Abstract
The ubiquity of social vocalization among animals provides the opportunity to identify conserved mechanisms of auditory processing that subserve vocal communication. Identifying auditory coding properties that are shared across vocal communicators will provide insight into how human auditory processing leads to speech perception. Here, we compare auditory response properties and neural coding of social vocalizations in auditory midbrain neurons of mammalian and avian vocal communicators. The auditory midbrain is a nexus of auditory processing because it receives and integrates information from multiple parallel pathways and provides the ascending auditory input to the thalamus. The auditory midbrain is also the first region in the ascending auditory system where neurons show complex tuning properties that are correlated with the acoustics of social vocalizations. Single unit studies in mice, bats and zebra finches reveal shared principles of auditory coding including tonotopy, excitatory and inhibitory interactions that shape responses to vocal signals, nonlinear response properties that are important for auditory coding of social vocalizations and modulation tuning. Additionally, single neuron responses in the mouse and songbird midbrain are reliable, selective for specific syllables, and rely on spike timing for neural discrimination of distinct vocalizations. We propose that future research on auditory coding of vocalizations in mouse and songbird midbrain neurons adopt similar experimental and analytical approaches so that conserved principles of vocalization coding may be distinguished from those that are specialized for each species.
Keywords: vocal communication, subcortical, auditory tuning, inferior colliculus, birdsong, comparative
Introduction
Vocal communication is common among animals. The ubiquity of this behavior provides the opportunity to identify conserved mechanisms of auditory processing that underlie perception of communication sounds. By identifying mechanisms of auditory-vocal processing that are shared across vocal communicators, we can gain insight into how human auditory processing leads to speech perception. In employing this comparative approach we can also distinguish shared mechanisms from those that are specialized for the demands of particular species, thereby providing a better understanding of the evolution of auditory processing mechanisms.
In this review, we compare auditory response properties and neural coding of social vocalizations in the auditory midbrain of laboratory mice (Mus mus), Mexican free-tailed bats (Tadarida brasiliensis) and zebra finches (Taeniopygia guttata). These animal groups are phylogenetically distant and have divergent behavioral repertoires, yet they all use acoustically complex vocal signals for social communication. Because these animal groups differ considerably in evolutionary history, mechanisms of vocalization processing that are common among groups are likely to represent conserved principles of auditory-vocal processing that support complex vocal communication.
Our focus is on the auditory midbrain because it is the first region in the ascending auditory system where individual neurons show complex tuning properties that are correlated with the acoustics of social vocalizations. This is true in mammals (Andoni et al., 2007; Holmstrom et al., 2007; Andoni and Pollak, 2011; Mayko et al., 2012) birds (Woolley et al., 2005; 2006, 2009; Schneider and Woolley, 2010, 2011) and frogs (Edwards et al., 2002, 2007; Elliott et al., 2011), and is therefore a general principle of auditory processing. The coding properties of auditory midbrain neurons are also important to understand because they provide the major input to the thalamus and cortex. Distinguishing between response properties that emerge in the cortex and those that are inherited from subcortical circuits requires an understanding of midbrain response properties. Indeed, it is well known that several response properties important for coding complex sounds emerge at the level of the auditory midbrain rather than the auditory cortex (Casseday et al., 1994; Portfors and Wenstrup, 2001; Nataraj and Wenstrup, 2005; Woolley et al., 2005, 2006; Xie et al., 2005; Schneider and Woolley, 2011).
Ascending inputs to the auditory midbrain in mammals and birds
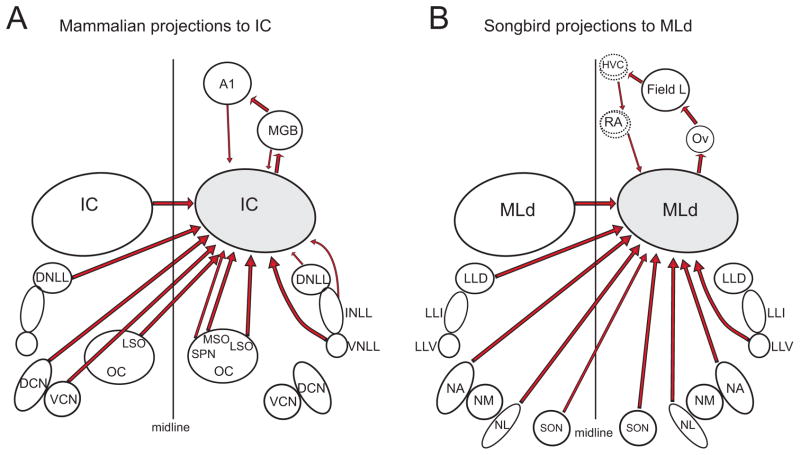
The ascending auditory pathways in mammals and birds are highly conserved (Butler and Hodos, 2005; Butler et al., 2011). The auditory midbrain is a nexus of auditory processing; it receives and integrates information from multiple parallel pathways and provides the ascending auditory input to the thalamus (Fig. 1). The avian auditory midbrain is traditionally called the lateral dorsal mesencephalon (MLd) because of its anatomical location, but this nucleus is homologous to the mammalian central nucleus of the inferior colliculus (ICc; Grothe et al., 2004; Covey and Carr, 2005). The ICc and MLd receive inputs directly from contralateral and ipsilateral cochlear nuclei, from lateral lemniscal nuclei and from the contralateral auditory midbrain (see Fig. 1 for details). The IC and MLd also receive ascending input from other brainstem nuclei, including the superior olivary complex and superior paraolivary nucleus in mammals (Winer and Schreiner, 2005 for review), and the superior olivary nucleus in songbirds (Wild et al., 2010).
Figure 1.
Schematic diagram of the major pathways to and from the right side auditory midbrain. To facilitate focusing solely on auditory midbrain projections, we have omitted all projections in the brainstem that do not go to the midbrain. A. Inferior colliculus (IC) of the mouse. Abbreviations are A1, primary auditory cortex; DCN, dorsal cochlear nucleus; DNLL, dorsal nucleus of the lateral lemniscus; INLL intermediate nucleus of the lateral lemniscus; LSO, lateral superior olive; MGB, medial geniculate body; MSO, medial superior olive; OC, olivocochlear nucleus; SPN, superior paraolivary nucleus; VCN, ventral cochlear nucleus; VNLL, ventral nucleus of the lateral lemniscus. B. Dorsal lateral mesencephalon (MLd) of the songbird. Abbreviations are LLD, dorsal nucleus of the lateral lemniscus; LLI intermediate nucleus of the lateral lemniscus; LLV, ventral nucleus of the lateral lemniscus; NA, nucleus angularis; NL, nucleus laminaris; NM, nucleus magnocellularis; Ov, nucleus ovoidalis; RA, robust nucleus of the arcopallium; SON, superior olivary nucleus. Note: HVC is the proper name of the primary vocal control nucleus. The dashed lines around HVC and RA refer to the “shelf” and “cup” regions, respectively. These regions and their projections form a descending pathway to MLd and the region surrounding MLd.
One major difference in the auditory systems of mammals and songbirds is the organization of descending projections to the midbrain. The IC receives descending input from the auditory thalamus and cortex (Saldana et al., 1996; Winer et al., 1998). The songbird MLd receives descending input from the pathway that parallels and surrounds the song motor pathway, specifically the “cup” surrounding the robust nucleus of the arcopallium (RA), a motor cortex-like forebrain region that is necessary for song production (Fig 1B; Mello et al., 1998). Thus, top-down influences on midbrain auditory processing may differ considerably between mammals and songbirds. Descending inputs to IC contribute to plasticity and learning (Gao and Suga, 2000; Zhang and Suga, 2005; Bajo et al., 2010), whereas the functional roles of descending inputs to MLd are unknown. The projections from RA cup to MLd and its surrounding region may convey information about vocal motor commands to the ascending auditory system. However, the sensory-motor interactions between descending vocal control circuits and subcortical auditory circuits remain to be studied.
Mice, bats and songbirds are good models for auditory processing
Our focus in this review is on mice, bats and songbirds because the neural mechanisms underlying vocalization coding in the auditory midbrain have been most well studied in these groups and, as described below, each group offers unique advantages for understanding mechanisms of auditory processing. A few studies in mammals with low frequency hearing (cat, guinea pig) have examined neural responses to vocalizations in the IC (Aitkin et al., 1994; Suta et al., 2003). In all of these studies, the stimuli were limited to a few representative vocalizations and analyses included only basic measures of response properties such as average firing rate. In general, vocalizations tended to evoke higher firing rates in the IC of cat and guinea pig compared to pure tones, noise or reversed-vocalizations. However, the neurons show little preference for some vocalizations over others (Aitkin et al., 1994; Suta et al., 2003). Further examination of neural response properties in the IC of mammals with low frequency hearing will contribute significantly to our understanding of common vertebrate auditory coding mechanisms engaged during vocalization processing.
Although mice vocalize in the ultrasonic frequency range (Sales, 1972; Nyby et al., 1979; Portfors, 2007), they are considered a hearing “generalist” with a typical mammalian auditory system, and as such provide a strong basis for understanding auditory coding mechanisms that could be common across mammals, including humans (Henry and McGinn, 1992). Moreover, genetic engineering in mice makes them an excellent model system for understanding neural and genetic mechanisms of auditory and communication disorders (Scattoni et al., 2009; Fischer and Hammerschmidt, 2011).
Currently, bats provide the best understanding of how complex sounds are encoded by auditory neurons. Mechanisms underlying auditory coding of bat echolocation signals have been studied for over 50 years (Suga, 1964; Suga et al., 1978; Pollak et al., 1978; O’Neill and Suga, 1979; Pollak and Schuller, 1981) and provide the basis for understanding how bat social vocalizations are encoded, as several mechanisms underlying selectivity to both echolocation and social vocalizations are similar (Kanwal et al., 1994; Leroy and Wenstrup, 2000; Portfors, 2004; Portfors and Felix, 2005). In addition, bats rely on hearing to a greater extent than other mammals and auditory specializations have evolved to support their exquisite echolocation abilities (Suga et al., 1975; Suga and Jen, 1976; Pollak and Bodenhamer, 1981). These auditory specializations may also be involved in processing social vocalizations. On the other hand, coding mechanisms for social vocalizations may not be strongly specialized in bats. Neural responses in frequency ranges representing social vocalizations rather than echolocation calls are similar to neural responses found in the IC of mice (Portfors and Felix, 2005), suggesting that the neural mechanisms for processing social vocalizations may have evolved before echolocation. Thus, comparing responses to vocalizations in the mouse and bat IC is informative for understanding the evolution of vocalization processing in mammals.
Less information about auditory coding exists in songbirds; aspects of how neurons respond to simple and complex sounds throughout the auditory pathway are just beginning to be a focus of songbird research (Woolley, 2012). Two advantages of using songbirds to study auditory coding of vocalizations are the detailed understanding of the functions of song and the well-characterized acoustics of their songs. A third and unique advantage is that songbirds learn their songs (Konishi, 1965). The behavior and neural mechanisms of song learning are well studied in zebra finches; we know a great deal about zebra finch song (Williams, 2004), how birds use song socially (Riebel, 2009), and the neural circuits involved in song learning and production (Konishi, 1994; Brainard and Doupe, 2002; Suthers and Margoliash, 2002; Fee et al., 2004; Mooney, 2009). The zebra finch’s abilities to learn song by vocal imitation and auditory-vocal practice and to recognize and remember the individual songs of social partners make this animal’s auditory coding mechanisms particularly interesting and applicable to the auditory mechanisms underlying processing of other complex vocalizations, such as speech.
Social vocalizations in mice, bats and songbirds
A common characteristic of male mice, bats and songbirds is that they produce complex vocalizations in the presence of females, supporting the general view that vocal cues are used in courtship and mating across taxa. Because courtship and mating vocalizations are behaviorally relevant, the majority of studies examining mechanisms underlying encoding of social vocalizations have used these types of sounds as stimuli. The role of complex vocalizations in attracting females is best understood in songbirds. Female songbirds are attracted to male song and use the species-specific and individual-specific acoustics of male songs as mate choice cues, presumably because they indicate reproductive fitness (Spencer, 2005; Holveck, 2008; Boogert, 2008; Riebel, 2009).
The functional role of vocalizations during courtship is less clear in mice and bats. Male mice emit sequences of vocalizations in the presence of a female or her urine (Sales, 1972; Nyby et al., 1976, 1979; Portfors, 2007) and these have even been described as “song” (Holy and Guo, 2005; Arriaga et al., 2012), although whether these vocalizations are truly song is debatable. While it has been shown that the types of syllables males emit change as males get closer to copulation (Wang et al., 2008; Hanson and Hurley, 2012), a number of behavioral questions remain unanswered. In contrast to songbirds, it is not clear that female mice use male vocalizations as a determinant of fitness. Nor is it clear what features of the vocalizations differ between males and what information is communicated to females. The same unanswered questions apply to bats. For example, during the mating season, Mexican free-tailed male bats establish territories and readily vocalize when females approach their territories. These songs contain multiple syllable types, with highly stereotyped sequencing (Bohn et al., 2008, 2009), which suggests they contain information that females may use for mate selection, but this has not been explicitly tested. Exploring these questions in mice and bats is an exciting and potentially rich area for future research.
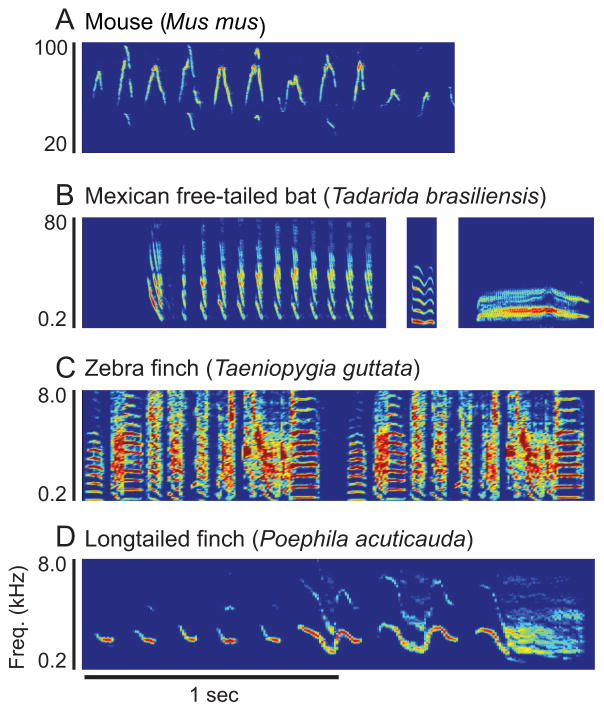
Mouse, bat and songbird social vocalizations are comprised of complex spectral and temporal features such as frequency modulation, amplitude modulation and harmonic stacks (Fig. 2). Vocal gestures often occur in bouts, with multiple, temporally distinct units of sound (syllables) emitted consecutively. Highly similar syllables that are repeated and grouped in time to form phrases are common, and individual syllables generally have significant frequency modulations (Konishi and Nottebohm, 1969; Kanwal et al., 1994; Bohn et al., 2008; Woolley, 2012; Holy and Guo, 2005; Portfors, 2007). Despite these fundamental similarities, the acoustics of social vocalizations differ considerably across taxa and among songbird species (Brenowitz and Beecher, 2005). Even closely related finch species have songs with highly divergent acoustics (Woolley and Moore, 2011). For example, zebra finches produce broadband songs composed of syllables with harmonic stacks and noise bursts, whereas long-tailed finches produce tonal songs with narrowband syllables and prominent frequency-modulated sweeps, similar to mouse social calls but much lower in frequency (Fig. 2).
Figure 2.
Spectrograms (frequency over time plots) showing the spectrotemporal features of mouse, bat and songbird social vocalizations. Color indicates intensity. Red is high and blue is low. A. Mouse social vocalization bout. B. Mexican free-tailed bat calls. C. Two motifs of zebra finch song. D. One longtailed finch song. Note that the zebra finch song is broadband and the longtailed finch song is tonal, similar to mouse social calls.
The most notable differences in mouse and bat versus songbird social vocalizations are in spectral content. First, mouse and bat calls contain a much higher and broader range of frequencies than do birdsongs, with most vocalizations occurring in the ultrasonic range (Fig. 2). Mouse courtship vocalizations are very high frequency, with most energy above 50 kHz (Figs. 2 and 3; Holy and Guo, 2005; Portfors, 2007) while the peak energy in most birdsongs is around 4 kHz. Second, mouse, bat and songbird social vocalizations differ in how much they are tonal versus noisy. Mouse and bat vocalizations are comparatively tonal, even when they contain harmonics. The majority of mouse vocalizations are highly tonal, with few harmonics. Some bird species’ songs are as tonal as mouse calls and as dominated by frequency-modulated sweeps as bat calls (Catchpole and Slater, 2008). In contrast, zebra finches sing noisy and harmonic songs, more like speech. Zebra finch songs are also highly stereotyped compared to mouse and bat social vocalizations. Songs are composed of spectrotemporally distinct syllables that are produced in stereotyped sequences, whereas sequences of mouse and bat vocalizations are more variable, although some rules of syntax have been identified in bats (Kanwal et al., 1994; Bohn et al., 2009). Comparing mouse and bat auditory response properties with those of zebra finches therefore provides the opportunity to differentiate between conserved coding principles in the auditory midbrains of vocally communicating vertebrates and coding properties that are tightly coupled with vocal acoustics.
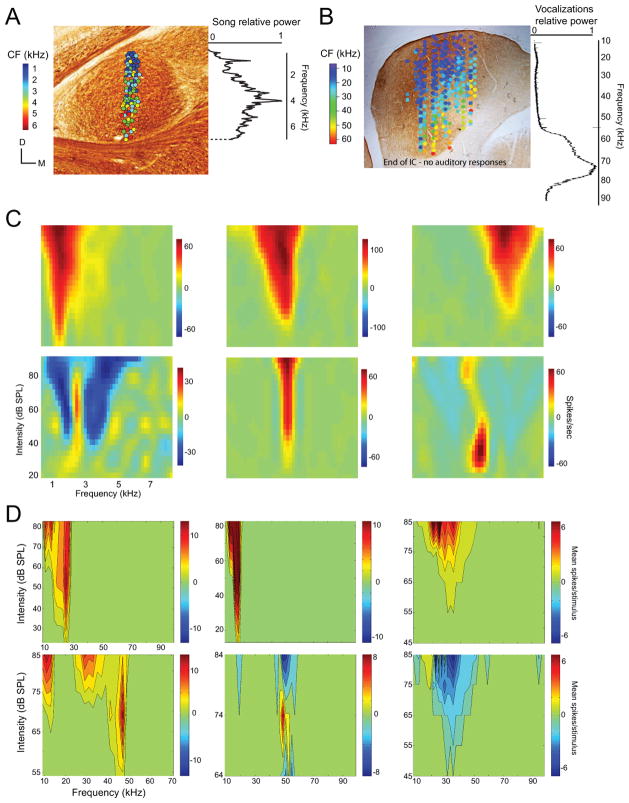
Figure 3.
Tonotopic organization and excitatory and inhibitory frequency tuning of single auditory midbrain neurons. A. Coronal section of zebra finch MLd with circles marking locations of single unit electrophysiological recordings superimposed and color coded according to the characteristic frequency (CF) of the neuron recorded at that location. Color coding of unit CFs and the average power of zebra finch songs to the right of the image show that frequency tuning and the frequencies represented in songs are closely matched. B. Coronal section of mouse IC with color-coded circles marking electrophysiological recording locations superimposed. The average power spectrum of mouse social vocalization is to the right of the image showing that the majority of power in vocalizations falls above the frequency tuning of most IC neurons. The top rows in panels C and D show classic V-shaped excitatory tuning. The bottom rows of panels C and D show tuning curves with excitatory and inhibitory tuning. C. Zebra finch single unit frequency/intensity tuning. D. Mouse single unit frequency/intensity tuning.
General features of auditory processing in IC and MLd
1. Tonotopy
The fundamental organizing principle of the auditory system is a mapping of frequency. A map of frequency begins in the cochlea and is relayed to each level of the ascending pathway such that particular frequencies excite neurons in specific locations in each nucleus. In the IC and MLd, ascending inputs give rise to a tonotopic neural map with low frequencies maximally exciting the most dorsal neurons and higher frequencies driving neurons in progressively more ventral locations (Fig. 3A and B; Clopfield et al., 1973, Merzenich et al., 1974, Schreiner and Langner, 1998; Woolley and Casseday, 2004). The range of frequencies mapped onto auditory structures depends on the species and the mechanics of the cochlea. Typically, the range of frequencies that drive auditory neurons matches the range of frequencies in behaviorally relevant signals, such as conspecific vocalizations, for each species (Konishi, 1969; Sach et al., 1980; Woolley and Casseday, 2004; Suga et al., 1975; Rübsamen et al., 1989; Schnitzler and Denzinger, 2011). This is demonstrated for the zebra finch in Fig. 3A where characteristic frequencies of MLd neurons are between 1 and 6 kHz.
In the mouse there is a mismatch between the frequency representation in the IC and the power spectra of their vocalizations, as illustrated in Fig. 3B (Portfors et al., 2009, Holmstrom et al., 2010, Portfors et al., 2011). This mismatch is not restricted to the IC. There is limited representation of the ultra-high frequencies used in mouse vocalizations (particularly those higher than 60 kHz) throughout the mouse auditory system (Liu and Schreiner, 2007; Muller et al., 2005; Stiebler and Ehret, 1985) and the sensitivity of those neurons that do respond to frequencies around 60 kHz is very low (Stiebler and Ehret, 1974; Portfors and Felix, 2005). The lack of neural representation of these frequencies matches the behavioral thresholds; behavioral responses to pure tones can be obtained up to 90 kHz but the thresholds are greater than 80 dB SPL (Ehret, 1974).
This mismatch predicts that the majority of neurons in the IC should not respond to most mouse vocalizations. However, many neurons with low frequency tuning that is far below the spectral content of the vocalizations respond to these vocalizations (Fig. 4A; Portfors et al., 2009; Holmstrom et al., 2010, Mayko et al., 2012). One proposed explanation for these responses is that IC neurons respond to the distortion products generated in the cochlea by the combination of ultrasonic frequencies present in vocalizations (Portfors et al., 2009). For example, a vocalization that contained two separate frequency elements of 60 and 80 kHz (Fig. 2A and 4A) would evoke a response from a 20 kHz neuron in the IC because presentation of that vocalization would generate an intermodulation distortion product on the basilar membrane in the 20 kHz region (80 kHz – 60 kHz = 20 kHz) and thus stimulate neurons in IC that were tuned to 20 kHz (Portfors et al., 2009). Responses to distortion products have been recorded in auditory nerve fibers (Goldstein et al., 1968) and in the IC (McAlpine, 2004; Portfors et al., 2009). Moreover, the responses recorded in the IC to combinations of high frequency elements contained in vocalizations are poorly predicted by responses to pure tones and are more accurately predicted by a nonlinear model of the cochlea (Lukashkin et al., 1998) that generates distortion products (Portfors et al., 2009). Thus, although the frequency representation in the auditory system of mice does not match the spectral content of their vocalizations, it appears that mice have evolved a strategy to utilize cochlear distortions to code at least some of their vocalizations, thus explaining the mismatch between the tonotopy of the auditory system and the spectral content of vocalizations. This strategy may be specialized to rodents or may be more conserved. A hint that this may be somewhat conserved is that mosquitoes are also thought to use distortion products to match wingbeat frequencies during courtship displays (Warren et al., 2009).
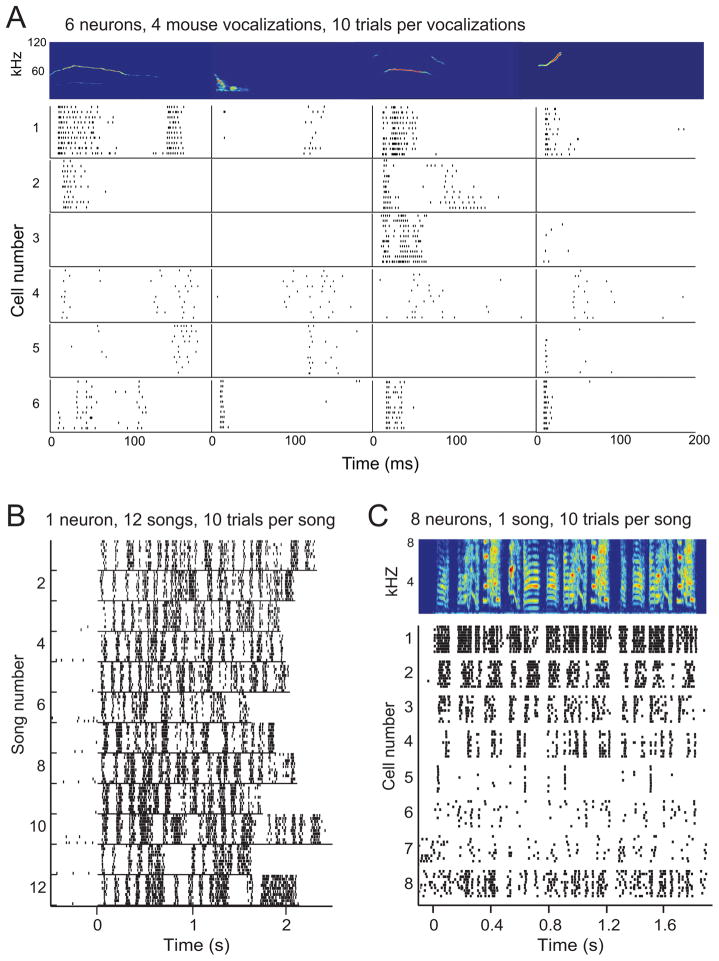
Figure 4.
Responses of mouse and songbird midbrain neurons illustrate response selectivity for syllables and the importance of spike timing in the coding of vocalizations. A. Raster plots of spike trains of six neurons in the IC of mice in response to four different vocalizations (10 presentations of each stimulus). Responses of different neurons show that each neuron responds differently to the individual vocalizations, and each neuron responds differently from the other neurons. B. Spike trains from a single neuron in response to 10 repetitions of 12 unique zebra finch songs. Each line shows a single spike train, and each tick represents the timing of a single action potential. Each group of 10 spike trains shows the responses to 10 presentations of a single song. Songs were presented pseudo-randomly and responses were organized by song to illustrate the temporal reliability of multiple responses to the same song and temporal differences in responses to different songs. C. The spectrogram (top) of a single zebra finch song. Below the spectrogram, raster plots show spike trains collected from eight neurons in response to 10 presentations of the song, illustrating differences in the responses of neurons with different spectrotemporal tuning to the same zebra finch song.
2. Diversity of frequency tuning curve shapes
A common feature of MLd and IC is the heterogeneity of frequency and intensity tuning among neurons (Fig. 3C and D). In the mouse IC, tuning curve shapes have been classified as broadly tuned on the low frequency side and sharply tuned on the high frequency side (class I), sharply tuned on both sides (class II), broadly tuned (classic V-shape, class III) or complexly tuned with multiple regions of excitation (class IV) (Egorova et al., 2001; Ehret et al., 2003). Class II and III tend to be most prevalent (~30 % each) (Egorova et al., 2001; Portfors et al., 2011). Zebra finch MLd neurons have these same tuning curve shapes but class II and IV neurons are less common than in mice and bats (Woolley and Casseday, 2004; Schumacher et al., 2011), and the relative proportions of neurons that fall into the four mammalian classes have not been rigorously quantified. In general, the majority of midbrain neurons in these three animal groups have classic V-shaped excitatory tuning; the range of tone frequencies that evoke firing above baseline rates widens as intensity increases (Fig. 3C and D top rows; Casseday and Covey, 1992; Egorova et al., 2001; Woolley and Casseday, 2004; Schneider and Woolley, 2011; Schumacher et al., 2011; Portfors et al., 2011).
The average breadth of spectral tuning in V-shaped tuning curves differs among songbirds, mice and bats. The width of frequency tuning relative to the full range of audible frequencies is broader in MLd neurons than in IC neurons. In zebra finch MLd neurons, the median spectral bandwidth at 20 dB above threshold is 1 kHz (Schumacher et al., 2011). Considering that zebra finches only hear frequencies between 0.2 and 7.0 kHz, the tuning of individual cells is broad. It is tempting to draw parallels between the broad tuning of zebra finch MLd neurons and the broadband acoustics of their songs. Testing correlations between auditory tuning and vocal acoustics in songbirds requires that multiple songbird species be examined, however, and those studies are just beginning (Woolley and Moore, 2011). In contrast, IC neurons in some species of bats are near the other extreme in sharpness of tuning. In bats that emit long constant frequency echolocation signals (the mustached bat is the most well studied species), a large population of neurons in the IC is extremely sharply tuned with maximum bandwidths much less than 500 Hz (Pollak and Bodenhammer, 1981). These neurons are specialized to detect frequency shifts in the returning echo due to the Doppler effect (Suga et al., 1975), and to detect small, rapid frequency modulations produced by insect wing flutter (Pollak and Schuller, 1981). Frequency tuning of neurons in the IC of the mustached bat in frequency ranges related to social vocalizations however, are not nearly as sharp and are more similar to tuning characteristics in the IC of mice (Leroy and Wenstrup, 2000; Portfors et al., 2009).
One contribution to the differences in sharpness of excitatory tuning observed in the midbrain of zebra finches and bats is the extent and strength of sideband inhibition. A common principle of the relationship between excitatory and inhibitory frequency tuning is that frequencies just above and/or below excitatory frequencies often suppress a neuron’s firing, both spontaneous and sound-evoked (Yang et al., 1992; Egorova et al., 2001; Egorova and Ehret, 2008; Mayko et al., 2012; Schneider and Woolley, 2011; Schumacher et al., 2011). This sideband inhibition is functionally similar to lateral inhibition in the visual system in that it sharpens tuning. The frequency-dependence of neural excitation and/or inhibition means that broadband sounds or those that have energy peaks at multiple points along the frequency axis (Fig. 2) can both excite and inhibit a single neuron. In bats, most (if not all) IC neurons that respond to pure tones exhibit sideband inhibition (Yang et al., 1992; Klug et al., 2002). In mice, 50 to 80% of IC neurons have inhibitory sidebands (Portfors and Felix, 2005; Mayko et al., 2012). In contrast, in zebra finches, 30% of MLd neurons show inhibitory sidebands (Schneider and Woolley, 2011; Schumacher et al., 2011). The larger influence of inhibition on tuning in bats and mice serves as a mechanism to sharpen frequency tuning in IC neurons and may relate to the narrowband frequency content of their vocalizations compared to those produced by zebra finches.
Interestingly, complex frequency tuning (Fig. 3C and D, bottom rows) is present in all three groups of animals, but it is more prevalent in mice and bats than in zebra finches. The majority of zebra finch midbrain neurons are most sensitive to one frequency (Woolley and Casseday, 2004; Schumacher et al., 2011), whereas a higher proportion of mouse and many bat IC neurons have secondary tuning curves (Fig. 3D; Portfors and Wenstrup, 2002; Holmstrom et al., 2007; Portfors and Felix, 2005; Portfors et al., 2011). It remains to be determined why mice and bats have more complex frequency tuning in the auditory midbrain than zebra finches.
3. Multi-frequency facilitation and suppression (nonlinear spectral interactions)
Nonlinear spectral interactions have been well studied in the auditory system of bats with respect to coding echolocation signals (Suga, 1978; Suga et al., 1978; Suga et al., 1979; O’Neill and Suga, 1979; O’Neill and Suga, 1982; Mittmann and Wenstrup, 1995; Portfors and Wenstrup, 1999; Wenstrup and Portfors, 2011; Wenstrup et al., 2012). Our focus here is on the role of nonlinear spectral interactions in the IC during coding of social vocalizations. Auditory midbrain neurons in mice, bats (these experiments have been done in mustached bats rather than Mexican free-tailed bats) and zebra finches show nonlinear responses to combinations of simultaneously presented tones at frequencies that are represented in social vocalizations (Leroy and Wenstrup, 2000, Portfors and Wenstrup, 2002; Portfors and Felix, 2005, Holmstrom et al., 2007; Portfors et al., 2009; Schneider and Woolley, 2011). Although the terminology used to describe these response properties in IC (combination sensitivity) and MLd (extra-classical receptive fields) is different, the basic features are similar. These nonlinear spectral interactions can be either facilitatory or inhibitory. In facilitatory interactions, the firing rate of a neuron is greater to the combination of two tones of different frequencies than to the sum of the individual tone responses, and in inhibitory interactions, the firing rate to an excitatory tone is suppressed by the simultaneous presentation of a second tone (Mittmann and Wenstrup, 1995; Portfors and Wenstrup, 2002; Portfors and Felix, 2005; Schneider and Woolley, 2011). In these combination-sensitive neurons, the frequency of the second (facilitating or suppressing) tone falls outside the neuron’s “classical” receptive field. In bats and mice, this modulating frequency is typically an octave or more away from the neuron’s characteristic frequency (CF), whereas in zebra finch MLd, extra-classical tuning consists of sideband excitation or inhibition (Schneider and Woolley, 2011). Response facilitation and suppression by frequencies that are far from the classical receptive field have not been found in songbirds.
Not only are the response features of these nonlinear neurons similar in mice, bats and zebra finches, their abundance is also similar. Between 30–50% of neurons in the IC of mice and mustached bats display combination sensitivity to frequencies contained in social vocalizations (Leroy and Wenstrup, 2000; Portfors and Wenstrup, 2002, Portfors and Felix, 2005), and about 60% of zebra finch MLd neurons have “extra-classical” receptive fields. These findings suggest that the modulation of responses by frequency information that falls outside of the classical receptive field is a shared coding mechanism that likely makes these neurons sensitive to the structure of spectrally correlated sounds that characterize the vocalizations of these different animals. The presence and similarity of nonlinear spectral interactions in mammals and birds suggests that combination sensitivity or extra-classical tuning is a conserved mechanism that shapes auditory coding of vocalizations.
4. Modulation tuning
Social vocalizations are characterized by spectral modulations, which are oscillations in sound energy across the frequency spectrum (e.g. harmonics), modulations in energy over time (e.g. syllable rates) and modulations in energy across frequency and time (e.g. frequency-modulated sweeps; Chi et al., 1999; Singh and Theunissen, 2003; Woolley et al., 2005; Andoni and Pollak, 2007; Portfors, 2007; Hoffmann et al., 2012; Mahrt et al., 2012) The spectral, temporal and joint spectrotemporal modulations in vocalizations are important for communication, as demonstrated by speech perception experiments (Shannon et al., 1995; Remez et al., 2001; Elliott and Theunissen, 2009). The dependence of speech perception on spectrotemporal modulations suggests that auditory neurons are tuned to the modulation frequencies represented in social vocalizations. While this hypothesis has yet to be directly addressed in mice, studies on modulation tuning in songbirds and bats suggest a strong relationship between midbrain tuning and the spectrotemporal modulation features of conspecific vocalizations. Zebra finch midbrain neurons are tuned for the spectral and temporal modulations that characterize song (Woolley et al., 2005). When a single complex sound containing spectral and temporal modulations that are in song and those that are absent from song is presented, neurons selectively respond to sound segments with spectrotemporal modulations that match those in song. This response selectivity for sounds that contain the spectrotemporal modulations represented in songs may serve as a tuning mechanism that facilitates the coding of acoustic information in song and filters out other acoustic features. Bat IC neurons are also tuned to spectrotemporal modulations that are prominent in their vocal signals; many neurons respond best to downward FM sweeps that match the sweep direction and velocity of bat social calls (Andoni and Pollak, 2007; 2011). Modulation tuning in both songbird and bat midbrain neurons facilitates the encoding of spectrotemporal features that distinguish individual songs and calls by failing to encode modulation frequencies that are redundant across different songs and calls (Woolley et al., 2005; Andoni and Pollak, 2011). Interestingly, speech perception experiments suggest that the human auditory neurons are also tuned to specific spectrotemporal modulations (Sabin et al., 2012). Moreover, tuning for specific spectrotemporal modulations in the human primary and secondary auditory cortex also appears to map onto the modulations in natural sounds (Schonwiesner and Zatorre, 2009). This tuning property may therefore be a conserved mechanism for auditory discrimination of social vocalizations in a wide range of vertebrates.
In summary, the diversity of responses to tones in the auditory midbrains of mice, bats and zebra finches are similar. These animals show the same complement of response properties, but to varying extents. The similarity of these general response features provides evidence that basic coding mechanisms for simple stimuli are conserved across phylogenetically diverse species. However, the observed species differences provide evidence for divergent evolutionary processes. For example, the evolution of bats to produce signals and hear in the high ultrasound range (up to at least 180 kHz) is directly related to the need for their echolocation signals to detect small insects (Conner and Corcoran, 2012). Mice may have evolved to emit vocalizations in the 60–120 kHz range to avoid predation and exploited the nonlinearities of the cochlea such that auditory neurons tuned to low frequencies can encode their social vocalizations. This eliminates the need for a specialized basilar membrane (as in bats) that can encode ultrasonic frequencies (Portfors et al., 2009). Thus, comparing auditory processing in phylogenetically diverse species can provide a more thorough understanding of conserved and specialized coding processes.
Auditory coding of social vocalizations
The diversity of frequency tuning curve shapes suggests that responses to vocalizations in the midbrain will also be diverse, and that the complex interplay between excitation and inhibition likely plays a role in creating selectivity to vocalizations.
Neural selectivity to vocalizations
The neural representation of vocalizations at the level of the auditory nerve can be described as temporal and rate-based codes for specific acoustic features in the signals (Sachs and Young, 1979; Young and Sachs, 1979), and responses to vocalizations are well explained by responses to pure tone stimuli in brainstem nuclei (Bauer et al., 2002; Xie et al., 2005). However, at the level of the auditory midbrain, selectivity to vocalizations is present in mice, bats and songbirds, and neurons in IC and MLd respond to vocalizations in diverse ways (Fig. 4).
While the responses of single neurons are reliable, meaning that they produce highly similar responses to the same sound presented multiple times (Holmstrom et al., 2010; Schneider and Woolley, 2010), the responses of different neurons to the same sound are highly variable (Fig. 4). Because of methodological differences in the presentation of vocal sounds between mammal and songbird studies, direct comparisons of neural selectivity for some vocalizations over others are difficult. Studies in mice and bats have recorded responses to individual syllables or calls independently and assessed selectivity based on responses to these individual stimuli (Fig 4A). Using this methodology, many IC neurons respond selectively to particular vocalizations. As shown in Fig. 4A, an individual neuron may respond to one or many vocalizations when presented with an array of different syllables (Klug et al., 2002; Portfors et al., 2009; Mayko et al., 2012). Moreover, different neurons respond to the same vocalizations in diverse ways (Fig. 4A). Thus, there is both response selectivity and response heterogeneity in the mouse and bat IC. In contrast, studies on songbirds typically record neural responses to entire song motifs and assess selectivity based on the strength of responses to whole songs (composed of multiple syllables).
Zebra finch MLd neurons respond to over 90% of presented zebra finch songs (generally the unique songs of 20 different males), but the number of syllables that each neuron responds to varies widely across neurons (Schneider and Woolley, 2012). Some neurons respond to only 1 or 2 syllables in a song of 5–8 syllables (Fig 4D cell 5). These response patterns, if assessed 1 syllable at a time, as is done in mammals, would show a good degree of selectivity but still not as high as in mouse or bat IC because zebra finch song syllables are more broadband than are mouse and bat calls (Fig. 2). Similarly, if IC response selectivity in mice and bats was assessed based on responses to vocalization bouts, individual neurons may respond to a high number of these bouts, in a manner similar to MLd. Thus, future work in which we adopt the same experimental and analytical approaches for studies in mammals and songbirds will lead to a more thorough understanding of similarities in response selectivity in the auditory systems of these different animals.
Mechanisms underlying vocalization coding
Recent studies have enriched our understanding of the mechanisms underlying selectivity to vocalizations in the IC of bats and mice (Klug et al., 2002; Xie et al., 2005; Mayko et al., 2012). In particular, inhibition plays a significant role in shaping selectivity to vocalizations. For example, blocking GABAergic and glycinergic receptors decreases selectivity to vocalizations in the IC of both bats and mice (Klug et al., 2002; Xie et al., 2005; Mayko et al., 2012; see Pollak this issue for review). There are potentially multiple ways that inhibition could affect selectivity to vocalizations (Mayko et al., 2012). Inputs that create inhibitory side-bands could sharpen the excitatory frequency tuning so that fewer vocalizations contain energy that falls within excitatory receptive fields. These side-bands could also enhance selectivity to vocalizations because any vocalization that contains spectral energy within the inhibitory bands would not evoke a response. For example, if two vocalizations shared spectral content that fell within the excitatory tuning curve but one also had energy within the inhibitory sidebands, the neuron would not respond to the one that evokes the inhibition. Thus, neurons with inhibitory sidebands could be more selective to vocalizations compared to those neurons with just a narrow excitatory region (Portfors, 2004).
The role that inhibition plays in coding of vocalizations in songbirds is unclear. Comparable experiments in which inhibitory receptors are pharmacologically blocked have not been conducted. However, considering that the prevalence and strength of inhibition in the zebra finch MLd (Schneider and Woolley, 2011) seems lower than in the bat and mouse IC, it is likely that inhibition plays a more limited role in songbird midbrain tuning. A detailed examination of the inputs to MLd would be a valuable contribution to understanding how excitatory and inhibitory inputs influence auditory coding in songbirds.
A nonlinear response property that impacts vocalization coding in songbirds is stimulus-dependent tuning, which is characterized by changes in a neuron’s spectral and/or temporal tuning measured from responses to complex sounds that differ in statistical structure. The tuning properties of many MLd neurons differ during the processing of different sound classes such as songs and noise (Woolley et al., 2005; 2006; Schneider and Woolley, 2011). Stimulus-dependent tuning is also found in mammalian auditory neurons (Escabi et al., 2003; Lesica and Grothe, 2008; David et al., 2009). Tuning differences observed in the same neuron during the processing of sounds that differ in spectrotemporal structure (e.g. song and noise), are measured by comparing the spectrotemporal receptive fields of the neuron calculated separately from responses to two sound classes (Woolley at et al., 2006). Stimulus-dependent tuning based on statistical differences among stimuli may maximize the information about a stimulus that is encoded in a response (Escabi et al., 2003; David et al., 2004; Woolley et al., 2005; Sharpee et al., 2006; Maravall et al., 2007), facilitate neural discrimination of vocalizations (Woolley et al., 2005, 2006) and correlate with changes in perceptual sensitivity (Webster et al., 2002; Dahmen et al., 2010). The mechanism for stimulus-dependent tuning in zebra finch midbrain neurons is the presence of extra-classical excitation and inhibition (Woolley and Schneider, 2011) Neurons with extra-classical tuning are sensitive to the structure of spectrally correlated sounds such as the noisy bursts of sound and harmonic stacks that characterize zebra finch song. MLd neurons, therefore, exhibit a simple non-linearity that can account for the stimulus-dependence of receptive fields estimated from the responses to sounds with natural and non-natural statistics. Whether or not similar mechanisms alter receptive field structure in the IC remains to be tested.
The role of spike timing in auditory coding of vocalizations
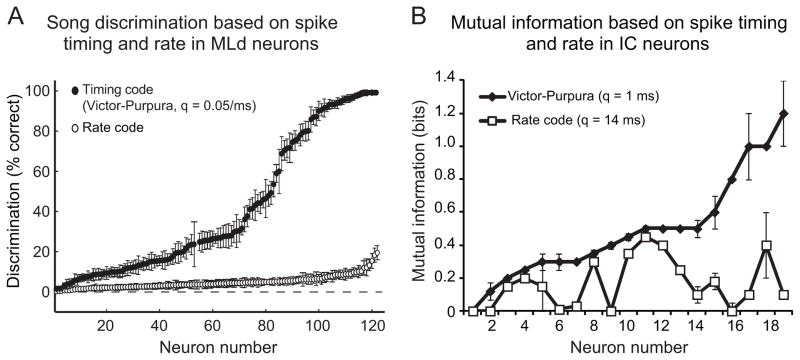
Because social vocalizations vary in frequency content and amplitude over time, neural responses to different vocalizations have unique temporal patterns (Fig. 4). Thus, the temporal patterns of spike trains carry information about the acoustic features of vocalizations. Both mouse and zebra finch midbrain neurons represent vocalizations with temporal codes (i.e. spike timing) rather than simply average firing rates. When the temporal patterns of responses to vocal sounds are included in the analysis of vocalization coding, the responses of single neurons can be used to discriminate among different vocalizations (Fig. 5). Both IC and MLd neurons encode vocalizations with precise and reliable temporally-patterned spike trains.
Figure 5.
Spike timing in addition to average spike rate is important for neural coding of social vocalizations. A. Neural discrimination of 20 zebra finch songs is significantly more accurate using a spike timing code such as the Victor-Purpura neurometric than using the average spike rate to discriminate among individual songs. B. Mutual information in responses of mouse IC neurons to vocalizations significantly increases when spike timing is taken into account. The value q is a measure of spike train similarity and indicates the cost of moving 1 spike 1 ms to match the temporal pattern of of two spike trains.
The responses of one MLd neuron to different songs have distinct temporal patterns because the neuron responds to short timescale acoustic features that occur at different points in each song (compare spike trains across rows in Fig. 4B). The temporal patterns of spike trains evoked by different songs differ more than do average firing rates in response to those songs (Schneider and Woolley, 2010). The similarity of spike trains evoked by the same song over multiple presentations and differences in the spike trains evoked by presentation of different songs can be quantified to estimate the “neural discrimination” of songs; spike trains can be used to predict which song a bird heard (Wang et al., 2007; Billimoria et al., 2008; Schneider and Woolley, 2010). Figure 5A shows the percent correct neural discrimination of 20 songs by single MLd neurons when a rate code (average firing rate to the whole song) and when a spike timing code (the temporal patterns of spike trains) are used to classify songs based on neural responses. For nearly all neurons, a rate code fails to discriminate among songs and a spike timing code significantly increases songs discrimination (Schneider and Woolley, 2010). However, the accuracy of song discrimination using a spike timing code varies considerably across neurons (Fig. 5A) and is closely correlated with the number of spikes in the response. The same neural discrimination of songs based on spike train temporal patterns is observed in primary forebrain neurons in field L (Wang et al., 2007). This coding principle may provide a basis for discriminating between songs sung by different males and potentially contribute to individual recognition during social interactions.
Because the responses of single MLd neurons are precisely timed, combining the responses of multiple, similarly-tuned neurons improves the accuracy of song neural discrimination (Schneider and Woolley, 2010). In addition, the responses of populations of midbrain neurons can be used to reconstruct the spectrograms of songs that evoked those responses (Ramirez et al., 2011). As a result, the combined responses of individual midbrain neurons tuned to different acoustic features in songs can represent the complete song. Similarly, population activity in the human auditory cortex can be used to accurately identify presented speech segments (Pasley et al., 2012; Mesgarani and Chang, 2012).
A similar spike timing principle seems to occur in mouse IC neurons. Although far fewer neurons have been examined in the mouse IC than in the songbird MLd, a spike timing code provides greater mutual information for many neurons compared to a rate code. In addition, there is similar diversity in spike timing versus rate codes in mouse IC and in MLd. In some neurons, spike timing provides the same amount of information as spike rate, whereas in other neurons, spike timing provides substantially more information (Dimitrov et al., 2012). Similar analyses have not yet been conducted in bats.
Future Direction
The conserved mechanisms of auditory coding described here suggest that more direct comparisons of songbirds and mammals will provide valuable information about the mechanisms of auditory processing in vocal communication. Specifically, research on the songbird and mouse auditory systems may make shared progress by adopting similar experimental and analytic approaches. First, behavioral studies on the roles of mouse vocalizations in mating and other social interactions may benefit from the behavioral experiments and analyses that established the functions of song in birds. In particular, including natural sequences of mouse syllables in behavioral experiments could facilitate the understanding of female responses to male vocalizations. Second, studies on songbirds that produce tonal songs (Fig. 2), resembling mouse vocalizations, may allow more direct comparisons of auditory tuning across taxa, and help identify those coding mechanisms that are tightly coupled to vocal acoustics. Third, cellular and circuit mechanisms of auditory tuning in songbirds may be approached with the pharmacological manipulations that have been used to determine how excitatory and inhibitory inputs shape tuning in mammalian auditory neurons. For example, the role of inhibition in songbird auditory coding should be tested based on its profound importance for mammalian auditory processing. Lastly, comparable analyses of population coding in the auditory systems of songbirds and mammals may facilitate discovery of fundamental mechanisms subserving vocal communication.
Highlights.
Mice, bats and songbirds produce and perceive complex social vocalizations
Auditory midbrains of these animals show common basic tuning properties
Mammal and songbird midbrain neurons have nonlinear coding properties
Nonlinear coding properties impact auditory coding of social vocalizations
Songbird and mouse research should share experimental and analytical approaches
Acknowledgments
We thank Svetlana Rosis for valuable comments on the manuscript. SMNW is supported by the NIH (R01-DC009810) and the NSF (IOS-0920081). CVP is supported by the NSF (IOS-0920060).
Abbreviations
- A1
primary auditory cortex
- CF
characteristic frequency
- dB
decibels
- DCN
dorsal cochlear nucleus
- DNLL
dorsal nucleus of the lateral lemniscus
- IC
inferior colliculus
- ICc
central nucleus of the inferior colliculus
- INLL
intermediate nucleus of the lateral lemniscus
- kHz
kilohertz
- LLD
dorsal nucleus of the lateral lemniscus
- LLI
intermediate nucleus of the lateral lemniscus
- LLV
ventral nucleus of the lateral lemniscus
- LSO
lateral superior olive
- MGB
medial geniculate body
- MLd
lateral dorsal mesencephalon
- MSO
medial superior olive
- ms
millisecond
- NA
nucleus angularis
- NL
nucleus laminaris
- NM
nucleus magnocellularis
- OC
olivocochlear nucleus
- Ov
nucleus ovoidalis
- RA
robust nucleus of the arcopallium
- SON
superior olivary nucleus
- SPN
superior paraolivary nucleus
- VCN
ventral cochlear nucleus
- VNLL
ventral nucleus of the lateral lemniscus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sarah M. N. Woolley, Email: sw2277@columbia.edu.
Christine V. Portfors, Email: portfors@vancouver.wsu.edu.
References
- Aitkin L, Tran L, Syka J. The responses of neurons in subdivisions of the inferior colliculus of cats to tonal, noise and vocal stimuli. Exp Brain Res. 1994;98:53–64. doi: 10.1007/BF00229109. [DOI] [PubMed] [Google Scholar]
- Andoni S, Pollak GD. Selectivity for spectral motion as a neural computation for encoding natural communication signals in bat inferior colliculus. J Neurosci. 2011;31:16529–40. doi: 10.1523/JNEUROSCI.1306-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andoni S, Li N, Pollak GD. Spectrotemporal receptive fields in the inferior colliculus revealing selectivity for spectral motion in conspecific vocalizations. J Neurosci. 2007;27:4882–93. doi: 10.1523/JNEUROSCI.4342-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriaga G, Zhou EP, Jarvis ED. Of mice, birds, and men: the mouse ultrasonic song system has some features similar to humans and song-learning birds. PLoS One. 2012;7:e46610. doi: 10.1371/journal.pone.0046610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo VM, Nodal FR, Moore DR, King AJ. The descending corticocollicular pathway mediates learning-induced auditory plasticity. Nat Neurosci. 2010;13:253–60. doi: 10.1038/nn.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer EE, Klug A, Pollak GD. Spectral determination of responses to species-specific calls in the dorsal nucleus of the lateral lemniscus. J Neurophysiol. 2002;88:1955–67. doi: 10.1152/jn.2002.88.4.1955. [DOI] [PubMed] [Google Scholar]
- Billimoria CP, Kraus BJ, Narayan R, Maddox RK, Sen K. Invariance and sensitivity to intensity in neural discrimination of natural sounds. J Neurosci. 2008;28:6304–8. doi: 10.1523/JNEUROSCI.0961-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn KM, Schmidt-French B, Ma ST, Pollak GD. Syllable acoustics, temporal patterns, and call composition vary with behavioral context in Mexican free-tailed bats. J Acoust Soc Am. 2008;124:1838–48. doi: 10.1121/1.2953314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn KM, Schmidt-French B, Schwartz C, Smotherman M, Pollak GD. Versatility and stereotypy of free-tailed bat songs. PLoS One. 2009;4:e6746. doi: 10.1371/journal.pone.0006746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boogert NJ, Giraldeau LA, Lefebvre L. Song complexity correlates with learning ability in zebra finch males. Animal Behaviour. 2008;76:1735–1741. [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–8. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Beecher MD. Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci. 2005;28:127–32. doi: 10.1016/j.tins.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Butler AB, Reiner A, Karten HJ. Evolution of the amniote pallium and the origins of mammalian neocortex. Ann N Y Acad Sci. 2011;1225:14–27. doi: 10.1111/j.1749-6632.2011.06006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casseday JH, Covey E. Frequency tuning properties of neurons in the inferior colliculus of an FM bat. J Comp Neurol. 1992;319:34–50. doi: 10.1002/cne.903190106. [DOI] [PubMed] [Google Scholar]
- Casseday JH, Ehrlich D, Covey E. Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science. 1994;264:847–50. doi: 10.1126/science.8171341. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird song: Biological themes and variations. 2. Cambridge University Press; Cambridge, U.K: 2008. [Google Scholar]
- Chi T, Gao Y, Guyton MC, Ru P, Shamma S. Spectro-temporal modulation transfer functions and speech intelligibility. J Acoust Soc Am. 1999;106:2719–32. doi: 10.1121/1.428100. [DOI] [PubMed] [Google Scholar]
- Clopton BM, Winfield JA. Tonotopic organization in the inferior colliculus of the rat. Brain Res. 1973;56:355–8. doi: 10.1016/0006-8993(73)90352-1. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Parks TN. Origin of ascending auditory projections to the nucleus mesencephalicus lateralis pars dorsalis in the chicken. Brain Res. 1986;367:96–113. doi: 10.1016/0006-8993(86)91583-0. [DOI] [PubMed] [Google Scholar]
- Covey E, Carr CE. The auditory midbrain in bats and birds. In: Winer JA, Schreiner CE, editors. The Inferior Colliculus. Springer; New York: 2005. pp. 493–538. [Google Scholar]
- Dahmen JC, Keating P, Nodal FR, Schulz AL, King AJ. Adaptation to stimulus statistics in the perception and neural representation of auditory space. Neuron. 2010;66:937–48. doi: 10.1016/j.neuron.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SV, Vinje WE, Gallant JL. Natural stimulus statistics alter the receptive field structure of v1 neurons. J Neurosci. 2004;24:6991–7006. doi: 10.1523/JNEUROSCI.1422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SV, Mesgarani N, Fritz JB, Shamma SA. Rapid synaptic depression explains nonlinear modulation of spectro-temporal tuning in primary auditory cortex by natural stimuli. J Neurosci. 2009;29:3374–86. doi: 10.1523/JNEUROSCI.5249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov A, Cummins G, Mayko ZM, Portfors CV. Influence of inhibition on encoding vocalizations in the mouse auditory midbrain. Cosyne; Salt Lake City, UT: 2012. [Google Scholar]
- Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu Rev Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Alder TB, Rose GJ. Auditory midbrain neurons that count. Nat Neurosci. 2002;5:934–6. doi: 10.1038/nn916. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Leary CJ, Rose GJ. Counting on inhibition and rate-dependent excitation in the auditory system. J Neurosci. 2007;27:13384–92. doi: 10.1523/JNEUROSCI.2816-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova M, Ehret G. Tonotopy and inhibition in the midbrain inferior colliculus shape spectral resolution of sounds in neural critical bands. Eur J Neurosci. 2008;28:675–92. doi: 10.1111/j.1460-9568.2008.06376.x. [DOI] [PubMed] [Google Scholar]
- Egorova M, Ehret G, Vartanian I, Esser KH. Frequency response areas of neurons in the mouse inferior colliculus. I. Threshold and tuning characteristics. Exp Brain Res. 2001;140:145–61. doi: 10.1007/s002210100786. [DOI] [PubMed] [Google Scholar]
- Ehret G. Age-dependent hearing loss in normal hearing mice. Naturwissenschaften. 1974;61:506–507. doi: 10.1007/BF00622976. [DOI] [PubMed] [Google Scholar]
- Ehret G, Egorova M, Hage SR, Muller BA. Spatial map of frequency tuning-curve shapes in the mouse inferior colliculus. Neuroreport. 2003;14:1365–9. doi: 10.1097/01.wnr.0000078545.07662.85. [DOI] [PubMed] [Google Scholar]
- Elliott TM, Theunissen FE. The modulation transfer function for speech intelligibility. PLoS Comput Biol. 2009;5:e1000302. doi: 10.1371/journal.pcbi.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TM, Christensen-Dalsgaard J, Kelley DB. Temporally selective processing of communication signals by auditory midbrain neurons. J Neurophysiol. 2011;105:1620–32. doi: 10.1152/jn.00261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escabi MA, Miller LM, Read HL, Schreiner CE. Naturalistic auditory contrast improves spectrotemporal coding in the cat inferior colliculus. J Neurosci. 2003;23:11489–504. doi: 10.1523/JNEUROSCI.23-37-11489.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee MS, Kozhevnikov AA, Hahnloser RH. Neural mechanisms of vocal sequence generation in the songbird. Ann N Y Acad Sci. 2004;1016:153–70. doi: 10.1196/annals.1298.022. [DOI] [PubMed] [Google Scholar]
- Felix RA, 2nd, Portfors CV. Excitatory, inhibitory and facilitatory frequency response areas in the inferior colliculus of hearing impaired mice. Hear Res. 2007;228:212–29. doi: 10.1016/j.heares.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer J, Hammerschmidt K. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes Brain Behav. 2011;10:17–27. doi: 10.1111/j.1601-183X.2010.00610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proc Natl Acad Sci U S A. 2000;97:8081–6. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J, Kiang N. Neural correlates of the aural combination tone 2f1-f2. Proc IEEE. 1968;56:981–992. [Google Scholar]
- Grothe B, Carr CE, Casseday JH, Fritzsch B, Köppl C. The evolution of central pathways and their neural processing patterns. In: Manley GA, Fay RR, editors. Spring Handbook of Auditory Research, Evolution of the Vertebrate Auditory System. Springer; New York: 2004. pp. 289–359. [Google Scholar]
- Hanson JL, Hurley LM. Female presence and estrous state influence mouse ultrasonic courtship vocalizations. PLoS One. 2012;7:e40782. doi: 10.1371/journal.pone.0040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haplea S, Covey E, Casseday JH. Frequency tuning and response latencies at three levels in the brainstem of the echolocating bat, Eptesicus fuscus. J Comp Physiol A. 1994;174:671–83. doi: 10.1007/BF00192716. [DOI] [PubMed] [Google Scholar]
- Henry KR, McGinn MD. The mouse as a model for human audition. A review of the literature. Audiology. 1992;31:181–9. doi: 10.3109/00206099209081653. [DOI] [PubMed] [Google Scholar]
- Hoffmann F, Musolf K, Penn DJ. Spectrographic analyses reveal signals of individuality and kinship in the ultrasonic courtship vocalizations of wild house mice. Physiol Behav. 2012;105:766–71. doi: 10.1016/j.physbeh.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Holmstrom L, Roberts PD, Portfors CV. Responses to social vocalizations in the inferior colliculus of the mustached bat are influenced by secondary tuning curves. J Neurophysiol. 2007;98:3461–72. doi: 10.1152/jn.00638.2007. [DOI] [PubMed] [Google Scholar]
- Holmstrom LA, Eeuwes LB, Roberts PD, Portfors CV. Efficient encoding of vocalizations in the auditory midbrain. J Neurosci. 2010;30:802–19. doi: 10.1523/JNEUROSCI.1964-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holveck M-J, de Castro ACV, Lachlan RF, ten Cate C, Riebel K. Accuracy of song syntax learning and singing consistency signal early condition in zebra finches. Behavioral Ecology. 2008:19. [Google Scholar]
- Holy TE, Guo Z. Ultrasonic songs of male mice. PLoS Biol. 2005;3:e386. doi: 10.1371/journal.pbio.0030386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal JS, Matsumura S, Ohlemiller K, Suga N. Analysis of acoustic elements and syntax in communication sounds emitted by mustached bats. J Acoust Soc Am. 1994;96:1229–54. doi: 10.1121/1.410273. [DOI] [PubMed] [Google Scholar]
- Kim DO, Molnar CE, Matthews JW. Cochlear mechanics: nonlinear behavior in two-tone responses as reflected in cochlear-nerve-fiber responses and in ear-canal sound pressure. J Acoust Soc Am. 1980;67:1704–21. doi: 10.1121/1.384297. [DOI] [PubMed] [Google Scholar]
- Klug A, Bauer EE, Hanson JT, Hurley L, Meitzen J, Pollak GD. Response selectivity for species-specific calls in the inferior colliculus of Mexican free-tailed bats is generated by inhibition. J Neurophysiol. 2002;88:1941–54. doi: 10.1152/jn.2002.88.4.1941. [DOI] [PubMed] [Google Scholar]
- Konishi M. An outline of recent advances in birdsong neurobiology. Brain Behav Evol. 1994;44:279–85. doi: 10.1159/000113582. [DOI] [PubMed] [Google Scholar]
- Konishi M, Nottebohm F. Experimental studies in the ontogeny of avian vocalizations. In: Hinde RA, editor. Bird vocalizations: their relations to current problems in biology and psychology; essays presented to W H Thorpe. Cambridge U.P; London: 1969. [Google Scholar]
- Krutzfeldt NO, Logerot P, Kubke MF, Wild JM. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. II. Projections of nucleus angularis and nucleus laminaris to the superior olive and lateral lemniscal nuclei. J Comp Neurol. 2010;518:2135–48. doi: 10.1002/cne.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy SA, Wenstrup JJ. Spectral integration in the inferior colliculus of the mustached bat. J Neurosci. 2000;20:8533–41. doi: 10.1523/JNEUROSCI.20-22-08533.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesica NA, Grothe B. Dynamic spectrotemporal feature selectivity in the auditory midbrain. J Neurosci. 2008;28:5412–21. doi: 10.1523/JNEUROSCI.0073-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. PLoS Biol. 2007;5(7):e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukashkin AN, Russell IJ. A descriptive model of the receptor potential nonlinearities generated by the hair cell mechanoelectrical transducer. J Acoust Soc Am. 1998;103:973–80. doi: 10.1121/1.421214. [DOI] [PubMed] [Google Scholar]
- Mahrt EJ, Perkel DJ, Tong L, Rubel EW, Portfors CV. Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. J Neurosci. 2013 doi: 10.1523/JNEUROSCI.5054-12.2013. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maravall M, Petersen RS, Fairhall AL, Arabzadeh E, Diamond ME. Shifts in coding properties and maintenance of information transmission during adaptation in barrel cortex. PLoS Biol. 2007;5:e19. doi: 10.1371/journal.pbio.0050019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayko ZM, Roberts PD, Portfors CV. Inhibition shapes selectivity to vocalizations in the inferior colliculus of awake mice. Front Neural Circuits. 2012;6:73. doi: 10.3389/fncir.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAlpine D. Neural sensitivity to periodicity in the inferior colliculus: Evidence for the role of cochlear distortions. J Neurophys. 2004;92:1295–1311. doi: 10.1152/jn.00034.2004. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vates GE, Okuhata S, Nottebohm F. Descending auditory pathways in the adult male zebra finch (Taeniopygia guttata) J Comp Neurol. 1998;395:137–60. [PubMed] [Google Scholar]
- Merzenich MM, Reid MD. Representation of the cochlea within the inferior colliculus of the cat. Brain Res. 1974;77:397–415. doi: 10.1016/0006-8993(74)90630-1. [DOI] [PubMed] [Google Scholar]
- Mesgarani N, Chang EF. Selective cortical representation of attended speaker in multi-talker speech perception. Nature. 2012;485:233–6. doi: 10.1038/nature11020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB. Long-term recognition of father’s song by female zebra finches. Nature. 1979a;280:389–391. [Google Scholar]
- Miller DB. The acoustic basis if mate recognition by female zebra finches (Taeniopygia guttata) Anim Behav. 1979b;27:376–380. [Google Scholar]
- Mittmann DH, Wenstrup JJ. Combination-sensitive neurons in the inferior colliculus. Hear Res. 1995;90:185–91. doi: 10.1016/0378-5955(95)00164-x. [DOI] [PubMed] [Google Scholar]
- Mooney R. Neural mechanisms for learned birdsong. Learn Mem. 2009;16:655–69. doi: 10.1101/lm.1065209. [DOI] [PubMed] [Google Scholar]
- Müller M, Hunerbein KV, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res. 2005;202:63–73. doi: 10.1016/j.heares.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Nataraj K, Wenstrup JJ. Roles of inhibition in creating complex auditory responses in the inferior colliculus: facilitated combination-sensitive neurons. J Neurophysiol. 2005;93:3294–312. doi: 10.1152/jn.01152.2004. [DOI] [PubMed] [Google Scholar]
- Nyby J, Dizinno GA, Whitney G. Social status and ultrasonic vocalizations of male mice. Behav Biol. 1976;18:285–9. doi: 10.1016/s0091-6773(76)92198-2. [DOI] [PubMed] [Google Scholar]
- Nyby J, Whitney G, Schmitz S, Dizinno G. Postpubertal experience establishes signal value of mammaliam sex odor. Behav Biol. 1978;22:545–52. doi: 10.1016/s0091-6773(78)92745-1. [DOI] [PubMed] [Google Scholar]
- Nyby J, Wysocki CJ, Whitney G, Dizinno G, Schneider J. Elicitation of male mouse (Mus musculus) ultrasonic vocalizations: I. Urinary cues. J Comp Physiol Psychol. 1979;93:957–975. [Google Scholar]
- O’Neill WE, Suga N. Target range-sensitive neurons in the auditory cortex of the mustache bat. Science. 1979;203:69–73. doi: 10.1126/science.758681. [DOI] [PubMed] [Google Scholar]
- O’Neill WE, Suga N. Encoding of target range and its representation in the auditory cortex of the mustached bat. J Neurosci. 1982;2:17–31. doi: 10.1523/JNEUROSCI.02-01-00017.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasley BN, David SV, Mesgarani N, Flinker A, Shamma SA, Crone NE, Knight RT, Chang EF. Reconstructing speech from human auditory cortex. PLoS Biol. 2012;10:e1001251. doi: 10.1371/journal.pbio.1001251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp R. Detectability threshold for combination tones. J Acoust Soc Am. 1965;37:1110–23. doi: 10.1121/1.1909532. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Bodenhamer RD. Specialized characteristics of single units in inferior colliculus of mustache bat: frequency representation, tuning, and discharge patterns. J Neurophysiol. 1981;46:605–20. doi: 10.1152/jn.1981.46.3.605. [DOI] [PubMed] [Google Scholar]
- Pollak GD, Schuller G. Tonotopic organization and encoding features of single units in inferior colliculus of horseshoe bats: functional implications for prey identification. J Neurophysiol. 1981;45:208–26. doi: 10.1152/jn.1981.45.2.208. [DOI] [PubMed] [Google Scholar]
- Pollak GK, Marsh DS, Bodenhamer R, Souther A. A single-unit analysis of inferior colliculus in unanesthetized bats: response patterns and spike-count functions generated by constant-frequency and frequency-modulated sounds. J Neurophysiol. 1978;41:677–91. doi: 10.1152/jn.1978.41.3.677. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Combination sensitivity and processing of communication calls in the inferior colliculus of the Moustached Bat Pteronotus parnellii. An Acad Bras Cienc. 2004;76:253–7. doi: 10.1590/s0001-37652004000200010. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. J Am Assoc Lab Anim Sci. 2007;46:28–34. [PubMed] [Google Scholar]
- Portfors CV, Wenstrup JJ. Delay-tuned neurons in the inferior colliculus of the mustached bat: implications for analyses of target distance. J Neurophysiol. 1999;82:1326–38. doi: 10.1152/jn.1999.82.3.1326. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Wenstrup JJ. Responses to combinations of tones in the nuclei of the lateral lemniscus. J Assoc Res Otolaryngol. 2001;2:104–17. doi: 10.1007/s101620010057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV, Wenstrup JJ. Excitatory and facilitatory frequency response areas in the inferior colliculus of the mustached bat. Hear Res. 2002;168:131–8. doi: 10.1016/s0378-5955(02)00376-3. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Felix RA., 2nd Spectral integration in the inferior colliculus of the CBA/CaJ mouse. Neuroscience. 2005;136:1159–70. doi: 10.1016/j.neuroscience.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Roberts PD, Jonson K. Over-representation of species-specific vocalizations in the awake mouse inferior colliculus. Neuroscience. 2009;162:486–500. doi: 10.1016/j.neuroscience.2009.04.056. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Mayko ZM, Jonson K, Cha GF, Roberts PD. Spatial organization of receptive fields in the auditory midbrain of awake mouse. Neuroscience. 2011;193:429–39. doi: 10.1016/j.neuroscience.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Ramirez AD, Ahmadian Y, Schumacher J, Schneider D, Woolley SM, Paninski L. Incorporating naturalistic correlation structure improves spectrogram reconstruction from neuronal activity in the songbird auditory midbrain. J Neurosci. 2011;31:3828–42. doi: 10.1523/JNEUROSCI.3256-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remez RE, Pardo JS, Piorkowski RL, Rubin PE. On the bistability of sine wave analogues of speech. Psychol Sci. 2001;12:24–9. doi: 10.1111/1467-9280.00305. [DOI] [PubMed] [Google Scholar]
- Riebel K. Song and female mate choices in zebra finches - a review. Adv Stud Behav. 2009;40:197–238. [Google Scholar]
- Rubsamen R, Neuweiler G, Marimuthu G. Ontogenesis of tonotopy in inferior colliculus of a hipposiderid bat reveals postnatal shift in frequency-place code. J Comp Physiol A. 1989;165:755–69. doi: 10.1007/BF00610874. [DOI] [PubMed] [Google Scholar]
- Sabin AT, Eddins DA, Wright BA. Perceptual learning evidence for tuning to spectrotemporal modulation in the human auditory system. J Neurosci. 2012a;32:6542–9. doi: 10.1523/JNEUROSCI.5732-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin AT, Clark CA, Eddins DA, Wright BA. Different Patterns of Perceptual Learning on Spectral Modulation Detection Between Older Hearing-Impaired and Younger Normal-Hearing Adults. J Assoc Res Otolaryngol. 2012b doi: 10.1007/s10162-012-0363-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MB, Young ED. Encoding of steady-state vowels in the auditory nerve: representation in terms of discharge rate. J Acoust Soc Am. 1979;66:470–9. doi: 10.1121/1.383098. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Woolf NK, Sinnott JM. Response properties of neurons in the avian auditory system: Comparisons with Mammalian homologues and consideration of the neural encoding of complex stimuli. In: Popper AN, Fay RR, editors. Comparative Studies of Hearing in Vertebrates. Springer; Berlin: 1980. pp. 223–253. [Google Scholar]
- Saldana E, Feliciano M, Mugnaini E. Distribution of descending projections from primary auditory neocortex to inferior colliculus mimics the topography of intracollicular projections. J Comp Neurol. 1996;371:15–40. doi: 10.1002/(SICI)1096-9861(19960715)371:1<15::AID-CNE2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav. 1972;20:88–100. doi: 10.1016/s0003-3472(72)80177-5. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Crawley J, Ricceri L. Ultrasonic vocalizations: a tool for behavioural phenotyping of mouse models of neurodevelopmental disorders. Neurosci Biobehav Rev. 2009;33:508–15. doi: 10.1016/j.neubiorev.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DM, Woolley SM. Discrimination of communication vocalizations by single neurons and groups of neurons in the auditory midbrain. J Neurophysiol. 2010;103:3248–65. doi: 10.1152/jn.01131.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DM, Woolley SM. Extra-classical tuning predicts stimulus-dependent receptive fields in auditory neurons. J Neurosci. 2011;31:11867–78. doi: 10.1523/JNEUROSCI.5790-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler HU, Denzinger A. Auditory fovea and Doppler shift compensation: adaptations for flutter detection in echolocating bats using CF-FM signals. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2011;197:541–59. doi: 10.1007/s00359-010-0569-6. [DOI] [PubMed] [Google Scholar]
- Schonwiesner M, Zatorre RJ. Spectro-temporal modulation transfer function of single voxels in the human auditory cortex measured with high-resolution fMRI. Proc Natl Acad Sci U S A. 2009;106:14611–6. doi: 10.1073/pnas.0907682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Laminar fine structure of frequency organization in auditory midbrain. Nature. 1997;388:383–6. doi: 10.1038/41106. [DOI] [PubMed] [Google Scholar]
- Schumacher JW, Schneider DM, Woolley SM. Anesthetic state modulates excitability but not spectral tuning or neural discrimination in single auditory midbrain neurons. J Neurophysiol. 2011;106:500–14. doi: 10.1152/jn.01072.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–4. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Sharpee TO, Sugihara H, Kurgansky AV, Rebrik SP, Stryker MP, Miller KD. Adaptive filtering enhances information transmission in visual cortex. Nature. 2006;439:936–42. doi: 10.1038/nature04519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NC, Theunissen FE. Modulation spectra of natural sounds and ethological theories of auditory processing. J Acoust Soc Am. 2003;114:3394–411. doi: 10.1121/1.1624067. [DOI] [PubMed] [Google Scholar]
- Spencer KA, Buchanan KL, Leitner S, Goldsmith AR, Catchpole CK. Parasites affect song complexity and neural development in a songbird. Proc Biol Sci. 2005;272:2037–43. doi: 10.1098/rspb.2005.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiebler I, Ehret G. Inferior colliculus of the house mouse. I. A quantitative study of tonotopic organization, frequency representation, and tone-threshold distribution. J Comp Neurol. 1985;238:65–76. doi: 10.1002/cne.902380106. [DOI] [PubMed] [Google Scholar]
- Suga N. Recovery cycles and responses to frequency modulated tone pulses in auditory neurones of echo-locating bats. J Physiol. 1964;175:50–80. doi: 10.1113/jphysiol.1964.sp007503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga N. Specialization of the auditory system for reception and processing of species-specific sounds. Fed Proc. 1978;37:2342–54. [PubMed] [Google Scholar]
- Suga N. Principles of auditory information-processing derived from neuroethology. J Exp Biol. 1989;146:277–86. doi: 10.1242/jeb.146.1.277. [DOI] [PubMed] [Google Scholar]
- Suga N, Jen PH. Disproportionate tonotopic representation for processing CF- FM sonar signals in the mustache bat auditory cortex. Science. 1976;194:542–4. doi: 10.1126/science.973140. [DOI] [PubMed] [Google Scholar]
- Suga N, Simmons JA, Jen PH. Peripheral specialization for fine analysis of doppler-shifted echoes in the auditory system of the “CF-FM” bat Pteronotus parnellii. J Exp Biol. 1975;63:161–92. doi: 10.1242/jeb.63.1.161. [DOI] [PubMed] [Google Scholar]
- Suga N, O’Neill WE, Manabe T. Cortical neurons sensitive to combinations of information-bearing elements of biosonar signals in the mustache bat. Science. 1978;200:778–81. doi: 10.1126/science.644320. [DOI] [PubMed] [Google Scholar]
- Suga N, O’Neill WE, Manabe T. Harmonic-sensitive neurons in the auditory cortex of the mustache bat. Science. 1979;203:270–4. doi: 10.1126/science.760193. [DOI] [PubMed] [Google Scholar]
- Suta D, Kvasnak E, Popelar J, Syka J. Representation of species-specific vocalizations in the inferior colliculus of the guinea pig. J Neurophys. 2003;90:3794–3808. doi: 10.1152/jn.01175.2002. [DOI] [PubMed] [Google Scholar]
- Suthers RA, Margoliash D. Motor control of birdsong. Curr Opin Neurobiol. 2002;12:684–90. doi: 10.1016/s0959-4388(02)00386-0. [DOI] [PubMed] [Google Scholar]
- Theunissen FE, Sen K, Doupe AJ. Spectral-temporal receptive fields of nonlinear auditory neurons obtained using natural sounds. J Neurosci. 2000;20:2315–31. doi: 10.1523/JNEUROSCI.20-06-02315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD. Spike train metrics. Curr Opin Neurobiol. 2005;15:585–92. doi: 10.1016/j.conb.2005.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liang S, Burgdorf J, Wess J, Yeomans J. Ultrasonic vocalizations induced by sex and amphetamine in M2, M4, M5 muscarinic and D2 dopamine receptor knockout mice. PLoS One. 2008;3:e1893. doi: 10.1371/journal.pone.0001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Narayan R, Grana G, Shamir M, Sen K. Cortical discrimination of complex natural stimuli: can single neurons match behavior? J Neurosci. 2007;27:582–9. doi: 10.1523/JNEUROSCI.3699-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren B, Gibson G, Russell IJ. Sex Recognition through midflight mating duets in Culex mosquitoes is mediated by acoustic distortion. Curr Biol. 2009;19:485–91. doi: 10.1016/j.cub.2009.01.059. [DOI] [PubMed] [Google Scholar]
- Washington SD, Kanwal JS. DSCF neurons within the primary auditory cortex of the mustached bat process frequency modulations present within social calls. J Neurophysiol. 2008;100:3285–304. doi: 10.1152/jn.90442.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MA, Georgeson MA, Webster SM. Neural adjustments to image blur. Nat Neurosci. 2002;5:839–40. doi: 10.1038/nn906. [DOI] [PubMed] [Google Scholar]
- Wenstrup JJ, Nataraj K, Sanchez JT. Mechanisms of spectral and temporal integration in the mustached bat inferior colliculus. Front Neural Circuits. 2012;6:75. doi: 10.3389/fncir.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup JJ, Portfors CV. Neural processing of target distance by echolocating bats: functional roles of the auditory midbrain. Neurosci Biobehav Rev. 2011;35:2073–83. doi: 10.1016/j.neubiorev.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Krutzfeldt NO, Kubke MF. Connections of the auditory brainstem in a songbird, Taeniopygia guttata. III. Projections of the superior olive and lateral lemniscal nuclei. J Comp Neurol. 2010;518:2149–67. doi: 10.1002/cne.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer JA, Schreiner CE. The Inferior Colliculus. Springer-Verlag Press; New York: 2005. [Google Scholar]
- Winer JA, Larue DT, Diehl JJ, Hefti BJ. Auditory cortical projections to the cat inferior colliculus. J Comp Neurol. 1998;400:147–74. [PubMed] [Google Scholar]
- Woolley SM. Early experience shapes vocal neural coding and perception in songbirds. Dev Psychobiol. 2012;54:612–31. doi: 10.1002/dev.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Casseday JH. Response properties of single neurons in the zebra finch auditory midbrain: response patterns, frequency coding, intensity coding, and spike latencies. J Neurophysiol. 2004;91:136–51. doi: 10.1152/jn.00633.2003. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Casseday JH. Processing of modulated sounds in the zebra finch auditory midbrain: responses to noise, frequency sweeps, and sinusoidal amplitude modulations. J Neurophysiol. 2005a;94:1143–57. doi: 10.1152/jn.01064.2004. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Moore JM. Coevolution in communication senders and receivers: vocal behavior and auditory processing in multiple songbird species. Ann N Y Acad Sci. 2011;1225:155–65. doi: 10.1111/j.1749-6632.2011.05989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Gill PR, Theunissen FE. Stimulus-dependent auditory tuning results in synchronous population coding of vocalizations in the songbird midbrain. J Neurosci. 2006;26:2499–512. doi: 10.1523/JNEUROSCI.3731-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley SM, Fremouw TE, Hsu A, Theunissen FE. Tuning for spectro-temporal modulations as a mechanism for auditory discrimination of natural sounds. Nat Neurosci. 2005b;8:1371–9. doi: 10.1038/nn1536. [DOI] [PubMed] [Google Scholar]
- Woolley SM, Gill PR, Fremouw T, Theunissen FE. Functional groups in the avian auditory system. J Neurosci. 2009;29:2780–93. doi: 10.1523/JNEUROSCI.2042-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie R, Meitzen J, Pollak GD. Differing roles of inhibition in hierarchical processing of species-specific calls in auditory brainstem nuclei. J Neurophysiol. 2005;94:4019–37. doi: 10.1152/jn.00688.2005. [DOI] [PubMed] [Google Scholar]
- Yang L, Pollak GD, Resler C. GABAergic circuits sharpen tuning curves and modify response properties in the mustache bat inferior colliculus. J Neurophysiol. 1992;68:1760–74. doi: 10.1152/jn.1992.68.5.1760. [DOI] [PubMed] [Google Scholar]
- Young ED, Sachs MB. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory-nerve fibers. J Acoust Soc Am. 1979;66:1381–1403. doi: 10.1121/1.383532. [DOI] [PubMed] [Google Scholar]
- Zann RA. The Zebra Finch: A Synthesis of Field and Laboratory Studies. Oxford University Press; Oxford: 1996. [Google Scholar]
- Zhang Y, Suga N. Corticofugal feedback for collicular plasticity evoked by electric stimulation of the inferior colliculus. J Neurophysiol. 2005;94:2676–82. doi: 10.1152/jn.00549.2005. [DOI] [PubMed] [Google Scholar]