Abstract
Electronic screening and brief intervention (e-SBI) approaches for substance use have shown early promise. This trial was designed to replicate previous findings from a single 20-minute e-SBI for drug use among postpartum women. A total of 143 postpartum, primarily low-income African-American women meeting criteria for drug use, were randomly assigned to either a tailored e-SBI or a time-matched control condition. Blinded follow-up evaluation 3- and 6-months following childbirth revealed strong effects for confirmed illicit drug use abstinence at the 3-month observation (OR = 3.3, p = .01), as did hair analysis at 6 months (OR = 4.8, p = .018). Additional primary outcomes suggested small to moderate effect sizes in favor of the e-SBI, but did not reach significance. This result replicates previous findings but fails to show durable effects. Assessment reactivity, e-SBI design, and possible extension of e-SBI via tailored messaging all merit careful consideration.
Keywords: Pregnancy, drug abuse screening, brief intervention, drug users, motivation, computers
1. Introduction
The perinatal period is an ideal point at which to identify and at least initially address problem drug use among women. According to 2010–2011 data from the National Survey on Drug Use and Health, 5.0% of pregnant women report current use of illicit drugs, compared to 10.8% of non-pregnant women of the same age (Substance Abuse and Mental Health Services Administration, 2012). Although this natural change is positive, most women who cut down during pregnancy return to previous levels of use in the year following childbirth (Office of Applied Studies, 2009). This suggests that the immediate post-partum period may be an opportunity to encourage maintenance of natural change.
Further, whereas the majority of pregnant women seek at least some prenatal care, virtually all women choose to deliver in hospitals. The post-partum period thus represents a unique opportunity in which to access high proportions of parenting women and facilitate new or continued behavior change, particularly given the potential of the postpartum period as a unique “teachable moment” (McBride, Emmons, & Lipkus, 2003). Success at this crucial juncture could reduce the many personal, social, and health-related consequences of drug use for the women themselves (Blumenthal, 1998; Kissin, Svikis, Morgan, & Haug, 2001; Mokdad, Marks, Stroup, & Gerberding, 2004) as well as for the children involved. Maternal substance use is associated with a wide range of negative prenatal and postnatal outcomes, including increased risk of child maltreatment (Besinger, Garland, Litrownik, & Landsverk, 1999; Chaffin, Kelleher, & Hollenberg, 1996; Ondersma, 2002), violence exposure (Ondersma, Delaney-Black, Covington, Nordstrom, & Sokol, 2006), asthma (Gergen, 2001; Mahabee-Gittens, 2002), depression (Reinherz, Giaconia, Hauf, Wasserman, & Paradis, 2000), behavioral problems (Weissman, Warner, Wickramaratne, & Kandel, 1999), and substance use in adolescence (Hoffmann & Su, 1998; Jacob et al., 2003; Kilpatrick et al., 2000).
Technology may facilitate the process of identifying and initially addressing drug use in the perinatal period, particularly given the clear challenges associated with person-delivered screening and brief intervention (SBI) in medical settings. For example, there are considerable time, financial, and logistic obstacles to integrating screening and brief intervention programs into ongoing medical practice (Aalto, Pekuri, & Seppa, 2003a; Beich, Gannik, & Malterud, 2002; Yarnall, Pollak, Ostbye, Krause, & Michener, 2003). Further, many medical professionals express discomfort with the screening and intervention process and report doubts about its effectiveness—even when voluntarily participating in a formal demonstration program (Beich, et al., 2002). This discomfort and skepticism may in part explain findings of very low levels of physician adherence to recommended brief intervention guidelines, even after training (Aalto, Pekuri, & Seppa, 2003b; DePue et al., 2002). Such challenges, documented with respect to single-focus SBI for alcohol or tobacco use, are likely to be exacerbated by attempts to address rarer and more stigmatized substances such as marijuana and cocaine.
Further, a number of recent systematic reviews and meta-analyses suggest that technology-delivered interventions are efficacious for substance abuse (Free et al., 2013; Moore, Fazzino, Garnet, Cutter, & Barry, 2011; Newman, Szkodny, Llera, & Przeworski, 2011b; Riper et al., 2011; Rooke, Thorsteinsson, Karpin, Copeland, & Allsop, 2010) as well as other mental health disorders (Newman, Szkodny, Llera, & Przeworski, 2011a) and health-related behaviors (e.g., Portnoy, Scott-Sheldon, Johnson, & Carey, 2008). For example, in a randomized trial with 107 postpartum women, Ondersma, Svikis, and Schuster (2007) showed that a single 20-minute e-SBI intervention plus two non-tailored mailings was associated with reductions in self-reported drug use at a 4-month follow-up.
The present study was designed to replicate the results of Ondersma et al. (2007) with respect to postpartum drug use. Replication studies are a crucial but greatly underutilized step in medical science (Ioannidis, 2012). We report here the results of a replication study, with a number of key extensions. First, the present study included a longer follow-up (6 months), allowing for better evaluation of the durability of any observed effects. Second, the present analysis used the Timeline Follow-Back (Sobell & Sobell, 1996) approach for evaluating days of drug use in the 90 days prior to each follow-up, rather than the simpler quantity-frequency approach used in the prior study. Third, the present study did not include the incentive for seeking treatment or the two motivational mailings used in the original study, thereby allowing for a more pure test of the e-SBI. In the present replication, a new and larger group of women reporting drug use in the post-partum period were randomly assigned to e-SBI vs. time control conditions, with concurrent trials for drug, alcohol, and tobacco use; only the drug use trial data are reported here. Drug use among study participants was re-evaluated 3- and 6-months following childbirth. We predicted less drug use in the e-SBI group as compared to the control group, as indicated by (a) greater abstinence per confirmed self-report of 7-day point-prevalence abstinence, and (b) fewer days of drug use in the 90 days preceding each follow-up assessment. Secondary outcomes included intervention-related differences in drug use consequences and in hair toxicology results at the 6-month follow-up.
2. Materials and Methods
2.1 Participants
Participants in this multi-site parallel-group randomized trial were 143 postpartum women, recruited during their inpatient hospitalization for childbirth at one of three hospitals in the Detroit area, all of which have only private rooms for childbirth and recovery. Exclusion criteria included inability to understand spoken English, frank cognitive impairment, and recent administration of prescription pain medication. Inclusion criteria included being age 18 or older, having slept since giving birth, and self-report of any illicit drug use in the month before becoming pregnant. The latter criterion has been shown in prior studies to be a far more sensitive and accurate predictor of verified drug use than a traditional screening tool (Grekin et al., 2010; Ondersma et al., 2012).
2.2 Procedure
Baseline data collection took place between January 16, 2008 and June 15, 2010, and follow-up data collection took place between April 16, 2008 and March 4, 2011, ending when overall recruitment goals were met. Women were approached in their private hospital rooms at one of three hospitals in Detroit, MI, and were given a brief description of the study. Those expressing potential interest were checked for preliminary eligibility (e.g., age, having slept). Women who remained eligible were then asked for verbal consent (using an information sheet) to complete an anonymous computer-delivered screen using a Tablet PC, with headphones for privacy. Participants received a small gift bag for their baby worth approximately $3 for completing the screener, regardless of the outcome of screening. All data collection procedures were approved by the Wayne State University IRB and by the Henry Ford Health System IRB. This trial was registered with ClinicalTrials.gov, NCT00685074.
Those who were eligible and provided informed consent were given the computer again, which presented an approximately 30-minute assessment and then randomly assigned participants in a 1:1 ratio to either the brief intervention or a time-control condition. Research assistants were thus blind to participant allocation to intervention vs. time control. Participants who completed the assessment and intervention or control process on the computer received a gift card for Target stores worth $40.
The research assistant then made arrangements for the two follow-up observations, which took place in the investigators' offices approximately 3- and 6-months post-baseline, and were conducted by a research assistant blind to experimental condition. Participants were contacted repeatedly by mail and later by phone following published tracking guidelines (Scott, 2004). They received a $75 gift certificate for completing the 3-month follow-up, assistance with transportation (either a $10 gas card or a taxi), and an additional $25 if they agreed to provide a urine sample. At the 6-month evaluation, in addition to assistance with transportation, participants received a $100 gift certificate plus an additional $25 for providing both urine and hair samples.
Given evidence of significant under-reporting of drug use among pregnant and post-partum women (in one study, 79.7% of postpartum women whose hair tested positive for illicit drugs denied any use in the past year; Grekin, et al., 2010), and of increased disclosure of stigmatized behaviors by postpartum women under conditions involving anonymity (Chase, Beatty, & Ondersma, 2011) we used a quasi-anonymous approach to protect participants in this study. In this approach, participant identifying information is collected in order to allow follow-up evaluations, but is not connected to responses via a linking table. Each participant's experimental condition (using a code for intervention vs. control to maintain blinding) was the only participant-level data to be connected to participants' names as well as their data, thus allowing longitudinal within-group comparisons between conditions. Although this approach has the disadvantage of precluding linking of individual cases over time, this drawback may be outweighed by increased accuracy of reporting (Chase, et al., 2011).
2.3 Measures
Most measures for this study were completed by the participant using the software and a Tablet PC. At baseline, participants completed the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST), a well-validated brief measure developed by the World Health Organization that evaluates frequency of use as well as consequences of use, separately for all categories of substances (Newcombe, Humeniuk, & Ali, 2005); the baseline ASSIST referred to drug use in the three months prior to pregnancy rather than the past three months, in order to promote disclosure and to establish a baseline more reflective of use when not pregnant. Participant baseline measures also included K6, a brief mental illness screener (Kessler et al., 2003) that measures general symptoms of distress over the past 30 days; and items relating to current relationships, demographics, and treatment history. Participants in the e-SBI condition also completed user experience/satisfaction ratings regarding their experience of using the software. These items, many of which have been used in previous research (Ondersma, Chase, Svikis, & Schuster, 2005), ask participants for ratings of the extent to which they found the software easy to use, respectful, helpful, likable, etc., on a 1–5 scale where 1 = not at all and 5 = very much. In this sample, these 9 items had an internal consistency (Cronbach's Alpha) of .78.
At each follow-up, participants completed the above measures (other than demographics and satisfaction items) as well as a computer-delivered Timeline Follow-Back (TLFB) interview evaluating drug use in the past week and past 90 days. In addition, participants provided urine samples at each follow-up evaluation, and provided 1.5 inch hair samples—giving an approximate 90-day window of drug use detection—at the 6-month evaluation only. Urine samples were tested by Redwood Toxicology Labs (Santa Rosa, CA) and hair samples were tested by Psychemedics, Inc. (Acton, MA) for evidence of cocaine, marijuana, amphetamine, or opiate use.
2.4 Intervention
The goal of the software used in this study was to facilitate self-change and/or treatment engagement regarding illicit drug use via a single 20-minute postpartum intervention session following Motivational Interviewing principles (Miller & Rollnick, 2002) to the extent possible, as well as the FRAMES brief intervention model (Miller, 1995; Miller, Zweben, DiClemente, & Rychtarik, 1994), with significant use of synchronous interactivity, user input, and empathic reflection. No keyboarding was required; all answers were provided by choosing responses from a list or by touching a visual analogue scale. Although many participants also smoked and/or met ASSIST criteria for a brief alcohol intervention, these substances were not addressed.
A mobile three-dimensional cartoon character capable of over 50 specific animated actions did the “talking” for the entire program. This character read each item for the participant, acted as narrator and guide throughout the process, and actively sought a non-judgmental, empathic, and non-threatening demeanor using reflections and self-deprecating humor. The experience of working with the software was intended to be highly interactive, with immediate responses to most input, occasional summaries, branching based on participant characteristics, responses, or preferences, and empathic reflections (e.g., “You really seem to feel two ways about this. On the one hand, you like the way that smoking marijuana helps you relax and have fun with your friends. But at the same time, you think you could really use that money on things for your baby”).
The overall intervention was broken down into components broadly focusing on (a) eliciting the participant's thoughts about change and their perceived advantages of doing so, if any; (b) reviewing feedback regarding how the participant's drug use compares to that of others, and of possible benefits of changing; and (c) optional goal-setting, including a menu of change options. The intervention allowed participant input (e.g., whether or not to see more information on a certain topic), and used different branches/approaches based on participant reports of current drug use and type of drug use, as well as on participants' stated plans regarding drug use after going home. Participants listened to the narrator via headphones to insure privacy. Earlier research with this platform showed very high acceptability (Ondersma, et al., 2005).
2.5 Control Condition
Control group participants completed the same assessment measures noted above. Then, in order to control for the time spent on the intervention, maintain blinding of research assistants, and mimic the interactivity of the intervention condition, participants assigned to the control condition were asked a number of questions about their preferences in music and television, were shown brief video clips consistent with their preferences, and were asked to provide feedback regarding their opinion of the various video clips. This relatively inactive control condition was intended to minimize, as much as possible in this two-arm study, the likelihood of assessment reactivity or of an unintended brief intervention effect.
2.6 Randomization
Participants were randomized by the software using simple randomization, stratified within substance use groups (marijuana vs. other illicit drug), to either intervention or time control conditions in a 1:1 ratio. Randomization by the software maintained research assistant blinding with respect to participant allocation into study conditions at the baseline data collection/intervention session.
2.7 Data Analysis
This study tested the efficacy of the e-SBI as reflected by 7-day point-prevalence abstinence at each follow-up point, per self-report (using a Timeline Follow-Back approach) and evidence of abstinence per toxicology tests (urine analysis at the 3-month follow-up, and urine plus hair analysis at the 6-month follow-up). Participants were considered abstinent only if they denied any drug use in the 7 days prior to the follow-up assessment, and also provided a urine sample that was negative for cocaine, amphetamines, marijuana, or opiates. As a co-primary outcome, this study also evaluated intervention effects on drug-using days in the 90 days prior to each follow-up. Power analyses suggested that a sample size of 140 was sufficient to detect a medium effect size. The primary outcome of 7-day point prevalence abstinence was evaluated at 3 and 6 months using either a χ2 test or Fisher's Exact test for variables with small numbers of events. The co-primary outcome of substance using days in the past 90 days was evaluated at 3- and 6-months using a non-parametric Wilcoxon Rank-Sum test. All analyses were conducted on an intent to treat basis considering participants in their originally assigned groups; loss to follow-up was handled by considering those lost to follow-up as positive for substance use in analyses of 7-day point prevalence, and also by simply conducting completer analysis, for comparison. Given the lack of an accepted standard for imputing missing data for continuous outcomes, as well as our use of a quasi-anonymous design (see below), the days of drug use outcome was analyzed only for participants who had completed follow-up.
Standardized effect sizes for point-prevalence were estimated via Odds Ratios and by Logit d, which was calculated using the formula where ln (OR) = the natural logarithm of the odds ratio, and π/3.5 = approximately 1.8138 (Hasselblad & Hedges, 1995). Effect sizes for days of use were calculated by where U is the Mann-Whitney statistic and na and nb are the sample sizes in the two groups (Grissom & Kim, 2012).
3.0 Results
3.1 Sample Characteristics
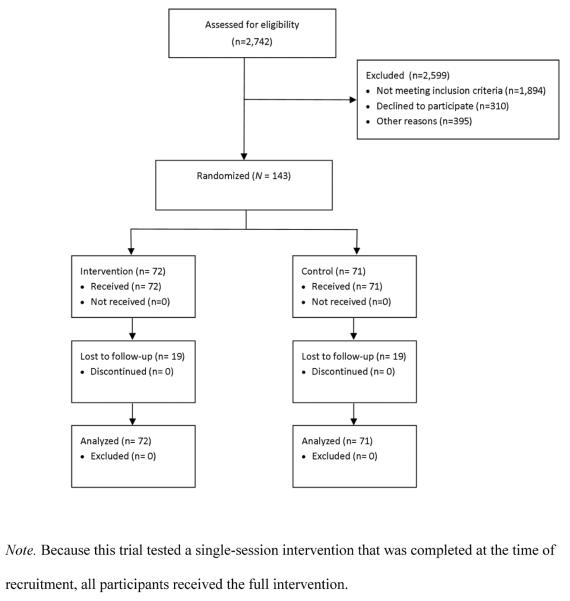
Participant flow is presented in Figure 1. A total of 2,742 participants were assessed for eligibility; 2,599 (94.8%) were excluded, with most of these (1,894, or 72.9%) being excluded due to denial of drug use and 310 (11.9%) declining to participate. Within the final sample of 143 participants, 72 were allocated to the e-SBI condition and 71 to the control condition. As seen in Table 1, participants were primarily African-American women, most of whom received some form of public assistance in the past year. As also seen in Table 1, the majority of participants did not report a prior history of either mental health or substance abuse treatment. A total of 110 (76.9%) participants scored at or above the ASSIST moderate risk range on the marijuana subscale, and 109 (76.2%) scored at or above the ASSIST moderate risk range on the alcohol subscale. Rates of moderate or higher risk for other substances were much lower (e.g., 9.1% for opiates, and 11% for cocaine). A total of 38 participants (26.6%) did not complete the 3-month follow-up, and 49 (34.3%) did not complete the 6-month evaluation. We did not find evidence that any participants experienced harm or unintended effects from participation in this study.
Figure 1.
Participant flow
Table 1.
Baseline sample characteristics (N = 143)
| Categorical variables (N, %) | Total sample (N = 143) | Control (n = 71) | Intervention (n = 72) | p |
|---|---|---|---|---|
| Hispanic ethnicity | 2 (1.5) | 1 (1.4) | 1 (1.6) | .957 |
| Race | .364 | |||
| African-American | 126 (90.6) | 66 (93.0) | 60 (88.2) | |
| White | 8 (5.8) | 4 (5.6) | 4 (5.9) | |
| Other | 5 (3.6) | 1 (1.4) | 4 (5.9) | |
| Receipt of food assistance | 126 (90.0) | 65 (91.5) | 61 (88.4) | .535 |
| High school graduate or higher | 84 (58.7) | 37 (52.1) | 47 (65.3) | .110 |
| Currently married | 10 (7.1) | 6 (8.5) | 4 (5.7) | .527 |
| Worked for pay in past 6 months | 64 (44.8) | 30 (42.3) | 34 (47.2) | .550 |
| Prior mental health medication | 27 (19.1) | 17 (23.9) | 10 (14.3) | .145 |
| Prior mental health counseling | 33 (23.4) | 17 (23.9) | 16 (22.9) | .879 |
| Prior treatment for alcohol use | 10 (7.2) | 7 (10.0) | 3 (4.3) | .197 |
| Prior treatment for drug use | 23 (16.5) | 13 (18.6) | 10 (14.5) | .518 |
| Daily/near daily marijuana use | 122 (86.5) | 61 (85.9) | 61 (87.1) | .831 |
| Pre-pregnancy binge drinking | 87 (61.7) | 42 (59.2) | 45 (64.3) | .531 |
| Continuous variables (N, SD) | ||||
| Age | 26.6 (6.0) | 27.1 (6.5) | 26.1 (5.6) | .365 |
| K6 mental illness screener | 21.4 (4.9) | 21.7 (5.5) | 21.2 (4.3) | .581 |
| ASSIST marijuana score | 14.4 (10.6) | 14.4 (10.5) | 14.5 (10.8) | .916 |
| ASSIST cocaine score | 1.9 (7.3) | 2.0 (7.7) | 1.7 (7.1) | .813 |
| ASSIST alcohol score | 18.4 (10.6) | 16.8 (11.3) | 20.0 (9.8) | .079 |
| ASSIST tobacco score | 13.1 (9.7) | 13.4 (9.5) | 12.9 (9.9) | .802 |
| ASSIST amphetamine score | 0.2 (1.7) | 0.2 (1.0) | 0.3 (2.2) | .706 |
| ASSIST opiates score | 2.0 (6.7) | 1.8 (6.6) | 2.2 (6.9) | .702 |
| ASSIST sedatives score | 1.1 (4.8) | 1.2 (5.0) | 0.9 (4.7) | .728 |
Note. ASSIST at baseline referred to drug use in the three months prior to pregnancy.
3.2 Success of Randomization
As seen in Table 1, no comparisons showed a significant difference at baseline between study arms. Of the 17 comparisons, only one—the ASSIST alcohol subscale score—yielded a value below .10, with participants in the intervention condition showing slightly higher scores on this measure.
3.3 Intervention Acceptability
The e-SBI was well-received. Using a 1–5 scale reflecting level of agreement (where 1 = “not at all” and 5 = “very much”), mean scores on key satisfaction items ranged from a low of 4.2 for whether the software resulted in reconsideration of marijuana/drug use, to highs of 4.9 for ease of use and respectfulness. A total of 61.2% of e-SBI participants indicated that they were more likely to change because of their interaction with the software. Of those asked, 55.8% said that they preferred using the software over talking with medical staff about their marijuana/drug use; 7.0% said they would have preferred working with medical staff, and 37.2% were unsure.
3.4 Intervention Effects—Primary Outcomes
As noted above, we analyzed data regarding to the first primary outcome (7-day point-prevalence abstinence) in two ways: presuming drug use among all of those lost to follow-up, and by completer analysis. As seen in Table 2, using the presumption of drug use among those lost to attrition, 19 intervention condition participants (26.4%) at the 3-month observation had been abstinent over the past 7 days, vs. 7 control group participants (9.9%); this difference was significant (χ2 [1] = 6.57, p = .01) with an OR of 3.3 (Table 2). At the 6-month follow-up, although participants in the e-SBI condition continued to be abstinent at a higher rate (13.9% vs. 9.9%; OR = 1.5), the difference was no longer significant (χ2 [1] = 0.55, p = .456). As also seen in Table 2, considering only participants who completed follow-up evaluation did not substantially change the strength of associations or the pattern of significance: 37.3% (e-SBI) vs. 13.7% (control) abstinence at the 3-month follow-up (χ2 [1] = 7.43, p = .006, OR = 3.7), and 21.7% (e-SBI) vs. 15.6% (control) at the 6-month follow-up (χ2 [1] = 0.57, p = .449, OR = 1.5).
Table 2.
Analysis of intervention effects on 7-day point-prevalence abstinence
| Observation | e-SBI (n, %) | Control (n, %) | OR (95% CI) | p | Logit d |
|---|---|---|---|---|---|
| Presuming those lost to follow-up are positive for drugs | |||||
| 3 month | 19 (26.4) | 7 (9.9) | 3.28 (1.3, 8.39) | .010 | 0.65 |
| 6 month | 10 (13.9) | 7 (9.9) | 1.47 (0.53, 4.12) | .456 | 0.21 |
| Restricting analyses to completers | |||||
| 3 month | 19 (37.3) | 7 (13.7) | 3.28 (1.3, 8.39) | .006 | 0.73 |
| 6 month | 10 (21.7) | 7 (15.6) | 1.47 (0.53, 4.12) | .449 | 0.23 |
Note. Abstinence at each observation was determined by self-report of use in the past 7 days, confirmed by urine drug screen. N = 143 for all analyses presuming drug use among those lost to follow-up; for completer analysis, N = 105 at 3 months and 94 at 6 months.
With regard to the second co-primary outcome, at the 3-month follow-up, participants in the intervention condition reported a median of 25.6 days of drug use in the past 90 days, vs. 51.4 for those in the control condition (d = .60); however, this difference failed to reach significance (p = .058). Participants in the intervention condition also reported fewer days of drug use at the 6-month follow-up (with a median of 31.6 vs. 77.2 days; d = .51), a difference that also failed to reach significance (p = .207).
3.5 Intervention Effects—Secondary Outcomes
Secondary outcomes from the ASSIST are presented in Table 3. As noted above, participants in this sample showed relatively low scores on this measure for substances other than marijuana, alcohol, and tobacco. Change in ASSIST subscales was modest and tended to show decreases over time from baseline through the two follow-up points. ASSIST subscale scores did not differ between groups.
Table 3.
Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) sub scale scores, by experimental condition
| Baseline |
3 months |
6 months |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| ASSIST subscale | Control | e-SBI | p | Control | e-SBI | p | Control | e-SBI | p |
| Alcohol | 16.8 | 20.0 | .079 | 15.6 | 15.3 | .766 | 15.6 | 15.1 | .906 |
| Marijuana | 14.4 | 14.5 | .916 | 10.4 | 8.6 | .141 | 12.1 | 10.1 | .355 |
| Tobacco | 13.4 | 12.9 | .802 | 13.7 | 13.7 | .936 | 12.0 | 12.1 | .581 |
| Opiates | 1.8 | 2.2 | .702 | 1.0 | 1.9 | .836 | 1.4 | 1.5 | .896 |
| Cocaine | 2.0 | 1.7 | .813 | 0.9 | 0.7 | .388 | 1.9 | 0.6 | .379 |
| Sedatives | 1.2 | 0.9 | .728 | 0.3 | 0.8 | .958 | 0.6 | 0.5 | .900 |
| Amphetamines | 0.2 | 0.3 | .706 | 0.1 | 1.0 | .306 | 0.2 | 0.3 | .957 |
Note. ASSIST at baseline referred to drug use in the three months prior to pregnancy. Significance was tested using Mann-Whitney U tests. N = 143 at baseline, 105 at 3 months, and 94 at 6 months.
Hair toxicology tests from the 6-month follow-up were also treated as a secondary outcome. Within the 76 available cases (valid hair samples were provided by 38 participants from each condition, or 80.9% of the 94 participants who completed 6-month follow-up assessment), 11 samples (28.9%) from the intervention group were negative for all drugs, vs. 3 (7.9%) from the control group (χ2 [1] = 5.6, p = .018, OR = 4.8, Logit d = 0.86).
4. Discussion
This study sought to replicate previous findings that were supportive of the efficacy of an e-SBI for postpartum drug use, and extended the original study by following participants to 6 months, using TLFB to measure drug-using days, and using only the e-SBI without any incentives or motivational mailings. Results support the short-term efficacy of the e-SBI for illicit drug use in this urban sample, with a biologically confirmed drug use abstinence rate of 26.4% in the intervention condition vs. 9.9% in the control condition. Three-month effect sizes in the present study are similar to those found in Ondersma et al., 2007: Logit d for the primary dichotomous outcome in the 2007 study was .48, compared to .65 in the present study. Similarly, the Cohen's d effect size for drug use frequency was .46 in the 2007 report (using a single item), compared to .60 in the current study (using a TLFB interview). Further, although only provided by a subset of participants at the 6-month follow-up, results from hair toxicology testing also suggested that abstinence was higher—in the 90 days prior to the 6-month observation—in participants who had received the e-SBI. However, rates of 7-day point-prevalence abstinence (as measured by self-report and confirmed by urine analyses) decreased by the 6-month observation and were no longer significant. Further, none of the analyses of days of drug use were statistically significant despite modest effects in the expected direction, and the intervention appears to have had no effect on drug use consequences as measured by ASSIST subscales. As with the prior study, the intervention was seen by participants as easy to use, helpful, and relevant; most participants said that they preferred the software over talking directly with medical staff.
The present findings are consistent with the larger literature on technology-based interventions for problem substance use, which suggests that the effects of such interventions are not large and often fail to reach significance, but nevertheless show positive aggregate effects in meta-analyses. For example, in the present study, the non-significant effect sizes ranged from d = .22 to .37 in favor of the e-SBI. Effect sizes from recent meta-analyses of technology-based interventions for problem substance use, all of which are statistically significant, include d = .20 (Rooke, et al., 2010), g = .44 (Riper, et al., 2011), d = .22 (Riper et al., 2009); and d = .24 (Portnoy, et al., 2008). Although generally in the small range, it is important to emphasize that effect sizes cannot be considered alone, but must be weighed against the overall cost and potential reach of the intervention. Brief interventions, particularly as delivered via technology, are designed to reach high proportions of at-risk populations with no degradation in fidelity when moving from research to practice, and as such may have substantial public health importance even when effect sizes are small. The high acceptability of these interventions could facilitate their widespread implementation in primary care and other medical settings.
The decline of the initially positive effects with respect to illicit drug use is consistent with other findings suggesting that effects for motivational interventions are often stronger in the short term (Hettema, Steele, & Miller, 2005; Vasilaki, Hosier, & Cox, 2006). As noted by McLellan (2002), medical treatments for many chronic conditions such as hypertension or diabetes are quite effective, but are not expected to continue to have an effect after treatment is removed. McLellan goes on to suggest that a continuing care approach, involving palatable treatment, ongoing monitoring, and integration of substance abuse services with health care, may be a more appropriate response than a single episode of treatment. Technology-based interventions could be an important part of such an approach: they can provide ongoing monitoring as part of ongoing health care, and can provide repeated interventions—always targeted at the behavior or behaviors that are most relevant at that visit, incorporating patient input regarding the style and focus of the intervention—and can help link patients to specialized services as needed. The clear willingness of participants to work with the software again supports the idea of repeated, spaced exposure to e-SBI.
Brief interventions, including computer-delivered brief interventions, have never been proposed as a replacement for extended treatment for those who want and need such treatment. Rather, they are seen as a way to (a) reach those who, despite needing treatment, neither receive nor want it (in 2011, this was 18.4 million persons, or 85.1% of all those needing treatment; Substance Abuse and Mental Health Services Administration, 2012); (b) promote treatment-seeking among those who could benefit from it; and (c) promote self-change among those whose substance use does not meet criteria for abuse or dependence, but nevertheless presents a health risk. Far from being in conflict with more intensive services, brief screening and intervention can promote identification and treatment-seeking among those who need it.
Point-prevalence data revealed an overall increase in drug use between the 3- and 6-month follow-up evaluations, except for 7-day point-prevalence abstinence in the control condition, which was unchanged between 3- and 6-months. This latter trend is in contrast to the tendency for substance use to increase throughout the first year postpartum (Office of Applied Studies, 2009), and may suggest a therapeutic effect of the extended 3-month assessment. This is consistent with evidence that extended research-related assessment and even mere study involvement can result in decreases in substance use (e.g., Epstein et al., 2005; Kypri, Langley, Saunders, & Cashell-Smith, 2007; McCambridge & Kypri, 2011; Ondersma, Winhusen, & Lewis, 2012), and that health-related behavior change may often be more sudden than gradual (Resnicow & Page, 2008). Although some studies have failed to find evidence for pre-treatment change (e.g., Daeppen et al., 2007), a screen-only control condition may be crucial to rigorously evaluating brief intervention effects (Donovan et al., 2012).
The present panoply of evidence-based, therapist-delivered interventions for problem substance use is the result of decades of research and theoretical evolution. Technology-delivered interventions are only just now gaining the critical mass needed to support meta-analyses and component analyses that can help optimize efficacy (e.g., Brouwer et al., 2011; Lehto & Oinas-Kukkonen, 2011; Lustria, Cortese, Noar, & Glueckauf, 2009; Morrison, Yardley, Powell, & Michie, 2012; Webb, Joseph, Yardley, & Michie, 2010). Although this is a critical milestone in the evolution of technology-delivered interventions, their development has in many ways only just begun. Their modularity and replicability may facilitate a unique opportunity for cumulative progress as work in this area continues. As one example of this, Webb et al. (2010) found that the efficacy of internet-delivered interventions can be enhanced through the use of subsequent text messaging. Such follow-up messaging can easily make use of data gleaned during the brief intervention (e.g., goals, concerns, motivating factors) and can reach patients in their natural environment using a widely available technology. Further research regarding augmentation of e-SBI interventions via text messaging is necessary, and is but one way in which research in this area can and should evolve.
Several limitations of this study should be noted. First, overall loss to follow-up ranged from 27% (at 3 months) to 34% (at 6 months) for this hard to follow population, and sample sizes within each substance use group were relatively small, limiting generalizability. The sample also primarily consisted of lower-income African-American women, thus potentially limiting relevance outside of similar populations. Second, as noted earlier, we believe that evidence of under-reporting in this population is significant enough to justify use of the quasi-anonymous approach, in which there is no link between participant data and identifying information such as names (thus potentially increasing the validity of self-report data). However, an important weakness of the quasi-anonymous approach is the inability to link subjects at the different time points and thus utilize a more powerful repeated measures analysis which would enable us to model both between subject (condition), within subject (time), and group by time interaction effects. Finally, it should be emphasized that although this study included participants regardless of the type of illicit drug reported, marijuana was the most commonly used drug. Although marijuana accounts for more abuse and dependence than any other drug, and is clearly an important topic for research and public health efforts, these results may not generalize to samples with different drug use patterns.
Despite these limitations, the current findings provide support for the previous trial (Ondersma, et al., 2007) and suggest that an e-SBI for drug use following pregnancy is feasible as well as efficacious in the short term. These findings and the observed effect sizes are consistent with the large and growing literature on e-Health interventions for substance use and other health-related behaviors. Future research should examine the ability of similar technology to screen for and address one or more of a broad range of behavioral targets, particularly in a sequential manner throughout healthcare settings. Future research should also carefully evaluate the relative strengths and weaknesses of e-SBI vs. traditional SBI, in terms of effectiveness and cost as well as reach (see Meyer et al., 2012 for an excellent example of reach analysis). Analysis of all three of these key outcomes can facilitate the all-important goal of population-wide decreases in drug use in the perinatal period.
Acknowledgements
This research was supported by NIH award DA021329 to Dr. Ondersma, and by Joseph Young, Sr. funds from the State of Michigan to Wayne State University. Dr. Thacker's efforts were supported in part by NIH award UL1TR00058 from The National Center for Advancing Translational Sciences. Dr. Ondersma is also part owner of Interva, Inc., the company that markets the intervention authoring tool used to develop the software used for this study. Neither NIH nor Interva, Inc. had any role in data analysis or write-up of results.
The authors also wish to gratefully acknowledge the invaluable assistance of the women who participated in this study, the medical staff from Hutzel Women's Hospital, Sinai-Grace Hospital, and the Henry Ford Health System who supported recruitment and data collection, and the data collection efforts of Nerissa Germain, Veronica Gorden, Lorna Mabunda, and Veronica Connors-Burge. Finally, none of this work would have been possible without the invaluable mentorship of the late Dr. Charles R. Schuster.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aalto M, Pekuri P, Seppa K. Obstacles to carrying out brief intervention for heavy drinkers in primary health care: a focus group study. Drug Alcohol Rev. 2003a;22(2):169–173. doi: 10.1080/09595230100100606. [DOI] [PubMed] [Google Scholar]
- Aalto M, Pekuri P, Seppa K. Primary health care professionals' activity in intervening in patients' alcohol drinking during a 3-year brief intervention implementation project. Drug Alcohol Depend. 2003b;69(1):9–14. doi: 10.1016/s0376-8716(02)00228-4. [DOI] [PubMed] [Google Scholar]
- Beich A, Gannik D, Malterud K. Screening and brief intervention for excessive alcohol use: qualitative interview study of the experiences of general practitioners. Bmj. 2002;325(7369):870. doi: 10.1136/bmj.325.7369.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besinger BA, Garland AF, Litrownik AJ, Landsverk JA. Caregiver substance abuse among maltreated children placed in out-of- home care. Child Welfare. 1999;78(2):221–239. [PubMed] [Google Scholar]
- Blumenthal SJ. Women and substance abuse: A new national focus. In: Wetherington CL, Roman Adele B., editors. Drug Addiction Research and the Health of Women. U.S. Department of Health and Human Services/National Institutes of Health; Rockville, MD: 1998. pp. 13–32. [Google Scholar]
- Brouwer W, Kroeze W, Crutzen R, de Nooijer J, de Vries NK, Brug J, Oenema A. Which intervention characteristics are related to more exposure to internet-delivered healthy lifestyle promotion interventions? A systematic review. [Review] J Med Internet Res. 2011;13(1):e2. doi: 10.2196/jmir.1639. doi: 10.2196/jmir.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaffin M, Kelleher K, Hollenberg J. Onset of physical abuse and neglect: psychiatric, substance abuse, and social risk factors from prospective community data. Child Abuse & Neglect. 1996;20(3):191–203. doi: 10.1016/s0145-2134(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Chase SK, Beatty JR, Ondersma SJ. A randomized trial of the effects of anonymity and quasi anonymity on disclosure of child maltreatment-related outcomes among postpartum women. Child Maltreat. 2011;16(1):33–40. doi: 10.1177/1077559510387659. [DOI] [PubMed] [Google Scholar]
- Daeppen JB, Gaume J, Bady P, Yersin B, Calmes JM, Givel JC, Gmel G. Brief alcohol intervention and alcohol assessment do not influence alcohol use in injured patients treated in the emergency department: a randomized controlled clinical trial. Addiction. 2007;102(8):1224–1233. doi: 10.1111/j.1360-0443.2007.01869.x. doi: 10.1111/j.1360-0443.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- DePue JD, Goldstein MG, Schilling A, Reiss P, Papandonatos G, Sciamanna C, Kazura A. Dissemination of the AHCPR clinical practice guideline in community health centres. Tob Control. 2002;11(4):329–335. doi: 10.1136/tc.11.4.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, Bogenschutz MP, Perl H, Forcehimes A, Adinoff B, Mandler R, Walker R. Study design to examine the potential role of assessment reactivity in the Screening, Motivational Assessment, Referral, and Treatment in Emergency Departments (SMART-ED) protocol. Addict Sci Clin Pract. 2012;7:16. doi: 10.1186/1940-0640-7-16. Epstein, E. E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapkin ML, Yusko DA, Cook SM, McCrady BS, Jensen NK. Is alcohol assessment therapeutic? Pretreatment change in drinking among alcohol-dependent women. J Stud Alcohol. 2005;66(3):369–378. doi: 10.15288/jsa.2005.66.369. [DOI] [PubMed] [Google Scholar]
- Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, Haines A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10(1):e1001362. doi: 10.1371/journal.pmed.1001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen PJ. Environmental tobacco smoke as a risk factor for respiratory disease in children. Respir Physiol. 2001;128(1):39–46. doi: 10.1016/s0034-5687(01)00263-8. [DOI] [PubMed] [Google Scholar]
- Grekin ER, Svikis DS, Lam P, Connors V, Lebreton JM, Streiner DL, Ondersma SJ. Drug use during pregnancy: validating the Drug Abuse Screening Test against physiological measures. Psychol Addict Behav. 2010;24(4):719–723. doi: 10.1037/a0021741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissom RJ, Kim JJ. Effect Sizes for Research: Univariate and Multivariate Applications. 2nd Edition Routledge/Taylor & Francis; New York, NY: 2012. [Google Scholar]
- Hasselblad V, Hedges LV. Meta-analysis of screening and diagnostic tests. Psychological Bulletin. 1995;117:167–178. doi: 10.1037/0033-2909.117.1.167. [DOI] [PubMed] [Google Scholar]
- Hettema J, Steele J, Miller WR. Motivational Interviewing. Annual Review of Clinical Psychology. 2005;1:91–111. doi: 10.1146/annurev.clinpsy.1.102803.143833. [DOI] [PubMed] [Google Scholar]
- Hoffmann JP, Su SS. Parental substance use disorder, mediating variables and adolescent drug use: a non-recursive model. Addiction. 1998;93(9):1351–1364. doi: 10.1046/j.1360-0443.1998.93913516.x. [DOI] [PubMed] [Google Scholar]
- Ioannidis JP. Why science is not necessarily self-correcting. Perspectives on Psychological Science. 2012;7:645–654. doi: 10.1177/1745691612464056. [DOI] [PubMed] [Google Scholar]
- Jacob T, Waterman B, Heath A, True W, Bucholz KK, Haber R, Fu Q. Genetic and Environmental Effects on Offspring Alcoholism: New Insights Using an Offspring-of-Twins Design. Arch Gen Psychiatry. 2003;60(12):1265–1272. doi: 10.1001/archpsyc.60.12.1265. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, Zaslavsky AM. Screening for serious mental illness in the general population. Arch Gen Psychiatry. 2003;60(2):184–189. doi: 10.1001/archpsyc.60.2.184. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. J Consult Clin Psychol. 2000;68(1):19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- Kissin WB, Svikis DS, Morgan GD, Haug NA. Characterizing pregnant drug-dependent women in treatment and their children. J Subst Abuse Treat. 2001;21(1):27–34. doi: 10.1016/s0740-5472(01)00176-3. [DOI] [PubMed] [Google Scholar]
- Kypri K, Langley JD, Saunders JB, Cashell-Smith ML. Assessment may conceal therapeutic benefit: findings from a randomized controlled trial for hazardous drinking. Addiction. 2007;102(1):62–70. doi: 10.1111/j.1360-0443.2006.01632.x. [DOI] [PubMed] [Google Scholar]
- Lehto T, Oinas-Kukkonen H. Persuasive features in web-based alcohol and smoking interventions: a systematic review of the literature. J Med Internet Res. 2011;13(3):e46. doi: 10.2196/jmir.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustria ML, Cortese J, Noar SM, Glueckauf RL. Computer-tailored health interventions delivered over the Web: review and analysis of key components. [Review] Patient Educ Couns. 2009;74(2):156–173. doi: 10.1016/j.pec.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Mahabee-Gittens M. Smoking in parents of children with asthma and bronchiolitis in a pediatric emergency department. Pediatr Emerg Care. 2002;18(1):4–7. doi: 10.1097/00006565-200202000-00002. [DOI] [PubMed] [Google Scholar]
- McBride CM, Emmons KM, Lipkus IM. Understanding the potential of teachable moments: the case of smoking cessation. Health Educ Res. 2003;18(2):156–170. doi: 10.1093/her/18.2.156. [DOI] [PubMed] [Google Scholar]
- McCambridge J, Kypri K. Can simply answering research questions change behaviour? Systematic review and meta analyses of brief alcohol intervention trials. PLoS One. 2011;6(10):e23748. doi: 10.1371/journal.pone.0023748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT. Have we evaluated addiction treatment correctly? Implications from a chronic care perspective. Addiction. 2002;97(3):249–252. doi: 10.1046/j.1360-0443.2002.00127.x. [DOI] [PubMed] [Google Scholar]
- Meyer C, Ulbricht S, Gross B, Kastel L, Wittrien S, Klein G, John U. Adoption, reach and effectiveness of computer-based, practitioner delivered and combined smoking interventions in general medical practices: a three-arm cluster randomized trial. Drug Alcohol Depend. 2012;121(1–2):124–132. doi: 10.1016/j.drugalcdep.2011.08.019. [DOI] [PubMed] [Google Scholar]
- Miller WR. Increasing motivation for change. In: Hester RK, Miller WR, editors. Handbook of alcoholism treatment approaches: Effective alternatives. 2nd ed. Allyn & Bacon; Needham Heights, MA, US: 1995. pp. 89–104. [Google Scholar]
- Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. Second ed. Guilford; New York: 2002. [Google Scholar]
- Miller WR, Zweben A, DiClemente CC, Rychtarik RG. Motivational Enhancement Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence. Vol. 2. National Institute on Alcohol Abuse and Alcoholism; Rockville, MD: 1994. [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Jama. 2004;291(10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Moore BA, Fazzino T, Garnet B, Cutter CJ, Barry DT. Computer-based interventions for drug use disorders: a systematic review. J Subst Abuse Treat. 2011;40(3):215–223. doi: 10.1016/j.jsat.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison LG, Yardley L, Powell J, Michie S. What design features are used in effective e-health interventions? A review using techniques from Critical Interpretive Synthesis. Telemed J E Health. 2012;18(2):137–144. doi: 10.1089/tmj.2011.0062. [DOI] [PubMed] [Google Scholar]
- Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24(3):217–226. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- Newman MG, Szkodny LE, Llera SJ, Przeworski A. A review of technology-assisted self-help and minimal contact therapies for anxiety and depression: is human contact necessary for therapeutic efficacy? Clin Psychol Rev. 2011a;31(1):89–103. doi: 10.1016/j.cpr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Newman MG, Szkodny LE, Llera SJ, Przeworski A. A review of technology-assisted self-help and minimal contact therapies for drug and alcohol abuse and smoking addiction: is human contact necessary for therapeutic efficacy? Clin Psychol Rev. 2011b;31(1):178–186. doi: 10.1016/j.cpr.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Office of Applied Studies Substance Use among Women During Pregnancy and Following Childbirth. The NSDUH Report. 2009 from http://www.samhsa.gov/data/2k9/135/PregWoSubUse.htm.
- Ondersma SJ. Predictors of neglect within low-SES families: the importance of substance abuse. Am J Orthopsychiatry. 2002;72(3):383–391. doi: 10.1037/0002-9432.72.3.383. [DOI] [PubMed] [Google Scholar]
- Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer-based brief motivational intervention for perinatal drug use. J Subst Abuse Treat. 2005;28(4):305–312. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Delaney-Black V, Covington CY, Nordstrom B, Sokol RJ. The association between caregiver substance abuse and self-reported violence exposure among young urban children. J Trauma Stress. 2006;19(1):107–118. doi: 10.1002/jts.20105. [DOI] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, LeBreton JM, Streiner DL, Grekin ER, Lam PK, Connors-Burge V. Development and preliminary validation of an indirect screener for drug use in the perinatal period. Addiction. 2012;107(12):2099–2106. doi: 10.1111/j.1360-0443.2012.03982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women. Am J Prev Med. 2007;32(3):231–238. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondersma SJ, Winhusen T, Lewis DF. Pre-treatment change in a randomized trial with pregnant substance-abusing women in community-based outpatient treatment. Contemp Clin Trials. 2012;33(5):1074–1079. doi: 10.1016/j.cct.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy DB, Scott-Sheldon LA, Johnson BT, Carey MP. Computer-delivered interventions for health promotion and behavioral risk reduction: a meta-analysis of 75 randomized controlled trials, 1988–2007. Prev Med. 2008;47(1):3–16. doi: 10.1016/j.ypmed.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz HZ, Giaconia RM, Hauf AM, Wasserman MS, Paradis AD. General and specific childhood risk factors for depression and drug disorders by early adulthood. J Am Acad Child Adolesc Psychiatry. 2000;39(2):223–231. doi: 10.1097/00004583-200002000-00023. [DOI] [PubMed] [Google Scholar]
- Resnicow K, Page SE. Embracing chaos and complexity: a quantum change for public health. American Journal of Public Health. 2008;98(8):1382–1389. doi: 10.2105/AJPH.2007.129460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riper H, Spek V, Boon B, Conijn B, Kramer J, Martin-Abello K, Smit F. Effectiveness of E-self-help interventions for curbing adult problem drinking: a meta-analysis. J Med Internet Res. 2011;13(2):e42. doi: 10.2196/jmir.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riper H, van Straten A, Keuken M, Smit F, Schippers G, Cuijpers P. Curbing problem drinking with personalized-feedback interventions: a meta-analysis. American Journal of Preventive Medicine. 2009;36(3):247–255. doi: 10.1016/j.amepre.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Rooke S, Thorsteinsson E, Karpin A, Copeland J, Allsop D. Computer-delivered interventions for alcohol and tobacco use: a meta-analysis. Addiction. 2010;105(8):1381–1390. doi: 10.1111/j.1360-0443.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- Scott CK. A replicable model for achieving over 90% follow-up rates in longitudinal studies of substance abusers. [Research Support, U.S. Gov't, P.H.S.] Drug Alcohol Depend. 2004;74(1):21–36. doi: 10.1016/j.drugalcdep.2003.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline FollowBack: A Calendar Method for Assessing Alcohol and Drug Use. Addiction Research Foundation; Toronto, Ontario: 1996. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health. Office of Applied Studies; Rockville, MD: 2012. pp. 12–4713. (NSDUH Series H-44). HHS Publication No. (SMA) [Google Scholar]
- Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol Alcohol. 2006;41(3):328–335. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- Webb TL, Joseph J, Yardley L, Michie S. Using the internet to promote health behavior change: a systematic review and meta-analysis of the impact of theoretical basis, use of behavior change techniques, and mode of delivery on efficacy. J Med Internet Res. 2010;12(1):e4. doi: 10.2196/jmir.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Warner V, Wickramaratne PJ, Kandel DB. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry. 1999;38(7):892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93(4):635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]