Abstract
The mineral phase of dentin is located primarily within collagen fibrils. During development, bone or dentin collagen fibrils are formed first and then water within the fibril is replaced with apatite crystallites. Mineralized collagen contains very little water. During dentin bonding, acid-etching of mineralized dentin solubilizes the mineral crystallites and replaces them with water. During the infiltration phase of dentin bonding, adhesive comonomers are supposed to replace all of the collagen water with adhesive monomers that are then polymerized into copolymers. The authors of a recently published review suggested that dental monomers were too large to enter and displace water from collagen fibrils. If that were true, the endogenous proteases bound to dentin collagen could be responsible for unimpeded collagen degradation that is responsible for the poor durability of resin-dentin bonds. The current work studied the size-exclusion characteristics of dentin collagen, using a gel-filtration-like column chromatography technique, using dentin powder instead of Sephadex. The elution volumes of test molecules, including adhesive monomers, revealed that adhesive monomers smaller than about 1000 Da can freely diffuse into collagen water, while molecules of 10,000 Da begin to be excluded, and bovine serum albumin (66,000 Da) was fully excluded. These results validate the concept that dental monomers can permeate between collagen molecules during infiltration by etch-and-rinse adhesives.
Keywords: size-exclusion, collagen, mineralized collagen, collagen water, resin-dentin bonding
1. Introduction
The poor durability of resin-dentin bonds has stimulated a great deal of research on its cause [1–3]. Soon after the development of the microtensile bond strength test [4–6] and nanoleakage techniques [7, 8], it became clear that when resin-bonded teeth were reduced to 1 × 1 × 6 mm sticks that were incubated in water over time, that this technique accelerated aging of the resin-dentin interface [1, 9, 10]. When adhesive resin sticks were stored in water or oil for 3–6 months, the results demonstrated that adhesives incubated in oil retained their mechanical properties, while specimens soaked in water showed 30–40% decreases in tensile properties [11]. This was correctly interpreted to be the result of water sorption causing plasticization of the resin polymers. When similar studies were done on resin-dentin bonds [12], the bond strengths fell 30–40% in 6 months of water storage and 50–70% after 12 months of water storage. In contrast, specimens incubated in oil showed no decrease in bond strength. Clearly, water is important in the poor durability of resin-dentin bonds [13]. The first transmission electron micrographs (TEMs) of control resin-dentin bonds versus those that had been incubated in water for 4 years demonstrated a loss of collagen fibrils from the hybrid layers [14]. Our group and others identified the presence of matrix metalloproteinases (MMPs) 2, 8 and 9 in dentin [15–17], and lead us to believe that the loss of collagen fibrils from the hybrid layer was the result of their hydrolytic activity in demineralized dentin matrices. When we incubated acid-etched dentin in buffer for up to 250 days, we saw the disappearance of collagen fibrils from the demineralized dentin [15]. If we added protease inhibitors to the buffer, there was much less degradation of collagen fibrils. Incubation of acid-etched dentin in oil instead of aqueous buffer blocked collagen degradation. When we incubated dentin powder in fluorescein-labeled soluble collagen substrate (DQ-collagen, EnzChek, Molecular Probes, Eugene, OR), the dentin released fluorescent collagen peptide fragments in the controls but not in the presence of 0.2% chlorhexidine (CHX) or other protease inhibitors [15].
We proposed [3,16–18] that acid-etching of dentin with weak acids removes all of the mineral crystallites from dentin to a depth of 6–10 μm, exposing the collagen fibrils of the matrix, increasing the porosity of the dentin surface, and activating proforms of endogenous dentin proteases. After rinsing acid-etched dentin with water, the dissolved minerals are extracted from the demineralized layer. Rinse-water replaces the mineral phase in the demineralized layer [19]. In etch-and-rinse adhesives, a mixture of adhesive comonomers in a volatile organic solvent is applied to the demineralized layer to replace that water with comonomers that are thought to envelope collagen fibrils, and their collagen-bound proteolytic enzymes that were inadvertently activated by acid-etching dentin. Theoretically, after light-induced polymerization of these copolymers, the dentin surface composition is 52% polymer and 48% collagen fibrils. If all the collagen water can be replaced by synthetic polymers there will be no water available to plasticize the polymer or to cleave collagen peptide bonds by collagenases, gelatinases and telopeptidases [20].
1.1 Replacement of collagen water by adhesive comonomers
Another complication in replacement of water by adhesive comonomers is the intrinsic permeability of collagen fibrils. The collagen fibrils in dentin matrix are about 100 nm in diameter. There is a 20–30 nm wide space around each fibril that is thought to be continuous with the water-filled microtubule network in demineralized dentin. This space serves as a diffusion channel for resin infiltration of demineralized dentin. Bertassoni et al. [21] hypothesized that the packing density of collagen molecules inside collagen fibrils is such that there too little space available for infiltration of adhesive resin comonomers into the collagen water of demineralized collagen fibrils. While resin may infiltrate the extrafibrillar spaces to provide mechanical retention of polymers, they may not be able to replace intrafibrillar water around all collagen molecules. As acid-etching does not remove matrix bound MMPs from the matrix, there is more than enough residual water to permit these acid-activated proteases that are technically hydrolases, to slowly hydrolyze collagen fibrils that are not enveloped by resin. This protease activity solubilizes collagen peptide fragments from the insoluble dentin matrix, thereby weakening its mechanical properties. If insoluble collagen fibrils are slowly solubilized over time, their ability to anchor resin composites to dentin will be lost. However, if resin monomers can infiltrate collagen water they may displace some or all of it and envelop the proteases bound to collagen, thereby stabilizing resin-dentin bonds. Thus, knowledge of the permeability characteristics of dentin collagen fibrils is critical to the determination of the mechanism(s) responsible for the poor durability of resin-dentin bonds [3].
1.2 Lack of adequate space in collagen water for comonomers
Bertassoni et al. [21] marshaled a good deal of evidence including small angle x-ray scattering, SEM, TEM and AFM studies to support their hypothesis that collagen molecule packing density in hydrated collagen leaves intermolecular spacing of only 1.8 nm, while claiming that the molecular size of adhesive monomers used in dentistry, such as triethyleneglycol dimethacrylate (TEGDMA) is about 2 nm in its extended length and 0.4 nm in diameter. Thus, the size of the TEGDMA molecule should fit within the available intermolecular space of hydrated collagen. Thus, the permeability properties of dentin to dental adhesive monomers are of more than academic interest, and knowledge of the molecular weight cut-off for molecules capable of infiltrating demineralized dentin collagen is very important. However, there is no dental literature on the permeability properties of dentin collagen fibrils to typical adhesive comonomers. The creation of perfect resin-dentin bonds presupposes that all dental comonomers can permeate into collagen water and displace that water so that each collagen molecule and their bound proteases, are enveloped by water-free polymerized resin.
1.3 How to measure the permeability properties of type I collagen
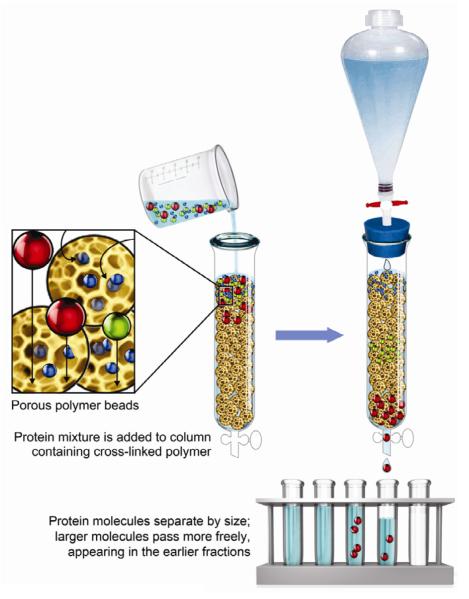
Others have thought about the permeability properties of bone matrix collagen fibrils to molecules necessary for bone mineralization [22]. Toroian et al. [23] hypothesized that the physical structure of tightly packed collagen molecules determines the size of the molecules that can diffuse into collagen water that lies within collagen fibrils, and thereby affects apatite crystal growth. They developed a unique experimental technique that can be used to investigate the size-exclusion characteristics of collagen fibrils. In gel-filtration chromatography, columns containing, for example Sephadex gel beads, are used to separate various sized molecules by their differential permeability into the water phase of hydrated Sephadex (Fig. 1). This novel, gel filtration-like procedure employed by Toroian et al. [23] used columns packed with type I collagen instead of Sephadex (Fig. 1). The elution volumes of test molecules showed the volume within the packed column that is accessible to the test molecules, and therefore, revealed the size-exclusion characteristics of collagen molecules in the bone powder that they studied [24]. Their results showed that in completely demineralized bone powder, molecules like phosphate, citrate, riboflavin and ostelcalcin (molecular weights 95–5700 Da) all eluted at the liquid volume of the column bed, meaning that these molecules are able to access all of the water within the packed demineralized bone powder. In contrast, larger molecules like fetuin (48,000 Da) or albumin (66,000 Da) eluted at smaller volumes indicating that they are excluded from entering collagen water [23]. We used this same technique to determine if dental adhesive monomers like HEMA and TEGDMA can access collagen water or if it is restricted to extrafibrillar water.
Figure 1.
Schematic showing gel-permeation or size-exclusion column chromatography. A column is filled with hydrophilic cross-linked polymers (for instance, dextrans) that allow small molecules access to polymer water, but do not allow access to larger molecules like albumin. The albumin comes through the column rapidly because it only has access to “interstitial” fluid between the polymer beads. Small molecules come out of the column later because they diffused into a larger volume. In the current study, mineralized dentin powder was used as the separating medium. Before demineralization, dentin powder contained very little water because collagen fibrils were filled with apatite crystallites. After complete demineralization of dentin, collagen fibrils became filled with water. (Modified from Fig. 3–17 in Lehninger's Principes of Biochemistry, 5th ed., by Nelson DL and Cox MM, W.H. Freeman and Co., New York, 2008, p. 87, with permission).
1.4 Purpose of study
The purpose of this work was to determine if HEMA and TEGDMA, as examples of hydrophilic adhesive monomers, can equilibrate with water within collagen molecules in demineralized dentin powder. The test null hypothesis was that HEMA and TEGDMA cannot equilibrate with collagen water.
2. Materials and Methods
2.1 Materials
Blue dextran (2 × 106 Da), bovine serum albumin (BSA) (6.6 × 104 Da), ovalbumin (albumin from chicken egg white) (4.5 × 104 Da), dextran 10,000 (104 Da), TEGDMA (286 Da), carbodiimide (192 Da) glucose (180 Da), HEMA (130 Da) and dimethyl sulfoxide (DMSO) (78 Da) were all obtained from Sigma/Aldrich (St. Louis, MO, USA), and were used as obtained.
2.2 Creation of dentin powder
In the first set of experiments, dentin powder was obtained from freshly extracted bovine incisors that were obtained at a local abattoir. Six hundred freshly extracted bovine incisor teeth which were stored in 0.9% NaCl containing 0.02% sodium azide at 4°C to prevent bacterial growth were used in this study. Then, dentin devoid of enamel, cementum and pulpal tissues were prepared from those teeth using dental burs in a highspeed handpiece and stainless steel files, with copious air-water spray. The apical 4 mm of each root segment was removed. Using an Isomet saw (Buehler Ltd., Lake Bluff, IL, USA) under water cooling, the dentin specimens were cut into two halves. The resulting tooth fragments were dehydrated in acetone for 20 min, placed in a 25 mL capacity stainless steel screw-top trituration jars containing 20 mm diameter stainless steel balls and immersed in liquid nitrogen for 15 min. After removal from the liquid nitrogen, the contents of the container were triturated to dentin powder in a Retsch ball-mill (Model MM201, Retsch Inc, Newtown, PA, USA) for 4 sec at 30 Hz. The resulting powder was passed through a series of stacked sieves. The powder that passed through 600 μm sieves but was retained on 425 μm sieves, similar to that used by Toroian et al. [23] was collected. Dentin powder particles larger than 600 μm in diameter were re-triturated and re-sieved to obtain enough dentin powder for the experiment. The mineralized dentin powder was kept frozen at −80 °C until use to prevent degradation of the collagen component.
Two batches of bovine dentin powder were prepared. One batch remained mineralized, while the second batch was completely demineralized in 10-fold excess of 0.1 M formic acid (pH 2.3) over three days at 4°C. When the completely demineralized dentin was ashed at high temperature (800°C) in a tared platinum container, atomic absorption spectrophometry analyses of the ash revealed that there were no traces of residual calcium, indicating demineralization had removed all traces of mineral from dentin powder.
One centimeter × 30 cm glass columns were weighed empty and after filling with dry mineralized or demineralized dentin powder. The columns were then filled with water. Excess water was removed to the surface of the packed dentin powder. The water-filled columns were reweighed to obtain the wet weight of the column. By subtracting the dry weight of the empty column, from the wet weight of the filled column, the wet weight of the dentin powder could be calculated (Table 1). The volume of the filled column was the volume of water that had to be placed in the empty column to reach a black mark made on the outside of the column when it was filled with mineralized or demineralized dentin powder. The amount of mineral in the dentin powder was determined by the differences in the dry weights before and after acid demineralization.
Table 1.
Characterization of column packed with mineralized and demineralized bovine dentin powder
| before demineralization | after demineralization | Change due to demineralization | |
|---|---|---|---|
| batch #1 (mineralized dentin) | |||
| A. Total volume of packed column | 22.8 ml | - | - |
| B. Dry weight of column contents | 23.9 g | - | - |
| C. Wet weight of column contents | 34.8 g | - | - |
| D. Weight of water in packed column | 10.9 g | - | - |
|
| |||
| batch #2 (demineralized dentin) | |||
| E. Total volume of packed column | 22.8 ml | 20.4 g | −2.4 ml |
| F. Dry weight of column contents | 24.4 g | 4.6 g | −19.8 g |
| G. Wet weight of column contents | 35.2 g | 21.9 g | −13.3 g |
| H. Weight of water in packed column | 10.8 g | 17.3 g | +6.5 g (+6.5 ml) |
| I. Weight of mineral in packed column | 19.8 g | 0.0 g | −19.8g |
|
| |||
| density of mineralized dentin | 2.0 g/ml | ||
| density of demineralized wet dentin | 1.1 g/ml | ||
In later experiments, we prepared dentin powder from extracted unerupted human third molars that were obtained with informed consent from 21–24 year old adults as part of the routine dental care in the clinics of the Georgia Regents University. These specimens were processed exactly like the bovine incisors.
2.3 Gel filtration procedures
Twenty-three cubic centimeters of mineralized or demineralized dentin powder were packed into a 1 × 30 cm glass column and equilibrated with 20 mM Tris buffer (pH 7.4) that contained 2 M NaCl to minimize nonspecific ionic interactions between test molecules and binding substrates [25–27]. That buffer was used to elute the column at a flow rate of 14.4 ml/hr using a precision syringe pump until the final effluent absorbance at 280 nm was less than 0.01, indicating that all extrafibrillar protein has been rinsed from the column. Sample sizes were 1.0 ml and were collected in a fraction collector set to change every 4.1 min.
Tracer samples were dissolved in 0.5 ml of column buffer and contained approximately 1.5 mg of blue dextran, or 2.5 mg of BSA, or 2.5 mg of ovalbumin, or 0.5 mg of dextran 10 K, or 3.13 × 10−2 mg of TEGDMA or 20 mg of glucose, or 6.25 × 10−2 mg of HEMA, or 0.4 mg of dimethyl sulfoxide. The volume of each fraction (1.01 ± 0.02 ml) was determined by weighing the fraction contents in a tared tube and divided by the density of the buffer (1.07 g/ml).
The elution of test tracers was determined as follows: blue dextran, absorbance at 620 nm; proteins (BSA and ovalbumin), absorbance at 280 nm; dextran 10k with anthrone reagent, as described [28], at 625 nm; TEGDMA, HEMA, carbodiimide and dimethyl sulfoxide, absorbance at 220 nm; glucose, glucose meter with glucose-specific stick (One Touch Ultra, Life Scan, Johnson & Johnson Co., Skillman, NJ, USA).
2.4 Statistical analysis
Because the distribution of the mean elution volume of all tracers with mineralized and demineralized powder was not normally distributed, the data were analyzed by Kruskal-Wallis with Dunn's multiple comparison tests. All statistical analysis were carried out with SPSS software version 16 (SPSS Inc., Chicago, IL, USA) at a 95% level of confidence.
3. Results
3.1 The size exclusion characteristics of mineralized bovine dentin powder
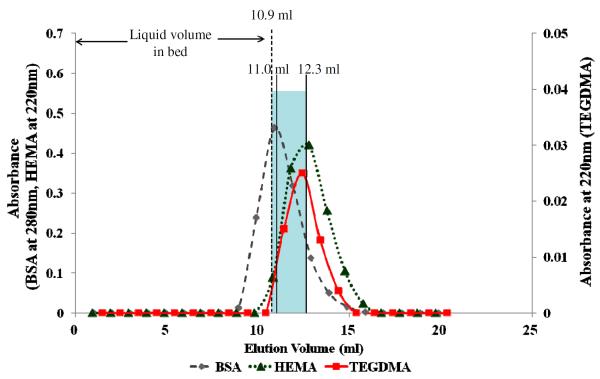
The initial experiments were done to determine if there was a measurable volume of water in hydrated mineralized dentin. This was done using the novel, gel filtration-like method developed by Toroian et al. [23]. Using a 1 × 30 cm column filled with mineralized dentin powder, the tracers BSA and HEMA were applied together in Tris buffer (pH 7.4) containing 2 M NaCl. Table 1 summarizes the important variables in mineralized versus demineralized bovine dentin. Note that the weight or volume of water in the column packed with mineralized dentin was 10.9 mL powder. Figure 2 shows that BSA and HEMA eluted at 11.0 ml and 12.3 ml, respectively. The mean elution volume of BSA is equivalent to the 10.9 ml volume of liquid in the column bed. BSA, with a molecular weight of 66,000 Da was too large to enter any of the mineralized collagen fibrils in the mineralized dentin powder. It could enter interstitial spaces between the dentin powder particles. However, HEMA, with a molecular weight of only 130 Da, should be able to diffuse into collagen water, but only if it was demineralized. It is thought that in the mineralized state, all collagen water has been displaced by apatite mineral crystallites [28]. The fact that the mean elution volume of HEMA (MW 130 Da) was 12.3 ml while that of BSA (MW 66000 Da) is 11.0 ml suggests that there is some water in the mineralized matrix [23] because HEMA was able to diffuse into a slightly larger volume than the volumes into which BSA passed. When mineralized dentin was exposed to tracers with different molecular weights, their elution volumes roughly followed their molecular size. Those with high molecular weights (i.e. > 10 kDa) formed one distinct group (mean elution volume of 10.8–11.0 mL), while those < 286 Da formed another statistically significantly different (11.2–12.2 mL) group (Table 2).
Figure 2.
Elution profile of BSA (◆), HEMA(▲) and TEGDMA(■) from mineralized dentin powder. The mean elution peak of BSA occurred at 11.0 ± 0.06 ml (n=4), while that of TEGDMA and HEMA occurred at 12.2 ± 0.12 and 12.3 ± 0.06 ml, respectively. The mean elution volumes for TEGDMA and HEMA were significantly larger (p<0.05) than for BSA, but the two monomer elution volumes were not significantly different from each other. Blue shading indicates that there was a small (1.3 ml) amount of water in the mineralized dentin.
Table 2.
The size exclusion properties of mineralized and demineralized bovine dentin
| Test molecule | Mass (Daltons) | Concentration (mg/ml) | Elution volume (ml) |
difference due to demineralization (ml) | |
|---|---|---|---|---|---|
| Mineralized dentin powder | demineralized dentin powder | ||||
| Blue Dextran | 2000000 | 3 | 10.8 (0.10)a | 10.1 (0.06)a | −0.7 |
| BSA | 67000 | 5 | 11.0 (0.06)a,b | 10.1 (0.10)a | −0.9 |
| Ovalbumin | 45000 | 5 | 10.9 (0.06)a,b | 10.4 (0.21)a | −0.6 |
| Dextran 10k | 10000 | 0.2 | 11.2 (0.25)b | 11.0 (0.21)b | −0.3 |
| TEGDMA | 286 | 0.0625 | 12.2 (0.12)c,d | 20.1 (0.21)c | 8.0 |
| Carbodiimide | 192 | 0.0768 | 12.0 (0.05)c | 20.2 (0.33)c | 8.2 |
| Glucose | 180 | 40 | 11.9 (0.10)c | 17.2 (0.21)d | 5.3 |
| HEMA | 130 | 0.125 | 12.3 (0.06)d | 19.6 (0.17)e | 7.3 |
| Dimethyl sulfoxide | 78 | 0.8 | 12.1 (0.10)c,d | 17.0 (0.10)d | 4.9 |
For each column, groups identified by different superscript letter are statistically significant (p < 0.05). (n = 4)
3.2 Characteristics of columns packed with demineralized dentin powder
When the mineralized dentin powder was completely demineralized using a 10-fold excess of 10% (v/v) formic acid for 3 days, the dentin lost 19.8 g of dry weight (Table 1). The wet weight of the demineralized dentin was only a fraction of its original mineralized wet weight because the acid had removed all mineral from the dentin. Mineralized dentin had a dry wt. of 24.4 g and a wet weight of 34.8 g giving it a solid content of 70.1 % and a water content of 100–70.1 = 29.9% water. The wet weight minus the dry weight of demineralized dentin was 21.9 g and 4.6 g, respectively, giving demineralized dentin a water content of 21.9 – 4.6 = 17.3 g. Dividing that 17.3 g of water by the 21.9 g of wet dentin gives, times 100 water content of wet dentin of 78.9%. Thus, complete demineralization of dentin increased its water content from 2.9% to 78.9%
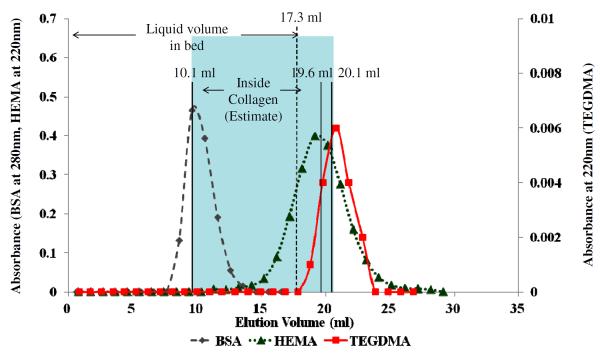
Figure 3 shows the elution volumes of the tracer molecules from a column of completely demineralized dentin. Note that the mean elution volume of BSA was still about 10.1 mL that was similar to Blue dextran and ovalbumin. However, the mean elution volume for HEMA was now 19.6 ml, which is somewhat above the 17.3 g of water in the demineralized dentin (Table 1), the predicted elution volume if the small tracers entered and equilibrated with all of the collagen water. The mean elution volumes of other tracers are listed in Table 2 and Fig. 3. In essence, the elution volumes of the larger tracers (blue dextran, BSA, ovalbumin and dextran 10 K) did not differ between mineralized and demineralized dentin powder, while the small tracers (TEGDMA, carbodiimide, glucose, HEMA and DMSO) showed elution volumes that were 1.5 × higher than that of mineralized dentin (Fig. 3).
Figure 3.
Elution profiles of BSA (◆), HEMA(▲) and TEGDMA(■) from completely demineralized dentin powder. The mean elution volume for BSA was 10.1 ± 0.10 mL (n=4), while that of HEMA and TEGDMA were 19.6 ± 0.17 and 20.1 ± 0.21 mL, respectively. The elution volumes of BSA, HEMA and TEGDMA were all significantly different (p<0.05) for each other. The blue shading indicates that the collagen water volume increased from 12.3–11.0 = 1.3 mL in mineralized dentin, to 20.1–10.1 = 10 mL in demineralized dentin powder.
The water solubility of TEGDMA is only 9.6 mg TEGDMA/ml water [29]. The water solubility of TEGDMA in 2 M NaCl was even less (0.03 mg/mL buffer). Indeed, when we tried to increase the TEGDMA load placed on the column, while the TEGDMA absorbance increased, the elution peak became unsymmetrical (data not shown). That is, TEGDMA diffused into collagen water but then the curve did not fall back to the original baseline but trailed off far above the baseline for several hours until the TEGDMA that came out of solution finally redissolved in elution water and was rinsed out of the column. Tracers that are applied to concentrations exceeding the solubility tend to produce unsymmetrical elution peaks. Such unsymmetrical peaks indicate that additional solvents must be added to the test monomer. In marketed dental adhesives, both TEGDMA and BisGMA are often solubilized in HEMA because they are so much more soluble in HEMA than water. We did not do that in the current experiments because the absorbance of HEMA would have interfered with the absorbance of TEGDMA at the same wave number. Future experiments will use HPLC to separate HEMA from TEGDMA so that they can both be analyzed in the same sample. HEMA itself is much more soluble in water than is its phosphate ester HEMA-phosphate. Similar results were obtained with HEMA-phosphate. The elusion peak became more unsymmetrical as higher doses of HEMA-phosphate were applied as tracers.
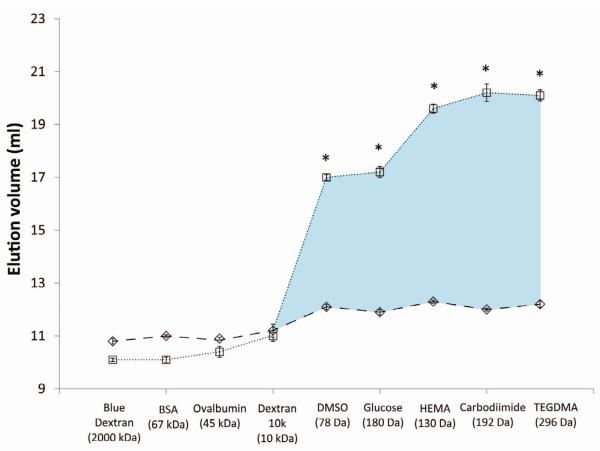
Figure 4 summarizes the large differences in elution volume of the various tracers in mineralized versus demineralized dentin.
Figure 4.
Summary of the elution volumes of all the tracers used in mineralized dentin powder (◇) versus demineralized dentin powder (□). * indicates significant differences (p<0.01) between mineralized vs. demineralized dentin. Values are means ± S.D. (n=4). Standard deviation brackets are smaller than the symbols. The blue shading indicates the increase in collagen water volume seen by each tracer molecule.
4. Discussion
The fact that HEMA and TEGDMA equilibrated with collagen water in demineralized dentin matrix indicates that these two common adhesive monomers are not too large to enter intermolecular spaces. That is, the packing density of collagen molecules in collagen fibrils does not exclude these monomers. These results require rejection of the null hypotheses that HEMA or TEGDMA cannot equilibrate with collagen water in completely demineralized dentin saturated with water. Bertassoni et al. [21] argued that, based upon small angle x-ray scattering and AFM imaging, there is insufficient space between collagen molecules to permit dental adhesive monomers such as TEGDMA or HEMA to occupy the available space. They calculated the size of TEGDMA based on extended states that are far from the equilibrium state that is much smaller due to torsion of single bonds. However, the work of Toroian et al. [23] using powdered bone indicated that bone collagen allowed molecules as large as 6 kDa to diffuse into and out of the collagen water. They showed that aggregated collagen molecules in bone collagen fibrils excluded molecules larger than 40 kDa.
Our results support the results of Toroian et al. [23] but extend those results to include the size exclusion characteristics of dentin collagen. The poor durability of resin-dentin bonds [3,13–14] does not seem to be the result of the inability of adhesive monomers like HEMA and TEGDMA from entering collagen water. If there had not been space for TEGDMA between collagen molecules, TEGDMA would have eluted somewhere between BSA and dextran 10,000 (Table 2). As this was not seen, it appears that TEGDMA and HEMA can easily enter collagen water.
There are other possible explanations for the poor durability of resin-dentin bonds. Many dimethacrylates like TEGDMA or bisphenol A diglycidylether dimethacrylate (BisGMA) are not very water soluble. When mixtures of HEMA and BisGMA dissolved in ethanol are applied to water-saturated dentin (a common procedure in adhesive dentistry), the two monomers undergo phase changes with HEMA and water in one phase, and BisGMA and ethanol in another phase [30,31]. This can even occur at a nanoscale [32]. Thus, the distribution of dental adhesives in collagen water may be different from their applied concentrations and may be less than homogeneous, not because of steric restrictions imposed by collagen molecules, but due to their poor water solubility and immiscibility issues.
Toroian et al. [23] suggested that collagen molecules have substantial freedom to rotate within collagen fibrils. They point out that13C nuclear magnetic resonance studies have shown that the polypeptide backbone of the collagen molecule is free to rotate within a fully hydrated collagen fibril in less than 0.1 msec. These motions are not observed in dry or mineralized collagen fibrils and are not affected by covalent cross-linking [33].
The idealistic molecular schematics of collagen fibrils shown in Fig. 2E of Bertassoni et al. [21] might be appropriate for reconstituted pure type I collagen, but are too regular for bone or dentin collagen that contain about 10% non-collagenous proteins that are periodically bound to collagen [34]. These include MMPs, cathepsins, proteoglycans, and growth factors [3]. Their periodic presence on collagen molecules must make the molecules somewhat “lumpy” and may interfere with uniform molecular packing.
In Fig. 3 are shown the elution volume of BSA and HEMA and TEGDMA in completely demineralized dentin. Note in Table 1 that demineralization removed 19.8 g of mineral or assuming a density of 2.0 g/ml (19.8 g ÷ 2.0 g/ml) or 9.9 ml of dentin volume, that became filled with water. That 9.9 ml of water that replaced the mineral phase of dentin plus the 10.1 ml of water that was originally in the dentinal tubules and their branches and in the water in the interstices between the dentin powder particles would give a total column water content of 20.8 ml in the column filled with demineralized dentin.
Figure 3 shows that BSA could only equilibrate with 10.1 ml of the water in the column containing completely demineralized dentin, yet HEMA and TEGDMA could equilibrate with 19.6 ml and 20.1 ml, respectively of the total water in the column of demineralized dentin. This means that these monomers are not sterically restricted from diffusing into collagen water.
A recent review article on the “complex organization features at the nanoscale and molecular levels” of type I collagen in dentin matrices [21] was very helpful in describing nanoscale barriers to successful infiltration of dental monomers during bonding procedures. The authors suggested that collagen molecules in microfibrils have an intermolecular spacing of 1.3 nm “which is too small to accommodate one single extended monomer (2 nm).” They also contend that the “intermolecular spaces have been shown to be fully occupied by tightly bound water molecules, which may be a critical contributor to the hydrolytic degradation of dental polymers.” Indeed, we would add that such water may contribute to the degradation of collagen, as the matrix metalloproteases (MMPs) that they discussed in their review are hydrolases. That is, they require water to hydrolyze collagen peptides. The presence of collagen degradation over time, assessed by decreases in bond strength of resin to dentin is used as indirect evidence that there is excessive residual water in degrading resin-dentin bonds, because of incomplete resin infiltration. That is, dimethacrylates like TEGDMA and BisGMA that are commonly found in dental adhesives apparently cannot displace all bound water from collagen molecules. Bertassoni et al. [21] interpreted that observation as evidence that dimethacrylates are too large to fit into intermolecular spaces in collagen occupied by water. However, the well-documented slow degradation of collagen fibrils from hybrid layers cited by Bertassoni et al. [21] may be due to the lack of solubility of dimethacrylates in water, not their size exclusion. The water solubility of TEGDMA and BisGMA is 9.5 × 10−3 [36] and 1 × 10−3 g/mL of water [Pashley, unpublished observations], respectively. At that solubility limit, their chemical concentrations would be unable to displace all intermolecular water. What is needed is replace collagen water with a water-miscible solvent such as ethanol or HEMA, in which TEGDMA and BisGMA are much more soluble.
Our use of water-saturated dentin powder was an attempt to simulate contemporary resin-dentin bonding in etch-and-rinse adhesives where demineralized dentin is saturated with water, a procedure that we believe exacerbates a critical barrier to creation of perfect resin-dentin bonds [33]. However, the results we obtained under dynamic flow conditions are far removed from passive clinical bonding conditions. Much more work is required before we can be certain that comonomers may displace water from collagen.
As pointed out by Bertassoni et al. [21], the introduction of ethanol wet-bonding by Pashley et al. [35] “could potentially overcome a number of limitations leading to degradation of dentin-bonded interfaces.” In this technique, ethanol is used to chemically dehydrate water-saturated, demineralized dentin matrices [36–38]. This results in the lateral shrinkage of collagen fibrils that increases the size of interfibrillar spaces. This shrinkage may be due to the loss of water from inter- and intrafibrillar proteoglycans caused by ethanol [37]. Ethanol-wet bonding tends to increase initial bond strength [38] but more importantly, these bond strengths do not fall over 12–18 months [36–37] and the collagen fibrils do not lose their cross-banding [39]. We believe that the long term-stability of resin-dentin bonds created by ethanol wet-bonding provides indirect evidence that there is little residual water left around collagen molecules. Without water, MMPs cannot hydrolyze collagen peptides. An alternative explanation for the stability of resin-dentin bonds created by wet bonding is that ethanol occupied the intra- and intermolecular spaces between collagen molecules and their bound MMPs. This may have permitted relatively high dimethacrylate concentrations in ethanol to form an intimate molecular-level interphase, resulting in the formation of a biocomposite that is half collagen and half synthetic polymer. Not only would this inactivate matrix bound MMPs thought to be responsible for hydrolysis of collagen, it would cover the collagen peptides with synthetic polymers preventing access of peptide bonds to any protease, whether intrinsic or extrinsic.
Bertassoni et al. [21] acknowledged that this may occur but then ask the question, what are the consequences of removing collagen water on the nanomechanical behavior of such collagen is dissipating mechanical forces during dentin strain? Clearly, there is an urgent need for more information on collagen-adhesive interactions.
4.1 Future experiments
Previous work indicates that solvents with Hoy's solubility parameters for hydrogen bonding cohesive forces (δh) of 15 (J/cm3)½ or less permit the formation of interpeptide hydrogen bonding between adjacent collagen peptides, creating a loss of internfibrillar spaces [35] and collagen shrinkage. Such solvents include acetone (δh = 11.0) Thus, in future experiments water-saturated demineralized dentin will be equilibrated with acetone to purposely shrink collagen as much as possible, to determine if this limits the ability of dimethacrylates to enter collagen intermolecular spaces. If that occurs, step-wise increments of water with a δh = 40 (J/cm3)½ will be added to acetone to permit stepwise expansions of collagen intermolecular spacing to see how that modifies dimethacrylate permeation.
Such experiments are important because some manufacturers solvate their adhesives in acetone, while others use ethanol or HEMA. It is important to determine the influence of such solvents on the permeability properties of type I collagen in dentin matrices.
We have shown that when ethanol replaces the layers of water around collagen molecules, that the collagen fibrils shrink in diameter, thereby enlarging the interfibrillar spaces [39], allowing more resin uptake. Under such conditions of dehydration, one might expect smaller intermolecular spacing. Rather than diffusing into intrafibrillar water, adhesive monomers would have to diffuse into intrafibrillar ethanol. Our new model of size-exclusion “chromatography” using columns filled with powdered dentin will allow us to test the hypothesis that in ethanol-saturated dentin, there is insufficient intermolecular spaces between collagen molecules to allow room for infiltration of dental adhesive resins. The experiments will be very similar to what we described above, but the column will be eluted with 100% ethanol into a fraction collector. If ethanol-dehydrated demineralized dentin does not permit uptake of dental adhesive monomers into collagen, we will determine how much water must be added to expand them enough for monomer permeation.
Conclusion
We have never before been this close to understanding how monomers may interact with collagen. Water-saturated demineralized dentin permits TEGDMA to equilibrate with collagen water. This does not mean that adhesive monomers can displace all water from collagen. In fact, because comonomers are usually solvated in ethanol of HEMA, these solvents may shrink intermolecular dimensions of collagen under more clinically relevant conditions. We agree with Bertassoni et al. [21] that “to date, there is no evidence that water in the intrafibrillar compartment of adhesive joints is completely replaced by resin.” Clearly, more fundamental research is required to investigate such questions.
Acknowledgments
This work was supported, in part, by R01 DE015306 from the NIDCR to DP (PI) and by the King Abdulazis University that supports DHP as a Highly Cited Scholar. The authors are grateful to Mrs. Michelle Barnes for her secretarial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None of the authors of this study received any corporate support for the use of products used in this work. None of the authors have any conflicts of interest with the products used in this study.
References
- [1].Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, et al. Durability of resin-dentin bonds. J Adhes Dent. 1999;1:211–8. [PubMed] [Google Scholar]
- [2].Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, Dorigo ED. Dental adhesion review: Aging and stability of the bonded interface. Dental Materials. 2008;24:90–101. doi: 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- [3].Tjäderhane L, Nascimento FD, Breschi L, Mazzoni A, Tersariol IL, Geraldeli S, et al. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent Mater. 2013;29:116–35. doi: 10.1016/j.dental.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sano H, Ciucchi B, Matthews WG, Pashley DH. Tensile properties of mineralized and demineralized human and bovine dentin. J Dent Res. 1994;73:1205–11. doi: 10.1177/00220345940730061201. [DOI] [PubMed] [Google Scholar]
- [5].Sano H, Shono T, Sonoda H, Takatsu T, Ciucchi B, Carvalho R, et al. Relationship between surface area for adhesion and tensile bond strength--evaluation of a micro-tensile bond test. Dent Mater. 1994;10:236–40. doi: 10.1016/0109-5641(94)90067-1. [DOI] [PubMed] [Google Scholar]
- [6].Sano H, Shono T, Takatsu T, Hosoda H. Microporous dentin zone beneath resin-impregnated layer. Oper Dent. 1994;19:59–64. [PubMed] [Google Scholar]
- [7].Sano H, Takatsu T, Ciucchi B, Horner JA, Matthews WG, Pashley DH. Nanoleakage: leakage within the hybrid layer. Oper Dent. 1995;20:18–25. [PubMed] [Google Scholar]
- [8].Sano H, Yoshiyama M, Ebisu S, Burrow MF, Takatsu T, Ciucchi B, et al. Comparative SEM and TEM observations of nanoleakage within the hybrid layer. Oper Dent. 1995;20:160–7. [PubMed] [Google Scholar]
- [9].Pashley DH, Carvalho RM, Sano H, Nakajima M, Yoshiyama M, Shono Y, Fernandes CA, Tay F. The microtensile bond test: a review. J Adhes Dent. 1999;1:299–309. [PubMed] [Google Scholar]
- [10].Shono Y, Ogawa T, Terashita M, Carvalho RM, Pashley EL, Pashley DH. Regional measurement of resin-dentin bonding as an array. J Dent Res. 1999;78:699–705. doi: 10.1177/00220345990780021001. [DOI] [PubMed] [Google Scholar]
- [11].Carrilho MR, Carvalho RM, Tay FR, Pashley DH. Effects of storage media on mechanical properties of adhesive systems. Am J Dent. 2004;17:104–8. [PubMed] [Google Scholar]
- [12].Carrilho MR, Tay FR, Pashley DH, Tjaderhane L, Carvalho RM. Mechanical stability of resin-dentin bond components. Dent Mater. 2005;21:232–41. doi: 10.1016/j.dental.2004.06.001. [DOI] [PubMed] [Google Scholar]
- [13].Brackett MG, Li N, Brackett WW, Sword RJ, Qi YP, Niu LN, et al. The critical barrier to progress in dentine bonding with the etch-and-rinse technique. J Dent. 2011;39:238–48. doi: 10.1016/j.jdent.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Armstrong SR, Vargas MA, Chung I, Pashley DH, Campbell JA, Laffoon JE, et al. Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Oper Dent. 2004;29:705–12. [PubMed] [Google Scholar]
- [15].Mazzoni A, Scaffa P, Carrilho M, Tjäderhane L, Di Lenarda R, Polimeni A, Tezvergil-Mutluay A, Tay FR, Pashley DH, Breschi L. Effects of etch-and-rinse and self-etch adhesives on dentin MMP-2 and MMP-9. J Dent Res. 2013;92:82–86. doi: 10.1177/0022034512467034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mazzoni A, Carrilho M, Papa V, Tjaderhane L, Gobbi P, Nucci C, et al. MMP-2 assay within the hybrid layer created by a two-step etch-and-rinse adhesive: biochemical and immunohistochemical analysis. J Dent. 2011;39:470–7. doi: 10.1016/j.jdent.2011.04.004. [DOI] [PubMed] [Google Scholar]
- [17].Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjaderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–7. doi: 10.1016/j.archoralbio.2006.08.009. [DOI] [PubMed] [Google Scholar]
- [18].Mazzoni A, Mannello F, Tay FR, Tonti GA, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and -9 forms in human sound dentin. J Dent Res. 2007;86:436–40. doi: 10.1177/154405910708600509. [DOI] [PubMed] [Google Scholar]
- [19].Nakabayashi N, Pashley DH. Hybridization of Dental Hard Tissues. Quintessence Publishing Co, Ltd; Chicago: 1998. p. 57. [Google Scholar]
- [20].Tezvergil-Mutluay A, Mutluay M, Seseogullari-Dirihan R, Agee KA, Key WO, Scheffel DL, et al. Effect of phosphoric acid on the degradation of human dentin matrix. J Dent Res. 2013;92:87–91. doi: 10.1177/0022034512466264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bertassoni LE, Orgel JP, Antipova O, Swain MV. The dentin organic matrix - limitations of restorative dentistry hidden on the nanometer scale. Acta Biomater. 2012;8:2419–33. doi: 10.1016/j.actbio.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tami AE, Schaffer MB, Knothe Tate ML. Probing the tissue to subcellular level structure underlying bone's molecular sieving function. Biorheology. 2003;40:577–590. [PubMed] [Google Scholar]
- [23].Toroian D, Lim JE, Price PA. The size exclusion characteristics of type I collagen: implications for the role of noncollagenous bone constituents in mineralization. J Biol Chem. 2007;282:22437–47. doi: 10.1074/jbc.M700591200. [DOI] [PubMed] [Google Scholar]
- [24].Tami AE, Schaffer MB, Knothe Tate ML. Probing the tissue to subcellular level structure underlying bone's molecular sieving function. Biorheaology. 2003;40:577–590. [PubMed] [Google Scholar]
- [25].Singh MP, Lumpkin JA, Rosenblatt J. Effect of electrostatic interactions on polylysine release rates from collagen matrices and comparison with model predictions. Journal of Controlled Release. 1995;35:165–79. [Google Scholar]
- [26].Blackburn RS, Harvey A, Kettle LL, Manian AP, Payne JD, Russell SJ. Sorption of chlorhexidine on cellulose: mechanism of binding and molecular recognition. J Phys Chem B. 2007;111:8775–84. doi: 10.1021/jp070856r. [DOI] [PubMed] [Google Scholar]
- [27].Kim J, Uchiyama T, Carrilho M, Agee KA, Mazzoni A, Breschi L, et al. Chlorhexidine binding to mineralized versus demineralized dentin powder. Dent Mater. 2010;26:771–8. doi: 10.1016/j.dental.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Scott TA, Jr., Melvin EH. Determination of dextran with anthrone. Analytical Chemistry. 1953;25:1656–61. [Google Scholar]
- [29].Sauro S, Toledano M, Augilera FS, Mannocci F, Pashley DH, Watson TF, Osorio R. Resin-dentin bonds to EDTA-treated vs. acid-etched dentin using 1 min ethanol wet-bonding. Dent Mater. 2010;26:368–379. doi: 10.1016/j.dental.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J Biomed Mater Res. 2002;62:447–56. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- [31].Ye Q, Park J, Laurence JS, Parthasarathy R, Misra A, Spencer P. Ternary phase diagram of model dentin adhesive exposed to over-wet environments. J Dent Res. 2011;90:1434–8. doi: 10.1177/0022034511423398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ye Q, Wang Y, Spencer P. Nanophase separation of polymers exposed to simulated bonding conditions. J Biomed Mater Res B Appl Biomater. 2009;88:339–48. doi: 10.1002/jbm.b.31047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Torchia DA. Solid-State Nmr-Studies of Molecular-Motion in Collagen Fibrils. Methods in Enzymology. 1982;82:174–86. [Google Scholar]
- [34].Beniash E, Tranb W, Veis A, Weinger S. A transmission electron microscopy study using vitrified ice sections of predentin: Structoral changes in dentin collagenous matrix prior to mineralization. J Struc. 2000;132:212–225. doi: 10.1006/jsbi.2000.4320. [DOI] [PubMed] [Google Scholar]
- [35].Pashley DH, Tay FR, Carvalho RM, Rueggeberg FA, Agee KA, Carrilho M, Donnelly A, Garcia-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am J Dent. 2007;20:7–21. [PubMed] [Google Scholar]
- [36].Sadek FT, Castellan CS, Braga RR, Mai S, Tjäderhane L, Pashley DH, Tay FR. One-year stability of resin-dentin bonds created with a hydrophobic ethanol-wet bonding technique. Dent Mater. 2010;26:380–386. doi: 10.1016/j.dental.2009.12.009. [DOI] [PubMed] [Google Scholar]
- [37].Sadek FT, Braga RR, Muench A, Liu L, Pashley DH, Tay FR. Ethanol wet-bonding challenges current anti-degradation strategy. J Dent Res. 2010a;89:1499–1504. doi: 10.1177/0022034510385240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pashley DH, Tay FR, Breschi L, Tjäderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art of etch-and-rinse adhesives. Dent Mater. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Tay FR, Pashley, Kapur RR, Carrilho MRO, Hur YB, Garrett LV, Tay KCY. Bonding BisGMA to dentin - a proof of concept. J Dent Res. 2007;86:1034–1039. doi: 10.1177/154405910708601103. [DOI] [PubMed] [Google Scholar]