Abstract
Objective
To determine the multidrug resistant (MDR) transporter activity in oocytes and their potential role in oocyte susceptibility to chemotherapy.
Design
Experimental laboratory study
Setting
University and Academic Center for reproductive medicine.
Patients/Animals
Women with eggs retrieved for ICSI cycles and adult female FVBN and B6C3F1 mouse strains.
Intervention
Inhibition of MDR activity in oocytes.
Main Outcome measure(s)
Efflux activity of MDRs using quantitative fluorescent dye efflux and oocyte cell death when exposed to chemotherapy.
Results
Oocytes effluxed fluorescent reporters and this activity was significantly reduced in the presence of the MDR inhibitor PSC 833. GV oocytes are more efficient at efflux compared to M2 oocytes. Human oocytes exposed to cyclophosphamide and PSC 833 showed cell death using two different viability assays compared to controls and those exposed to cyclophosphamide alone. Immunoblots detected MDR-1 in all oocytes with the greatest accumulation in the GV stage.
Conclusions
Oocytes have a vast repertoire of active MDRs. The implications of this study are that these protective mechanisms are important during oogenesis, and these activities change with maturation, increasing susceptibility to toxicants. Future directions may exploit the up regulation of these transporters during gonadotoxic therapy.
Keywords: ATP binding cassette (ABC), multidrug resistant transporter (MDR), P-glycoprotein, oocyte, chemotherapy
Introduction
An estimated 790,740 women in the United States were diagnosed in 2012 with some form of cancer. Approximately 8%, or 60,000, of these women were under the age of 40 (National Cancer Institute SEER Database 2005-2009). Current treatment regimens utilize aggressive chemotherapy aimed at cure. Unfortunately, the alkylating agents used, such as busulfan, carboplatin, chlorambucil, cisplatin, cyclophosphamide, dacarbazine, ifosfamide and thiotepa, can often induce premature ovarian failure, rendering the patient infertile and hormonally compromised (1). Women have the option to preserve their fertility through IVF followed by embryo cryopreservation, ovarian tissue cryopreservation, MII oocyte cryopreservation, and the administration of GnRH agonists (2) However, women are less likely to be offered fertility preservation options compared to men (3) and this is also true in the pediatric cancer population (4). The opportunity to preserve fertility may also be limited by financial constraints and lack of insurance coverage (5). Therefore, the gonadotoxicity of the chemotherapy occurs before any discussion of the current standards of care can be presented to the patient. This has resulted in chemotherapy-induced toxicity quickly becoming a leading cause of ovarian failure, which predisposes the patient to early onset of menopause-associated risks such as accelerated bone loss, increased cardiovascular risk, and sexual dysfunction in addition to infertility severely affecting their quality of life after surviving cancer (6).
The current thinking is that chemotherapeutic agents deplete the pool of primordial follicles and immature oocytes in a drug- and dose-dependent manner. However, work in mice showed that whole ovaries exposed to doxorubicin, an anthracycline, underwent apoptosis through acute injury of the vasculature and that apoptosis occurred in all follicular stages, but only significantly in those at the secondary stage (7). Soleimani et al., 2012 (8) reported that doxorubicin treated ovaries showed double strand DNA breakage-mediated apoptosis in primordial follicles, oocytes, and granulosa cells in a dose-dependent manner. Their data also showed that stromal and vascular damage was implicated in ovarian failure post-treatment with doxorubicin. In a recent review by Morgan et al; (9); 17 studies were selected to ascertain whether the mammalian oocyte or the granulosa cells were the main target of various chemotherapeutics including cisplatin, doxorubicin, and cyclophosphamide. No conclusion could be made from these studies as to whether the germ or somatic cell demise is truly responsible for the observed ovarian damage. A very different perspective has emerged in a study by Kalich-Philosoph et al, (10) which proposes a new mechanism for ovarian insufficiency that suggests that accelerated primordial cell activation may result in aneffective “burn out” and follicle depletion. A definite understanding of the mechanism of ovarian insufficiency has yet to be universally accepted.
It is known that protection from chemotherapy drugs is often observed from multidrug resistant (MDR) transporters, though our understanding of these gene products in the ovarian context is poor. Plasma membranes control the flow of molecules between cells and their environment and the ATP-binding cassette (ABC) transporters, also dubbed multidrug resistant (MDR) transporters, are transmembrane proteins found in cells that provide protection by exporting toxins and xenobiotics (11-14). MDR transporters also function to protect cells from toxic substances including chemotherapeutics, stimulating a second tier approach to block their activity to increase effectiveness of the therapy. P-glycoprotein (P-gp) or MDR-1 is the archetypal transporter with the most clinical importance and overexpression of P-gp is believed to be responsible for drug resistance in many tumors. MDR transporters are expressed in a variety of reproductive cell types including oocytes. Murine oocytes and early embryos express P-gp which is thought to protect them against toxicants (14). However, the relative activity levels and role of multidrug resistant transporters in the oocyte and somatic cells of the ovary has yet to be identified. Studies in oocytes of sea stars (starfish) and pigs have found that MDR transporter activity increase as they mature from germinal vesicle (GV) stage to mature eggs in meiosis II (15-17). The phenomenon of P-glycoprotein up regulation has also been observed in porcine oocytes and granulosa cells matured by in vitro maturation (17). Further, ABC efflux transporters have been implicated in fertility since mutations of ABCC7 (CFTR) have been found to be associated with abnormalities in ovulation and/or oocyte maturation in mice (18). Here we demonstrate the expression of MDR transporters in mouse and human oocytes at the GV, MI, and MII stages by observing directly the efflux of fluorescent dye substrates of MDRs. We also show that Novartis PSC 833, a cyclosporine analog, effectively blocks reporter efflux in the mouse and human oocyte in vitro, further clarifying the MDR activity. We also demonstrate that the inhibition of MDR transporters leads to increased susceptibility of the oocyte to the toxicity of chemotherapy, particularly cyclophosphamide, a drug that alkylates and crosslinks DNA. These data could help to elucidate which stage oocytes are most sensitive to chemotherapy and what role MDR transporters may play in the protection of the oocyte in addition to its supportive granulosa cells.
Material and Methods
Oocyte Sources
Ovaries were harvested from murine B6C3F1 strains from Embryotech in Haverhill, MA by mechanically disrupting follicles with a tuberculin 25 gauge needle. A Drummond pipette was then used to denude oocytes, which were then separated and pooled for experimentation. MII oocytes were recovered from the oviducts of superovulated mice given 5U of PMSG and 75 IU of hCG. Deidentified human oocytes deemed unsuitable for ICSI due to GV or MI stage were collected from the IVF Laboratory at the Center for Reproduction and Infertility at Women and Infants Hospital after obtaining IRB approval for residual tissue studies. These oocytes were either treated immediately or placed into in vitro maturation media supplemented with 7.5IU FSH/7.5IU LH. When necessary oocytes were denuded chemically with 80U hyaluronidase and/or mechanically with micro pipettes.
Immunoblot
For immunoblots with sc-C19 (sc-1517), samples of 50 pooled oocytes of each stage were placed in 20 μl of 2x SDS loading buffer and stored at −20 degrees Celsius. Protein samples were prepared by adding 1 mM DTT to the sample and the solutions were then boiled for 2min taking care to avoid evaporation. Samples were loaded onto a 4–20% gradient Tris–glycine polyacrylamide gel (Invitrogen, Carlsbad, CA) and run at 125 V for 106 min at room temperature. Proteins were transferred to nitrocellulose for 70 minutes at 30 V in a cooled chamber. Blots were blocked for 2-3 h in 4% non-fat milk in Tris-buffered saline (10 μM Trisbase, 150 μM NaCl, pH 7.4) and stored overnight in Tris-buffered saline with 1% Tween. P-gp protein was labeled by incubating blots overnight at 4 degrees Celsius with goat polyclonal antibodyC19 at 1:500, followed by horseradish peroxidase (HRP)-conjugated donkey anti- goat (diluted 1:3000) for 1 h. HRP-labeled P-gp protein was visualized by incubation in a chemiluminescence solution (1.25 mM luminol, 68 μM coumeric acid, 0.0093% hydrogen peroxide and 0.1 M Tris pH 8.6) for 1 min, exposed to film and developed. M2 eggs from the sea star P. miniata, which were previously shown to express MDR-1, were used as positive control (15).
Quantative PCR
Primers to the unique regions of the MDR-1 transcript (F: 5’cacagcttgtccagccaat 3’ and R: 5’acttctcgaagatgggcaa 3’) as well as to the transcripts for β-actin and 18S rRNA in mice were designed. Oocytes were pooled in RNA Lysis Buffer and stored at −80 degrees Celsius until pools of 25-30 oocytes of each stage could be assembled. Using the RNA Easy microkit (Qiagen), RNA was extracted and reverse transcription was utilized to make cDNA using the Taqman Kit (Applied Biosystems). RT-PCR was then performed.
Fluorescence Efflux Activity
All dye and chemotherapy experiments were recorded using confocal microscopy (Zeiss 510 meta) equipped with a 37° C degree temperature stage and Trigas chamber (composed of 90 % Nitrogen, 5% CO2 and 5% O2 mimicking IVF-incubator conditions). Oocytes from the GV to the MII stage were incubated with Calcein AM dye for 1 hour and washed. Under confocal microscopy, oocyte fluorescence intensity was monitored longitudinally and recorded with an excitation of 495 nm and 518 nm emission for Calcein AM. Images were then quantified over time using MetaMorph.
Oocyte Chemotherapy Susceptibility
After performing a dose curve, mouse oocytes were incubated with 25mM of cyclophosphamide and +/25 microM of PSC 833. In addition, human oocytes were incubated with 80 mM of cyclosphosphamide for 12 hours +/− 10 microM PSC 833. To determine cell death 0.04% Trypan blue was used as a dye exclusion viability test as well as The LIVE/DEAD Viability/Cytotoxicity Assay (Invitrogen) to quantitate cell death in these oocytes. Human oocyte fluorescence results were quantified using Metamorph imaging software (Universal Imaging Corporation, Downingtown, PA).
Immunofluorescence
Isolated mouse oocytes were fixed in 4% paraformaldehyde in 0.1 M PBS and stored at 4 degrees Celsius until ready for use. The oocytes were then washed in IF buffer (PBS and 0.4% Triton) three times for 10 minutes. After establishing a titration curve with the primary antibodies, the oocytes were incubated with the MDR-1 (Santa Cruz sc-1517; diluted 1:250) at 4 degrees overnight followed by three 10 minute washes in IF buffer. The oocytes were then incubated with secondary antibodies (Alexa 488 donkey anti-goat 1:5000), also diluted in PBS and 0.4% Triton, for 1 hour at room temperature, followed by three 10-minute washes. A secondary control (no primary) of oocytes was also prepared and processed simultaneously. Finally, the oocytes were mounted between slide and coverslip and visualized with a 510 LSM laser scanning confocal microscope (Zeiss) (Carl Zeiss Incorporated, Thornwood, NY). Optical sections of 1 micrometer were obtained, and the imaging parameters were set using the greyscale function in the Zen software (Zeiss) so that all the images were recorded equally and under the point of saturation. The images were then exported as TIFF files and analyzed with Metamorph.
Data analysis
Fluorescence intensity was measured for individual oocytes with MetaMorph software. All absolute measurements were averaged (± 1 SD). Data were reported as average fluorescence intensity with differences between treatments analyzed using two sample paired t-tests of means (with a significance cutoff of P < 0.05). Chemotherapeutic-induced cell death was quantified by using Trypan blue dye exclusion test and the LIVE/DEAD Viability/Cytotoxicity Assay according to manufacturer's instructions.
Results
Diversity of MDRs in the oocyte
We used recently acquired transcriptomic results to computationally probe the repertoire of mRNAs present in mouse and human oocytes (19,20). We find from both single cell analysis of largely MI staged oocytes ~25 different MDR-genes present in both mouse and humans representing a large diversity of ABC subfamily members (Supplemental figures 1 and 2). Although each transporter has not been fully characterized, and their precise substrates for efflux have not been defined, we believe the results presented here likely represent a much larger set of transporters present in the cell. Further, it is of interest that in the mouse Taf4b knock-out, a model for POI, that transcripts of some ABC transporters are significantly higher in WT compared to the knockout (e.g. ABCa1 WT = 5.3, compared to the KO=1.48) whereas several transporter transcripts are markedly elevated in the KO compared to WT (e.g. ABCf3 WT=0, KO=40.29; ABCb1b WT=1.31, KO=15.48; ABCb4 WT=17.91, KO=49.87 (Supplemental Figure 1).
A dynamic expression of MDR protein and mRNA during oocyte maturation
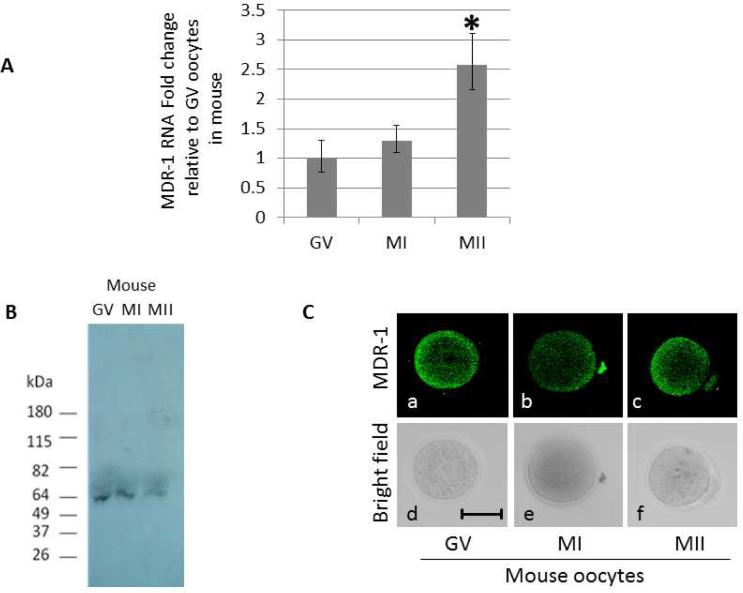
When transcript levels of MDR-1 were tested by qPCR, M2 oocytes showed an increase compared to GV (Figure 1A). Immunoblots of oocytes of various stages documented a single band of 65kDa in GV, M1, and M2 stages. When normalized to number of oocytes, this result revealed more signal intensity in the GV pool of oocytes compared to M1 and M2 pools (Figure 1B). This result is supported by immunolocalization in oocytes – using the same antibody between blots and in situ immunolabeling, the fluorescence signal in GV oocytes is markedly more intense when compared to M2 oocytes (Figure 1C, Supplemental figures 3 and 4). These results may mean that the mRNA increase for MDR-1 seen in MII oocytes is not translated, but perhaps stored for use later, after fertilization and during embryogenesis. Recently, many mRNAs in the oocyte have been shown to be under translational control by a variety of means (21), and the results obtained here suggest that at least MDR-1 is also regulated post-transcriptionally. These results may offer new approaches to therapeutic promise in the future.
Figure 1.
Dynamic expression of MDR mRNA and protein during oocyte maturation in mouse. (A) MDR-1 RNA levels increase during oogenesis. Quantitative PCR was used to measure the RNA levels of MDR-1 in mouse oocytes at the indicated developmental stages: GV, MI and MII. All values were normalized against the β-actin RNA and represented as a fold change relative to the amount of RNA present in the GV oocytes. Significance was assessed between each developmental stage using Student's ttest, P<0.05. Significant differences (*) were obtained between GV and MII, and MI and MII. (B) MDR-1 protein expression. Western blot using an antibody against MDR-1 on GV, MI, and MII mouse oocytes. 50 oocytes were loaded in each group. (C) MDR-1 is expressed throughout the oocytes. Immunofluorescence using an antibody against MDR-1 on mouse oocytes, at GV (a), MI (b), and MII (c).The corresponding differential interference contrast images are respectively shown in d to f. Pictures were taken at 200x magnification. Scale bars, 50 μm.
Oocytes exhibit significant and dynamic MDR Efflux Activity during Maturation
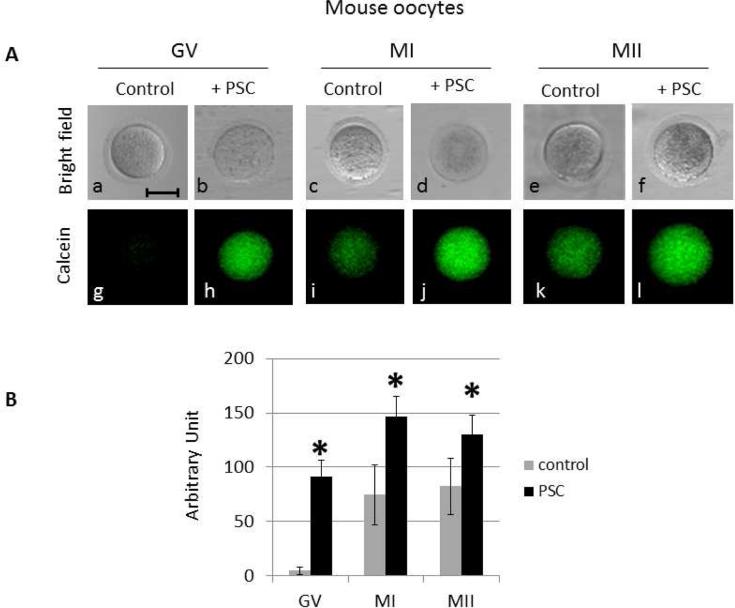
Calcein-AM is a valuable reporter for MDR activity in a cell. It enters the cell by diffusion as a result of its AM-group, which is then cleaved off by endogenous esterase activity, trapping the fluorescent marker in the cell. Effluxing of this reporter is selective for MDR-1/Pgp in the oocyte's plasma membrane and is the gold standard for studying transporter function. Efflux activity with calcein-AM was detected in all stages of oocytes, and the relative activity decreased in M2 oocytes compared to the earlier stages of germinal vesicles (Figure 2, Supplemental figure 5). Polar bodies of the M2 oocytes also appeared to efflux the dyes although somewhat less efficiently than its sibling oocyte. The rate of calcein efflux for GV stages is higher than that seen in M2 oocytes, with P-value significance great than 0.05. Interestingly, in the germinal vesicle stage dye does not enter the germinal vesicle itself instead it remains in the cytoplasm. That removal of the calcein reporter from the cell is mediated by MDR is supported by use of the P-gp inhibitor PSC833. This inhibitor is specific for Pgp MDRs, and the effect on the oocyte is a dramatic loss (~10-fold) of efflux capability in the oocytes at GV and MI stage. MII staged oocytes are much less efficient at effluxing in general, so the inhibitory effect of PSC833 is significantly less.
Figure 2.
MDR activity changes with oocyte maturation stage. (A) Mouse oocytes were incubated with calcein AM without (control: g, i,k) or with PSC 833 (PSC: h,j,l). The corresponding differential interference contrast images are respectively shown in a to f, at 200x magnification. Scale bar, 50 μm.(B) The fluorescence resulting from the calcein in the whole oocytes was quantified using metamorph. 12 GV, 7 MI and 8 MII were used for the quantification in the control, 9 GV, 4 MI and 7 MII were analyzed after the PSC833 treatment. Significance was assessed between control (without PSC) and PSC833 treated oocytes for each developmental stage using Student's ttest, P<0.005. Significant differences (*) were obtained for each stage.
MDR-1 functions in Chemotherapy Susceptibility
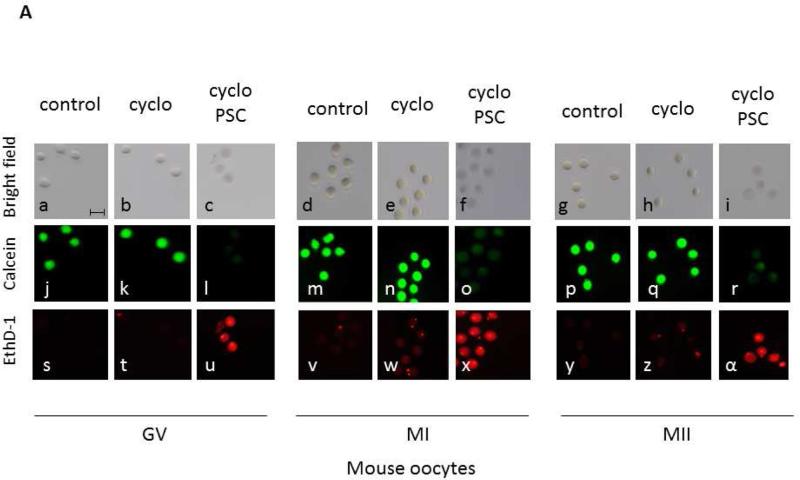
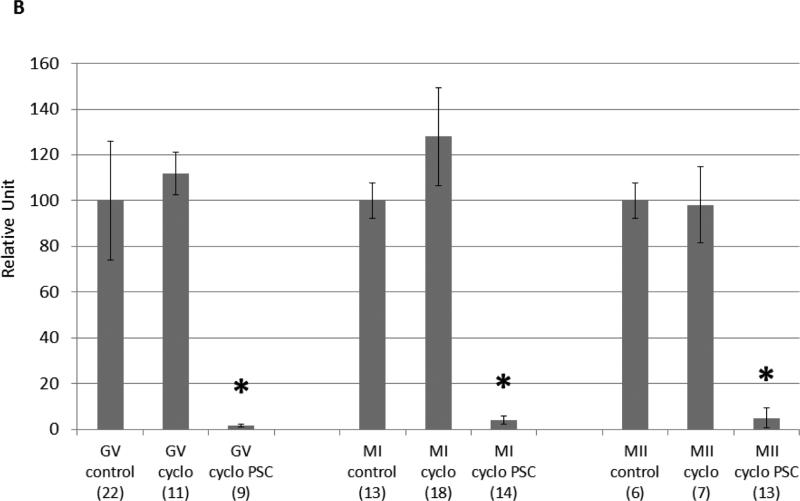
Oocytes at all maturational stages show significantly decreased MDR-1 activity when treated with the specific MDR-1 inhibitor PSC 833. This result supports the contention that calcein efflux is indeed occurring through the MDR-1 channel. Both mouse and human oocytes exposed to cyclophosphamide and PSC 833 showed cell death with The LIVE/DEAD Viability/Cytotoxicity assays in comparison to oocytes treated with media alone, PSC alone, or cyclophosphamide alone (Figures 3 and 4). Cell death was also observed by trypan blue on human oocytes treated with cyclophosphamide and PSC833 (Supplemental figure 6). Here, to better understand the role of MDRs in oocytes, we purposely chose concentrations of cyclophosphamide that did not induce apoptosis on its own. Although the internal concentrations of this drug in the oocyte are unknown, the concentrations used herein are likely much higher than oocytes experience, forming a test of MDR activity of high stringency.
Figure 3.
MDR activity is essential for the survival of mouse oocytes exposed to a chemotherapeutic agent. (A) Cell death was measured using the live/dead assay. GV, MI and MII mouse oocytes were incubated for 12 hours with 25mM cyclophosphamide only (b,k,t; e,n,w; h,q,z) or in presence of 25mM cyclophosphamide and 25μM PSC 833 (c,l,u; f,o,x; i,r,α). Untreated oocytes were used as control (a,j,s; d,m,v; g,p,y). The live/dead assay was performed by simultaneously monitoring the fluorescence after addition of calcein AM (j,k,l,m,n,o,p,q,r) and ethidium homodimer-1 (s,t,u,v,w,x,y,z,α). The calcein AM (green) is retained in live cells, while the ethidium homodimer-1 (red) enters dead cells. The corresponding differential interference contrast images are respectively shown in a to i. Scale bar, 100 μm. (B) The fluorescence was quantified using metamorph. For each oocyte, only a small area was considered for the fluorescence measurement in order to avoid the surrounding granulosa cells. Cell viability is represented on the graph using a relative unit: ratio of green/red fluorescence measured in each oocyte. The number of oocytes used for each condition is indicated in parenthesis. Significance was assessed between oocytes treated with cyclophosphamide without or with PSC 833 for each developmental stage using Student's ttest, P<0.005. Significant differences (*) were obtained for each stage.
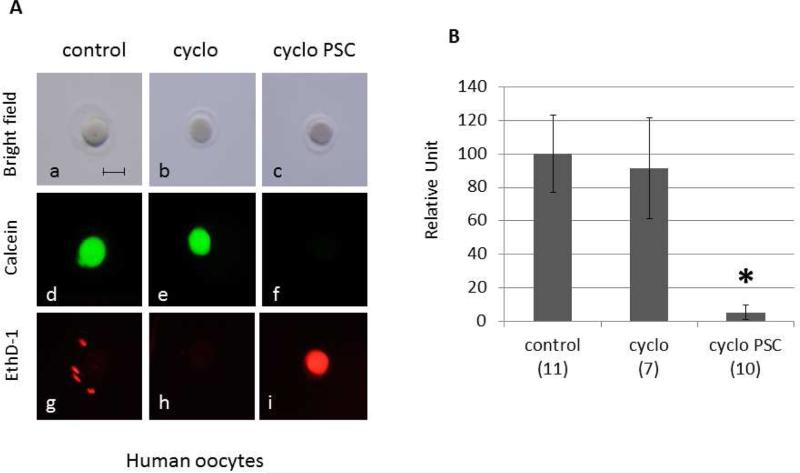
Figure 4.
MDR activity is essential for the survival of human oocytes exposed to a chemotherapeutic agent. (A) Cell death was measured using the live/dead assay. Human oocytes were incubated for 12 hours with 80mM cyclophosphamide only (b,e,h) or in presence of 80mM cyclophosphamide and 10μM PSC 833 (c,f,i). Untreated oocytes were used as control (a,d,g). The live/dead assay was performed by simultaneously monitoring the fluorescence after addition of calcein AM (d,e,f) and ethidium homodimer-1 (g,h,i). The calcein AM (green) is retained in live cells, while the ethidium homodimer-1 (red) enters dead cells. The corresponding differential interference contrast images are respectively shown in a to c. Scale bar, 100 μm. (B) The fluorescence was quantified using metamorph. For each oocyte, only a small area was considered for the fluorescence measurement in order to avoid the surrounding granulosa cells. Cell viability is represented on the graph using a relative unit: ratio of green/red fluorescence measured in each oocyte. The number of oocytes used for each condition is indicated in the parenthesis. Significance was assessed between oocytes treated with cyclophosphamide without or with PSC 833 using Student's ttest, P<0.005. Significant difference (*) was obtained in presence of PSC 833.
Discussion
The chemotherapeutic cost in treatment of reproductive aged women is premature loss of primordial follicles or perhaps accelerated activation leading to early onset of menopause. Chemotherapy-induced toxicity is rapidly becoming a leading cause of ovarian sufficiency, which predisposes the patient to early onset of menopause-associated risks such as accelerated bone loss, increased cardiovascular risk, and sexual dysfunction in addition to infertility (6). Protection from xenobiotics in cells often results from MDR transporters, and P-glycoprotein is the best understood MDR transporter in mammalian cells. The exact role of the MDR transporter in oocytes has yet to be determined although investigators (15) have shown in oocytes of the sea star that their function is not in maturation of the oocyte. Yet, they did find that these transporters are mobilized as the oocyte matures. MDRs appear to be highly conserved transporters, perhaps reflecting their crucial roles in effluxing of metabolites, xenobiotics, and perhaps even signaling molecules (22,23). In the case of sea stars, which broadcast spawn their oocytes into the environment, it is plausible that the transporters served as protection to their germ line. Perhaps, mammalian oocytes may have retained the capacity to accomplish this given that the oocytes must endure the often lengthy reproductive life of the animal and which may include exposure to toxicants such as metabolites, chemicals, and chemotherapeutics. Our transcriptomic results suggest that the MDR profile of cells can be dramatically changed either directly or indirectly by transcriptional activity of the cell(19). This may reflect a potential target for modulating MDR activity and differences of such activities naturally in cells may explain why some oocytes have better xenobiotic resistance than others. At least for this study, it suggests a much broader level of MDR presence than previously considered.
We have shown here that mouse and human oocytes have active effluxers, and that MDR-dependent dye is removed from the cell in GV stage oocytes more effectively than M2 stage oocytes. This result in comparative activity is different than seen in the sea star oocyte as well as the pig oocyte (15,16,24), which has more efflux activity as it matures. Perhaps in starfish, which spawn their mature eggs from a continuous stem cell supply, there may be an advantage to upregulating MDR activity at the M2 stage which will be exposed to environmental conditions in the benthos as opposed to mammals who have a finite reserve of long lived oocytes with ootoxic exposures occurring over prolonged periods. This ‘limited supply’ while being challenged by recent work (24) may be why immature oocytes are preferentially protected because they are present in the animal for the duration of its reproductive years. While it is clear that GV do not have significantly more transporters, it appears that their efflux activity is more efficient compared to later stages. This is evidenced by increases in Calcein-AM fluorescence retention in more mature M1 and M2 oocytes. We believe that most, if not all of the reporter efflux is via MDRs since use of only the MDR-1 transport inhibitor, PSC833, retains dye significantly. Calein-AM dye in particular works by first diffusing into the cell as a result of the AM-conjugated group. However, since the AM-conjugation is labile to endogenous esterase activity, the AM-cleaved product results in calcein now trapped in the cell. Its only exit mechanism known is effluxing through the MDRs, and this conclusion is supported in both human and mouse oocytes by use of the MDR-1 inhibitor PSC833, in which the dye no longer leaves the cell and instead remains trapped within the cytoplasm.
When oocytes are exposed to cyclophosphamide levels used herein, they survive. However, if the MDR-1 is inhibited and the oocytes are exposed to the chemodrugs, the oocytes are no longer viable. This is supportive of the work Kalich-Philosoph et al (10) who also showed that cyclophosphamide does not destroy primordial follicle oocytes directly through apoptosis. Although the level of each drug that actually reaches the oocyte in vivo is unknown, we used a range of concentrations to test the protection provided by the MDR repertoire in the cell. The results suggest that MDR transport may be vital to oocyte protection especially in crucial transition of an oocyte during maturation. Further study is needed to comprehend the basic physiologic process of MDR in primordial follicles and maturing oocytes so that this transporter can be understood to maximally protect primordial follicles and growing oocytes from toxicity. Additionally, MDR activity may be critical for the survival of the entire follicles since it is also detected in the granulosa cells (Supplemental figure 7), which merits further investigation. MDR function in oocytes and whole follicles could serve as a model for assessing environmental reproductive toxicants as well as other chemotherapeutics.
Supplementary Material
Supplemental figure 1: Oocyte MDR Transcriptome in WT Mice and Taf4B (KO; subfertile mice). RPKM=Reads Per Kilobase length of transcript per Million reads. KO=Taf4B mice who are subfertile
Supplemental figure 2: MDR Transcriptome in Human Oocytes
Supplemental figure 3: Titration of the MDR-1 antibody on mouse oocytes. GV oocytes were used to define the optimal concentration of MDR-1 antibody. The control represents oocytes that were only treated with the secondary antibody, in absence of MDR-1 primary antibody (A). Three dilutions of MDR-1 antibody were tested: 1:100 (B), 1:250 (C) and 1:500 (D). The corresponding differential interference contrast images are respectively shown in E to H, at 200x magnification. Scale bar, 50 μm.
Supplemental figure 4: Immunofluorescence control on mouse oocytes. Immunofluorescence without MDR-1 primary antibody on GV (A), MI (B) and MII (C) oocytes. The corresponding differential interference contrast images are respectively shown in D to F. Pictures were taken using the same microscope settings as Figure 3 (laser intensity, pin-hole opening) at 200x magnification. Scale bar, 50 μm.
Supplemental figure 5: MDR activity changes with oocyte maturation stage in FVBN mouse. Oocytes were incubated with calcein AM without (control) or with PSC 833 (PSC). Pictures were taken at 200x magnification. Scale bar, 50 μm. (B) The fluorescence was quantified using metamorph. 10 GV and 6 MI were used for the quantification in the control, 8 GV and 10 MI were analyzed after the PSC treatment. Significance was assessed between control (without PSC) and PSC treated oocytes for each developmental stage using Student's ttest, P<0.05. Significant differences (*) were obtained in GV and MI oocytes.
Supplemental figure 6: Human oocytes die in presence of cyclophosphamide and PSC. Human oocytes were treated for 12 hours with 80mM cyclophosphamide only (A), or 80mM cyclophosphamide and 10μM PSC 833 (B). Cell death was analyzed after incubation of the oocytes in 0.04% trypan blue. Scale bar, 100 μm.
Supplemental figure 7: MDR activity in granulosa cells. (A) GV oocytes surrounded by granulosa cells were incubated with calcein AM. The corresponding differential interference contrast image is shown in (B) at 200x magnification. Scale bar, 50 μm.
Acknowledgements
We thank the members of PRIMO for a rich work environment. In particularly we thank Adrian Reich for help in interpreting transcriptomic data for MDR sequences. Support for this work was provided in part by the National Institutes of Health (2R01HD028152 to GMW).
Abbreviations
- MDR
multidrug resistant
- ABC
ATP binding cassette
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: There are no financial disclosures
References
- 1.Meirow D. Reproduction post-chemotherapy in young cancer patients. Mol Cell Endocrinol. 2000;169:123–31. doi: 10.1016/s0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 2.Blumenfeld Z. How to Preserve Fertility in Young Women Exposed to Chemotherapy? The Role of GnRH Agonist Cotreatment in Addition to Cryopreservation of Embrya, Oocytes, or Ovaries. Oncologist. 2007;12:1044–54. doi: 10.1634/theoncologist.12-9-1044. [DOI] [PubMed] [Google Scholar]
- 3.Armuand GM, Rodriguez-Wallberg KA, Wettergren L, Ahlgren J, Enblad G, Hoglund M, et al. Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol. 2012;30:2147–53. doi: 10.1200/JCO.2011.40.6470. [DOI] [PubMed] [Google Scholar]
- 4.Kohler TS, Kondapalli LA, Shah A, Chan S, Woodruff TK, Brannigan RE. Results from the survey for preservation of adolescent reproduction (SPARE) study: gender disparity in delivery of fertility preservation message to adolescents with cancer. J Assist Reprod Genet. 2011;28:269–77. doi: 10.1007/s10815-010-9504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basco D, Campo-Engelstein L, Rodriguez S. Insuring against infertility: expanding state infertility mandates to include fertility preservation technology for cancer patients. J Law Med Ethics. 2010;38:832–9. doi: 10.1111/j.1748-720X.2010.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebar RW. Premature ovarian failure. Obstet Gynecol. 2009;113:1355–63. doi: 10.1097/AOG.0b013e3181a66843. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Aharon I, Bar-Joseph H, Tzarfaty G, Kuchinsky L, Rizel S, Stemmer SM, et al. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol. 2010;8:20. doi: 10.1186/1477-7827-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging. 2011;3:782–93. doi: 10.18632/aging.100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18:525–35. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 10.Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, et al. Cyclophosphamide Triggers Follicle Activation and “Burnout”; AS101 Prevents Follicle Loss and Preserves Fertility. Sci Transl Med. 2013;5(185) doi: 10.1126/scitranslmed.3005402. [DOI] [PubMed] [Google Scholar]
- 11.Dean M, Allikmets R. Complete characterization of the human ABC gene family. J Bioenerg Biomembr. 2001;33:475–9. doi: 10.1023/a:1012823120935. [DOI] [PubMed] [Google Scholar]
- 12.Deeley RG, Cole SP. Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS lett. 2006;580:1103–11. doi: 10.1016/j.febslet.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 14.Elbling L, Berger W, Rehberger A, Waldhor T, Micksche M. P-glycoprotein regulates chemosensitivity in early developmental stages of the mouse. FASEB J. 1993;7:1499–506. doi: 10.1096/fasebj.7.15.7903262. [DOI] [PubMed] [Google Scholar]
- 15.Roepke TA, Hamdoun AM, Cherr GN. Increase in multidrug transport activity is associated with oocyte maturation in sea stars. Dev Growth Differ. 2006;48:559–73. doi: 10.1111/j.1440-169X.2006.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arai M, Yamauchi N, Fukuda H, Soh T, Hattori MA. Development of multidrug resistance type I P-glycoprotein function during in vitro maturation of porcine oocyte. Reprod Toxicol. 2006;21:34–41. doi: 10.1016/j.reprotox.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Yokota K, Hirano T, Urata N, Yamauchi N, Hattori MA. Upregulation of P-glycoprotein activity in porcine oocytes and granulosa cells during in vitro maturation. J Reprod Dev. 2011;57:322–6. doi: 10.1262/jrd.10-137m. [DOI] [PubMed] [Google Scholar]
- 18.Hodges CA, Palmert MR, Drumm ML. Infertility in females with cystic fibrosis is multifactorial: evidence from mouse models. Endocrinology. 2008;149:2790–7. doi: 10.1210/en.2007-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reich A, Neretti N, Freiman RN, Wessel GM. Transcriptome variance in single oocytes within, and between, genotypes. Mol Reprod Dev. 2012;79:502–3. doi: 10.1002/mrd.22061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reich A, Klatsky P, Carson S, Wessel G. The transcriptome of a human polar body accurately reflects its sibling oocyte. J Biol Chem. 2011;286:40743–9. doi: 10.1074/jbc.M111.289868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Melton C, Suh N, Oh JS, Horner K, Xie F, et al. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011;25:755–66. doi: 10.1101/gad.2028911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campanale JP, Hamdoun A. Programmed reduction of ABC transporter activity in sea urchin germline progenitors. Development. 2012;139:783–92. doi: 10.1242/dev.076752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricardo S, Lehmann R. An ABC transporter controls export of a Drosophila germ cell attractant. Science. 2009;323:943–6. doi: 10.1126/science.1166239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18:413–21. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1: Oocyte MDR Transcriptome in WT Mice and Taf4B (KO; subfertile mice). RPKM=Reads Per Kilobase length of transcript per Million reads. KO=Taf4B mice who are subfertile
Supplemental figure 2: MDR Transcriptome in Human Oocytes
Supplemental figure 3: Titration of the MDR-1 antibody on mouse oocytes. GV oocytes were used to define the optimal concentration of MDR-1 antibody. The control represents oocytes that were only treated with the secondary antibody, in absence of MDR-1 primary antibody (A). Three dilutions of MDR-1 antibody were tested: 1:100 (B), 1:250 (C) and 1:500 (D). The corresponding differential interference contrast images are respectively shown in E to H, at 200x magnification. Scale bar, 50 μm.
Supplemental figure 4: Immunofluorescence control on mouse oocytes. Immunofluorescence without MDR-1 primary antibody on GV (A), MI (B) and MII (C) oocytes. The corresponding differential interference contrast images are respectively shown in D to F. Pictures were taken using the same microscope settings as Figure 3 (laser intensity, pin-hole opening) at 200x magnification. Scale bar, 50 μm.
Supplemental figure 5: MDR activity changes with oocyte maturation stage in FVBN mouse. Oocytes were incubated with calcein AM without (control) or with PSC 833 (PSC). Pictures were taken at 200x magnification. Scale bar, 50 μm. (B) The fluorescence was quantified using metamorph. 10 GV and 6 MI were used for the quantification in the control, 8 GV and 10 MI were analyzed after the PSC treatment. Significance was assessed between control (without PSC) and PSC treated oocytes for each developmental stage using Student's ttest, P<0.05. Significant differences (*) were obtained in GV and MI oocytes.
Supplemental figure 6: Human oocytes die in presence of cyclophosphamide and PSC. Human oocytes were treated for 12 hours with 80mM cyclophosphamide only (A), or 80mM cyclophosphamide and 10μM PSC 833 (B). Cell death was analyzed after incubation of the oocytes in 0.04% trypan blue. Scale bar, 100 μm.
Supplemental figure 7: MDR activity in granulosa cells. (A) GV oocytes surrounded by granulosa cells were incubated with calcein AM. The corresponding differential interference contrast image is shown in (B) at 200x magnification. Scale bar, 50 μm.