Abstract
NOD.H-2h4 mice given NaI in their drinking water develop iodine-accelerated spontaneous autoimmune thyroiditis (ISAT) with chronic inflammation of the thyroid by T and B cells and production of anti-mouse thyroglobulin (MTg) autoantibody. CD28−/− NOD.H-2h4 mice, which have reduced numbers of CD4+FoxP3+ regulatory T cells (Treg), were developed to examine the role of Treg in ISAT development. CD28−/− NOD.H2-h4 mice develop more severe ISAT than wild-type (WT) mice, with collagen deposition (fibrosis) and low serum T4. CD28−/− mice have increased expression of proinflammatory cytokines IFNγ and IL-6 consistent with increased mononuclear cell infiltration and tissue destruction in thyroids. Importantly, transferring purified CD4+FoxP3+ Treg from WT mice reduces ISAT severity in CD28−/− mice without increasing the total number of Treg, suggesting that endogenous Treg in CD28−/− mice are functionally ineffective. Endogenous CD28−/− Treg have reduced surface expression of CD27, TNFR2 p75, and Glucocorticoid-induced TNFR-related protein (GITR) compared to transferred CD28+/+ Treg. Although anti-MTg autoantibody levels generally correlate with ISAT severity scores in WT mice, CD28−/− mice have lower anti-MTg autoantibody responses than WT mice. The percentages of follicular B-cells are decreased and marginal zone B cells increased in spleens of CD28−/− mice, and they have fewer thyroid-infiltrating B cells than WT mice. This suggests that CD28 deficiency has direct and indirect effects on the B cell compartment. B-cell deficient (B−/−) NOD.H-2h4 mice are resistant to ISAT, but CD28−/−B−/− mice develop ISAT comparable to WT mice and have reduced numbers of Treg compared to WT B−/− mice.
Keywords: Treg, autoimmunity, CD28
Introduction
NOD.H-2h4 mice given NaI in their drinking water develop iodine-accelerated spontaneous autoimmune thyroiditis (ISAT) (1–4). ISAT is characterized by infiltration of the thyroid by T and B cells, with destruction of thyroid follicles and production of antibodies to mouse thyroglobulin (MTg) (1, 4, 5). Although B-cell deficient (B−/−) mice are resistant to ISAT, they develop ISAT after transient depletion of CD4+CD25+ regulatory T cells (Treg) (6, 7), suggesting an important role for Treg in ISAT. Our earlier studies indicated that transient depletion of CD25+ cells in which CD4+CD25+ Treg were depleted for 7–10 days had little effect on subsequent ISAT severity scores in wild-type (WT) NOD.H-2h4 mice (7), but Treg depleted WT mice had increased anti-MTg autoantibody responses compared to controls (our unpublished results). Others have shown that more prolonged Treg depletion in which anti-CD25 antibody was administered repeatedly to maintain Treg depletion for more than 3 weeks in WT NOD.H-2h4 mice resulted in more severe ISAT and increased production of proinflammatory cytokines (8). In addition, Treg depletion for >3 wk in ISAT resistant IL-17 deficient mice resulted in susceptibility to ISAT (9). These results suggest that Treg play an important role in ISAT, but depletion for at least several weeks is needed to reveal their role.
CD28 signaling is important for the development and peripheral homeostasis of CD4+CD25+ Treg (10). CD28 costimulation promotes IL-2 production by conventional T cells, and IL-2 is important for Treg survival (11). CD28-deficient mice have reduced numbers of CD4+CD25+ Treg, and CD28−/− NOD mice develop earlier and more severe diabetes than WT NOD mice (12, 13). CD28 was originally described as an important costimulator of T cell activation (14, 15). CD28 signaling is important for activation of naïve T cells following their interaction with APCs presenting foreign antigens (15), and for induction of most experimentally induced models of autoimmune disease including thyroiditis (13, 16–18)(our unpublished observations). However, NOD mice lacking CD28 develop spontaneous autoimmune diseases, such as diabetes and autoimmune pancreatitis (10, 13, 15, 16, 19), indicating that CD28/B7 interactions are not required for activation of autoreactive T cells in a Treg deficient environment and in mice with a genetic predisposition to develop autoimmune disease (13, 16). The reasons for the differences in requirements for development of experimentally induced vs. spontaneous autoimmune diseases are not known, but may be because CD28 costimulation is less critical when there is chronic stimulation by self antigen, or because other costimulatory molecules are used in spontaneous autoimmune diseases (10, 13, 16, 20).
Since NOD.H-2h4 mice are closely related to NOD mice that develop diabetes, we hypothesized that an early permanent deficiency in Treg, as in NOD mice (10, 13, 16), would lead to increased activation of autoreactive effector CD4+ T cells and increased ISAT severity in WT and B−/− CD28−/− NOD.H-2h4 mice. CD28−/− NOD.H-2h4 mice were developed to test this hypothesis. The results presented here suggest that in addition to having reduced Treg compared to WT NOD.H-2h4 mice, CD28-deficient mice have Treg that are less effective at suppressing autoimmune thyroiditis. Also of note, B cell function and/or the effectiveness of T cell help were affected by the lack of CD28.
Materials and Methods
Mice
NOD.H-2h4 mice express H-2Kk, I-Ak, and Db on the NOD background (21). Mice were bred and maintained in the animal facility at the University of Missouri. All animal protocols were approved by the University of Missouri Animal Care and Use Committee. CD28−/− NOD male mice, obtained from Jackson Laboratories (Bar Harbor, ME), were crossed with WT NOD.H-2h4 females. The F1 mice were crossed and F2 mice were selected for expression of the NOD.H-2h4 MHC by flow cytometry and for deficiency of CD28 by PCR of tail DNA using the primer sequences and protocol provided on the Jackson Laboratories web site. CD28−/− NOD.H-2h4 WT mice were crossed with B−/− NOD.H-2h4 mice to generate CD28−/−B−/− NOD.H-2h4 mice. FoxP3-GFP NOD.H-2h4 mice, were used for sorting and transfer of CD28+ Treg. NOD.FoxP3:GFP mice (22) were crossed with WT or B−/− NOD.H-2h4 mice. F1 mice were crossed and F2 mice were selected for expression of NOD.H-2h4 MHC by flow cytometry and for homozygosity of the FoxP3:GFP reporter gene in females and the presence of the gene in males by PCR of tail DNA.
Assessment of thyroiditis
At 8 wks of age, mice were given 0.08% NaI in their drinking water. Thyroids were removed 8–9 wk later, and one thyroid lobe from each mouse was fixed in formalin, sectioned, and stained with hematoxylin and eosin as described previously (1, 5). Thyroid destruction and inflammatory cell infiltration was scored using a scale of 0 to 4+ as described previously (1, 4, 5). A score of 0 indicates a normal thyroid. A 1+ is defined as having one or several foci consisting of at least 125 cells. Thyroids having 10–20 larger foci of cellular infiltration, with destruction of up to one fourth of the gland are given a score of 2+. A 3+ score indicates that one fourth to one half of the thyroid follicles are destroyed or replaced by infiltrating inflammatory cells, and a 4+ score indicates that greater than one half of the gland is replaced by infiltrating inflammatory cells. Thyroid lesions in NOD.H-2h4 mice reach maximal severity 8 weeks after mice are given NaI in their drinking water beginning at 2 months of age (1, 5).
Autoantibody determination
MTg-specific IgG autoantibodies were determined by ELISA using serum from individual mice diluted 1/50 or 1/100 as previously described (23).
Serum T4 determination
Serum thyroxine (T4) levels were determined by ELISA as previously described using Leinco T4 ELISA test kit (Leinco, St. Louis, MO) (24). Results are expressed as μg T4/dL serum. Values for normal mouse serum range from 4 to 8 μg T4/dL, and values >3 are considered normal (25).
Isolation and transfer of Treg
Splenocytes from FoxP3-GFP NOD.H-2h4 mice were incubated with anti-CD4 (RM4-5 APC) (eBioscience, San Diego, CA) for 30 min at 4°C. The cells were then washed and sorted using the DAKO MoFlo XDP cell sorter (Beckman Coulter Inc, Fullerton, CA). CD28−/− NOD.H-2h4 recipients were given 106 purified CD4+GFP+(FoxP3+) Treg i.v. every two weeks for a total of three injections. Controls were given sorted CD4+GFP−(FoxP3−) T cells i.v. every two weeks for a total of three injections. Mice were irradiated (300 Gy) using an X-RAD 320 Irradiator (Precision X-ray, North Branford, CT) prior to the first transfer. Mice were given NaI water starting at the time of the first cell transfer, and thyroids were removed after 7 wks.
Isolation of thyroid-infiltrating cells and intracellular cytokine staining
Thyroid-infiltrating cells were isolated from single thyroid lobes of individual mice by treating thyroids with Liberase (0.08 U/ml) (Roche, Indianapolis, IN) for 45 min at 37°C. For intracellular analysis of cytokine production by flow cytometry, thyroid-infiltrating cells were isolated and stimulated with PMA (50 ng/ml) and ionomycin (1 μM)(Alexis Biochemicals, San Diego, CA) plus LPS (10 μg/ml) for 12 hours. Brefeldin A (1 μg/ml) was added after 4 hours of stimulation. Cells from WT or CD28−/− NOD.H-2h4 mice were then incubated (1 × 106 cells/100μl) with antibodies against CD45 (30-F11, FITC) and CD3 specific antibody (RA3-6B2, APC) or with isotype control as previously described (26). Cells were washed and then fixed and permeabilized using the eBioscience FoxP3 buffer set (eBioscience, San Diego, CA) and stained for IFNγ (XMG1.2, PE). Cells were washed and the data collected using the DAKO CyAN flow cytometer and analyzed using Summit software version 5.2 (Beckman Coulter Inc, Fullerton, CA). Antibodies for flow cytometry were purchased from eBioscience and BioLegend (San Diego, CA)
Cell surface and FoxP3 staining for flow cytometry
For determination of Treg cell numbers, cells from spleens or cervical lymph nodes of WT and CD28−/− NOD.H-2h4 mice were incubated (1 × 106 cells/100μl) with antibodies against CD4 (RM4-5 PerCP-Cy5.5) and CD25 (PC61 FITC) or isotype control antibody for 30 min at 4°C, then stained for FoxP3 (FJK-16s APC) using the eBioscience Mouse Regulatory T Cell Staining Kit (eBioscience). Cells were washed and the data collected using the DAKO CyAN flow cytometer and analyzed using Summit software version 5.2. For characterization of Treg in recipients of sorted Treg or control cells, splenocytes from Treg recipients and control mice were incubated (1 × 106 cells/100μl) with antibodies against CD4 (RM4-5 PerCP-Cy5.5), CD28 (E18 FITC), and TNFR2 p75 (TR75-89 PE), GARP (F011-5 PE), GITR (DTA-1 PE), or CD27 (LG.7F9 PE) or isotype control antibody for 30 min at 4°C, then stained for FoxP3 (FJK-16s APC) using the eBioscience Mouse Regulatory T Cell Staining Kit. Thyroid-infiltrating cells were isolated from single thyroid lobes of individual recipeint mice by treating thyroids with Liberase (0.08 U/ml) (Roche, Indianapolis, IN) for 45 min at 37°C. Cells were then incubated (1 × 106 cells/100μl) with antibodies against CD4 (RM4-5 PerCP-Cy5.5), CD28 (E18 FITC), and CD45 (30-F11, PE) or isotype control antibody for 30 min at 4°C, then stained for FoxP3 (FJK-16s APC) using the eBioscience Mouse Regulatory T Cell Staining Kit. Antibodies for flow cytometry were purchased from eBioscience and BioLegend. Data was collected and analyzed as above. For determination of plasma cell numbers, splenocytes or thyroid infiltrating cells from WT or CD28−/− NOD.H-2h4 mice were incubated (1 × 106 cells/100μl) with antibodies against either CD45 (30-F11, FITC), B220 (RA3-6B2, PerCP-Cy5.5), and CD138 (281.2, APC) or B220 (RA3-6B2, FITC) and CD138 (281.2, APC). Cells were washed, and the data collected using the DAKO Cyan flow cytometer. For determination of B cell subsets in the spleen, cells were stained with antibodies against either B220 (RA3-6B2, APC), CD21 (eBio8D9, PE), and CD23 (B3B4, FITC) or with isotype control as previously described (26). Cells were washed, and the data collected using the BD FACSCalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA) and analyzed using FlowJo software version 8.8.6 (Tree Star, Inc., Ashland, OR).
Semiquantitative RT PCR
Total RNA was isolated from thyroids using TRIzol, and cDNA was generated as previously described (4, 27). Semiquantitative RT-PCR was performed as previously described (4, 27–29), using β-actin to correct for sample-to-sample variations in amounts of RNA. Samples were electrophoresed, stained with ethidium bromide, and densitometry analysis was performed. Densitometric units were normalized to the corresponding β-actin band (27). Results are expressed as ratios of gene of interest / the housekeeping gene β-actin. A value of 100 indicates a 1:1 ratio between a particular gene of interest and β-actin.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software version 4.0 (GraphPad Software, La Jolla, CA) with Student’s t-test or the nonparametric Mann-Whitney test. A value of p < 0.05 was considered statistically significant.
Results
CD28−/− NOD.H-2h4 mice have reduced numbers of Treg
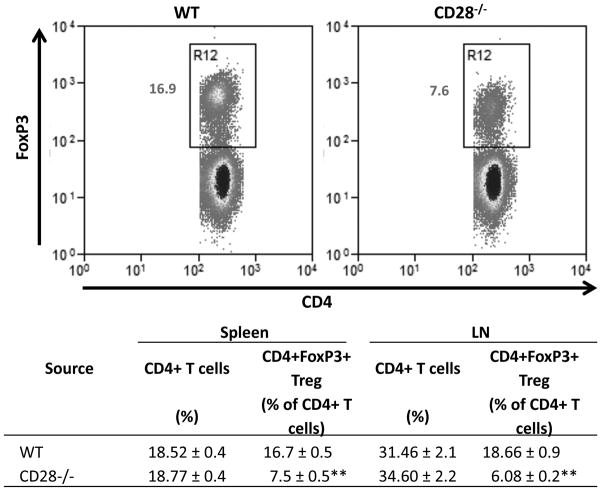
CD28 is important for generation and homeostasis of CD4+CD25+ Treg, and CD28−/− NOD mice have reduced numbers of CD4+CD25+ Treg (11–13). To determine if Treg numbers are reduced in CD28−/− NOD.H-2h4 mice, spleen and cervical lymph node (CLN) cells from adult WT or CD28−/− NOD.H-2h4 mice were stained for CD4, CD25, and FoxP3 and analyzed by flow cytometry (Fig. 1). CD28−/− mice had reduced numbers of CD4+FoxP3+ Treg in both spleen and CLN compared to WT NOD.H-2h4 mice (p < 0.01). Because the number of cells in spleen and CLN were comparable for CD28−/− and CD28+/+ mice, both the absolute numbers and percentages of Treg were reduced in CD28−/− mice (Fig. 1 and data not shown).
Figure 1.
CD28−/− NOD.H-2h4 mice have fewer Treg than WT NOD.H-2h4 mice. CD4+FoxP3+ cells in spleens and lymph nodes of WT and CD28−/− NOD.H-2h4 mice were determined by flow cytometry. Dot plots of representative mice from each group indicating the percentages of splenic CD4+ cells that express FoxP3 are shown (top). Data are shown as mean ± SEM of N = 6(Spleen B−/− and CD28−/−B−/−) and 3(LN B−/− and CD28−/−) per group and are pooled from 2 experiments. ** p<0.01 compared with respective control CD28+ group, Student’s t-test.
CD28−/− NOD.H-2h4 WT mice develop severe ISAT and fibrosis and have reduced serum T4
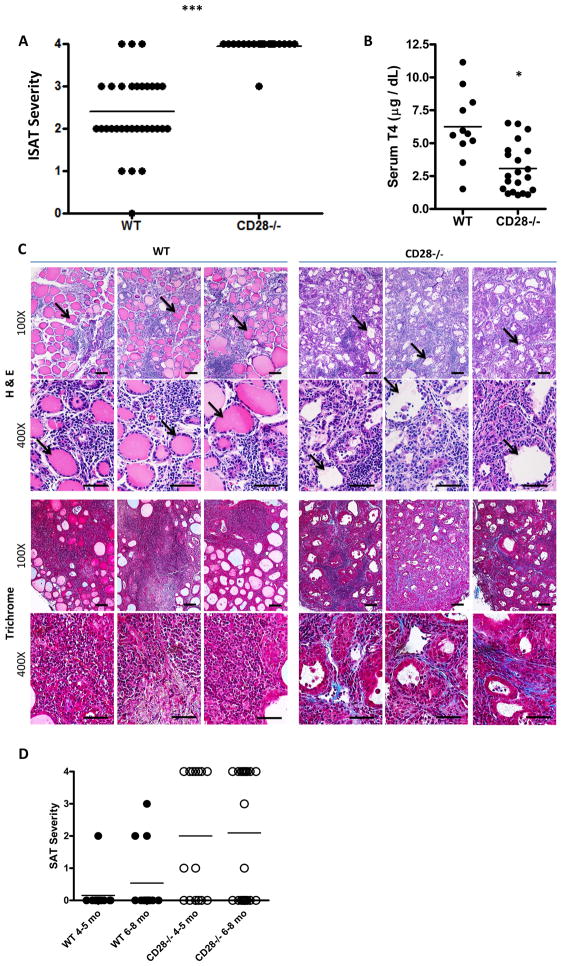
Most WT NOD.H-2h4 mice given NaI in their drinking water develop ISAT, and transient (7–10 days) depletion of Treg in WT NOD.H-2h4 mice has little effect on ISAT severity (6). To test the hypothesis that an earlier and sustained reduction in Treg would have a greater effect on ISAT development, adult WT and CD28−/− NOD.H-2h4 mice were given NaI water, and their thyroids were removed 8 wk later. CD28−/− mice had significantly higher ISAT severity scores than WT mice, with the vast majority of them having severity scores of 4+ (Fig. 2A). Thyroids of CD28−/− mice with ISAT had increased numbers of thyroid infiltrating CD4+ T cells and reduced numbers of FoxP3+ Treg compared to WT mice (data not shown). Many CD28−/− mice had low serum T4 levels, whereas WT mice almost always have normal serum T4 (3.08 ± 0.41 compared to 6.25 ± 0.81) (Fig. 2B). The histologic features of ISAT in CD28−/− mice also differed from those in WT mice. Specifically, most thyroid follicles appear ‘empty’, indicating that they lack colloid (Fig. 2C), whereas thyroid follicles in WT NOD.H-2h4 mice were always filled with colloid (Fig. 2C). This is consistent with the T4 results, as colloid stores thyroglobulin and other proteins used in production of thyroid hormones such as T4 and T3 (30, 31). Thyroids of CD28−/− NOD.H-2h4 mice also had fibrosis, which was verified using Trichrome staining (Fig. 2C), whereas fibrosis was rarely seen in thyroids of WT NOD.H-2h4 mice (Fig. 2C). As previously reported, ISAT is chronic and with increasing time after NaI water, ISAT severity scores in WT NOD.H-2h4 mice remain relatively unchanged with minimal or no fibrosis and maintenance of normal serum T4 levels (1). ISAT severity scores remained constant at 4+ in most CD28−/− mice, but fibrosis increased substantially over time and most CD28−/− mice had low serum T4 when they were 7–9 months of age (data not shown). Moreover, while spontaneous autoimmune thyroiditis (SAT) develops in only a small percentage of aged WT NOD.H-2h4 mice in our colony if they are not given NaI in the drinking water (Fig. 2D) (1), more than half of CD28−/− mice developed SAT by 4–8 mo of age without administration of NaI in the drinking water (Fig. 2D). SAT severity scores of CD28−/− mice were ususally 3–4+, i.e. very few of them developed the milder 1–2+ scores that are common in WT mice (Fig. 2D). The histologic features of SAT in CD28−/− mice were indistinguishable from those of the CD28−/− mice given NaI water in Fig. 2C (data not shown).
Figure 2.
Comparison of ISAT severity, serum T4, and histology of thyroids from WT and CD28−/−NOD.H-2h4 mice. WT and CD28−/− NOD.H-2h4 were given NaI in their drinking water at 8 wk of age. After 8 weeks, thyroids were removed, fixed, sectioned, and either stained with hematoxylin and eosin or for collagen by Trichrome staining. (A) ISAT severity scores 8 wks after NaI water. p < 0.001 N= 35(WT) and 21(CD28−/−). Results are pooled from 4 experiments and are representative of multiple experiments involving >100 mice. (B) Serum T4 levels from individual mice. p = 0.0005 N= 11(WT) and 21(CD28−/−) from 7 independent experiments. (C) H&E stained thyroid sections demonstrating increased infiltration and follicle destruction in CD28−/− compared with WT mice. Note empty follicles (arrows) in thyroids of CD28−/− mice compared to colloid filled follicles in WT thyroids. ISAT scores (WT) – 2, 3, 2; (CD28−/−) – 4, 4, 4. Trichrome staining shows collagen deposition (blue) in thyroids of CD28−/− mice with severe (4+) ISAT. Fibrosis is absent in thyroids of WT mice with 3+ ISAT. Results are representative of at least eight thyroids per group from five experiments. 100× (bar = 0.01mm); 400× (bar = 0.005mm). (D) SAT severity scores of 4–8 month old WT and CD28−/− mice not given NaI water. N = 13(WT 4–5 mo) and 13(WT 6–8 mo) from 4 experiments, 15(CD28−/− 4–5 mo) from 3 experiments, and 21(CD28−/− 6–8 mo) from 3 experiments.
CD28−/− mice have increased expression of proinflammatory cytokines in the thyroid
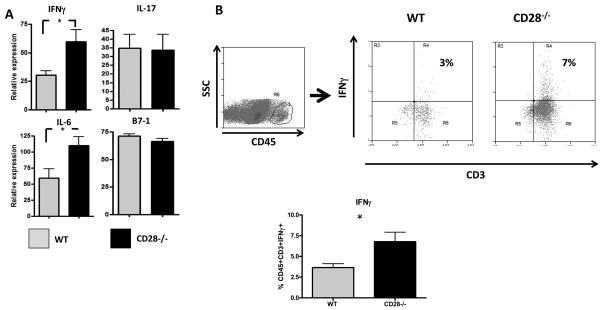
Because CD28−/− mice develop more severe ISAT than CD28+ mice, it was of interest to determine if this was associated with alterations in expression of particular cytokines in the thyroid. CD28−/− mice have increased expression of IFNγ and IL-6 compared to WT mice (Fig. 3A). There is no difference in expression of the costimulatory molecule B7-1, which is a binding partner for CD28, and expression of IL-17 did not differ for WT vs. CD28−/− mice. The increased expression of IFNγ and IL-6 mRNA is consistent with the increased inflammation and tissue damage in CD28−/− compared to WT mice, and with our previous studies indicating that IFNγ is an essential cytokine for development of ISAT (27). Intracellular cytokine staining of thyroid-infiltrating cells also showed that more IFNγ was produced, primarily by CD45+CD3+ cells, in thyroids of CD28−/− mice compared to WT mice (Fig. 3B). Thyroids of CD28−/− mice had higher numbers of total CD45+ infiltrating cells compared to WT mice and therefore they also had higher total numbers of CD45+CD3+IFN-γ + cells (data not shown). Intracellular IL-6 was not detected (data not shown).
Figure 3.
Differential expression of proinflammatory cytokines in thyroids of CD28−/− and WT NOD.H-2h4 mice. (A) WT and CD28−/− NOD.H-2h4 mice were given NaI in their drinking water at 8 weeks of age. Thyroids were removed 8 wk later, snap frozen and RNA was isolated from individual thyroid lobes. Expression of IFNγ, IL-6, IL-17 and B7-1 were determined by semiquantitative RT PCR as described in Methods. Results represent the ratio of particular cytokine:β-actin densitometric units ± SEM of individual thyroids from 5 mice per group, and are representative of two separate experiments. (B) Thyroid infiltrating cells were stained for the presence of intracellular IFNγ as described in Methods. Dot plots show expression of CD3 and IFNγ by CD45+ thyroid infiltrating cells. Dot plots are representative of the data in the bar graph. The bar graph represents mean ± SEM of CD45+CD3+IFNγ+ cells per thyroid from 4(WT) and 8(CD28−/−) mice per group pooled from two experiments. * p<0.05, Student’s t-test.
WT Treg suppress development of ISAT in CD28−/− NOD.H-2h4 mice, and differ phenotypically from Treg in CD28−/− mice
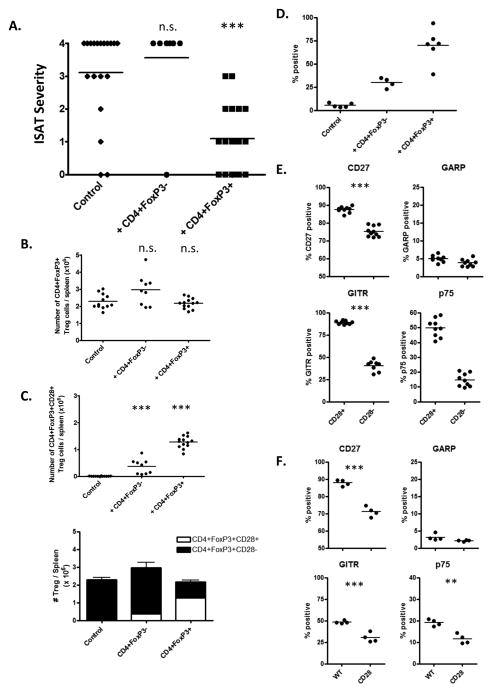
Given that CD28−/− mice had reduced Treg numbers and percentages compared to WT mice, we hypothesized that the increased ISAT severity in CD28−/− mice was due, at least in part, to decreased function and/or numbers of Treg in CD28−/− mice. If so, CD4+FoxP3+ Treg from CD28+/+ mice should reduce ISAT severity after transfer to CD28−/− mice. To test this, CD4+FoxP3+ Treg were sorted from spleens of FoxP3-GFP CD28+/+ mice and transferred into 6–7 wk old CD28−/− NOD.H-2h4 mice. All mice were given NaI in their drinking water, and recipient mice were given three i.v. injections of 106 sorted Treg two weeks apart. Controls included mice that did not receive Treg or mice given the same number of CD4+FoxP3− T cells. When thyroids were removed 3 wk after the third injection of Treg, recipients of CD28+ Treg had significantly reduced ISAT severity scores compared to controls (Fig. 4A) (p = 0.0005). CD28+CD4+FoxP3-negative cells had no effect on ISAT development (Fig. 4A), indicating that expression of FoxP3 and not simply expression of CD28 was important for suppression of ISAT.
Figure 4.
CD28+/+ Treg suppress ISAT development in CD28−/− mice. (A) CD28−/− mice were given three i.v. injections of 106 sorted CD4+FoxP3+ Treg or CD4+FoxP3− T cells from CD28+/+ FoxP3−GFP NOD.H-2h4 mice as described in Methods. Mice were given NaI in their drinking water at the time of the first injection of Treg, and ISAT severity was determined 7 wks later. N = 19(Control), 9(+ CD4+FoxP3−), and 20 (+ CD4+FoxP3+) mice per group pooled from 3 experiments. *** p < 0.001, Mann-Whitney nonparametric test. (B, C) Splenocytes from CD4+FoxP3+ Treg, CD4+FoxP3− T cell recipients and control mice were stained for expression of CD4, CD28, and FoxP3. Plots represent the total number of CD4+FoxP3+ Treg (B) or CD4+FoxP3+CD28+ Treg (C) per spleen. Bar graph represents total of CD28−CD4+FoxP3+ and CD28+CD4+FoxP3+ Treg per recipient. N = 12(Control), 9(+ CD4+FoxP3−) and 13(+ CD4+FoxP3+) mice per group pooled from 2 independent experiments. (D) Thyroids from the Treg groups in A–C were stained for expression of CD45, CD4, FoxP3, and CD28 and analyzed by flow cytometry. Plots represent the percentage of CD45+CD4+FoxP3+ cells that express CD28. N = 5(Control), 4(+ CD4+FoxP3−) and 6(+ CD4+FoxP3+) mice per group and are representative of 3 independent experiments) (E) Splenocytes from CD4+FoxP3+ Treg recipients were stained for the presence of CD4, CD28, FoxP3 and CD27, GARP, GITR, or TNFR2 p75. Plots represent the percentage of CD28+CD4+FoxP3+CD28+ or CD28−CD4+FoxP3+ cells positive for the indicated marker. (N=9 mice per group; representative of 3 independent experiments.) (F) Splenocytes from WT or CD28−/− mice given NaI water for 8 wks were stained for the presence of CD4, CD28, FoxP3 and CD27, GARP, GITR, or TNFR2 p75. Plots represent the percentage of CD4+FoxP3+ cells positive for the indicated marker. (N=4 mice per group and are representative of 2 independent experiments) n.s. = not significant, ** p < 0.01; *** p < 0.001, Student’s t-test.
Importantly, recipients of sorted CD28+CD4+FoxP3+ Treg did not have significantly increased overall CD4+FoxP3+ Treg numbers compared to controls, but the CD28+/+ donor Treg comprised more than half of the total CD4+FoxP3+ Treg (Fig. 4B, C) (1.2 × 106 CD4+FoxP3+CD28+ Treg of 2.1 × 106 total Treg). These results suggest the transferred CD28+/+ Treg may have a survival advantage over CD28−/− Treg, so that many of the endogenous Treg are replaced by the transferred Treg, as recently reported by others (32). As seen in Figure 4D, the majority of CD45+CD4+FoxP3+ Treg in the thyroids of the CD28 −/− recipients of CD28+ Treg express CD28, indicating that they can effectively migrate to the thyroid and could therefore potentially exert some function at the site of inflammation. It is also of interest that recipients of CD28+CD4+FoxP3− T cells had some splenic CD28+CD4+FoxP3+ cells in their spleens at the end of the experiment. These cells may have been present and undetectable in the transferred cells, or they may have been induced to express FoxP3 in the recipient environment. These cells were present in relatively low numbers, and were unable to suppress ISAT. Also, approximately 40% of CD28+ T cells in recipients of CD28+CD4+FoxP3+ Treg no longer expressed FoxP3 by 7–8 weeks after the initial transfer (data not shown).
CD28−/− recipients of Treg from CD28+/+ donors developed less severe ISAT even though the total Treg numbers were similar to those of CD28−/− mice not given CD28+ Treg, suggesting that the transferred CD28+ Treg differ functionally from the endogenous CD28-negative Treg. To determine if CD28-positive and CD28-negative Treg had phenotypic differences that might explain their functional differences, flow cytometry was used to compare expression of various markers by CD28+ and CD28− Treg in the CD28−/− recipients of CD28+ Treg or control CD28+CD4+FoxP3− T cells. The results (Fig. 4E, F) indicate that donor CD28+CD4+FoxP3+ Treg have significantly higher expression of CD27, TNFR2 p75, and glucocorticoid-induced TNFR-related protein (GITR) compared to the endogenous CD28-negative Treg (Fig. 4E). These TNF receptor superfamily members TNFR2 p75, CD27, and GITR have been reported by others to identify effective Treg in other models (33–36). Expression of the surface protein glycoprotein A repititions predominant (GARP) on activated Treg has been shown to be required for surface TGFβ expression (37), and GARP expression did not differ for CD28+ and CD28-negative Treg (Fig. 4E). These results suggest that Treg from CD28+/+ mice were able to suppress ISAT in CD28−/− mice because they differ functionally from CD28-negative Treg. These differences are not simply due to the CD28+ Treg having been sorted and transferred, as Treg from WT and CD28−/− mice with ISAT also have differences in expression of CD27, p75, and GITR (Fig. 4E). Although there were some CD28+CD4+FoxP3+ cells in recipients of control FoxP3-negative cells, those CD28+ Treg did not differ phenotypically from endogenous CD28-negative Treg (data not shown). The reason for this is not known.
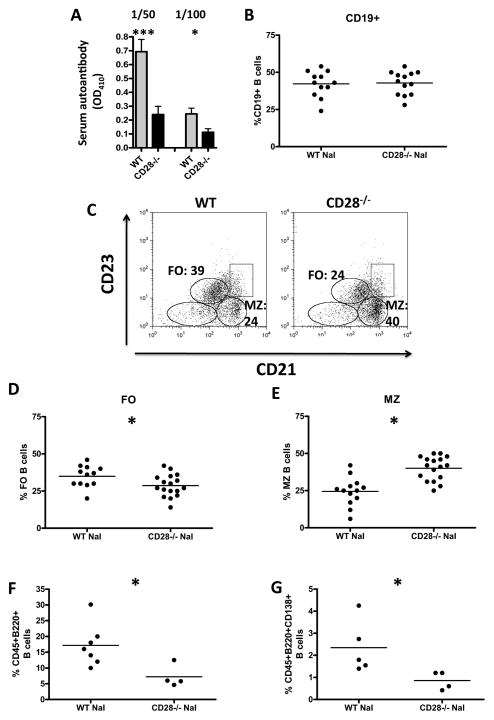
CD28−/− mice have reduced anti-MTg autoantibody responses, increased numbers of splenic marginal zone B cells and fewer thyroid infiltrating B cells compared to WT NOD.H-2h4 mice
Our previous studies indicate that serum anti-MTg autoantibody levels generally correlate with ISAT severity scores (1, 7, 26, 27). Although CD28−/− NOD.H-2h4 mice develop more severe ISAT than WT NOD.H-2h4 mice, they always have much lower anti-MTg autoantibody responses than their WT counterparts (Fig. 5A). These results indicate that severe ISAT can develop even if autoantibody responses are low, and are consistent with results of others showing that B7/CD28 interactions are critical for the ability of CD4+ T cells to provide help to B cells for antibody production (38–40).
Figure 5.
Differences in serum autoantibody levels and changes in the B cell compartment in CD28−/− mice. WT and CD28−/− NOD.H-2h4 mice were given NaI in their drinking water beginning at 8 weeks of age. After 8 weeks, serum anti-MTg autoantibodies were determined (A) and splenocytes were stained for CD19, B220, CD21, and CD23 (B–E) and analyzed by flow cytometry. Thyroids were stained for B220, CD45, and CD138. (F, G) (A) Serum anti-MTg autoantibody production from was determined using 1/50 and 1/100 dilution of serum from individual mice and is expressed as mean OD410 ± SEM. * p < 0.05, *** < 0.001, Student’s t-test N= 20(WT), 18(CD28−/−) from 3 experiments. (B) CD19+ cells as a percentage of live gated cells. (C) Dot plots show CD21 and CD23 expression on B220+ cells. Representative dot plots from mice in D and E (D) Follicular B cells as a percentage of B220+ cells. (E) Marginal zone B cells as a percentage of B220+ cells. N = 12 (WT) and 17(CD28−/−) mice pooled from 6 experiments. (F) B220+ cells as a percentage of CD45+ cells. (G) CD138+ cells as a percentage of CD45+B220+ cells. Results are pooled from two independent experiments with N = 5(WT) and 4(CD28−/−) mice per group. Each symbol represents a single mouse and bars represent the mean of each group. * p < 0.05, Student’s t-test.
Because CD28−/− mice have reduced anti-MTg autoantibody responses, it was important to determine if total B cell numbers or B cell subsets differed in WT vs. CD28−/− mice. To determine if there were differences in B cells in CD28+/+ and CD28−/− mice, WT and CD28−/− mice were given NaI water for 8 weeks, and splenic B cell numbers and subsets were evaluated by flow cytometry as previously described (26). There were no differences in the relative percentages or absolute numbers of CD19+ B cells in spleens of WT and CD28−/− mice (Fig. 5B and data not shown). However, spleens of CD28−/− mice had increased percentages and absolute numbers of MZ B cells compared to WT mice (40.06 ± 1.9 vs. 24.5 ± 2.6) (p<0.001) and a concomitant decrease in FO B cells (28.65 ± 1.8 vs. 34.92 ± 2.1) (p<0.05) (Fig. 5C–E; data not shown). This is in contrast to young naïve CD28−/− and WT NOD.H-2h4 mice, which did not have differences in B cell subsets (unpublished results). Importantly, CD28−/− mice had significantly fewer thyroid infiltrating B220+ B cells than WT mice, as well as fewer thyroid infiltrating CD138+ plasma cells (Fig. 5F–G). There were no differences in splenic plasma cell numbers between WT and CD28−/− mice (data not shown). These results are consistent with the reduced autoantibody responses in CD28−/− mice.
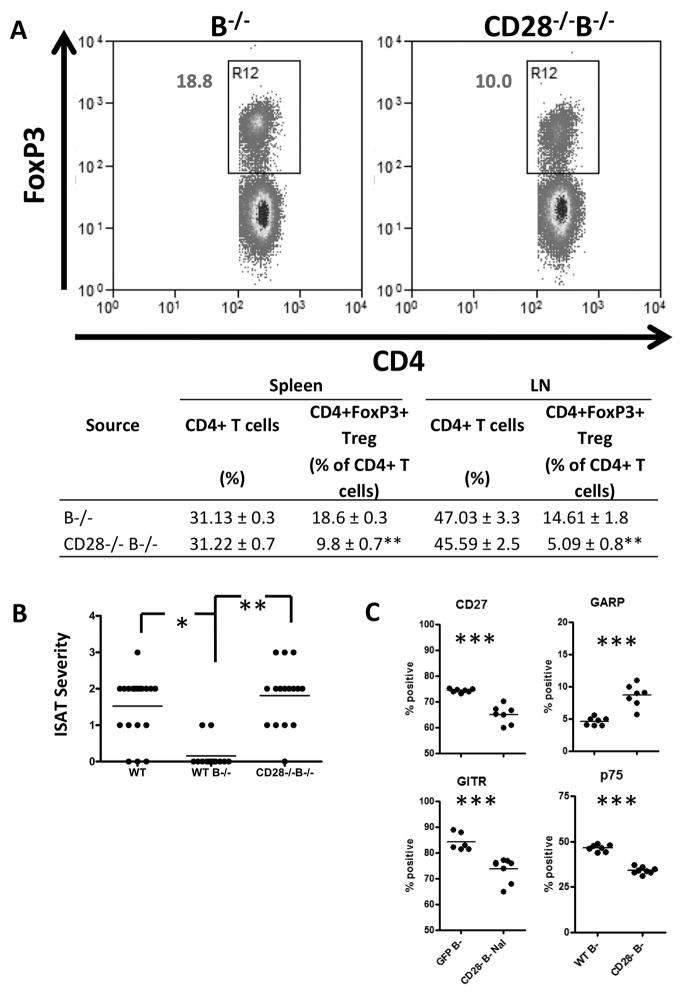
CD28−/−B−/− NOD.H-2h4 mice develop ISAT
The results presented above indicate that CD28−/− NOD.H-2h4 WT mice develop severe ISAT even though they have greatly reduced anti-MTg autoantibody responses and reduced infiltration of B cells and plasma cells in their thyroids. We previously showed that B−/− NOD.H-2h4 mice are resistant to ISAT, but they develop ISAT when Treg are transiently depleted (6). Because CD28−/− mice have fewer and functionally defective Treg, we hypothesized that CD28−/− B−/− mice might develop ISAT without a requirement for Treg depletion. To test this hypothesis, we first determined whether CD28−/−B−/− have fewer Treg than CD28+/+B−/−mice (Fig. 6A). As total numbers of splenic and LN cells were similar in CD28+/+ and CD28−/−B−/− mice, CD28−/−B−/− mice also have significantly reduced numbers of CD4+FoxP3+ Treg compared to CD28+/+B−/− mice. To determine if CD28−/− B−/− mice developed ISAT, WT and CD28+/+B−/−, and CD28−/−B−/− mice were given NaI water. Thyroids were removed 8 wk later, and ISAT severity was determined. The results (Fig. 6B) indicate that CD28+/+B−/− mice develop minimal or no ISAT as shown previously (6), while CD28−/−B−/− NOD.H-2h4 mice develop ISAT comparable to that of WT NOD.H-2h4 mice (p = 0.412). These results are consistent with our previous results using anti-CD25 to transiently deplete Treg in B−/− mice and indicate that decreasing functional Treg through CD28 depletion results in development of ISAT in B−/− mice comparable to that seen in WT NOD.H-2h4 mice. However, CD28−/− mice that have B cells always develop more severe ISAT indicating that B cells have some positive function in ISAT development. Whether this reflects a need for B cells as effective APC as suggested earlier or to some other function of B cells cannot be determined from these results. Splenic Treg from CD28−/−B−/− mice with ISAT differ phenotypically from WT B−/− Treg with respect to expression of CD27, GITR and p75 (Fig. 6C), as shown above for B cell sufficient mice (Fig. 4F). There is also an increased percentage of CD4+FoxP3+GARP+ Treg in CD28−/−B−/− mice compared to WT B−/− mice.
Figure 6.
CD28 deficient B−/− mice have reduced Treg numbers and develop ISAT similar to WT mice. (A) Splenocytes from WT B−/− and CD28−/−B−/− mice were analyzed by flow cytometry for the presence of CD4+FoxP3+ Treg. Dot plots of representative mice from each group indicating the percentages of splenic CD4+ cells that express FoxP3 are shown (top). Data are shown as mean ± SEM of N = 6(Spleen B−/− and CD28−/−B−/−) and 3(LN B−/− and CD28−/−) per group and are pooled from 2 experiments. * p<0.05, ** p<0.01 compared with respective control CD28+ group, Student’s t-test. (B) WT, CD28+/+B−/−, or CD28−/−B−/− mice were given NaI in their drinking water for 8 weeks, and ISAT severity was determined. (N= 19(WT), 13(WT B−/−), and 16(CD28−/−B−/−) mice per group) * p < 0.05, ** p < 0.01, Mann-Whitney nonparametric test. (C) Splenocytes from WT B−/− or CD28−/−B−/− mice given NaI water for 8 wks were stained for the presence of CD4, CD28, FoxP3 and CD27, GARP, GITR, or TNFR2 p75. Plots represent the percentage of CD4+FoxP3+ cells positive for the indicated marker. (N=7 mice per group combined from two experiments) ** p < 0.01; * p < 0.05, Student’s t-test.
Discussion
CD28−/− NOD.H-2h4 mice were developed in order to test the hypothesis that an early and permanent deficiency in Treg would result in more severe ISAT in WT and B−/− NOD.H-2h4 mice. CD28 is known to be an important costimulator of T cell receptor signaling (14, 15). However, a more important role for CD28 costimulation in development of spontaneous autoimmune diseases may be in aiding the generation of regulatory T cells that limit overactive immune responses and block autoimmunity (10, 13, 16, 20). Although CD28 costimulation is essential for development of immune responses to most foreign antigens and for experimentally induced autoimmune diseases (13, 15–18)(our unpublished observations), it is not required for spontaneous development of diabetes or pancreatic exocrine disease in autoimmune-prone NOD mice (10, 13, 15, 19). NOD mice have reduced numbers of CD4+CD25+ Treg (11–13, 41), and they develop an early and aggressive form of diabetes compared to WT NOD mice (10, 13, 15) due to the reduction in Treg (13).
In the current study, CD28 deficient WT and B−/− mice on the autoimmune thyroiditis-prone NOD.H-2h4 background developed more severe ISAT than their CD28+/+ counterparts (Fig. 2A and 6B). CD28−/− B cell+ NOD.H-2h4 mice almost uniformly developed severe ISAT with 4+ severity scores that are relatively rare in WT NOD.H-2h4 mice (Fig. 2A). Moreover, administration of NaI in the drinking water is important for SAT development in WT NOD.H-2h4 mice (1), whereas many CD28−/− mice develop severe SAT if they are not given NaI in their drinking water (Fig. 2D). Importantly, thyroid lesions in CD28−/− mice differed histologically from those in WT CD28+/+ mice, and many CD28−/− mice were clinically hypothyroid (Fig. 2B, C). The more severe ISAT scores in CD28−/− mice were accompanied by increased infiltration of the thyroid by CD4+ T cells and increased expression of proinflammatory cytokines including IFNγ and IL-6 in thyroids (Fig. 3) as determined by semiquantitative PCR. Intracellular cytokine staining confirmed that IFNγ producing CD45+CD3+ T cells are increased in thyroids of CD28−/− mice (Fig. 3B). IFNγ is required for development of ISAT (27), and depletion of CD4+CD25+ Treg results in increased expression of IFNγ in ISAT (8, 9). Diabetogenic effector T cells in CD28−/− NOD mice were also reported to have increased IFNγ responses to autoantigen (40), whereas Th2 and Th17 responses are reported to be reduced in CD28−/− mice (40, 42). IL-6 and IFNγ may be upregulated as a result of the increased inflammation and tissue destruction in thyroids of CD28−/− mice due to their inherent Treg deficit.
As hypothesized, CD28−/− NOD.H-2h4 mice had fewer CD4+FoxP3+ Treg in CLN, spleens and thyroids compared to WT NOD.H-2h4 mice (Fig. 1 and data not shown). While CD4+CD25+ Treg were reduced by 75–80% in CD28−/− NOD mice (10, 11, 13), Treg were reduced by only about 50% in the spleen and 65–70% in LN in NOD.H-2h4 mice (Fig. 1 and Fig. 6A). It is not known why Treg numbers were reduced to a lesser extent in CD28−/− NOD.H-2h4 mice compared to CD28−/− NOD mice, but development of ISAT was profoundly affected, despite the relatively modest reduction in total Treg numbers. The diminished Treg numbers explain, at least in part, the increased ISAT severity in CD28−/− compared to WT NOD.H-2h4 mice because transfer of Treg from CD28+/+ mice suppresses ISAT development in CD28−/− mice (Fig. 4A).
The results of our Treg transfer experiments are consistent with those reported by others in the NOD diabetes model (13, 19), and they provide new information regarding the function of Treg in CD28−/− mice. First, the endogenous Treg in CD28−/− NOD.H-2h4 mice presumably differ functionally from those in CD28+/+ mice since transfer of WT Treg into CD28−/− mice significantly reduced ISAT severity without increasing the overall Treg pool (Fig. 4A–C). The transferred CD28+CD4+FoxP3+ Treg make up greater than half of the total Treg pool 7 wks after the first transfer (Fig. 4B–C), suggesting that Treg in CD28−/− mice could have a survival defect that allows for their replacement by CD28+ Treg.
The idea that CD28+ Treg differ functionally from those in CD28−/− mice is supported by the finding that CD28+ Treg transferred to CD28−/− recipients differ from the recipients’ endogenous Treg in expression of several surface TNF receptor superfamily member markers that were shown to identify functionally distinct subsets of Treg in other models. TNFR2 p75 has been reported to identify highly suppressive Treg by several groups (33, 43, 44), and CD27 plays a role in T cell differentiation and is reported to be expressed on effective Treg in humans (35, 36). GITR can play a role in Treg suppression of effector responses, and CD4+FoxP3+GITR+ cells were reduced when CD28/B7 interactions are blocked (11, 34). All three of these TNF receptor superfamily members are expressed on significantly more CD28+/+ WT donor Treg than on recipient CD28-negative Treg (Fig. 4E), consistent with the hypothesis that CD28-negative Treg are inherently less effective suppressors than CD28+ Treg. Regulatory T cells lacking CD28 have recently been reported to have phenotypic differences and a survival disadvantage compared to WT Treg in a model where CD28 was specifically deleted from FoxP3 expressing cells (32). Treg in these mice had markedly reduced CTLA4, CCR6, and PD-1 expression (32), and expression of CTLA4 on Treg was also reduced when CD28 deletion is induced in adult mice (45). The phenotypic differences in WT and CD28−/− Treg are not an artifact of the transfer system, as splenic Treg from WT and CD28−/− mice with ISAT have similar differences in CD27, GITR, and p75 (Fig. 4F), and Treg in B−/− CD28+ and CD28− mice also had differential expression of these molecules (Fig. 6C). Splenic Treg in naïve WT and CD28−/− mice also differ in expression of CD27, GITR, and p75 (data not shown), although the differences are less pronounced than in mice with ISAT (Fig. 4F). This suggests that natural Treg in WT and CD28−/− mice are inherently different. It is interesting that recipients of CD4+FoxP3− T cells had some CD28+CD4+FoxP3+ Treg 7 wk after transfer (Fig. 4C), but they had no effect on ISAT severity (Fig. 4A). These CD28+CD4+FoxP3+ Treg could have expanded as the result of CD4+FoxP3+ Treg contamination in the sorted CD4+FoxP3− pool, or they could be induced Treg that upregulated FoxP3 expression after transfer. Combined with the transfer experiments, the phenotyping results suggest that Treg in CD28+/+ and CD28−/− mice differ from one another both phenotypically and functionally. The transfer experiments did not provide a direct comparison of the function of CD28−/− and CD28+/+ Treg, they indicate that Treg from CD28+/+ mice are capable of suppressing SAT development, whereas the endogenous Treg in CD28−/− mice permit development of severe ISAT.
Although anti-MTg autoantibody responses generally correlate with ISAT severity scores in WT NOD.H-2h4 mice (1, 27), CD28−/− NOD.H-2h4 mice have greatly reduced serum anti-MTg autoantibodies compared to WT mice (Fig 5A). There are several possible explanations for the reduced autoantibody responses in CD28−/− NOD.H-2h4 mice. First, CD28/B7 interactions are important for activation of B cells by CD4+ T cells (9, 46), and for development of germinal centers (38). CD28−/− mice have reduced antibody responses following immunization with foreign antigens (40, 46), and CD28−/− NOD.H-2h4 mice produce minimal anti-MTg autoantibody following immunization with MTg and LPS (our unpublished results). CD28+/+ and CD28−/− mice had comparable numbers of CD19+ B cells, but CD28−/− mice had more splenic MZ B cells and fewer FO B cells compared to WT mice (Fig. 6B–E). MZ B cells are found primarily in the splenic marginal sinus (47), and have been implicated as the primary autoantibody producers in mouse models of lupus (48–50). They also increase in number and are important APC in pancreatic lymph nodes of NOD mice developing diabetes (51). However, FO B cells, the major subset of splenic B cells and the major circulating B cell population, are the primary B cell subset infiltrating thyroids of mice with ISAT, and when FO B cells are depleted by anti-CD20, ISAT is inhibited (SH Hong and H Braley-Mullen submitted for publication). These results suggest that FO B cells are the major B cell subset in this model, and it is evident that the modest reduction in FO B cells in CD28−/− mice was not sufficient to reduce ISAT severity. B220+ B cells in thyroids of CD28−/− mice are diffuse and reduced in number compared to those in thyroids of WT mice (Fig. 5F and data not shown). Although WT and CD28−/− NOD.H-2h4 mice had comparable numbers of splenic CD138+ cells (our unpublished results), CD28−/− NOD.H-2h4 mice have fewer thyroid infiltrating CD138+ plasma cells (Fig. 5G). These results may be consistent with those reported recently by another group indicating that long-term survival of bone marrow plasma cells and subsequent humoral immunity was reduced in CD28−/− mice (46). However another group reported that both short and long-lived plasma cells produced more antibody in CD28−/− compared to WT mice (52). Therefore, further studies are needed to determine how CD28 regulates B cell and plasma cell numbers and function.
The results of this study demonstrate that lack of CD28 in NOD.H-2h4 mice leads to more severe ISAT than that seen in WT mice, with increased thyroid follicle destruction, low serum T4 and collagen deposition (fibrosis) in thyroids (Fig. 2). The increased thyroid destruction and inflammation was shown to be due, at least in part, to the fact that CD28−/− mice have fewer Treg compared to WT mice. Importantly, CD28−/− Treg appear to differ both functionally and phenotypically from those in WT NOD.H-2h4 mice, and WT CD4+FoxP3+ Treg can suppress ISAT after transfer to CD28−/− mice even though overall numbers of Treg are not increased.
Acknowledgments
Funding. This work was supported by National Institute of Health grant RO1 AI 076395.
Abbreviations used in this article
- B−/−
B-cell deficient
- MTg
mouse thyroglobulin
- CLN
cervical lymph node
- FO
follicular B cell
- MZ
marginal zone B cell
- SAT
spontaneous autoimmune thyroiditis
- ISAT
iodine-accelerated spontaneous autoimmune thyroiditis
- Treg
regulatory T cells
- GITR
Glucocorticoid-induced TNFR-related protein
- GARP
glycoprotein A repititions predominant
References
- 1.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun. 1999;12:157–165. doi: 10.1006/jaut.1999.0272. [DOI] [PubMed] [Google Scholar]
- 2.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81:287–292. doi: 10.1006/clin.1996.0191. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Hutchings P, Guo J, McLachlan S, Rapoport B, Cooke A. Role of MHC class I expression and CD8(+) T cells in the evolution of iodine-induced thyroiditis in NOD-H2(h4) and NOD mice. Eur J Immunol. 2000;30:1191–1202. doi: 10.1002/(SICI)1521-4141(200004)30:4<1191::AID-IMMU1191>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 4.Yu S, Medling B, Yagita H, Braley-Mullen H. Characteristics of inflammatory cells in spontaneous autoimmune thyroiditis of NOD.H-2h4 mice. J Autoimmun. 2001;16:37–46. doi: 10.1006/jaut.2000.0458. [DOI] [PubMed] [Google Scholar]
- 5.Braley-Mullen H, Yu S. Early requirement for B cells for development of spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2000;165:7262–7269. doi: 10.4049/jimmunol.165.12.7262. [DOI] [PubMed] [Google Scholar]
- 6.Yu S, Maiti PK, Dyson M, Jain R, Braley-Mullen H. B cell-deficient NOD.H-2h4 mice have CD4+CD25+ T regulatory cells that inhibit the development of spontaneous autoimmune thyroiditis. J Exp Med. 2006;203:349–358. doi: 10.1084/jem.20051438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu S, Ellis JS, Dunn R, Kehry MR, Braley-Mullen H. Transient depletion of B cells in young mice results in activation of regulatory T cells that inhibit development of autoimmune disease in adults. Int Immunol. 2012;24:233–242. doi: 10.1093/intimm/dxs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagayama Y, Horie I, Saitoh O, Nakahara M, Abiru N. CD4+CD25+ naturally occurring regulatory T cells and not lymphopenia play a role in the pathogenesis of iodide-induced autoimmune thyroiditis in NOD-H2h4 mice. J Autoimmun. 2007;29:195–202. doi: 10.1016/j.jaut.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Horie I, Abiru N, Sakamoto H, Iwakura Y, Nagayama Y. Induction of autoimmune thyroiditis by depletion of CD4+CD25+ regulatory T cells in thyroiditis-resistant IL-17, but not interferon-{gamma} receptor, knockout nonobese diabetic-H2h4 mice. Endocrinology. 2011;152:4448–4454. doi: 10.1210/en.2011-1356. [DOI] [PubMed] [Google Scholar]
- 10.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 12.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 14.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 15.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 16.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girvin AM, Dal Canto MC, Rhee L, Salomon B, Sharpe A, Bluestone JA, Miller SD. A critical role for B7/CD28 costimulation in experimental autoimmune encephalomyelitis: a comparative study using costimulatory molecule-deficient mice and monoclonal antibody blockade. J Immunol. 2000;164:136–143. doi: 10.4049/jimmunol.164.1.136. [DOI] [PubMed] [Google Scholar]
- 18.Tada Y, Nagasawa K, Ho A, Morito F, Ushiyama O, Suzuki N, Ohta H, Mak TW. CD28-deficient mice are highly resistant to collagen-induced arthritis. J Immunol. 1999;162:203–208. [PubMed] [Google Scholar]
- 19.Meagher C, Tang Q, Fife BT, Bour-Jordan H, Wu J, Pardoux C, Bi M, Melli K, Bluestone JA. Spontaneous development of a pancreatic exocrine disease in CD28-deficient NOD mice. J Immunol. 2008;180:7793–7803. doi: 10.4049/jimmunol.180.12.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Podolin PL, Pressey A, DeLarato NH, Fischer PA, Peterson LB, Wicker LS. I-E+ nonobese diabetic mice develop insulitis and diabetes. J Exp Med. 1993;178:793–803. doi: 10.1084/jem.178.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tartar DM, VanMorlan AM, Wan X, Guloglu FB, Jain R, Haymaker CL, Ellis JS, Hoeman CM, Cascio JA, Dhakal M, Oukka M, Zaghouani H. FoxP3+RORgammat+ T helper intermediates display suppressive function against autoimmune diabetes. J Immunol. 2010;184:3377–3385. doi: 10.4049/jimmunol.0903324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braley-Mullen H, Chen K, Wei Y, Yu S. Role of TGFbeta in development of spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2001;167:7111–7118. doi: 10.4049/jimmunol.167.12.7111. [DOI] [PubMed] [Google Scholar]
- 24.Yu S, Sharp GC, Braley-Mullen H. Thyroid epithelial cell hyperplasia in IFN-gamma deficient NOD.H-2h4 mice. Clin Immunol. 2006;118:92–100. doi: 10.1016/j.clim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 25.McLachlan SM, Braley-Mullen H, Chen CR, Aliesky H, Pichurin PN, Rapoport B. Dissociation between iodide-induced thyroiditis and antibody-mediated hyperthyroidism in NOD.H-2h4 mice. Endocrinology. 2005;146:294–300. doi: 10.1210/en.2004-1126. [DOI] [PubMed] [Google Scholar]
- 26.Yu S, Dunn R, Kehry MR, Braley-Mullen H. B cell depletion inhibits spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2008;180:7706–7713. doi: 10.4049/jimmunol.180.11.7706. [DOI] [PubMed] [Google Scholar]
- 27.Yu S, Sharp GC, Braley-Mullen H. Dual roles for IFN-gamma, but not for IL-4, in spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2002;169:3999–4007. doi: 10.4049/jimmunol.169.7.3999. [DOI] [PubMed] [Google Scholar]
- 28.Tang H, Sharp GC, Peterson KE, Braley-Mullen H. Induction of granulomatous experimental autoimmune thyroiditis in IL-4 gene-disrupted mice. J Immunol. 1998;160:155–162. [PubMed] [Google Scholar]
- 29.Peterson KE, Sharp GC, Tang H, Braley-Mullen H. B7.2 has opposing roles during the activation versus effector stages of experimental autoimmune thyroiditis. J Immunol. 1999;162:1859–1867. [PubMed] [Google Scholar]
- 30.Berndorfer U, Wilms H, Herzog V. Multimerization of thyroglobulin (TG) during extracellular storage: isolation of highly cross-linked TG from human thyroids. J Clin Endocrinol Metab. 1996;81:1918–1926. doi: 10.1210/jcem.81.5.8626858. [DOI] [PubMed] [Google Scholar]
- 31.Gerber H, Studer H, Conti A, Engler H, Kohler H, Haeberli A. Reaccumulation of thyroglobulin and colloid in rat and mouse thyroid follicles during intense thyrotropin stimulation. A clue to the pathogenesis of colloid goiters. J Clin Invest. 1981;68:1338–1347. doi: 10.1172/JCI110381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Subleski JJ, Kopf H, Howard OM, Mannel DN, Oppenheim JJ. Cutting edge: expression of TNFR2 defines a maximally suppressive subset of mouse CD4+CD25+FoxP3+ T regulatory cells: applicability to tumor-infiltrating T regulatory cells. J Immunol. 2008;180:6467–6471. doi: 10.4049/jimmunol.180.10.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 35.Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793–1803. doi: 10.1084/jem.20050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107:2409–2414. doi: 10.1182/blood-2005-06-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal center formation. J Immunol. 1996;156:4576–4581. [PubMed] [Google Scholar]
- 39.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 40.Lenschow DJ, Herold KC, Rhee L, Patel B, Koons A, Qin HY, Fuchs E, Singh B, Thompson CB, Bluestone JA. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 41.Lio CW, Dodson LF, Deppong CM, Hsieh CS, Green JM. CD28 facilitates the generation of Foxp3(−) cytokine responsive regulatory T cell precursors. J Immunol. 2010;184:6007–6013. doi: 10.4049/jimmunol.1000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouguermouh S, Fortin G, Baba N, Rubio M, Sarfati M. CD28 co-stimulation down regulates Th17 development. PLoS One. 2009;4:e5087. doi: 10.1371/journal.pone.0005087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Housley WJ, Adams CO, Nichols FC, Puddington L, Lingenheld EG, Zhu L, Rajan TV, Clark RB. Natural but not inducible regulatory T cells require TNF-alpha signaling for in vivo function. J Immunol. 2011;186:6779–6787. doi: 10.4049/jimmunol.1003868. [DOI] [PubMed] [Google Scholar]
- 44.Tsakiri N, Papadopoulos D, Denis MC, Mitsikostas DD, Kollias G. TNFR2 on non-haematopoietic cells is required for Foxp3+ Treg-cell function and disease suppression in EAE. Eur J Immunol. 2012;42:403–412. doi: 10.1002/eji.201141659. [DOI] [PubMed] [Google Scholar]
- 45.Gogishvili T, Luhder F, Goebbels S, Beer-Hammer S, Pfeffer K, Hunig T. Cell-intrinsic and -extrinsic control of Treg-cell homeostasis and function revealed by induced CD28 deletion. Eur J Immunol. 2013;43:188–193. doi: 10.1002/eji.201242824. [DOI] [PubMed] [Google Scholar]
- 46.Rozanski CH, Arens R, Carlson LM, Nair J, Boise LH, Chanan-Khan AA, Schoenberger SP, Lee KP. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med. 2011;208:1435–1446. doi: 10.1084/jem.20110040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pillai S, Cariappa A, Moran ST. Marginal zone B cells. Annu Rev Immunol. 2005;23:161–196. doi: 10.1146/annurev.immunol.23.021704.115728. [DOI] [PubMed] [Google Scholar]
- 48.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167:1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 49.Wither JE, Loh C, Lajoie G, Heinrichs S, Cai YC, Bonventi G, MacLeod R. Colocalization of expansion of the splenic marginal zone population with abnormal B cell activation and autoantibody production in B6 mice with an introgressed New Zealand Black chromosome 13 interval. J Immunol. 2005;175:4309–4319. doi: 10.4049/jimmunol.175.7.4309. [DOI] [PubMed] [Google Scholar]
- 50.Zhou Z, Niu H, Zheng YY, Morel L. Autoreactive marginal zone B cells enter the follicles and interact with CD4+ T cells in lupus-prone mice. BMC immunology. 2011;12:7. doi: 10.1186/1471-2172-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marino E, Batten M, Groom J, Walters S, Liuwantara D, Mackay F, Grey ST. Marginal-zone B-cells of nonobese diabetic mice expand with diabetes onset, invade the pancreatic lymph nodes, and present autoantigen to diabetogenic T-cells. Diabetes. 2008;57:395–404. doi: 10.2337/db07-0589. [DOI] [PubMed] [Google Scholar]
- 52.Njau MN, Kim JH, Chappell CP, Ravindran R, Thomas L, Pulendran B, Jacob J. CD28-B7 interaction modulates short- and long-lived plasma cell function. J Immunol. 2012;189:2758–2767. doi: 10.4049/jimmunol.1102728. [DOI] [PMC free article] [PubMed] [Google Scholar]