Abstract
BACKGROUND
STEP is a brain-specific protein tyrosine phosphatase that opposes the development of synaptic strengthening and the consolidation of fear memories. In contrast, stress facilitates fear memory formation, potentially by activating corticotrophin releasing factor (CRF) neurons in the anterolateral cell group of the bed nucleus of the stria terminalis (BNSTALG).
METHODS
Here, using dual-immunofluorescence, single-cell RT-PCR, quantitative RT-PCR, Western blot, and whole cell patch-clamp electrophysiology, we examined the expression and role of STEP in regulating synaptic plasticity in rat BNSTALG neurons, and its modulation by stress.
RESULTS
STEP was selectively expressed in CRF neurons in the oval nucleus of the BNSTALG. Following repeated restraint stress (RRS), animals displayed a significant increase in anxiety-like behavior, which was associated with a down-regulation of STEP mRNA and protein expression in the BNSTALG as well as selectively enhanced magnitude of long-term potentiation (LTP) induced in Type III, putative CRF neurons. To determine if the changes in STEP expression following RRS were mechanistically related to the facilitation of synaptic strengthening, we examined the effects of intracellular application of STEP on the induction of LTP. STEP completely blocked the RRS-induced facilitation of LTP in BNSTALG neurons.
CONCLUSIONS
Hence, STEP acts to buffer CRF neurons against excessive activation, while down-regulation of STEP after chronic stress may result in pathological activation of CRF neurons in the BNSTALG and contribute to prolonged states of anxiety. Thus, targeted manipulations of STEP activity might represent a novel treatment strategy for stress-induced anxiety disorders.
Keywords: striatal-enriched protein tyrosine phosphatase, STEP, corticotrophin releasing factor, CRF, bed nucleus of the stria terminalis, BNST, chronic stress, anxiety
Introduction
Striatal-enriched protein tyrosine phosphatase (STEP; also known as protein tyrosine phosphatase non-receptor type 5, PTPN5), is a brain-specific tyrosine phosphatase that is highly expressed in regions involved in learning and memory, such as the striatum, neocortex, amygdala, and hippocampus (1, 2), where it contributes to the regulation of synaptic plasticity and cognitive function (for review see (3)). Functionally, STEP inactivates several kinases by dephosphorylating a regulatory tyrosine (Tyr) within their activation loop. Target substrates include extracellular signal regulated kinase 1 and 2 (ERK1/2) (4), the stress-activated protein kinase p38 (4, 5), the Src family tyrosine kinase Fyn (6), and proline-rich tyrosine kinase 2 (7). In addition, STEP dephosphorylates regulatory Tyr residues in subunits of NMDA (GluN2B, formerly known as NR2B) and AMPA receptors, promoting internalization of GluN1/GluN2B and AMPA receptor complexes (3, 8, 9). Dephosphorylation of these proteins significantly attenuates the development of synaptic plasticity and the consolidation of memories (10–14).
Consistent with these findings, STEP blocks long-term potentiation (LTP) in amygdala slices (15), while STEP-deficient mice show facilitated LTP and amygdala-dependent fear conditioning (16). Moreover, knockdown of STEP in the dorsal hippocampus delayed physiological recovery from stress, whereas STEP overexpression enhanced resilience to stress (17). Consequently, it has been suggested that down-regulation of STEP function plays a role in the etiology of stress-induced anxiety disorders, such as post-traumatic stress disorder (PTSD) and generalized anxiety disorder (17).
Two regions that play a critical role in regulating fear and anxiety-like behavior in response to stress stimuli are the central nucleus of the amygdala (CeA) and the bed nucleus of the stria terminalis (BNST) (18). Both regions contain a high density of neurons that express the stress hormone, corticotrophin releasing factor (CRF) (19, 20). CRF neurons in the paraventricular nucleus of the hypothalamus (PVN) mediate the classic endocrine response to stress; while activation of CRF neurons in the BNST is thought to initiate the behavioral response to stress (18), and stress is known to modulate synaptic plasticity in the BNST (21, 22). The BNST is a heterogeneous structure consisting of several distinct nuclei, which differentially regulate the stress axis (23) and anxiety-like behavior (24). Here, we have focused on the anterolateral cell group of the BNST (BNSTALG), which contains a high density of CRF neurons (19, 25, 26) and is the only division showing somatodendritic immunoreactivity for STEP.
Three distinct neuronal subtypes exist in the BNSTALG, Type I-III (27), of which Type III neurons are thought to be CRF neurons (26, 28). However, little is known about the mechanisms that regulate synaptic plasticity in Type I-III neurons or the potential role of STEP in these mechanisms. In the current study, we employed a combination of immunohistochemical, molecular, electrophysiological, and behavioral techniques to examine the relative expression, distribution and potential function of STEP in Type I-III neurons of the BNSTALG from control and stressed animals.
Material and Methods
Animal subjects
All experiments were performed in adult (45–60 days old, n=78) male, Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA). For stereotaxic surgery and colchicine injections, rats were anaesthetized with an intraperitoneal injection of dexdormitor (0.16 mg/kg; Pfizer Animal Health, New York, NY, USA) and ketamine hydrochloride (48 mg/kg; Butler-Schein Animal Health, Dublin, OH, USA). All procedures were approved by the Institutional Animal Care and Use Committees of Emory University and were in compliance with National Institutes of Health guidelines. Animals were housed in same sex groups, 4 animals per cage and were maintained on a 12:12-hr light–dark cycle with ad libitum access to food and water. Animals were housed at least 1 week after arrival. Separate cohorts of control, non-stressed (NS) and repeated restraint stressed (RRS) rats were used for each experiment.
Detailed information on immunofluorescence, dual-immunofluorescence protocols and confocal microscopy, single-cell RT-PCR (scRT-PCR) from physiologically defined Type I-III BNSTALG neurons, acoustic startle response (ASR) testing, quantitative RT-PCR (qtRT-PCR) of BNSTALG tissue samples from control and stressed rats, Western blot on the BNSTALG from control and stressed rats, scRT-PCR of physiologically defined Type I-III BNSTALG neurons from control and stressed rats, subcellular fractionation and NMDAR subunits expression (GluN1/GluN2B) in BNSTALG of control and stressed rats, in vitro whole cell patch clamp electrophysiology in BNST of control and stressed rats is provided in Supplementary Information.
Repeated restraint stress (RRS) experiments
A standard RRS protocol was utilized to mimic the behavioral effects of chronic stress. RRS rats (n=36) were first tested for their baseline acoustic startle response (pretest ASR, day 1) and then subjected to 1-hour per day restraint in wire-mesh restrainers for 4 consecutive days (days 1–4), and then left undisturbed in their home cages for 6 days until behavioral testing on day 10. Control non-stressed rats (NS, n=36) were tested for ASR and were then left undisturbed. On day 10, all RRS and NS animals were retested for their ASR (posttest) and then BNST samples were collected (following decapitation after isoflurane anesthesia) and assayed for protein expression (n=4 per group), qtRT-PCR (n=4), scRT-PCR (n=4), subcellular fractionation (n=8), and patch-clamp electrophysiology (n=16).
Statistical analysis
All values were expressed as mean ± SEM. Statistical analyses for Western blots and quantitative RT-PCR were performed using t-Test. Two-way ANOVA was performed for the behavioral experiments with treatment (NS and RRS) and time (pre-test and post-test) as factors. Two-way ANOVA with repeated measures was performed for the electrophysiological experiments with treatment (NS and RRS), and time as factors using Graphpad Prism 4.0 software.
Results
STEP is expressed in the extended amygdala
We first conducted an immunofluorescence study to determine the relative distribution of STEP protein in the extended amygdala. Consistent with previous studies (15), high levels of somatodendritic labeling were observed in the lateral subdivision of the CeA (CeAl, Fig. 1A′). High levels of somatodendritic STEP labeling was also observed in the BNSTALG, where the highest immunoreactivity was seen in its subdivisions, the oval (BNSTov) and juxtacapsular nuclei (BNSTjxt, Fig. 1B′). High levels of STEP-immunolabeling were also observed in the postero-lateral division of the BNST, where it was mainly restricted to the neuropil. As expected, very high levels of STEP labeling were also observed in the adjacent striatum (Fig. 1B′), as well as in the lateral septum (data not shown).
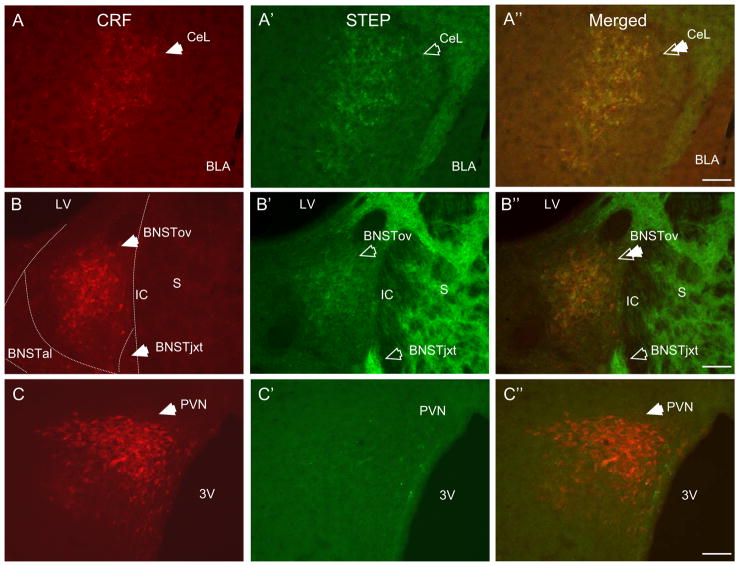
Figure 1. STEP and CRF immunoreactivity co-localize in neurons of the CeAl, BNSTALG, but not PVN.
A-A″ CRF (A, red, closed arrow) and STEP (A′, green, open arrow) show high somatodendritic immunoreactivity in the CeAl. Dual-label immunofluorescence revealed almost complete co-localization of STEP with CRF in neurons of the CeAl (A″, double arrow). B-B″ A similar pattern of co-localization was observed in neurons of the BNSTALG subdivision BNSTov, but not BNSTjxt. C-C″ STEP did not co-localize with CRF in neurons of the PVN. (S-Striatum, LV-Lateral ventricle, IC-Internal capsule, AC-Anterior commissure, BNSTALG-Anterolateral cell group of the BNST, BNSTov-Oval nucleus of the BNST, BNSTjxt-Juxtacapsular nucleus of the BNST, BNSTal-Anterolateral nucleus of the BNST, CeAl-Lateral division of the central nucleus of the amygdala, BLA-Basolateral nucleus of the amygdala, 3V-3rd ventricle, 10x, scale bar 100 μm).
STEP is co-localized with CRF in neurons of the extended amygdala
Since the CeAl and the BNSTALG are two regions that contain a high density of CRF neurons (19, 25), we next performed a dual-immunofluorescence study to determine whether STEP was co-localized with CRF in neurons of the extended amygdala. The paraventricular nucleus of the hypothalamus (PVN) also contains high numbers of CRF neurons. Hence, we compared STEP expression in the PVN and the extended amygdala. Consistent with previous studies (19, 20), high somatodendritic CRF immunoreactivity was observed in the CeAl (Fig. 1A), BNSTALG subdivisions - the BNSTov and fusiform nuclei (Fig. 1B), and the parvocellular PVN (Fig. 1C). Little or no expression of CRF immunoreactivity was observed in the BNSTjxt (19) (Fig. 1B). Dual-immunolabeling revealed almost complete co-localization of STEP with CRF in neurons of the BNSTov and CeAl, such that STEP was co-expressed by 98% of CRF neurons in the BNSTov, and by 94% of CRF neurons in the CeAl. Moreover, 97% of STEP-positive neurons co-expressed CRF in the BNSTov, and 96% in the CeAl (Fig. 1A″, 1B″, 2A″). In contrast, limited STEP labeling was observed in the PVN (Fig. 1C′).
The high level of STEP expression in BNSTjxt indicated that STEP in the BNSTALG might also be expressed in subpopulations of non-CRF neurons. Several neuropeptides including, but not limited to, NPY, ENK, and SOM are expressed by BNSTALG neurons (29, 30). However, although high levels of NPY- and ENK-immunolabeling were observed in the BNSTov and BNSTjxt (Fig. 2B, 2C), neither peptide was seen to co-localize with STEP (Fig. 2B″, 2C″). In contrast, ENK-positive processes in the BNSTov and BNSTjxt were often seen to make perisomatic contacts with STEP-positive neurons (Fig. 2C″).
Figure 2. In the BNSTov STEP immunoreactivity co-localizes with CRF but not NPY or ENK.
A-A″ At higher magnification CRF-positive cells (A, closed arrows) and STEP-positive (A′, open arrows) showed almost complete co-localization in the BNSTov (A″, double arrows). However, there are occasional STEP-immunoreactive neurons which do not appear to co-localize CRF (A″, open arrows). B-B″ STEP-positive neurons (B′, green, open arrows) do not co-localize with NPY (B, red, closed arrows), or ENK (C-C″). ENK-positive processes are observed to make perisomatic contacts with STEP-expressing neurons (C″, double arrow). (NPY-Neuropeptide Y, ENK-Enkephalin, 63x, scale bar 10 μm).
STEP is co-localized with its downstream substrates in the BNSTALG
We next examined the co-localization of STEP protein with its substrates and associated proteins. High levels of GluN1, p38, ERK1/2 (Fig. 3A′, B′, C′), and Fyn immunolabeling were observed throughout the BNSTALG, and dual-immunofluorescence revealed that nearly all STEP-positive neurons in the BNSTov co-localized with GluN1 (Fig. 3A″), p38 (Fig. 3B″), and ERK1/2 (Fig. 3C″). Fyn-immunolabeling was mainly observed in the neuropil of the BNSTALG (not shown) and, hence, it was difficult to unequivocally co-localize Fyn with STEP. Significantly, the most intense immunolabeling for ERK1/2 was observed in BNSTov neurons that did not co-localize STEP (Fig. 3C-C″). Similarly, numerous GluN1- and p38 MAPK-positive neurons were observed that did not express STEP (Fig. 3A″, 3B″). Nevertheless, these results indicate that STEP-positive neurons in the BNSTov express many STEP substrates.
Figure 3. STEP-positive neurons in the BNSTov co-localize several known STEP substrates.
A-A″ All STEP-positive neurons (A, green, open arrows) co-localize GluN1 (A′, red, closed arrows) in the BNSTALG (A″, merged, double arrows) but the majority of GluN1-immunoreactive neurons do not co-localize STEP (A″, merged, closed arrows). B-B″ Virtually all STEP-positive neurons in the BNSTALG (B, open arrows) co-localize p38 (B′, red, closed arrows). However, numerous p38-positive neurons do not co-localize STEP (B″, closed arrows). C-C″ Nearly all STEP-positive neurons of the BNSTALG (green, open arrows, C) co-express ERK1/2 (C′, closed arrows). However, the highest ERK1/2 immunoreactivity was observed in BNST neurons that do not express STEP (C″, closed arrows). In addition, some STEP-positive cells do not express ERK 1/2 (C″, open arrows).
STEP is co-expressed with its substrates in Type III BNSTALG neurons
We then used scRT-PCR to examine mRNA transcript expression in electrophysiologically defined Type I-III neurons of the BNSTALG. Results from scRT-PCR confirmed the dual-immunofluorescence results and showed that 100% of Type III, putative CRF neurons, expressed mRNA transcripts for STEP, whereas 30% of Type II neurons, and no Type I neurons expressed STEP mRNA. Notably, in contrast to our immunofluorescence study, STEP mRNA was found in 71% of PVN neurons that also co-expressed CRF mRNA.
Examination of mRNA transcript expression for STEP substrates and associated proteins revealed that transcripts for three subunits of the NMDA receptor (GluN1, GluN2A, and GluN2B) and STEP substrates (p38, ERK1/2, and Fyn), showed a differential expression pattern in Type I-III neurons. Thus, transcripts for GluN1 and GluN2A were preferentially expressed in Type III putative CRF neurons (60%), while transcripts for GluN2B subunit were found only in 10% of these neurons. In contrast, GluN2B subunit was expressed in 50% of Type II neurons. Moreover, the majority of Type I (70%) and II neurons (80%) expressed mRNA for ERK 1/2, while transcripts for Fyn and p38 were not detected in Type I neurons and were found at low levels in Type II neurons. In contrast, 100% of Type III neurons expressed mRNA transcripts for ERK1/2, 70% expressed transcripts for Fyn, and 30% expressed transcripts for p38. The results of the scRT-PCR study, summarized in Table S2 (see Supplementary Information), suggest that STEP in Type III, putative CRF neurons may act to selectively regulate the activity of GluN1, GluN2A, ERK1/2, and Fyn.
RRS down-regulates STEP mRNA levels in the BNSTALG
Previously, we have shown that RRS (31) and repeated unpredictable shock stress (32) cause an increase in anxiety-like behavior in rats, as measured by an increase in the acoustic startle response (ASR) on day 10 following stress onset. We now examined whether this effect was associated with alterations in STEP levels (mRNA and protein) in the BNSTALG. Consistent with previous observations (31), there was a significant effect of treatment (RRS), such that on day 10, stressed rats exhibited a significant increase in ASR as compared to NS rats (F(59,180)=1.94, p=0.0005, n=24, two-way ANOVA, NS pos-test 0.797±0.14, RRS posttest 0.911±0.06). There was also a significant effect of time on ASR (RRS pre-test 0.671±0.13, RRS post-test 0.911±0.06, F(1,180)=14.70, p=0.0002, n=24, two-way ANOVA), but there was no significant interaction between time and treatment (F(29,180)=0.64, p=0.9748, two-way ANOVA, Fig. 4A).
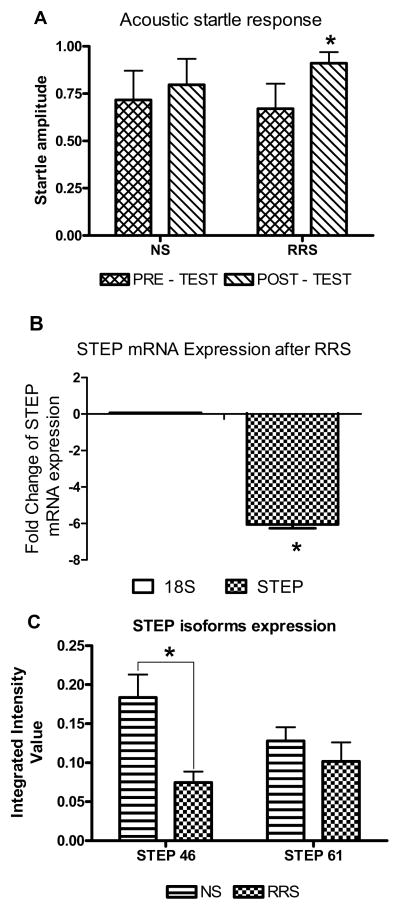
Figure 4. Repeat restraint stress (RRS) causes an increase in anxiety-like behavior and a concomitant decrease in STEP mRNA and protein expression in the BNSTALG.
A: A plot of the acoustic startle response (ASR) before (pretest, day 0) and 10 days after RRS onset (posttest). On day 10 (six days after the termination of RRS), stressed rats exhibited a significant increase in ASR as compared to NS rats (F(59, 180)=1.94, p=0.0005, n=24, two-way ANOVA). There was also a significant effect of time on ASR (F(1, 180)=14.70, p=0.0002, n=24, two-way ANOVA), but there was no significant interaction between time and treatment (F(29, 180)=0.64, p=0.9748, two-way ANOVA). B: A histogram showing the effects of RRS on STEP mRNA expression levels in the BNSTALG. STEP mRNA was measured by real-time RT-PCR normalized to 18S RNA expression, thus we have analyzed mean fold change expressed as ΔΔCts between NS and RRS groups. Quantitative RT-PCR revealed that RRS caused a significant (6-fold) decrease in STEP mRNA levels compared to non-stressed rats (p<0.05, n=4). C: A histogram showing the effect of RRS of STEP protein levels in the BNSTALG. Western blot analysis showed that RRS significantly reduced expression of the cytosolic 46 kDa STEP isoform, but not the 61 kDa membrane-bound STEP isoform (p<0.005, n=4).
Next, we ran qtRT-PCR on mRNA isolated from the BNSTALG of NS and RRS rats to determine the effects of stress on STEP mRNA expression on day 10. STEP mRNA was measured by real-time RT-PCR normalized to 18S RNA and the mean fold change in STEP expression was calculated according to the formula ΔΔCT= (CT STEP - CT18S)RRS - (CT STEP - CT18S)NS (33). RRS caused a significant (6-fold, p<0.05) reduction in STEP mRNA transcript expression in the BNSTALG compared to NS animals on day 10 following the stress onset (Fig. 4B).
RRS down-regulates STEP mRNA expression in Type III BNSTALG neurons
Next, we ran scRT-PCR experiments on samples obtained from BNSTALG neurons of NS and RRS rats to determine if RRS could differentially regulate STEP mRNA expression in Type I-III neurons. Consistent with our previous observations, STEP mRNA was expressed in 100% of Type III neurons in the NS group (14/14), and in 27% of Type II neurons (7/26), but was not detected in Type I neurons (0/15). After RRS the number of Type III neurons expressing STEP mRNA decreased significantly to 29% of all Type III neurons tested (4/14). The number of Type II neurons expressing STEP also decreased to 12% of all neurons tested (3/25). RRS had no effect on STEP mRNA expression in Type I neurons (0/12). These results suggest that RRS reduced STEP mRNA expression preferentially in Type III, putative CRF neurons.
RRS down-regulates STEP46 protein content in the BNSTALG
We then examined if RRS had a similar effect on STEP protein levels and/or its substrates. Alternative splicing of the STEP gene produces two main products, a 46-kDa cytosolic isoform and a 61-kDa membrane associated isoform (2). Although both STEP isoforms were present in BNSTALG tissue from NS and RRS rats (Fig. 5A), RRS caused a significant reduction (2-fold) in levels of STEP46 but not STEP61 (NS STEP46 IIV=0.18±0.03, RRS IIV=0.07±0.01, p<0.008; NS STEP61 IIV=0.13±0.02, RRS IIV=0.10±0.02, p=0.2, Fig. 4C, Fig. 5A). We also analyzed protein expression of STEP substrates in BNSTALG samples obtained from the same NS and RRS rats. No significant difference was observed in the total protein level for any of the STEP substrates following RRS (Supplementary Information). Finally, we examined whether protein levels for STEP46 correlated with individual measures of ASR on day 10 (post-test). No significant correlation was observed between STEP46 and the ASR in the control NS rats (r2=0.428, p>0.05). However, in RRS-rats, STEP46 levels showed a significant negative correlation with the post-test ASR (r2=−0.932, p<0.05), such that the highest ASR amplitudes were observed in rats with the lowest STEP46 protein levels, suggesting that reduced STEP levels might lead to increased ASR.
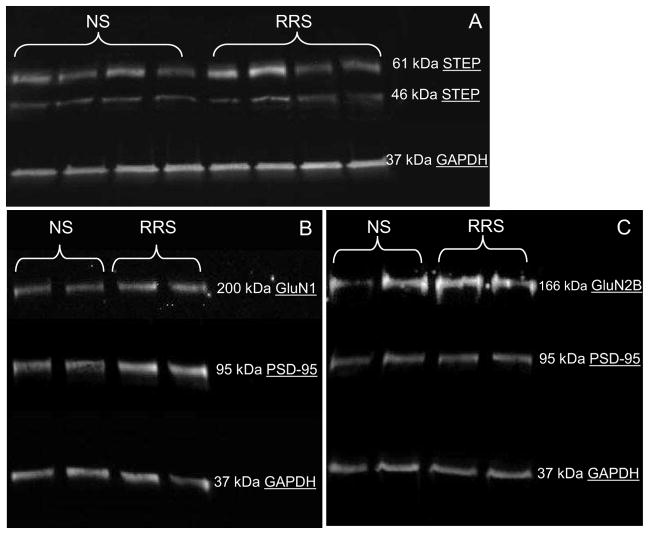
Figure 5. A . RRS significantly down-regulates STEP protein content in the BNSTALG. B–C. RRS up-regulates protein content of the NMDAR subunits in the synaptic membranes of the BNSTALG neurons.
Representative Western blot showing that RRS significantly reduced expression of the cytosolic STEP46 isoform (p<0.005, n=4, A). RRS caused a significant up-regulation in protein expression of the GluN1 subunit of the NMDAR in synaptic membranes fraction of the BNSTALG neurons on day 10 (n=8, p<0.05, B), and non-significant increase in GluN2B subunit of the NMDAR (n=8, p=0.08, C).
RRS up-regulates protein expression of NMDAR subunits in synaptic membranes of the BNSTALG neurons
Although we did not observe an effect of RRS on total protein levels of STEP substrates in the BNSTALG, we next examined whether RRS could alter the subcellular localization of GluN1 and GluN2B subunits of NMDAR in the BNSTALG neurons. RRS caused significant increase in protein expression of the GluN1 subunit of the NMDAR in synaptic membranes fraction of the BNSTALG neurons on day 10 (NS IIV=0.50±0.05, RRS IIV=0.82±0.10, p<0.05, Fig. 5B) and non-significant increase in GluN2B subunit of the NMDAR (NS IIV=0.91±0.22, RRS IIV=1.32±0.28, p=0.08, Fig. 5C).
RRS facilities synaptic plasticity of Type III neurons of the BNSTALG
We next examined whether RRS could directly affect either basic membrane properties of Type I-III neurons, or basal excitatory synaptic transmission onto these neurons. As shown in Table S3 (see Supplementary Information), RRS had no significant effect on any intrinsic membrane property measured in Type I-III neurons (p>0.05). Similarly, RRS had no significant effect on the amplitude of the eEPSC evoked in response to low frequency stimulation (0.2 Hz) of the afferent input (NS=136±19 pA, n=13; RRS=141±23 pA, n=6, p>0.05). Consequently, we next examined if RRS-induced alterations in STEP protein expression had any correlation with the induction of LTP in Type I-III BNSTALG neurons.
As illustrated in Figure 6, HFS induced a significant increase in the amplitude of the eEPSC in all neurons recorded (183%, 185%, and 169% of baseline in Type I-III neurons, respectively, Fig. 6A–C). Moreover, two-way ANOVA revealed a significant difference in the magnitude of LTP achieved in Type III neurons compared to Type I (F(1,400)=4.9, p<0.05) and Type II neurons (F(1,560)=7.11, p<0.01). These data suggest that STEP might buffer Type III CRF neurons against excessive activation. Notably, significant differences were also observed in the magnitude of the LTP response of Type I-III neurons following RRS (176%, 192%, and 230% of baseline, respectively). Hence, RRS caused no significant change in the magnitude of LTP achieved at any time point tested in either Type I (F(39,520)=0.19, p=1.00, NS n=7, RRS n=8, Fig. 6A), or in Type II neurons (F(39,520)=0.31, p=1.00, NS n=5, RRS n=10, two-way ANOVA, Fig. 6B) compared to the NS group. However, in Type III neurons RRS caused a significant increase in the magnitude of LTP achieved (F(1,440)=93.88, p<0.001, NS n=5, RRS n=8, two-way ANOVA, Fig. 6C), although there was no significant interaction between time and LTP amplitude compared to NS group (F(39,440)=0.27, p=1.00, two-way ANOVA). Thus, reduced STEP expression in Type III neurons following RRS may make them susceptible to hyperactivation by strong synaptic input.
Figure 6. RRS significantly facilitates long-term potentiation (LTP) in Type III but not Type I or II neurons, and this effect could be blocked by exogenous STEP application.
A: Effect of high frequency stimulation of the stria terminalis (HFS, five 100 Hz, 1s trains, interval 20s) on EPSC amplitude in Type I BNSTALG neurons. EPSC amplitude was normalized to the mean amplitude of the 15 min baseline eEPSC and expressed as the percentage of baseline. In Type I neurons RRS had no effect on the magnitude of LTP level at any time point (F (39, 520) = 0.19, p=1.00, Two-way ANOVA). B: RRS caused no enduring change in LTP magnitude at any time point in Type II neurons (F (39, 520) = 0.31, p=1.00, Two-way ANOVA) in comparison to NS group. C: In Type III neurons, RRS significantly increased the magnitude of LTP (F (1, 440) = 93.88, p<0.001, Two-way ANOVA). D: Administration of exogenous wild-type STEP protein (300 nM) in the patch-recording pipette completely blocked the RRS-induced facilitation of LTP (p<0.05). Application of the wild-type STEP protein had no effect on the magnitude of LTP induced in non-stressed rats.
STEP blocks RRS-induced facilitation of the synaptic plasticity of BNSTALG neurons
We next examined whether intracellular administration of exogenous STEP protein via the patch-recording pipette might attenuate LTP induction in BNSTALG neurons. HFS induced a significantly greater LTP in neurons from RRS rats (214% of baseline values, n=7, Fig. 6D) as compared to control, NS rats (166%, n=6, p<0.05). Intracellular infusion of wild-type STEP protein (TAT-WT-STEP46; 300 nM) had no significant effect on either the induction or maintenance of LTP in neurons recorded from NS rats (156%, n=7). However, inclusion of TAT-WT-STEP46 abolished the facilitation of LTP magnitude induced by RRS (157% of baseline, n=7, p<0.01 Fig. 6D). Therefore, reduced STEP expression in Type III neurons in response to RRS may result in a significant and long-lasting facilitation of LTP, and that this effect could be reversed by intracellular application of exogenous STEP46.
Discussion
We demonstrate for the first time that the protein tyrosine phosphatase STEP is expressed at high levels in the subdivisions of the BNSTALG, especially in the BNSTov and BNSTjxt nuclei. STEP appears to selectively co-localize with CRF neurons of the BNSTov and in the CeAl, but not in CRF neurons of the PVN. Furthermore, STEP neurons in the BNSTov express mRNA and protein for several downstream substrates of STEP, including the synaptic plasticity-related kinases ERK1/2, p38, and Fyn. Thus, STEP may function to selectively regulate synaptic plasticity in CRF neurons of the BNSTALG. Notably, we have shown that RRS caused an elevation in ASR on day 10 following the stress onset, which was associated with a concomitant 6-fold reduction in STEP mRNA expression and a 2-fold reduction in STEP protein levels in the BNSTALG, as well as up-regulation of GluN1 subunit of NMDAR in the synaptic membranes of BNSTALG neurons, suggesting that RRS may significantly impair the ability of STEP to regulate synaptic plasticity in CRF neurons of the BNSTALG. We further show that RRS was associated with a cell-type selective reduction of STEP mRNA and facilitation of LTP in Type III CRF neurons, while intracellular application of exogenous STEP prevented the RSS-induced facilitation of synaptic plasticity in BNSTALG neurons.
STEP co-expression with peptide neurotransmitters and target substrates
Dual-immunofluorescence and scRT-PCR indicate that STEP was preferentially expressed in CRF neurons and not with other neuropeptides in the BNSTov. STEP protein was also co-localized with CRF in the CeAl but not in the PVN, suggesting that STEP expression is a marker for CRF neurons in the extended amygdala. However, scRT-PCR showed that the majority of CRF neurons in the PVN expressed STEP mRNA transcripts, suggesting that in the PVN STEP mRNA might be translated into mature protein only under certain physiological conditions, e.g. following prolonged activation during stress. Discrepancy between mRNA expression (34) and mature protein levels (35) has been shown in certain neuronal populations before.
STEP requires NMDA-dependent dephosphorylation by calcineurin/PP1 to become associated with its substrates (5). Once activated, STEP reduces NMDA receptor function by dephosphorylating GluN2B subunit at Tyr1472 (36). However, our scRT-PCR demonstrated that in contrast to GluN1A and GluN2A subunits, which were expressed by majority of Type III, putative CRF neurons, the GluN2B subunit was only expressed at low levels in Type III neurons. Although it is not yet known if the GluN1 or GluN2A subunits are also STEP substrates, in vitro studies have shown that STEP promotes internalization of GluN1/GluN2A receptor complexes (37).
STEP-positive neurons in the BNSTALG express several STEP substrates, such as ERK1/2, Fyn, and p38. Each of these kinases is activated by the NMDA receptor-signaling cascade and normally facilities synaptic plasticity (5, 9, 36). These data suggest that the presence of STEP in CRF neurons of the extended amygdala could function to suppress the activity of key modulators of synaptic plasticity (Fig. 7).
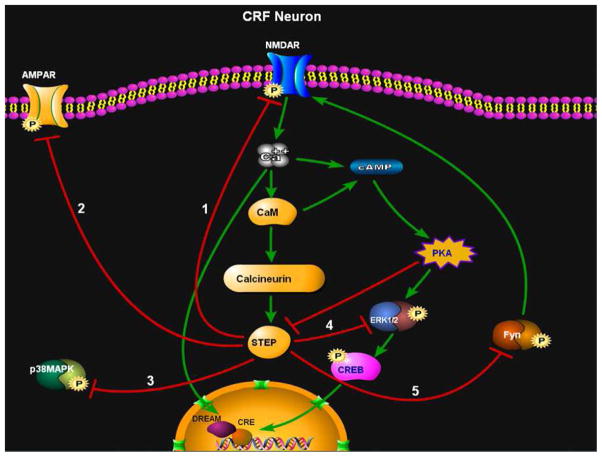
Figure 7.
A schematic showing the proposed signaling cascade by which activation of STEP could regulate the development of synaptic plasticity in CRF neurons of the BNSTALG. Following NMDA receptor-dependent activation, STEP dephosphorylates tyrosine residues in the activation domains of 1) NMDA receptors, 2) AMPA receptors, 3) p-38, 4) ERK1/2, and 5) Fyn kinase. Dephosphorylation of these key STEP substrates would put a significant temporal constraint on the induction of synaptic plasticity in these neurons. Green arrows = activation. Red lines = inhibition.
Stress modulation of STEP signaling in the BNSTALG
Glutamatergic activation of neurons in the BNSTALG mediates sustained anxiety-like responses to long-duration, but not short-duration, threats (38). Notably, CRF neurons in the BNSTALG are associated with the affective component of the stress response (39–41), and chronic over-expression of CRF in the BNSTALG is reported to increase depressive- (42), as well as anxiety-like behaviors in rats (43). Thus, stress-induced activation of CRF neurons in the BNSTALG could result in long-lasting anxiety-like behavior. Here, we have shown that RRS increased the baseline ASR in rats 10 days after stress onset, which was strongly positively correlated with a reduction in STEP protein expression in the BNSTALG. However, while a correlation between reduced STEP levels and ASR potentiation following RRS is apparent, we have not yet proven a cause-effect relationship. Confounds associated with post-surgical stress have hindered progress on this issue.
Importantly, while RRS resulted in a 6-fold reduction in STEP mRNA expression and a 2-fold reduction in STEP46 expression, our scRT-PCR indicated that STEP mRNA was preferentially down-regulated in Type III, putative CRF neurons in the BNSTALG. In agreement with our results, stress-susceptible rats express lower levels of STEP mRNA and protein, and show lower STEP enzymatic activity in the hippocampus compared to non-stressed and stress-unsusceptible rats (17).
At a functional level, we showed that RRS had no effect on basal synaptic transmission, but significantly increased the magnitude of LTP induced in Type III, putative CRF neurons of the BNSTALG. Consistent with our findings, STEP-KO mice show normal basal synaptic transmission in the amygdala, but enhanced LTP (16), and inhibition of endogenous STEP enhanced both stimulus-evoked NMDA-mediated EPSCs and LTP in the hippocampus (44). Moreover, the enhanced LTP after RRS is consistent with decreased STEP expression in the BNSTALG and an associated up-regulation in the expression of GluN1 subunit of NMDAR in synaptic membranes of the BNSTALG neurons. A possible cause-effect relationship between STEP expression and the modulation of postsynaptic NMDAR is suggested by the observation that intracellular STEP46 infusion completely blocked the facilitation of LTP observed in RRS rats.
Consistent with this observation, administration of a substrate-trapping STEP protein (STEP C-S), which prevents ERK translocation to the nucleus, disrupted LTP formation in the amygdala, as well as fear memory consolidation in vivo (15), suggesting that STEP plays a key role in regulating experience-dependent synaptic plasticity. We propose that in the BNSTALG, STEP selectively buffers CRF neurons against hyper-activation by acute stress. However, chronic stress-induced down-regulation of STEP could facilitate synaptic plasticity, prolong activation of CRF neurons, and contribute to an increase in anxiety-like behavior. Consistent with this observation, selective ablation of the GABA(A)α1 subunit in CRF neurons increased anxiety-like behavior and impaired extinction of conditioned fear, coincident with elevated plasma corticosterone levels (45).
Intriguingly, acute stressors are reported to preferentially activate enkephalinergic and not CRF neurons in the BNST (46). Our results suggest that, unlike ENK or NPY neurons, STEP may selectively buffer CRF neurons from significant activation in response to acute stressors. Indeed, only chronic and not acute stress is reported to increase CRF mRNA and peptide expression in the BNST (47, 48).
In conclusion, the present study demonstrates that STEP expression is uniquely associated with CRF neurons of the BNSTov and CeAl, and that STEP-positive neurons in the BNSTov also co-localize key molecules necessary for the regulation of synaptic plasticity. Notably, we demonstrate the behavioral (increased anxiety), molecular (down-regulation of STEP expression and up-regulation of GluN1 expression), as well as physiological (facilitated synaptic plasticity of CRF neurons) changes in the BNSTALG in response to RRS. We propose that chronic stress leads to activation of CRF neurons in the BNSTALG, which could then initiate anxiety-like behavior as a behavioral manifestation of chronic stress. Hence, therapy targeted at increasing levels of STEP46 in the BNSTALG neurons might have potential implications for treatment of chronic stress-induced anxiety disorders.
Supplementary Material
Acknowledgments
We would like to thank laboratory members for helpful discussions and critical reading of the manuscript. Especially, we would like to thank David E. Ehrlich and Steven J. Ryan for helping with rat brains dissection. This work was supported by MH-072908 to DGR, and MH-01527 to PJL from the National Institute of Mental Health (NIMH), and by the National Center for Research Resources P51RR169, which is supported by the Office of Research Infrastructure Programs/OD P51OD11107. JD is supported by NIMH K99MH-096746 grant.
Footnotes
Conflict of interest statement
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lombroso PJ, Murdoch G, Lerner M. Molecular characterization of a protein-tyrosine-phosphatase enriched in striatum. Proc Natl Acad Sci U S A. 1991;88:7242–7246. doi: 10.1073/pnas.88.16.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goebel-Goody SM, Baum M, Paspalas CD, Fernandez SM, Carty NC, Kurup P, et al. Therapeutic implications for striatal-enriched protein tyrosine phosphatase (STEP) in neuropsychiatric disorders. Pharmacol Rev. 2012;64:65–87. doi: 10.1124/pr.110.003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Munoz JJ, Tarrega C, Blanco-Aparicio C, Pulido R. Differential interaction of the tyrosine phosphatases PTP-SL, STEP and HePTP with the mitogen-activated protein kinases ERK1/2 and p38alpha is determined by a kinase specificity sequence and influenced by reducing agents. Biochem J. 2003;372:193–201. doi: 10.1042/BJ20021941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen TH, Liu J, Lombroso PJ. Striatal enriched phosphatase 61 dephosphorylates Fyn at phosphotyrosine 420. J Biol Chem. 2002;277:24274–24279. doi: 10.1074/jbc.M111683200. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Kurup P, Bartos JA, Patriarchi T, Hell JW, Lombroso PJ. Striatal-enriched Protein-tyrosine Phosphatase (STEP) Regulates Pyk2 Kinase Activity. J Biol Chem. 2012;287:20942–20956. doi: 10.1074/jbc.M112.368654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang R, Yamada J, Hayashi Y, Wu Z, Koyama S, Nakanishi H. Inhibition of NMDA-induced outward currents by interleukin-1beta in hippocampal neurons. Biochem Biophys Res Commun. 2008;372:816–820. doi: 10.1016/j.bbrc.2008.05.128. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Kurup P, Xu J, Carty N, Fernandez SM, Nygaard HB, et al. Genetic reduction of striatal-enriched tyrosine phosphatase (STEP) reverses cognitive and cellular deficits in an Alzheimer’s disease mouse model. Proc Natl Acad Sci U S A. 2010;107:19014–9019. doi: 10.1073/pnas.1013543107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fadool DA, Holmes TC, Berman K, Dagan D, Levitan IB. Tyrosine phosphorylation modulates current amplitude and kinetics of a neuronal voltage-gated potassium channel. J Neurophysiol. 1997;78:1563–1573. doi: 10.1152/jn.1997.78.3.1563. [DOI] [PubMed] [Google Scholar]
- 11.Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Trepanier CH, Jackson MF, MacDonald JF. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2012;279:12–19. doi: 10.1111/j.1742-4658.2011.08391.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu YF, Kojima N, Tomizawa K, Moriwaki A, Matsushita M, Obata K, et al. Enhanced synaptic transmission and reduced threshold for LTP induction in fyn-transgenic mice. Eur J Neurosci. 1999;11:75–82. doi: 10.1046/j.1460-9568.1999.00407.x. [DOI] [PubMed] [Google Scholar]
- 15.Paul S, Olausson P, Venkitaramani DV, Ruchkina I, Moran TD, Tronson N, et al. The striatal-enriched protein tyrosine phosphatase gates long-term potentiation and fear memory in the lateral amygdala. Biol Psychiatry. 2007;61:1049–1061. doi: 10.1016/j.biopsych.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olausson P, Venkitaramani DV, Moran TD, Salter MW, Taylor JR, Lombroso PJ. The tyrosine phosphatase STEP constrains amygdala-dependent memory formation and neuroplasticity. Neuroscience. 2012;225:1–8. doi: 10.1016/j.neuroscience.2012.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang CH, Huang CC, Hsu KS. A critical role for protein tyrosine phosphatase nonreceptor type 5 in determining individual susceptibility to develop stress-related cognitive and morphological changes. J Neurosci. 2012;32:7550–7562. doi: 10.1523/JNEUROSCI.5902-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- 19.Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- 20.Cummings S, Elde R, Ells J, Lindall A. Corticotropin-releasing factor immunoreactivity is widely distributed within the central nervous system of the rat: an immunohistochemical study. J Neurosci. 1983;3:1355–1368. doi: 10.1523/JNEUROSCI.03-07-01355.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McElligott ZA, Klug JR, Nobis WP, Patel S, Grueter BA, Kash TL, et al. Distinct forms of Gq-receptor-dependent plasticity of excitatory transmission in the BNST are differentially affected by stress. Proc Natl Acad Sci U S A. 2010;107:2271–2276. doi: 10.1073/pnas.0905568107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conrad KL, Louderback KM, Gessner CP, Winder DG. Stress-induced alterations in anxiety-like behavior and adaptations in plasticity in the bed nucleus of the stria terminalis. Physiol Behav. 2011;104:248–256. doi: 10.1016/j.physbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007;27:2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim SY, Adhikari A, Lee SY, Marshel JH, Kim CK, Mallory CS, et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature. 2013;496:219–223. doi: 10.1038/nature12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merchenthaler I, Vigh S, Petrusz P, Schally AV. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am J Anat. 1982;165:385–396. doi: 10.1002/aja.1001650404. [DOI] [PubMed] [Google Scholar]
- 26.Dabrowska J, Hazra R, Ahern TH, Guo JD, McDonald AJ, Mascagni F, et al. Neuroanatomical evidence for reciprocal regulation of the corticotrophin-releasing factor and oxytocin systems in the hypothalamus and the bed nucleus of the stria terminalis of the rat: Implications for balancing stress and affect. Psychoneuroendocrinology. 2011;36:1312–1326. doi: 10.1016/j.psyneuen.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammack SE, Mania I, Rainnie DG. Differential expression of intrinsic membrane currents in defined cell types of the anterolateral bed nucleus of the stria terminalis. J Neurophysiol. 2007;98:638–656. doi: 10.1152/jn.00382.2007. [DOI] [PubMed] [Google Scholar]
- 28.Hazra R, Guo JD, Ryan SJ, Jasnow AM, Dabrowska J, Rainnie DG. A transcriptomic analysis of type I-III neurons in the bed nucleus of the stria terminalis. Mol Cell Neurosci. 2011;46:699–709. doi: 10.1016/j.mcn.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chronwall BM, DiMaggio DA, Massari VJ, Pickel VM, Ruggiero DA, O’Donohue TL. The anatomy of neuropeptide-Y-containing neurons in rat brain. Neuroscience. 1985;15:1159–1181. doi: 10.1016/0306-4522(85)90260-x. [DOI] [PubMed] [Google Scholar]
- 30.Wamsley JK, Young WS, 3rd, Kuhar MJ. Immunohistochemical localization of enkephalin in rat forebrain. Brain Res. 1980;190:153–174. doi: 10.1016/0006-8993(80)91166-x. [DOI] [PubMed] [Google Scholar]
- 31.Hammack SE, Guo JD, Hazra R, Dabrowska J, Myers KM, Rainnie DG. The response of neurons in the bed nucleus of the stria terminalis to serotonin: Implications for anxiety. Prog Neuropsychopharmacol Biol Psychiatry. 2009 doi: 10.1016/j.pnpbp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hazra R, Guo JD, Dabrowska J, Rainnie DG. Differential distribution of serotonin receptor subtypes in BNST(ALG) neurons: modulation by unpredictable shock stress. Neuroscience. 2012;225:9–21. doi: 10.1016/j.neuroscience.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Sugino K, Hempel CM, Miller MN, Hattox AM, Shapiro P, Wu C, et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- 35.Dabrowska J, Rainnie DG. Expression and distribution of Kv4 potassium channel subunits and potassium channel interacting proteins in subpopulations of interneurons in the basolateral amygdala. Neuroscience. 2010;171:721–733. doi: 10.1016/j.neuroscience.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 37.Braithwaite SP, Adkisson M, Leung J, Nava A, Masterson B, Urfer R, et al. Regulation of NMDA receptor trafficking and function by striatal-enriched tyrosine phosphatase (STEP) Eur J Neurosci. 2006;23:2847–2856. doi: 10.1111/j.1460-9568.2006.04837.x. [DOI] [PubMed] [Google Scholar]
- 38.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liang KC, Chen HC, Chen DY. Posttraining infusion of norepinephrine and corticotropin releasing factor into the bed nucleus of the stria terminalis enhanced retention in an inhibitory avoidance task. Chin J Physiol. 2001;44:33–43. [PubMed] [Google Scholar]
- 41.Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997;17:6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, et al. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- 43.Sink KS, Walker DL, Freeman SM, Flandreau EI, Ressler KJ, Davis M. Effects of continuously enhanced corticotropin releasing factor expression within the bed nucleus of the stria terminalis on conditioned and unconditioned anxiety. Mol Psychiatry. 2012 doi: 10.1038/mp.2011.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, et al. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 45.Gafford GM, Guo JD, Flandreau EI, Hazra R, Rainnie DG, Ressler KJ. Cell-type specific deletion of GABA(A)alpha1 in corticotropin-releasing factor-containing neurons enhances anxiety and disrupts fear extinction. Proc Natl Acad Sci U S A. 2012;109:16330–16335. doi: 10.1073/pnas.1119261109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kozicz T. Met-enkephalin immunoreactive neurons recruited by acute stress are innervated by axon terminals immunopositive for tyrosine hydroxylase and dopamine-alpha-hydroxylase in the anterolateral division of bed nuclei of the stria terminalis in the rat. Eur J Neurosci. 2002;16:823–835. doi: 10.1046/j.1460-9568.2002.02129.x. [DOI] [PubMed] [Google Scholar]
- 47.Kim SJ, Park SH, Choi SH, Moon BH, Lee KJ, Kang SW, et al. Effects of repeated tianeptine treatment on CRF mRNA expression in non-stressed and chronic mild stress-exposed rats. Neuropharmacology. 2006;50:824–833. doi: 10.1016/j.neuropharm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Chappell PB, Smith MA, Kilts CD, Bissette G, Ritchie J, Anderson C, et al. Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci. 1986;6:2908–2914. doi: 10.1523/JNEUROSCI.06-10-02908.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.