Abstract
Purpose
To investigate the effect of cocoa powder supplementation on obesity-related inflammation in high fat (HF)-fed obese mice.
Methods
Male C57BL/6J (n = 126) were fed with either low-fat (LF, 10 % kcal from fat) or HF (60 % kcal from fat) diet for 18 weeks. After 8 weeks, mice from HF group were randomized to HF diet or HF diet supplemented with 8 % cocoa powder (HF–HFC group) for 10 weeks. Blood and tissue samples were collected for biochemical analyses.
Results
Cocoa powder supplementation significantly reduced the rate of body weight gain (15.8 %) and increased fecal lipid content (55.2 %) compared to HF-fed control mice. Further, cocoa supplementation attenuated insulin resistance, as indicated by improved HOMA-IR, and reduced the severity of obesity-related fatty liver disease (decreased plasma alanine aminotransferase and liver triglyceride) compared to HF group. Cocoa supplementation also significantly decreased plasma levels of the pro-inflammatory mediators interleukin-6 (IL-6, 30.4 %), monocyte chemoattractant protein-1 (MCP-1, 25.2 %), and increased adiponectin (33.7 %) compared to HF-fed mice. Expression of pro-inflammatory genes (Il6, Il12b, Nos2, and Emr1) in the stromal vascular fraction (SVF) of the epididymal white adipose tissue (WAT) was significantly reduced (37–56 %) in the cocoa-supplemented mice.
Conclusions
Dietary supplementation with cocoa ameliorates obesity-related inflammation, insulin resistance, and fatty liver disease in HF-fed obese mice, principally through the down-regulation of pro-inflammatory gene expression in WAT. These effects appear to be mediated in part by a modulation of dietary fat absorption and inhibition of macrophage infiltration in WAT.
Keywords: Theobroma cacao, Cocoa, Polyphenols, Inflammation, Obesity
Introduction
Obesity is defined as a phenotypic manifestation of abnormal or excessive fat accumulation that alters health and increases mortality [1]. Today, more than 35 % of Americans are obese and if the current trajectory continues, the rate will reach 51 % by 2030 [2]. Indeed, obesity is a multifactorial disorder that is a significant risk factor for type 2 diabetes, cardiovascular disease, obesity-related fatty liver disease (ORFLD), and certain cancers [1, 3, 4]. Obesity and the associated metabolic pathologies are the most common and detrimental metabolic diseases, affecting over 50 % of the adult population [5]. It is becoming more evident that obesity is a chronic inflammatory state, also known as low-grade or systemic inflammation, which represents the important link between obesity and its comorbidities [1, 3, 4].
Obesity-related inflammation is characterized by macrophage infiltration into adipose tissue, abnormal cytokine production, and activation of inflammatory signaling pathways [5–7]. Compared with white adipose tissue (WAT) from lean individuals, WAT from obese individuals expresses increased amounts of pro-inflammatory mediators including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), inducible nitric oxide synthase (iNOS, also known as NOS2), and monocyte chemoattractant protein-1 (MCP-1), as well as decreased levels of adiponectin [8, 9]. Adipose tissue-associated macrophages (ATMs) are responsible for nearly all TNF-α released by WAT and approximately 50 % of WAT-derived IL-6 in obese mice [8, 10]. Most cytokines produced by WAT are closely associated with obesity-induced insulin resistance and hepatic steatosis (fatty liver), the excess accumulation of triglycerides in hepatocytes [11], which is commonly reflected by abnormal circulating concentrations of hepatic enzymes (e.g., alanine aminotransferase (ALT)).
Recently, a growing number of studies have reported the beneficial health effects of cocoa (Theobroma cacao) and cocoa polyphenols, including modulation of atherosclerosis and hypertension [12, 13]. A number of potential mechanisms have been proposed for these effects, including the inhibition of platelet aggregation, as well as antioxidant and anti-inflammatory effects [14, 15]. Relatively few studies have investigated the preventive or therapeutic effects of cocoa and cocoa constituents against obesity and metabolic syndrome [16–20]. For example, treatment of high fat (HF)-fed rats with 12.5 % cocoa powder for 3 weeks significantly decreased final body weights, mesenteric WAT weights, and modulated the expression of genes related to fatty acid metabolism [17]. Tomaru et al. [18] and Yamashita et al. [20] have both reported that dietary supplementation with cocoa liquor proanthocyanidins (PACs) suppressed HF diet-induced hyperglycemia, glucose intolerance, and fat accumulation in WAT in diabetic obese mice.
Several in vitro studies have shown that certain isolated flavan-3-ols and PACs from cocoa exert anti-inflammatory activities by modulating the transcription and secretion of pro-inflammatory cytokines in human peripheral blood mononuclear cells and macrophages, although the effects of these compounds vary depending on the degree of polymerization (DP) of the test compound [21–25]. For example, epicatechin and cocoa PACs have been found to reduce the secretion of TNF-α, MCP-1, and nitric oxide (NO) in macrophages in vitro [24]. We have previously reported that cocoa-derived PACs potently inhibit the activity of secreted phospholipase A2 in a cell-free system [26]. Inhibitory potency increased as a function of DP with higher-molecular weight compounds (DP > 7) having the most potent activity. These studies would suggest that cocoa has anti-inflammatory effects in vivo but further experimental evidence is needed to establish effective dose levels, demonstrate efficacy, and establish the underlying mechanisms of action.
In the present study, we investigated the effects of cocoa powder supplementation on the markers of obesity-related inflammation and co-pathologies in HF-fed obese C57BL/ 6J mice. Herein, we report the results of our studies.
Materials and methods
Diets and chemicals
Unsweetened cocoa powder was generously provided by Blommer Chocolate Co. (Chicago, IL). The composition of the cocoa powder including polyphenol content is shown in Table 1. Total polyphenols in cocoa powder were quantified by Folin–Ciocalteu method with gallic acid as a standard, and the contents of flavan-3-ols (from monomers to decamer) were determined by diol HPLC, described by a previous method [27]. All other chemicals were of the highest grade commercially available. Low-fat (LF, 10 % kcal from fat, D12450B), high-fat (HF, 60 % kcal from fat, D12492) and HF diet supplemented with 80 g/kg cocoa (HFC, D10052503) diet were prepared by Research Diets (New Brunswick, NJ). The composition of the diets is given in Table 2.
Table 1.
Composition in unsweetened cocoa powder
| Energy content | 4 kcal/g |
|---|---|
|
| |
| Content (mg/g) | |
| Macronutrients | |
| Total fat | 100 |
| Total carbohydrate | 600 |
| Dietary fiber | 200 |
| Sugar | 0 |
| Total protein | 200 |
| Polyphenol | |
| Total monomers | 71.0 |
| Epicatechin | 4.6 |
| Catechin | 2.2 |
| Proanthocyanidins | |
| Dimer | 3.3 |
| Trimer | 3.1 |
| Tetramer | 3.8 |
| Pentamer | 3.2 |
| Hexamer | 2.7 |
| Heptamer | 2.2 |
| Octamer | 2.6 |
| Nonamer | 1.5 |
| Decamer | 1.7 |
Table 2.
Composition of mouse diets
| LF | HF | HFC | |
|---|---|---|---|
| Macronutrient composition | |||
| Protein, % of energy | 20.0 | 20.0 | 21.0 |
| Carbohydrate, % of energy | 70.0 | 20.0 | 21.0 |
| Fat, % of energy | 10.0 | 60.0 | 59.0 |
| Energy (MJ/kg) | 15.9 | 21.8 | 21.4 |
| Ingredient (g/kg) | |||
| Casein | 189.6 | 258.4 | 237.8 |
| L-cystine | 2.8 | 3.9 | 3.6 |
| Corn starch | 298.6 | 0.0 | 0.0 |
| Maltodextrin | 33.2 | 161.5 | 148.6 |
| Sucrose | 331.7 | 88.9 | 81.8 |
| Cellulose | 47.4 | 64.6 | 59.4 |
| Soybean oil | 23.7 | 32.3 | 29.7 |
| Lard | 19.0 | 316.6 | 291.3 |
| Mineral mixa | 9.5 | 12.9 | 11.9 |
| Vitamin mixb | 9.5 | 12.9 | 11.9 |
| Choline bitartrate | 1.9 | 2.6 | 2.4 |
| Cocoa | 0.0 | 0.0 | 80.0 |
Mineral mix adds the following components (per g mineral mix): sodium chloride, 259 mg; magnesium oxide, 41.9 mg; magnesium sulfate, 257.6 mg; chromium K sulfate, 1.925 mg; cupric carbonate, 1.05 mg; sodium fluoride, 0.2 mg; potassium iodate, 0.035 mg; ferric citrate, 21 mg; manganous carbonate, 12.25 mg; ammonium molyb-date, 0.3 mg; sodium selenite, 0.035 mg; zinc carbonate, 5.6 mg
Vitamin mix adds the following components (per g vitamin mix): vitamin A palmitate, 400 IU; vitamin D3, 100 IU; vitamin E acetate, 5 IU; menadione sodium bisulfite, 0.05 mg; biotin, 0.02 mg; cyanocobalamin, 1 μg; folic acid, 0.2 mg; nicotinic acid 3 mg; calcium pantothenate, 1.6 mg; pyridoxine–HCl, 0.7 mg; riboflavin, 0.6 mg; thiamin HCl, 0.6 mg
Animals and treatment
All animal experiments were conducted in accordance with a protocol (IACUC# 28962 and 37115) approved by the Institutional Animal Care and Use committee at the Pennsylvania State University (University Park, PA). Male C57BL/6J mice (4 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME) and maintained on 12 h light/dark with access to food and water ad libitum. After a two-week acclimatization period, mice were randomized to LF diet (n = 12) or HF diet (n = 46) treatments. After 8 weeks, HF-fed mice were randomized again into two groups based on body weight: half were maintained on the HF diet (HF group, n = 23) and half were fed with HFC diet (HF–HFC group, n = 23) for 10 weeks. Body weight and food intake were recorded weekly. At the end of week 18, mice were food-deprived for 7 h (7 am–2 pm), anesthetized, and killed by exsanguination via cardiac puncture. Hearts, livers, spleens, kidneys, and visceral fat depots (epididymal, retroperitoneal, and mesenteric) were harvested, rinsed, and weighed. Plasma samples were isolated by centrifugation at 3200g for 15 min. All samples were snap-frozen and stored at −80 °C until further analysis. The study was repeated (Exp 2) a year after the original study (Exp 1) using the same experimental design, where n = 23 for LF, and n = 21 for HF, and n = 24 for HF–HFC.
Glycemic markers
Fasting blood glucose measurements were recorded on weeks 0, 4, 8, 10, 12, 14, 16, and 18 for each treatment group using a handheld Contour glucose monitor (Bayer Healthcare, Tarrytown, NY). Mice were food-deprived for 7 h after the cage bedding was changed (to prevent coprophagy) and blood was sampled from the tail vein. Fasting plasma insulin was determined at the end of the experiment using an ELISA kit (Crystal Chem, Downers Grove, IL) according to the manufacturer’s protocol. Insulin resistance was estimated based on the final blood glucose and insulin values using the homeostasis model assessment of insulin resistance (HOMA-IR) [28].
Fecal lipid content
Samples were weighed, pulverized in liquid nitrogen, and then extracted twice with an equal volume of methanol/ chloroform (2:1, v:v). The organic phase was filtered through a 0.45 μm PTFE membrane and dried under vacuum. The residue was weighed and normalized to fecal wet weight.
Biochemical analysis of plasma samples
Plasma ALT levels were measured using a spectrophotometric method (λmax = 340 nm) from Catachem (Bridge-port, CT) according to the manufacture’s protocol. Plasma levels of TNF-α, IL-6, MCP-1, and adiponectin were determined using commercially available ELISAs for mice from R&D Systems (Minneapolis, MN) according to the manufacturer’s protocols.
Liver triglycerides
Liver triglycerides were determined by homogenizing liver tissue (50–100 mg) in 2 mL isopropanol. The homogenate was centrifuged at 2000g for 10 min and the supernatant was analyzed with an L-type triglycerides kit (Wako, Diagnostics, VA). Lipid concentrations were normalized to tissue wet weight.
Stromal vascular fraction (SVF) isolation
Epididymal WAT was excised, minced into small (<10 mg) pieces, and placed into digestion media consisting of Dulbecco’s modified Eagle’s medium (DMEM, Mediatech, Manassas, VA) supplemented with 2.5 % HEPES, 10 mg/ mL bovine serum albumin, and Collagenase Type II (0.3 %, Sigma-Aldrich, St. Louis, MO). Following incubation in a shaking 37 °C water bath for 45 min, samples were filtered through a 70-μm cell strainer to remove debris and centrifuged at 4 °C at 300g for 8 min. The pellet, consisting of the stromal vascular fraction (SVF), was washed with DMEM and centrifuged at 4 °C at 300g for 8 min. The supernatant was discarded and erythrocytes were lysed by incubation in 1 mL ACK lysis buffer (NH4Cl 150 mM, KHCO3 10 mM, EDTA·Na2·2H2O 10 μM) for 1 min on ice before the addition of 4 mL of DMEM to stop the reaction. Samples were then centrifuged at 4 °C at 300g for 10 min. The pellet was frozen at −80 °C for RNA isolation.
Real-time PCR
Total RNA was extracted and genomic DNA contamination was removed using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol. Total RNA was quantified with a Nanodrop 2000 spectrophotometer and reverse-transcribed to cDNA using a RT2 HT First Strand Kit (SA Biosciences, Valencia, CA). Real-time PCR was performed using an Applied Biosystems 7900HT Fast Real-Time PCR System (San Francisco, CA). The reactions included 5 μL perfeCTa® qPCR SuperMix, ROX™ (Quanta BioSciences, Gaithersburg, MD), 0.5 μL TaqMan® probe (Applied Biosystems, Table 3), and 4.5 μL diluted cDNA. PCRs were incubated in a 384-well plate at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. Data were recorded and analyzed with Sequence Detector Software (Applied Biosystems). Relative quantification or fold change in gene expression was determined using the 2−ΔΔCT method, where ΔΔCT = (CT,target − CT,reference)HF or HF-HFC − (CT,target − CT,reference)LF, with Gapdh as the reference gene.
Table 3.
Taqman® probes used in this study
| Symbol | Gene name | Accession ID | Manufacture’s no. | Length |
|---|---|---|---|---|
| Gapdh | Glyceraldehyde-3-phosphate dehydrogenase | NM_008084.2 | Mm99999915_g1 | 107 |
| Il6 | Interleukin 6 | NM_031168.1 | Mm00446190_m1 | 78 |
| Il12b | Interleukin 12b | NM_008352.2 | Mm00434174_m1 | 75 |
| Nos2 | Nitric oxide synthase 2, inducible | NM_010927.3 | Mm00440502_m1 | 66 |
| Tnfa | Tumor necrosis factor | NM_013693.2 | Mm00443260_g1 | 61 |
| Ccl2 | Chemokine (C–C motif) ligand 2 (MCP1) | NM_011333.3 | Mm00441242_m1 | 74 |
| Emr1 | Egf-like module containing, mucin-like, hormone receptor-like sequence 1 | NM_010130.4 | Mm00802529_m1 | 92 |
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). Two-way ANOVA with Bonferroni’s posttest was used for body weight, food intake, and blood glucose comparisons over the course of the study. One-way ANOVA with Dunnet’s posttest was used for all other data comparisons. A P <0.05 was considered statistically significant. All analyses were performed using GraphPad Prism 5.0 (San Diego, CA).
Results
Effect of cocoa on body weight, body fat mass, and organ weights
After 8 weeks of treatment, the average body weight of the HF-treated mice was 1.3-fold higher (P < 0.001) than that of the LF-treated mice (Fig. 1a). Cocoa supplementation for 10 weeks significantly decreased final body weight by 4.7 % (P < 0.05) compared to HF-fed controls, and significantly decreased the rate of body weight gain by 15.8 % (P < 0.001, Fig. 1b) without affecting the food and energy intake (Table 4). Cocoa-supplemented mice also displayed lower gross heart, liver, and retroperitoneal WAT weights compared to HF-fed mice (P < 0.05, Fig. 1c, d). The experiment was repeated twice, and the body weight, tissue weight, and food intake data were not significantly different between the two studies. We therefore combined the data from the two experiments.
Fig. 1.
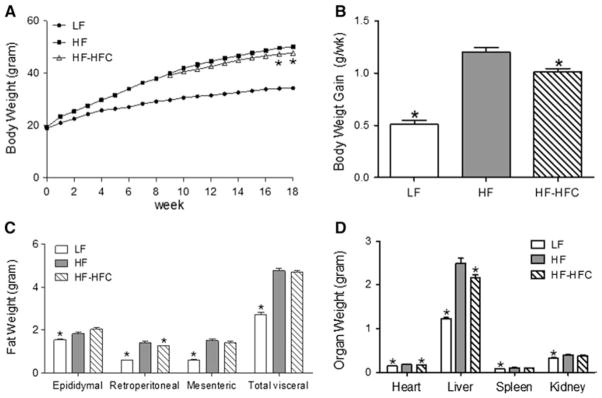
Effect of cocoa supplementation on body weight, body fat mass, and organ weight. a Body weights were determined over the course of 18 weeks. b The rate of body weight gain was calculated after cocoa supplementation (week 9–18). c Visceral fat mass and d gross organ weights were determined at the end of the experiment. Data were pooled from two identical studies (Experiment 1 and Experiment 2). LF, n = 35; HF, n = 44; and HF-HFC, n = 47. Values are expressed as mean ± SEM. Body weights were compared by two-way ANOVA with Bonferroni’s posttest (an asterisk indicates P < 0.05 compared to HF group). Mice body weights in LF group were significantly lower than HF mice since week 1. All other parameters were compared by one-way ANOVA with Dunnett’s posttest (an asterisk indicates P < 0.05 compared to HF-fed controls)
Table 4.
Effect of cocoa treatment on energy intake and excretion by high fat-fed obese mice
| LF | HF | HF-HFC | |
|---|---|---|---|
| Food intake (g/mouse/week) | 20 ± 0.2 | 20.5 ± 1.0 | 20.7 ± 1.0 |
| Energy intake (kcal/week) | 75.7 ± 0.8* | 106.3 ± 1.3 | 105.2 ± 1.3 |
| Fecal lipid content (% wet weight) | 8.4 ± 1.3 | 9.2 ± 0.7 | 14.3 ± 1.9* |
Values are expressed as mean ± SEM. Data on food and energy intake were pooled from both Experiment 1 and Experiment 2. Fecal samples were collected from each cage at week 15 in Experiment 1 in duplicate. Statistical significance was determined using the one-way ANOVA with Dunnet’s posttest
P < 0.05 compared to HF
Effect of cocoa on food intake and fecal lipid content
Fecal samples were taken after 7 weeks of treatment with the cocoa-supplemented diet. Cocoa treatment increased the fecal lipid content by 55.2 % (P < 0.05) compared to the HF obese mice without affecting food and energy intake (Table 4).
Effect of cocoa on glycemic markers and insulin resistance
No significant differences were found between mean fasting blood glucose (data not shown) and final fasting blood glucose (Table 5) in cocoa-supplemented mice and HF-fed controls. Fasting plasma insulin was determined at the completion of the experiment, and cocoa-supplemented mice had 26.7 % lower (P < 0.001) plasma insulin levels than HF-treated mice (Table 5). Moreover, HF-fed obese mice had increased HOMA-IR scores (P < 0.001) compared to LF-fed lean mice (Table 5). This increase was ameliorated in cocoa-supplemented mice (P < 0.001).
Table 5.
Effect of cocoa treatment on glycemic markers in high fat-fed obese mice
| LF | HF | HF-HFC | |
|---|---|---|---|
| Final fasting blood glucose (mmol/L) | 7.29 ± 0.18* | 9.96 ± 0.17 | 9.53 ± 0.25 |
| Fasting plasma insulin (pmol/L) | 165.47 ± 15.49* | 1176.40 ± 64.11 | 862.58 ± 61.42* |
| HOMA-IR | 7.74 ± 0.83* | 73.76 ± 4.32 | 50.32 ± 4.17* |
Values are expressed as mean ± SEM. Data on fasting blood glucose were pooled from both Experiment 1 and Experiment 2. Plasma insulin was from Experiment 2 and HOMA-IR was calculated from Experiment 2. Statistical significance was determined using the one-way ANOVA with Dunnet’s posttest
P < 0.05 compared to HF
Effect of cocoa on the markers of ORFLD
High-fat diet increased the plasma ALT levels by 2.2-fold (P < 0.001) compared to LF-fed controls. Cocoa supplementation attenuated this increase, resulting in 41.1 % lower (P < 0.001) plasma ALT levels than HF-fed mice (Fig. 2a). Liver triglyceride levels were also elevated in HF-fed mice (2.1-fold increase, P < 0.001) compared to LF-fed mice. Cocoa supplementation reduced the liver triglyceride levels by 32.3 % (P < 0.001) compared to HF-fed mice (Fig. 2b).
Fig. 2.
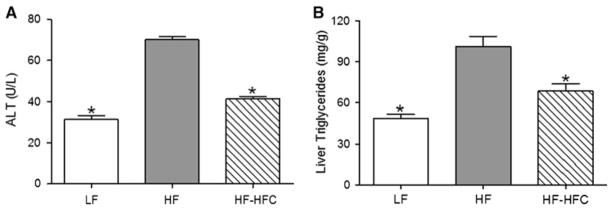
Effect of cocoa supplementation on the markers of ORFLD. a Plasma alanine aminotransferase (ALT) and b liver triglyceride levels were determined at the end of the experiment. Values are expressed as mean ± SEM. Plasma samples were taken from Experiment 1. LF, n = 12; HF, n = 23 and HF-HFC, n = 23. Lipid was extracted from liver samples from Experiment 2. LF, n = 23; HF, n = 21 and HF-HFC, n = 24. Means were compared to the HF-fed controls by one-way ANOVA with Dunnett’s posttest (an asterisk indicates P < 0.05)
Effect of cocoa on plasma cytokine levels
Plasma levels of MCP-1 and IL-6 were significantly elevated (P < 0.05) in HF-fed obese mice compared to the LF-fed lean mice. Supplementation with cocoa reduced plasma MCP-1 and IL-6 production by 25.3 % (P < 0.01) and 30.4 % (P < 0.01), respectively, compared to HF-fed mice (Fig. 3a, b). There was no significant difference in TNF-α level among the three groups (P = 0.27), although the cocoa-supplemented mice tended to have lower mean levels than the HF-fed controls (Fig. 3c). Conversely, plasma adiponectin levels were significantly lower (P < 0.05) in HF group compared to LF-fed controls, and cocoa treatment increased adiponectin levels by 33.7 % (P < 0.05) compared to HF-fed mice (Fig. 3d).
Fig. 3.
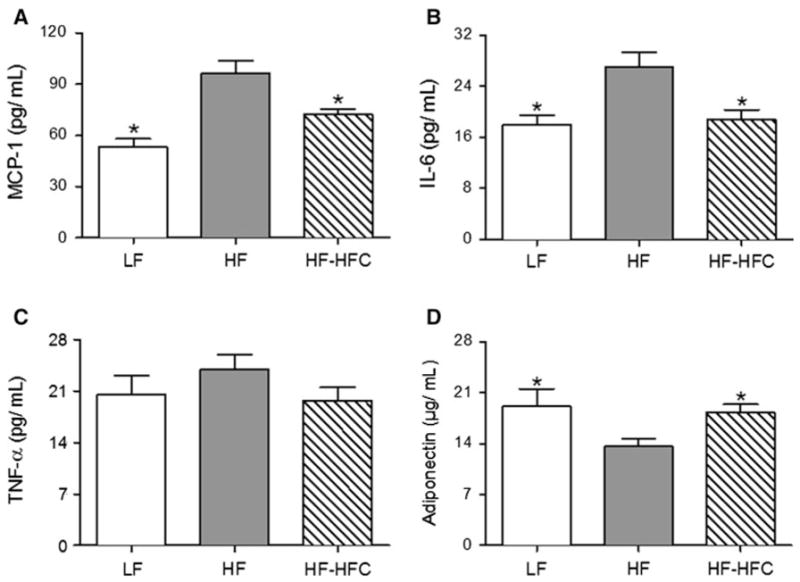
Effect of cocoa supplementation on systemic circulating levels of cytokines, chemokines, and adipokines. Plasma levels of a MCP-1, b IL-6, c TNF-α, and d adiponectin. Plasma samples were taken from Experiment 1. LF, n = 12; HF, n = 23; and HF-HFC, n = 23. Values are expressed as mean ± SEM. Means were compared to the HF-fed controls by one-way ANOVA with Dunnett’s posttest (an asterisk indicates P < 0.05)
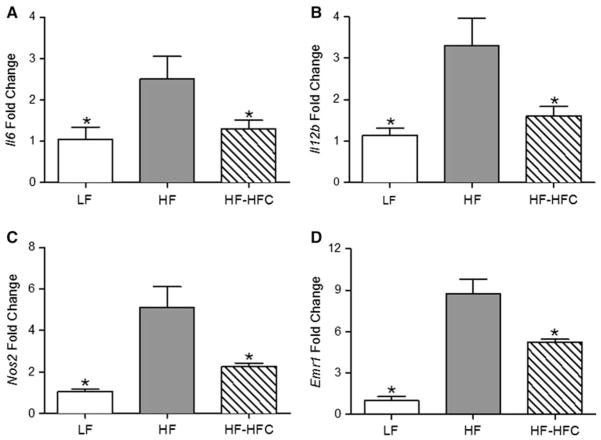
Effect of cocoa on pro-inflammatory gene expression
The SVF of WAT is enriched in macrophages, which are related to inflammatory processes in obesity. The mRNA levels of Il6, Il12b, Nos2, and Emr1 in the SVF of epididymal WAT of the HF-fed mice were increased by 2.4-(P < 0.05), 2.9- (P < 0.05), 4.8- (P < 0.01), and 8.5-fold (P < 0.001), respectively, compared to LF-fed lean mice. Cocoa supplementation reduced the expression of these genes by 37–56 % (P < 0.05, Fig. 4). There was no significant difference in the expression of Tnfa and Ccl2 (Mcp1) among the three groups (Suppl. Fig. 1).
Fig. 4.
Effect of cocoa supplementation on the expression of pro-inflammatory genes in SVF of WAT. Expression of a Il6, b Il12b, c Nos2, and d Emr1 was determined at the end of the experiment using RNA isolated from the epididymal SVF from a set of representative mice from Experiment 2. LF, n = 10; HF, n = 18; and HF-HFC, n = 18. Values are expressed as mean ± SEM. Means were compared to the HF-fed controls by one-way ANOVA with Dunnett’s posttest (an asterisk indicates P < 0.05)
Discussion
Obesity is accompanied by systemic inflammatory responses, and factors believed to contribute to this process include increased levels of circulating cytokines, as well as tissue-specific derangements, such as hepatic inflammation and the accumulation of inflammatory macrophages in adipose tissue [8, 29, 30]. In this study, we report for the first time the therapeutic effect of dietary supplementation with cocoa on obesity-related inflammation, insulin resistance, and fatty liver disease in HF-fed C57BL/6J mice.
In the present study, dietary supplementation of HF-fed, obese C57BL/6J mice with 8 % cocoa powder for 10 weeks significantly reduced the rate of body weight gain (16 % decrease), as well as final body weight (5 % decrease) and retroperitoneal WAT weight (11 % decrease) compared to HF-fed controls. This dose of cocoa showed no signs of toxicity and did not affect food intake. Dietary supplementation with 8 % cocoa powder supplementation in mice is equivalent to an approximate daily dose of 54 g of cocoa powder consumption in humans based on a 2,000 kcal daily energy intake. This amount of cocoa powder is enough to make four cups of hot cocoa according to typical preparation methods (15 g in 250 mL). Our results are similar to those reported previously in HF-fed rats supplemented with 12.5 % cocoa for 3 weeks [17]. We found that these effects on body weight and body fat were related to a 55 % increase in fecal lipid output by cocoa-supplemented mice. This result suggests that the effect of cocoa powder on the rate of body weight gain is related to the inhibition of lipid absorption. A recent study from our laboratory indicates that cocoa extracts and their component polyphenols can inhibit the activity of pancreatic lipase and secreted phospholipase A2 in vitro suggesting a potential mechanism for the inhibition of dietary fat absorption [26].
Increasing evidence suggests that chronic inflammation is a key mediator of obesity-related pathologies including insulin resistances and fatty liver disease. There is growing evidence that obesity-related inflammation results from increased macrophage infiltration in WAT, as well as increased expression, production, and release of a number of pro-inflammatory cytokines [31]. Adipose tissue macrophages (ATMs) are highly inflammatory, which have been identified as the primary source of many of the circulating cytokines that are detected in the obese state, such as tumor necrosis factors (e.g., TNF-α), interleukins (e.g., IL-1, IL-6, IL-12), and contribute to the recruitment of additional macrophages by secreting chemokines including MCP-1.
Here, we found that plasma pro-inflammatory cytokine levels are dramatically elevated in obese HF mice compared to the lean LF mice, and that these increases were ablated by cocoa supplementation. Furthermore, the expression of pro-inflammatory genes (Il6, Il12b, and Nos2) was elevated in HF-fed obese mice in SVF of WAT, where macrophages reside. Cocoa supplementation suppressed their expression levels by nearly 50 %. However, there was no significant difference in the levels of Tnfa and Ccl2 among three groups. Interestingly, although we observed a dramatic decrease in the expression of several pro-inflammatory genes in the epididymal fat depot of cocoa-treated obese mice, there was no decrease in the mass of that depot. These results suggest that the effects on gene expression are not secondary to decreased adipose tissue mass, but may be due to some more direct mechanism. We also found that Emr1 (macrophage F4/80-specific gene) was increased in the HF group but decreased in the HF–HFC group, which suggests a possible anti-inflammatory mechanism of cocoa through the inhibition of macrophage infiltration. Although ours is the first study to examine the effect of cocoa on obesity-induced increases in plasma inflammatory cytokines, Kanamoto et al. [7] have reported that the administration of a PAC-rich black soybean seed coat extract for 14 week remarkably decreased plasma leptin level, as well as Tnfa, Ccl2, and Il6 expression in mesenteric WAT in HF diet-fed mice. Similar results were reported for a study of HF-fed rats supplemented with a PAC-rich grape seed preparation [6].
In the present study, we found that cocoa supplementation significantly decreased the fasting plasma insulin level in obese mice and improved HOMA-IR score, but did not affect fasting blood glucose levels, compared to HF-fed obese mice. Previous studies have shown that cocoa and cocoa products can exert hypoglycemic properties and improve insulin resistance. Yamashita et al. [20] have showed that a PAC-rich cocoa liquor extract suppressed HF diet-induced hyperglycemia through the activation of AMP-activated protein kinase α, and translocation of glucose transporter 4 in HF diet-fed obese mice. Grassi et al. [32] reported that the short-term administration of dark chocolate improved insulin resistance in terms of improved HOMA-IR and quantitative insulin sensitivity check index (QUICKI) in healthy subjects. Increasing evidence from human population studies and animal research has established correlative as well as causative links between obesity-induced chronic inflammation and insulin resistance [29, 33]. Circulating levels of pro-inflammatory cytokines are correlated with insulin resistance through indirect inhibition of insulin signal transduction [7, 10, 33, 34] and possibly via the activation of Jun N-terminal kinase and inhibitor of κB kinase β [35]. Based on this, we speculate that the effects of cocoa supplementation on insulin resistance may, in part, be secondary to the observed anti-inflammatory effects in terms of suppression of cytokine production and pro-inflammatory gene expression.
Finally, in addition to the effects on insulin resistance, cocoa supplementation ameliorated the symptoms of ORFLD compared to HF-fed obese mice. One of the consequences of insulin resistance is elevated adipose lipolysis, which results in enhanced free fatty acid flux to the liver that leads to excess esterification to triglycerides and hepatic lipid accumulation [11]. Hepatic steatosis is the hallmark of ORFLD, characterized by elevated concentrations of markers of liver injury, including ALT, aspartate aminotransferase (AST), and γ-glutamyl-transferase (GGT) [36]. Of these liver enzymes, ALT is most closely related to liver fat accumulation and is often used in epidemiological studies as a surrogate marker for ORFLD [37]. In the present study, we observed a decrease in plasma ALT levels, hepatic triglyceride levels, and gross liver weight in cocoa-treated mice compared to HF group. Recent studies also demonstrated a key hepatoprotective role for adiponectin, an adipokine with known anti-inflammatory activities. Buecher et al. [38] reported that adiponectin antagonizes excess lipid storage in the liver and protects from inflammation and fibrosis. Interestingly, cocoa supplementation significantly increased systemic adiponectin levels in mice compared to HF-fed controls, which may contribute to the decreased triglyceride accumulation in liver. As mentioned earlier, we observed that cocoa supplementation can increase fecal lipid content, perhaps by modifying lipid digestion. These two activities may work together to produce the observed liver protective effect of cocoa.
In summary, we have observed that dietary supplementation with cocoa ameliorates obesity-related inflammation, insulin resistance, and fatty liver disease in HF-fed obese mice, principally mediated by down-regulation of pro-inflammatory gene expression in WAT. These effects appear to be due in part to the modulation of dietary fat absorption and the inhibition of macrophage infiltration in adipose tissue. Our results provide support for future human intervention studies on the anti-inflammatory effects of cocoa at nutritionally relevant doses of cocoa powder. Future studies are needed to identify the active chemical components in cocoa and to more clearly delineate the mechanistic relationship between modulation of dietary fat absorption and the observed anti-inflammatory effects.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Dr. Sarah Forester, Ms. Sudathip Sae-tan, Ms. Tongtong Xu, Ms. Ling Tao, Mr. Zachary Bitzer, and Ms. Amy Brownschidle for technical assistance.
Abbreviations
- ALT
Alanine aminotransferase
- ATM
Adipose tissue-associated macrophage
- DP
Degree of polymerization
- HF
High fat
- HFC
High-fat diet supplemented with 8 % cocoa powder
- HOMA-IR
Homeostasis model assessment of insulin resistance
- IL
Interleukin
- iNOS
Inducible nitric oxide synthase
- LF
Low fat
- MCP-1
Monocyte chemoattractant protein-1
- NO
Nitric oxide
- ORFLD
Obesity-related fatty liver disease
- PAC
Proanthocyanidin
- SVF
Stromal vascular fraction
- TNF-α
Tumor necrosis factor-α
- WAT
White adipose tissue
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00394-013-0510-1) contains supplementary material, which is available to authorized users.
Conflict of interest None.
Contributor Information
Yeyi Gu, Center of Excellence for Plant and Mushroom Foods and Health, Department of Food Science, The Pennsylvania State University, 332 Food Science Building, University Park, PA 16802, USA.
Shan Yu, Department of Veterinary and Biomedical Sciences, Intercollege Graduate Program in Physiology, The Pennsylvania State University, University Park, PA 16802, USA.
Joshua D. Lambert, Email: jdl134@psu.edu, Center of Excellence for Plant and Mushroom Foods and Health, Department of Food Science, The Pennsylvania State University, 332 Food Science Building, University Park, PA 16802, USA
References
- 1.González-Castejón M, Rodriguez-Casado A. Dietary phytochemicals and their potential effects on obesity: a review. Pharmacol Res. 2011;64:438–455. doi: 10.1016/j.phrs.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Finkelstein EA, Khavjou OA, Thompson H, Trogdon JG, Pan L, Sherry B, Dietz W. Obesity and severe obesity forecasts through 2030. Am J Prev Med. 2012;42:563–570. doi: 10.1016/j.amepre.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Terra X, Pallarés V, Ardèvol A, Bladé C, Fernández-Larrea J, Pujadas G, Salvadó J, Arola L, Blay M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J Nutr Biochem. 2011;22:380–387. doi: 10.1016/j.jnutbio.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Emanuela F, Grazia M, Marco DR, Maria Paola L, Giorgio F, Marco B. Inflammation as a link between obesity and metabolic syndrome. J Nutr Metab. 2012;2012:476380. doi: 10.1155/2012/476380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terra X, Montagut G, Bustos M, Llopiz N, Ardèvol A, Bladé C, Fernández-Larrea J, Pujadas G, Salvadó J, Arola L, Blay M. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J Nutr Biochem. 2009;20:210–218. doi: 10.1016/j.jnutbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Kanamoto Y, Yamashita Y, Nanba F, Yoshida T, Tsuda T, Fukuda I, Nakamura-Tsuruta S, Ashida H. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J Agric Food Chem. 2011;59:8985–8993. doi: 10.1021/jf201471p. [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, Mccann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11:886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 10.Eder K, Baffy N, Falus A. The major inflammatory mediator interleukin-6 and obesity. Inflamm Res. 2009;58:727–736. doi: 10.1007/s00011-009-0060-4. [DOI] [PubMed] [Google Scholar]
- 11.Clément S, Juge-Aubry C, Sgroi A, Conzelmann S, Pazienza V, Pittet-Cuenod B, Meier CA, Negro F. Monocyte chemo-attractant protein-1 secreted by adipose tissue induces direct lipid accumulation in hepatocytes. Hepatol. 2008;48:799–807. doi: 10.1002/hep.22404. [DOI] [PubMed] [Google Scholar]
- 12.Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 13.Selmi C, Mao TK, Keen CL, Schmitz HH, Gershwin ME. The anti-inflammatory properties of cocoa flavanols. Inflammation. 2006;47:163–171. doi: 10.1097/00005344-200606001-00010. [DOI] [PubMed] [Google Scholar]
- 14.Cooper KA, Donovan JL, Waterhouse AL, Williamson G. Cocoa and health: a decade of research. Br J Nutr. 2008;99:1–11. doi: 10.1017/S0007114507795296. [DOI] [PubMed] [Google Scholar]
- 15.Monagas M, Khan N, Andres-Lacueva C, Casas R, Urpí-Sardà M, Llorach R, Lamuela-Raventós RM, Estruch R. Effect of cocoa powder on the modulation of inflammatory biomarkers in patients at high risk of cardiovascular disease. Am J Clin Nutr. 2009;90:1144–1150. doi: 10.3945/ajcn.2009.27716. [DOI] [PubMed] [Google Scholar]
- 16.Ruzaidi A, Amin I, Nawalyah AG, Hamid M, Faizul HA. The effect of Malaysian cocoa extract on glucose levels and lipid profiles in diabetic rats. J Ethnopharmacol. 2005;98:55–60. doi: 10.1016/j.jep.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Matsui N, Ito R, Nishimura E, Yoshikawa M, Kato M, Kamei M, Shibata H, Matsumoto I, Abe K, Hashizume S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition. 2005;21:594–601. doi: 10.1016/j.nut.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Tomaru M, Takano H, Osakabe N, Yasuda A, Inoue K, Yanagisawa R, Ohwatari T, Uematsu H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition. 2007;23:351–355. doi: 10.1016/j.nut.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Jalil AMM, Ismail A, Pei CP, Hamid M, Kamaruddin SHS. Effects of cocoa extract on glucometabolism, oxidative stress, and antioxidant enzymes in obese-diabetic (Ob-db) rats. J Agric Food Chem. 2008;56:7877–7884. doi: 10.1021/jf8015915. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita Y, Okabe M, Natsume M, Ashida H. Prevention mechanisms of glucose intolerance and obesity by cacao liquor procyanidin extract in high-fat diet-fed C57BL/6 mice. Arch Biochem Biophys. 2012;2012:1–10. doi: 10.1016/j.abb.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 21.Mao T, Water JVD, Keen CL, Schmitz HH, Gershwin ME. Cocoa Procyanidins and Human Cytokine Transcription and Secretion. J Nutr. 2000;130:2093–2099. doi: 10.1093/jn/130.8.2093S. [DOI] [PubMed] [Google Scholar]
- 22.Mao TK, Powell J, Water JVD, Keen CL, Schmitz HH, John F, Gershwin ME. The Effect of cocoa procyanidins on the transcription and secretion of interleukin 1beta in peripheral blood mononuclear cells. Life Sci. 2000;66:1377–1386. doi: 10.1016/s0024-3205(00)00449-5. [DOI] [PubMed] [Google Scholar]
- 23.Mao TK, van de Water J, Keen CL, Schmitz HH, Gershwin ME. Modulation of TNF-α secretion in peripheral blood mononuclear cells by cocoa flavanols and procyanidins. Dev Immunol. 2002;9:135–141. doi: 10.1080/1044667031000137601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramiro E, Franch A, Castellote C, Pérez-Cano F, Permanyer J, Izquierdo-Pulido M, Castell M. Flavonoids from theobroma cacao down-regulate inflammatory mediators. J Agric Food Chem. 2005;53:8506–8511. doi: 10.1021/jf0511042. [DOI] [PubMed] [Google Scholar]
- 25.Kenny TP, Keen CL, Schmitz HH, Gershwin ME, Enny THPK, Een CARLLK, Chmitz HAHS. Immune effects of procyanidin oligomers on peripheral blood mononuclear cells. Exp Biol Med. 2007;232:293–300. [PubMed] [Google Scholar]
- 26.Gu Y, Hurst WJ, Stuart DA, Lambert JD. Inhibition of key digestive enzymes by cocoa extracts and procyanidins. J Agric Food Chem. 2011;59:5305–5311. doi: 10.1021/jf200180n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelm MA, Johnson JC, Robbins RJ, Hammerstone JF, Schmitz HH. High-performance liquid chromatography separation and purification of cacao (Theobroma cacao L.) procyanidins according to degree of polymerization using a diol stationary phase. J Agric Food Chem. 2006;54:1571–1576. doi: 10.1021/jf0525941. [DOI] [PubMed] [Google Scholar]
- 28.Mlinar B, Marc J, Janez A, Peifer M. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta. 2007;375:20–35. doi: 10.1016/j.cca.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 31.Trayhurn P, Wood IS. Signalling role of adipose tissue: adipokines and inflammation in obesity. Biochem Soc Trans. 2005;33:1078–1081. doi: 10.1042/BST0331078. [DOI] [PubMed] [Google Scholar]
- 32.Grassi D, Lippi C, Necozione S, Desideri G, Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 33.de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 36.Clark J, Brancati F, Diehl A. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 37.Schindhelm RK, Diamant M, Dekker JM, Teerlink T, Heine RJ. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22:437–443. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- 38.Buechler C, Wanninger J, Neumeier M. Adiponectin, a key adipokine in obesity related liver diseases. World J Gastroenterol. 2011;17:2801–28011. doi: 10.3748/wjg.v17.i23.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.