Abstract
Intake of lycopene, a red, tetraterpene carotenoid found in tomatoes is epidemiologically associated with a decreased risk of chronic disease processes, and lycopene has demonstrated bioactivity in numerous in vitro and animal models. However, our understanding of absorption, tissue distribution, and biological impact in humans remains very limited. Lycopene absorption is strongly impacted by dietary composition, especially the amount of fat. Concentrations of circulating lycopene in lipoproteins may be further influenced by a number of variations in genes related to lipid absorption and metabolism. Lycopene is not uniformly distributed among tissues, with adipose, liver, and blood being the major body pools, while the testes, adrenals, and liver have the greatest concentrations compared to other organs. Tissue concentrations of lycopene are likely dictated by expression of and genetic variation in lipoprotein receptors, cholesterol transporters, and carotenoid metabolizing enzymes, thus impacting lycopene accumulation at target sites of action. The novel application of genetic evaluation in concert with lycopene tracers will allow determination of which genes and polymorphisms define individual lycopene metabolic phenotypes, response to dietary variables, and ultimately determine biological and clinical outcomes. A better understanding of the relationship between diet, genetics, and lycopene distribution will provide necessary information to interpret epidemiological findings more accurately and to design effective, personalized clinical nutritional interventions addressing hypotheses regarding health outcomes.
Keywords: Lycopene, pharmacokinetics, biodistribution, bioavailability, genetics, tissue accumulation
Introduction
Lycopene is a bioactive carotenoid found in tomatoes, watermelon, pink grapefruit, guava and several other red fruits. In the United States, men and women consume an average of 6.8 and 4.6 mg of lycopene/d, respectively [1]. The majority of dietary lycopene comes from tomatoes, thus plasma lycopene concentration is a robust biomarker of tomato intake. The role of lycopene as a natural bioactive has been examined relative to cancer risk [2], cardiovascular disease risk [3, 4], and other disease processes [5–8]. The expanding literature regarding lycopene is dominated by a focus upon epidemiologic investigations and experimental laboratory systems. In comparison, human intervention studies are limited; much less is known regarding lycopene absorption, distribution, metabolism, mechanisms of bioactivity, and excretion. Emerging data clearly indicate that both environmental and genetic factors have major impacts on these processes. Relevant environmental factors include the dietary source of lycopene, its geometric isomeric configuration, food processing and cooking, and nutrient composition of the co-consumed meal. Variations in host genes and nutrient status that influence body lycopene pools and bioactivity are just beginning to be elucidated. In this review, the current knowledge regarding lycopene absorption, tissue distribution, and metabolism will be addressed.
Blood lycopene concentrations in human populations
For the sake of brevity, we will focus upon epidemiological reports of blood (plasma or serum) lycopene and cancer risk, but the commentary is relevant to similar human epidemiologic studies regarding other disease processes. Interest in the relationship of dietary lycopene to cancer risk began with associations between estimated intake of tomato products and risk, with approximate lycopene exposure being defined based upon an average concentration in tomato-containing food products. While this approach is fraught with challenges and assumptions, due to imprecision of dietary assessment tools over the life span and remarkable heterogeneity of lycopene content within specific tomato-based foods, many epidemiologic studies have reported a protective relationship, while as expected, many of limited statistical power fail to detect any relationship. Based upon these hypothesis-generating studies, laboratory efforts to define lycopene as an anticancer agent in vitro and in rodent models have also suggested a potential beneficial effect [7, 9]. In parallel, more sophisticated epidemiological approaches have been pursued. Most critically, studies correlating plasma or serum lycopene concentrations and cancer risk provide some insight. For example, recent reviews illustrate the current status of the literature with an emphasis on prostate and breast cancer [10–12]. With regards to prostate cancer risk, upper quartiles/quintiles of plasma lycopene concentrations inversely associated with risk are observed to be from >0.40 to >0.92 [micro]mol/L, while, the higher risk groups fall within the lowest plasma lycopene concentration quintiles/quartiles from <0.20 to <0.26 [micro]mol/L [13–19]. Similarly, based on a meta-analysis of eight cohort studies of serum carotenoids and breast cancer risk, plasma lycopene concentrations >0.838 [micro]mol/L (the highest quintile) were inversely associated with breast cancer risk compared to plasma lycopene concentrations <0.292 [micro]mol/L (the lowest quintile) [10]. Nonetheless, these studies typically examine blood carotenoid concentrations in adult life, often prospectively as nested case-control studies, close to the diagnosis of cancer. Yet, if dietary impact on cancer risk is more closely related to earlier life events, such as during organ development, puberty, or reproductive years, correlations of earlier blood lycopene concentrations with later disease risk may be of greater interest.
Changes in plasma lycopene concentrations within the above ranges associated with decreased cancer risk can be achieved by dietary changes. A cross-over tomato product feeding study [20] in men and women demonstrated 0.2 and 0.8 [micro]mol/L changes in plasma lycopene in as little as one week of daily soup (12 mg lycopene from a 1 cup serving) or tomato sauce (21 mg lycopene from a 0.5 cup serving) consumption, respectively [20]. Interestingly, though easily changed by diet, a single measure of plasma lycopene concentrations is a robust measure of usual plasma lycopene [21], suggesting that habitual tomato intake dietary patterns are relatively constant, with blood lycopene concentrations being strongly correlated (0.63; P<0.001) in samples collected four years apart with an intra-pair coefficient of variation of ~9% [21].
Human blood lycopene pharmacokinetics
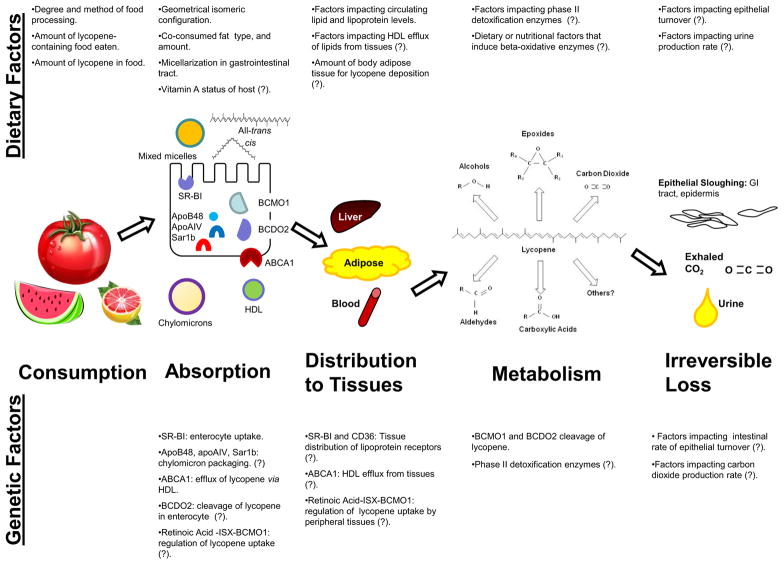
In order to examine the relevant mechanisms by which lycopene impacts disease, or serves as a biomarker for tomato intake, the pharmacokinetics of lycopene must be determined. A better understanding of the absorption, distribution, metabolism, and excretion of lycopene provides an enhanced ability to interpret epidemiologic findings, plan physiologically-relevant animal and in vitro mechanistic investigations, and to design interventions for clinical evaluation. Many aspects of the pharmacokinetics of lycopene are of interest, such as how the diet impacts circulating lycopene concentrations, absorption and biodistribution, tissue concentrations, how disease state can impact distribution, metabolic breakdown, and lastly, which human genetic variables impact these processes (Figure 1).
Figure 1. Known and Proposed Dietary and Genetic Factors Impacting Lycopene Absorption and Metabolism.
Multiple dietary and genetic factors have been demonstrated to impact the absorption and distribution of dietary lycopene and other carotenoids. In this scheme, factors for which there evidence exists connecting them to lycopene absorption and distribution are listed. Additional hypothesized factors are listed based on relationships with other carotenoids or tenuous relationships with lycopene metabolism and are denoted by a (?) and should be the subject of future investigations.
One important question concerns the half-life of lycopene in the body. Plasma lycopene half-life can provide insight into how frequently lycopene should be consumed in order to maintain a desired plasma concentration. In one study in which 20 mg of lycopene from tomato soup or from synthetic lycopene tablets was consumed for 8 sequential days, plasma lycopene half-life was found to be 6.4 and 5.6 d, respectively [22]. cis-Lycopene isomers tend to be the predominant form of lycopene seen in plasma and tissues [21, 23–26], even though these are minor constituents in red tomatoes, and therefore comparing the metabolism of these geometric isomers is of interest. The half-lives of all-trans versus 5-cis lycopene were 5.4 and 7.4 d, respectively, suggesting an underlying pharmacokinetic difference which may contribute to distinctive tissue and plasma lycopene isomeric patterns [22]. In a study of two individuals, the half-life of a microdose of total 14C-lycopene was 5.5 d in one subject, with a shorter half-life of all-trans lycopene (4.8 d) and a longer half-life of 5-cis lycopene (6.6 d), while in the second subject, 5-cis had a shorter half-life (2.9 d) than all-trans (4.2 d) [27]. Gustin, et al., reported a much shorter half-life in a study of unlabeled lycopene of ~1.2 d in response to 10 and 30 mg doses of lycopene from a tomato product [28], though this difference may have been influenced by the time points employed to calculate half-life and the absence of a labeled tracer.
Differences in half-lives for lycopene geometrical isomers are likely due to a number of factors including the higher bioavailability of cis versus trans [29–32], endogenous isomerization, which may or may not be enzymatic [33], and greater thermodynamic stability of cis at elevated temperatures [34, 35]. Based on the prevalence of lycopene cis isomers in plasma and tissues [23], emerging evidence suggesting their greater antioxidant potential than trans lycopene [36], and their hypothesized role as a preferred substrate for beta-carotene 9′,10′ dioxygenase 2 (BCDO2; an enzyme responsible for eccentric cleavage of carotenoids, discussed later) [37], future investigations should seek to describe the metabolism of cis versus trans lycopene. Based on the heterogeneity observed in these limited studies, this area of research could benefit from larger sample sizes, the use of isotopically-labeled tracer lycopene, and accounting for relevant genetic variation.
Compartmental modeling is a mathematical approach to pharmacokinetic investigation which allows tracing the movement of a compound of interest through a number of conceptual or physiological compartments described through a series of equations [38, 39]. Using a mathematical compartmental modeling approach, additional kinetic variables can be calculated including tissue lycopene pool sizes, elimination rates from kinetically distinct compartments, and nutrient bioavailability [39]. One such study of lycopene has been published and provides insight into lycopene absorption, distribution, and clearance in a study of 25 men consuming between 10 – 120 mg of lycopene from a tomato paste formulation [40]. A 7-compartment model with two kinetically distinct tissue compartments, one slow-turnover pool of lycopene and one fast-turnover pool of lycopene connected through the plasma compartment, could accurately describe plasma lycopene pharmacokinetics [40]. Irreversible loss of lycopene was described in this model from the slow turnover lycopene pool, which most likely represents the large mass of lycopene found in adipose and other extrahepatic tissues [40]. The model also revealed that the total mass of lycopene absorbed was not significantly different between different dose levels over a wide range, and was on average 4.69 ± 0.55 mg. This finding supports the hypothesis that lycopene absorption is a saturable, and perhaps active, protein mediated, or otherwise limited, process [40].
Lycopene Metabolism
Conceptually, considering lycopene metabolism via enzymatic and non-enzymatic pathways is a reasonable approach at present. Ross, et al. demonstrated that after a single microdose of 14C-lycopene, radioactivity was detected in exhaled carbon dioxide within 4 hours after dosing and within 12 hr in urine [27]. About 18% of the total radioactivity provided was detected in the total urine collected from two subjects, and ~3% was detected in the exhaled carbon dioxide over the 1008 hr study duration [27]. The presence of radioactivity in exhaled CO2 suggests beta-oxidation of lycopene [27], and it is important to consider that other, volatile, eccentric cleavage products of lycopene might also be exhaled. Furthermore, while the half-lives of intact 14C-lycopene in the two subjects of this study were 5.5 and 4.6 d, the half-life of total 14C in the plasma differed remarkably (15.8 and 21.4 d, respectively), suggesting extensive metabolism and sustained circulation of resultant lycopene metabolites [27]. These data, along with a previous report that indicated that much of the radioactivity in 14C-lycopene dosed rats was found in polar, non-lycopene fractions of tissues [41], suggested that lycopene is rapidly and extensively metabolized to a number of products that ultimately are excreted in urine or are exhaled. Intact lycopene has not been found in either exhaled air or urine, as expected, for a large and very nonpolar molecule.
As new analytical chemistry technologies develop, investigators are making progress in characterizing lycopene metabolites found in foods, experimental models, and humans. In parallel, in vitro and rodent studies are beginning to discover biological activities of several metabolites [6, 42–49]. With the availability of modern mass spectrometric methods and synthesis of lycopene metabolite standard materials, a series of chain-shortened lycopene aldehydes were discovered to be present in both lycopene-containing foods as well as in the plasma of subjects consuming tomato products [50]. Interestingly, two of these aldehydes, apo-8′-lycopenal and apo-12′-lycopenal have been detected in the livers of rats fed lycopene [51]. In addition to the aldehydes, several lycopene epoxides and diols have also been reported in human serum and milk as well as in food products [52, 53]. It is now clear that some of the metabolites may be found in food and absorbed while endogenous production in situ by enzymatic or non-enzymatic processes may also occur [50, 54]. Isotopically-labeled lycopene can be very useful in both animal models and humans to examine these processes. In several model systems, BCDO2 has been shown to cleave lycopene [37, 55], and loss of the BCDO2 enzyme is associated with greater blood intact lycopene concentrations in murine models [56]. Thus, we can postulate that variability in BCDO2 activity in humans, either due to genetic polymorphisms or to presently undefined enzyme regulatory processes may account for variability in lycopene plasma responses and also account for variability in lycopene cleavage products seen between individuals (Figure 1) [50].
Human tissue lycopene concentrations
Lycopene may indirectly impact tissue functions by modulating systemic immune responses or the hormonal environment [57–60]. However, lycopene or its metabolites may act directly at the target tissue site as well. Studies of lycopene concentrations in human tissue are limited, due to the challenges of procuring tissue samples from healthy or diseased individuals for analysis. Nonetheless, there is a diverse body of literature resulting from such human biopsy and tissue donation studies that can assist in gauging what levels of lycopene may be found in healthy versus diseased tissues and how dietary interventions can impact tissue lycopene concentrations.
Eighteen publications identified at the time of this publication have reported tissue in addition to blood lycopene concentrations. We have presented weighted averages of tissue and plasma lycopene concentrations in Table 1 based on the total number of samples in all available studies for each tissue, irrespective of study design or dietary intervention. Data was obtainable for 14 different tissues and for plasma or serum; weighted averages for tissue lycopene concentrations were then multiplied by the average tissue masses available for reference adult males and females [published by the International Commission on Radiological Protection [61]] to yield an approximate lycopene pool size in each tissue. The majority of body lycopene (60–72% in males and females) is found in the adipose tissue, with most of the remaining lycopene being found in the liver (17–23%) and serum or plasma (5–6%). Lycopene appears to be particularly concentrated in the liver (3.6 nmol/g) and adrenals (14.5 nmol/g), as well as in the testes in males (15.2 nmol/g). As a crude estimate, the mass of lycopene in adult males and females consuming a modern diet may be approximately 30 [micro]mol.
Table 1.
Weighted average tissue and plasma or serum lycopene concentrations and pool sizes1 for men and women, based on published literature
| Compartment | References | Human Weighted Average Concentration ([micro]mol/L) (min-max range) |
Pooled Sample Size (n) | Lycopene Mass in Plasma or Serum, Males ([micro]mol) | % in Serum or Plasma, Males | Lycopene Mass in Plasma or Serum, Females ([micro]mol) | % in Serum or Plasma, Females |
|---|---|---|---|---|---|---|---|
| Serum or Plasma ([micro]mol/L ) | [23, 62, 70, 75–77, 106–109] | 0.597 (0.09–1.43) | 743 | 1.79 | 6.35 | 1.43 | 4.87 |
| Compartment | References | Human Weighted Average Concentration (nmol/g) (min-max range) |
Pooled Sample Size (n) | Lycopene Mass, Males(nmol) | % Total Body Lycopene, Males | Lycopene Mass, Females (nmol) | % Total Body Lycopene, Females |
|---|---|---|---|---|---|---|---|
| Testes | [110, 111] | 15.210 (4.34–21.2) | 25 | 532 | 1.88 | n/a | n/a |
| Adrenals | [110, 111] | 14.487 (1.90–21.57) | 25 | 203 | 0.72 | 188 | 0.64 |
| Liver | [53, 110–112] | 3.552 (0.66–6.90) | 42 | 6394 | 22.59 | 4973 | 16.86 |
| Adipose | [75, 110, 111, 113] | 0.943 (0.20–2.62) | 135 | 17156 | 60.61 | 21209 | 71.92 |
| Prostate | [23, 53, 62, 76, 77, 109] | 0.482 (0.24–0.82) | 236 | 8 | 0.03 | n/a | n/a |
| Lung | [53, 112, 114] | 0.438 (0.22–0.66) | 33 | 512 | 1.81 | 388 | 1.31 |
| Kidney | [110–112] | 0.419 (0.15–0.62) | 39 | 130 | 0.46 | 115 | 0.39 |
| Colon | [53, 114] | 0.418 (0.31–1.00) | 19 | 155 | 0.55 | 150 | 0.51 |
| Heart | [110, 111] | 0.351 (n/a) | 16 | 116 | 0.41 | 88 | 0.3 |
| Skin | [53, 75, 107, 114, 115] | 0.333 (0.13–0.48) | 209 | 1100 | 3.9 | 766 | 2.61 |
| Thyroid | [110] | 0.289 (n/a) | 16 | 6 | 0.02 | 5 | 0.02 |
| Ovary | [110] | 0.280 (n/a) | 16 | n/a | n/a | 3 | 0.01 |
| Breast Adipose | [70, 106, 114] | 0.151 (0.09–0.78) | 180 | n/a | n/a | 76 | 0.26 |
| Spleen | [110] | 0.187 (n/a) | 16 | 28 | 0.1 | 24 | 0.08 |
| Brain | [116] | 0.007 (n/a) | 5 | 10.3 | 0.04 | 9.23 | 0.03 |
| Total Lycopene in Body | 28.2 [micro]mol | 29.4 [micro]mol |
Weighted averages of these tissue concentrations were based on the total number of samples in referenced studies for each tissue are presented. Weighted averages for tissue lycopene concentrations were then multiplied by the average tissue masses available for reference adult males and females [published by the International Commission on Radiological Protection [61]] to yield an approximate lycopene pool size in each tissue. To convert from nmol to ng or from [micro]mol to ug, multiply by 536.873.
Lycopene concentrations in cancer tissue
The development of a malignancy within a tissue is associated with a change in the structure of the microenvironment and cellular metabolism, thus carotenoid uptake and biological impact may be different between a cancer and the adjacent “normal” tissue. Prostate lycopene concentrations in tissue collected by prostatectomy from men with localized prostate cancer [23] differed significantly between cancerous and normal prostate tissues (P = 0.04; 0.91 +/− 0.13 nmol/g of cancer vs. 0.63 +/− 0.09 nmol/g of non-cancerous prostate, respectively). The effect of prostate cancer on lycopene accumulation in the prostate was examined in a randomized, double-blinded, placebo-controlled supplementation trial [62]. Men with either prostate cancer or benign prostatic hyperplasia were treated for 3-wk with placebo or lycopene (30 mg lycopene/day as tomato extract supplement) [62]. While the cancerous vs. benign tissue lycopene concentrations were not statistically compared in the original publication, they were numerically presented for the placebo-control group as 0.52 ± 0.66 vs. 0.39 ± 0.42 nmol/g, for prostate cancer and benign prostatic hyperplasia (BPH) respectively and for the tomato extract-fed group as 0.71 ± 0.60 vs. 0.46 ± 0.43 nmol/g, in tissues from subjects with prostate cancer vs. BPH, respectively [62].
Cancerous cells are typically more metabolically active than their non-cancerous counterparts. Perhaps an increased demand for preformed cholesterol by prostate tumors stimulates lipoprotein uptake [63], resulting in elevated lycopene co-transport into cancerous prostate tissue. Lycopene is generally understood to be distributed in the same manner as cholesterol (discussed later in this review), thus it may be hypothesized that lycopene is deposited extensively in cancerous prostate tissues as a result of perturbed cholesterol and lipoprotein homeostasis. Though the cellular uptake and efflux of lycopene and its metabolites is still poorly understood, it is known that prostate cancers have elevated levels of cholesterol in the epithelial cells [64], and cholesterol uptake via scavenger receptor class B type I (SR-BI), a receptor also involved in lycopene uptake in some tissues [65], is required for optimal LNCaP cell growth in vitro [63]. Furthermore, recently it was demonstrated that in vitro prostate cancer cells did not express ATP-binding cassette transporter, subfamily 1, ABCA1, a protein involved in cholesterol efflux, and supplying LDL in the media increased proliferation [66]. Whether BCDO2 activity or expression in prostate cancer cells compared to normal prostate cells contributes to changes in lycopene concentrations remains to be clearly demonstrated, though it is plausible. Perturbations in BCDO2 expression have been shown to result in large changes in tissue lycopene accumulation such that BCDO2 −/− mice fed lycopene had greater lycopene concentrations in the liver and testis than wild-type mice [56].
When colonic tissue was evaluated, lycopene concentrations in cancerous, adenomatous polyps vs. surrounding non-cancerous colorectal mucosa were not significantly different (P=0.062) in a small investigation of individuals with colon polyps (n = 7), though lycopene concentrations were numerically greater in uninvolved mucosa than in colonic adenomas (0.34 versus 0.21 pmol/ug DNA) [67]. When rectal and/or colonic mucosa, polyps, and cancerous tissues were examined in healthy subjects, those with benign polyps, and those with cancer, respectively, it was found that the polyp tissues of benign polyp patients had greater lycopene concentrations than normal surrounding colon and rectal tissues (P < 0.001 and P < 0.05, respectively), while lycopene concentrations in cancerous tissue (0.027 ± 0.048 [micro]mol lycopene/kg) was not different from normal surrounding rectal tissue (0.017 ± 7.1 [micro]mol lycopene/g) in patients with cancer [68]. In women who were healthy, had precancerous lesions, or had cervical cancer, the lycopene concentrations of healthy, pre-cancerous, and cancerous cervical tissue did not significantly differ (0.24 ± 0.16, 0.25 ± 0.15, and 0.25 ± 0.17 nmol/g, respectively) [69]. At this point in time, it is premature to make any conclusions regarding lycopene concentrations in cancer compared to nonmalignant counterparts.
Correlation between plasma and tissue lycopene
Evaluating the correlation between plasma and tissue lycopene is of tremendous interest. Breast adipose trans-lycopene concentrations were correlated with serum lycopene concentrations in Korean women with either benign breast disease or breast cancer [70]. A correlation coefficient of 0.614 was found for unadjusted breast adipose and serum lycopene concentrations, which improved slightly when plasma was adjusted for triglyceride content (0.683) [70]. In a study to evaluate the correlation between lycopene concentrations at different adipose tissue sites (abdomen, buttock, and thigh) with serum concentrations and dietary lycopene intake, abdominal adipose tissue was found to have the strongest correlation with dietary intake of carotenoids (0.514 Pearson correlation coefficient) and serum carotenoid concentrations (0.420 Pearson correlation coefficient), [71]. Other studies have found adipose lycopene concentrations and lycopene intake to be more weakly correlated. Specifically, while estimated total dietary lycopene intake was poorly correlated with serum lycopene concentrations (0.078), abdominal adipose had a stronger correlation with dietary lycopene intake (0.408) and serum lycopene (0.384) [71]. Similarly, another study found lycopene intake to be quite weakly correlated with plasma lycopene (r=0.19 and 0.35 for women and men, respectively) and buttock adipose tissue (r=0.14 and 0.26, women and men, respectively) [72]. Another group reported that the correlation between dietary lycopene intake and buttock adipose lycopene was low (0.28), and similar to the weak correlation between serum lycopene and dietary intake (0.30) [73]. These studies clearly reinforce the concept that estimated dietary exposure from food diaries and food frequency questionnaires (FFQ) are correlated rather weakly with serum and adipose tissue lycopene concentrations. Correlations between FFQ /food diary-estimated lycopene intake with plasma lycopene are poor and can range from 0.16–0.54, with high levels of variability, emphasizing a need to understand other factors that contribute to resultant physiological lycopene concentrations [74]. However, in carefully controlled human intervention trials, changes in dietary exposure will significantly impact serum and tissue concentrations over a period of a few days to a month [20, 62, 75, 76].
Changes in tissue lycopene concentrations with dietary lycopene interventions
A small number of studies examined changes in lycopene concentrations in the prostate, adipose, skin, and buccal mucosal cells of individuals with or without cancer in response to a dietary lycopene intervention. First, to understand the supplement-plasma-tissue lycopene relationship, men scheduled for prostate biopsies consumed 30 mg of lycopene/day from a tomato oleoresin gel capsule supplement for three weeks, and experienced significant changes in circulating plasma lycopene (P < 0.0001) from 0.74 ± 0.39 [micro]mol/L up to 1.43 ± 0.61 [micro]mol/L. Compared to men consuming a lycopene-free placebo, the prostates of the men who consumed lycopene had significantly greater (P = 0.005) lycopene concentrations (0.59 ± 0.47 nmol/g; n = 49) than the placebo group (0.446 ± 0.530 nmol/g; n = 46) [62]. A similar change in plasma lycopene, from 0.43 ± 0.22 to 1.24 ± 0.31 [micro]mol/L was achieved with a longer, 6 month, supplementation period with 15 mg lycopene /day [77]. To evaluate changes in prostatic lycopene in men with localized prostatic adenocarcinoma in response to a 3 wk feeding regimen of daily tomato pasta sauce (30 mg lycopene/day from ¾ c sauce), biopsies were taken before the intervention and tissue samples were collected by prostatectomy after the intervention [76]. Prostatic lycopene changed significantly (P < 0.001) from 0.28 nmol/g at baseline to 0.82 nmol/g after the 21 d intervention [76]. A similar impact of supplementation was observed in the non-malignant prostatic tissue of men with prostate cancer who consumed 30 mg of lycopene/d from a tomato-oleoresin capsule for 3 weeks and underwent a radical prostatectomy [78]. When compared with prostate tissue from men provided a placebo, the tomato oleoresin supplemented group had significantly greater final prostatic lycopene concentrations (P = 0.02; 0.99 ± 0.06 vs. 0.67 ± 0.11 pmol/g) [78]
A tissue that is more easily obtained and has often been examined as a surrogate for general tissue lycopene exposure is the oral buccal mucosa. Buccal mucosal cell (BMC) lycopene changed from 0.06 ± 0.1 to 0.59 ± 0.58 pmol/[micro]g DNA with consumption of 15 mg of synthetic lycopene/day for 6 months (P<0.0001) [77]. In a much shorter study, the effect of consuming three different tomato-containing foods for two weeks on BMC lycopene concentrations was evaluated. Consuming tomato sauce (21 mg lycopene from a 0.5 cup serving), juice (17 mg lycopene from a 8 oz serving), or soup (12 mg lycopene from a 1 cup serving) raised baseline BMC lycopene concentrations from 1.26 to 3.34 (P< 0.005), 1.82 (non-significant increase), and 1.72 (non-significant increase) nmol lycopene/[micro]g protein, respectively [20]. The effect of matrix and delivery method on BMC lycopene concentrations was also quite apparent when subjects were fed daily lycopene (70–75 mg/d) from tomato juice (2 cups), tomato oleoresin (4 capsules), or lycopene beadlets (15 capsules) for 4 weeks [79]. While lycopene concentrations increased in all three treatment groups, this increase was only significant with the oleoresin supplementation (P<0.001; 3.8 ± 0.54 to 9.22 ± 1.0 nmol/g protein), beadlet supplementation was marginally significant (P<0.053; from 3.6 ± 0.67 to 7.0 ± 1.1 nmol/g protein), and tomato juice led to only a minor increase (from 4.9 ± 0.7 to 6.1 ± 1.3 nmol/g protein) [79]. These studies clearly show that BMC lycopene concentrations do respond to lycopene consumption and that heterogeneity in response within a population is quite large.
Changes in adipose and skin lycopene concentration after lycopene feeding have been investigated in several studies. Walfisch, et al. investigated the effect of tomato oleoresin supplementation (30 mg lycopene/d for 1–7 wk) compared to placebo on patients with planned hemorrhoidectomy or perianal fistulotomy [75]. After a variable duration of tomato oleoresin consumption (average 24 ± 3 days) or placebo (average 25 ± 2 days), serum, skin, and adipose lycopene concentrations were significantly greater in the tomato-oleoresin supplemented group than in the placebo group. Serum lycopene in the placebo group was 0.24 ± 0.12 versus 0.52 ± 0.25 [micro]mol/L (P<0.0001) in the oleoresin group, skin lycopene concentrations of the placebo group were 0.30 ± 0.18 versus 0.48 ± 0.20 nmol/g (P=0.0003) in the oleoresin dosed group, and adipose lycopene concentrations of the placebo group were 0.23 +/− 0.16 versus 0.34 ± 0.23 nmol/g (P =0.02) in the oleoresin group. In this study, serum and skin were more responsive to supplementation than adipose tissue [75], supporting the concept that adipose is a much larger pool of lycopene with slower turnover [40]. A 12 wk time course study of lactolycopene supplementation (an unique preparation containing lycopene in a whey protein matrix, which provided 25 mg lycopene/d) or placebo resulted in similar findings to those of Walfisch, et al. [80]. Compared to skin lycopene concentrations after a 4 wk lycopene wash-out, skin lycopene concentrations were significantly increased after 4 wk of lactolycopene supplementation on the back (~0.1 vs. ~0.2 nmol/g; P=0.007), forearm (~0.15 vs. ~0.2 nmol/g; P=0.037), and forehead (0.2 vs. ~0.29 nmol/g; P=0.077), but with a return to washout levels for the remaining 8 wk of supplementation [80]. Serum lycopene concentrations were more sensitive to lycopene supplementation, with significant elevations after 4, 8, and 12 weeks (~0.2 [micro]mol/L before supplementation, and ~0.75, ~0.78, and ~0.81 [micro]mol/L, at 4, 8, and 12 wk; P<0.0001 for all time points) of supplementation compared to baseline [80]. Recent advances in Raman resonance spectroscopy offers a non-invasive method to measure skin lycopene, and these measurements are shown to be highly correlated (0.74) with invasive biopsy HPLC measurements [81, 82]. As a result of this technological advance, more information linking dietary lycopene, skin, and plasma concentrations are expected.
Dietary factors influencing lycopene biodistribution
Lycopene bioavailability in the proximal intestine is understood to be influenced by food processing and cooking, meal composition, mastication, and the isomeric configurations of lycopene (Figure 1) [9, 83]. Absorption and lycopene plasma concentrations are then influenced by the ability of transporters on absorptive intestinal cells to facilitate lycopene uptake and basolateral efflux from the enterocyte, an individual’s lipid metabolic profile, the uptake and efflux transporters present on different cell types, and the activity of putative lycopene metabolizing enzymes. Several comprehensive and relevant reviews of absorption and transport of the array of fat soluble vitamins as well as carotenoids have been recently published [25, 26, 84]. Lycopene is a very lipophilic molecule, and therefore to be absorbed from a food it must be solubilized, first by emulsification in the gastric contents, then incorporation into mixed micelles in the duodenum as facilitated by bile acid surfactants and lipases [84]. Co-consumed dietary fat is important, if not essential, for effectively solubilizing lycopene, stimulating the excretion of bile acids, and providing lipids to form the micelles. Characteristics of specific lipids make this process more or less efficient. Triglyceride fatty acyl chain length is inversely correlated with lycopene solubility, however the opposite is true for mixed micelle formation [85], while the fatty acyl chain length does not impact enterocyte uptake of carotenoids. The impact of degree of fatty acid saturation on lycopene incorporation into chylomicrons is currently unknown [85]. Lycopene, although a lipophilic compound, is poorly soluble in most food-grade oils [24], however, the amount of co-consumed dietary lipid can significantly impact the amount of lycopene absorbed as determined in the post-prandial triglyceride rich lipoprotein (TRL) fraction of the plasma after consuming a salad of raw vegetables and either soybean oil, butter, or canola oil-containing dressings [86]. In particular, 20 g of co-consumed lipid increased the amount of lycopene absorbed as determined by plasma lycopene area under the curve (AUC) response, regardless of lipid type, compared to 8 or 3 g of co-consumed lipids [86]. However, a dose-response effect was not observed with two different amounts of avocado fruit or oil (contributing 12 or 24 g of lipid) on lycopene bioavailability from salsa or salad, though avocado or avocado oil consumption at either level significantly increased the triglyceride rich lipoprotein (TRL) fraction lycopene AUC response [87]. A clearer understanding of how much and what species of lipid is required to impact lycopene absorption is needed, as it apparently is not a linear relationship. In addition to enhancing solubilization and micellarization, dietary lipids may also enhance lycopene absorption via specific upregulation of lycopene uptake and effluxing proteins which may be in part regulated by dietary fat exposure.
Genetic and cellular physiological factors influencing lycopene distribution
Emerging evidence from human genetic variant association studies as well as mouse genetic studies have indicated the important role that genes encoding proteins involved in absorption and metabolism of lipids and carotenoids may play in defining serum and tissue concentrations of lycopene and biological activity (Figure 1) [1, 25, 26, 56, 88–90]. We currently have limited knowledge on which transporters may be involved in active lycopene uptake and efflux from the enterocyte, but several in vitro studies suggest that transporters involved in cholesterol metabolism may play a role [26]. The observation that lycopene uptake and secretion in Caco-2 cells (an in vitro model used to study intestinal epithelial transport processes) is reduced by about 20% when treated with a cholesterol uptake inhibitor, ezetimibe, supports this hypothesis [65]. Ezetimibe acts by decreasing expression of the cholesterol receptors scavenger receptor class B, type I (SR-BI), Niemann-Pick type C1 Like1 (NPC1L1), and ABCA1 [65], and it was determined that SR-BI was responsible for up to 60% of lycopene uptake in Caco-2 cells and NPC1L1 was not involved in lycopene uptake [91]. This point was supported when a study in mice overexpressing SR-BI in the intestine were fed lycopene-containing diets and had nearly 10-fold greater plasma lycopene concentrations than wild-type mice [91]. Interestingly, Lobo, et al. discovered that the expression of SR-BI is mediated by a homeobox transcription factor, ISX, which is in turn regulated by retinoic acid, thus it is quite plausible that vitamin A status has an indirect effect on lycopene (and -carotene) absorption via intestinal SR-BI expression [89]. Carotenoid cleavage enzymes, BCMO1 and BCDO2, are present in the intestinal epithelium, and while BCMO1 appears to be negative feedback-regulated by ISX-mediated retinoic acid signaling [89], the regulation of BCDO2 remains unknown. Carotenoids that are not cleaved by BCMO1 or BCDO2 in the intestinal epithelial cells are primarily packaged into chylomicrons and transported in the lymphatic circulation to the thoracic duct and into the venous circulation, or may be processed into HDL particles of intestinal origin [25]. While little is directly known about the basolateral efflux of lycopene from the enterocyte, it is generally thought that the proteins involved in chylomicron assembly, such as microsomal triglyceride transfer protein, apoB48, apoAIV, and Sar1b, are involved in the incorporation of lycopene into chylomicrons for excretion [25]. The protein involved in HDL secretion, ABCA1, may also be involved indirectly in lycopene efflux from intestinal cells [25]. Clearly, much more research is necessary to elucidate the intracellular handling of lycopene in the enterocyte and how polymorphisms in these genes may be relevant to humans.
In fasting plasma, lycopene is found primarily in LDL (76%), followed by HDL (17%) and VLDL (7%) [92], thus it is plausible that genetic factors impacting lipoprotein metabolism are likely to impact lycopene plasma pharmacokinetics and tissue distribution. In fact, plasma cholesterol levels were significantly associated with plasma lycopene levels (P= 0.0001) and in a stepwise regression analysis, accounted for 14% of the variability in plasma lycopene in a study population (n = 111) [93]. Dietary lycopene, plasma triglycerides, and dietary vitamin C were also significantly correlated with plasma lycopene levels in this population (P=0.002, 0.005, and 0.03, respectively) [93]. Thus, genetic variation related to lipid metabolism, drugs which impact lipids, and dietary lipid profiles likely impact lycopene status in a complex interactive manner that remains to be clearly explained.
While the involvement of SR-BI in lycopene absorption has been demonstrated for intestinal cells, the role of this protein in non-intestinal tissue uptake of lycopene has not yet been confirmed, although this transporter is found in liver, testes, ovaries, and macrophages [25]. SR-BI thus appears to have broad substrate specificity, including primarily HDL, as well as LDL, and VLDL, permitting uptake of esterified cholesterol from HDL, but also intestinal uptake of free cholesterol, esterified cholesterol, phospholipids, and triacylglycerol hydrolysis products [25]. While SNPs in SR-BI have not yet been reported to be related to plasma lycopene, one SNP in this protein (rs11057841) was associated with increased serum lutein, a xanthophyll carotenoid also transported by this protein, such that each T allele confers a 24% increase in plasma lutein [94]. Because SR-BI is already believed to be involved in lycopene uptake, genetic variants of this protein are of significant interest with regards to lycopene as well. Another scavenger receptor, cluster determinant 36 (CD36), is known to facilitate the uptake of long chain fatty acids into both muscle and adipose cells, and was also estimated to mediate ~20–50% of lycopene uptake into adipose in vitro cultured cells and ex vivo white adipose tissue from wild-type and CD36 −/− mice [95]. The determination of which transporters are important for lycopene uptake on cell types other than adipocytes and enterocytes has yet to be investigated.
The subcellular localization of lycopene in cells may also impact biological activity. In cultured prostate cancer cells (LNCaP, DU145, and PC-3), LNCaP cells accumulated the greatest amount of lycopene from the media, and the majority of this lycopene is found in the nuclear membranes (55%), followed by the nuclear matrix (26%), and microsomal fractions (19%), with lycopene being undetectable in the cytosol [96]. In adipocytes, lycopene was nearly evenly distributed between lipid droplets (32–51%), the plasma membrane (32–37%), and the nuclear membrane (19–29%) [97], while in hepatic stellate cells the majority of lycopene, 65%, was in cellular membranes, followed by 22% in the cytoplasm, 8% in the lipid droplets, and 5% in the nuclei [33]. These studies indicate that lycopene subcellular localization may vary substantially in differing cell types, each of which has unique functions. This concept may suggest unique bioactivities of lycopene in various tissues.
Carotenoid Metabolizing Enzymes, BCMO1 and BCDO2
Variation in the expression and activity carotenoid metabolizing enzymes may have a significant impact on in vivo lycopene concentrations and bioactivity (Figure 1). Most of this research has focused on genetic variants of the beta-carotene 15,15′-monooxygenase 1 (BCMO1) enzyme, and impact upon beta-carotene absorption and vitamin A status. BCMO1 is responsible for the central cleavage of provitamin A carotenoids like beta-carotene and alpha-carotene, to yield retinaldehyde. The BCMO1 metabolizing enzyme is negatively feedback regulated by retinoic acid, one of the downstream metabolites of retinaldehyde, in the intestine [89]. BCMO1 is a cytoplasmic protein [98] found in relatively high amounts in the epithelium of a number of tissues and organs including the stomach, small intestine, colon, and endometrium, as well as the skin epidermis, the liver hepatocytes, and pancreatic acinar cells [99]. It is more weakly present in the kidney proximal and distal convoluted tubules, testicular Leydig and Sertoli cells, adrenal zona glomerulosa, fasciculata, reticularis, prostatic stroma and epithelium, ovarian granulosa and theca interna, and skeletal muscle fibers [99]. While BCMO1−/− mice have lower concentrations of lycopene in some tissues (liver, spleen, and thymus) than wild-type mice fed lycopene, and a polymorphism in the human BCMO1 gene is related to lower plasma lycopene concentrations [100, 101], more work is necessary to define whether lycopene is a substrate of the BCMO1 enzyme. Interestingly, dietary lycopene in rats down-regulated expression of BCMO1 in several tissues, although the role of BCMO1 in metabolism of lycopene in humans is uncertain [1, 6, 102]. There are ten reported polymorphisms in the BCMO1 gene that result in amino acid substitutions, though the frequency of only three of these is known (4–36%) for individuals from the United States with European ancestry [103]. Some of these variants were associated with decreased efficiency of beta-carotene conversion to vitamin A [103]. Furthermore, the G-allele of one SNP, rs6564851, was found in two large populations to be associated with lower plasma lycopene [101]. In a small clinical trial, the effect of single nucleotide polymorphisms, or SNPs, in the carotenoid metabolizing enzymes, BCMO1 and BCDO2, on plasma lycopene response was directly investigated for the first time [1]. Subjects consumed lycopene-containing watermelon juice or tomato juice and were then classified as either strong or weak responders based upon serum lycopene response and the effect of SNPs in the BCMO1 gene was evaluated [1]. Two SNPs in the BCMO1 coding region were investigated, rs12934922 (0.448 heterozygosity) and rs7501331 (0.235 heterozygosity), and while differing genotypes in either SNP alone were not correlated with lycopene plasma response, a combination of genotypes for the two SNPs (AA/CT for rs12934922 and rs7501331, respectively) was associated with being a lycopene responder.
BCDO2 has broader substrate specificity and is responsible for the eccentric cleavage of both pro-vitamin A and non-provitamin A carotenoids [104]. Limited information exists on the expression patterns of BCDO2 in different tissues, however it is known to vary broadly, with higher expression in the liver and duodenum and lower expression in the testes and prostate of both mice and humans [6]. BCDO2 mRNA is also detected in the kidneys, adrenals, stomach, pancreas, eye, muscle, prostate, and endometrium [105]. How the expression patterns may effect tissue lycopene concentrations is not yet fully understood, but should be examined further. BCDO2 SNPs were not able to be investigated in the aforementioned clinical trial of SNPs and lycopene plasma response because of a low frequency of heterozygosity in their population (<10%) [1]. In fact, SNPs in BCDO2 are generally of very low frequency in the population, with only 2 of 13 found in the exon to occur in >5% of a population with European ancestry [104]. However, 19 splice variants have been identified, and thus Lietz and colleagues have suggested that such splice variants may be a more common source of differences in enzymatic carotenoid cleavage activity than SNPs [104]. A correlation between BCDO2 SNPs and decreased fasting HDL concentrations in women has been reported, though this finding will require further investigation [104].
Summary
Studies from the epidemiological literature and a growing number of human clinical studies, coupled with accumulating evidence from the laboratory support the hypothesis that lycopene may have biological activity in humans and impact health or disease risk. Yet, definitive causal relationships are very uncertain, and it is not possible to recommend a specific dose of lycopene or foods containing lycopene in order to enhance health outcomes or prevent disease. Studies in humans suggest that lycopene exposure, absorption, metabolism, and bioactivity is quite complex. By synthesizing and building upon existing knowledge on how dietary lycopene intake interacts with host genetics to result in unique tissue and plasma lycopene profiles will yield a better understanding of lycopene bioactivity. Plant foods, such as tomatoes, contain highly variable amounts of lycopene due to plant genetics and environmental conditions. Food processing and cooking may have a dramatic impact on bioavailability. Many genes may impact lycopene absorption and metabolism and human variation in expression of these genes, due to regulatory factors or inherited polymorphisms, is only beginning to be understood. In addition, the molecular and cellular mechanisms whereby lycopene or its metabolites impact biology in various tissues are uncertain. Indeed, a great scientific effort is imperative if we are to move forward and provide answers to these relevant questions. As the understanding of how dietary and genetic variables interact emerges, so will the potential to better interpret epidemiological and experimental findings in order to develop appropriate dietary strategies to reduce disease risk.
Highlights.
There is wide tissue and plasma lycopene variability in response to dietary consumption.
Dietary factors include fat amount and type, food matrix, and geometric isomeric configuration.
Genetic factors may be lipid transporters, lipoproteins, and carotenoid cleavage enzymes.
Most intact lycopene is found in the adipose, liver, and serum.
Lycopene products include epoxides, alcohols, aldehydes, carboxylic acids, and carbon dioxide.
Acknowledgments
Funding
Support provided by NIH NCCAM R21 AT005166, and NEM was supported by a Pelotonia Postdoctoral Fellowship from the Ohio State University Comprehensive Cancer Center.
Abbreviations
- BCMO1
Beta-carotene 15, 15′ monooxygenase
- BCDO2
Beta-carotene 9′,10′ dioxygenase
- SR-B1
Scavenger receptor class B type1
- NPC1L1
Niemann-Pick type C1 Like1
- ABCA1
ATP-binding cassette transporter, subfamily A
- SNP
Single nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang TT, Edwards AJ, Clevidence BA. J Nutr Biochem. 2013 doi: 10.1016/j.jnutbio.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 2.WCRF and AICR. Summary: food, nutrition, physical activity and the prevention of cancer: a global perspective. American Institute for Cancer Research; Washington, D.C: 2007. [Google Scholar]
- 3.Voutilainen S, Nurmi T, Mursu J, Rissanen TH. Am J Clin Nutr. 2006;83:1265–1271. doi: 10.1093/ajcn/83.6.1265. [DOI] [PubMed] [Google Scholar]
- 4.Mordente A, Guantario B, Meucci E, Silvestrini A, Lombardi E, Martorana GE, Giardina B, Bohm V. Curr Med Chem. 2011;18:1146–1163. doi: 10.2174/092986711795029717. [DOI] [PubMed] [Google Scholar]
- 5.Clinton SK. Nutr Rev. 1998;56:35–51. doi: 10.1111/j.1753-4887.1998.tb01691.x. [DOI] [PubMed] [Google Scholar]
- 6.Ford NA, Erdman JW., Jr Acta Biochim Pol. 2012;59:1–4. [PubMed] [Google Scholar]
- 7.Story EN, Kopec RE, Schwartz SJ, Harris GK. Annu Rev Food Sci Technol. 2010;1:189–210. doi: 10.1146/annurev.food.102308.124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canene-Adams K, Campbell JK, Zaripheh S, Jeffery EH, Erdman JW., Jr J Nutr. 2005;135:1226–1230. doi: 10.1093/jn/135.5.1226. [DOI] [PubMed] [Google Scholar]
- 9.Tan HL, Thomas-Ahner JM, Grainger EM, Wan L, Francis DM, Schwartz SJ, Erdman JW, Jr, Clinton SK. Cancer Metastasis Rev. 2010;29:553–568. doi: 10.1007/s10555-010-9246-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eliassen AH, Hendrickson SJ, Brinton LA, Buring JE, Campos H, Dai Q, Dorgan JF, Franke AA, Gao YT, Goodman MT, Hallmans G, Helzlsouer KJ, Hoffman-Bolton J, Hulten K, Sesso HD, Sowell AL, Tamimi RM, Toniolo P, Wilkens LR, Winkvist A, Zeleniuch-Jacquotte A, Zheng W, Hankinson SE. J Natl Cancer Inst. 2012;104:1905–1916. doi: 10.1093/jnci/djs461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei MY, Giovannucci EL. J Oncol. 2012;2012:271063. doi: 10.1155/2012/271063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etminan M, Takkouche B, Caamano-Isorna F. Cancer Epidemiol Biomarkers Prev. 2004;13:340–345. [PubMed] [Google Scholar]
- 13.Giovannucci E. Cancer Causes Control. 2011;22:1055–1059. doi: 10.1007/s10552-011-9776-x. [DOI] [PubMed] [Google Scholar]
- 14.Key TJ, Appleby PN, Allen NE, Travis RC, Roddam AW, Jenab M, Egevad L, Tjonneland A, Johnsen NF, Overvad K, Linseisen J, Rohrmann S, Boeing H, Pischon T, Psaltopoulou T, Trichopoulou A, Trichopoulos D, Palli D, Vineis P, Tumino R, Berrino F, Kiemeney L, Bueno-de-Mesquita HB, Quiros JR, Gonzalez CA, Martinez C, Larranaga N, Chirlaque MD, Ardanaz E, Stattin P, Hallmans G, Khaw KT, Bingham S, Slimani N, Ferrari P, Rinaldi S, Riboli E. J Clin Nutr Am. 2007;86:672–681. doi: 10.1093/ajcn/86.3.672. [DOI] [PubMed] [Google Scholar]
- 15.Lu QY, Hung JC, Heber D, Go VL, Reuter VE, Cordon-Cardo C, Scher HI, Marshall JR, Zhang ZF. Cancer Epidemiol Biomarkers Prev. 2001;10:749–756. [PubMed] [Google Scholar]
- 16.Zhang J, Dhakal I, Stone A, Ning B, Greene G, Lang NP, Kadlubar FF. Nutr Cancer. 2007;59:46–53. doi: 10.1080/01635580701385900. [DOI] [PubMed] [Google Scholar]
- 17.Vogt TM, Mayne ST, Graubard BI, Swanson CA, Sowell AL, Schoenberg JB, Swanson GM, Greenberg RS, Hoover RN, Hayes RB, Ziegler RG. Am J Epidemiol. 2002;155:1023–1032. doi: 10.1093/aje/155.11.1023. [DOI] [PubMed] [Google Scholar]
- 18.Hsing AW, Comstock GW, Abbey H, Polk BF. J Natl Cancer Inst. 1990;82:941–946. doi: 10.1093/jnci/82.11.941. [DOI] [PubMed] [Google Scholar]
- 19.Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, Helzlsouer KJ. Am J Epidemiol. 2003;157:335–344. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 20.Allen CM, Schwartz SJ, Craft NE, Giovannucci EL, De Groff VL, Clinton SK. Nutr Cancer. 2003;47:48–56. doi: 10.1207/s15327914nc4701_6. [DOI] [PubMed] [Google Scholar]
- 21.Wu K, Schwartz SJ, Platz EA, Clinton SK, Erdman JW, Jr, Ferruzzi MG, Willett WC, Giovannucci EL. J Nutr. 2003;133:1930–1936. doi: 10.1093/jn/133.6.1930. [DOI] [PubMed] [Google Scholar]
- 22.Cohn W, Thurmann P, Tenter U, Aebischer C, Schierle J, Schalch W. Eur J Nutr. 2004;43:304–312. doi: 10.1007/s00394-004-0476-0. [DOI] [PubMed] [Google Scholar]
- 23.Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, Erdman JW., Jr Cancer Epidemiol Biomarkers Prev. 1996;5:823–833. [PubMed] [Google Scholar]
- 24.Spernath A, Yaghmur A, Aserin A, Hoffman RE, Garti N. J Agric Food Chem. 2002;50:6917–6922. doi: 10.1021/jf025762n. [DOI] [PubMed] [Google Scholar]
- 25.Borel P. Mol Nutr Food Res. 2012;56:228–240. doi: 10.1002/mnfr.201100322. [DOI] [PubMed] [Google Scholar]
- 26.Reboul E, Borel P. Prog Lipid Res. 2011;50:388–402. doi: 10.1016/j.plipres.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Ross AB, Vuong LT, Ruckle J, Synal HA, Schulze-König T, Wertz K, Rümbeli R, Liberman RG, Skipper PL, Tannenbaum SR, Bourgeois A, Guy PA, Enslen M, Nielsen ILF, Kochhar S, Richelle M, Fay LB, Williamson G. The American Journal of Clinical Nutrition. 2011;93:1263–1273. doi: 10.3945/ajcn.110.008375. [DOI] [PubMed] [Google Scholar]
- 28.Gustin DM, Rodvold KA, Sosman JA, Diwadkar-Navsariwala V, Stacewicz-Sapuntzakis M, Viana M, Crowell JA, Murray J, Tiller P, Bowen PE. Cancer Epidemiol Biomarkers Prev. 2004;13:850–860. [PubMed] [Google Scholar]
- 29.Unlu NZ, Bohn T, Francis DM, Nagaraja HN, Clinton SK, Schwartz SJ. Br J Nutr. 2007;98:140–146. doi: 10.1017/S0007114507685201. [DOI] [PubMed] [Google Scholar]
- 30.Unlu NZ, Bohn T, Francis D, Clinton SK, Schwartz SJ. J Agric Food Chem. 2007;55:1597–1603. doi: 10.1021/jf062337b. [DOI] [PubMed] [Google Scholar]
- 31.Boileau AC, Merchen NR, Wasson K, Atkinson CA, Erdman JW., Jr J Nutr. 1999;129:1176–1181. doi: 10.1093/jn/129.6.1176. [DOI] [PubMed] [Google Scholar]
- 32.Boileau TW, Boileau AC, Erdman JW., Jr Exp Biol Med (Maywood) 2002;227:914–919. doi: 10.1177/153537020222701012. [DOI] [PubMed] [Google Scholar]
- 33.Teodoro AJ, Perrone D, Martucci RB, Borojevic R. Eur J Nutr. 2009;48:261–268. doi: 10.1007/s00394-009-0001-6. [DOI] [PubMed] [Google Scholar]
- 34.Schiedt K, Liaaen-Jensen S. In: Carotenoids: Isolation and Analysis. Britton G, Liaaen-Jensen S, Pfander H, editors. Birkhauser Verlag; Basel: 1995. p. 81. [Google Scholar]
- 35.Hadley CW, Miller EC, Schwartz SJ, Clinton SK. Exp Biol Med (Maywood) 2002;227:869–880. doi: 10.1177/153537020222701006. [DOI] [PubMed] [Google Scholar]
- 36.Muller L, Goupy P, Frohlich K, Dangles O, Caris-Veyrat C, Bohm V. J Agric Food Chem. 2011;59:4504–4511. doi: 10.1021/jf1045969. [DOI] [PubMed] [Google Scholar]
- 37.Hu KQ, Liu C, Ernst H, Krinsky NI, Russell RM, Wang XD. J Biol Chem. 2006;281:19327–19338. doi: 10.1074/jbc.M512095200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green MH. J Nutr. 1992;122:690–694. doi: 10.1093/jn/122.suppl_3.690. [DOI] [PubMed] [Google Scholar]
- 39.Novotny JA. J Nutr. 2005;135:2048S–9S. doi: 10.1093/jn/135.8.2048S. [DOI] [PubMed] [Google Scholar]
- 40.Diwadkar-Navsariwala V, Novotny JA, Gustin DM, Sosman JA, Rodvold KA, Crowell JA, Stacewicz-Sapuntzakis M, Bowen PE. J Lipid Res. 2003;44:1927–1939. doi: 10.1194/jlr.M300130-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Zaripheh S, Boileau TW, Lila MA, Erdman JW., Jr J Nutr. 2003;133:4189–4195. doi: 10.1093/jn/133.12.4189. [DOI] [PubMed] [Google Scholar]
- 42.Chung J, Koo K, Lian F, Hu KQ, Ernst H, Wang XD. J Nutr. 2012;142:405–410. doi: 10.3945/jn.111.150052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang CM, Hu TY, Hu ML. Nutr Cancer. 2012;64:274–285. doi: 10.1080/01635581.2012.643273. [DOI] [PubMed] [Google Scholar]
- 44.Yang CM, Huang SM, Liu CL, Hu ML. J Agric Food Chem. 2012;60:1576–1585. doi: 10.1021/jf204451n. [DOI] [PubMed] [Google Scholar]
- 45.Ford NA, Elsen AC, Zuniga K, Lindshield BL, Erdman JW., Jr Nutr Cancer. 2011;63:256–263. doi: 10.1080/01635581.2011.523494. [DOI] [PubMed] [Google Scholar]
- 46.Gouranton E, Aydemir G, Reynaud E, Marcotorchino J, Malezet C, Caris-Veyrat C, Blomhoff R, Landrier JF, Ruhl R. Biochim Biophys Acta. 2011;1811:1105–1114. doi: 10.1016/j.bbalip.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Mein JR, Lian F, Wang XD. Nutr Rev. 2008;66:667–683. doi: 10.1111/j.1753-4887.2008.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lian F, Smith DE, Ernst H, Russell RM, Wang XD. Carcinogenesis. 2007;28:1567–1574. doi: 10.1093/carcin/bgm076. [DOI] [PubMed] [Google Scholar]
- 49.Lindshield BL, Canene-Adams K, Erdman JW., Jr Arch Biochem Biophys. 2007;458:136–140. doi: 10.1016/j.abb.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Kopec RE, Riedl KM, Harrison EH, Curley RW, Hruszkewycz DP, Clinton SK, Schwartz SJ. J Agric Food Chem. 2010;58:3290–3296. doi: 10.1021/jf100415z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gajic M, Zaripheh S, Sun F, Erdman JW., Jr J Nutr. 2006;136:1552–1557. doi: 10.1093/jn/136.6.1552. [DOI] [PubMed] [Google Scholar]
- 52.Khachik F, Pfander H, Traber B. J Agric Food Chem. 1998;46:4885–4890. [Google Scholar]
- 53.Khachik F, Carvalho L, Bernstein PS, Muir GJ, Zhao DY, Katz NB. Exp Biol Med (Maywood) 2002;227:845–851. doi: 10.1177/153537020222701002. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez EB, Rodriguez-Amaya DB. J Food Sci. 2009;74:C674–82. doi: 10.1111/j.1750-3841.2009.01353.x. [DOI] [PubMed] [Google Scholar]
- 55.Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. J Biol Chem. 2001;276:14110–14116. doi: 10.1074/jbc.M011510200. [DOI] [PubMed] [Google Scholar]
- 56.Ford NA, Clinton SK, von Lintig J, Wyss A, Erdman JW., Jr J Nutr. 2010;140(12):2134. doi: 10.3945/jn.110.128033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campbell JK, Stroud CK, Nakamura MT, Lila MA, Erdman JW., Jr J Nutr. 2006;136:2813–2819. doi: 10.1093/jn/136.11.2813. [DOI] [PubMed] [Google Scholar]
- 58.Ford NA, Moran NE, Smith JW, Clinton SK, Erdman JW., Jr Int J Cancer. 2012;131:E143–8. doi: 10.1002/ijc.26446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Briviba K, Kulling SE, Moseneder J, Watzl B, Rechkemmer G, Bub A. Carcinogenesis. 2004;25:2373–2378. doi: 10.1093/carcin/bgh249. [DOI] [PubMed] [Google Scholar]
- 60.Watzl B, Bub A, Briviba K, Rechkemmer G. Ann Nutr Metab. 2003;47:255–261. doi: 10.1159/000072397. [DOI] [PubMed] [Google Scholar]
- 61.Valentin J. Ann ICRP. 2002;32:1–277. doi: 10.1016/S0146-6453(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 62.van Breemen RB, Sharifi R, Viana M, Pajkovic N, Zhu D, Yuan L, Yang Y, Bowen PE, Stacewicz-Sapuntzakis M. Cancer Prev Res (Phila) 2011;4:711–718. doi: 10.1158/1940-6207.CAPR-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Twiddy AL, Cox ME, Wasan KM. Prostate. 2012;72:955–965. doi: 10.1002/pros.21499. [DOI] [PubMed] [Google Scholar]
- 64.Krycer JR, Brown AJ. Biochim Biophys Acta. 2013;1835:219–229. doi: 10.1016/j.bbcan.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 65.During A, Dawson HD, Harrison EH. J Nutr. 2005;135:2305–2312. doi: 10.1093/jn/135.10.2305. [DOI] [PubMed] [Google Scholar]
- 66.Murtola TJ, Syvala H, Pennanen P, Blauer M, Solakivi T, Ylikomi T, Tammela TL. PLoS One. 2012;7:e39445. doi: 10.1371/journal.pone.0039445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muhlhofer A, Buhler-Ritter B, Frank J, Zoller WG, Merkle P, Bosse A, Heinrich F, Biesalski HK. Clin Nutr. 2003;22:65–70. doi: 10.1054/clnu.2002.0598. [DOI] [PubMed] [Google Scholar]
- 68.Pappalardo G, Maiani G, Mobarhan S, Guadalaxara A, Azzini E, Raguzzini A, Salucci M, Serafini M, Trifero M, Illomei G, Ferro-Luzzi A. Eur J Clin Nutr. 1997;51:661–666. doi: 10.1038/sj.ejcn.1600457. [DOI] [PubMed] [Google Scholar]
- 69.Peng YM, Peng YS, Childers JM, Hatch KD, Roe DJ, Lin Y, Lin P. Cancer Epidemiol Biomarkers Prev. 1998;7:347–350. [PubMed] [Google Scholar]
- 70.Yeum KJ, Ahn SH, Rupp de Paiva SA, Lee-Kim YC, Krinsky NI, Russell RM. J Nutr. 1998;128:1920–1926. doi: 10.1093/jn/128.11.1920. [DOI] [PubMed] [Google Scholar]
- 71.Chung HY, Ferreira AL, Epstein S, Paiva SA, Castaneda-Sceppa C, Johnson EJ. Am J Clin Nutr. 2009;90:533–539. doi: 10.3945/ajcn.2009.27712. [DOI] [PubMed] [Google Scholar]
- 72.El-Sohemy A, Baylin A, Kabagambe E, Ascherio A, Spiegelman D, Campos H. Am J Clin Nutr. 2002;76:172–179. doi: 10.1093/ajcn/76.1.172. [DOI] [PubMed] [Google Scholar]
- 73.Kabagambe EK, Baylin A, Allan DA, Siles X, Spiegelman D, Campos H. Am J Epidemiol. 2001;154:1126–1135. doi: 10.1093/aje/154.12.1126. [DOI] [PubMed] [Google Scholar]
- 74.Miller EC, Giovannucci E, Erdman JW, Jr, Bahnson R, Schwartz SJ, Clinton SK. Urol Clin North Am. 2002;29:83–93. doi: 10.1016/s0094-0143(02)00020-4. [DOI] [PubMed] [Google Scholar]
- 75.Walfisch Y, Walfisch S, Agbaria R, Levy J, Sharoni Y. Br J Nutr. 2003;90:759–766. doi: 10.1079/bjn2003955. [DOI] [PubMed] [Google Scholar]
- 76.Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, van Breemen R, Ashton D, Bowen PE. J Natl Cancer Inst. 2001;93:1872–1879. doi: 10.1093/jnci/93.24.1872. [DOI] [PubMed] [Google Scholar]
- 77.Schwarz S, Obermuller-Jevic UC, Hellmis E, Koch W, Jacobi G, Biesalski HK. J Nutr. 2008;138:49–53. doi: 10.1093/jn/138.1.49. [DOI] [PubMed] [Google Scholar]
- 78.Kucuk O, Sarkar FH, Djuric Z, Sakr W, Pollak MN, Khachik F, Banerjee M, Bertram JS, Wood DP., Jr Exp Biol Med (Maywood) 2002;227:881–885. doi: 10.1177/153537020222701007. [DOI] [PubMed] [Google Scholar]
- 79.Paetau I, Rao D, Wiley ER, Brown ED, Clevidence BA. Am J Clin Nutr. 1999;70:490–494. doi: 10.1093/ajcn/70.4.490. [DOI] [PubMed] [Google Scholar]
- 80.Blume-Peytavi U, Rolland A, Darvin ME, Constable A, Pineau I, Voit C, Zappel K, Schafer-Hesterberg G, Meinke M, Clavez RL, Sterry W, Lademann J. Eur J Pharm Biopharm. 2009;73:187–194. doi: 10.1016/j.ejpb.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 81.Scarmo S, Cartmel B, Lin H, Leffell DJ, Welch E, Bhosale P, Bernstein PS, Mayne ST. Arch Biochem Biophys. 2010;504:34–39. doi: 10.1016/j.abb.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mayne ST, Cartmel B, Scarmo S, Lin H, Leffell DJ, Welch E, Ermakov I, Bhosale P, Bernstein PS, Gellermann W. Am J Clin Nutr. 2010;92:794–800. doi: 10.3945/ajcn.2010.29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maiani G, Caston MJ, Catasta G, Toti E, Cambrodon IG, Bysted A, Granado-Lorencio F, Olmedilla-Alonso B, Knuthsen P, Valoti M, Bohm V, Mayer-Miebach E, Behsnilian D, Schlemmer U. Mol Nutr Food Res. 2009;53(Suppl 2):S194–218. doi: 10.1002/mnfr.200800053. [DOI] [PubMed] [Google Scholar]
- 84.Britton G, Liaaen-Jensen S, Pfander H. SpringerLink. 2009;5 [Google Scholar]
- 85.Conlon LE, King RD, Moran NE, Erdman JW., Jr J Agric Food Chem. 2012;60:8386. doi: 10.1021/jf301902k. [DOI] [PubMed] [Google Scholar]
- 86.Goltz SR, Campbell WW, Chitchumroonchokchai C, Failla ML, Ferruzzi MG. Mol Nutr Food Res. 2012;56:866–877. doi: 10.1002/mnfr.201100687. [DOI] [PubMed] [Google Scholar]
- 87.Unlu NZ, Bohn T, Clinton SK, Schwartz SJ. J Nutr. 2005;135:431–436. doi: 10.1093/jn/135.3.431. [DOI] [PubMed] [Google Scholar]
- 88.Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. FASEB J. 2009;23:1041–1053. doi: 10.1096/fj.08-121962. [DOI] [PubMed] [Google Scholar]
- 89.Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. FASEB J. 2010;24:1656–1666. doi: 10.1096/fj.09-150995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Borel P, Lietz G, Goncalves A, Szabo de Edelenyi F, Lecompte S, Curtis P, Goumidi L, Caslake MJ, Miles EA, Packard C, Calder PC, Mathers JC, Minihane AM, Tourniaire F, Kesse-Guyot E, Galan P, Hercberg S, Breidenassel C, Gonzalez Gross M, Moussa M, Meirhaeghe A, Reboul E. J Nutr. 2013;143:448–456. doi: 10.3945/jn.112.172734. [DOI] [PubMed] [Google Scholar]
- 91.Moussa M, Landrier JF, Reboul E, Ghiringhelli O, Comera C, Collet X, Frohlich K, Bohm V, Borel P. J Nutr. 2008;138:1432–1436. doi: 10.1093/jn/138.8.1432. [DOI] [PubMed] [Google Scholar]
- 92.Paetau I, Khachik F, Brown ED, Beecher GR, Kramer TR, Chittams J, Clevidence BA. Am J Clin Nutr. 1998;68:1187–1195. doi: 10.1093/ajcn/68.6.1187. [DOI] [PubMed] [Google Scholar]
- 93.Mayne ST, Cartmel B, Silva F, Kim CS, Fallon BG, Briskin K, Zheng T, Baum M, Shor-Posner G, Goodwin WJ., Jr J Nutr. 1999;129:849–854. doi: 10.1093/jn/129.4.849. [DOI] [PubMed] [Google Scholar]
- 94.McKay GJ, Loane E, Nolan JM, Patterson CC, Meyers KJ, Mares JA, Yonova-Doing E, Hammond CJ, Beatty S, Silvestri G. Ophthalmology. 2013 doi: 10.1016/j.ophtha.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moussa M, Gouranton E, Gleize B, Yazidi CE, Niot I, Besnard P, Borel P, Landrier JF. Mol Nutr Food Res. 2011;55:578–584. doi: 10.1002/mnfr.201000399. [DOI] [PubMed] [Google Scholar]
- 96.Liu A, Pajkovic N, Pang Y, Zhu D, Calamini B, Mesecar AL, van Breemen RB. Mol Cancer Ther. 2006;5:2879–2885. doi: 10.1158/1535-7163.MCT-06-0373. [DOI] [PubMed] [Google Scholar]
- 97.Gouranton E, Yazidi CE, Cardinault N, Amiot MJ, Borel P, Landrier JF. Food Chem Toxicol. 2008;46:3832–3836. doi: 10.1016/j.fct.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 98.Lindqvist A, Andersson S. J Biol Chem. 2002;277:23942–23948. doi: 10.1074/jbc.M202756200. [DOI] [PubMed] [Google Scholar]
- 99.Lindqvist A, Andersson S. J Histochem Cytochem. 2004;52:491–499. doi: 10.1177/002215540405200407. [DOI] [PubMed] [Google Scholar]
- 100.Lindshield BL, King JL, Wyss A, Goralczyk R, Lu CH, Ford NA, Erdman JW., Jr J Nutr. 2008;138:2367–2371. doi: 10.3945/jn.108.099663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ferrucci L, Perry JR, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C, Fried LP, Albanes D, Corsi AM, Cherubini A, Guralnik J, Bandinelli S, Singleton A, Virtamo J, Walston J, Semba RD, Frayling TM. Am J Hum Genet. 2009;84:123–133. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zaripheh S, Nara TY, Nakamura MT, Erdman JW., Jr J Nutr. 2006;136:932–938. doi: 10.1093/jn/136.4.932. [DOI] [PubMed] [Google Scholar]
- 103.Lietz G, Lange J, Rimbach G. Arch Biochem Biophys. 2010;502:8–16. doi: 10.1016/j.abb.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 104.Lietz G, Oxley A, Boesch-Saadatmandi C, Kobayashi D. Mol Nutr Food Res. 2012;56:241–250. doi: 10.1002/mnfr.201100387. [DOI] [PubMed] [Google Scholar]
- 105.Lindqvist A, He YG, Andersson S. J Histochem Cytochem. 2005;53:1403–1412. doi: 10.1369/jhc.5A6705.2005. [DOI] [PubMed] [Google Scholar]
- 106.Shim E, Yeum KJ, Tang G, Ahn SH, Hwang J, Lee-Kim YC. Nutr Cancer. 2012;64:956–963. doi: 10.1080/01635581.2012.717678. [DOI] [PubMed] [Google Scholar]
- 107.Peng YM, Peng YS, Lin Y, Moon T, Baier M. Cancer Epidemiol Biomarkers Prev. 1993;2:145–150. [PubMed] [Google Scholar]
- 108.Bowen P, Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, Kim HS, Christov-Tzelkov K, van Breemen R. Exp Biol Med (Maywood) 2002;227:886–893. doi: 10.1177/153537020222701008. [DOI] [PubMed] [Google Scholar]
- 109.Freeman VL, Meydani M, Yong S, Pyle J, Wan Y, Arvizu-Durazo R, Liao Y. Am J Epidemiol. 2000;151:109–118. doi: 10.1093/oxfordjournals.aje.a010175. [DOI] [PubMed] [Google Scholar]
- 110.Kaplan LA, Lau JM, Stein EA. Clin Physiol Biochem. 1990;8:1–10. [PubMed] [Google Scholar]
- 111.Stahl W, Schwarz W, Sundquist AR, Sies H. Arch Biochem Biophys. 1992;294:173–177. doi: 10.1016/0003-9861(92)90153-n. [DOI] [PubMed] [Google Scholar]
- 112.Schmitz HH, Poor CL, Wellman RB, Erdman JW., Jr J Nutr. 1991;121:1613–1621. doi: 10.1093/jn/121.10.1613. [DOI] [PubMed] [Google Scholar]
- 113.Parker RS. Am J Clin Nutr. 1988;47:33–36. doi: 10.1093/ajcn/47.1.33. [DOI] [PubMed] [Google Scholar]
- 114.Nierenberg DW, Nann SL. Am J Clin Nutr. 1992;56:417–426. doi: 10.1093/ajcn/56.2.417. [DOI] [PubMed] [Google Scholar]
- 115.Peng YM, Peng YS, Lin Y, Moon T, Roe DJ, Ritenbaugh C. Nutr Cancer. 1995;23:233–246. doi: 10.1080/01635589509514378. [DOI] [PubMed] [Google Scholar]
- 116.Craft NE, Haitema TB, Garnett KM, Fitch KA, Dorey CK. J Nutr Health Aging. 2004;8:156–162. [PubMed] [Google Scholar]