Abstract
Objective
Hypertension, a common modifiable cardiovascular risk factor, is more common in patients with rheumatoid arthritis (RA), but the underlying mechanisms are unclear. We examined the hypothesis that mediators of inflammation and markers of cardiovascular risk are associated with hypertension in RA.
Methods
We compared measures of inflammation (serum C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), homocysteine and leptin concentrations) and insulin resistance (homeostasis model assessment index (HOMA)) in RA patients with (n=90) and without hypertension (n=79). Hypertension was defined as blood pressure ≥140/90 mmHg or treatment with antihypertensive therapy. The independent association of markers of interest with hypertension was examined using multivariable logistic regression.
Results
Hypertensive patients were significantly older and had longer disease duration than those without hypertension (both P<0.001). Concentrations of homocysteine (11.1[8.5–13.5] μmol/L vs. 9.3[7.8–11.0] μmol/L were significantly higher in hypertensive patients (P<0.001). After adjustment for age, sex, race, smoking, body mass index, and corticosteroid and NSAID use, increased concentrations of homocysteine (OR 2.9, 95%CI: 1.5–5.5, P=0.001), and leptin (OR 2.0, 95%CI: 1.0–3.8, P=0.046) were significantly associated with hypertension, but the 28-joint Disease Activity Score, IL-6, CRP, TNF-α and HOMA index were not (all P values >0.05).
Conclusion
Hypertension in patients with RA is not associated with generalized systemic inflammation or insulin resistance, but is associated with increasing concentrations of homocysteine and leptin. The pathogenesis of hypertension in RA may involve pathways more likely usually associated with fat and vascular homeostasis.
Keywords: rheumatoid arthritis, inflammation, hypertension, blood pressure, homocysteine, leptin, insulin resistance
Patients with rheumatoid arthritis (RA) have increased cardiovascular morbidity and mortality.(1) The mechanisms underlying increased cardiovascular risk are unclear, but are likely to include an increased prevalence of some traditional cardiovascular risk factors as well as additional risk factors specific to RA. Hypertension is one of the most common modifiable cardiovascular risk factors in the general population. In patients with RA hypertension may represent a traditional cardiovascular risk factor that is both increased in prevalence and also modified by the presence of the disease.(2)
Not all studies are concordant, but several, including our own, have found that the hypertension is more common in patients with RA, particularly in those with long-standing disease, than in the general population.(3, 4) As is the case in the general population, hypertension in patients with RA is associated with increased atherosclerosis and cardiovascular risk.(1, 5) Thus, identifying factors that contribute to hypertension in RA is important, particularly where these can be modified and thus targeted interventions could improve outcomes.
The factors that account for the increased prevalence of hypertension in RA are not well defined, but there are several possible contributors; these include medications, inflammation, oxidative stress and insulin resistance.(4, 6) In addition, concentrations of homocysteine and leptin are increased in patients with RA,(7, 8) and are associated with hypertension in the general population.(9, 10)
There is limited information about hypertension in patients with RA. One study found that hypertension was associated with age, body mass index (BMI) and daily prednisolone dose ≥7.5 mg and ≤30 mg, but not with other medications, inflammation or insulin sensitivity.(11) We have previously reported that blood pressure in patients with RA was not associated with the use of medications(12) or insulin resistance,(13) and that hypertension was associated with osteoprotegerin concentrations,(7) but not with oxidative stress.(14) Considering the importance of hypertension as a cardiovascular risk factor, and its unexplained increased prevalence in patients with RA, we examined the hypothesis that inflammation, homocysteine and leptin are independently associated with the presence of hypertension.
Methods
Patients
One hundred and sixty-nine patients who are part of a study of cardiovascular risk in RA were recruited as previously described.(15) Consecutive eligible patients older than 18 years of age who met the ACR classification criteria for RA(16) and had duration of disease less than 5 years or more than 10 years were enrolled. Controls did not meet classification criteria for rheumatoid arthritis or any other inflammatory disease. Control subjects were frequency matched for age, sex and race with the entire group of rheumatoid arthritis patients so as to ensure that the control group would not differ markedly from either the early or established rheumatoid arthritis groups with respect to these variables. Patients were recruited from a registry of patients with early rheumatoid arthritis, local rheumatologists and by advertisements. Control subjects were recruited from patients’ acquaintances, by advertisement, and from a database of volunteers maintained by the General Clinical Research Center. The study was approved by the Institutional Review Board of Vanderbilt University Hospital and all subjects gave written informed consent.
Clinical assessment
Details regarding recruitment and study procedures have been published.(15) Briefly, information was obtained from interviews, review of the medical records, self-report questionnaires, physical examination and laboratory tests. Blood pressure was measured by a trained study coordinator using an appropriate cuff size and using a semiautomated device (DINAMAP® PRO Series 200). Blood pressure was determined as the average of two measurements obtained 5 minutes apart after subjects had rested in the supine position for at least 10 minutes. Hypertension was defined as systolic blood pressure ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg or currently taking antihypertensive drugs.
Height and weight were measured and body mass index (BMI) calculated by dividing weight (kg) by the square of the height (m2). RA disease activity was measured using the Disease Activity Score based on the evaluation 28 joints (DAS28).(17) DAS28 is a composite index containing a 28-joint count for tenderness, a 28-joint count for swelling, erythrocyte sedimentation rate (ESR), and the overall assessment of well-being.
Laboratory tests
Blood was collected after an overnight fast. Glucose, homocysteine and C-reactive protein (CRP) concentrations, and Westergren ESR were measured by the hospital laboratory. Before 2003, the laboratory did not use a high-sensitivity CRP assay, and low concentrations were reported as <3 mg/l; in 40 patients with RA who had CRP concentrations <3 mg/l, CRP concentrations were measured by multiplex ELISA. Serum concentrations of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), insulin, and leptin were measured by multiplex ELISA (LincoPlex Multiplex Immunoassy kit; Millipore). Homeostasis model assessment (HOMA) index was calculated with the following formula: [fasting glucose (mmol/l) × fasting insulin (μU/ml)/22.5].(18)
Statistical analysis
Data are presented as median and interquartile range (IQR). Univariate analyses were performed to compare differences between patients with and without hypertension using Pearson’s Chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables.
The independent association of each variable with the presence or absence of hypertension was assessed using separate multivariable logistic regression models. Covariates for adjustment were chosen a priori based on factors known to be associated with blood pressure in RA and non-RA populations (4, 19, 20) and included age, sex, race, smoking, BMI, and current use of corticosteroids and non-steroidal antiinflammatory drugs (NSAIDs). All inflammation measures were natural logarithm-transformed to improve normality. Goodness-of-fit tests for logistic regression models were assessed using Hosmer-Lemeshow tests. Statistical analysis was performed with R 2.9.1(http://www.r-project.org) and 2-sided p-values <0.05 were considered statistically significant.
Results
The demographic and clinical characteristics of patients with RA with and without hypertension are summarized in Table 1. Hypertension was present in 90 patients (53.3%) who were significantly older than those without hypertension (n=79) (median [IQR] age 58.5 [51.2–67.0] years vs. 46.0 [41.0–56.0] years, P<0.001) and had longer duration of RA (P<0.001). Gender, BMI, smoking history and serum creatinine did not differ significantly among patients with and without hypertension (all P values >0.05). Despite the fact that 64 of the 90 patients with hypertension were receiving antihypertensive treatment, their systolic and diastolic blood pressure was significantly higher (145 [136–157] vs. 120 [112–130] mm Hg and 78 [71–87] vs.71 [66–78] mm Hg, respectively) than that of patients without hypertension (both P values <0.001).
Table 1.
Clinical characteristics of patients with RA with and without hypertension
| Hypertension present* (n = 90) | Hypertension absent* (n = 79) | P-value+ | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 58.5 (51.2–67.0) | 46.0 (41.0–56.0) | <0.001 |
| Sex (% female) | 73 | 65 | 0.22 |
| Race (% white) | 87 | 90 | 0.61 |
| Systolic blood pressure (mmHg) | 145 (136–157) | 120 (112–130) | <0.001 |
| Diastolic blood pressure (mmHg) | 78 (71–87) | 71 (66–78) | <0.001 |
| Body mass index (kg/m2) | 28.4 (24.0–33.4) | 27.9 (23.8–32.4) | 0.71 |
| Smoking (pack-years) | 0 (0–28) | 0 (0–18) | 0.63 |
| Serum creatinine (mg/dl) | 0.8 (0.7–1.0) | 0.8 (0.7–0.9) | 0.06 |
| Characteristics of rheumatoid arthritis | |||
| Disease duration of RA (years) | 11.0 (2.0–20.0) | 2.0 (1.6–11.5) | <0.001 |
| Current use of glucocorticoids (%) | 52 | 57 | 0.54 |
| Current use of NSAIDs (%) | 58 | 65 | 0.37 |
Values are the median (interquartile range) unless otherwise indicated.
Wilcoxon’s rank sum test was used for comparing continuous variables and Pearson’s Chi-square test for comparison of categorical variables.
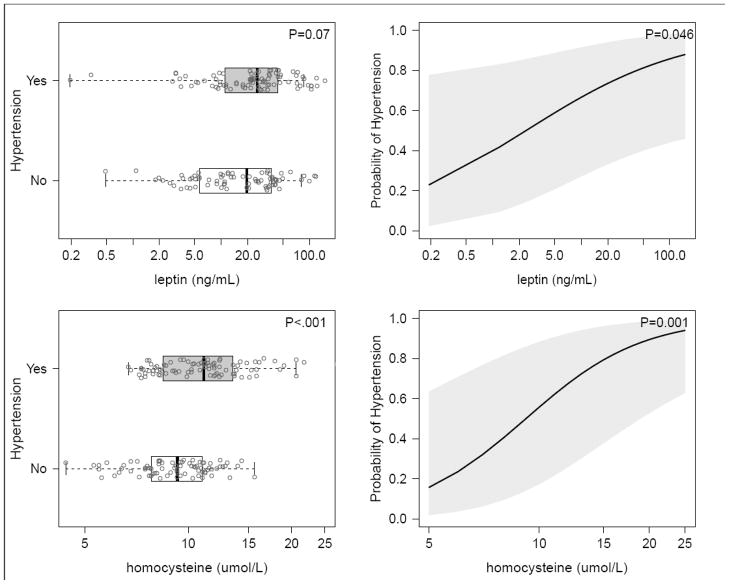
In unadjusted analyses, disease characteristics including DAS28 score (P=0.04) and ESR (P=0.02) were higher, and methotrexate use was less frequent (P=0.045), in patients with hypertension compared to those without, but CRP, TNF-α, and IL-6 and HOMA index were not significantly associated with hypertension (Table 2). Homocysteine concentrations (11.1 [8.5–13.5] μmol/L vs. 9.3 [7.8–11.0] μmol/L were significantly higher among hypertensive patients (p<0.001), and leptin concentrations were marginally higher (25.7 [11.0–43.8] ng/ml vs. 19.5 [5.7–36.9] pg/ml, P=0.07, Table 2, Figure 1).
Table 2.
Disease characteristics and biomarkers in patients with and without hypertension
| Hypertension present* (n = 90) | Hypertension absent * (n = 79) | P-value** | Adjusted Odds Ratio (95% CI)*** | P-value*** | |
|---|---|---|---|---|---|
| Disease duration of RA (years) | 11.0 (2.0–20.0) | 2.0 (1.6–11.5) | <0.001 | 1.3 (0.7–2.4) | 0.35 |
| DAS28 scores | 4.1 (2.8–5.0) | 3.6 (2.4–4.5) | 0.04 | 1.2 (0.7–2.0) | 0.45 |
| M-HAQ scores | 0.5 (0.1–0.9) | 0.4 (0.0–0.8) | 0.19 | 1.3 (0.7–2.5) | 0.48 |
| Current methotrexate use† (%) | 64 | 78 | 0.045 | 0.4 (0.2–1.0) | 0.04 |
| Current leflunomide use† (%) | 20 | 16 | 0.55 | 1.8 (0.7–4.6) | 0.25 |
| Current hydroxychloroquine use† (%) | 21 | 29 | 0.23 | 0.8 (0.3–1.9) | 0.59 |
| Current anti-TNF drug use† (%) | 17 | 25 | 0.17 | 0.5 (0.2–1.3) | 0.15 |
| ESR (mm/hour) | 19 (10–39) | 11 (5–28) | 0.02 | 1.2 (0.7–2.1) | 0.47 |
| CRP (mg/L) | 4.5 (2.0–11.0) | 3.0 (1.0–10.0) | 0.16 | 1.3(0.7–2.3) | 0.42 |
| TNF-α (pg/ml) | 6.6 (3.2–11.5) | 4.8 (2.5–9.6) | 0.12 | 1.2 (0.8–1.8) | 0.35 |
| IL-6 (pg/ml) | 16.1 (6.3–38.8) | 12.4 (3.5–44.9) | 0.13 | 1.2 (0.7–2.1) | 0.56 |
| HOMA index | 2.5 (1.1–5.4) | 1.9 (1.3–3.5) | 0.18 | 1.1 (0.7–1.7) | 0.68 |
| Leptin (ng/ml) | 25.7 (11.0–43.8) | 19.5 (5.7–36.9) | 0.07 | 2.0 (1.0–3.8) | 0.046 |
| Homocysteine (μmol/L) | 11.1 (8.5–13.5) | 9.3 (7.8–11.0) | <0.001 | 2.9 (1.5–5.5) | 0.001 |
DAS28=Disease Activity Score 28-joint assessment; M-HAQ=Modified Health Assessment Questionnaire; ESR=erythrocyte sedimentation rate; CRP=C-reactive protein.
Values are the median (interquartile range) unless otherwise indicated.
Wilcoxon rank sum test was used for comparing continuous variables and Chi-square test for comparison of categorical variables (unadjusted analyses).
Separate multivariable logistic regression models adjusted for age, sex, race, BMI, smoking status, current corticosteroid use and current NSAIDs were used to estimate the association of each factor with hypertension. Biomarkers were natural log transformed. For continuous variables, the odds ratio (OR) with 95% confidence interval (95% CIs) is presented per interquartile range increment.
Non-users represent reference group
Figure 1.
Leptin and homocysteine distribution according to hypertension status (left panels) and adjusted probability of hypertension (right panels)
Data are presented as box plots (left panels), where the boxes represent the interquartile range (IQR), the lines within boxes represent the median, and the lines outside the boxes represent the lower quartile minus 1.5 times the IQR or the upper quartile plus 1.5 times the IQR. The right panels shows the increasing adjusted probability of hypertension with increasing concentrations of leptin and homocysteine from separate multivariable logistic regression model adjusted for age, sex, race, BMI, smoking status, current use of corticosteroid or NSAIDs.
After adjustment for age, sex, race, smoking, BMI, and current use of corticosteroids and NSAIDs, concentrations of homocysteine (OR 2.9, 95% CI: 1.5–5.5, P=0.001) and leptin (OR 2.0, 95% CI 1.0–3.8, P=0.046) were statistically significantly associated with hypertension. After adjustment for covariates, there were no significant differences as regards disease duration, DAS28 scores, ESR and CRP among patients with and without hypertension (Table 2). The less frequent current use of methotrexate in hypertensive patients remained statistically significant (P=0.04) after adjustment (Table 2).
Discussion
The major new finding of this study is that hypertension in patients with RA is associated with increased concentrations of homocysteine and leptin but not with markers of inflammation.
Most studies, including our own,(3) have found that the proportion of patients with hypertension is higher in RA than in the general population. In one of the largest studies involving 28,208 patients with RA and 112,832 control subjects the prevalence of hypertension was 31% in patients with RA compared to 23.4% in controls.(21) In our study hypertension was present in 53.3% (95%CI: 45.7%, 60.6%) of patients, and this compares with 71% in a study of 400 patients with RA that was performed in an older population (median age 65 years vs. 58.5 years in the current study).(11) The high prevalence of hypertension reported in patients with RA suggests that factors related to RA or its treatment may play a role in the pathogenesis of hypertension. Factors most commonly proposed as risk factors for hypertension in RA include medications, inflammation, oxidative stress and insulin resistance.
Several medications used to treat RA affect blood pressure. For example, corticosteroids, NSAIDs and leflunomide(22) can increase blood pressure. However, we have previously reported that blood pressure did not differ among patients currently taking or not taking these medications.(12) Similarly, in the present study we found no association between current use of NSAIDs, corticosteroids or leflunomide and hypertension. This may be because the changes in blood pressure associated with medications are small and variable, or that physicians avoid prescribing medications that can increase blood pressure to patients at risk of hypertension. Methotrexate use was less frequent in patients with hypertension. However, another study found no relationship between methotrexate use and hypertension,(23) and our findings could be confounded by the selection of patients for methotrexate therapy if physicians avoided methotrexate in patients with hypertension because of concerns about future renal impairment. Prospective studies to define the effect of methotrexate on blood pressure will be required to specifically determine if it protects against hypertension. We have previously found that both systolic and diastolic blood pressure tended to be lower in patients receiving hydroxychloroquine,(12) but in the present study the frequency of hydroxychloroquine use did not differ among patients with and without hypertension.
Inflammation, mediated through cytokines, is directly associated with increased arterial stiffness and endothelial dysfunction, and could thus predispose to the development of hypertension.(24, 25) Inflammation can also result in increased oxidative stress, and thus decreased nitric oxide bioavailability and consequently endothelial dysfunction, impaired vasodilatation and increased arterial stiffness.(26) Indeed, in keeping with the theory that inflammation plays a role in the pathogenesis of hypertension, increased concentrations of markers of inflammation such as ESR, CRP, TNF-α and IL-6 were significantly associated with hypertension in non-RA populations.(27–29) Although concentrations of these inflammatory markers are significantly increased in patients with RA, we found no differences in concentrations among patients with and without hypertension. These findings are concordant with another study that found no significant association between DAS28, CRP or ESR and hypertension in RA,(11) and they suggest that generalized systemic inflammation itself may not be the major factor underlying increased risk of hypertension in RA.
We have observed that insulin resistance is more common in patients with RA than control subjects.(3) Insulin resistance is associated with hypertension in the general population and may affect blood pressure through mechanisms that include enhanced renal tubular sodium reabsorption, endothelial dysfunction, and increased sympathetic activity.(30) As was the case in another study,(11) we found no difference in insulin sensitivity in patients with and without hypertension. These finding suggest that it is unlikely that insulin resistance is a key factor in the development of hypertension in RA.
Higher concentrations of homocysteine are known to be associated with atherosclerosis and thrombosis, but their association with hypertension is less widely recognized. Several studies have reported that higher concentrations of homocysteine are associated with increased blood pressure and hypertension.(31, 32) Additionally, some studies have shown that treatment to lower homocysteine can be associated with a reduction in both systolic and diastolic blood pressure.(33, 34) Homocysteine is thought to affect blood pressure regulation through several mechanisms including impaired vascular endothelial and smooth muscle cell function, oxidative stress, and increased renal sodium reabsorption.(35)
Homocysteine concentrations are elevated in patients with RA compared to control subjects,(3) and in our cohort, homocysteine concentrations were not affected by current methotrexate, perhaps because concurrent folic acid use was almost universal.(12) We found that higher homocysteine concentrations were associated with hypertension in RA, but interestingly, we previously observed that oxidative stress, as determined by urinary F2-isoprostane excretion, was not.(14) This suggests that increased oxidative stress is not the mechanism through which homocysteine contributes to hypertension in RA.
It is unclear why homocysteine concentrations are increased, or how they might contribute to hypertension, in RA. There are several possibilities. It appears unlikely that altered renal elimination of homocysteine accounts for higher concentrations in RA because we have previously shown in this cohort that creatinine clearance did not differ significantly among RA and control subjects.(36) However, homocysteine production or clearance may be altered because increased homocysteine levels after a methionine load, and an association between homocysteine levels and inflammation, have been observed in RA.(37, 38) The adverse effects of homocysteine on endothelial function(39) may be particularly important in RA since endothelial function is often impaired.(40)
Leptin is a peptide hormone secreted by adipocytes that suppresses appetite. In addition, there are several mechanisms whereby chronically increased leptin concentrations could increase blood pressure. These include increased sympathetic nervous system activity and impaired natriuresis.(41) Leptin concentrations are strongly associated with obesity and several studies in the general population have found that higher leptin concentrations are associated with hypertension.(41) We have reported that leptin concentrations were higher in patients with RA than control subjects, and that this difference was independent of BMI and correlated with the degree of inflammation.(8) Our finding that leptin concentrations are associated with hypertension in RA, independent of BMI and other demographic confounders, raises the possibility that leptin, in addition to a positive association with insulin resistance,(42) and a negative association with joint damage,(8) may play a direct role in the pathogenesis of hypertension in RA.
Our study has a number of limitations; the cross-sectional, observational design can establish association but not causality. Furthermore, we cannot exclude the possibility that factors associated with the early development of hypertension may no longer be evident in established hypertension. Also, in an observational study it is difficult to define the role of specific medications since they may be differentially prescribed to patients with and without hypertension. The number of patients studied, although large for RA studies, is small compared to studies performed in a more general population.
In conclusion, hypertension in patients with RA is associated with higher concentrations of homocysteine and leptin, but not with insulin resistance or markers of inflammation. The pathogenesis of hypertension in RA may involve pathways more usually associated with the maintenance of fat and vascular homeostasis.
Acknowledgments
Sources of Funding: Supported by NIH grants, P60 AR056116, HL65082, HL67964, GM07569, UL1RR024975 from NCRR/NIH and the Dan May Chair in Medicine.
Footnotes
Disclosures: None of the authors has a conflict of interest related to this work.
Reference List
- 1.Wolfe F, Freundlich B, Straus WL. Increase in cardiovascular and cerebrovascular disease prevalence in rheumatoid arthritis. J Rheumatol. 2003 Jan;30(1):36–40. [PubMed] [Google Scholar]
- 2.Dessein PH, Joffe BI, Veller MG, Stevens BA, Tobias M, Reddi K, et al. Traditional and nontraditional cardiovascular risk factors are associated with atherosclerosis in rheumatoid arthritis. J Rheumatol. 2005 Mar;32(3):435–42. [PubMed] [Google Scholar]
- 3.Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008 Feb;196(2):756–63. doi: 10.1016/j.atherosclerosis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 4.Panoulas VF, Metsios GS, Pace AV, John H, Treharne GJ, Banks MJ, et al. Hypertension in rheumatoid arthritis. Rheumatology (Oxford) 2008 Sep;47(9):1286–98. doi: 10.1093/rheumatology/ken159. [DOI] [PubMed] [Google Scholar]
- 5.Bergstrom U, Jacobsson LT, Turesson C. Cardiovascular morbidity and mortality remain similar in two cohorts of patients with long-standing rheumatoid arthritis seen in 1978 and 1995 in Malmo, Sweden. Rheumatology (Oxford) 2009 Dec;48(12):1600–5. doi: 10.1093/rheumatology/kep301. [DOI] [PubMed] [Google Scholar]
- 6.Panoulas VF, Douglas KM, Stavropoulos-Kalinoglou A, Metsios GS, Nightingale P, Kita MD, et al. Long-term exposure to medium-dose glucocorticoid therapy associates with hypertension in patients with rheumatoid arthritis. Rheumatology (Oxford) 2008 Jan;47(1):72–5. doi: 10.1093/rheumatology/kem311. [DOI] [PubMed] [Google Scholar]
- 7.Asanuma Y, Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, et al. Serum osteoprotegerin is increased and independently associated with coronary-artery atherosclerosis in patients with rheumatoid arthritis. Atherosclerosis. 2007 Dec;195(2):e135–e141. doi: 10.1016/j.atherosclerosis.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rho YH, Solus J, Sokka T, Oeser A, Chung CP, Gebretsadik T, et al. Adipocytokines are associated with radiographic joint damage in rheumatoid arthritis. Arthritis Rheum. 2009 Jul;60(7):1906–14. doi: 10.1002/art.24626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim U, Cassano PA. Homocysteine and blood pressure in the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2002 Dec 15;156(12):1105–13. doi: 10.1093/aje/kwf157. [DOI] [PubMed] [Google Scholar]
- 10.Agata J, Masuda A, Takada M, Higashiura K, Murakami H, Miyazaki Y, et al. High plasma immunoreactive leptin level in essential hypertension. Am J Hypertens. 1997 Oct;10(10 Pt 1):1171–4. doi: 10.1016/s0895-7061(97)00310-5. [DOI] [PubMed] [Google Scholar]
- 11.Panoulas VF, Douglas KM, Milionis HJ, Stavropoulos-Kalinglou A, Nightingale P, Kita MD, et al. Prevalence and associations of hypertension and its control in patients with rheumatoid arthritis. Rheumatology (Oxford) 2007 Sep;46(9):1477–82. doi: 10.1093/rheumatology/kem169. [DOI] [PubMed] [Google Scholar]
- 12.Rho YH, Oeser A, Chung CP, Milne GL, Stein CM. Drugs Used in the Treatment of Rheumatoid Arthritis: Relationship between Current Use and Cardiovascular Risk Factors. Arch Drug Inf. 2009 Jun;2(2):34–40. doi: 10.1111/j.1753-5174.2009.00019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, Avalos I, et al. Inflammation-associated insulin resistance: differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008 Jul;58(7):2105–12. doi: 10.1002/art.23600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rho YH, Chung CP, Oeser A, Solus JF, Gebretsadik T, Shintani A, et al. Interaction between oxidative stress and high-density lipoprotein cholesterol is associated with severity of coronary artery calcification in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010 Oct;62(10):1473–80. doi: 10.1002/acr.20237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung CP, Oeser A, Raggi P, Gebretsadik T, Shintani AK, Sokka T, et al. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheum. 2005 Sep 30;52(10):3045–53. doi: 10.1002/art.21288. [DOI] [PubMed] [Google Scholar]
- 16.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis & Rheumatism. 1988 Mar;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 17.Prevoo ML, van ‘t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995 Jan;38(1):44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 18.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 19.Lakoski SG, Herrington DM, Siscovick DM, Hulley SB. C-reactive protein concentration and incident hypertension in young adults: the CARDIA study. Arch Intern Med. 2006 Feb 13;166(3):345–9. doi: 10.1001/archinte.166.3.345. [DOI] [PubMed] [Google Scholar]
- 20.Lakoski SG, Cushman M, Siscovick DS, Blumenthal RS, Palmas W, Burke G, et al. The relationship between inflammation, obesity and risk for hypertension in the Multi-Ethnic Study of Atherosclerosis (MESA) J Hum Hypertens. 2011 Feb;25(2):73–9. doi: 10.1038/jhh.2010.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006 Nov;33(11):2167–72. [PubMed] [Google Scholar]
- 22.Rozman B, Praprotnik S, Logar D, Tomsic M, Hojnik M, Kos-Golja M, et al. Leflunomide and hypertension. Ann Rheum Dis. 2002 Jun;61(6):567–9. doi: 10.1136/ard.61.6.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van HV, Nurmohamed MT, Twisk JW, Dijkmans BA, Voskuyl AE. Disease-modifying antirheumatic drugs are associated with a reduced risk for cardiovascular disease in patients with rheumatoid arthritis: a case control study. Arthritis Res Ther. 2006;8(5):R151. doi: 10.1186/ar2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roman MJ, Devereux RB, Schwartz JE, Lockshin MD, Paget SA, Davis A, et al. Arterial stiffness in chronic inflammatory diseases. Hypertension. 2005 Jul;46(1):194–9. doi: 10.1161/01.HYP.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- 25.Mahmud A, Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005 Nov;46(5):1118–22. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- 26.Landmesser U, Harrison DG. Oxidative stress and vascular damage in hypertension. Coron Artery Dis. 2001 Sep;12(6):455–61. doi: 10.1097/00019501-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Rafnsson V, Bengtsson C. Erythrocyte sedimentation rate and cardiovascular disease. Results from a population study of women in Goteborg, Sweden. Atherosclerosis. 1982 Mar;42(1):97–107. doi: 10.1016/0021-9150(82)90130-7. [DOI] [PubMed] [Google Scholar]
- 28.Sesso HD, Wang L, Buring JE, Ridker PM, Gaziano JM. Comparison of interleukin-6 and C-reactive protein for the risk of developing hypertension in women. Hypertension. 2007 Feb;49(2):304–10. doi: 10.1161/01.HYP.0000252664.24294.ff. [DOI] [PubMed] [Google Scholar]
- 29.Cottone S, Mule G, Nardi E, Vadala A, Guarneri M, Briolotta C, et al. Relation of C-reactive protein to oxidative stress and to endothelial activation in essential hypertension. Am J Hypertens. 2006 Mar;19(3):313–8. doi: 10.1016/j.amjhyper.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Ferrannini E. Is insulin resistance the cause of the metabolic syndrome? Ann Med. 2006;38(1):42–51. doi: 10.1080/07853890500415358. [DOI] [PubMed] [Google Scholar]
- 31.Nygard O, Vollset SE, Refsum H, Stensvold I, Tverdal A, Nordrehaug JE, et al. Total plasma homocysteine and cardiovascular risk profile. The Hordaland Homocysteine Study. JAMA. 1995 Nov 15;274(19):1526–33. doi: 10.1001/jama.1995.03530190040032. [DOI] [PubMed] [Google Scholar]
- 32.Sutton-Tyrrell K, Bostom A, Selhub J, Zeigler-Johnson C. High homocysteine levels are independently related to isolated systolic hypertension in older adults. Circulation. 1997 Sep 16;96(6):1745–9. doi: 10.1161/01.cir.96.6.1745. [DOI] [PubMed] [Google Scholar]
- 33.van Dijk RA, Rauwerda JA, Steyn M, Twisk JW, Stehouwer CD. Long-term homocysteine-lowering treatment with folic acid plus pyridoxine is associated with decreased blood pressure but not with improved brachial artery endothelium-dependent vasodilation or carotid artery stiffness: a 2-year, randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol. 2001 Dec;21(12):2072–9. doi: 10.1161/hq1201.100223. [DOI] [PubMed] [Google Scholar]
- 34.Mangoni AA, Sherwood RA, Swift CG, Jackson SH. Folic acid enhances endothelial function and reduces blood pressure in smokers: a randomized controlled trial. J Intern Med. 2002 Dec;252(6):497–503. doi: 10.1046/j.1365-2796.2002.01059.x. [DOI] [PubMed] [Google Scholar]
- 35.van Guldener GC, Nanayakkara PW, Stehouwer CD. Homocysteine and blood pressure. Curr Hypertens Rep. 2003 Feb;5(1):26–31. doi: 10.1007/s11906-003-0007-z. [DOI] [PubMed] [Google Scholar]
- 36.Lertnawapan R, Bian A, Rho YH, Kawai VK, Raggi P, Oeser A, et al. Cystatin C, Renal Function, and Atherosclerosis in Rheumatoid Arthritis. J Rheumatol. 2011 Aug 15; doi: 10.3899/jrheum.110168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roubenoff R, Dellaripa P, Nadeau MR, Abad LW, Muldoon BA, Selhub J, et al. Abnormal homocysteine metabolism in rheumatoid arthritis. Arthritis & Rheumatism. 1997 Apr;40(4):718–22. doi: 10.1002/art.1780400418. [DOI] [PubMed] [Google Scholar]
- 38.Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, et al. Hyperhomocysteinemia: a cardiovascular risk factor in autoimmune diseases? Lupus. 2007;16(11):852–62. doi: 10.1177/0961203307084176. [DOI] [PubMed] [Google Scholar]
- 39.Welch GN, Loscalzo J. Homocysteine and atherothrombosis [see comments] New England Journal of Medicine. 1998 Apr 9;338(15):1042–50. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 40.Khan F, Galarraga B, Belch JJ. The role of endothelial function and its assessment in rheumatoid arthritis. Nat Rev Rheumatol. 2010 May;6(5):253–61. doi: 10.1038/nrrheum.2010.44. [DOI] [PubMed] [Google Scholar]
- 41.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008 Jun 24;117(25):3238–49. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rho YH, Chung CP, Solus JF, Raggi P, Oeser A, Gebretsadik T, et al. Adipocytokines, insulin resistance, and coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2010 May;62(5):1259–64. doi: 10.1002/art.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]