%) | 45 (77.6%) | 0.02 |
| INR day of TEE | 2.2±0.7 | 2.2±0.7 | 2.3±0.9 | 0.33 |
| Average INR (3 weeks prior to TEE) | 2.2±0.8 | 2.3±0.9 | 2.2±0.9 | 0.34 |
| LA volume (ml) | 116±43 | 113±41 | 122±46 | 0.18 |
| LA tissue fibrosis (%) | 17.3±11.3 | 11.1±4.7 | 30.4±9.5 | <0.01 |
| LA appendage thrombus [n (%)] | 12 (6.7%) | 4 (3.3%) | 8 (13.8%) | <0.01 |
| LA SEC [n (%)] | 19 (10.7%) | 9/120 (7.5%) | 10/58 (17.2%) | 0.04 |
No significant difference in atrial fibrosis was observed between patients with a CHADS2 of 0 or 1 (17.8±11.5%) and patients with a CHADS2 of 2 or more (17.1±11.9%; p=0.69). Atrial fibrosis was higher in the CHADS2-VASc ≥2 group (18.6±11.8%) compared to CHADS2-VASc <2 (15.0±9.8%; p=0.04).
Atrial fibrillation was the presenting rhythm at the time of TEE evaluation in 123 patients (69%). Another indication for referral for TEE was the absence of properly documented therapeutic INRs for at least 3 consecutive weeks leading up to cardioversion or ablation. A therapeutic INR in the 2.0–3.0 range was present in 111 patients (62.4%) on the day of TEE.
LA fibrosis and TEE findings
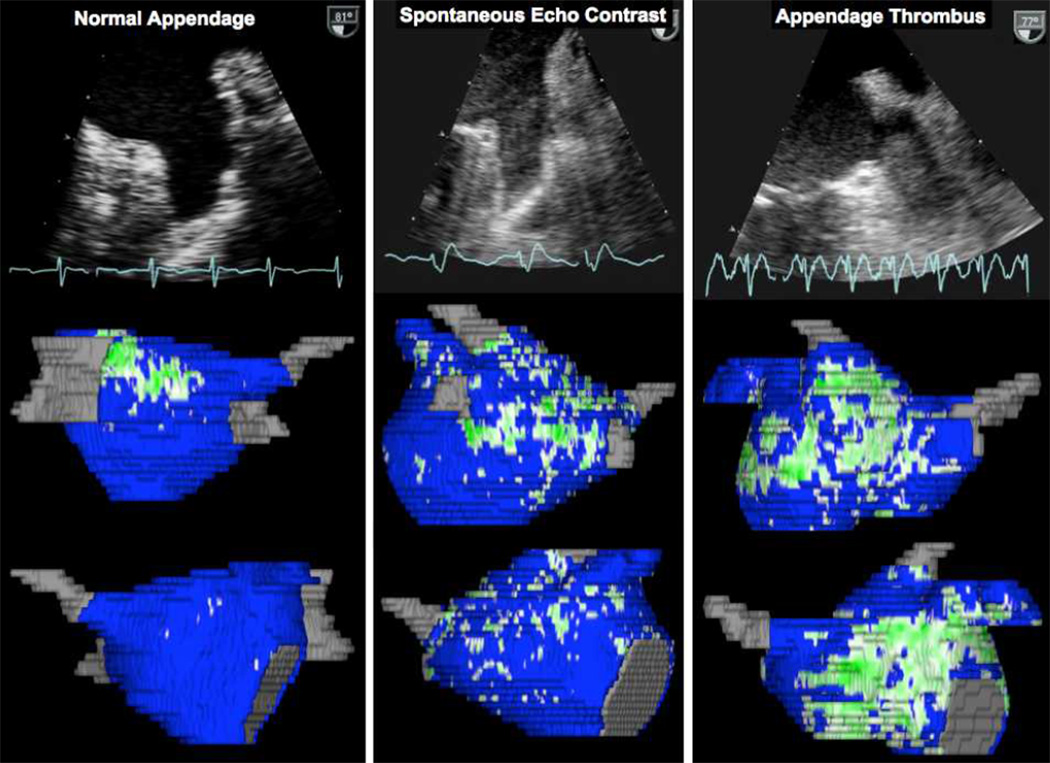
LAA thrombus was identified in 12 patients (6.7%) while left atrial SEC was observed in 19 patients (10.7%). Figure 2 shows examples of patients with normal LA appendage, LAA SEC and LAA thrombus with their corresponding left atrial images demonstrating atrial fibrosis.
Figure 2.
Sample transesophageal echocardiographic still frames representing patients with a normal LA appendage, SEC and a LAA thrombus with their respective three-dimensional reconstructions illustrating atrial fibrosis derived from LGE-MRI imaging.
Patients with LAA thrombus had significantly higher atrial fibrosis compared to patients without LAA thrombus (26.9±16.6 vs 16.8±10.5; p<0.01). A nonsignificant trend for a higher average CHADS2 score was found between patients with and without LAA thrombus (1.2±1.1 vs 1.7±0.9; p=0.07). No such trend was noted for the CHA2DS2-VASc score (2.5±1 for thrombus patients vs 2.05±1.34 for no thrombus; p=0.26). Patients with a CHADS2 score ≥2 were more likely to have a LAA thrombus (8 of 59; 13.6%) compared to patients with a CHADS2 score <2 (4 of 116; 3.5%; p=0.02). For the CHA2DS2-VASc score, 10 of 113 patients with a CHA2DS2-VASc ≥2 had evidence of thrombus (8.9%) compared to 2 of 61 patients with a CHA2DS2-VASc <2 (3.3%; p=0.17).
For LA spontaneous echo contrast, a higher degree of tissue fibrosis was found in patients with SEC (23.3±13.7%) compared to patients without SEC (16.7±10.8%; p=0.01). No significant difference in the CHADS2 score was appreciated between the SEC groups (1.2±1.1 vs 1.5±0.9; p=0.21) but a higher average CHA2DS2-VASc score in the SEC group (2.72±1.07) compared to the no-SEC group (2.01±1.34; p=0.03).
The average INR over 3 weeks preceding the TEE was not significantly lower between patients who did have a thrombus (1.9±0.7) and patients who did not (2.2±0.8; p=0.21). A similar observation was made for patients with LA SEC (average INR 2.1±0.7) and patients without LA SEC (average INR 2.2±0.8; p=0.53).
Predictors of abnormal TEE findings
In univariate analysis, fibrosis >20% and CHADS2 score ≥2 were associated with a statistically significant increase in risk of LAA thrombus (Odds ratios of 3.4 for fibrosis >20%; p<0.01 and Odds ratio of 3.5 for a CHADS2 score ≥2; p<0.01) and LA spontaneous echo contrast (odds ratio of 2.5 for fibrosis >20%; p<0.01 and odds ratio of 2.6 for CHADS2 score ≥2; p<0.05). CHA2DS2-VASc and persistent AF showed a nonsignificant trend towards increased risk of LAA thrombus and SEC. A therapeutic INR at the time of TEE showed a trend towards reduced risk of TEE abnormalities; however, this did not reach statistical significance (Table 2).
Table 2.
Univariate logistic regression analysis to identify predictors LA spontaneous echo contrast (A) and LA appendage thrombus (B).
| Uni-variate Analysis | A- Spontaneous Contrast | B- LAA thrombus | ||||
|---|---|---|---|---|---|---|
| Odds Ratio |
95% CI | P value | Odds Ratio |
95% CI | p value | |
| Fibrosis >20% | 2.6 | 0.9–6.7 | 0.06 | 4.6 | 1.3–16.1 | 0.02 |
| CHADS2 ≥2 | 2.4 | 0.9–6.3 | 0.07 | 4.4 | 1.3–15.3 | 0.02 |
| CHA2DS2-VASc ≥2 | 3.0 | 0.8–10.8 | 0.09 | 2.9 | 0.6–13.5 | 0.18 |
| Persistent AF | 2.0 | 0.6–6.3 | 0.24 | 2.6 | 0.6–12.3 | 0.23 |
| INR at TEE | 0.9 | 0.48–2.0 | 0.96 | 0.7 | 0.3–1.7 | 0.40 |
We performed multivariate logistic regression to identify predictors of abnormal TEE findings (LAA thrombus or spontaneous echocardiographic contrast). Three variables were included in the multivariate models including fibrosis >20%, CHADS2 score ≥2, and persistent AF for the first model and fibrosis >20%, CHA2DS2-VASc score ≥2, and persistent AF for the second model. A fibrosis >20% was a significant predictor of TEE abnormalities in both models. The results of the multivariate regression are shown in table 3. A receiver operating characteristics (ROC) analysis using this multivariate model showed an area under the curve of 0.73 for both models. This is compared to an area under the curve of 0.63 for the CHADS2 score alone and 0.65 for the CHA2DS2-VASc score alone.
Table 3.
Univariate and multivariate logistic regression analysis to identify predictors for abnormal TEE findings (Thrombus or spontaneous echocardiographic contrast).
| Abnormal TEE (Thrombus or SEC) |
Uni-variate Analysis | Multi-variate Analysis with CHA2DS2-VASc ≥2 |
Multi-variate Analysis with CHADS2 ≥2 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio |
95% CI | p value |
Odds Ratio |
95% CI | p value |
Odds Ratio |
95% CI | P value |
|
| Fibrosis >20% | 3.4 | 1.5–7.9 | 0.004 | 2.6 | 1.1–6.3 | 0.03 | 3.6 | 1.5–8.6 | 0.004 |
| CHADS2 ≥2 | 3.5 | 1.4–7.4 | 0.006 | -- | -- | -- | 3.5 | 1.4–8.3 | 0.005 |
| CHA2DS2-VASc ≥2 | 3.5 | 1.1–10.6 | 0.03 | 2.7 | 0.9–8.5 | 0.09 | -- | -- | -- |
| Persistent AF | 2.6 | 0.9–7.2 | 0.07 | 2.9 | 0.9–9.0 | 0.07 | 2.4 | 0.8–7.0 | 0.10 |
| INR at TEE | 0.8 | 0.4–1.5 | 0.49 | ||||||
Discussion
Our study evaluated the association of atrial fibrosis with the presence of left atrial appendage thrombus and left atrial spontaneous echo contrast on transesophageal examination in patients with atrial fibrillation. We demonstrated the presence of an independent association between atrial tissue changes in AF and echocardiographic findings linked to thromboembolism and stroke.
Our group has previously demonstrated, in a retrospective analysis, the association of atrial fibrosis and clinical strokes.9 We showed that AF patients with previous history of stroke had a significantly more advanced degree of atrial tissue structural remodeling compared to patients without a prior history of stroke, without verifying whether all strokes were embolic or cardiac in origin. In the current study, we focused on the left atrial appendage as it is considered the most common site of thrombus formation in the LA10.
Current risk assessment and treatment interventions to reduce cerebral thromboembolic events in AF patients were derived from hard clinical events of TIA or stroke and oral anticoagulation strategies address the long-term prevention of stroke or thrombo-embolism. In the acute clinical setting, decisions on whether to perform direct current cardioversion, initiate an antiarrhythmic drug or proceed with catheter ablation procedure are often preceded by a TEE to exclude the presence of an appendage thrombus. When the CHADS2 and CHADS2VASc are applied to the preclinical events, i.e., atrial thrombus formation, they do not demonstrate a very good predictive accuracy. A study by Scherr and coworkers demonstrated a CHADS2 score ≥2 was associated with an Odds ratio of 5.7 for the presence of LA appendage thrombus.11 Another study by Providencia and coworkers, both CHADS2 and CHADS2VASc demonstrated modest accuracy in predicting the presence of LA thrombi in AF patients undergoing TEE evaluation. The area under the curve for CHADS2 was 0.62 and that of the CHADS2-VASc was 0.63.12
In our study, the predictive accuracy of the CHADS2 score for TEE abnormalities was also found to be 0.63 and that of the CHA2DS2-VASc score was 0.65. When we evaluated atrial fibrotic changes assessed around the time of TEE acquisition, we found it to be significantly associated with the presence of TEE abnormalities, independent from the CHADS2 and CHA2DS2-VASc scores. Another parameter that is not included in the CHADS2 score, the AF phenotype (paroxysmal vs persistent), was examined and did not reach statistical significance. When atrial fibrosis was added to the prediction model for TEE abnormalities, the area under the curve improved to 0.73.
While clinical factors encompassed in the CHADS2 and the CHA2DS2-VASc scores have been linked to stroke, the mechanism by which these individual factors contribute to thrombus formation is not known. Atrial fibrosis appears to be an independent predictor of thrombus formation and can potentially be mechanistically linked to that process. Replacement of healthy atrial tissue with fibrotic tissue in AF can lead to reduced atrial contractile function and blood stasis. In addition, atrial fibrosis may constitute tissue injury that can also contribute to the thrombogenesis cascade. This is supported by the finding that some AF patients have have thrombi outside the left atrial appendage.
Study Limitations
We included a group of patients at a relatively high thromboembolic risk with greater than 35.4% CHADS2 score ≥2, and a high prevalence of persistent AF (66.9%), who commonly presented for transesophageal echocardiography in atrial fibrillation (69% of patients). This group, however, represents AF patients commonly referred for TEE evaluation prior to catheter ablation according to current guidelines13. None of our patients developed subsequent clinical neurological events. This was likely due to heightened vigilance to maintain therapeutic anticoagulation and possibly due to embolization to sites that are more clinically silent than the cerebral circulation. The MRI acquisition was not focused on the atrial appendage and therefore could not be used to reliably assess for thrombi or a quantitative assessment of appendageal enhancement.
Conclusions
The risk of thromboembolism in AF patients not only depends on clinical comorbidities but also on atrial structural changes including atrial tissue remodeling that may predispose to thrombus formation. Future prospective studies are needed to validate the value of this measure and incorporate it into risk assessment and interventions aimed at reducing this risk.
Acknowledgments
This work was made possible in part by the NIH/NCI 1KM1CA156723 (NA), as well as grants from the National Institute of General Medical Sciences (8 P41 GM103545-14) from the National Institutes of Health through the Center for Integrative Biomedical Computing (CIBC).
BW reports participation on research grants supported by GE and Astellas. NM is co-founder & president of MARREK Inc, in which he holds stock options.
Footnotes
Other authors: No disclosures.
References
- 1.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The framingham heart study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dulli DA, Stanko H, Levine RL. Atrial fibrillation is associated with severe acute ischemic stroke. Neuroepidemiology. 2003;22:118–123. doi: 10.1159/000068743. [DOI] [PubMed] [Google Scholar]
- 4.Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovascular Research. 2002;54:230–246. doi: 10.1016/s0008-6363(02)00258-4. [DOI] [PubMed] [Google Scholar]
- 5.Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M. Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation. 1997;96:3157–3163. doi: 10.1161/01.cir.96.9.3157. [DOI] [PubMed] [Google Scholar]
- 6.Oakes RS, Badger TJ, Kholmovski EG, Akoum N, Burgon NS, Fish EN, Blauer JJE, Rao SN, DiBella EVR, Segerson NM, Daccarett M, Windfelder J, McGann CJ, Parker D, MacLeod RS, Marrouche NF. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–1767. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akoum N, Daccarett M, McGann C, Segerson N, Vergara G, Kuppahally S, Badger T, Burgon N, Haslam T, Kholmovski E, Macleod R, Marrouche N. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: A de-mri guided approach. J Cardiovasc Electrophysiol. 2011;22:16–22. doi: 10.1111/j.1540-8167.2010.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aschenberg W, Schluter M, Kremer P, Schroder E, Siglow V, Bleifield W. Transesophageal two-dimentional echocardiography for the detection of left atrial appendage thrombus. J Am Coll Cardiol. 1986;7:4. doi: 10.1016/s0735-1097(86)80275-3. [DOI] [PubMed] [Google Scholar]
- 9.Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, Kholmovski E, McGann CJ, Parker D, Brachmann J, Macleod RS, Marrouche NF. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. Journal of the American College of Cardiology. 2011;57:831–838. doi: 10.1016/j.jacc.2010.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Saady N, Obel O, Camm A. Left atrial appendage: Structure, function, and role in thromboembolism. Heart. 1999;82:8. doi: 10.1136/hrt.82.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scherr D, Dalal D, Chilukuri K, Dong JUN, Spragg D, Henrikson CA, Nazarian S, Cheng A, Berger RD, Abraham TP, Calkins H, Marine JE. Incidence and predictors of left atrial thrombus prior to catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1357–1363. doi: 10.1111/j.1540-8167.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- 12.Providencia R, Botelho A, Trigo J, Quintal N, Nascimento J, Mota P, Leitao-Marques A. Possible refinement of clinical thromboembolism assessment in patients with atrial fibrillation using echocardiographic parameters. Europace. 2012;14:10. doi: 10.1093/europace/eur272. [DOI] [PubMed] [Google Scholar]
- 13.Calkins H, Kuck KH, Cappato R, Brugada J, Camm AJ, Chen SA, Crijns HJ, Damiano RJ, Jr, Davies DW, DiMarco J, Edgerton J, Ellenbogen K, Ezekowitz MD, Haines DE, Haissaguerre M, Hindricks G, Iesaka Y, Jackman W, Jalife J, Jais P, Kalman J, Keane D, Kim YH, Kirchhof P, Klein G, Kottkamp H, Kumagai K, Lindsay BD, Mansour M, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Nakagawa H, Natale A, Nattel S, Packer DL, Pappone C, Prystowsky E, Raviele A, Reddy V, Ruskin JN, Shemin RJ, Tsao HM, Wilber D. 2012 hrs/ehra/ecas expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2012;9:632–696. e621. [Google Scholar]