Abstract
Resonance Raman Spectroscopy (RRS) is a non-invasive method that has been developed to assess carotenoid status in human tissues including human skin in vivo. Skin carotenoid status has been suggested as a promising biomarker for human studies. This manuscript describes research done relevant to the development of this biomarker, including its reproducibility, validity, feasibility for use in field settings, and factors that affect the biomarker such as diet, smoking, and adiposity. Recent studies have evaluated the response of the biomarker to controlled carotenoid interventions, both supplement-based and dietary [e.g., provision of a high-carotenoid fruit and vegetable (F/V)-enriched diet], demonstrating consistent response to intervention. The totality of evidence supports the use of skin carotenoid status as an objective biomarker of F/V intake, although in the cross-sectional setting, diet explains only some of the variation in this biomarker. However, this limitation is also a strength in that skin carotenoids may effectively serve as an integrated biomarker of health, with higher status reflecting greater F/V intake, lack of smoking, and lack of adiposity. Thus, this biomarker holds promise as both a health biomarker and an objective indicator of F/V intake, supporting its further development and utilization for medical and public health purposes.
Keywords: carotenoids, skin, resonance Raman spectroscopy, beta-carotene, biomarker
1.0 INTRODUCTION TO SKIN CAROTENOIDS: HEALTH EFFECTS
Carotenoids accumulate in human skin, with the levels of carotenoids reflecting dietary intake and bioavailability from food sources.1 The most common carotenoids in the Western diet are alpha-carotene, beta-carotene, beta-cryptoxanthin, lycopene, lutein, and zeaxanthin.2 After absorption in the intestine, carotenoids are transported through the bloodstream by lipoproteins to various target tissues,3,4 including skin. Cholesterol transporters, such as scavenger receptor class B1 type 1 protein (SR-B1) and Cluster of Differentiation 36 membrane protein (CD 36), appear to facilitate absorption of carotenoids in the intestine,5 and these transporters may also facilitate carotenoid absorption in the epidermal layers of the skin.6 Some have suggested that sweat and sebum may also transport carotenoids to the skin surface, allowing the carotenoids to subsequently penetrate back into the skin.7 Carotenoids are lipophilic molecules found in many tissues, including skin--especially in skin sites where the stratum corneum, the upper-most skin layer, is thick.8 Body sites highest in total skin carotenoid levels include the sole of the foot, forehead, and palm of the hand9 (e.g., vegetarians are commonly noted to have “yellow” palms due to visible carotenoid accumulation). All of the major carotenoids have been detected in human skin using chemical (e.g., HPLC) analysis.8,10
There has been and continues to be considerable interest in possible health effects of carotenoids in skin as reviewed elsewhere.11–13 Arguably the best-studied potential health effect of carotenoids beyond a role in provitamin A activity is a role in photoprotection, that is, the protection against erythema and sunlight damage.11–15 Beta-carotene has established efficacy in the treatment of erythropoietic protoporphyria, a photosensitivity disease.16,17 In humans without this disease, there is also evidence from controlled studies that carotenoids such as beta-carotene have efficacy in the protection from sunburn,18 although the sun protection factor (SPF) is modest (i.e., approximately equal to 2). Carotenoids are known to quench singlet oxygen and other free radical species, which are generated in the skin by exposure to UVA and can cause skin damage.16 Several recent studies have examined the potential protective effects of carotenoids against premature photoaging of the skin, marked by signs such as wrinkling, pigmentation, dryness, and inelasticity. There is suggestive evidence for a protective effect of beta-carotene on photoaging.19 It has also been suggested that other carotenoids, such as lycopene20 and astaxanthin21 may also protect against photoaging. Tobacco smoking, which also generates free radical species, is another cause of premature skin aging,22 further suggesting that free radical quenching ability of carotenoids may contribute to photoprotection/reducing premature skin aging.
1.1 INTRODUCTION TO SKIN CAROTENOIDS AS A BIOMARKER OF FRUIT AND VEGETABLE INTAKE
Not only is skin carotenoid assessment of interest for studying the direct effects of carotenoids on skin, but there is great interest in assessing skin carotenoids as a biomarker of fruit and vegetable intake. Higher relative to lower fruit and vegetable intake has been associated with a reduction in the risk of a number of chronic diseases, including various cancers,23 cardiovascular disease,24 age-related degenerative diseases,25 and obesity.26 Many countries, including the U.S., are supporting interventions to increase fruit and vegetable intake, and the recent Dietary Guidelines for Americans 2010 27 recommendation states that individuals should “increase vegetable and fruit intake.” In order to identify populations at particular risk for inadequate intake of fruits and vegetables, and to evaluate the success of interventions aimed at increasing fruit and vegetable intake, objective indicators of fruit and vegetable intake are critically needed. This is especially true in studies involving children, where it is extraordinarily difficult to obtain valid dietary intake data.28 Parents and caregivers typically do not observe all meals consumed, and young children lack the cognitive ability to self-report diet.
Fruits and vegetables are concentrated sources of carotenoids. Carotenoids can be measured in blood and in other tissues, and levels in blood and tissues are correlated with dietary intake of both total and specific carotenoids.29 Plasma concentrations of carotenoids also increase significantly in response to fruit/vegetable behavioral interventions.30,31 Given their widespread distribution in fruits and vegetables, carotenoids have been used as an objective biomarker of fruit and vegetable intake. The National Academy of Sciences2 stated “blood concentrations of carotenoids are the best biological markers for consumption of fruits and vegetables.”
Thus, blood concentrations of carotenoids can and have served as concentration biomarkers of fruit and vegetable intake, and are used in nutritional surveillance (e.g., NHANES biochemical components), in observational research studies, and as a marker of adherence in relevant behavioral interventions. Concentration biomarkers can be used to calibrate and correct for measurement error in self-reported nutrient intake data, improving the quality of dietary exposure data for epidemiologic research.32 Despite these advantages of blood carotenoids as a biomarker, there are real and significant disadvantages to the use of plasma or serum carotenoid concentrations, including cost (of sample collection, processing, storage, and chemical analysis, usually by HPLC); the need for study participants to agree to submit to venipuncture, which may introduce participation bias; sample lability during processing and analysis; and the relatively short half-life of carotenoids in blood.33 Adipose tissue is thought to be a more stable depot of carotenoids,34 and a few epidemiologic studies have utilized adipose tissue to assess carotenoid status. However, this approach requires biopsies and extensive sample preparation prior to HPLC analysis (to remove lipid) and thus is even more prohibitive to use in the setting of large population studies. Despite the costs and other limitations, blood carotenoids have been the biomarker of choice for fruit and vegetable intake for human studies.
1.2 NONINVASIVE ASSESSMENT OF SKIN CAROTENOIDS
As described above, skin carotenoids are of current interest as a biomarker associated with better health, as well as a potential biomarker of fruit and vegetable intake. Both of these areas of research would be greatly facilitated by the availability of non-invasive approaches to rapidly assess carotenoid status in living human skin. Because of some of the unique properties of carotenoids, both reflectance based methods35 as well as methods utilizing resonance Raman light scattering spectroscopy (RRS) have been evaluated.
RRS is a form of laser spectroscopy that detects the characteristic vibrational/rotational energy levels of a molecule. Carotenoids are particularly well suited to RRS, as all have a conjugated carbon backbone molecular structure, strongly absorbing in the blue wavelength region and thus providing the basis for efficient resonant laser excitation of the molecules with visible laser lines. The backbone consists of alternating carbon double- and single-bonds, with the conjugation length differing between particular carotenoid species. The stretch vibration frequencies of the carbon double and single bonds can be detected with RRS, where they appear as sharp spectral lines that are shifted by the vibration frequencies relative to the frequency of the excitation laser.9 In homogeneous, optically thin solvent systems, the intensity of the resonance Raman scattered light is linearly related to the carotenoid concentration, thus serving as an optical measure for carotenoid content.
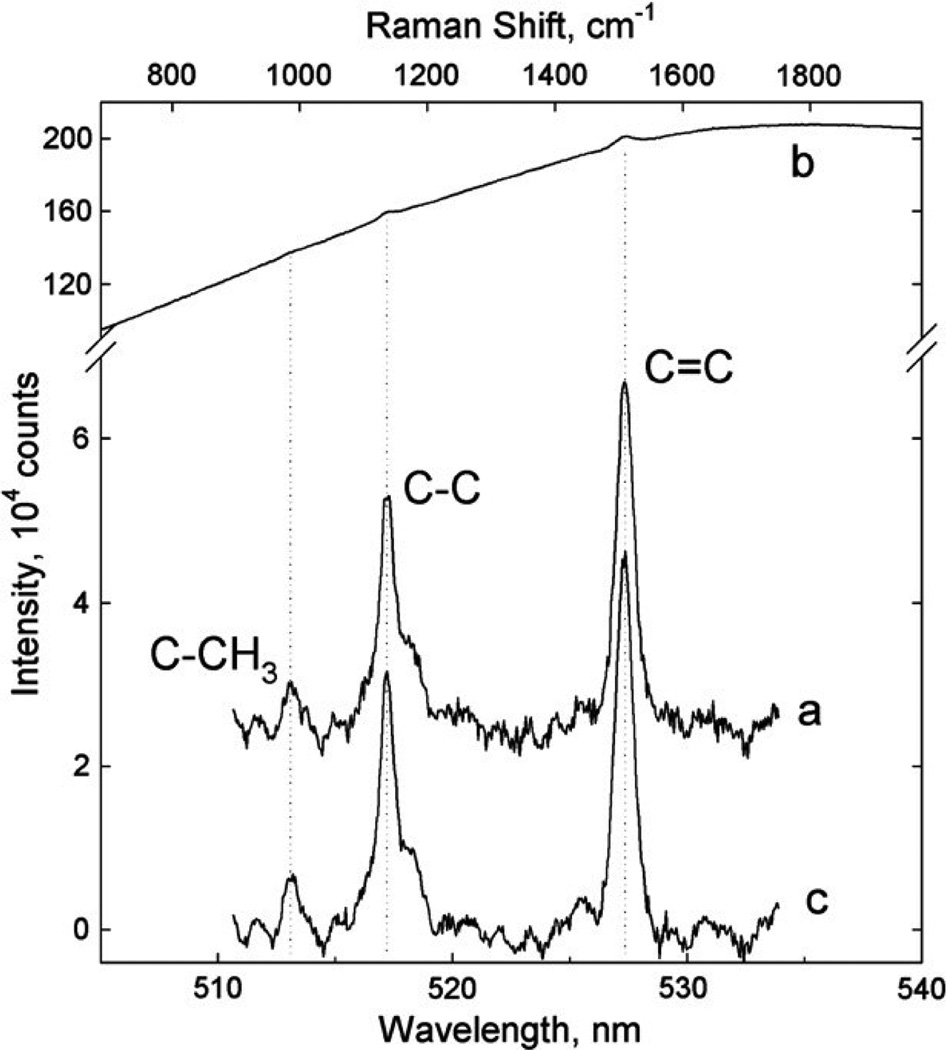
These light scattering properties have led us to explore the use of RRS for the non-invasive quantitative optical measurement of carotenoids and their spatial distributions in living human tissue, initially in the human macula (retina),36–38 and shortly after also in human skin8,9,39–41 and oral mucosal tissue.9 Typical spectra for skin are shown in Fig. 1 and compared with an RRS spectrum for a beta-carotene solution. A growing number of studies, done by our group as well as other research groups over the past several years, support the promise of this approach. Below we summarize what is known about skin carotenoid status as assessed by RRS, including reproducibility, validity, feasibility of use in field settings, and factors known to affect skin carotenoid status. We conclude with a discussion of future research needs with regard to assessment of skin carotenoid status.
Figure 1.
RRS spectrum of a solution of beta-carotene in acetone (a); typical raw skin spectrum (b) featuring weak carotenoid RRS peaks superimposed on a strong fluorescence background; processed carotenoid RRS spectrum (c) obtained after subtraction of fluorescence background. Skin carotenoid C-C and C=C RRS peaks are detectable with high signal-to- noise ratios. Taken with permission from Ermakov et al.47
2.0 BIOMARKER DEVELOPMENT: INTRA- VERSUS INTER-SUBJECT VARIABILITY
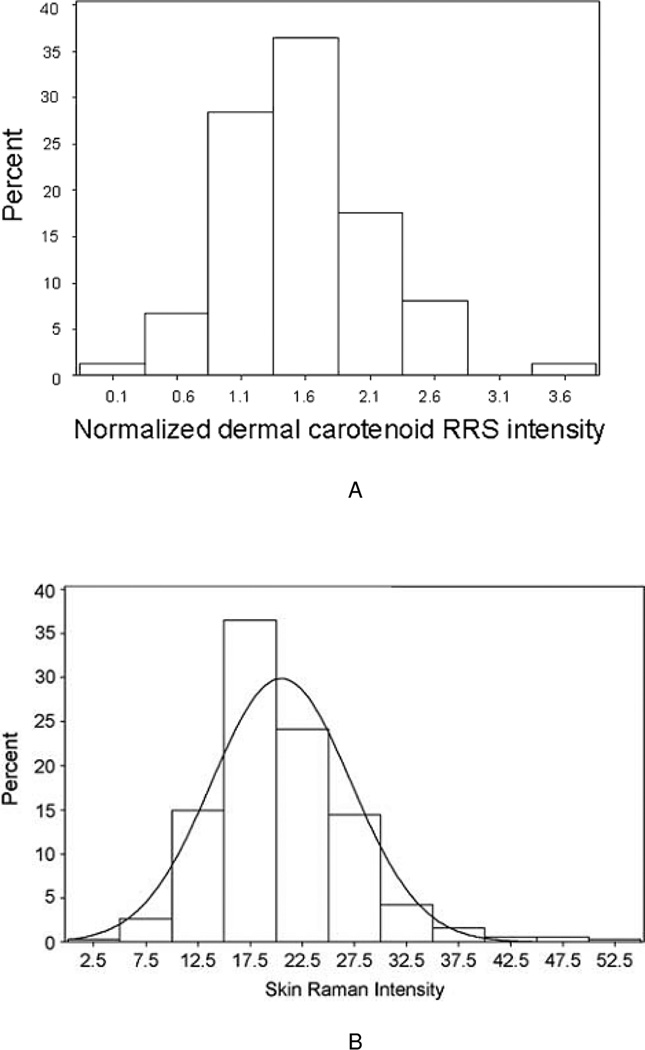
An early step in the development of any biomarker is characterizing the intra-(within) subject variability as well as the inter- (between) subject variability. Ideal biomarkers would vary widely across different individuals within a population, but be relatively constant over time within an individual. To understand the variability in skin carotenoids as assessed by RRS, we conducted two initial surveys in which we recruited 57 and 1,375 healthy adults, respectively, and measured the carotenoid status by RRS, choosing the palm of the hand as a convenient tissue site. Wide distributions were evident, with large variation throughout the populations.9,42 More recently, 74 healthy adults were recruited and their carotenoid status assessed by RRS longitudinally six times over the ensuing six months.43 As shown in Figure 2A, we observed again a wide distribution of skin carotenoid status that was nearly normal (slight right skew). We conducted a third, large study (N=381) in 3 to 4 year old children,44 which showed a similarly wide distribution (Figure 2B). Thus, there is adequate inter-subject variation in skin carotenoid status as assessed by RRS.
Figure 2.
A. Histogram showing distribution of skin total carotenoids in the palm, n = 74 healthy adults. Taken with permission from Mayne et al.43
B. Histogram showing distribution of skin total carotenoids in the palm, n = 381 preschool children. Taken with permission from Scarmo et al.44 Note that the scales in Figures 2A and 2B are not comparable as one was normalized to an external standard calibrator.
We assessed the reproducibility of the skin carotenoid biomarker over time with intraclass correlation coefficients (ICCs) across three body sites (palm, inner forearm, and outer forearm) and over each of six time points.43 ICCs for total carotenoids for each of the six time points ranged from 0.85 to 0.89, indicating that participants who had high Raman counts in skin of one body site were similarly high in the skin of other body sites assessed. ICCs for total carotenoids within each body site over time were as follows: palm = 0.97; inner forearm = 0.95; outer forearm = 0.93, showing that biomarker levels were highly consistent over time within each body site, with the highest reproducibility shown for the palm. Similarly, another research group assessed skin carotenoids longitudinally in 10 subjects over a one-year period. They noted some seasonal variation (amounting to a 26% overall increase in skin carotenoids) from winter/spring values to summer/autumn values.45 They also commented on some significant variation within just a single day; however, our group has not observed this in multiple human studies suggesting that a significant artifactual instrument variation rather than biological variation cannot be ruled out as a source of this variation.
Another method to assess reproducibility for biomarkers is to assess agreement between a single biomarker measure of skin carotenoid status, and the mean of multiple measures of skin carotenoid status. We performed this analysis using data from our longitudinal study in adults (six scans over six months), and observed good agreement between skin carotenoid status measured by RRS at baseline and multiple time points.46 Of the 18 subjects categorized in the highest quartile at baseline, 14 remained in the highest quartile when carotenoid status was estimated as the average of six time points. Sixteen of the 18 subjects categorized as the lowest quartile of carotenoid status at baseline remained in the lowest quartile when their status was averaged over time. Overall, 56 of the total 74 subjects remained in the same category of skin carotenoid status, and the weighted Kappa showed an 80% agreement (95% CI = 0.72–0.88) between the measures, supporting the feasibility of obtaining a single measure as an index of usual carotenoid status, at least over six months (a period with marked seasonal variation in climate and availability of fresh fruit and vegetables where the study was conducted).
2.1 BIOMARKER DEVELOPMENT: VALIDITY
Living human skin presents a highly heterogeneous tissue structure, is highly scattering, and also contains several strongly absorbing and spectrally overlapping chromophores. In view of the resulting highly complex light-tissue interaction scenario, it is necessary to validate that the RRS device is assessing skin carotenoid status accurately. Details of the validation work have been described previously47; here we describe two human studies in which we compared RRS measured values in human skin against chemical (HPLC) analyses of skin obtained from healthy adults. In the first study, we recruited a sample of 28 healthy adults who agreed to undergo dermal posterior hip biopsy (3 mm punch), after being scanned at the site of the biopsy using RRS. We then compared correlations between the RRS biomarker (total carotenoids) and the relative levels in the dermal skin biopsies using HPLC. Total carotenoid level in skin by RRS was significantly correlated with total carotenoid level in skin by HPLC (r = 0.66, p = 0.0001).43 Because we were working with very small tissue samples (with corresponding analytical challenges for HPLC analyses) and with punch geometries in which the biopsied tissue thickness exceeded the light penetration depth, we performed a second study, where heel skin carotenoid levels were measured in vivo using the RRS method in eight subjects, and compared to HPLC results of relatively thin heel skin slices (which produce more tissue than skin biopsies). The resulting correlation coefficient was 0.95,47 indicating accurate quantitation.
2.2 BIOMARKER DEVELOPMENT: DETERMINANTS
Prior to using skin carotenoid status as a biomarker for human studies, a clear understanding of factors that affect the biomarker is needed. Below we summarize determinants as reported in the literature. Of note, some of these factors are known to be correlated with each other; for example, smokers are known to consume fewer fruits and vegetables than non-smokers.48–51 Thus, we have employed both univariate and multivariate analyses to understand the significance and impact of various potentially correlated factors on skin carotenoid status.
Diet
An analysis of our early survey of 1,375 healthy subjects indicated a pronounced cross-sectional relationship between self-reported fruit and vegetable intake (a source of carotenoids) and skin carotenoid RRS status.40,42 Subsequent cross sectional studies are also quite consistent in showing significant associations between self-reported consumption of fruits/vegetables and/or dietary carotenoid intake and skin carotenoid status, as measured by RRS. This has been observed both in adults43,45,52,53 and children.44 Interestingly, in the study of children, both parental report of the child’s frequency of consumption of fruits and vegetables, as well as parental report of the child’s preference for carotenoid-rich fruits and vegetables, was significantly correlated with skin carotenoid status. Thus, it is clear that skin carotenoid status is a biomarker of diet; however, as is the case with diet in relation to plasma carotenoids, the correlation coefficients are modest with substantial variation in skin carotenoid status (cross-sectionally) unexplained by dietary intake. Note that this is typical for many concentration biomarkers of nutrition in current use; the only biomarkers for which diet explains most of the variance are reference or recovery biomarkers such as doubly-labeled water and urinary nitrogen.32,54,55
Smoking
Our early survey of 1,375 healthy subjects revealed that smokers had much lower levels of skin carotenoids than non-smokers.40,42 This has subsequently been observed in other studies.43,56 More specifically, smokers had about a 20–30% reduction in skin carotenoid levels when compared to non-smokers. Because some of this effect is likely due to lower intake of carotenoids (in addition to the direct effects of tobacco-induced oxidation of carotenoids), we performed multivariate analyses in one of our studies, which showed, after correcting for dietary intake, that smoking was still associated with lower carotenoid status at baseline, although the p value was marginally significant (p = 0.07).46 In our study of children, having a smoker in the home (and therefore having the child exposed to environmental tobacco smoke) was also associated with a modest reduction (7%) in skin carotenoid status (p < 0.10) although this was not significant in multivariate analyses.44 These findings are consistent with a large body of evidence linking smoking with substantial reductions in plasma carotenoid levels57–61 and with research indicating that some, but not all, of the reduction is due to lower intakes of carotenoids.60
Adiposity
Body mass index (BMI, or weight in kg divided by height in meters squared) is a proxy measure of body fatness commonly used in human studies and is weakly and inconsistently associated with lower skin carotenoid status. More specifically, Meinke et al. observed that obese subjects (BMI ≥ 30) had 13% lower skin carotenoids, as measured by RRS, compared to normal/overweight subjects (BMI < 30), p < 0.05.56 Rerksuppaphol and Rerksuppaphol also observed lower skin carotenoid status in obese subjects relative to normal weight subjects, but the association with obesity was not significant.52 In our own work in adults, the highest skin carotenoid values were observed in subjects who were underweight or normal body weight, although there was no trend and the association was not significant.43 Similarly, in children, the highest skin carotenoid status was observed in underweight and normal body weight children, although the trend was inconsistent and the differences not statistically significant.44 Lower skin carotenoid status in persons of greater adiposity may reflect that adipose tissue is serving as a reservoir for fat-soluble carotenoids, may be a consequence of obesity-mediated inflammation (e.g., plasma carotenoids are inversely correlated with biomarkers of inflammation such as C-reactive protein and IL-6 62–64) or may reflect dietary intake differences between groups with different BMIs. while we attempted to adjust for self-reported dietary differences in our statistical models, diet is prone to measurement error and it remains uncertain whether lower carotenoid status in the setting of obesity reflects poorer diet, inflammation, a greater adipose tissue reservoir, or multiple effects.
Sunlight/Ultraviolet Radiation
Carotenoids are photolabile compounds, which may explain our early observation that persons with habitual high sunlight exposure have significantly lower skin carotenoid levels than people with little sunlight exposure.40,42 We followed up on this cross-sectional association with a longitudinal study where we asked subjects at each visit to self-report the number of hours during the past three days that their skin had been exposed to the sun without sunscreen. We then performed statistical analyses (linear mixed effects regression) to examine how the time-dependent measure of skin-carotenoid status was associated with the time- dependent measure of recent sun exposure. These analyses also indicated that recent sun exposure was a significant predictor of lower skin carotenoid status over time (p=0.01).46 In this same study, we observed some seasonal variation in skin carotenoid status, but in contrast to the results of Darvin et al.,45 who observed higher carotenoid status in summer/autumn than spring, we observed lower skin carotenoid status in the summer in this study.46 This may be because skin carotenoids reflect dietary carotenoid intake over many weeks prior, and carotenoid intake in spring would be expected to be relatively low in our climate (northeastern United States); however, it is also plausible that sunlight/ultraviolet radiation may have accounted for some of the seasonal variation observed in our study.
Further evidence for an effect of UV radiation on skin carotenoids comes from intervention studies as summarized by Lademann et al. 65 UV irradiation of the skin of the forearm resulted in significant decreases in beta-carotene and lycopene. In addition, infrared irradiation also led to reductions in skin carotenoids. The authors speculate that solar irradiation in the UV, IR and visible regions produced free radicals in skin that deplete carotenoids.
While sun exposure does appear to affect skin carotenoid status, it is worth noting that the overall impact on carotenoids may be fairly modest (e.g., fully adjusted beta-coefficients were small in our study),46 although we are unable to rule out a more substantial effect under more extreme conditions of sun exposure than what were experienced in our free-living study subjects.
Skin pigmentation
Most of the studies that have used RRS for skin carotenoid status measures have been done in largely Caucasian populations, so there is limited information available about the effect of skin pigmentation on RRS measures. In our adult study, we did not have quantitative measures of melanin content in skin available, but instead assessed skin color on the inner arm by matching it to a color wheel of skin tone samples used for prosthetic devices (Steeper USA©, San Antonio, TX, USA). RRS measures were lowest in the few subjects with the darkest skin tone,43 but this could reflect many other factors that vary by race/ethnicity. When we performed multivariate analyses, skin tone was not significantly associated with skin carotenoid status,46 although we had a small sample size, and therefore limited power to detect an effect should one exist. More research is needed in diverse populations, with quantitative measures of melanin content, to determine if skin pigmentation is a determinant of skin carotenoid status. If so, correction of spectrophotometrically assessed melanin in RRS devices is feasible for tissue locations containing melanin. Generally, the influence of melanin on RRS scores can be largely avoided by choosing measurement sites such as the palm of the hand9 or heel of the foot47 where melanin levels are typically low independent of ethnicity.
Genetic Factors
The enzyme that controls the metabolism of provitamin A carotenoids into retinal (precursor to retinol), beta-carotene 15,15’-monoxygenase 1 (BCMO1), is known to be polymorphic. In 2009, Leung et al.66 reported that two common polymorphisms in this gene were associated with fasting plasma beta-carotene concentrations. Ferrucci et al. used a genome-wide association approach to search for genetic variants that were associated with circulating carotenoid levels; 4 single nucleotide polymorphisms (SNPs) near the BCMO1 gene were identified.67 One SNP (rs6564851) showed the strongest association with plasma beta-carotene; persons with the G allele had higher plasma beta-carotene concentrations, although this SNP explained <2% of the variance in plasma beta-carotene concentrations. This variant was also associated with plasma concentrations of other carotenoids; more specifically, higher concentrations of alpha-carotene, but lower concentrations of lutein, zeaxanthin, and lycopene. Hendrickson et al. also examined SNPs in BCMO1 in relation to plasma carotenoid concentrations, and calculated weighted gene scores in relation to individual carotenoids.68 The weighted gene scores developed for each individual carotenoid were significantly associated with plasma concentrations of other carotenoids--in some cases higher, and in some cases lower concentrations. Thus, it appears that genetic variation is a significant determinant of individual carotenoid concentrations in blood; however, the impact on total carotenoids in blood would be expected to be less. Relevant to the skin carotenoid biomarker, we are unaware of any published studies examining genetic variation in relation to skin carotenoid status, cross-sectionally or in response to intervention, although such studies are underway. Of note, the skin carotenoid biomarker as typically used (with blue light excitation at λ=488 nm) assesses total carotenoids, so results from plasma studies of single carotenoids may not apply to the skin carotenoid biomarker.
Oxidative stress
When analyzed by a chemical assay based on urinary malondialdehyde excretion, an indicator of oxidative lipid damage, people with high oxidative stress had significantly lower skin carotenoid levels than people with low oxidative stress.40,42 Another study used electron paramagnetic resonance spectroscopy to assess reduction of nitroxides as an indicator of antioxidative capacity of human skin, and found that skin carotenoid status as assessed by RRS was correlated with the rate constant of nitroxide decrease.69 These observations suggest that skin carotenoid RRS scores might be useful as a surrogate marker for general antioxidant status. General stress factors (such as fatigue, illness) have also been suggested to influence skin carotenoid status,45 but have not been quantitatively or systematically assessed in multivariate models controlling for other factors.
Other factors
Age does not appear to be an important determinant of skin carotenoid status in adults, although a wide age range has not been systematically evaluated. In babies and young children, however, those who are older on average have higher skin carotenoid status (see Section 2.3 below). In terms of gender, on average, women have higher skin carotenoid status than men,43,56 consistent with what is known about adipose tissue70 and plasma carotenoids,59 likely reflecting greater intake of fruits and vegetables in women along with smaller body size.
2.3 BIOMARKER DEVELOPMENT: FEASIBILITY
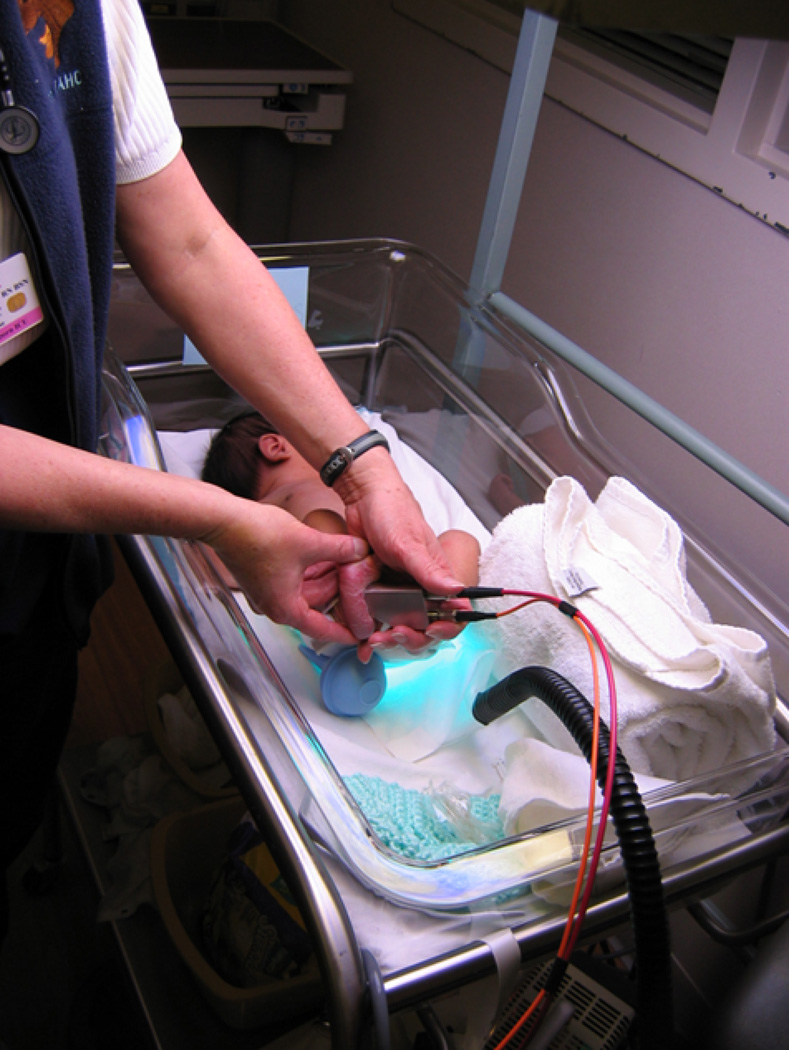
As summarized above, RRS measures of skin carotenoid status appear to be reproducible and valid, with known determinants in addition to carotenoid intake from foods. However, before this method can be widely used in field research, the feasibility of assessment merits consideration. Our first adult studies used a prototype RRS device that was not sufficiently portable to enable its use in field settings.42,43 However, our group subsequently developed and evaluated portable 488 nm solid state laser based compact instruments in field settings. One such evaluation included our study of 3 and 4 year-old children who were assessed in a preschool setting in Connecticut (Northeastern USA).44 We found it quite feasible to scan a large number of children (nearly 400 total) in a relatively short time-frame with no difficulties (e.g., up to 60 children were assessed in a two-hour window of data collection). More recently, some of our team members have used the RRS device to assess skin carotenoid status in the heel of infants and premature babies, as illustrated in Fig. 3.71 Results showed that preterm newborns had the lowest skin carotenoid scores, with RRS values increasing across categories of age (e.g., term newborns and older infants). This is consistent with our earlier work with 3 and 4 year-old children which found that skin carotenoids were higher in the older than younger preschool children.44
Figure 3.
Clinical use of portable RRS scanner with fiber optical module for heel skin carotenoid measurements in infants. Taken with permission from Ermakov et al.71
2.4 BIOMARKER DEVELOPMENT: RESPONSE TO DIETARY INTERVENTION
As described earlier, there is considerable interest in using the RRS method to evaluate whether or not interventions designed to improve fruit and vegetable intake actually change intake behaviors and improve nutritional status. This is a critical research need, because without objective verification that behavior change has occurred, results of trials (of health outcomes, as well as of intake) are difficult to interpret. The ideal method for assessing response to intervention is in the setting of controlled intervention studies.
Beginning with carotenoid supplementation trials, it is well known and accepted that carotenoid supplementation leads to measurable increases in carotenoids in human skin. 35,41,65 This obviously underlies much of the work done in the setting of using dietary sources of carotenoids for sun protection.11,14,15 This also underlies the licensing of the RRS method to the nutritional supplement industry (“Biophotonic Scanner”, NuSkin/Pharmanex Inc., Provo, Utah), based on the finding that carotenoid-containing vitamin supplements produced an increase in skin carotenoid values within a relatively short time frame.41 These studies clearly indicate that providing relatively bioavailable carotenoids through supplementation impacts skin carotenoid status.
Considering other studies that used RRS methodologies, Meinke et al. evaluated a commercial oil extract of kale (Lutrex™), which was rich in various carotenoids, versus a placebo oil to assess carotenoid bioavailability in 22 subjects (11 per group).72 Results showed an increase in both skin and blood carotenoids with Lutrex™ supplementation. The blood values increased faster in the first 14 days as compared to the subsequent 14 days of supplementation, suggesting saturation. In contrast, skin did not appear to saturate (reach equilibrium) during the four weeks of Lutrex™ supplementation. When Lutrex™ supplementation ceased, carotenoid decreases were much more rapid in serum than in skin. This is consistent with a much longer half-life of carotenoids in skin.
In contrast to supplementation trials, there has been a lack of information on skin carotenoid response to food-based interventions (e.g., fruit and vegetable interventions). To address this, our research group conducted a controlled feeding study, done in partnership with the U.S. Department of Agriculture, in which we assessed skin carotenoid status in response to several different dietary interventions including a carotenoid depletion phase (provision of minimal carotenoid-rich foods for six weeks), followed by a high fruit and vegetable-provided diet (eight weeks), followed by another depletion diet (six weeks), and then subjects returned to their self-selected diets for an additional eight weeks. Preliminary and final results have been presented73,74 and a manuscript is in preparation; findings parallel those seen for the carotenoid-rich oil extract intervention.72 That is, skin carotenoid levels decreased during depletion and increased during high-carotenoid feeding, with skin carotenoid status tracking similarly to plasma carotenoids although the rates of decrease (during depletion) were faster in plasma versus skin. Skin carotenoids had not yet plateaued by 8 weeks post-intervention, suggesting that they reflect intake over at least the prior 2+ months. Thus, multiple lines of evidence now show that skin carotenoid status is responsive to carotenoid intervention involving supplements, vegetable oil extracts given as dietary supplements, and carotenoid-rich fruits and vegetables consumed in typical US diets.
3.0 FUTURE NEEDS
The work, to date, on this biomarker is highly promising and supports further development of skin carotenoids as a biomarker. However, before it can be adopted more widely, some barriers need to be addressed.
The first concerns the impact of melanin on the biomarker. As noted above, there is a real need to evaluate this biomarker in large populations with diverse skin pigmentation with objective quantitation of dermal melanin in order to directly assess the effect of melanin on the carotenoid biomarker. It is plausible that melanin may absorb some of the laser excitation and RRS light, and thus “dampen” the resulting RRS response, resulting in an artifactual reduction in values obtained. Alternatively, it is also plausible that higher melanin density in skin may help to protect photolabile carotenoids from the adverse effects of UV irradiation, producing somewhat higher skin carotenoid scores in persons with dark skin pigmentation as compared to persons with light skin holding all other factors constant. The lack of knowledge on the impact of melanin is most limiting when attempting to using the skin carotenoid biomarker cross-sectionally, rather than in an intervention setting, where each person’s skin response is compared against their baseline measures.
Next, the RRS methodology for assessing skin carotenoid status led to large-scale commercial production of low-power devices for proprietary use in the nutritional supplement market. This was facilitated by a recently developed relatively inexpensive instrument configuration that uses excitation with a spectrally narrowed 473 nm LED light source instead of a 488 nm laser, and also by RRS light detection via filter instead of spectrograph.75 However, production has not been similarly scaled up for higher-grade research devices for use in medical and public health research76 and devices adequate for research remain relatively costly at this time. Recognizing that the solid state lasers used in portable research-grade RRS devices are still relatively expensive, we have been exploring alternative optical methodologies to assess skin carotenoid status noninvasively but at reduced cost. For example, skin reflectance-based methodologies have been evaluated by other groups, including the group of Sies and Stahl, for many years.77,78 We have developed a variation on this method using topical pressure, which temporarily squeezes blood out of the tissue volume to reduce the influence of hemoglobin on the reflection spectra, and which holds promise as a simple and inexpensive method.76 In contrast to our RRS method, it does not require narrow- band light sources for excitation, or high-resolution spectrophotometers for detection of spectrally narrow Raman line features, and minimizes effects of blood and melanin that can impact reflection spectroscopy methods.76 We have been evaluating the performance of this new method in comparison to our existing RRS method with early promising results (e.g., a correlation coefficient of 0.9).76 Thus, we anticipate that ongoing technological advances will continue to enable valid and reproducible assessment of skin carotenoids, with affordable/available devices to facilitate broader uptake in the medical and public health research fields.
4.0 CONCLUSIONS
As summarized in this review, skin carotenoid status as assessed by RRS is an attractive biomarker for use in human studies. It is highly reproducible and valid, and with the development of portable scanners, feasible for use in field settings. Skin carotenoid status is an objective biomarker of dietary intake of fruits and vegetables, although in the cross-sectional setting, diet explains only part of the variation in this biomarker with other factors such as smoking, adiposity, and UV potentially affecting carotenoid status. Thus, as is the case with many concentration-based biomarkers in nutrition, skin carotenoid status is an imperfect measure of fruit and vegetable intake for observational research, but adds value as an objective indicator and for incorporation into dietary measurement error correction models.32 Also, because higher status reflects greater fruit and vegetable intake, lack of smoking, and lack of adiposity, this may facilitate its use as an integrated biomarker of overall health that is predictive of future chronic disease risk, although longitudinal studies are needed to test this hypothesis. In the intervention setting, skin carotenoid status responds predictably and consistently to carotenoid intervention, both from supplements and from foods, although the numbers of subjects evaluated is still relatively small. Thus, use of skin carotenoid status as an objective biomarker of change should be considered for inclusion in fruit and vegetable-based intervention trials. Commercialization and scale-up of devices to assess skin carotenoid status, including less expensive devices based on alternative technologies, will enable more broad-based use of skin carotenoids for research and public health purposes in the future.
ABB Highlights.
We describe resonance Raman spectroscopy to measure skin carotenoid status
We describe skin carotenoid status as a promising biomarker for human health research
We review reproducibility, validity and feasibility of skin carotenoids as a biomarker
Factors that influence skin carotenoids in humans are considered
ACKNOWLEDGMENTS
We wish to thank the participants in our numerous human studies. Some of the research described in this review was supported by an R01 CA96838 grant from the National Cancer Institute; National Institutes of Health. Additional support was provided by an R01 EY-11600 grant from the National Eye Institute, National Institutes of Health; and The USDA/Agricultural Research Service, USDA 5450-51000-049-00D. The contents of this publication do not necessarily reflect the views or policies of the USDA or the Agricultural Research Service, nor does mention of trade names, commercial products, or organizations imply endorsement from the U.S. government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Canene-Adams K, Erdman JJW. Absorption, Transport, Distribution in Tissues and Bioavailability. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids Volume 5: Nutrition and Health. Basel, Switzerland: Birkhauser Verlag; 2009. [Google Scholar]
- 2.Institute of Medicine, National Academy of Sciences, Food and Nutrition Board, Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington: National Academy Press; 2000. [Google Scholar]
- 3.Lin S, Quaroni L, White WS, et al. Localization of carotenoids in plasma low-density lipoproteins studied by surface-enhanced resonance Raman spectroscopy. Biopolymers. 2000;57:249–256. doi: 10.1002/1097-0282(2000)57:4<249::AID-BIP6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 4.Lowe GM, Bilton RF, Davies IG, et al. Carotenoid composition and antioxidant potential in subfractions of human low-density lipoprotein. Ann Clin Biochem. 1999;36(Pt 3):323–332. doi: 10.1177/000456329903600304. [DOI] [PubMed] [Google Scholar]
- 5.van Bennekum A, Werder M, Thuahnai ST, et al. Class B scavenger receptor-mediated intestinal absorption of dietary beta-carotene and cholesterol. Biochemistry. 2005;44:4517–4525. doi: 10.1021/bi0484320. [DOI] [PubMed] [Google Scholar]
- 6.Tsuruoka H, Khovidhunkit W, Brown BE, et al. Scavenger receptor class B type I is expressed in cultured keratinocytes and epidermis. Regulation in response to changes in cholesterol homeostasis and barrier requirements. J Biol Chem. 2002;277:2916–2922. doi: 10.1074/jbc.M106445200. [DOI] [PubMed] [Google Scholar]
- 7.Darvin ME, Fluhr JW, Schanzer S, et al. Dermal carotenoid level and kinetics after topical and systemic administration of antioxidants enrichment strategies in a controlled in vivo study. J Dermatol Sci. 2011;64:53–58. doi: 10.1016/j.jdermsci.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Hata TR, Scholz TA, Ermakov IV, et al. Non-invasive raman spectroscopic detection of carotenoids in human skin. J Invest Dermatol. 2000;115:441–448. doi: 10.1046/j.1523-1747.2000.00060.x. [DOI] [PubMed] [Google Scholar]
- 9.Ermakov IV, Ermakova MR, McClane RW, et al. Resonance Raman detection of carotenoid antioxidants in living human tissues. Opt Lett. 2001;26:1179–1181. doi: 10.1364/ol.26.001179. [DOI] [PubMed] [Google Scholar]
- 10.Scarmo S, Cartmel B, Lin H, et al. Significant correlations of dermal total carotenoids and dermal lycopene with their respective plasma levels in healthy adults. Arch Biochem Biophys. 2010;504:34–39. doi: 10.1016/j.abb.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goralczyk R, Wertz K. Skin Photoprotection by Carotenoids. In: Britton G, Liaaen-Jensen S, Pfander H, editors. Carotenoids Volume 5: Nutrition and Health. Basel, Switzerland: Birkhauser Verlag; 2009. pp. 335–362. [Google Scholar]
- 12.Stahl W, Sies H. beta-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96:1179S–1184S. doi: 10.3945/ajcn.112.034819. [DOI] [PubMed] [Google Scholar]
- 13.Sies H, Stahl W. Nutritional protection against skin damage from sunlight. Annu Rev Nutr. 2004;24:173–200. doi: 10.1146/annurev.nutr.24.012003.132320. [DOI] [PubMed] [Google Scholar]
- 14.Stahl W, Heinrich U, Wiseman S, et al. Dietary tomato paste protects against ultraviolet light-induced erythema in humans. Journal of Nutrition. 2001;131:1449–1451. doi: 10.1093/jn/131.5.1449. [DOI] [PubMed] [Google Scholar]
- 15.Stahl W, Heinrich U, Jungmann H, et al. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. American Journal of Clinical Nutrition. 2000;71:795–798. doi: 10.1093/ajcn/71.3.795. [DOI] [PubMed] [Google Scholar]
- 16.Mathews-Roth MM. Photoprotection by carotenoids. Federation Proceedings. 1987;46:1890–1893. [PubMed] [Google Scholar]
- 17.Mathews-Roth MM. Treatment of erythropoietic protoporphyria with beta-carotene. Photo-Dermatology. 1984;1:318–321. [PubMed] [Google Scholar]
- 18.Kopcke W, Krutmann J. Protection from sunburn with beta-Carotene--a metaanalysis. Photochemistry & Photobiology. 2008;84:284–288. doi: 10.1111/j.1751-1097.2007.00253.x. [DOI] [PubMed] [Google Scholar]
- 19.Eicker J, Kurten V, Wild S, et al. Betacarotene supplementation protects from photoaging-associated mitochondrial DNA mutation. Photochem Photobiol Sci. 2003;2:655–659. doi: 10.1039/b300808h. [DOI] [PubMed] [Google Scholar]
- 20.Darvin M, Patzelt A, Gehse S, et al. Cutaneous concentration of lycopene correlates significantly with the roughness of the skin. Eur J Pharm Biopharm. 2008;69:943–947. doi: 10.1016/j.ejpb.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 21.Suganuma K, Nakajima H, Ohtsuki M, et al. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J Dermatol Sci. 2010;58:136–142. doi: 10.1016/j.jdermsci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Morita A. Tobacco smoke causes premature skin aging. J Dermatol Sci. 2007;48:169–175. doi: 10.1016/j.jdermsci.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 23.World Cancer Research Fund, American Institution for Cancer Research. The Second Expert Report. Washington D.C: AICR; 2007. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. [Google Scholar]
- 24.Liu S, Manson JE, Lee IM, et al. Fruit and vegetable intake and risk of cardiovascular disease: the Women's Health Study. Am J Clin Nutr. 2000;72:922–928. doi: 10.1093/ajcn/72.4.922. [DOI] [PubMed] [Google Scholar]
- 25.Cho E, Seddon JM, Rosner B, et al. Prospective study of intake of fruits, vegetables, vitamins, and carotenoids and risk of age-related maculopathy. Arch Ophthalmol. 2004;122:883–892. doi: 10.1001/archopht.122.6.883. [DOI] [PubMed] [Google Scholar]
- 26.Tohill BC, Seymour J, Serdula M, et al. What epidemiologic studies tell us about the relationship between fruit and vegetable consumption and body weight. Nutr Rev. 2004;62:365–374. doi: 10.1111/j.1753-4887.2004.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans. 2010 www.dietaryguidelines.gov.
- 28.Livingstone MB, Robson PJ. Measurement of dietary intake in children. Proc Nutr Soc. 2000;59:279–293. doi: 10.1017/s0029665100000318. [DOI] [PubMed] [Google Scholar]
- 29.Peng YM, Peng YS, Lin Y, et al. Concentrations and plasma-tissue-diet relationships of carotenoids, retinoids, and tocopherols in humans. Nutrition & Cancer. 1995;23:233–246. doi: 10.1080/01635589509514378. [DOI] [PubMed] [Google Scholar]
- 30.McEligot AJ, Rock CL, Flatt SW, et al. Plasma carotenoids are biomarkers of long-term high vegetable intake in women with breast cancer. Journal of Nutrition. 1999;129:2258–2263. doi: 10.1093/jn/129.12.2258. [DOI] [PubMed] [Google Scholar]
- 31.Lanza E, Schatzkin A, Daston C, et al. Implementation of a 4-y, high-fiber high-fruit-and-vegetable low-fat dietary intervention results of dietary changes in the Polyp Prevention Trial. American Journal of Clinical Nutrition. 2001;74:387–401. doi: 10.1093/ajcn/74.3.387. [DOI] [PubMed] [Google Scholar]
- 32.Prentice RL, Sugar E, Wang CY, et al. Research strategies and the use of nutrient biomarkers in studies of diet and chronic disease. Public Health Nutr. 2002;5:977–984. doi: 10.1079/PHN2002382. [DOI] [PubMed] [Google Scholar]
- 33.Rock CL, Swendseid ME, Jacob RA, et al. Plasma carotenoid levels in human subjects fed a low carotenoid diet. Journal of Nutrition. 1992;122:96–100. doi: 10.1093/jn/122.1.96. [DOI] [PubMed] [Google Scholar]
- 34.Parker RS. Carotenoid and tocopherol composition of human adipose tissue. Am J Clin Nutr. 1988;47:33–36. doi: 10.1093/ajcn/47.1.33. [DOI] [PubMed] [Google Scholar]
- 35.Stahl W, Heinrich U, Jungmann H, et al. Increased dermal carotenoid levels assessed by noninvasive reflection spectrophotometry correlate with serum levels in women ingesting Betatene. Journal of Nutrition. 1998;128:903–907. doi: 10.1093/jn/128.5.903. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein PS, Yoshida MD, Katz NB, et al. Raman detection of macular carotenoid pigments in intact human retina. Investigative Ophthalmology & Visual Science. 1998;39:2003–2011. [PubMed] [Google Scholar]
- 37.Ermakov IV, McClane RW, Gellermann W, et al. Resonant Raman detection of macular pigment levels in the living human retina. Opt Lett. 2001;26:202–204. doi: 10.1364/ol.26.000202. [DOI] [PubMed] [Google Scholar]
- 38.Sharifzadeh M, Zhao DY, Bernstein PS, et al. Resonance Raman imaging of macular pigment distributions in the human retina. J Opt Soc Am A Opt Image Sci Vis. 2008;25:947–957. doi: 10.1364/josaa.25.000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ermakov IV, Ermakova MR, Gellermann W, et al. Noninvasive selective detection of lycopene and beta-carotene in human skin using Raman spectroscopy. J Biomed Opt. 2004;9:332–338. doi: 10.1117/1.1646172. [DOI] [PubMed] [Google Scholar]
- 40.Ermakov IV, Sharifzadeh M, Ermakova M, et al. Resonance Raman detection of carotenoid antioxidants in living human tissue. J Biomed Opt. 2005;10:064028. doi: 10.1117/1.2139974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ermakov IV, Sharifzadeh M, Bernstein PS, et al. Application of Resonance Raman Spectroscopy to the Detection of Carotenoids In Vivo. In: Landrum JT, editor. Carotenoids -- Physical, Chemical and Biological Functions and Properties. Atlanta: CRC Press; 2009. pp. 87–109. [Google Scholar]
- 42.Gellermann W, Zidichouski J, Smidt C, et al. Raman detection of carotenoids in human tissue. In: Packer L, et al., editors. Carotenoids and Retinoids. Champlain, Ill: AOCS Press; 2005, pp. pp. 86–114. [Google Scholar]
- 43.Mayne ST, Cartmel B, Scarmo S, et al. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010;92:794–800. doi: 10.3945/ajcn.2010.29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scarmo S, Henebery K, Peracchio H, et al. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. Eur J Clin Nutr. 2012;66:555–560. doi: 10.1038/ejcn.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Darvin ME, Patzelt A, Knorr F, et al. One-year study on the variation of carotenoid antioxidant substances in living human skin influence of dietary supplementation and stress factors. J Biomed Opt. 2008;13:044028-1–044028-9. doi: 10.1117/1.2952076. [DOI] [PubMed] [Google Scholar]
- 46.Scarmo S, Cartmel B, Lin H, et al. Single v. multiple measures of skin carotenoids by resonance Raman spectroscopy as a biomarker of usual carotenoid status. Br J Nutr. 2013:1–7. doi: 10.1017/S000711451200582X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ermakov IV, Gellermann W. Validation model for Raman based skin carotenoid detection. Arch Biochem Biophys. 2010;504:40–49. doi: 10.1016/j.abb.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 48.Birkett NJ. Intake of fruits and vegetables in smokers. Public Health Nutr. 1999;2:217–222. doi: 10.1017/s1368980099000270. [DOI] [PubMed] [Google Scholar]
- 49.Agudo A, Pera G. Vegetable and fruit consumption associated with anthropometric, dietary and lifestyle factors in Spain. EPIC Group of Spain. European Prospective Investigation into Cancer. Public Health Nutr. 1999;2:263–271. doi: 10.1017/s136898009900035x. [DOI] [PubMed] [Google Scholar]
- 50.Baer Wilson D, Nietert PJ. Patterns of fruit, vegetable, and milk consumption among smoking and nonsmoking female teens. Am J Prev Med. 2002;22:240–246. doi: 10.1016/s0749-3797(02)00418-x. [DOI] [PubMed] [Google Scholar]
- 51.Billson H, Pryer JA, Nichols R. Variation in fruit and vegetable consumption among adults in Britain. An analysis from the dietary and nutritional survey of British adults. Eur J Clin Nutr. 1999;53:946–952. doi: 10.1038/sj.ejcn.1600877. [DOI] [PubMed] [Google Scholar]
- 52.Rerksuppaphol S, Rerksuppaphol L. Effect of fruit and vegetable intake on skin carotenoid detected by non-invasive Raman spectroscopy. J Med Assoc Thai. 2006;89:1206–1212. [PubMed] [Google Scholar]
- 53.Hesterberg K, Lademann J, Patzelt A, et al. Raman spectroscopic analysis of the increase of the carotenoid antioxidant concentration in human skin after a 1-week diet with ecological eggs. J Biomed Opt. 2009;14:024039. doi: 10.1117/1.3119257. [DOI] [PubMed] [Google Scholar]
- 54.Potischman N. Biologic and methodologic issues for nutritional biomarkers. J Nutr. 2003;133(Suppl 3):875S–880S. doi: 10.1093/jn/133.3.875S. [DOI] [PubMed] [Google Scholar]
- 55.Potischman N, Freudenheim JL. Biomarkers of nutritional exposure and nutritional status: an overview. J Nutr. 2003;133(Suppl 3):873S–874S. doi: 10.1093/jn/133.3.873S. [DOI] [PubMed] [Google Scholar]
- 56.Meinke M, Lauer A, Taskoparan B, et al. Influence on the carotenoid levels of skin arising from age, gender, body mass index in smoking/non-smoking individuals. Free Radicals and Antioxidants. 2011:15–20. [Google Scholar]
- 57.Wang L, Gaziano JM, Norkus EP, et al. Associations of plasma carotenoids with risk factors and biomarkers related to cardiovascular disease in middle-aged and older women. Am J Clin Nutr. 2008;88:747–754. doi: 10.1093/ajcn/88.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Comstock GW, Menkes MS, Schober SE, et al. Serum levels of retinol, beta-carotene, and alpha-tocopherol in older adults. Am J Epidemiol. 1988;127:114–123. doi: 10.1093/oxfordjournals.aje.a114771. [DOI] [PubMed] [Google Scholar]
- 59.Brady WE, Mares-Perlman JA, Bowen P, et al. Human serum carotenoid concentrations are related to physiologic and lifestyle factors. J Nutr. 1996;126:129–137. doi: 10.1093/jn/126.1.129. [DOI] [PubMed] [Google Scholar]
- 60.Stryker WS, Kaplan LA, Stein EA, et al. The relation of diet, cigarette smoking, and alcohol consumption to plasma beta-carotene and alpha-tocopherol levels. Am J Epidemiol. 1988;127:283–296. doi: 10.1093/oxfordjournals.aje.a114804. [DOI] [PubMed] [Google Scholar]
- 61.Galan P, Viteri FE, Bertrais S, et al. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr. 2005;59:1181–1190. doi: 10.1038/sj.ejcn.1602230. [DOI] [PubMed] [Google Scholar]
- 62.Hu P, Reuben DB, Crimmins EM, et al. The effects of serum beta-carotene concentration and burden of inflammation on all-cause mortality risk in high-functioning older persons MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2004;59:849–854. doi: 10.1093/gerona/59.8.m849. [DOI] [PubMed] [Google Scholar]
- 63.Ford ES, Liu S, Mannino DM, et al. C-reactive protein concentration and concentrations of blood vitamins, carotenoids, and selenium among United States adults. Eur J Clin Nutr. 2003;57:1157–1163. doi: 10.1038/sj.ejcn.1601667. [DOI] [PubMed] [Google Scholar]
- 64.Kritchevsky SB, Bush AJ, Pahor M, et al. Serum carotenoids and markers of inflammation in nonsmokers. Am J Epidemiol. 2000;152:1065–1071. doi: 10.1093/aje/152.11.1065. [DOI] [PubMed] [Google Scholar]
- 65.Lademann J, Meinke MC, Sterry W, et al. Carotenoids in human skin. Exp Dermatol. 2011;20:377–382. doi: 10.1111/j.1600-0625.2010.01189.x. [DOI] [PubMed] [Google Scholar]
- 66.Leung WC, Hessel S, Meplan C, et al. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15'-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009;23:1041–1053. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 67.Ferrucci L, Perry JR, Matteini A, et al. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids a genome-wide association study. American Journal of Human Genetics. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hendrickson SJ, Hazra A, Chen C, et al. beta-Carotene 15,15'-monooxygenase 1 single nucleotide polymorphisms in relation to plasma carotenoid and retinol concentrations in women of European descent. Am J Clin Nutr. 2012;96:1379–1389. doi: 10.3945/ajcn.112.034934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haag SF, Taskoparan B, Darvin ME, et al. Determination of the antioxidative capacity of the skin in vivo using resonance Raman and electron paramagnetic resonance spectroscopy. Exp Dermatol. 2011;20:483–487. doi: 10.1111/j.1600-0625.2010.01246.x. [DOI] [PubMed] [Google Scholar]
- 70.Virtanen SM, van't Veer P, Kok F, et al. Predictors of adipose tissue carotenoid and retinol levels in nine countries. The EURAMIC Study. Am J Epidemiol. 1996;144:968–979. doi: 10.1093/oxfordjournals.aje.a008867. [DOI] [PubMed] [Google Scholar]
- 71.Ermakov IV, Ermakova MR, Bernstein PS, et al. Resonance Raman based skin carotenoid measurements in newborns and infants. J Biophotonics. doi: 10.1002/jbio.201200195. published online 29 NOV 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meinke MC, Darvin ME, Vollert H, et al. Bioavailability of natural carotenoids in human skin compared to blood. Eur J Pharm Biopharm. 2010;76:269–274. doi: 10.1016/j.ejpb.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Jahns L, Wigham L, Johnson L, et al. Dermal carotenoids as measured by resonance Raman spectroscopy as a biomarker of response to a fruit/vegetable intervention study. Experimental Biology abstract. 2012 [Google Scholar]
- 74.Jahns L, Whigham L, Johnson L, et al. Skin total carotenoids predict plasma carotenoid levels during a 28-week experimental feeding study with varying levels of vegetables and fruit. Experimental Biology abstract. 2013 [Google Scholar]
- 75.Bergeson SD, Peatross JB, Eyring NJ, et al. Resonance Raman measurements of carotenoids using light-emitting diodes. J Biomed Opt. 2008;13:044026. doi: 10.1117/1.2952075. [DOI] [PubMed] [Google Scholar]
- 76.Ermakov IV, Gellermann W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J Biophotonics. 2012;5:559–570. doi: 10.1002/jbio.201100122. [DOI] [PubMed] [Google Scholar]
- 77.Stahl W, Heinrich U, Jungmann H, et al. Carotenoids in human skin: noninvasive measurement and identification of dermal carotenoids and carotenol esters. Methods Enzymol. 2000;319:494–502. doi: 10.1016/s0076-6879(00)19046-9. [DOI] [PubMed] [Google Scholar]
- 78.Alaluf S, Heinrich U, Stahl W, et al. Dietary carotenoids contribute to normal human skin color and UV photosensitivity. J Nutr. 2002;132:399–403. doi: 10.1093/jn/132.3.399. [DOI] [PubMed] [Google Scholar]