Abstract
In the fruit fly Drosophila melanogaster, a network of circadian pacemaker neurons drives daily rhythms in rest and activity. The ion channel NARROW ABDOMEN (NA), orthologous to the mammalian sodium leak channel NALCN, functions downstream of the molecular circadian clock in pacemaker neurons to promote behavioral rhythmicity. To better understand the function and regulation of the NA channel, we have characterized two putative auxiliary channel subunits in Drosophila, unc79 (aka dunc79) and unc80 (aka CG18437). We have generated novel unc79 and unc80 mutations that represent strong or complete loss-of-function alleles. These mutants display severe defects in circadian locomotor rhythmicity that are indistinguishable from na mutant phenotypes. Tissue-specific RNA interference and rescue analyses indicate that UNC79 and UNC80 likely function within pacemaker neurons, with similar anatomical requirements to NA. We observe an interdependent, post-transcriptional regulatory relationship among the three gene products, as loss of na, unc79, or unc80 gene function leads to decreased expression of all three proteins, with minimal effect on transcript levels. Yet despite this relationship, we find that the requirement for unc79 and unc80 in circadian rhythmicity cannot be bypassed by increasing NA protein expression, nor can these putative auxiliary subunits substitute for each other. These data indicate functional requirements for UNC79 and UNC80 beyond promoting channel subunit expression. Immunoprecipitation experiments also confirm that UNC79 and UNC80 form a complex with NA in the Drosophila brain. Taken together, these data suggest that Drosophila NA, UNC79, and UNC80 function together in circadian clock neurons to promote rhythmic behavior.
Introduction
Circadian rhythms are daily patterns of behavior and physiology driven by cellular clocks. Circadian clocks in metazoans consist of interdependent transcriptional feedback loops and post-translational modifications that produce ∼24 hour molecular oscillations. At the core of the Drosophila circadian clock, the transcription factor partners CLOCK (CLK) and CYCLE (CYC) upregulate the expression of period (per) and timeless (tim). PER and TIM proteins accumulate in the cytoplasm and later translocate to the nucleus, where they inhibit CLK-CYC activity and their own expression. This mechanism and others result in ∼24 hour rhythms in CLK-CYC transcription factor activity and in PER/TIM expression. This molecular clock is highly conserved in animals, and homologs of several Drosophila clock genes exhibit similar functions in mammals [1]. In Drosophila, the molecular clocks essential for daily activity rhythms are found in roughly 150 pacemaker neurons in the adult brain, and specific groups of these neurons have been shown to be important for different aspects of behavioral rhythmicity. A subset of pacemaker neurons express the neuropeptide Pigment-Dispersing Factor (PDF), and the PDF+ cells communicate to a broader group of pacemaker neurons to synchronize and enhance molecular clock oscillations [2].
An important component of circadian pacemaker neuronal output in Drosophila is NARROW ABDOMEN (NA), a putative sodium leak channel orthologous to mammalian NALCN. Drosophila na mutants exhibit strong defects in circadian locomotor behavior, as well as increased anesthetic sensitivity and “hesitant” walking [3]. Despite disruptions in circadian behavior, oscillations of the clock protein PER remain essentially intact in na mutants, indicating function primarily downstream of the molecular clock [4]. NA protein is expressed broadly in the adult Drosophila brain, and gene expression likely includes multiple groups of pacemaker neurons [3], [4]. Tissue-specific expression of na in most or all pacemaker neurons using the GAL4/ UAS system fully rescues rhythmic behavior in na mutants. Moreover, rescue of na rhythmicity phenotypes using the pan-neuronal driver elavGAL4 is blocked when the GAL4 inhibitor GAL80 is expressed specifically in circadian neurons (cryptochromeGAL80) [4]. In mammals, electrophysiological characterization has demonstrated that NALCN functions as a voltage-insensitive, non-selective cation channel that contributes sodium leak conductance. NALCN knockout mice display severe defects in respiratory rhythm and die within a day of birth, and mutant hippocampal neurons exhibit hyperpolarized resting membrane potential [5].
Two putative auxiliary channel subunits for NA/NALCN, known as UNC79 and UNC80, were first identified in C. elegans, where mutants display anesthetic sensitivity phenotypes that resemble Drosophila na mutants [6]. Like NA/NALCN, UNC79 and UNC80 orthologs are found in all animals. These large proteins (> = 300 Kd in most animals) are unrelated to each other and have no clear functional domains [7], [8]. In C. elegans, loss of function of any of the putative subunits (unc-79, unc-80, or the NA/NALCN homologs nca-1/2) produces similar phenotypes, including defects in crawling and swimming behavior as well as anesthetic sensitivity [7], [9]. In Drosophila, mutation of the UNC79 ortholog (unc79/ dunc79) causes anesthetic sensitivity and hesitant walking phenotypes [7], while the Drosophila UNC80 ortholog (CG18437) has not been characterized. In C. elegans, the three putative subunits exhibit interdependence, as loss of function of one of these genes is associated with either decreased expression or disrupted localization of each of the corresponding proteins. This regulatory relationship may be post-transcriptional, as nca-1 transcript levels are unaffected in either unc-80 or unc-79 mutants [7], [10]. Notably, Drosophila unc79 mutants also express decreased levels of NA protein but not transcript, suggesting a possible conserved regulatory relationship [7]. Yet some differences in this relationship are evident in mammals, where UNC79 mutant mice lack detectable levels of UNC80 protein but retain NALCN [11], [12].
Data from mammals has provided further insight into the function and regulation of this subfamily of ion channels. Co-immunoprecipitation experiments demonstrate that NALCN, UNC79 and UNC80 proteins form a complex in the mouse brain [11], [13]. Moreover, electrophysiological data indicate that NALCN channel activity is regulated through multiple G-protein coupled receptor (GPCR) signaling pathways. The substance P receptor TACR1 increases channel activity in a G-protein independent manner; this activation requires the UNC80 subunit and SRC kinase activity [13], [14]. The M3 muscarinic receptor may activate NALCN in pancreatic islet cells in a similar manner [15]. In contrast, the calcium sensing receptor (CaSR) GPCR appears to provide a tonic inhibition of NALCN channel activity that is G-protein dependent [11]. Notably, CaSR-mediated inhibition is disrupted in hippocampal neurons isolated from UNC79 knockout mice, which express NALCN protein but not UNC80. Inhibition can be restored by transfection of UNC80 into UNC79 mutant neurons, suggesting that the requirement for UNC79 in channel regulation can be bypassed by UNC80 [11].
Here we show that both UNC79 and UNC80 are important for promoting circadian behavioral rhythmicity in Drosophila. Similar to C. elegans, the three putative channel subunits in Drosophila exhibit an interdependent regulatory relationship that is primarily post-transcriptional. The anatomical requirements for all three gene products in circadian rhythms are similar or identical, and co-immunoprecipitation experiments demonstrate that these proteins form a complex in the Drosophila head. Transgenic expression of unc79 or unc80 in pacemaker neurons can restore rhythmicity to the corresponding mutant strain. However, transgenic expression of one subunit does not restore circadian behavior in the absence of another. Thus, both UNC79 and UNC80 likely have functional requirements in Drosophila beyond promoting expression of the other channel subunits. These requirements may include the modulation of NA channel activity, which may serve as an important mechanism for controlling the activity of circadian pacemaker neurons.
Materials and Methods
Drosophila strains
The unc79x25 allele was generated by imprecise excision of P-element insertion GS9462 (Drosophila Genetics Resource Center). Genomic analysis indicates that unc79x25 contains a 1305 bp genomic deletion in unc79, while retaining 12 bp of the P-element (Figure S1A). Transcript analysis was performed by cloning reverse transcriptase PCR (RT-PCR) products from unc79x25/ Df(3R)ED5942 and +/ Df(3R)ED5942 adult heads (TRIzol reagent, Superscript III Reverse Transcriptase, TOPO TA cloning; Invitrogen). All clones isolated from unc79x25/ Df(3R)ED5942 (11/11) lacked the 57 bp and 80 bp exons corresponding to the deleted sequence (Figure S1A, exons 12–13); some of these clones (3/11) contained a 16 bp exon in lieu of the 57/80 bp exons. Conceptual translation confirms that all 11 unc79x25 clones produce a shift in reading frame compared to the predicted wild-type UNC79 protein. The unc80x42 allele was produced by imprecise excision of GS12792 (Drosophila Genetics Resource Center), an insertion into a predicted unc80 coding exon (Figure S1B, exon 18). Genomic sequence analysis indicates that unc80x42 retains 14 bp of GS12792, but lacks the UAS repeat sequence. Conceptual translation of unc80x42 indicates that the 14 bp insertion introduces 2 stop codons and shifts the reading frame of any read-through products. Transgenic expression strains were produced by cloning unc79 or unc80 cDNAs into pUAST [16], followed by injection into Drosophila embryos (Bestgene Inc). Full-length unc79 cDNA was subcloned from two partially overlapping cDNAs (RE64326, GH05210), and a 6X MYC tag was added at the C-terminus. Full-length unc80 cDNA was generated by RT-PCR from wild-type head extracts (TRIzol reagent, Superscript III Reverse Transcriptase, TOPO TA cloning; Invitrogen), and a 3X HA tag was added at the N-terminus. For UAS-unc79MYC, two independent insertions (designated 23 and 24) were recombined on chromosome II to increase expression. All RNA interference (RNAi) strains and relevant controls were obtained from the Vienna Drosophila RNAi Center (VDRC) [17]. The unc79 and unc80 deficiency strains were obtained from the Bloomington Drosophila stock center [18], [19]. Other strains have been previously described: pdfGAL4 [20], timGAL4 [21], elavGAL4 [22], UAS-dcr2 [17], UAS-na U3 [4], nahar [3], w1118 iso31 [19], nae04385, unc79f03453, and unc79f01615 [7]. For the experiments indicated, na, unc79, and unc80 mutant strains were backcrossed to w1118 iso31 for 6-8 generations. After the final backcross, stable lines were generated from both wild-type and mutant individuals.
Cross schemes and behavior analyses
Drosophila crosses were maintained at 25°C in 12 hr Light: 12 hr Dark (LD) conditions, except some elavGAL4 UAS-dicer2 X RNAi crosses were raised at room temperature to increase viability. To produce unc79 and unc80 homozygous mutant progeny, most mutant strains were maintained over the TM3 hs-hid balancer [23]. These crosses (plus controls) were heat-shocked for 1-2 hrs at 37°C after 4–6 days in order to kill TM3 hs-hid progeny. The TM3 hs-hid balancer was also backcrossed to w1118 iso31 for 8 generations for use in balancing backcrossed unc79 and unc80 mutant and control strains.
For behavior analyses, locomotor activity levels were assayed from 0-7 day old adults for 5 days LD conditions followed by 7 days constant darkness (DD) at 25°C using the Drosophila Activity Monitor system (Trikinetics). The behavior data presented were analyzed from male flies, except where indicated. To produce LD activity profiles, activity levels of individual flies were normalized and averaged within genotypes over the last four days of LD conditions. Morning anticipation index (MAI) and evening anticipation index (EAI) were calculated from LD data by determining the largest 2–3 hour increase in normalized average activity of each genotype over the last 5 hours of dark phase (MAI) or the last 5 hours of light phase (EAI; modified from [4]). For DD analyses, chi-squared periodogram measurements were performed on individual flies using ClockLab analysis software (Actimetrics). Flies were considered rhythmic if the chi-squared power was > = 10 above significance, as previously described [4].
Antibodies and Western blot
Anti-UNC79 and UNC80 sera were produced by expressing His-tagged polypeptides of either unc79 (aa1843-1992, NP_001163652.1) or unc80 (aa 3092-3187, NP_651577.3) from BL21(DE3) cells using the pET28 plasmid and His-Bind column purification system (EMD Millipore). Unconjugated protein was used to inject rabbits, sera were tested by ELISA, and terminal bleeds used for protein detection. Rabbit anti-NA was described previously [3]. Mouse anti-α-spectrin (3A9; [24]) was obtained from the Developmental Studies Hybridoma Bank (University of Iowa, Department of Biology). Western blots were performed as previously described, using adult head extracts from mixed light phase (ZT 0–10) samples [4]. For experiments in which protein levels were compared between genotypes, additional steps were taken to improve the reliability of quantitation [25]. Equal amounts of head extract (4–10 µg, depending on experiment) were loaded in each lane as determined by Bradford protein assay (Bio-Rad), and similar protein levels were confirmed after transfer using SYPRO Ruby Protein Blot staining (Lonza) or Novex Reversible Membrane Protein Stain (Invitrogen). A minimum of three biological replicates was performed for each comparison made, and lane order was varied between experiments to control for uneven transfer. Protein levels were measured using NIH ImageJ gel analysis (http://rsbweb.nih.gov/ij/). To account for intensity differences based on exposure, protein levels were normalized to the average intensity of all samples within the blot; in most cases identical samples were loaded in each biological replicate. For NA, UNC79, and UNC80, the antisera used recognize both transgenic and endogenous proteins. Transgenic NA protein (derived from UAS-na) migrates more quickly on Western blot than the endogenous doublet, perhaps due to the use of a different start methionine [4]. For UNC79, a prominent band was often detected just below the endogenous and transgenic bands, but our evidence indicates that this band is non-specific. The intensity of this band varies depending on strain, rearing conditions, and immunoblotting procedures, and it is generally much more prominent when PVDF membrane is used as compared to nitrocellulose. Levels of this band are not consistently decreased using an RNAi strain that targets unc79 transcript near the region of the anti-UNC79 antigen (VDRC 108132; data not shown). Moreover, RNA-seq analyses have not identified any unc79 splice variants likely to produce a detectable protein of this size in both unc79+ and unc79x25 strains (Drosophila modENCODE project).
Co-immunoprecipitation
Protein was extracted from adult heads using homogenization buffer (0.25M sucrose, 10 mM Tris pH 7.4, 1 mM EDTA) with protease inhibitors (Roche). Nuclei and cellular debris were pelleted at 1000 x g for 10 min and the protein concentration of the cleared supernatant was measured using Bradford reagent (Bio-Rad). Supernatents were then subject to a second spin at 48,000 x g for 40 min in order to pellet membranes. This membrane pellet was resuspended in 150 mM NaCl, 50 mM Tris pH 7.4, 1 mM EDTA plus protease inhibitors. Incubation of resuspended membranes at a concentration of 2 mg/ml in 1% n-Dodecyl-β-D-Maltopyranoside, 1% Anapoe-58 (Affymetrix) in resuspension buffer for 40 minutes on ice was followed by a 20 minute spin at 133,000 x g (Beckman Coulter Airfuge) to pellet unsolubilized particles. Solubilized protein (80 µl) was incubated with 2 µl rabbit or mouse antiserum in a total volume of 0.5 ml and rocked at room temperature for one hour. A 2% bed volume of Protein-A:Sepharose beads (GE Healthcare) were added and the tube was rocked for one hour at 4°C. Beads were pelleted with a short spin, supernatant removed, and beads washed 3x in resuspension buffer with the above detergents. Elution was with 1/10th volume LDS running buffer with reducing agent (Invitrogen) and 20 µl was loaded per gel lane.
Statistical analysis
LD anticipation index values (MAI/EAI) and DD period values were compared among genotypes by Kruskal-Wallis one-way ANOVA followed by Dunn’s post-hoc test, using Sigmaplot (Systat Software). For DD rhythmicity data, the proportion of rhythmic flies was determined as described above, and comparisons were made between genotypes using Fisher’s exact test (Sigmaplot or Graphpad Quickcalcs, http://www.graphpad.com/quickcalcs/). For protein and RNA expression comparisons, significance was determined using Student’s t-test (Microsoft Excel).
Results
Drosophila unc79 and unc80 are required for robust behavioral rhythmicity
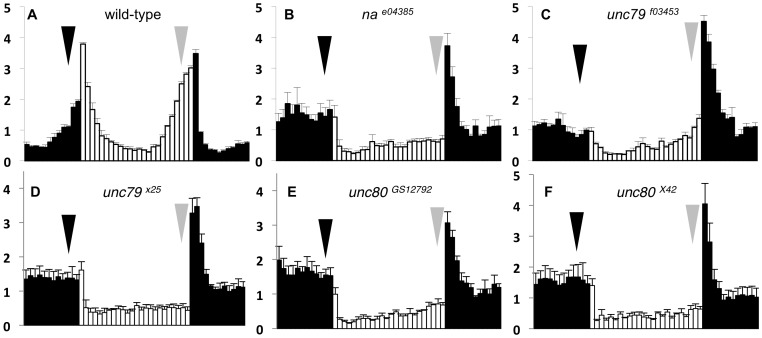
Drosophila unc79 and CG18437 (herein referred to as unc80) encode putative auxiliary subunits of the NA ion channel. We have previously shown that the pore-forming subunit NA is an important component of circadian pacemaker neuronal output [4]. To determine whether unc79 and unc80 also contribute to circadian rhythmicity, we assessed locomotor activity rhythms in strains mutated for either gene. An intronic insertion allele of unc79, unc79f03453 (Figure S1A), was previously shown to exhibit anesthetic sensitivity phenotypes similar to loss of na [7]. We observe that unc79f03453 mutants exhibit decreased morning and evening anticipatory behavior during 12 hr light: 12 hr dark conditions (LD), similar to na mutants ( Figure 1A-C , arrows). Yet the evening anticipation phenotype observed in unc79f03453 mutants (EAI = 1.0+/−0.1) is less severe than in nae04385 mutants ( Figure 1B-C , gray arrows; EAI = 0.5+/−0.1, P<0.05). Furthermore, unc79f03453 flies exhibit only a moderate loss of rhythmicity in constant dark conditions (DD) relative to a wild-type control strain (P = 0.015); this phenotype is also less severe than that of na mutants (nahar, nae04385) maintained in the same genetic background ( Table 1 , P<0.001). Evaluation of unc79f03453 by RT-PCR suggests that some wild-type transcript is produced in this strain (data not shown), indicating that this allele is likely hypomorphic. Therefore, we used transposon excision to generate a novel unc79 mutant strain. This strain, unc79x25, contains a ∼1300 bp genomic deletion that encompasses two putative coding exons (Figure S1A). Sequencing of RT-PCR products derived from unc79x25 reveals that a 57 bp exon and a 80 bp exon are always absent, and all products isolated contain a shift in the predicted reading frame (see Materials and Methods). We find that unc79x25 mutants exhibit severe defects in both anticipatory behavior ( Figure 1D, P<0.05) and free-running rhythmicity ( Table 1 , P<0.001) in comparison to wild-type flies; these behavioral phenotypes are indistinguishable from strong loss-of-function alleles of na (P = 1.00 for DD rhythmicity). All of the mutants assayed were backcrossed to an isogenic w1118 strain (iso31) for six to eight generations, minimizing the influence of genetic background differences on the observed phenotypes ( Figure 1 , Table 1 ). Notably, unc79x25 mutants exhibit similarly strong phenotypes when assayed in trans to a chromosomal deficiency (Df) that deletes the unc79 locus (Df(3R) ED5942; Table S1, P = 1.00 and data not shown). Thus, unc79x25 likely represents a complete or severe loss of unc79 gene function.
Figure 1. Drosophila unc79 and unc80 mutants display defects in anticipatory behavior.
(A-F) Normalized locomotor activity profiles from adult male populations, averaged over four days of 12 hr light: 12 hr dark (LD) entrainment conditions. White bars indicate light phase; black bars indicate dark phase. Error bars represent standard error of the mean. Arrows indicate morning anticipation (black) and evening anticipation (gray). All strains were backcrossed to w1118 (iso31) for 6–8 generations. Morning anticipation index (MAI) and evening anticipation index (EAI) are indicated for each genotype (see Materials and Methods). (A) Representative wild-type strain (n = 52), MAI = 1.5+/−0.1, EAI = 2.1+/−0.1; (B) nae04385 (n = 75), MAI = 0.8+/−0.2, EAI = 0.5+/−0.1; (C) unc79f03453 (n = 44), MAI = 0.5+/−0.1, EAI = 1.0+/−0.1; (D) unc79x25 (n = 93), MAI = 0.7+/−0.1, EAI = 0.4+/−0.1; (E) unc80GS12792 (n = 64), MAI = 0.7+/−0.2, EAI = 0.5+/−0.1; (F) unc80x42 (n = 67), MAI = 0.9+/−0.2, EAI = 0.5+/−0.1. Comparison of MAI values using Kruskal-Wallis one-way ANOVA indicates a significant difference among groups (P<0.001, 5 degrees of freedom), with each of the mutant genotypes (B-F) differing from the wild-type strain (A; Dunn’s method, P<0.05). Significant differences among genotypes are also observed for EAI values (Kruskal-Wallis one-way ANOVA, P<0.001, 5 degrees of freedom). Each mutant strain again differs from wild-type, and unc79f03453 (C) also differs significantly from the other mutant genotypes (B, D-F; Dunn’s method, P<0.05). No significant differences in MAI or EAI are observed among the strong mutant alleles nae04385 (B), unc79x25 (D), unc80GS12792 (E) or unc80x42 (F), as determined by Dunn’s method.
Table 1. unc79 and unc80 mutants exhibit severe defects in free-running rhythmicity.
| Genotype 1 | Period (hrs) | Power | Rhythmic (%) | n |
| na [har+] | 23.9+/−0.1 | 66+/−6 | 97 | 29 |
| na [har] | NA | 1+/−0 | 0 | 19 |
| na [e04385+] | 24.1+/−0.1 | 81+/−5 | 98 | 50 |
| na[e04385] | 31.5 2 | 1+/−0 | 3 | 33 |
| unc79 [f03453+] | 23.9+/−0.0 | 74+/−6 | 96 | 52 |
| unc79 [f03453] | 24.6+/−0.2 2 | 40+/−8 | 75 | 20 |
| unc79 [x25+] | 23.9+/−0.1 | 65+/−7 | 95 | 41 |
| unc79 [x25] | 33.5 2 | 1+/−1 | 3 | 31 |
| unc80 [GS12792+] | 23.9+/−0.0 | 81+/−4 | 99 | 84 |
| unc80 [GS12792] | 22.0 2 | 1+/−0 | 4 | 27 |
| unc80 [x42+] | 23.8+/−0.1 | 53+/−6 | 79 | 48 |
| unc80 [x42] 3 | NA | 0+/−0 | 0 | 9 |
All strains were backcrossed to iso31 for > = 6 generations, and stable lines were established from both mutant and wild-type (+) individuals.
Period length in unc79 [f03453] differs significantly from wild-type (Kruskal-Wallis one-way ANOVA, 1 degree of freedom, P<0.001). Period measurements in na[e04385], unc79[x25], and unc80[GS12792] are based on single weakly rhythmic flies, and were thus excluded from statistical analysis.
Few flies survived to the end of DD; refer to Figure 1F for LD phenotype.
For unc80, we assayed circadian behavior in a strain that contains a transposon insertion in a putative coding exon, unc80GS12792 (Figure S1B). unc80GS12792 mutants exhibit strong defects in both anticipatory behavior ( Figure 1E, P<0.05) and free-running rhythmicity ( Table 1 , P<0.001) when compared to wild-type controls. We generated an additional unc80 allele by incomplete excision of the GS12792 P-element insertion. This strain, unc80x42, retains 14 bp of the transposon insertion but lacks a UAS element present in unc80GS12792 (see Materials and Methods). Like unc80GS12792, unc80x42 mutants exhibit severe disruptions in circadian behavior ( Figure 1F, P<0.05; Table 1 , P<0.001). To confirm that the observed phenotypes map to the unc80 locus, we generated unc80GS12792/Df and unc80x42/Df trans-heterozygotes; these flies exhibit phenotypes similar to homozygous unc80GS12792 or unc80x42 mutants (Table S1, P = 1.00; data not shown). Both alleles were also backcrossed for eight generations to the iso31 strain. All strains that retain an unc80 insertion element exhibit a similar, strong circadian phenotype while all unc80+ backcrossed strains exhibit normal rhythmicity (compiled in Figure 1 and Table 1 , data not shown). Thus, our data indicate that unc80GS12792 and unc80x42 retain little or no unc80 function. For both unc79 and unc80, strong mutant alleles exhibit circadian behavioral phenotypes that are indistinguishable from na mutants, suggesting that both genes may be required for na function.
To assess potential genetic interactions among na, unc79, and unc80, we also examined circadian behavior in single versus double heterozygotes. Neither unc79x25/+ nor unc79x25/ unc8042 males exhibit significant DD rhythmicity defects when compared to wild-type controls (Table S2, P > = 0.082), although a subtle decrease in rhythmicity is observed in unc80x42/+ single heterozygotes (Table S2, P = 0.049). As the na locus is on the X chromosome, we assayed na/+ heterozygous combinations in females. Neither nae04385/+;; unc79x25/+ nor nae04385/+;; unc8042/+ flies exhibit strong deficits in DD rhythmicity compared to single heterozygotes (Table S2, P > = 0.380). However, baseline rhythmicity of females in this genetic background (iso31) is not very robust, so subtle defects might be difficult to assess.
UNC79, UNC80, and NA have similar anatomical requirements
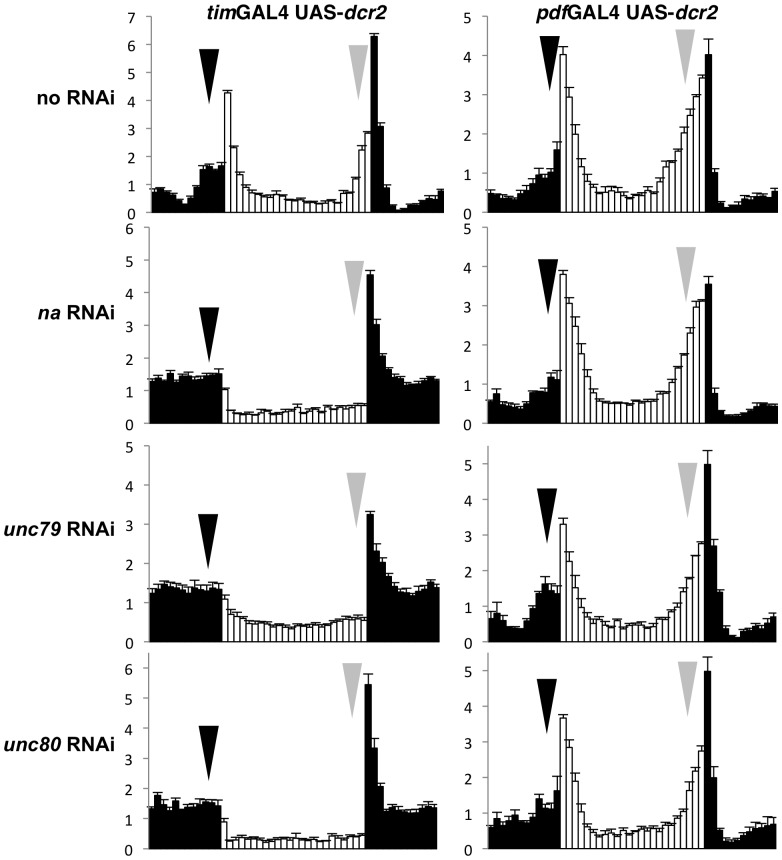
To determine the anatomical requirements for unc79 and unc80 in circadian behavior, we utilized tissue-specific RNA interference (RNAi). We find that pan-neuronal knockdown of na, unc79, or unc80 using elavGAL4 driven expression of UAS-RNAi constructs results in a strong decrease in protein expression for the targeted gene, particularly when these constructs are co-expressed with the RNAi component dicer2 (UAS-dcr2; Figure S2A). To address whether unc79 and unc80 function is required in pacemaker neurons, we crossed these UAS-RNAi lines to circadian GAL4 strains. Expression of unc79, unc80, or na RNAi using the broad circadian driver timelessGAL4 (timGAL4) plus UAS-dcr2 produces strong LD and DD rhythmicity defects ( Figure 2, left panels, P<0.05; Table 2 , P<0.001), which are comparable to the strongest phenotypes observed in the corresponding mutant strains ( Figure 1 ; Table 1 , P > = 0.385). Restricting RNAi expression to the PDF+ pacemaker neurons using pdfGAL4 UAS-dcr2 produces strong defects in DD rhythmicity compared to control strains ( Table 2 , P<0.001), but does not clearly alter LD behavior ( Figure 2, right panels, P > = 0.146). These data suggest that both unc79 and unc80 are required broadly among pacemaker neurons to promote locomotor rhythmicity. Moreover, both genes appear to have similar anatomical requirements as na itself.
Figure 2. RNAi knockdown of na, unc79, or unc80 in all pacemaker neurons results in anticipation defects.
Normalized activity profiles from adult male populations averaged over four days of LD entrainment. White bars represent light phase; black bars indicate dark phase. Error bars represent standard error of the mean. Arrows indicate morning anticipation (black) and evening anticipation (gray). The genotypes represented in the left panels are timGAL4/ +; UAS-dcr2/ +, heterozygous for the following insertions from the Vienna Drosophila RNAi Center (VDRC): (top panel) no RNAi = control strain attp VIE-260B (n = 55), MAI = 1.5+/−0.1, EAI = 2.6+/−0.1; (second panel) na = 103754 (n = 43), MAI = 0.5+/−0.1, EAI = 0.4+/−0.1; (third panel) unc79 = 108132 (n = 44), MAI = 0.3+/−0.1, EAI = 0.3+/−0.1; (bottom panel) unc80 = 108934 (n = 42), MAI = 0.7+/−0.1, EAI = 0.3+/−0.1. Genotypes represented in the right panels are pdfGAL4 UAS-dcr2/+ heterozygous for the same VDRC strains: (top panel) attp VIE-260B (n = 32), MAI = 1.3+/−0.1, EAI = 2.4+/−0.2; (second panel) na = 103754 (n = 33), MAI = 1.0+/−0.1, EAI = 2.3+/−0.2; (third panel) unc79 = 108132 (n = 37), MAI = 1.5+/−0.2, EAI = 2.1+/−0.2; (bottom panel) unc80 = 108934 (n = 35), MAI = 1.3+/−0.2, EAI = 2.3+/−0.2. Both MAI and EAI differ significantly among the timGAL4/ +; UAS-dcr2/ + genotypes (Kruskal-Wallis one-way ANOVA, 3 degrees of freedom, P<0.001), and each RNAi genotype exhibits significantly lower MAI and EAI than the control strain (Dunn’s method, P<0.05). MAI and EAI values for each timGAL4 UAS-dcr2 RNAi strain are either lower or not significantly different from values calculated from strong mutant alleles for the corresponding gene (Kruskal-Wallis one-way ANOVA, 1-2 degrees of freedom). No significant differences in MAI or EAI are observed among pdfGAL4 UAS-dcr2/ + genotypes (Kruskal-Wallis one-way ANOVA, 3 degrees of freedom, P > = 0.146).
Table 2. RNAi knockdown of na, unc79, or unc80 in circadian pacemaker neurons decreases DD rhythmicity.
| Genotype | Period (hrs) | Power | Rhythmic (%) | n |
| UAS-naRNAi[103754]/+; UAS-dcr2/ + | 23.9+/−0.1 | 73+/−5 | 100 | 24 |
| UAS-unc79RNAi[108132]/+; UAS-dcr2/ + | 23.8+/−0.1 | 104+/−6 | 100 | 23 |
| UAS-unc80RNAi[108934]/+; UAS-dcr2/ + | 24.0+/−0.1 | 69+/−6 | 100 | 27 |
| timGAL4/ VIE-260B; UAS-dcr2/ + | 24.3+/−0.0 | 64+/−5 | 94 | 53 |
| timGAL4/ UAS-naRNAi[103754]; UAS-dcr2/ + | 22.5 | 1+/−0 | 2 | 41 |
| timGAL4/ UAS-unc79RNAi[108132]; UAS-dcr2/ + | NA | 0+/−0 | 0 | 35 |
| timGAL4/ UAS-unc80RNAi[108934]; UAS-dcr2/ + | NA | 0+/−0 | 0 | 40 |
| pdfGAL4 UAS-dcr2/ VIE-260B | 24.4+/−0.1 | 70+/−7 | 97 | 30 |
| pdfGAL4 UAS-dcr2/ UAS-naRNAi[103754] | 23.1+/−0.5 | 5+/−2 | 19 | 31 |
| pdfGAL4 UAS-dcr2/ UAS-unc79RNAi[108132] | 24.3+/−0.7 | 8+/−2 | 38 | 32 |
| pdfGAL4 UAS-dcr2/ UAS-unc80RNAi[108934] | 24.5+/−1.0 | 4+/−1 | 13 | 31 |
Drosophila na, unc79, and unc80, exhibit an interdependent, post-transcriptional regulatory relationship
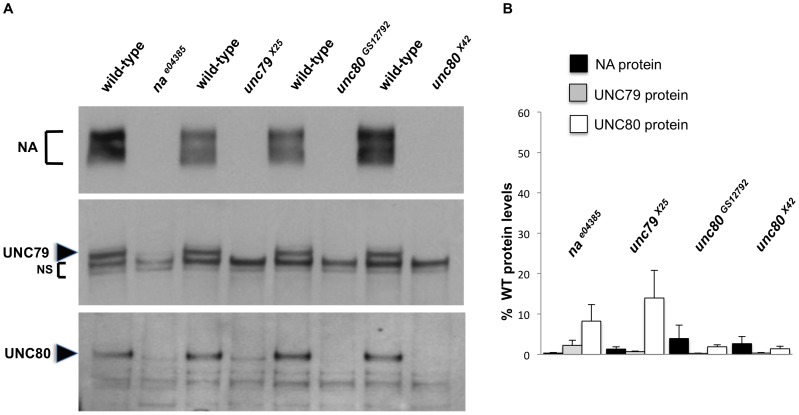
To examine the regulatory relationship between NA and the putative channel subunits UNC79 and UNC80, we assessed protein and transcript levels in head extracts obtained from na, unc79, and unc80 mutants. It has previously been shown that unc79f03453 mutants express decreased levels of NA protein, but normal levels of na transcript [7]. Consistent with this finding, we observe that unc79x25 mutants express little or no detectable NA protein ( Figure 3A , top panel, lanes 3–4; Figure 3B , black bars, P<0.01 compared to unc79+ control), but only a minor decrease in na transcript (Figure S3A, black bars, P<0.05). We also find that unc79x25 flies express strongly decreased levels of UNC80 protein ( Figure 3A bottom panel; Figure 3B , white bars, P<0.01), but no significant change in unc80 transcript (Figure S3A, white bars). These data support a post-transcriptional regulatory relationship among the putative subunits. Similarly, we find that unc80x42 and unc80GS12792 mutants express very little NA or UNC79 protein relative to wild-type control strains ( Figure 3A , lanes 5-8; Figure 3B , black and gray bars, P<0.01), while unc80x42 mutants express normal levels of na and unc79 transcript (Figure S3B). In addition, we assessed the dependence of UNC79 and UNC80 on na. UNC79 and UNC80 protein levels are strongly decreased in nae04385 mutants ( Figure 3A , lanes 1–2; Figure 3B , gray and white bars, P<0.05). While unc79 and unc80 transcript levels are also somewhat lower in nae04385 mutants than controls (Figure S3C, P<0.05), the observed decreases in transcript levels (∼23%) appear insufficient to explain the decreases in protein expression (> = 86%). Notably, pan-neuronal RNAi knockdown of na, unc79, or unc80 is associated with reduced expression of the other two proteins (Figure S2B). Taken together, these data indicate that the expression of NA, UNC79, and UNC80 proteins is strongly interdependent.
Figure 3. Drosophila NA, UNC79, and UNC80 exhibit an interdependent regulatory relationship.
(A) Representative Western blot analyses, performed from adult Drosophila head extracts. The strains assayed were generated by backcrossing nae04385 (Lanes 1–2), unc79x25 (Lanes 3–4), unc80x42 (Lanes 5–6), or unc80GS12792 (Lanes 7–8) to iso31 for 6–8 generations. NS = non-specific UNC79 bands; see Materials and Methods for more details. (B) Quantitation of NA, UNC79, and UNC80 protein levels in each mutant strain, as a percentage of the level observed in the corresponding wild-type strain. Black bars indicate NA protein, gray bars UNC79 protein, and white bars UNC80 protein. Error bars indicate standard error of the mean, determined from 3 independent experiments. NA, UNC79, and UNC80 protein levels are significantly lower in each mutant strain compared to the corresponding control strain, as determined by Student’s t-test (all P<0.01, except UNC80 levels in nae04358, P = 0.012).
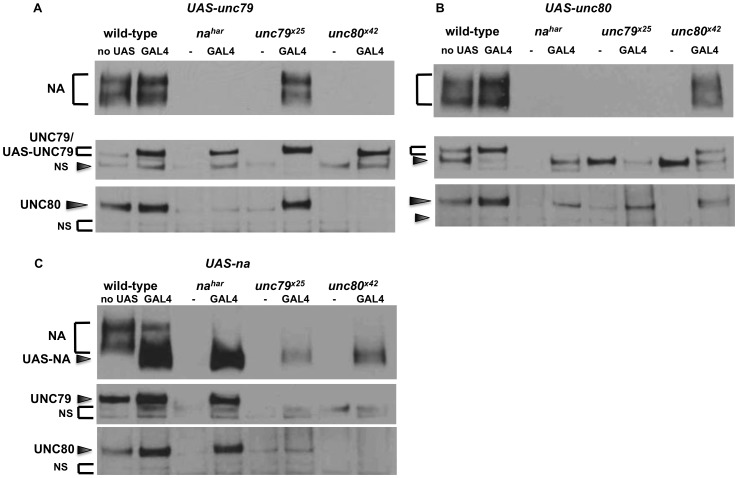
Expression of full-length or truncated UNC79 or UNC80 restores rhythmicity and channel subunit expression to the corresponding mutant strain
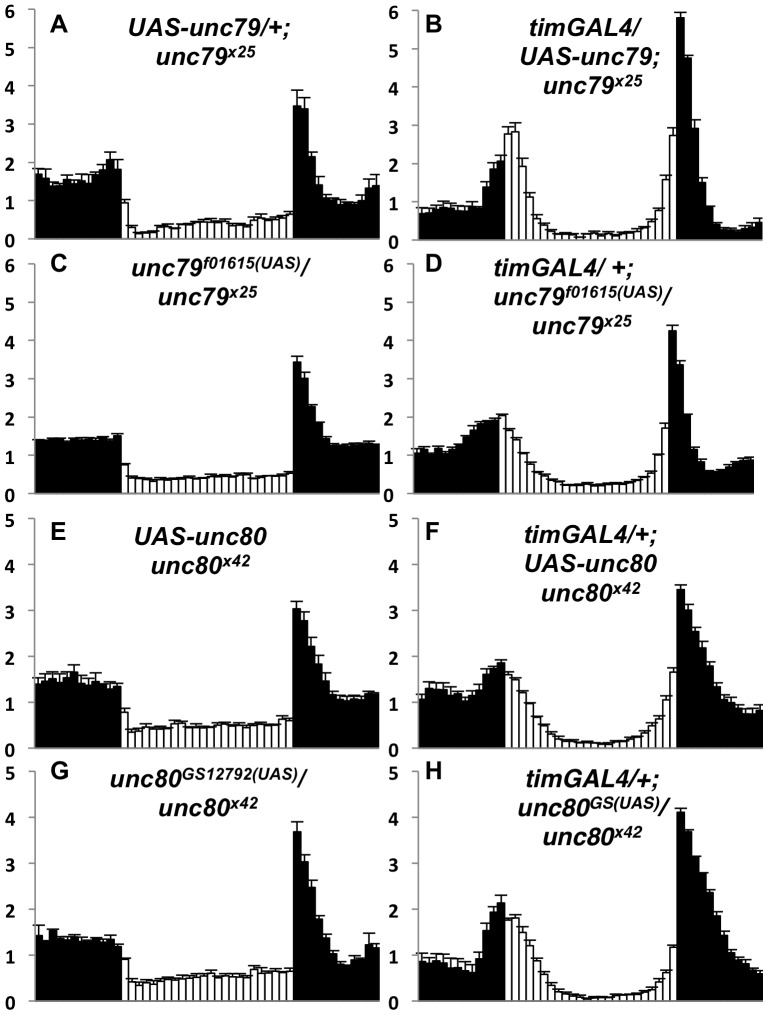
To further assess the functional requirements for unc79 and unc80, we performed tissue-specific transgenic expression experiments. Expression of full-length unc79 cDNA in pacemaker neurons using timGAL4 strongly restores circadian behavior in unc79x25 mutants in both LD and DD conditions ( Figure 4A, B, P<0.05; Table 3 , P<0.001). Moreover, an intronic insertion in unc79 that contains a UAS element (unc79f01615) also rescues behavior in the presence of timGAL4 ( Figure 4C, D, P<0.05; Table 3 , P<0.001). Based on the position of this insertion, we predict that this UAS element initiates the production of functional, truncated form(s) of UNC79 (Figure S1A). Indeed, at least two truncated UNC79 protein bands can be detected in GAL4/ unc79f01615 flies via Western blot that are not evident in wild-type flies (data not shown). These data suggest that UNC79 function in pacemaker neurons is sufficient to promote rhythmicity. We also assessed whether transgenic expression of UNC79 restores NA and UNC80 expression in unc79 mutants. For these assays we utilized elavGAL4, as this pan-neuronal driver is likely more representative of endogenous channel complex expression than timGAL4 [3]. As described earlier, elavGAL4 driven expression of RNAi to na, unc79, or unc80 leads to strong knockdown of the corresponding proteins in adult head extracts, indicating that this driver encompasses the major sources of expression of all three gene products in the Drosophila head (Figure S2A). We find that pan-neuronal expression of either full-length or truncated UNC79 strongly restores NA and UNC80 protein levels to unc79 mutants ( Figure 5A , lanes 5-6, P<0.01; data not shown).
Figure 4. Transgenic rescue of unc79 and unc80 anticipation phenotypes.
(A-H) Normalized activity profiles from adult males averaged over four days of LD conditions. White bars indicate light phase; black bars indicate dark phase. Error bars represent standard error of the mean. (A) UAS-unc79MYC 23-24/+; unc79x25 (n = 42), MAI = 1.1+/0.2, EAI = 0.5+/−0.1; (B) timGAL4/ UAS-unc79MYC 23-24; unc79x25 (n = 68), MAI = 1.7+/−0.1, EAI = 2.6+/−0.2; (C) unc79f01615/ unc79x25 (n = 117), MAI = 0.4+/−0.1, EAI = 0.26+/−0.03; (D) timGAL4/ +; unc79f01615/ unc79x25 (n = 74), MAI = 1.1+/−0.1, EAI = 1.6+/−0.1; (E) UAS-HAunc80 1M unc80x42/ unc80x42 (n = 52), MAI = 0.4+/−0.1, EAI = 0.3+/−0.1; (F) timGAL4/ +; UAS-HAunc80 1M unc80x42/ unc80x42 (n = 48), MAI = 1.1+/−0.2, EAI = 1.5+/−0.1; (G) unc80GS12792 (UAS)/ unc80x42 (n = 71), MAI = 0.5+/−0.1, EAI = 0.3+/−0.1; (H) timGAL4/ +; unc80GS12792 (UAS)/ unc80x42 (n = 43), MAI = 1.7+/−0.1, EAI = 1.1+/−0.1. MAI and EAI values differ significantly between each mutant (A, C, E, G) and the corresponding rescue genotype (B, D, F, H), as determined by Kruskal-Wallis one-way ANOVA (all comparisons P<0.001, 1 degree of freedom).
Table 3. Transgenic rescue of unc79 and unc80 rhythmicity phenotypes.
| Genotype | Period (hrs) | Power | Rhythmic (%) | n |
| timGAL4/ + | 24.6+/−0.0 | 40+/−3 | 76 | 135 |
| timGAL4/ UAS-na | 25.3+/−0.1 | 21+/−6 | 55 | 22 |
| timGAL4/ UAS-unc79MYC | 25.0+/−0.1 | 59+/−7 | 88 | 33 |
| timGAL4/ +; unc79[UAS-f01615]/ + | 25.0+/−0.1 | 85+/−7 | 92 | 36 |
| timGAL4/ +; UAS-HAunc80/ + | 25.0+/−0.2 | 38+/−7 | 69 | 35 |
| timGAL4/ +; unc80[UAS-GS12792]/ + | 24.6+/−0.1 | 83+/−9 | 95 | 19 |
| timGAL4/ +; unc79[x25] | 25.7+/−3.1 | 2+/−1 | 7 | 56 |
| UAS-unc79MYC/+; unc79[x25] | 24.1+/−2.4 | 3+/−1 | 13 | 30 |
| unc79[x25]/ unc79[UAS-f01615] | 24.6+/−0.3 | 2+/−1 | 6 | 100 |
| timGAL4/ UAS-unc79MYC; unc79[x25] | 23.8+/−0.0 | 108+/−6 | 97 | 61 |
| timGAL4/ +; unc79[x25]/ unc79[UAS-f01615] | 24.4+/−0.1 | 58+/−5 | 82 | 66 |
| timGAL4/ UAS-na; unc79[x25] 1 | N/A | 1+/−1 | 0 | 2 |
| timGAL4/ +; UAS-HAunc80 unc79[x25]/ unc79[x25] | 21.8+/−5.8 | 1+/−0 | 3 | 75 |
| timGAL4/ +; unc79[x25] unc80[UAS-GS12792]/ unc79[x25] | 22.4+/−1.6 | 3+/−1 | 12 | 50 |
| timGAL4/ UAS-na; UAS-HAunc80 unc79[x25]/ unc79[x25] | 24.0 | 1+/−0 | 4 | 28 |
| timGAL4/ +; unc80[x42] | 25.0+/−1.0 | 1+/−0 | 3 | 66 |
| unc80[x42] UAS-HAunc80/ unc80[x42] | 19.5+/−0.0 | 1+/−0 | 3 | 66 |
| unc80[x42]/ unc80[UAS-GS12792] | N/A | 0+/−0 | 0 | 54 |
| timGAL4/ +; UAS-HAunc80 unc80[x42]/ unc80[x42] | 24.5+/−0.1 | 94+/−7 | 93 | 42 |
| timGAL4/ +; unc80[x42]/ unc80[UAS-GS12792] | 24.3+/−0.1 | 134+/−5 | 100 | 38 |
| timGAL4/ UAS-na; unc80[x42] 1 | N/A | 2+/−2 | 0 | 5 |
| timGAL4/ UAS-unc79MYC; unc80[x42] | 17.8+/−1.5 | 2+/−0 | 5 | 61 |
| timGAL4/ +; unc79[UAS- f01615] unc80[x42]/ unc80[x42] | 19.5 | 1+/−0 | 2 | 48 |
Few flies survived to the end of DD; refer to Figures 6B,E for LD phenotype.
Figure 5. Transgenic unc79, unc80 or na expression produces increased protein levels in wild-type and mutant backgrounds.
(A-C) Western blot analyses were used to label UNC79, UNC80, and NA proteins from adult head extracts; representative blots are shown. For all blots shown, lane 1 = elavGAL4/+ (no UAS); lanes 3,5,7 = UAS/+ (no GAL4); lanes 2,4,6,8 = elavGAL4; UAS/+. (A) Pan-neuronal expression of UAS-unc79MYC 23-24 in wild-type (lane 2), nahar (lane 4), unc79x25 (lane 6), or unc80x42 (lane 8) backgrounds. (B) Expression of UAS-HAunc80 1M in wild-type (lane 2), nahar (lane 4), unc79x25 (lane 6), or unc80x42 (lane 8) backgrounds. (C) Expression of UAS-na U3 in wild-type (lane 2), nahar (lane 4), unc79x25 (lane 6), or unc80x42 (lane 8) backgrounds.
Similarly, expression of full-length unc80 cDNA using timGAL4 strongly rescues LD and DD rhythmicity in unc80x42 mutant flies ( Figure 4E, F, P<0.05; Table 3 , P<0.001). Surprisingly, we also find that timGAL4 restores LD and DD rhythmic behavior in unc80GS12792/ unc80x42 trans-heterozygotes ( Figure 4G, H ; P<0.05; Table 3 , P< 0.001). As described, unc80GS12792 contains a UAS element near the middle of the presumptive UNC80 coding sequence (Figure S1B). We predict that this UAS element is capable of initiating the production of functional UNC80 protein, which likely contains < = 1790 of the ∼3310 amino acids predicted for the full length protein. Consistent with this prediction, we detect at least one truncated (∼180 Kd) UNC80 band in GAL4/ unc80GS12792 flies (data not shown). These data indicate that broad circadian expression of either full-length or C-terminal UNC80 protein is sufficient to restore rhythmicity to unc80 mutants. In addition, we find that pan-neuronal expression of either full-length or truncated UNC80 restores NA and UNC79 protein expression to unc80 mutants ( Figure 5B , lanes 7–8, P<0.01; data not shown).
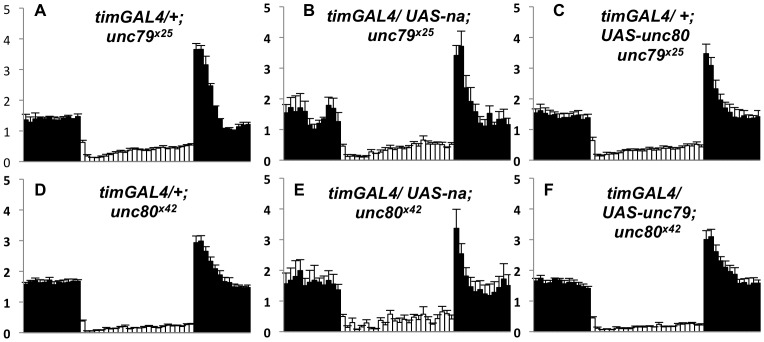
Transgenic expression of NA, UNC79, or UNC80 does not relieve the requirement for other channel subunits in the Drosophila brain
Given the interdependent regulatory relationship among na, unc79, and unc80, we asked whether transgenic expression of any putative subunits could restore rhythmicity in the absence of another. Notably, transfection experiments in mouse primary brain culture indicate that expression of mouse UNC80 can bypass the requirement for UNC79 [11]. In contrast, we find that circadian or pan-neuronal expression of either NA or UNC80 in vivo is not sufficient to restore rhythmicity to unc79x25 mutants ( Figure 6A-C ; Table 3 , P = 0.401; data not shown), nor is co-expression of both NA and UNC80 ( Table 3 , P = 0.661; data not shown). Similarly, circadian or pan-neuronal expression of either NA or UNC79 does not restore rhythmicity to unc80x42 mutants ( Figure 6D-F ; Table 3 , P > = 0.671; data not shown). Because each mutant is deficient in the expression of all three proteins, we also assessed whether pan-neuronal expression of one subunit restores protein expression in another mutant background. We find that transgenic UAS-na cDNA expression in unc79 or unc80 mutants produces a detectable increase in NA protein levels ( Figure 5C , top panel; Figure S4C, P<0.01), but much less than what is observed when expressed in a na mutant background ( Figure 5C , top panel; Figure S4C, P<0.05). This indicates that transgenic NA expression is strongly, but not completely, dependent on unc79 and unc80. Transgenic NA expression in unc79x25 mutants results in a small but significant increase in UNC80 protein levels ( Figure 5C , bottom panel; Figure S4C, right panel, P<0.05), suggesting that NA/UNC80 regulation may occur independently of UNC79. However, transgenic NA does not restore a significant increase in UNC79 to unc80x42 mutants ( Figure 5C , middle panel; Figure S4C, right panel, P = 0.12). Expression of UAS-HAunc80 cDNA results in increased levels of UNC80 protein in nahar and unc79x25 mutants ( Figure 5B , bottom panel; Figure S4B, P<0.01). While transgenic unc80 produces no clear increase in NA levels in unc79x25 mutants (P = 0.65), it does produce a significant increase in UNC79 levels in nahar mutants ( Figure 5B , top and middle panels; Figure S4B, right panel; P<0.01). This suggests that regulation of UNC79 expression by UNC80 can occur independently of NA. Finally, transgenic expression of UAS-unc79 also produces a clear increase in UNC79 protein expression in both na and unc80 mutants ( Figure 5A , middle panel; Figure S4A, P<0.01). However, unc79 expression does not result in increased UNC80 expression in na mutants, nor does it restore NA expression to unc80 mutants ( Figure 5A , top and bottom panels; Figure S4A, right panel, P > = 0.25). Taken together, these data indicate that increasing expression of one or two putative channel subunit proteins in the absence of a third does not improve behavioral rhythmicity.
Figure 6. Transgenic expression of other subunits does not restore anticipatory behavior to unc79 or unc80 mutants.
. (A-F) Normalized activity profiles from adult males averaged over four days of LD conditions. White bars indicate light phase; black bars indicate dark phase. Error bars represent standard error of the mean. (A) timGAL4/ +; unc79x25 (n = 73), MAI = 0.6+/−0.1, EAI = 0.3+/−0.1; (B) timGAL4/ UAS-na U3; unc79x25 (n = 25), MAI = 1.2+/−0.2, EAI = 0.3+/−0.1; (C) timGAL4/+; UAS-HAunc80 1M unc79x25/ unc79x25 (n = 90), MAI = 0.4+/−0.1, EAI = 0.32+/−0.04; (D) timGAL4/ +; unc80x42 (n = 78), MAI = 0.3+/−0.1, EAI = 0.16+/−0.03; (E) timGAL4/ UAS-na U3; unc80x42 (n = 21), MAI = 0.6+/−0.2, EAI = 0.5+/−0.2; (F) timGAL4/ UAS-unc79MYC 23-24; unc80x42 (n = 74), MAI = 0.4+/−0.1, EAI = 0.19+/−0.04. For unc79x25 genotypes (A-C), EAI values do not differ significantly (Kruskal-Wallis one-way ANOVA, 2 degrees of freedom, P = 0.567). MAI values for genotypes A-C exhibit significant variation (Kruskal-Wallis one-way ANOVA, 2 degrees of freedom, P = 0.0006), but neither of the transgenic genotypes (B,C) differs significantly from the mutant control (A; Dunn’s method). For unc80x42 genotypes (D-F), no significant difference is detected in MAI values (Kruskal-Wallis one-way ANOVA, 2 degrees of freedom, P = 0.391). Significant variation is observed for EAI values for genotypes D-F (Kruskal-Wallis one-way ANOVA, 2 degrees of freedom, P = 0.022), but no pairwise differences are significant (Dunn’s method).
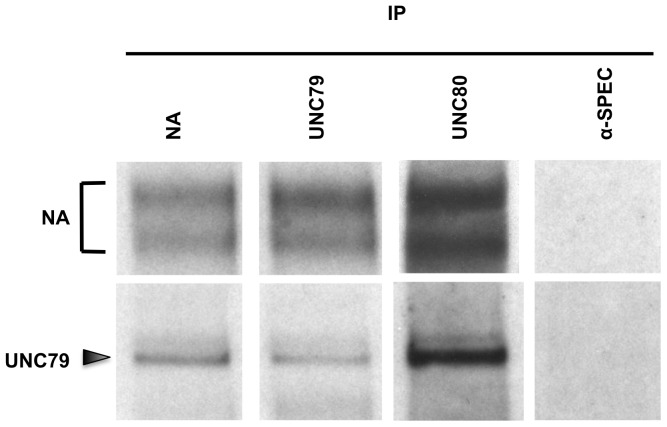
NA, UNC79, and UNC80 proteins are present in a complex in the Drosophila head
The mammalian orthologs of NA, UNC79, and UNC80 are believed to function in a protein complex [11], [13]. To determine whether the Drosophila proteins also interact with each other, we performed co-immunoprecipitation experiments from adult head extracts. We find that NA protein can be immunoprecipitated using anti-UNC79 or anti-UNC80, while UNC79 protein is immunoprecipitated using either anti-NA or anti-UNC80 ( Figure 7 ). These data support a model in which Drosophila NA, UNC79, and UNC80 proteins form a channel complex, similar to the proposed relationship of their mammalian orthologs.
Figure 7. NA, UNC79, and UNC80 proteins form a complex in the Drosophila head.
Western blot analyses of immunoprecipitated complexes. Immunoprecipitations were performed from membrane preparations of adult Drosophila head extracts using the antibodies indicated (anti-NA, anti-UNC79, anti-UNC80, and anti-α-SPECTRIN; see Materials and Methods).
Discussion
Here we demonstrate that the Drosophila genes unc79 and unc80 (aka CG18437) are required for robust circadian locomotor rhythms. We find that a previously described unc79 mutant allele, unc79f03453, exhibits circadian phenotypes that are less severe than strong loss-of-function na mutants ( Figure 1 , Table 1 ). It was initially unclear whether the differences in phenotypic severity represented partial unc79 gene function in the unc79f03453 mutant or residual NA channel function in the absence of unc79. Notably, UNC79 knockout mice retain NALCN expression and some basal channel activity [11]. For unc79f03453, transcript analysis indicates that this allele is likely hypomorphic. A novel deletion allele, unc79x25, represents a complete or near complete loss of unc79 function, and these mutants exhibit severe circadian phenotypes that are indistinguishable from na mutants. Similarly, mutants that contain a coding disruption in the Drosophila UNC80 ortholog (unc80/ CG18437), exhibit strong rhythmicity defects that are very similar to na and unc79x25 mutants ( Figure 1 , Table 1 ).
Expression data from na, unc79, and unc80 mutants indicate an interdependent, post-transcriptional regulatory relationship between these gene products in Drosophila ( Figure 3 , Figure S3). This is similar to previous findings from C. elegans, perhaps reflecting conserved regulatory mechanisms [7], [10]. Notably, residual UNC79 and UNC80 protein is observed in nae04385 mutants and some UNC80 protein is detected in unc79x25 mutants. The nae04385 allele contains a transposon insertion in the middle of the NA coding sequence, and behavioral analyses suggest that this represents a severe loss of function allele ( Figure 1 , Table 1 ; [7]). We have no evidence of phenotypic differences among the three mutants that might correspond to the residual protein expression. Strong na, unc79, and unc80 alleles in the iso31 background exhibit a similar array of phenotypes in addition to circadian behavioral defects, including decreased locomotor activity, poor female fertility, and poor viability in locomotor assays (data not shown). Different isogenized strains retain some variability in the severity of these phenotypes, but this does not appear to correlate with mutation of a particular gene (data not shown). The regulatory relationship among these gene products in Drosophila appears to be somewhat different than in mammals. In mice, UNC79 knockouts lack UNC80 expression but retain NALCN. While both NALCN and UNC79 knockouts exhibit breathing rhythm defects and die shortly after birth, slight differences in neonatal lethality between the two mutants may reflect residual NALCN activity in the UNC79 knockout [11].
Our data support the hypothesis that UNC79 and UNC80 function in a complex with NA in circadian pacemaker neurons to promote rhythmic behavior. The anatomical requirements for the channel complex includes the PDF-expressing (PDF+) subset of pacemaker neurons, since pdfGAL4 driven RNAi knockdown of na, unc79, or unc80 results in decreased DD rhythmicity ( Table 2 ). It is notable that pdfGAL4 driven knockdown of na, unc79, or unc80 does not cause strong defects in LD anticipatory behavior ( Figure 2 ). This phenotype is less severe than what is observed in pdf neuropeptide mutants [20], suggesting that PDF release is not completely compromised upon loss of NA channel complex function. These findings complement previous na tissue-specific rescue data, in which expression of the GAL4 inhibitor GAL80 in PDF+ neurons (pdfGAL80) blocks elavGAL4 and timGAL4 mediated rescue of DD rhythmicity but does not block LD rescue [4].
Transgenic rescue experiments indicate that either full length or C-terminal regions of UNC79 and UNC80 fully restore behavioral rhythmicity to the corresponding mutant strains ( Figure 4 , Table 3 ). For both unc79 and unc80, a UAS insertion after the presumed start methionine can initiate the production of a functional, truncated protein. The robust rescue observed using the unc80GS12792-UAS strain was particularly surprising, as conceptual translation indicates that the protein produced would include, at most, ∼1790 of the ∼3310 amino acids predicted for full-length Drosophila UNC80 (Figure S1B). Notably, this C-terminal region exhibits greater homology to mammalian UNC80 than does the N-terminal region preceding the GS12792 insertion (data not shown). These rescue data imply that the C-terminal ∼half of UNC80 protein may be sufficient to promote circadian behavior and NA/UNC79 protein expression. However, we cannot rule out cooperative function with residual N-terminal UNC80 protein that may be present in unc80x42 and unc80GS12792 mutants. Similarly, the C-terminal truncated protein produced in GAL4/ unc79f01615 flies (containing < = 1871 of the ∼2776 aa) could potentially cooperate with residual N-terminal UNC79 protein in unc79x25 and unc79f01615 mutants.
We also used transgenic expression experiments to address whether both unc79 and unc80 are required for more than regulating subunit protein levels. In mice, UNC80 has been implicated in the regulation of NALCN channel activity, whereas the requirement for UNC79 in cultured hippocampal neurons can be bypassed by UNC80 transfection [11], [13]. In contrast, we find that the in vivo requirement for one subunit in the Drosophila circadian system cannot be bypassed by expression of another. Transgenic expression of Drosophila na and/or unc80 promotes significant expression of the corresponding protein(s) to unc79x25 mutants but provides no measureable improvement in rhythmic behavior ( Figure 5 , Figure 6 , Table 3 , data not shown). In contrast, some hypomorphic alleles (unc79f03453) and RNAi combinations have been identified in which little subunit protein is detected but significant rhythmicity is observed ( Figure 1 , Table 1 , data not shown; [7]). Taken together, these data suggest that Drosophila UNC79 and UNC80 each have functional requirements beyond promoting the expression of other subunits. These putative auxiliary subunits could be required for proper localization of the channel complex and/or regulation of channel activity. As UNC80 is known to mediate NALCN activity regulation in mammals, it will be valuable to determine whether similar or distinct mechanisms modulate channel activity in circadian pacemaker neurons. Such regulatory mechanisms may be relevant to the mammalian circadian pacemaker, the suprachiasmatic nucleus (SCN), as electrophysiological analyses suggest that NALCN may indeed function in this tissue [26], [27], [28].
Finally, we note that transgenic expression of UNC80 in na mutants produces a significant increase in UNC79 expression, and transgenic NA expression in unc79 mutants produces a small but significant increase in UNC80 expression ( Figure 5 , Figure S4). If the regulatory relationship among these gene products is mediated by protein-protein interaction, this would suggest that UNC79 and UNC80 can interact in the absence of NA, and that NA and UNC80 can interact in the absence of UNC79. This model for channel subunit interactions is supported by co-immunoprecipitation data in mammalian cell culture, where UNC80 was found to mediate the physical interaction between NALCN and UNC79 [11].
Supporting Information
Drosophila unc79 and unc80 gene loci. Schematic representation of the Drosophila unc79 (A) and unc80 (B) gene loci, both located on Chromosome 3R. Transcript and protein predictions are based on Drosophila genome annotation 5.1. Triangles represent the approximate locations of relevant transposable elements insertions. P = P-element transposon insertion; PBac = Piggybac transposon insertion. Black triangles represent insertions that were evaluated as mutant alleles and/or were used to generate novel alleles. Gray triangles represent insertions that contain a 5’- 3’ UAS element; both of these insertions likely decrease gene function in the absence of GAL4 but produce functional proteins in the presence of GAL4. (A) unc79 gene locus. Bracket indicates the genomic sequence deleted in the unc79x25 allele. This includes 57 bp and 80 bp coding exons. M = approximate location of the first 3 predicted start methionines after the unc79 f01615-UAS insertion. (B) unc80/ CG18437 gene locus. M(4) = exon containing the first 4 predicted start methionines after the unc80GS12792-UAS insertion.
(TIF)
Pan-neuronal expression of na , unc79 , or unc80 RNAi decreases expression of channel complex proteins. Quantitation of NA (black bars), UNC79 (gray bars), and UNC80 (white bars) protein levels upon pan-neuronal (elavGAL4) driven expression of RNAi, relative to control strains (elavGAL4; attp VIE260/+ with or without UAS-dcr2). Protein levels were measured from Western blot data using NIH Image J gel analysis. Error bars represent standard error of the mean (n = 4 experiments). Statistical significance was determined using Student’s t-test. n.s. = no significant difference; * = P<0.05; ** = P<0.01. (A) Decreased levels of the targeted protein are detected upon pan-neuronal expression of na RNAi (VDRC 103754), unc79 RNAi (VDRC 108132), or unc80 RNAi (VDRC 108934). For unc79 and unc80, knockdown of protein levels is significantly enhanced upon co-expression of UAS-dicer2 (dcr2). (B) Pan-neuronal RNAi knockdown of na, unc79, or unc80 results in decreased expression of the other putative subunit proteins.
(TIF)
Drosophila na , unc79 , and unc80 mutants display minimal differences in transcript expression of other subunits. mRNA expression levels of na (black bars), unc79 (gray bars), and/or unc80 (white bars) in (A) unc79x25 mutants, (B) unc80x42 mutants, and (C) nae04385 mutants relative to expression in the corresponding wild-type strains, as determined by qPCR. The strains assayed were backcrossed to w1118 iso31 for 6–8 generations. Samples were normalized to RP49 expression and analysed using theΔΔCt method, as described in Materials and Methods. Error bars represent standard error of the mean. Statistical significance was determined using Student’s t-test. n.s. = no significant difference; * = P<0.05; ** = P<0.01.
(TIF)
Quantitation of protein levels upon transgenic unc79 , unc80 or na expression. Quantitation of NA (black bars), UNC79 (gray bars), and UNC80 (white bars) protein levels in the genotypes indicated, as determined using NIH ImageJ analyses of Western blot data (n = 3–5 experiments). Error bars indicate standard error of the mean. In left panels, protein levels are reported as a percent of wild-type (elavGAL4/ +). In right panels, the % increase in expression reflects the increase in protein levels observed in elavGAL4 UAS/+ strains as compared to UAS/+ alone, as determined within each experiment and mutant background. Statistical significance was determined using Student’s t-test. n.s. = no significant difference; * = P<0.05; ** = P<0.01. (A) Pan-neuronal expression of UAS-unc79MYC (elavGAL4; UAS-unc79MYC 23–24/+) in the backgrounds indicated. In the right panel, UNC80 and NA levels were decreased upon transgenic expression of unc79, but these changes were not significant. (B) Pan-neuronal expression of UAS-HAunc80 (elavGAL4;; UAS-HA-unc80 1M/+). (C) Pan-neuronal expression of UAS-na (elavGAL4; UAS-na U3 /+).
(TIF)
unc79 and unc80 complementation assays.
(DOCX)
DD rhythmicity in unc79 and unc80 single and double heterozygotes.
(DOCX)
(DOCX)
Acknowledgments
We thank Xiaomei Li, Erin Petrik, Ashley Lipinski, Ramu Annamalai, and Ajay Dwivedi for technical assistance.
Funding Statement
This work was supported by NIH grant R00 GM080107 to B.C.L, NIH R01 NS052903 to R.A., and a NARSAD Young Investigator Award to B.C.L. The funders had no role in study, design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lear BC, Allada R (2012) Circadian Rhythms. Encyclopedia of Life Sciences. Chichester: John Wiley & Sons, Ltd. pp. 1–7.
- 2. Allada R, Chung BY (2010) Circadian organization of behavior and physiology in Drosophila. Annual review of physiology 72: 605–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nash HA, Scott RL, Lear BC, Allada R (2002) An unusual cation channel mediates photic control of locomotion in Drosophila. Current biology : CB 12: 2152–2158. [DOI] [PubMed] [Google Scholar]
- 4. Lear BC, Lin JM, Keath JR, McGill JJ, Raman IM, et al. (2005) The ion channel narrow abdomen is critical for neural output of the Drosophila circadian pacemaker. Neuron 48: 965–976. [DOI] [PubMed] [Google Scholar]
- 5. Lu B, Su Y, Das S, Liu J, Xia J, et al. (2007) The neuronal channel NALCN contributes resting sodium permeability and is required for normal respiratory rhythm. Cell 129: 371–383. [DOI] [PubMed] [Google Scholar]
- 6. Morgan PG, Sedensky MM, Meneely PM, Cascorbi HF (1988) The effect of two genes on anesthetic response in the nematode Caenorhabditis elegans. Anesthesiology 69: 246–251. [DOI] [PubMed] [Google Scholar]
- 7. Humphrey JA, Hamming KS, Thacker CM, Scott RL, Sedensky MM, et al. (2007) A putative cation channel and its novel regulator: cross-species conservation of effects on general anesthesia. Current biology : CB 17: 624–629. [DOI] [PubMed] [Google Scholar]
- 8. Jospin M, Watanabe S, Joshi D, Young S, Hamming K, et al. (2007) UNC-80 and the NCA ion channels contribute to endocytosis defects in synaptojanin mutants. Current biology : CB 17: 1595–1600. [DOI] [PubMed] [Google Scholar]
- 9. Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, et al. (2008) Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proceedings of the National Academy of Sciences of the United States of America 105: 20982–20987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yeh E, Ng S, Zhang M, Bouhours M, Wang Y, et al. (2008) A putative cation channel, NCA-1, and a novel protein, UNC-80, transmit neuronal activity in C. elegans. PLoS biology 6: e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu B, Zhang Q, Wang H, Wang Y, Nakayama M, et al. (2010) Extracellular calcium controls background current and neuronal excitability via an UNC79-UNC80-NALCN cation channel complex. Neuron 68: 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Speca DJ, Chihara D, Ashique AM, Bowers MS, Pierce-Shimomura JT, et al.. (2010) Conserved role of unc-79 in ethanol responses in lightweight mutant mice. PLoS genetics 6. [DOI] [PMC free article] [PubMed]
- 13. Lu B, Su Y, Das S, Wang H, Wang Y, et al. (2009) Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature 457: 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang H, Ren D (2009) UNC80 functions as a scaffold for Src kinases in NALCN channel function. Channels 3: 161–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Swayne LA, Mezghrani A, Varrault A, Chemin J, Bertrand G, et al. (2009) The NALCN ion channel is activated by M3 muscarinic receptors in a pancreatic beta-cell line. EMBO reports 10: 873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415. [DOI] [PubMed] [Google Scholar]
- 17. Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, et al. (2007) A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448: 151–156. [DOI] [PubMed] [Google Scholar]
- 18. Cook RK, Christensen SJ, Deal JA, Coburn RA, Deal ME, et al. (2012) The generation of chromosomal deletions to provide extensive coverage and subdivision of the Drosophila melanogaster genome. Genome biology 13: R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ryder E, Blows F, Ashburner M, Bautista-Llacer R, Coulson D, et al. (2004) The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167: 797–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99: 791–802. [DOI] [PubMed] [Google Scholar]
- 21. Kaneko M, Hall JC (2000) Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J Comp Neurol 422: 66–94. [DOI] [PubMed] [Google Scholar]
- 22. Lin DM, Goodman CS (1994) Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron 13: 507–523. [DOI] [PubMed] [Google Scholar]
- 23. Grether ME, Abrams JM, Agapite J, White K, Steller H (1995) The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes & development 9: 1694–1708. [DOI] [PubMed] [Google Scholar]
- 24. Dubreuil R, Byers TJ, Branton D, Goldstein LS, Kiehart DP (1987) Drosophilia spectrin. I. Characterization of the purified protein. The Journal of cell biology 105: 2095–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ (2008) The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. Journal of neuroscience methods 172: 250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jackson AC, Yao GL, Bean BP (2004) Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 24: 7985–7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LeSauter J, Silver R, Cloues R, Witkovsky P (2011) Light exposure induces short- and long-term changes in the excitability of retinorecipient neurons in suprachiasmatic nucleus. Journal of neurophysiology 106: 576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ren D (2011) Sodium leak channels in neuronal excitability and rhythmic behaviors. Neuron 72: 899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Drosophila unc79 and unc80 gene loci. Schematic representation of the Drosophila unc79 (A) and unc80 (B) gene loci, both located on Chromosome 3R. Transcript and protein predictions are based on Drosophila genome annotation 5.1. Triangles represent the approximate locations of relevant transposable elements insertions. P = P-element transposon insertion; PBac = Piggybac transposon insertion. Black triangles represent insertions that were evaluated as mutant alleles and/or were used to generate novel alleles. Gray triangles represent insertions that contain a 5’- 3’ UAS element; both of these insertions likely decrease gene function in the absence of GAL4 but produce functional proteins in the presence of GAL4. (A) unc79 gene locus. Bracket indicates the genomic sequence deleted in the unc79x25 allele. This includes 57 bp and 80 bp coding exons. M = approximate location of the first 3 predicted start methionines after the unc79 f01615-UAS insertion. (B) unc80/ CG18437 gene locus. M(4) = exon containing the first 4 predicted start methionines after the unc80GS12792-UAS insertion.
(TIF)
Pan-neuronal expression of na , unc79 , or unc80 RNAi decreases expression of channel complex proteins. Quantitation of NA (black bars), UNC79 (gray bars), and UNC80 (white bars) protein levels upon pan-neuronal (elavGAL4) driven expression of RNAi, relative to control strains (elavGAL4; attp VIE260/+ with or without UAS-dcr2). Protein levels were measured from Western blot data using NIH Image J gel analysis. Error bars represent standard error of the mean (n = 4 experiments). Statistical significance was determined using Student’s t-test. n.s. = no significant difference; * = P<0.05; ** = P<0.01. (A) Decreased levels of the targeted protein are detected upon pan-neuronal expression of na RNAi (VDRC 103754), unc79 RNAi (VDRC 108132), or unc80 RNAi (VDRC 108934). For unc79 and unc80, knockdown of protein levels is significantly enhanced upon co-expression of UAS-dicer2 (dcr2). (B) Pan-neuronal RNAi knockdown of na, unc79, or unc80 results in decreased expression of the other putative subunit proteins.
(TIF)
Drosophila na , unc79 , and unc80 mutants display minimal differences in transcript expression of other subunits. mRNA expression levels of na (black bars), unc79 (gray bars), and/or unc80 (white bars) in (A) unc79x25 mutants, (B) unc80x42 mutants, and (C) nae04385 mutants relative to expression in the corresponding wild-type strains, as determined by qPCR. The strains assayed were backcrossed to w1118 iso31 for 6–8 generations. Samples were normalized to RP49 expression and analysed using theΔΔCt method, as described in Materials and Methods. Error bars represent standard error of the mean. Statistical significance was determined using Student’s t-test. n.s. = no significant difference; * = P<0.05; ** = P<0.01.
(TIF)
Quantitation of protein levels upon transgenic unc79 , unc80 or na expression. Quantitation of NA (black bars), UNC79 (gray bars), and UNC80 (white bars) protein levels in the genotypes indicated, as determined using NIH ImageJ analyses of Western blot data (n = 3–5 experiments). Error bars indicate standard error of the mean. In left panels, protein levels are reported as a percent of wild-type (elavGAL4/ +). In right panels, the % increase in expression reflects the increase in protein levels observed in elavGAL4 UAS/+ strains as compared to UAS/+ alone, as determined within each experiment and mutant background. Statistical significance was determined using Student’s t-test. n.s. = no significant difference; * = P<0.05; ** = P<0.01. (A) Pan-neuronal expression of UAS-unc79MYC (elavGAL4; UAS-unc79MYC 23–24/+) in the backgrounds indicated. In the right panel, UNC80 and NA levels were decreased upon transgenic expression of unc79, but these changes were not significant. (B) Pan-neuronal expression of UAS-HAunc80 (elavGAL4;; UAS-HA-unc80 1M/+). (C) Pan-neuronal expression of UAS-na (elavGAL4; UAS-na U3 /+).
(TIF)
unc79 and unc80 complementation assays.
(DOCX)
DD rhythmicity in unc79 and unc80 single and double heterozygotes.
(DOCX)
(DOCX)