Abstract
While high resolution peripheral quantitative computed tomography (HRpQCT) and central quantitative computed tomography (QCT) studies have shown bone structural differences between Chinese-American (CH) and white (WH) women, these techniques are not readily available in the clinical setting. The trabecular bone score (TBS) estimates trabecular microarchitecture from DXA spine images. We assessed TBS in CH and WH women and investigated whether TBS is associated with QCT and HRpQCT indices. Areal BMD (aBMD) by DXA, lumbar spine (LS) TBS, QCT of the LS and hip, and HRpQCT of the radius and tibia were performed in 71 pre- (37 WH and 34 CH) and 44 postmenopausal (21 WH and 23 CH) women. TBS did not differ by race in either pre- or postmenopausal women. In the entire cohort, TBS positively correlated with LS trabecular volumetric BMD (vBMD) (r=0.664); femoral neck (FN) integral (r=0.651), trabecular (r=0.641) and cortical vBMD (r=0.346) and cortical thickness (C/I; r=0.540) by QCT (p<0.001 for all). TBS also correlated with integral (r=0.643), trabecular (r=0.574) and cortical vBMD (r=0.491) and C/I (r=0.541) at the total hip (TH) (p<0.001 for all). The combination of TBS and LS aBMD predicted more of the variance in QCT measures than aBMD alone. TBS was associated with all HRpQCT indices (r=0.20–0.52) except radial cortical thickness and tibial trabecular thickness. Significant associations between TBS and measures of HRpQCT and QCT in WH and CH pre- and postmenopausal women demonstrated here suggest that TBS may be a useful adjunct to aBMD for assessing bone quality.
Keywords: Trabecular Bone Score, QCT, HRpQCT, microarchitecture, Chinese, white
Introduction
Measurement of areal bone mineral density (aBMD) by dual X-ray absorptiometry (DXA) is the gold standard for fracture risk assessment. Despite its widespread use and utility, DXA has several limitations. It provides a two-dimensional (areal) depiction of BMD rather than a three-dimensional or volumetric bone mineral density (vBMD). aBMD is affected by bone size - it is underestimated in those with smaller bones and overestimated in those with larger bones (1). Importantly, DXA does not distinguish between cortical and trabecular bone, nor can it discern bone microarchitecture.
Newer methods such as central quantitative computed tomography (QCT) and high resolution peripheral quantitative computed tomography (HRpQCT) can non-invasively assess vBMD, which is not affected by bone size. Further, these technologies provide separate measures for the cortical and trabecular compartments, and can characterize bone strength (2–4). HRpQCT has the advantage of non-invasively evaluating bone microarchitecture and has been shown to distinguish those with and without fracture in cross-sectional studies (4). QCT assesses central skeletal sites where the most important clinical fractures occur and can predict new vertebral fractures (3).
These techniques have provided insight into racial differences in bone quality and fracture risk. Asian-Americans exhibit lower aBMD by DXA (5), but have lower rates of hip and forearm fractures than white women (6, 7). HRpQCT studies indicate that Chinese-Americans have smaller bone size but denser and thicker cortices and more plate-like trabeculae at the radius and tibia than white women (8–11). QCT has also demonstrated cortical advantages at the hip among Chinese-American women but similar trabecular vBMD at both central skeletal sites between races (12). Further, using micro finite element analysis (μFEA), we demonstrated that these characteristics in Chinese-Americans are associated with increased bone strength, despite smaller bone size (10, 13) compared to white women. These findings help explain lower rates of non-vertebral fractures in Chinese Americans, despite lower aBMD.
While QCT and HRpQCT have improved the assessment of bone quality, they are not yet widely available. The Trabecular Bone Score (TBS), a novel gray-scale textural analysis of spine DXA images, provides a quantitative estimate of trabecular microarchitecture using variograms of 2D projection images (14). TBS is independent of bone size and highly correlated with trabecular number, separation and connectivity density by micro-computed tomography (μCT), the gold standard for microarchitectural assessment (14). TBS utilizes the same region of interest (ROI) as DXA and can be performed on previously acquired images from GE Lunar and Hologic densitometers. In prospective studies, TBS predicts fracture risk in postmenopausal women (15, 16). Now that the United States Food and Drug Administration (FDA) has approved TBS (17), it might be applied more widely as an adjunct to aBMD testing.
In this study, we sought to assess racial differences in TBS and to determine whether TBS reflects vBMD and microarchitecture at central and peripheral skeletal sites as measured by QCT and HRpQCT in our cohort of Chinese-American and white women.
Materials and Methods
Subjects
As previously described, pre- and postmenopausal Chinese-American and white women were evaluated cross-sectionally using DXA, QCT and HRpQCT (8–10, 12, 13) at Columbia University Medical Center (CUMC). Participants enrolled in our prior studies were included in this analysis if measurements from all 4 modalities (TBS, DXA, QCT and HRpQCT) were available. Forty-four postmenopausal (21 White and 23 Chinese American) and 71 premenopausal women (37 White and 34 Chinese American) were studied. The terms Chinese-American and white refer to women of full Chinese or Caucasian descent, respectively (defined by both parents and both sets of grandparents). Premenopausal women ages 29–40, with a history of regular menses with (> 6 cycles per year) were eligible. Postmenopausal (amenorrhea for >1 year) women, ages 59–70, were included. Exclusion criteria included conditions/medications that affect bone metabolism, detailed elsewhere (12). All participants gave written, informed consent. The study was approved by the CUMC Institutional Review Board.
Clinical and Biochemical Evaluation
Past medical history, medications, lifestyle factors, and dietary information by questionnaires (18) were assessed as previously described (8, 9, 12). Intact PTH and 25-hydroxyvitamin D were measured by chemiluminescence assay and liquid chromatography tandem mass spectrometry, respectively.
Areal Bone Mineral Densitometry
aBMD was measured at the LS (L1–L4); total hip (TH); femoral neck (FN); and one-third radius (1/3 Radius) using a QDR 4500A (Hologic, Waltham, MA, USA). In vivo precision at this facility is 1.28%, 1.36% and 0.70% at the LS, hip, and one-third radius respectively (19). The manufacturer’s Caucasian reference curves were used to calculate T-scores.
Trabecular Bone Score
Site-matched spine TBS parameters were extracted from DXA images using TBS iNsight software (v1.9, medimaps group, Geneva, Switzerland). TBS measurements were performed at the University of Lausanne (Switzerland) using de-identified spine DXA files from scans obtained at CUMC. TBS was evaluated by determining the variogram of the trabecular bone projected image, calculated as the sum of the squared gray-level differences between pixels at a specific distance and angle. As previously described, TBS was then calculated as the slope of the log-log transform of this variogram (14). The slope characterizes the rate of gray-level amplitude variations in the trabecular bone. Therefore, a steep variogram slope with a high TBS value is associated with better bone microarchitecture (more numerous, more connected and less sparse trabeculae). A low TBS score indicates worse bone microstructure (low trabecular number and connectivity, and high trabecular separation) (14). The mean value of the individual measurements for L1–L4 represents the LS TBS (unitless). The precision of TBS ranges from 1.12 to 1.9%, as previously reported (20, 21).
Central QCT
Helical CT images (GE LightSpeed 64 VCT Scanner; GE Medical Systems, Milwaukee, WI, USA) were acquired at L1–L2 and the hips as previously described (22, 23) at CUMC. Images were processed to extract vBMD and bone size measures using analysis techniques described previously (22, 23). The processing task included calibration of the CT images from the native scanner Hounsfield Units to equivalent concentration (g/cm3) of calcium hydroxyapatite. The following BMD and geometric measures were determined according to published methods (22, 23): L1–L2 vertebral cross-sectional area (CSA); average mid-vertebra (central 10 mm of the vertebra between the two endplates); integral, trabecular and cortical vBMD at the FN and TH; minimum FN CSA; and the ratio of tissue volume in the cortical region of the TH and FN to total tissue volume within the periosteal boundaries (C/I, the proportion of total bone volume that is cortical bone). The techniques used to define the FN ROI and the cortical component are described elsewhere (24). Precision values for QCT vBMD are 1.76–2.93%, 1.56–5.85%, and 0.72–1.36% at the LS, FN, and TH respectively, and 1.44–2.41% for CSA.
HRpQCT
vBMD and microarchitecture were measured at the nondominant distal radius and tibia using a HRpQCT instrument (XtremeCT; Scanco Medical, Brüttisellen, Switzerland) as described (2). The following variables were assessed at both sites: total area, total, cortical (Ct.), and trabecular (Tb.) vBMD; cortical thickness (Ct.Th) and trabecular thickness (Tb.Th); bone volume to tissue volume (BV/TV); trabecular number (Tb.N), trabecular spacing (Tb.Sp), and trabecular distribution (Tb.Sp.SD). In vivo precision values at our center are 0.7–1.0% and 1.0–7.3% for density and trabecular architecture respectively.
Statistics
Descriptive statistics are expressed as mean ± SD. Continuous variables were compared using Student’s t-test. Criterion values were adjusted for unequal variances where appropriate. The correlation of TBS with QCT parameters and DXA measurements was assessed by the Pearson correlation test, while the correlation of HRpQCT with TBS was assessed by the Spearman correlation test. Partial correlation was used to adjust for body weight and BMI. Linear regression was used to estimate the variability in QCT and HRpQCT parameters when TBS, LS aBMD or both were entered as the explanatory variables. For the QCT models, residuals of univariate analyses of LS aBMD, and the combination of TBS with LS aBMD were extracted and compared for better model fitting. Differences in the model residuals were assessed by a paired t-test. Agreement between TBS and the QCT measure of trabecular vBMD in mid vertebra was assessed using the Bland-Altman plot, considering the QCT measure as the gold standard (25). Multiple regression was performed to further assess the relationship between TBS and clinical characteristics, LS aBMD, QCT and HRpQCT indices. Potential predictors were entered into the model in a stepwise fashion with TBS as the dependent variable. The stepwise selection process criterion for entry to the model was a univariate P<0.3. The criterion for retention in the model was a multivariate P<0.05. For all analyses, a two-tailed p<0.05 was considered to indicate statistical significance. Statistical analysis was performed using SAS, version 9.2 (SAS Institute, Inc., Cary, NC, USA), and the Bland-Altman plot was generated using MedCalc version 11.4.0 (MedCalc, Belgium).
Results
As shown in Table 1, the racial groups did not differ in age or years since menopause. Compared to white women, Chinese-Americans weighed less, were shorter, and tended to have lower calcium intake and serum 25-hydroxyvitamin D levels. Premenopausal Chinese-Americans had higher PTH levels than white women. aBMD at the LS and one-third radius did not differ by race in premenopausal women, but it was lower at the TH and FN in Chinese-Americans. White and Chinese-American postmenopausal participants had comparable aBMD at all sites. There were no racial differences in TBS values, even after adjusting for height, weight, calcium intake, PTH, and 25-hydroxy vitamin D. TBS was higher in premenopausal than postmenopausal women in both races (Table 1).
Table 1.
Anthropometric, biochemical and densitometric characteristics of the cohort
| Characteristics | Entire Group | Postmenopausal | Postmenopausal | ||
|---|---|---|---|---|---|
| (n=115) | White (n=37) |
Chinese- American (n=34) |
White (n=21) |
Chinese- American (n=23) |
|
| Age (years) | 45.5 ± 13.6 | 34.8 ± 3.3 | 35.6 ± 3.4 | 62.8 ± 2.9# | 61.8 ± 2.3# |
| Weight (kg) | 62 ± 14 | 65 ± 19 | 56 ± 6* | 69 ± 14 | 59 ± 8* |
| Height (cm) | 162 ± 6 | 166 ± 7 | 162 ± 5* | 163 ± 5 | 157 ± 4*# |
| BMI (kg/m2) | 23.6 ± 4.8 | 23.7 ± 6.2 | 21.6 ± 2.4 | 26 ± 5.7 | 24 ± 2.7 |
| Years since menopause | 11.2 ± 4.6† | NA | NA | 12.0 ± 4.5 | 10.4 ± 4.6 |
| Calcium intake (mg/day) | 1168 ± 1143 | 1427 ± 1783 | 831 ± 512 | 1530 ± 631 | 907 ± 539* |
| Vitamin D intake (IU/day) | 635 ± 608 | 769 ± 739 | 380 ± 270* | 995 ± 731 | 461 ± 359* |
| Serum 25-hydroxy vitamin D (ng/mL) | 32.2 ± 13.5 | 36.5 ± 14.0 | 24.0 ± 9.6* | 39 ± 15 | 31 ± 10*# |
| Serum PTH (pg/mL) | 35.6 ± 12.3 | 30.2 ± 12.3 | 38.1 ± 12.8* | 41.4 ± 10.9 | 35.3 ± 10.0 |
| Lumbar spine TBS (unitless) | 1.413 ± 0.108 | 1.465 ± 0.091 | 1.467 ± 0.064 | 1.336 ± 0.097# | 1.318 ± 0.089# |
| L1–L4 aBMD (g/cm2) | 0.999 ± 0.132 | 1.030 ± 0.119 | 1.029 ± 0.137 | 0.964 ± 0.134 | 0.937 ± 0.119# |
| Lumbar spine T-score | −0.4 ± 1.2 | −0.1 ± 1.1 | −0.2 ± 1.2 | −0.8 ± 1.2 | −1.0 ± 1.1 |
| Total hip aBMD (g/cm2) | 0.896 ± 0.114 | 0.947 ± 0.129 | 0.895 ± 0.086* | 0.862 ± 0.123# | 0.847 ± 0.088# |
| Total Hip T-score | −0.4 ± 0.9 | 0.0 ± 1.1 | −0.4 ± 0.7* | −0.7 ± 1.0# | −0.8 ± 0.7# |
| Femoral neck aBMD (g/cm2) | 0.767 ± 0.111 | 0.824 ± 0.116 | 0.77 ± 0.081* | 0.702 ± 0.103# | 0.702 ± 0.077# |
| Femoral Neck T-score | −0.7 ± 1.0 | −0.1 ± 1.0 | −0.6 ± 0.7* | −1.3 ± 0.9# | −1.3 ± 0.7# |
| Distal 1/3 radius aBMD (g/cm2) | 0.673 ± 0.057 | 0.699 ± 0.050 | 0.694 ± 0.046 | 0.642 ± 0.049# | 0.627 ± 0.052# |
| Distal 1/3 radius T-score | −0.3 ± 1.0 | 0.1 ± 0.8 | 0.0 ± 0.8 | −0.9 ± 0.8# | −1.1 ± 0.9# |
PTH: parathyroid hormone; TBS: trabecular bone score; aBMD: areal bone mineral density
p value <0.05 (White vs. Chinese-American within menopausal status)
p value <0.05 (Pre- vs. Postmenopausal women within race)
Postmenopausal women only
The Relationship between TBS and QCT indices
As shown in Table 2, TBS was positively correlated with QCT vBMD measures at the LS, FN and TH. In contrast, bone size measures, including vertebral body cross-sectional area (CSA) and minimum FN cross-sectional area (Minimal CSA), were not associated with TBS. C/I, an estimate of cortical thickness, at the TH and FN was also positively correlated with TBS. The strongest association between TBS and QCT variables was at the LS. Adjustment for weight or BMI did not change the direction or significance of the correlations except for the weight-adjusted association between TBS and vertebral body CSA (r=0.237, p=0.02).
Table 2.
Correlations (r values) of TBS and Lumbar Spine aBMD with QCT variables (n=115)
| QCT parameters | LS aBMD | TBS | P-value Comparison of correlations |
|---|---|---|---|
| Lumbar Spine (L1, L2): | |||
| Vertebral Body CSA | 0.295** | 0.128 | 0.11 |
| Average trabecular vBMD mid vertebra | 0.689*** | 0.664*** | 0.94 |
| Femoral Neck: | |||
| Minimal CSA | 0.356*** | 0.010 | 0.23 |
| Integral vBMD | 0.508*** | 0.651*** | 0.01 |
| Trabecular vBMD | 0.633*** | 0.641*** | 0.88 |
| Cortical vBMD | 0.081 | 0.346*** | 0.33 |
| C/I | 0.459*** | 0.540*** | 0.15 |
| Total Hip: | |||
| Integral vBMD | 0.593*** | 0.643*** | 0.23 |
| Trabecular vBMD | 0.571*** | 0.574*** | 0.96 |
| Cortical vBMD | 0.359*** | 0.491*** | 0.3 |
| C/I | 0.355*** | 0.541*** | 0.07 |
LS aBMD: lumbar spine areal bone mineral density; TBS: trabecular bone score;
CSA: cross-sectional area; vBMD: volumetric bone mineral density;
C/I: an estimate of cortical thickness
p<.05;
p<.01;
p<.001
LS aBMD was positively correlated with all QCT vBMD measures at the LS, FN and TH with the exception of FN cortical vBMD (Table 2). The strongest association was with trabecular vBMD at the LS. The strength of the association (r) between FN integral vBMD by QCT and TBS was greater than that between FN integral vBMD by QCT and LS aBMD (p=0.01).
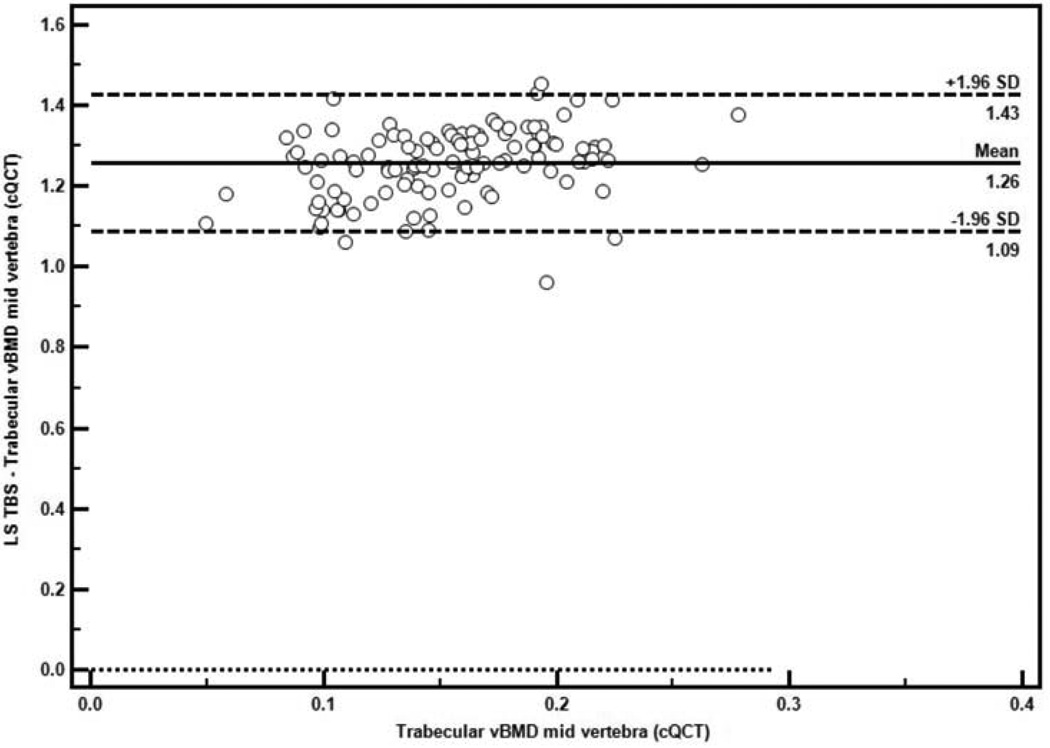
Using the QCT measure as the gold standard, the Bland-Altman plot indicated good agreement between TBS and trabecular vBMD at the mid vertebra by QCT, with uniform scatter around the mean difference (limits of agreement 1.09 to 1.43) and no evidence of systematic error (Figure 1).
Figure 1.
Bland-Altman Plot of the agreement between TBS and the QCT measure of trabecular vBMD at the mid vertebra (n=115). The solid horizontal line represents the mean difference, and the dashed lines represent the limits of agreement (mean difference ±SD).
As shown in Table 3, TBS accounted for 44% of the variance in trabecular vBMD at the LS, 12–42% of the variance in QCT vBMD measurements at the FN, 24–41% of the variance in TH vBMD and 29% of the variance in FN and TH cortical thickness. TBS was not a predictor in the models for QCT bone size variables (Vertebral Body CSA and Minimal CSA). LS aBMD explained 47% of the variance in trabecular vBMD at the LS, 26–40% of the variance in integral and trabecular vBMD at the FN, and 13–35% of the variance in vBMD at the TH. LS aBMD was not associated with FN cortical vBMD. In contrast to TBS, LS aBMD predicted bone size (Vertebral Body CSA and Minimal CSA) but accounted for little of the variability. TBS and aBMD together explained more of the variance in all QCT indices than aBMD alone, except indices of bone size (Vertebral Body CSA and Minimal CSA), cortical vBMD measures, and trabecular vBMD at the TH (Table 3).
Table 3.
Univariate or multiple linear regression analysis to predict the variability in QCT indices (n=115)
| QCT parameters | LS aBMD (R2) | TBS (R2) | TBS + LS aBMD (R2) |
P-value Combination vs. aBMD alone |
|---|---|---|---|---|
| Lumbar Spine (L1, L2) | ||||
| Vertebral Body CSA | 0.087** | 0.016 | 0.088** | 0.50 |
| Average trabecular vBMD mid vertebra | 0.474*** | 0.441*** | 0.605*** | 0.005 |
| Femoral Neck | ||||
| Minimal CSA | 0.126*** | 0.000 | 0.165*** | 0.98 |
| Integral vBMD | 0.258*** | 0.424*** | 0.467*** | 0.0003 |
| Trabecular vBMD | 0.401*** | 0.411*** | 0.540*** | 0.01 |
| Cortical vBMD | 0.007 | 0.120*** | 0.131*** | 0.24 |
| C/I | 0.210*** | 0.292*** | 0.338*** | 0.006 |
| Total Hip | ||||
| Integral vBMD | 0.352*** | 0.414*** | 0.510*** | 0.005 |
| Trabecular vBMD | 0.326*** | 0.330*** | 0.436*** | 0.09 |
| Cortical vBMD | 0.129*** | 0.241*** | 0.258*** | 0.13 |
| C/I | 0.126*** | 0.292*** | 0.301*** | 0.04 |
LS aBMD: lumbar spine areal bone mineral density; TBS: trabecular bone score;
CSA: cross-sectional area; vBMD: volumetric bone mineral density; C/I: an estimate of cortical thickness
p<.05;
p<.01;
p<.001
The Relationship between TBS and HRpQCT indices
TBS was weakly to moderately associated with all vBMD and microarchitectural indices by HRpQCT at the radius except cortical thickness (Table 4). As expected, there were positive associations between TBS and BV/TV, Tb.Th and Tb.N and negative correlations with Tb.Sp and Tb.Sp.SD. At the radius, the strongest association was observed for trabecular vBMD. At the tibia, TBS correlated with all HRpQCT parameters except for Tb.Th, and the strongest association was between TBS and cortical vBMD. TBS was not associated with bone size (total area) at the radius or tibia. Adjusting TBS for weight or BMI did not change the direction or significance of correlations at either site. TBS and LS aBMD alone or in combination explained little of the variance in HRpQCT parameters (Supplemental Table 1).
Table 4.
Correlations (r values) between TBS and HRpQCT parameters at the radius and at the tibia (n=115):
| HRpQCT parameters | TBS | |
|---|---|---|
| Radius | Total Area | 0.116 |
| Total vBMD | 0.224* | |
| Ct.vBMD | 0.226* | |
| Ct.Th | 0.142 | |
| Tb.vBMD | 0.342*** | |
| BV/TV | 0.342*** | |
| Tb.N | 0.207* | |
| Tb.Th | 0.266** | |
| Tb.Sp | −0.253** | |
| Tb.Sp.SD | −0.253** | |
| Tibia | Total Area | 0.011 |
| Total vBMD | 0.392*** | |
| Ct.vBMD | 0.524*** | |
| Ct.Th | 0.294** | |
| Tb.vBMD | 0.330*** | |
| BV/TV | 0.328*** | |
| Tb.N | 0.202* | |
| Tb.Th | 0.135 | |
| Tb.Sp | −0.244** | |
| Tb.Sp.SD | −0.254** |
TBS: trabecular bone score; Total vBMD: total volumetric bone mineral density; Ct.vBMD: cortical volumetric bone mineral density; Ct.Th: cortical thickness; Tb.vBMD: trabecular volumetric bone mineral density; BV/TV: bone volume to tissue volume; Tb.N: trabecular number; Tb.Th: trabecular thickness; Tb.Sp: trabecular separation; Tb.Sp.SD: trabecular distribution;
p<.05;
p<.01;
p<.001
The Relationship between TBS, clinical characteristics, LS aBMD, QCT and HRpQCT parameters
A stepwise multiple regression model (with candidate predictors age, BMI, race, menopausal status, calcium intake, serum PTH and 25-hydroxyvitamin D, LS aBMD, and all QCT and HRpQCT indices) indicated that the combination of LS aBMD, integral vBMD at the TH, race, age, and BMI explained 72% of the variance in TBS (Table 5). The parameter estimate for race demonstrates that Caucasian race is associated with a 0.035 increase in LS TBS.
Table 5.
Multiple regression model of TBS (n=115):
| Variable | β | SE | P-value | Model R2 and P-value |
|---|---|---|---|---|
| LS aBMD | 0.3752 | 0.0676 | <0.0001 | 0.719 p<0.0001 |
| Total Hip Integral vBMD | 0.5454 | 0.2449 | 0.028 | |
| Caucasian Race | 0.0345 | 0.0126 | 0.007 | |
| Age | −0.0025 | 0.0005 | <0.0001 | |
| BMI | −0.0102 | 0.0015 | <0.0001 |
SE: standard error; LS aBMD: lumbar spine areal bone mineral density;
vBMD: volumetric bone mineral density; BMI: body mass index
Discussion
To our knowledge, this is the first investigation examining racial differences in trabecular microarchitectural texture using TBS, and the associations between TBS and QCT indices. We found no differences in spine TBS between races, but TBS correlated with vBMD and microarchitecture by QCT and HRpQCT in white and Chinese-American women. Our results suggest that TBS is generally reflective of skeletal indices measured by higher resolution imaging.
The finding that TBS was comparable between races is consistent with our prior QCT study indicating no racial differences in unadjusted trabecular vBMD at any site (LS, FN or TH) in either pre- or postmenopausal women in this cohort (12). While Chinese-Americans tended to have covariates (lower weight, vitamin D levels, calcium and vitamin D intake) predisposing to worse BMD and microarchitecture than their white counterparts, TBS remained comparable between the racial groups when adjusted for these differences. Our observations utilizing TBS are generally congruent with vertebral fracture data, which suggest similar or slightly higher rates in Chinese versus Caucasians (26, 27). Of note, while TBS was not different between the racial groups, race was a significant predictor of TBS in our regression model.
In agreement with theses findings, TBS correlated with all QCT indices of vBMD, with the strongest association at the LS trabecular vBMD. Further, using QCT as the gold standard (25), the Bland-Altman analysis demonstrated good agreement between TBS and LS trabecular vBMD across the spectrum of observed values, suggesting that TBS may be an acceptable alternative when QCT is not available or if radiation exposure is a concern. LS aBMD also correlated with all QCT indices except FN cortical vBMD. The association between integral vBMD at the FN and TBS was stronger than its association with LS aBMD. Of note, while LS aBMD was positively correlated with QCT measures of bone size (CSA) at the LS and FN, TBS was not. aBMD underestimates true BMD in those with small bone size (1). Previous studies have shown that Chinese-Americans have smaller bone size than white women, which may explain their lower aBMD (8, 11, 12). Since bone size influences bone strength, a measurement such as aBMD that encompasses both BMD and size could be advantageous. However, this may not be so for Chinese-Americans, whose lower aBMD seems to disproportionately reflect their smaller bone geometry rather than their higher vBMD and greater bone strength (10, 13). To this end, TBS could offer advantages over aBMD since TBS, as opposed to aBMD, is not influenced by bone size. In line with this observation, we found that the combination of TBS and LS aBMD tended to predict the variance in the QCT indices better than LS aBMD alone.
Similarly, TBS correlated with HRpQCT measures of vBMD and microarchitecture at the radius and tibia (except radial cortical thickness and tibial Tb.Th), but the strength of associations between TBS and HRpQCT tended to be of lower magnitude than those between TBS and QCT. Previous ex vivo studies indicate that TBS of lumbar vertebra is highly associated with trabecular microarchitecture assessed by μCT of the same bony region (14). We speculate that the weaker correlations between TBS and HRpQCT than between TBS and QCT are due to differences in trabecular microstructure between central and peripheral sites (28, 29). Similarly, our previous HRpQCT findings indicating thicker, more plate-like trabeculae and thicker, denser cortices and greater bone stiffness at peripheral sites in Chinese-American versus white women (13) were not evident by spine TBS. Although this could also be explained by site-to-site differences, we can not rule out the possibility that we may have detected differences in TBS with a larger cohort. However, we had 80% power with a two-tailed α of 5% to detect inter-racial differences in TBS of 0.68SD (or ~3.7%) in premenopausal women and a 0.86SD (6%) among postmenopausal women. In the Manitoba study (15), each SD decrease in TBS conferred a 35% increase in the risk for any major osteoporotic fracture. Applying these data, our study allowed us to detect TBS differences that would translate into a 24–30% difference in fracture risk.
Our study has limitations. Since we studied a small, convenience sample rather than a population-based sample, our results may not be generalizable to all Chinese-American and white women. Additionally, the small sample size and study design did not allow us to evaluate the association between TBS and QCT or HRpQCT among the different race-menopause subgroups or to assess the association between TBS and fracture. This study, however, has several important strengths. This is the first study to assess racial differences in TBS and to report correlations between TBS and QCT. Further, we studied both pre- and postmenopausal women, whose bone quality has been well characterized by multiple methodologies, allowing us to compare findings across modalities.
In conclusion, TBS is comparable between Chinese-American and white women, regardless of menopausal status, which is consistent with previous QCT data in this cohort. Moreover, TBS correlated with vBMD and microarchitecture measures by QCT and HRpQCT. TBS has the advantage of being available from DXA images through the application of specific software. While TBS may not fully capture some aspects of trabecular microstructure, the associations between TBS and measures of HRpQCT and QCT demonstrated here suggest that TBS may be a useful adjunct to aBMD for assessing bone quality when higher resolution imaging technologies are not available.
Supplementary Material
Acknowledgements
This work was supported by NIH K23 AR053507, a National Osteoporosis Foundation grant, and the Mary and David Hoar Fellowship Program of the New York Community Trust and the New York Academy of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Didier Hans is a co-owner of the patent for TBS. All the other authors state that they have no conflicts of interest.
References
- 1.Carter DR, Bouxsein ML, Marcus R. New approaches for interpreting projected bone densitometry data. J Bone Miner Res. 1992;7(2):137–145. doi: 10.1002/jbmr.5650070204. [DOI] [PubMed] [Google Scholar]
- 2.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–6515. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 3.Wang X, Sanyal A, Cawthon PM, et al. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res. 2012;27(4):808–816. doi: 10.1002/jbmr.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein EM, Liu XS, Nickolas TL, et al. Abnormal microarchitecture and reduced stiffness at the radius and tibia in postmenopausal women with fractures. J Bone Miner Res. 2010;25(12):2572–2581. doi: 10.1002/jbmr.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiaoge D, Eryuan L, Xianping W, et al. Bone mineral density differences at the femoral neck and Ward's triangle: a comparison study on the reference data between Chinese and Caucasian women. Calcif Tissue Int. 2000;67(3):195–198. doi: 10.1007/s002230001139. [DOI] [PubMed] [Google Scholar]
- 6.Lauderdale DS, Jacobsen SJ, Furner SE, et al. Hip fracture incidence among elderly Asian-American populations. Am J Epidemiol. 1997;146(6):502–509. doi: 10.1093/oxfordjournals.aje.a009304. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Lu A, Zhao X, Chen X, Cummings SR. Very low rates of hip fracture in Beijing, People's Republic of China the Beijing Osteoporosis Project. Am J Epidemiol. 1996;144(9):901–907. doi: 10.1093/oxfordjournals.aje.a009024. [DOI] [PubMed] [Google Scholar]
- 8.Walker MD, Liu XS, Stein E, et al. Differences in bone microarchitecture between postmenopausal Chinese-American and white women. J Bone Miner Res. 2011;26(7):1392–1398. doi: 10.1002/jbmr.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker MD, McMahon DJ, Udesky J, Liu G, Bilezikian JP. Application of high-resolution skeletal imaging to measurements of volumetric BMD and skeletal microarchitecture in Chinese-American and white women: explanation of a paradox. J Bone Miner Res. 2009;24(12):1953–1959. doi: 10.1359/JBMR.090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu XS, Walker MD, McMahon DJ, et al. Better skeletal microstructure confers greater mechanical advantages in Chinese-American women versus white women. J Bone Miner Res. 2011;26(8):1783–1792. doi: 10.1002/jbmr.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang XF, Wang Q, Ghasem-Zadeh A, et al. Differences in macro- and microarchitecture of the appendicular skeleton in young Chinese and white women. J Bone Miner Res. 2009;24(12):1946–1952. doi: 10.1359/jbmr.090529. [DOI] [PubMed] [Google Scholar]
- 12.Walker MD, Saeed I, McMahon DJ, et al. Volumetric bone mineral density at the spine and hip in Chinese American and White women. Osteoporos Int. 2012;23(10):2499–2506. doi: 10.1007/s00198-011-1855-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walker MD, Liu XS, Zhou B, et al. Pre- and postmenopausal differences in bone microstructure and mechanical competence in Chinese-American and white women. J Bone Miner Res. 2013;28(6):1308–1318. doi: 10.1002/jbmr.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hans D, Barthe N, Boutroy S, et al. Correlations between trabecular bone score, measured using anteroposterior dual-energy X-ray absorptiometry acquisition, and 3-dimensional parameters of bone microarchitecture: an experimental study on human cadaver vertebrae. Journal of clinical densitometry : the official journal of the International Society for Clinical Densitometry. 2011;14(3):302–312. doi: 10.1016/j.jocd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Hans D, Goertzen AL, Krieg MA, Leslie WD. Bone microarchitecture assessed by TBS predicts osteoporotic fractures independent of bone density: the Manitoba study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(11):2762–2769. doi: 10.1002/jbmr.499. [DOI] [PubMed] [Google Scholar]
- 16.Boutroy S, Hans D, Sornay-Rendu E, et al. Trabecular bone score improves fracture risk prediction in non-osteoporotic women: the OFELY study. Osteoporos Int. 2013;24(1):77–85. doi: 10.1007/s00198-012-2188-2. [DOI] [PubMed] [Google Scholar]
- 17. [Accessed on October 22, 2012];FDA.gov. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn_template.cfm?id=k121716.
- 18.Hertzler AA, Frary RB. A dietary calcium rapid assessment method (RAM) Topics in clinical nutrition. 1994;9(3):76–85. [Google Scholar]
- 19.Bonnick SL, Johnston CC, Jr, Kleerekoper M, et al. Importance of precision in bone density measurements. J Clin Densitom. 2001;4(2):105–110. doi: 10.1385/jcd:4:2:105. [DOI] [PubMed] [Google Scholar]
- 20.Popp AW, Guler S, Lamy O, et al. Effects of zoledronate versus placebo on spine bone mineral density and microarchitecture assessed by the trabecular bone score in postmenopausal women with osteoporosis: A three-year study. J Bone Miner Res. 2013;28(3):449–454. doi: 10.1002/jbmr.1775. [DOI] [PubMed] [Google Scholar]
- 21.Dufour R, Winzenrieth R, Heraud A, Hans D, Mehsen N. Generation and validation of a normative, age-specific reference curve for lumbar spine trabecular bone score (TBS) in French women. Osteoporos Int. 2013 doi: 10.1007/s00198-013-2384-8. Epub ahead of print May 17. [DOI] [PubMed] [Google Scholar]
- 22.Cheng X, Li J, Lu Y, Keyak J, Lang T. Proximal femoral density and geometry measurements by quantitative computed tomography: association with hip fracture. Bone. 2007;40(1):169–174. doi: 10.1016/j.bone.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Lang T, LeBlanc A, Evans H, et al. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19(6):1006–1012. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 24.Marshall LM, Lang TF, Lambert LC, et al. Dimensions and volumetric BMD of the proximal femur and their relation to age among older U.S. men. J Bone Miner Res. 2006;21(8):1197–1206. doi: 10.1359/jbmr.060506. [DOI] [PubMed] [Google Scholar]
- 25.Krouwer JS. Why Bland-Altman plots should use X, not (Y+X)/2 when X is a reference method. Stat Med. 2008;27(5):778–780. doi: 10.1002/sim.3086. [DOI] [PubMed] [Google Scholar]
- 26.Bow CH, Cheung E, Cheung CL, et al. Ethnic difference of clinical vertebral fracture risk. Osteoporos Int. 2012;23(3):879–885. doi: 10.1007/s00198-011-1627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau EM, Chan HH, Woo J, et al. Normal ranges for vertebral height ratios and prevalence of vertebral fracture in Hong Kong Chinese: a comparison with American Caucasians. J Bone Miner Res. 1996;11(9):1364–1368. doi: 10.1002/jbmr.5650110922. [DOI] [PubMed] [Google Scholar]
- 28.Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14(7):1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 29.Cohen A, Dempster DW, Muller R, et al. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: comparison with transiliac bone biopsy. Osteoporos Int. 2010;21(2):263–273. doi: 10.1007/s00198-009-0945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.