Abstract
Background
Lofexidine, an α2-adrenergic agonist, is being investigated as a treatment for reducing opioid withdrawal symptoms and blocking stress-induced relapse to cocaine taking. Opioid abusers are often polydrug abusers and cocaine is one frequent drug of choice. However, relatively little is known about lofexidine interactions with cocaine. The present study investigated the effects of acute and chronic treatment with lofexidine in a pre-clinical model of cocaine self-administration.
Methods
Male rhesus monkeys were trained to respond for food (1 g) and cocaine (0.01 mg/kg/inj) under a fixed ratio 30 (FR30) or a second order FR2 (VR16:S) schedule of reinforcement. Systematic observations of behavior were conducted during and after chronic treatment with lofexidine.
Results
Acute treatment with lofexidine (0.1 or 0.32 mg/kg, IM) significantly reduced cocaine self-administration but responding for food was less effected. In contrast, chronic treatment (7–10 days) with lofexidine (0.1–0.32 mg/kg/hr, IV) produced a leftward shift in the cocaine self-administration dose-effect curve, but had no effect on food-maintained responding. Lofexidine did not produce any observable side effects during or after treatment.
Conclusions
Lofexidine potentiated cocaine’s reinforcing effects during chronic treatment. These data suggest that it is unlikely to be effective as a cocaine abuse medication and could enhance risk for cocaine abuse in polydrug abusers.
Keywords: self-administration, cocaine, adrenergic agonists, lofexidine, behavioral observations
1. INTRODUCTION
Norepinephrine (NE) is one of the most abundant neurotransmitters in the brain with key roles in such behaviors as attention, arousal, stress responses, learning and memory, neuronal excitability, etc. (Sofuoglu and Sewell, 2009). Lofexidine, 2-[1-(2,6-dichlorophenoxy)ethyl]-4,5-dihydro-1H-Imidazole, is an analog of the FDA-approved α2 adrenergic agonist clonidine. Lofexidine is available in the United Kingdom as an anti-hypertensive agent, and as a medication to alleviate opioid withdrawal symptoms. Lofexidine is not yet available in the United States, though clinical trials are underway (www.clinicaltrials.gov).
Lofexidine and other α2 agonists have received considerable attention as potential pharmacotherapies for several aspects of drug addiction. For example, clonidine has been useful in treating opioid withdrawal signs. Lofexidine is as effective as clonidine in attenuating opiate withdrawal symptoms (Lin et al., 1997; Shearman et al., 1980; Yu et al., 2008) but has a more favorable side effect profile and less hypotension and sedation (Gowing et al., 1996; 2002). Lofexidine is well tolerated clinically (Welberg, 2012), even at supratherapeutic doses (Walsh et al., 2003). Clinical trials to evaluate lofexidine’s efficacy alone and in conjunction with methadone to attenuate opiate withdrawal signs are ongoing (Gorodetzky and Longstreth, 2012). Preclinical and clinical studies suggest that lofexidine also may be effective in reducing relapse to cocaine and cocaine + heroin abuse. In rats, lofexidine blocked stress-induced reinstatement of responding for cocaine (Erb et al., 2000) and “speedball” (heroin + cocaine; Highfield, 2001) injections, and other α2 agonists prevented cue-induced cocaine seeking in rats (Smith and Aston-Jones, 2011). Further, α2 antagonists such as yohimbine and RS-79948 reinstate cocaine-seeking behavior in squirrel monkeys, and these effects are dose-dependently blocked by clonidine (Lee et al., 2003). In clinical studies, several α2 agonists also have been shown to block stress- and cue-induced cocaine or opiate craving (Fox et al., 2012; Jobes et al., 2011; Sinha et al., 2006).
Little is known about how lofexidine treatment influences on-going cocaine self-administration. Acute treatment with UK-14304, an α2 agonist, had no effect on cocaine self-administration in rats with a history of short- or long-access to cocaine, however, only one dose of cocaine was studied (Wee et al., 2008). Repeated lofexidine injections for 5 consecutive days also did not affect on-going “speedball” (cocaine + heroin) self-administration in rats (Highfield, 2001). Finally, pretreatment with several α2 agonists (i.e., clonidine, lofexidine, guanabenz) did not attenuate cocaine-induced reinstatement of cocaine seeking (Erb et al., 2000).
The present study was designed to examine how lofexidine influences cocaine self-administration in rhesus monkeys. This question is important because cocaine use increases during methadone treatment in some patients (Condelli et al., 1991; Kosten et al., 1989; Schottenfeld et al., 1993). Polydrug abuse, particularly cocaine + heroin (“speedball”), is a common form of drug abuse (World Drug Report, 2010). Thus, risk for cocaine use by “speedball” abusers is high and the influence of chronic lofexidine treatment is unknown. We studied the effects of acute and chronic lofexidine treatment on cocaine self-administration in nonhuman primate models of cocaine self-administration. The effects of lofexidine treatment on behavior maintained by a non-drug reinforcer (i.e., food) were also evaluated to determine if any effects of lofexidine were specific to cocaine or reflected a general disruption of operant behavior. Finally, systematic observations of behavior were conducted to characterize potential side effects of chronic treatment with lofexidine.
2. METHODS AND MATERIALS
2.1 Animals
Seven adult male rhesus monkeys (Macaca mulatta) that weighed between 6 and 10 kg were studied. Monkeys received Lab Diet Jumbo Monkey Biscuits (PMI Feeds Inc., St. Louis, MO), a multiple vitamin, and fresh fruit and vegetables daily. Water was continuously available from an automatic watering system. A 12-hr light-dark cycle was in effect (lights on 8:00AM – 8:00PM) except where noted below. Animal maintenance and research were conducted in accordance with the guidelines provided by the Institute of Laboratory Animal Resources (ILAR-NRC, 2010) and the NIH Office of Laboratory Animal Welfare (OLAW). The facility is licensed by the U.S. Department of Agriculture and the McLean Hospital Institutional Animal Care and Use Committee approved all protocols. Consultant veterinarians monitored the health of the colony. Enrichment was provided through mirrors and toys in the home-cage, interaction with technical staff, and the opportunity to manipulate their environment during operant food and drug procedures (Lutz and Novak, 2005).
Monkeys lived in stainless steel chambers (64 × 64 × 79 cm) equipped with a custom-designed operant response panel. Operant panels contained three square translucent response keys (5.1 × 5.1 cm) arranged 3.5 cm apart in a horizontal row 9 cm from the top of the operant panel. Each key could be transilluminated with red or green stimulus lights (SuperBright LEDs; Fairchild Semiconductor, San Jose, CA). Three circular translucent keys were illuminated with stimulus lights (1.9 cm in diameter) and located in a vertical column below the center response key and could also be transilluminated by red or green stimulus lights. A pellet dispenser (Gerbrands Model G5210, Arlington, MA) and two syringe pumps (Model 981210, Harvard Apparatus, Inc., South Natick, MA), one for each lumen of the double-lumen catheter, were mounted on shelving above the chamber. Banana-flavored food pellets (Formula 4TUR, Purina Mills Test Diet, Richmond, IN) were delivered to a food cup attached to the lower left front of the chamber. All experimental events were custom programmed on a Hewlett-Packard (model 8100 Elite CMT PC) desktop PC connected to the chambers via a Med Associates (Georgia, VT) Interface.
2.2 Venous Catheter Implantation
Details of our surgical procedures have been described previously (Mello et al., 2012; Mello and Newman, 2011; Newman et al., 2010; Mello et al, 2013). Briefly, double lumen Silicone catheters (I.D. 0.028 in, O.D. 0.088 in; Saint Gobain Performance Plastics, Beaverton, MI) were surgically implanted in a jugular or femoral vein under aseptic conditions. The double lumen catheters permitted IV cocaine self-administration through one lumen and concurrent IV saline or lofexidine administration during chronic treatment studies through the second lumen. The intravenous catheter exited in the mid-scapular region and was protected by a tether system consisting of a custom-fitted nylon vest connected to a flexible stainless-steel cable and fluid swivel (Lomir Biomedical, Inc., Malone, NY). Catheter patency was evaluated periodically by administration of a short-acting barbiturate, methohexital sodium (4 mg/kg, IV). If muscle tone was decreased within 10 s after drug administration, the catheter was considered patent.
2.3 Acute Lofexidine Effects on Cocaine Self-Administration
The effects of acute lofexidine (0.1 or 0.32 mg/kg) treatment on cocaine self-administration were studied in four cocaine-experienced monkeys. Each daily 2-hr session consisted of three response components separated by 5-min time-out (TO) periods. During the first and last components (Food 1 and Food 2), red lights illuminating the center response key signaled the availability of 1-g banana-flavored food pellets under a fixed ratio 30 (FR30): TO 10-s schedule of reinforcement for 5-min. During the cocaine self-administration component, green lights illuminating the center response key signaled the availability of intravenous (IV) injections of cocaine or saline for 100-min under an FR30: TO 60-s schedule. This component was immediately preceded by illumination of a yellow light for 10-s and the non-contingent delivery of a single ‘priming’ injection of saline or cocaine at the dose that was subsequently available. During the TO following each reinforcer delivery (food or cocaine), the center response key was illuminated with yellow lights. During inter-component TO periods, all lights were off and responding had no scheduled consequences.
During training sessions, saline (S) or a unit dose of cocaine (C) was available under a double alternation schedule (e.g, SS CC, SS, CC, etc) that varied irregularly between subjects. Once responding was stable, the cocaine self-administration dose–effect curve was determined over a dose range of 0.0032 – 0.1 mg/kg/inj cocaine. Responding was considered stable if the number of injections on the day preceding a test was within 15% of the mean of all baseline sessions at that cocaine dose within the past 3 months. Next, pretreatment with lofexidine (IM, 15-min) was studied in test sessions that were routinely conducted on the second day of the double alternation schedule, and following a session during which control rates and patterns of self-administration behavior were near baseline levels. The 15-min pretreatment time was determined during preliminary drug discrimination studies (Kohut, unpublished). Initially, dose-ranging experiments were conducted to determine the effects of lofexidine on self-administration of 0.032 mg/kg/inj cocaine. This unit dose of cocaine produces reliable and stable levels of self-administration behavior. These experiments sought to identify pretreatment doses of lofexidine that decreased cocaine self-administration by at least 50% while maintaining some selectivity in decreasing cocaine over food-maintained responding. An effective dose of lofexidine was then studied against a range of cocaine doses (0.0032–0.1 mg/kg/inj) in each monkey. The acute effects of a single dose of lofexidine (0.1 mg/kg in three subjects (R-009, R-105, and R082) and 0.32 mg/kg in one subject (R-166) were then examined when either saline or various doses of cocaine were available for self-administration (see Table 2 and below).
Table 2. Lofexidine dose-ranging studies.
The acute effects of pretreatment with lofexidine (0.032–0.32 mg/kg, IM) on cocaine self-administration and food-maintained behavior in individual monkeys
| Monkey | Lofexidine (mg/kg) | 1 Inj | 2 Inj | 3 Inj | 4 Inj | Total Inj | Food 1 Pellets | Food 2 Pellets |
|---|---|---|---|---|---|---|---|---|
| R-009 | 0 | 9 (± 4) | 7 (± 5) | 11 (± 5) | 11 (± 4) | 37 (± 13) | 12 (± 6) | 16 (± 1) |
| 0.032 | 14 | 16 | 17 | 13 | 60 | 12 | 10 | |
| 0.1 | 0 | 0 | 0 | 0 | 0 | 10 | 9 | |
| 0.32 | 0 | 0 | 0 | 0 | 0 | 14 | 9 | |
|
| ||||||||
| R-082 | 0 | 10 (± 3) | 11 (± 1) | 9 (± 2) | 9 (± 2) | 39 (± 4) | 13 (± 2) | 0 (± 0) |
| 0.032 | - | - | - | - | - | - | - | |
| 0.1 | 1 | 10 | 13 | 12 | 36 | 0 | 0 | |
| 0.32 | 0 | 4 | 13 | 11 | 28 | 0 | 0 | |
|
| ||||||||
| R-105 | 0 | 7 (± 4) | 7 (± 1) | 6 (± 2) | 5 (± 1) | 25 (± 5) | 16 (± 1) | 0 (± 0) |
| 0.032 | 5 | 7 | 4 | 4 | 20 | 13 | 11 | |
| 0.1 | 0 | 0 | 0 | 0 | 0 | 10 | 10 | |
| 0.32 | 0 | 0 | 0 | 0 | 0 | 10 | 11 | |
|
| ||||||||
| R-166 | 0 | 12 (± 9) | 7 (± 3) | 8 (± 4) | 10 (± 2) | 37 (± 16) | 15 (± 0) | 13 (± 1) |
| 0.032 | - | - | - | - | - | - | - | |
| 0.1 | 14 | 13 | 14 | 12 | 53 | 15 | 9 | |
| 0.32 | 0 | 0 | 0 | 0 | 0 | 10 | 11 | |
2.4 Chronic Lofexidine Effects on Cocaine Self-Administration
Once the acute effects of lofexidine on cocaine- and food-maintained responding were examined, chronic lofexidine was studied to determine if treatment effects were sustained over time. Saline or lofexidine was administered every 20 minutes for 23-hr each day (10:30AM–9:30AM). Each treatment dose was studied for 7–10 days until responding was stable. Successive treatment doses were separated by an interval of saline treatment until drug- and food-maintained responding returned to baseline levels. The saline treatment interval was used to prevent any carryover effects from the preceding treatment. Two doses of lofexidine (0.1 and 0.32 mg/kg/hr) were tested against the cocaine dose-effect curve in each monkey. This chronic treatment procedure was used to permit comparisons with other candidate treatment medications (e.g., modafinil, buspirone, d-amphetamine) previously studied in this laboratory (see Mello et al., 2012; Negus and Mello, 2003; Newman et al., 2010).
The effect of chronic lofexidine (0.1 – 0.32 mg/kg/hr) treatment on cocaine self-administration was studied in three cocaine-experienced monkeys. Each 2-hr daily session consisted of 1-hr of food and 1-hr of cocaine access. Food was available at 11am, 3pm, 7pm, and 6am the next morning, and drug was available at 12pm, 4pm, 8pm, and 7am the next morning. During the food components, a red light illuminating the center response key signaled the availability of 1-g banana-flavored food pellets under a Fixed Ratio 2, Variable Ratio 16 (FR2; VR16:S]) second order schedule of reinforcement. During cocaine self-administration components, a green light signaled cocaine availability, and completion of the response requirement resulted in delivery of 0.1 ml of a cocaine solution over 1-sec through one lumen of the double-lumen catheter. A 10-sec TO period followed delivery of each food pellet or drug injection during which stimulus lights were turned off. If 25 food pellets or 20 cocaine injections were delivered before the end of the 1-hr session, then all stimulus lights were turned off, and responding had no scheduled consequences for the remainder of that session. Extinction training consisted of sessions in which no injection was substituted for 0.01 mg/kg IV cocaine and all stimulus lights operated as described above.
Training continued until monkeys met the following criteria for stable food and cocaine responding: 1) three consecutive days during which the number of drug injections/day varied by no more than 20% of the three-day mean with no upward or downward trend in performance, and 2), the mean number of food pellets and injections delivered per day was equal to or greater than 60. Once responding was stable, self-administration of saline and cocaine (0.001 – 0.1 mg/kg/inj) were studied in an irregular order for each monkey. Each dose was substituted for 7–10 days until responding was stable according to the above criteria. Following each substitution test, monkeys were returned to the maintenance dose of cocaine, 0.01 mg/kg/inj, for at least three days or until responding was stable to ensure reliable baseline responding before the next substitution test. Increasing doses of lofexidine were initially tested against the maintenance dose of cocaine (0.01 mg/kg/inj). Cocaine (0.001 – 0.1 mg/kg/inj) was delivered by computer-controlled variations in pump infusion duration (Fivel, 2011).
2.5 Behavioral Observations During and After Chronic Lofexidine Treatment
Behavioral changes from the saline treatment baseline were studied during chronic treatment and withdrawal from lofexidine. Behavioral observations were conducted at about 1PM each day during (7–10 days) and after treatment (3 days) with lofexidine (0.1 – 0.32 mg/kg/hr) during tests with 0.01 mg/kg/inj cocaine. Table 1 summarizes the behaviors rated (Kato and Yanagita, 1981). This scale has been used to compare the effects of drugs from several pharmacological classes on gross behavioral observations in rhesus monkeys (Kato and Yanagita, 1981).
Table 1.
Behavioral Observation Scale
| Behavior | Scale | Operational Definition |
|---|---|---|
|
| ||
Apprehension/Arousal
|
0 | Not shown even on handling |
| 1 | Shown on handling | |
| 2 | Shown on opening cage | |
| 3 | Shown on touching cage door | |
| 4 | Shown on standing before cage | |
| 5 | Shown on looking into monkey’s eyes | |
|
| ||
| Spontaneous Motor Activity | 0 | Sitting with head in tucked position |
| 1 | Sitting without large movement | |
| 2 | Sitting with movements of head and arms | |
| 3 | Sitting and walking in cage | |
| 4 | Walking continuously in cage | |
|
| ||
| Attention | 0 | Does not follow treat |
| 1 | Follows treat for 1 second | |
| 2 | Follows treat for 2 seconds | |
| 3 | Follows treat for 3 seconds | |
| 4 | Follows treat for 4 seconds | |
|
| ||
| Gross Motor Function | 0 | No movement |
| 1 | Unable to mount perch | |
| 2 | Nearly unable to stay on perch | |
| 3 | Slowed movement | |
| 4 | Slightly slowed movement | |
| 5 | No slowing of movement | |
|
| ||
| Tremor | 0 | None |
| 1 | Visible in fingers when moving | |
| 2 | Visible in fingers and limbs when moving | |
| 3 | Visible markedly in fingers and limbs when moving | |
|
| ||
| Salivation/ Pupils | 0 | None / Small Pupils (Needlepoint) |
| 1 | Moistening of lips / Normal Pupils | |
| 2 | Recognizable but no dribbling/ Large Pupils (Dime) | |
| 3 | Dribbling from mouth | |
|
| ||
| Stereotypy | 0 | Absent |
| 1 | Present | |
|
| ||
| Sedation | 0 | No Sedation alert to environement |
| 1 | Slightly suppressed, little movement, quieter than usual | |
| 2 | Moderately sedated: sitting, not reaching for pellets Responds to noise in the room. | |
| 3 | Heavily sedated; lying on floor of cage, no response to Exp. | |
2.6 Data Analysis
The primary dependent variable was the total number of drug or saline injections and food pellets earned per session. In chronic treatment studies, the number of injections self-administered and food pellets earned during the last three days of each treatment condition were averaged. Changes in drug-maintained responding from saline-treatment during lofexidine treatment at each dose of cocaine were evaluated using a two-way repeated measures analysis of variance (ANOVA). A significant ANOVA was followed by Bonferroni multiple comparison post-hoc tests. One-way ANOVA with Dunnett’s post-hoc tests were used to determine which points differed from saline self-administration.
Behavioral observations were recorded before, during, and after chronic treatment with lofexidine. Ratings during the last three days of chronic treatment and during the first three days after treatment were averaged. Friedman’s Test was used to compare ratings at the two doses of lofexidine to baseline levels for each behavior measured. Because baselines differed between animals, the data is presented graphically as the mean difference from baseline.
All figures and statistical analyses were conducted using GraphPad Prism 6.0 for Macintosh (GraphPad Software Inc.).
2.7 Drugs
Cocaine HCl was provided by the National Institute on Drug Abuse (Rockville, MD, USA). Lofexidine HCl was purchased from Santa Cruz Biosciences (Santa Cruz, Ca). All doses are expressed as the salt. All drugs were dissolved in sterile water and were sterile-filtered with a syringe-driven 0.22-micron filter (Millipore Corporation, Billerica, MA).
3. RESULTS
3.1 Acute Treatment with Lofexidine Decreased Cocaine Self-Administration
Table 2 shows individual data from lofexidine dose-ranging tests with 0.032 mg/kg/inj cocaine. Dose-ranging tests identified pretreatment doses of lofexidine that decreased IV cocaine self-administration by at least 50% while maintaining some selectivity in decreasing cocaine over food-maintained responding. Low doses of lofexidine increased responding for cocaine in 2 of 4 monkeys (R-009 at 0.032 mg/kg and R-166 at 0.1 mg/kg). However, increasing their respective doses by 1/2–log unit completely eliminated cocaine self-administration. In another monkey (R-105), lofexidine dose-dependently decreased cocaine self-administration when low doses of lofexidine (0.032 and 0.1 mg/kg) were administered with little effect on food-maintained responding. Finally, cocaine self-administration was only moderately affected by lofexidine pretreatment yet food-maintained responding was eliminated at 0.1 mg/kg lofexidine in Monkey R-082. It should be pointed out that responding during the second food component was eliminated under baseline conditions in two monkeys (R-082 and R-105). In the acute procedure, we often find that monkeys vary in sensitivity to the rate decreasing effects of cocaine, which is most prominent after self-administration of high doses of cocaine.
A dose of 0.1 mg/kg lofexidine was subsequently tested during determination of a complete cocaine dose-effect curve in monkeys R-009, R-105, and R-082 while monkey R-166 received 0.32 mg/kg lofexidine. Self-administration of cocaine (0.0032 - 0.1 mg/kg/inj) under baseline conditions followed an inverted-U shaped function. A dose of 0.01 mg/kg/inj cocaine (mean 47.5 ± 4.44 injections/session; range 49–56 inj) maintained the highest level of responding in three monkeys and 0.032 mg/kg maintained the highest level of responding in the fourth monkey (R-166; 41 injections/session). When saline was substituted for cocaine, responding rapidly extinguished to 8.75 ± 0.48 injections/session.
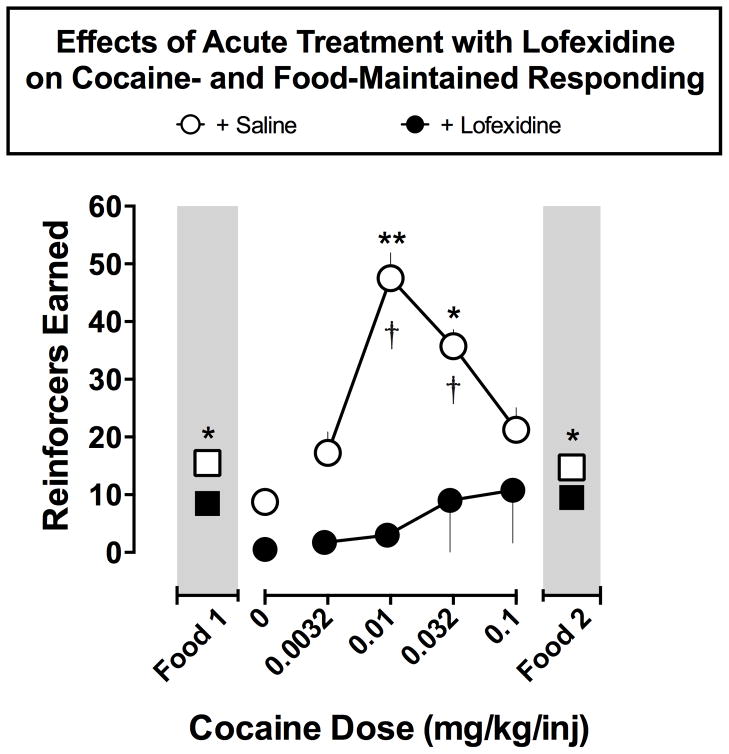
As shown in Figure 1, acute treatment with lofexidine (0.1 or 0.32 mg/kg, IM) produced a flattening of the cocaine self-administration dose-effect curve with significant decreases at 0.01 and 0.032 mg/kg/inj (both p’s <0.05). The number of cocaine injections was not significantly different from saline-maintained responding across cocaine doses of 0.0032, 0.01, 0.032, and 0.1 mg/kg/inj, respectively. On average, monkeys earned 15.5 ± 0.29 and 14.75 ± 0.48 food pellets during Food Sessions 1 and 2, respectively. Lofexidine treatment moderately reduced the number of pellets earned during the first food session by about 45% while food was decreased by about 35% in the second food session (both p’s <0.05).
Figure 1. Effects of Acute Treatment with Lofexidine on Cocaine- and Food-Maintained Responding.
Abscissa, cocaine dose in mg/kg/inj (log-scale). Ordinate, total cocaine injections earned per day. All data points show the mean ± SEM in four monkeys. Repeated measures ANOVA found significant main effects of Treatment (F(3,18)=3.39; p<0.05) and Cocaine Dose (F(1,6)=45.40; p<0.001) as well as a significant Treatment X Cocaine Dose Interaction (F(3,18)=3.92; p<0.05). Dunnett’s post-hoc analysis showed that cocaine self-administration with lofexidine treatment was significantly lower than baseline when 0.01 and 0.032 mg/kg/inj cocaine was available (both p’s <0.01). Self-administration of each dose of cocaine was also compared to saline self-administration to determine which doses elicited significantly more behavior than when saline was available. Under baseline conditions, a significant effect of cocaine dose was found (F(4,12)=20.96; p<0.0001) and Dunnett’s post-hoc tests showed that 0.01 and 0.032 mg/kg/inj produced significantly more self-administration than saline. There was no main effect of cocaine dose with lofexidine treatment (F (4,12)=0.46; p>0.05). Dunnett’s tests indicate that cocaine self-administration did not differ from saline levels at any dose when lofexidine was administered as a pretreatment (all p’s >0.05). Paired t-tests on number of pellets earned found that responding for food was slightly, but significantly reduced during both food sessions (both p’s<0.05). * p<0.05 lofexidine treatment vs. cocaine baseline; † p<0.05 cocaine vs. saline self-administration.
3.2 Chronic Treatment with Lofexidine Enhanced Cocaine Self-Administration
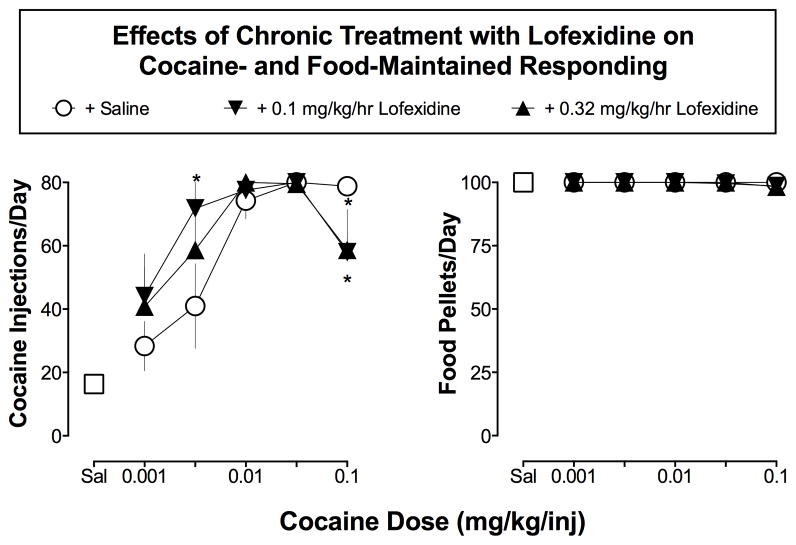
As shown in Figure 2, peak cocaine-maintained responding occurred at a unit dose of 0.032 mg/kg/inj (i.e., 80 inj/day) during saline treatment. Monkeys earned about 50% of the maximal number of cocaine injections (i.e., 41.00 ± 13.37) when a dose of 0.0032 mg/kg/inj was available and about 35% of the maximal number of injections at the lowest dose of cocaine (0.001 mg/kg/inj; 28.33 ± 7.81). When saline was substituted for cocaine, responding decreased to 16.33 ± 2.46 inj/day. Monkeys earned all of the available food pellets/day during saline or cocaine self-administration sessions under saline treatment.
Figure 2. Effects of Chronic Treatment with Lofexidine on Cocaine- and Food-Maintained Responding.
Abscissa, cocaine dose in mg/kg/hr (log-scale). Left Panel Ordinates, total cocaine injections earned per day (out of 80 possible). Right Panel Ordinates, total food pellets earned per day (out of 100 possible). All data points show the mean ± SEM of the last three days of 7–10 days treatment in three monkeys. A 3×5 repeated measures ANOVA with factors of Treatment and Cocaine Dose found a main effect of Cocaine Dose (F(4,8)=6.120; p=0.01) but no main effect of Treatment (F(2,4)=0.896; p=0.48). However, there was a significant Treatment X Cocaine Dose interaction (F(6,12)=3.445; p=0.03). Post-hoc tests found that 0.1 mg/kg/hr lofexidine increased cocaine self-administration when 0.0032 mg/kg/inj cocaine was available (p=0.003). Conversely, both 0.1 and 0.32 mg/kg/hr lofexidine, decreased the number of injections earned when 0.1 mg/kg/inj cocaine was available for self-administration (both p’s<0.05). * p<0.05 lofexidine treatment vs. cocaine baseline
The maintenance dose of 0.01 mg/kg/inj cocaine was relatively insensitive to chronic treatment with lofexidine. The number of cocaine (0.01 mg/kg/inj) injections earned were 77.34 ± 2.67, 80 ± 0, 77.56 ± 2.28, and 80 ± 0 at lofexidine doses of 0.01, 0.032, 0.1, and 0.32 mg/kg/hr, respectively. Subsequently, the highest doses of 0.1 and 0.32 mg/kg/ lofexidine were chosen to test against the complete cocaine dose-effect curve. Figure 2 shows that chronic treatment with 0.1 and 0.32 mg/kg/hr lofexidine both shifted the cocaine dose-effect curve leftward. When low doses of cocaine were available for self-administration (0.001 and 0.0032 mg/kg/inj), 0.1 mg/kg/hr lofexidine produced about a 55 and 75% increase in number of cocaine injections earned, respectively, and about a 43% increase at both cocaine doses when 0.32 mg/kg/hr lofexidine was administered. Chronic lofexidine had little effect on intermediate doses of cocaine (0.01–0.032 mg/kg/inj) but decreased the number of injections when the highest dose of cocaine was available (0.1 mg/kg/inj) by about 25% (both p’s<0.05). The number of food pellets earned per day was not significantly altered by chronic lofexidine treatment.
3.3 Behavioral Effects of Lofexidine During and After Chronic Treatment
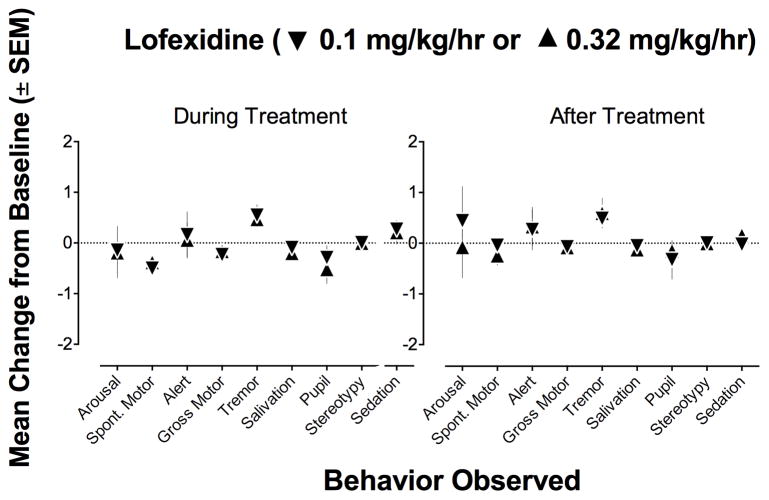
Behavioral observations were conducted during and after chronic treatment with saline and lofexidine. Figure 3 shows the average difference from baseline behavioral ratings during the final three days of treatment and for the first three days after cessation of treatment with lofexidine. None of the behaviors measured were significantly different from baseline during or after lofexidine treatment.
Figure 3. Behavioral Observations During and After Chronic Treatment with Lofexidine.
Abscissa, mean change from baseline. Ordinates, behavior scored during daily observation periods. All data points show the mean ± SEM of three monkeys. Friedman’s test found that chronic lofexidine treatment did not significantly change the behaviors observed different from baseline (all p’s > 0.167). Cessation of lofexidine treatment also did not change any behavioral ratings from saline (all p’s > 0.167).
4. DISCUSSION
This is the first evaluation of the effects of acute and chronic (7–10 days) treatment with the α2 adrenergic agonist, lofexidine, on cocaine self-administration in nonhuman primates. Our major findings were that acute lofexidine significantly attenuated cocaine self-administration whereas chronic lofexidine significantly potentiated cocaine self-administration. The contrast between the acute and chronic effects of lofexidine on cocaine self-administration was unexpected. Some factors that may have contributed to these findings are discussed below.
Although lofexidine has not been studied extensively, several preclinical reports in rodents suggest that α2 agonists do not modulate the behavioral effects of cocaine. Previous studies in rats have found no effect of α2 agonists on cocaine or cocaine + heroin self-administration (Erb et al., 2000; Highfield, 2001; Wee et al., 2008). However, acute treatment with an α2 antagonist (dexefaroxan) increased locomotor activity induced by a selective DAT inhibitor (GBR 12783; Villégier et al., 2003)) and enhanced circling behavior induced by methylphenidate or apomorphine (Chopin et al., 1999). In the current study, acute administration of the α2 agonist lofexidine, attenuated cocaine self-administration in rhesus monkeys and was less effective at decreasing responding for food.
The evaluation of chronic lofexidine treatment was designed to model clinical treatment. In contrast to our findings with acute lofexidine treatment, chronic treatment with lofexidine potentiated cocaine self-administration. Increases in cocaine self-administration at low doses were evident on Day 1 of the lofexidine treatment regimen and persisted throughout the duration of treatment. For example, when a low minimally reinforcing dose of cocaine (0.001 mg/kg/inj) was available for self-administration during saline treatment, a burst of responding typical of extinction was seen in the first 3–4 days, then gradually decreased to saline levels. Conversely, during lofexidine treatment, 0.001 mg/kg/inj cocaine maintained stable levels of self-administration similar to a higher dose of cocaine (0.0032 mg/kg/inj) during saline treatment.
4.1 Possible Mechanisms of Lofexidine’s Effect on Cocaine Self-Administration
The mechanisms underlying the differential effects of acute and chronic lofexidine treatment on cocaine self-administration are unclear. Several lines of evidence suggest a reciprocal interaction between α2 receptors and dopamine activity in brain regions that modulate the behavioral effects of psychomotor stimulants such as cocaine. For example, radioligand binding studies have found that striatal brain regions and prefrontal cortex (Ordway et al., 1993; Nicholas et al., 1993; Uhlen et al., 1997) each contain a high density of α2 receptors. Interestingly, the affinity of dopamine and norepinephrine for α2 receptors is similar and dopamine has been shown to inhibit forskolin-stimulated accumulation of cyclic-adenylyl cyclase in α2 transfected NRK cells (Zhang et al., 1999). Decreased α2 receptor responsiveness also occurs after repeated cocaine exposure in rats (Baumann et al., 2004). Further, α2 receptors have been shown to regulate DA release in both the nucleus accumbens (Ihalainen and Tanila, 2004) and prefrontal cortex (Ihalainen and Tanila, 2002).
The complex regulation of dopaminergic activity by α2 adrenergic receptor activation changes with repeated α2 agonist exposure. α2 receptors primarily exist as presynaptic autoreceptors in the central nervous system (Docherty, 1998) and microdialysis studies have found that α2 receptor agonism regulates both norepinephrine and dopamine levels in prefrontal and striatal brain areas. For example, acute administration of clonidine or dexmedetomidine (an α2 receptor agonist) decreased basal levels of extracellular norepinephrine and dopamine in the prefrontal cortex (Ihalainen and Tanila, 2002; Tanda et al., 1996) and nucleus accumbens (Ihalainen and Tanila, 2004). Further, clonidine blocked cocaine- (Florin et al., 1994) and stress-induced (Erb et al., 2000) increases in extracellular norepinephrine levels in rats (see also Anden et al, 1970). Conversely, yohimbine, an α2 antagonist, increased extracellular dopamine and norepinephrine in the prefrontal cortex (Tanda et al., 1996). The neurochemical effects of α2 agonists and antagonists parallel the differences between the acute and chronic effects of lofexidine on cocaine self-administration in the present study. For example, decreases in cocaine self-administration after acute administration of lofexidine (Fig. 1) likely reflect decreases in basal levels of extracellular dopamine after α2 receptor agonist administration (see Arnsten and Dudley, 2005; Tanda et al., 1996). The rightward and downward shift in the cocaine self-administration dose-effect curve during pretreatment with high doses of lofexidine (see Fig. 1) and increases in responding observed during pretreatment with low doses of lofexidine (see Table 1) is consistent with functional antagonism. In contrast, “tolerance” or desensitization of α2 adrenergic receptors (Baumann et al., 2004; Jimenez-Rivera et al., 2006) may develop during chronic lofexidine treatment which leads to decreased inhibitory tone and enhancement of prefrontal cortical or striatal activity (Gamo et al., 2010; Dennis et al., 1987; Fox et al., 2012). In the present study, this is reflected in the leftward shift in cocaine self-administration (Fig. 2) where lower doses of cocaine maintained higher levels of responding. Consistent with this notion, previous studies suggest that when norepinephrine is blocked acutely through α2 receptor activation, dopamine transmission and behavioral responses to stimulants appear to be attenuated (Jimenez-Rivera et al., 2006; Tanda et al., 1996). However, over time the dopamine system compensates for decreased dopamine levels by up-regulating high-affinity postsynaptic dopamine receptors (Schank et al., 2005; Seeman, 2005), resulting in behavioral hypersensitivity to psychostimulants and other direct dopamine agonists. In support of this, dopamine β-hydroxylase knockout mice, which lack norepinephrine, show hypersensitive responses to the locomotor, rewarding, and aversive effects of cocaine and amphetamine (Schank et al., 2005; Weinshenker, 2002).
Lofexidine has a half-life of about 11.5-hr in clinical studies (Al-Ghananeem, 2009) suggesting that delivery of the drug at the doses used in the present study (0.1 – 0.32 mg/kg/hr) on a mg/kg/hr basis may have resulted in cumulative, steady state doses that were significantly higher than those administered in the acute study. Interestingly, the development of tolerance during chronic lofexidine treatment appears to have developed rapidly as the leftward shift in cocaine self-administration was evident on Day 1 and stable throughout the course of lofexidine treatment (see above). Further, it should be noted that lower doses of lofexidine (0.01 – 0.032 mg/kg/hr) that would have resulted in mg/kg/day doses similar to the acute study were initially tested against the maintenance dose of cocaine with no evidence of antagonism. Thus, understanding the factors (e.g., route of administration, frequency of dosing, etc.) responsible for the development of tolerance or desensitization of α2 receptors during chronic agonist (i.e., lofexidine) treatment is an important direction for future studies to address.
4.2 Translational Implications for Lofexidine in Addiction Treatment
As noted earlier, the α2 agonist clonidine is often used to attenuate opioid withdrawal signs and symptoms during detoxification. However, it is generally recognized that clonidine is associated with several undesirable side effects such as sedation and hypotension. Lofexidine was not associated with adverse side effects during and after treatment, and may be an acceptable alternative to clonidine (Gowing et al., 1996). Further, preliminary studies suggest that α2 agonists such as lofexidine, may decrease stress- and cue-induced craving in cocaine (Fox et al., 2012; Jobes et al., 2011) and opiate abusers (Sinha et al., 2006). Alternatively, our finding that chronic lofexidine treatment increased cocaine self-administration by non-human primates suggests it may increase risk for cocaine abuse in cocaine and polydrug (cocaine + heroin) abusers. More research is necessary to understand how and under what conditions lofexidine may increase this risk.
Acknowledgments
Role of Funding Source
Funding for this study was provided in part by NIDA Grants R01-DA002519 and R01-DA026892; the NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
We thank Dr. Jack Bergman for helpful discussions on an earlier version of the manuscript. We thank Sherilyn Pappel, Meredith Mahnke, George Anderson, Olga Smirnova, and Kevin Costa for excellent technical assistance.
Footnotes
Contributors
Authors SJK and NKM designed the study and wrote the protocol. Authors SJK and PAF managed data collection. Author SJK undertook the statistical analyses, managed literature searches, and wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
No conflict declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anden NE, Corrodi H, Fuxe K, Hokfelt B, Kokfelt T, Rydin C, Svensson T. Evidence for a central noradrenaline receptor stimulation by clonidine. Life Sci. 1970;9:513–523. doi: 10.1016/0024-3205(70)90207-9. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Dudley AG. Methylphenidate improves prefrontal cortical cognitive function through α2 adrenoceptor and dopamine D1 receptor actions: relevance to therapeutic effects in Attention Deficit Hyperactivity Disorder. Behav Brain Funct. 2005;1:2. doi: 10.1186/1744-9081-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Milchanowski AB, Rothman RB. Evidence for alterations in α2-adrenergic receptor sensitivity in rats exposed to repeated cocaine administration. Neuroscience. 2004;125:683–690. doi: 10.1016/j.neuroscience.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Chopin P, Colpaert FC, Marien M. Effects of alpha-2 adrenoceptor agonists and antagonists on circling behavior in rats with unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway. J Pharmacol Exp Ther. 1999;288:798–804. [PubMed] [Google Scholar]
- Condelli WS, Fairbank JA, Dennis ML, Rachal J. Cocaine use by clients in methadone programs: significance, scope, and behavioral interventions. J Subst Abuse Treat. 1991;8:203–212. doi: 10.1016/0740-5472(91)90040-h. [DOI] [PubMed] [Google Scholar]
- Dennis T, L’Heureux R, Carter C, Scatton B. Presynaptic alpha-2 adrenoceptors play a major role in the effects of idazoxan on cortical noradrenaline release (as measured by in vivo dialysis) in the rat. J Pharmacol Exp Ther. 1987;241:642–649. [PubMed] [Google Scholar]
- Docherty JR. Subtypes of functional alpha1- and alpha2-adrenoceptors. Eur J Pharmacol. 1998;362:1–15. doi: 10.1016/s0014-2999(98)00682-7. [DOI] [PubMed] [Google Scholar]
- Erb SE, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-Induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Fivel PA. Computer-controlled drug doses for IV drug self-administration. Exp Clin Psychopharmacol. 2011;19:131–133. doi: 10.1037/a0023037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin SM, Kuczenski R, Segal DS. Regional extracellular norepinephrine responses to amphetamine and cocaine and effects of clonidine pretreatment. Brain Res. 1994;654:53–62. doi: 10.1016/0006-8993(94)91570-9. [DOI] [PubMed] [Google Scholar]
- Fox HC, Seo D, Tuit K, Hansen J, Kimmerling A, Morgan PT, Sinha R. Guanfacine effects on stress, drug craving and prefrontal activation in cocaine dependent individuals: preliminary findings. J Psychopharmacol (Oxford) 2012;26:958–972. doi: 10.1177/0269881111430746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo NJ, Wang M, Arnsten AFT. Methylphenidate and atomoxetine enhance prefrontal function through α2-adrenergic and dopamine D1 receptors. J Am Acad Child Adolesc Psychiatry. 2010;49:1011–1023. doi: 10.1016/j.jaac.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Gowing L, Farrell M, Ali R, White JM. Alpha2 Adrenergic Agonists for the Management of Opioid Withdrawal. John Wiley & Sons, Ltd; Chichester, UK: 1996. [DOI] [PubMed] [Google Scholar]
- Gowing LR, Farrell M, Ali RL, White JM. α 2-Adrenergic agonists in opioid withdrawal. Addiction. 2002;97:49–58. doi: 10.1046/j.1360-0443.2002.00037.x. [DOI] [PubMed] [Google Scholar]
- Highfield D. Repeated lofexidine treatment attenuates stress-induced, but not drug cues-induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology. 2001;25:320–331. doi: 10.1016/S0893-133X(01)00227-5. [DOI] [PubMed] [Google Scholar]
- Ihalainen JA, Tanila H. In vivo regulation of dopamine and noreadrenaline release by α 2A-adrenoceptors in the mouse prefrontal cortex. Eur J Neurosci. 2002;15:1789–1794. doi: 10.1046/j.1460-9568.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- Ihalainen JA, Tanila H. In vivo regulation of dopamine and noradrenaline release by alpha2A-adrenoceptors in the mouse nucleus accumbens. J Neurochem. 2004;91:49–56. doi: 10.1111/j.1471-4159.2004.02691.x. [DOI] [PubMed] [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology. 2011;218:83–88. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasanetz F, Lafourcade M, Deroche-Gamonet V, Revest JM, Berson N, Balado E, Fiancette JF, Renault P, Piazza PV, Manzoni OJ. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol Psychiatry. 2012;18:729–737. doi: 10.1038/mp.2012.59. [DOI] [PubMed] [Google Scholar]
- Kato S, Yanagita T. Comparison of the effects of several psychotropic drugs by gross behavioral observation on rhesus monkeys. Yakubutsu Seishin Kodo. 1981;1:29–37. [PubMed] [Google Scholar]
- Kosten TR, Kleber HD, Morgan C. Treatment of cocaine abuse with buprenorphine. Biol Psychiatry. 1989;26:637–639. doi: 10.1016/0006-3223(89)90090-5. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Xie X, Ramirez DR, Fuchs RA. Prefrontal cortical regulation of drug seeking in animal models of drug relapse. Curr Top Behav Neurosci. 2009;3:101–117. doi: 10.1007/7854_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmacological blockade of α2-adrenoceptors induces reinstatement of cocaine-seeking behavior in squirrel monkeys. Neuropsychopharmacology. 2003;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Lin SK, Strang J, Su LW, Tsai CJ, Hu WH. Double-blind randomised controlled trial of lofexidine versus clonidine in the treatment of heroin withdrawal. Drug Alcohol Depend. 1997;48:127–133. doi: 10.1016/s0376-8716(97)00116-6. [DOI] [PubMed] [Google Scholar]
- Lu H, Chefer S, Kurup PK, Guillem K, Vaupel DB, Ross TJ, Moore A, Yang Y, Peoples LL, Stein EA. fMRI response in the medial prefrontal cortex predicts cocaine but not sucrose self-administration history. NeuroImage. 2012;62:1857–1866. doi: 10.1016/j.neuroimage.2012.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz CK, Novak MA. Environmental enrichment for nonhuman primates: theory and application. ILAR J. 2005;46:178–191. doi: 10.1093/ilar.46.2.178. [DOI] [PubMed] [Google Scholar]
- Mello NK, Fivel PA, Kohut SJ, Bergman J. Effects of chronic buspirone treatment on cocaine self-administration. Neuropsychopharmacology. 2012;38:455–467. doi: 10.1038/npp.2012.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Newman JL. Discriminative and reinforcing stimulus effects of nicotine, cocaine, and cocaine + nicotine combinations in rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:203–214. doi: 10.1037/a0023373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Mello NK. Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend. 2003;70:39–52. doi: 10.1016/s0376-8716(02)00339-3. [DOI] [PubMed] [Google Scholar]
- Newman JL, Negus SS, Lozama A, Prisinzano TE, Mello NK. Behavioral evaluation of modafinil and the abuse-related effects of cocaine in rhesus monkeys. Exp Clin Psychopharmacol. 2010;18:395–408. doi: 10.1037/a0021042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone B, Hokfelt T. Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp Neurol. 1993;328:575–594. doi: 10.1002/cne.903280409. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Jaconetta SM, Halaris AE. Characterization of subtypes of alpha-2 adrenoceptors in the human brain. J Pharmacol Exp Ther. 1993;264:967–976. [PubMed] [Google Scholar]
- Jimenez-Rivera CAJ, Mojer MF, Torres RV. Alpha-noradrenergic receptors modulate the development and expression of cocaine sensitization. Ann N Y Acad Sci. 2006;1074:390–402. doi: 10.1196/annals.1369.039. [DOI] [PubMed] [Google Scholar]
- Schank JR, Ventura R, Puglisi-Allegra S, Alcaro A, Cole CD, Liles LC, Seeman P, Weinshenker D. Dopamine β-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2005;31:2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- Schottenfeld RS, Pakes J, Ziedonis D, Kosten TR. Buprenorphine: dose-related effects on cocaine and opioid use in cocaine-abusing opioid-dependent humans. Biol Psychiatry. 1993;34:66–74. doi: 10.1016/0006-3223(93)90258-f. [DOI] [PubMed] [Google Scholar]
- Seeman P. Dopamine supersensitivity correlates with D2High states, implying many paths to psychosis. Proc Natl Acad Sci. 2005;102:3513–3518. doi: 10.1073/pnas.0409766102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman GT, Lal H, Ursillo RC. Effectiveness of lofexidine in blocking morphine-withdrawal signs in the rat. Pharmacol Biochem Behav. 1980;12:573–575. doi: 10.1016/0091-3057(80)90191-4. [DOI] [PubMed] [Google Scholar]
- Sinha R, Kimmerling A, Doebrick C, Kosten TR. Effects of lofexidine on stress-induced and cue-induced opioid craving and opioid abstinence rates: preliminary findings. Psychopharmacology. 2006;190:569–574. doi: 10.1007/s00213-006-0640-8. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Aston-Jones G. α2 Adrenergic and Imidazoline Receptor Agonists Prevent Cue- Induced Cocaine Seeking. Biol Psychiatry. 2011;70:712–719. doi: 10.1016/j.biopsych.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suska A, Lee BR, Huang YH, Dong Y, Schlüter OM. Selective presynaptic enhancement of the prefrontal cortex to nucleus accumbens pathway by cocaine. Proc Natl Acad Sci. 2013;110:713–718. doi: 10.1073/pnas.1206287110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G. Chronic desipramine and fluoxetine differentially affect extracellular dopamine in the rat prefrontal cortex. Psychopharmacology. 1996;127:83–87. doi: 10.1007/BF02805978. [DOI] [PubMed] [Google Scholar]
- Tanda G, Bassareo V, Chiara D. Mianserin markedly and selectively increases extracellular dopamine in the prefrontal cortex as compared to the nucleus accumbens of the rat. Psychopharmacology. 1996;123:127–130. doi: 10.1007/BF02246169. [DOI] [PubMed] [Google Scholar]
- Uhlen S, Lindblom J, Tiger G, Wikberg JE. Quantification of alpha2A and alpha2C adrenoceptors in the rat striatum and in different regions of the spinal cord. Acta Physiol Scand. 1997;160:407–412. doi: 10.1046/j.1365-201X.1997.00175.x. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Drouin C, Bizot JC, Marien M, Glowinski J, Colpaërt F, Tassin JP. Stimulation of postsynaptic alpha1b- and alpha2-adrenergic receptors amplifies dopamine-mediated locomotor activity in both rats and mice. Synapse. 2003;50:277–284. doi: 10.1002/syn.10267. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Strain EC, Bigelow GE. Evaluation of the effects of lofexidine and clonidine on naloxone-precipitated withdrawal in opioid-dependent humans. Addiction. 2003;98:427–439. doi: 10.1046/j.1360-0443.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- Wee S, Mandyam CD, Lekic DM, KOOB GF. α1-Noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur Neuropsychopharmacol. 2008;18:303–311. doi: 10.1016/j.euroneuro.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinshenker D. Mice with chronic norepinephrine deficiency resemble amphetamine- sensitized animals. Proc Natl Acad Sci. 2002;99:13873–13877. doi: 10.1073/pnas.212519999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welberg L. Psychiatric disorders: CRF flicks a motivational switch. Nat Rev Neurosci. 2012;13:741–741. doi: 10.1038/nrn3364. [DOI] [PubMed] [Google Scholar]
- World Drug Report. United Nations Office on Drugs and Crime; New York: 2010. [Google Scholar]
- Yu E, Miotto K, Akerele E, O’Brien CP, Ling W, Kleber H, Fischman MW, Elkashef A, Herman BH, Al-Ghananeem AM. Clinical pharmacokinetics of lofexidine, the α 2-adrenergic receptor agonist, in opiate addicts plasma using a highly sensitive liquid chromatography tandem mass spectrometric analysis. Am J Drug Alcohol Abuse. 2008;34:611–616. doi: 10.1080/00952990802308122. [DOI] [PubMed] [Google Scholar]
- Zhang W, Klimek V, Farley JT, Zhu MY, Ordway GA. alpha2C adrenoceptors inhibit adenylyl cyclase in mouse striatum: potential activation by dopamine. J Pharmacol Exp Ther. 1999;289:1286–1292. [PubMed] [Google Scholar]