Abstract
Background
High attrition rates among African-Americans (AA) volunteers are a persistent problem that makes clinical trials less representative and complicates estimation of treatment outcomes. Many studies contrast AA with other ethnic/racial groups, but few compare the AA volunteers who remain in treatment with those who leave. Here, in addition to comparing patterns of attrition between African Americans and whites, we identify predictors of overall and early attrition among African Americans.
Method
Sample comprised non-Hispanic African-American (n=673) and white (n=2,549) participants in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Chi-square tests were used to examine racial group differences in reasons for exit. Multivariate logistic regression was used to examine predictors of overall attrition, early attrition (by Level 2) and top reasons cited for attrition among African Americans.
Results
For both African-American and white dropouts, non-compliance reasons for attrition were most commonly cited during the earlier phases of the study while reasons related to efficacy and medication side effects were cited later in the study. Satisfaction with treatment strongly predicted overall attrition among African Americans independent of socioeconomic, clinical, medical or psychosocial factors. Early attrition among African American dropouts was associated with less psychiatric comorbidity, and higher perceived physical functioning but greater severity of clinician-rated depression.
Conclusions
The decision to drop out is a dynamic process that changes over the course of a clinical trial. Strategies aimed at retaining African Americans in such trials should emphasize engagement with treatment and patient satisfaction immediately following enrollment and after treatment initiation.
Keywords: Research Volunteers, Ethnic Groups, Blacks, Depression, Disparities, Treatment
Introduction
Participant retention in clinical trials for the treatment of Major Depressive Disorder remains a significant public health and scientific issue. Depression treatment studies have linked attrition to incomplete remission (1–3). This association is especially evident among racial and ethnic minorities who demonstrate significantly higher dropout and lower remission relative to non-minorities in clinical trials (2, 4).
The disparity in treatment completion is particularly salient for African-Americans who typically exhibit higher dropout rates relative to other minority groups (1, 5, 6) even when education levels are taken into account. Specific attitudes and beliefs about depression and antidepressants have been suggested as additional drivers of the disparity in treatment seeking and compliance (7–13). Initial attitudinal and access barriers, however strong, evidently do not always prevent minority participants from enrolling. Although many factors influence both retention and recruitment, retention remains conceptually distinct. Treatment experiences and other factors may take on greater importance after enrollment (14–16).
The current literature on study retention and attrition tends to focus broadly on factors associated with dropout, with relatively less attention paid to cited reasons for dropping out. To effectively address study attrition among minorities, more direct data on the specific reasons for dropout and their relationship to the broader factors associated with attrition is needed. This is especially challenging in view of the paucity of empirical studies with large and representative samples of minority participants.
The Sequenced Treatment Alternatives to Relieving Depression (STAR*D) study was a multi-site clinical trial that was designed to develop a feasible set of options for treating depressed patients in real-life psychiatric and medical settings (17–18). The study boasted a healthy recruitment of non-Hispanic African-Americans (18%), but earlier studies on attrition in STAR*D showed that African-Americans were significantly more likely to drop out of the study prematurely than whites. In addition, socio-demographic disadvantages such as lower income or lower education status, and higher medical burden such as greater severity of depression and more comorbid physical and psychiatric illness, were associated with higher dropout rates (3, 20). Although these analyses have helped to shed considerable light on broader issues related to dropout in depression treatment studies, relatively less information on the specific issues pertinent to ethnic minorities who enroll in these studies has been reported (21). Consequently, gaps remain in our understanding of why some African-Americans leave the study early while others remain to completion.
In view of the known higher attrition rates among African-American participants in STAR*D, we sought to (1a) identify the main reasons for study attrition among African-Americans across the four treatment levels; (1b) compare those reasons with that of white participants who also dropped out of the study; (2a) identify the strongest predictors of overall attrition and early attrition among African Americans, and (2b) determine whether and how any of these predictors are associated with the top reasons cited for dropping out of the study.
Materials and Method
The study comprised outpatients from 18 primary care and 23 psychiatric specialty care facilities. Treatment options were sequenced through 4 distinct levels of the trial. The study, including methods, has been described extensively elsewhere (17–18, 22).
Sample
Race and ethnicity were based on participants’ self-report and were assessed as part of a package of baseline measures in the 4041 adult patients enrolled in STAR*D. Our sample included 673 participants who self-identified as African-American or Black (non-Hispanic), and who did not have missing or ambiguous exit data. In addition, we used a comparison sample of 2,549 non-Hispanic whites who also had non-missing exit data. The 21 African Americans with missing study exit data had a non-significant trend of lower monthly household income but otherwise did not differ from significantly from the cases used in our analyses on any of the predictors in the study. The 65 missing white cases were more likely to report a family history of committed suicide, but otherwise did not differ significantly from the cases used in our comparison group.
Study Levels
The trial was divided into four predetermined phases (Levels 1–4), each with different treatment options, ranging from one single medication (citalopram), to increasingly complex augmentation or switching strategies, including cognitive behavioral psychotherapy (17, 18). Participants were treated at a given level for up to 14 weeks. Those sufficiently improved were referred out of the sequence for follow-up (up to 12 months). The rest were sent on to the next treatment level.
Participants could exit a study level by improving (responding or remitting), being referred for follow up, moving to the next level, or dropping out of the study. Participants who exited the study prematurely were asked to ascribe their withdrawal to one or more reasons among a list of 14 (See Supplemental Table 4a). The reasons were not mutually exclusive and a participant could have 2 or more reasons for dropout.
Study Measures
At the onset and during the course of treatment, participants completed a variety of clinician-administered or self-reported assessments. At baseline, demographic, eligibility, diagnostic (26), medical illness inventory (27–28), family history of psychiatric illness, and baseline depressive symptom measures were administered (29–31). During clinician visits, symptom severity measures (e.g., QIDS-C) (32) and medication side-effects profiles (33) were obtained at approximately two-week intervals. Research outcome measures which also included depressive symptom severity, medication side effects current psychosocial and physical functioning (23–25), and participant satisfaction measures were obtained at baseline, at week 6 of each level, and at exit from each level (when possible). Three questions on patient baseline attitudes towards help-seeking from professionals and helpfulness of family or friends in patients’ ability to cope with their depression, and two questions on patient satisfaction with clinician and treatment (administered at baseline and up to two regularly spaced intervals during each level) are included in the supplement information – Appendix 1. For a detailed description of all the study measures used as well as the administration schedule and modality, see (18, 22).
Statistical Analyses
Reasons for attrition and related constructs
We used descriptive analyses to identify the top reasons for attrition among African Americans and whites, across levels 1–4. For each study level, we considered all of the reported reasons for exit, as a participant could have more than one reason attributed for exit. Using Chi-square tests we examined whether there were any racial group differences in the top reasons cited for dropout at each level.
Predictors of overall and early attrition among African Americans
Overall attrition was defined as leaving the study unremitted at any time, and early attrition was defined as leaving the study unremitted by the end of level 2. Multivariate logistic regressions were used to assess the relationship between the predictors and overall attrition or early attrition.
Analyses for overall and early attrition were done in two separate steps. First, predictors were grouped under various headings (e.g., Sociodemographic, Attitudes towards professional help and informal support, etc.), and each group was entered into a separate model. Significant predictors from each group were then entered simultaneously into a final regression model. Because of the smaller the sample sizes for the early attrition analyses, we decided to include in the final regression model predictors with p-values ≤ .010, and odds ratios ≥ 1.50 or ≤ 0.50. (See Supplemental Table 2).
Results
Racial and ethnic group differences in the STAR*D study have been detailed elsewhere (4), so here only group differences in attrition at each level (Table 1), predictors used in this study (Supplemental Table 1), and reasons cited for attrition (Figure 1) are presented. For categorical predictors, the reference category is noted in the table; for dichotomous predictors, the reference category is the absence of the variable, and for continuous predictors, the scores are averaged for the duration of the study if they are taken at different time points or baseline scores are indicated if used (means and standard deviations are presented in the table).
Table 1.
Attrition among African-Americans and Whites at levels 1–4
| Level | African-American | White | ||
|---|---|---|---|---|
|
| ||||
| n | Dropped out (%) | n | Dropped out (%) | |
| 1 | 673 | 258 (39) | 2,549 | 603 (24) |
| 2 | 220 | 100 (46) | 889 | 234 (26) |
| 3 | 56 | 30 (56) | 242 | 109 (45) |
| 4 | 15 | 9 (60) | 68 | 34 (54) |
Note. Odds Ratio (O.R.) reflects odds of dropping out of the study for African-American vs. White race. Chi-square (χ2) Fisher exact tests null hypothesis of equal proportions (two-tailed).
Level 1: O.R. = 2.01 (1.68–2.40); χ2 = 58.67, p <.0001
Level 2: O.R. = 2.27 (1.67–3.07); χ2 = 28.77, p <.0001
Level 3: O.R. = 1.57 (0.87–2.83); χ2 = 2.28, p =.138
Level 4: O.R. = 1.36 (0.45–4.16); χ2 = 0.29, p =.781
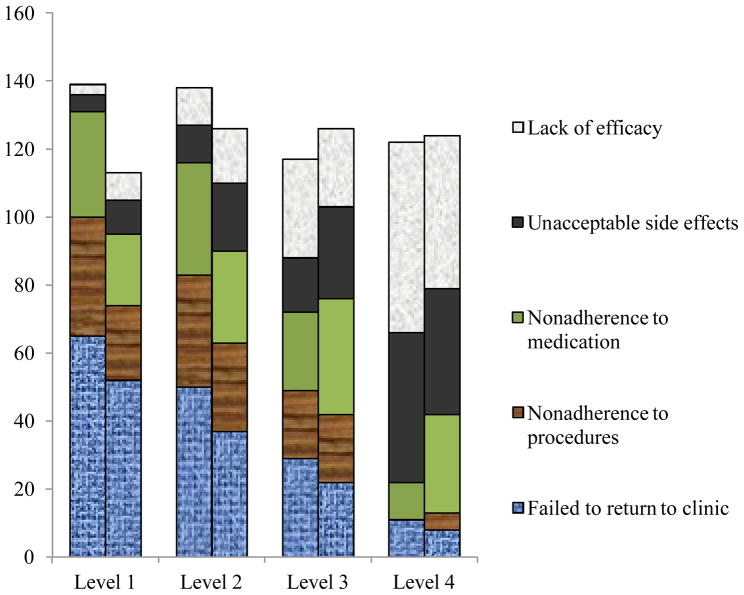
Figure 1. Top reasons for exit at levels 1–4 among African-American and white participants.
Note. At each level, left column represents African Americans; right column represents whites. Figures represent % out of all reasons ascribed for dropping out at that level. Reasons ascribed are not mutually exclusive so columns do not add to 100%.
There were no significant racial group differences in the proportion of dropouts who cited Failed to return to clinic at level 1 (χ2 df1 = 1.01, p =.178), level 2 (χ2 df1 = 0.47, p = .293), level 3 (χ2 df1 = 0.33, p = .543), and level 4 (χ2 df1 = 0.27, p = 0.27).
There were no significant racial group differences in the proportion of dropouts who cited Lack of efficacy at level 1 (χ2 df1 = 3.46, p =.080), level 2 (χ2 df1 = 1.19, p =.310), level 3(χ2 df1 = 0.82, p =.467), and level 4 (χ2 df1 = 0.26, p =.718).
Non adherence to study medication was cited more frequently by African Americans than whites at level 1 only (χ2 df1 = 14.88, p <.0001). There were no racial group differences at level 2 (χ2 df1 = 2.14, p =.145), level 3(χ2 df1 = 1.03, p =.376), or level 4 (χ2 df1 = 0.99, p =.299).
Non adherence to study procedures was cited more frequently by African Americans than whites at level 1 only (χ2 df1 = 24.42, p < .0001). There were no racial group differences at level 2 (χ2 df1 = 2.35, p =.138), level 3 (χ2 df1 = 0.20, p =.785), or level 4 (χ2 df1 = 0.50, p = 1.00).
Unacceptable side effects of medication was cited more frequently by whites than African Americans at levels 1 (χ2 df1 = 5.39, p < .05) and 2 (χ2 df1 = 3.78, p =.058) only. There were no racial group differences at level 3(χ2 df1 = 1.25, p =.341) or level 4 (χ2 df1 = 0.13, p =.721).
As shown in Table 1, African-American attrition exceeded that of whites at every level of the study, and was significantly higher at levels 1 and 2. As has been reported previously and shown in Supplemental Table 1, African-Americans exhibited greater sociodemographic disadvantage, baseline severity of depressive symptoms, and more psychiatric and medical comorbidity. However, they did not differ from whites in their baseline attitudes towards professional help-seeking or helpfulness of family and friends in coping with their depression, although they reported lower average satisfaction than whites with their clinicians.
Reasons cited for dropping out of the study
Figure 1 shows the top reasons endorsed by African Americans and whites for study exit at each level. African Americans who dropped out at levels 1 and 2 were most commonly ascribed Failed to return to the clinic (for 65% of dropouts at level 1 and 50% at level 2), Non adherence to study procedures (for 35% of dropouts at level 1 and 33% at level 2), and Non adherence to study medication (for 31% of dropouts at level 1 and 33% at level 2). By level 3, Lack of efficacy cited by 29% of dropouts, along with Failed to return to the clinic (29%) and Non adherence to medication (20%), were the top reasons. At level 4, Lack of efficacy (56%) was the top reason cited followed by Unacceptable medication side effects (44%).
The trends in top reasons cited among whites were similar to those of African Americans. At levels 1 and 2, whites who dropped out most commonly endorsed Failed to return to the clinic, followed by Non adherence to study medication and Non adherence to study procedures, in some order. By level 4, whites who dropped out most commonly cited Lack of efficacy (45%) and Unacceptable side effects (37%).
There were no racial group differences at any time in the proportion of dropouts who cited Failed to return to the clinic or Lack of efficacy. However, at level 1, significantly more African-American dropouts cited Non adherence to medication and Non adherence to study procedures compared to white dropouts, and at levels 1 and 2 significantly more white than African-American dropouts cited Unacceptable side effects. For a complete list of all the possible reasons cited and how they were distributed among African-American dropouts at each level, see Supplemental Tables 4a-d.
Predictors of overall attrition among African Americans
As depicted in Supplemental Table 2, all the significant predictors in each of the categories were selected for the final analysis. When all these predictors were simultaneously considered in one model, as shown in Table 2, the strongest independent predictors of overall attrition were lower satisfaction with treatment and lower perceived physical and mental functioning. To determine whether lower satisfaction with treatment was better accounted for by other sociodemographic, clinical, or psychosocial factors, we assessed the impact of treatment satisfaction controlling for these groups of variables in separate regression models. Satisfaction with treatment remained a significant predictor of overall attrition with odds ratios ranging from 0.58 to 0.74 and all p-values remaining less than or equal to .001. (Data is not shown in tables but available upon request)
Table 2.
Predictors of overall attrition among African Americans
| Predictor | B | (S.E.) | Odds Ratio | (95% C.I.) | Wald Z-statistic |
|---|---|---|---|---|---|
| Age (in years) | −0.001 | 0.01 | 1.00 | (0.97–1.03) | 0.01 |
| Employed vs. unemployed | −0.21 | 0.32 | 0.81 | (0.45–1.52) | 0.42 |
| Retired vs. unemployed | −0.23 | 0.61 | 0.79 | (0.24–2.63) | 0.14 |
| Satisfaction with clinician1 | −0.18 | 0.15 | 0.84 | (0.62–1.13) | 1.37 |
| Satisfaction with treatment1 | −0.34 | 0.13 | 0.72 | (0.56–0.92) | 7.06 ** |
| HRSD17 baseline severity of depression | 0.07 | 0.04 | 1.07 | (0.99–1.16) | 2.53 |
| Family history of suicide | −0.94 | 0.86 | 0.39 | (0.07–2.09) | 1.21 |
| Having more than one major depressive episode | −0.40 | 0.31 | 0.67 | (0.36–1.23) | 1.67 |
| Concurrent alcohol abuse/dependence | −1.31 | 0.67 | 0.27 | (0.07–0.99) | 3.88* |
| Concurrent drug abuse/dependence | 0.50 | 0.94 | 1.64 | (0.26–10.30) | 0.28 |
| QIDS-C score (depressive symptoms at week 0) | 0.01 | 0.06 | 1.01 | (0.90–1.14) | 0.02 |
| Quality of Life Enjoyment & Satisfaction (QLESQ) | −0.01 | 0.02 | 1.01 | (0.98–1.04) | 0.14 |
| Perceived physical functioning | −0.07 | 0.02 | 0.94 | (0.90–0.97) | 12.27*** |
| Perceived mental functioning | −0.09 | 0.03 | 0.91 | (0.87–0.96) | 13.48*** |
| GRSEB –impairment from medication side effects | 0.14 | 0.06 | 1.15 | (0.88–1.50) | 1.09 |
All estimates obtained from multivariate logistic regression, two-tailed significance,
p < .05,
p < .01,
p < .001.
Note. All predictors are entered simultaneously in logistic regression model. For continuous variables (e.g., Perceived physical functioning), regression coefficient (B) represents the unit change in predictor associated with dropping out vs. not dropping out of the study. For dichotomous variables (e.g., concurrent alcohol abuse/dependence), odds ratios are the odds of dropping out of the study in the presence of these conditions. For categorical variable (employment status) odds ratios represent the odds of dropping out early if one is employed, or if one is retired vs. unemployed.
Only 56% of the African-American sample responded to these two items, (83% of non-dropouts and 36% of dropouts). Pearson correlation between the two items for African-American sample = .55, p < .001.
Predictors of early attrition among African Americans
As was done for overall attrition, we selected the strongest predictors of early attrition based on initial estimates from the regression models (see Supplemental Table 2) and entered them simultaneously in a single final model. Table 3 shows the regression coefficients and odds ratios for early vs. late attrition. The strongest predictors of early attrition were having greater baseline depressive symptoms when rated by the clinician, but lower self-rated baseline depressive symptoms, absence of comorbid anxiety and higher perceived physical functioning.
Table 3.
Predictors of early attrition among African-Americans who dropped out of the study
| Predictor | B | (S.E.) | Odds Ratio | (95% C.I.) | Wald Z-statistic |
|---|---|---|---|---|---|
| Marital status | |||||
| Married | −0.75 | 0.55 | 0.47 | (0.16–1.93) | 1.85 |
| Divorced/separated/widowed | −0.85 | 0.52 | 0.43 | (0.15–1.18) | 1.18 |
| Comorbid anxiety disorder | −0.90 | 0.40 | 0.41 | (0.18–0.90) | 4.99* |
| Concurrent alcohol abuse/dependence | 0.20 | 0.84 | 1.22 | (0.24–6.30) | 0.06 |
| QIDS-C (depressive symptoms at week 0) | 0.22 | 0.08 | 1.24 | (1.07–1.45) | 7.96** |
| QIDS-SR (depressive symptoms at week 0) | −0.14 | 0.06 | 0.87 | (0.77–0.98) | 4.93* |
| Perceived physical functioning | 0.06 | 0.02 | 1.06 | (1.03–1.10) | 12.09*** |
All estimates obtained from multivariate logistic regression, two-tailed significance,
p < .05,
p < .01,
p < .001
Note. Early attrition is defined as leaving the study by level 2 without remission of depression. All predictors are entered simultaneously in logistic regression model. For continuous variables (QIDS scores and perceived physical functioning, regression coefficient (B) represents the unit change in predictor associated with dropping out early vs. later in the study. For dichotomous variables (comorbid anxiety disorder and concurrent alcohol abuse/dependence), odds ratios are the odds of dropping out early vs. late in the presence of these conditions. For categorical variable (marital status), odds ratios represent the odds of dropping out early if one is married, or if one is separated/divorced/widowed vs. never married.
Factors associated with top reasons cited for attrition among African Americans
Among the African Americans who did not return to the clinic, 115 failed to return to the clinic with no other reason ascribed for their leaving the study. Among those who did not adhere to medication, 31 did so uniquely and among those who did not adhere to study procedures, 16 did so uniquely. As shown in Supplemental Table 3, those who did not return to the clinic were more likely to be married, less likely to believe that their friends and family were helpful in coping with their illness, less likely to have had more than one major depressive episode, less likely to have alcohol abuse or dependence, and less likely to have comorbid anxiety, and more likely to have higher perceived physical functioning than those who dropped out of the study with cited reasons.
There were no sociodemographic, clinical or medical factors strongly associated with non- adherence to study medication or procedures. Only one psychosocial measure (greater perceived work and social adjustment) was found to be significant, with a non-significant trend of younger age. Medication side effects was not predictive of non-adherence to medication, when this non-adherence was examined separately (data not shown but available upon request) or in combination with non-adherence to study procedures (Supplemental Table 3).
Discussion
This is the first study from the STAR*D trial that addresses participant differences within an ethnic group identified as having relatively high dropout rate with poorer treatment outcome.
Previous research, including earlier reports on attrition in the STAR*D study, has highlighted the role of socioeconomic and related demographic factors in premature termination of treatment (2, 7, 38, 39). While sociodemographic disadvantage may partly explain the higher dropout rates for African Americans relative to whites, by addressing within-group variation in African Americans, this study identified additional contributors to study attrition outside of sociodemographic disadvantage that otherwise might have been overlooked.
A key contributor to overall attrition among African Americans was satisfaction with treatment which was not better explained by sociodemographic status, clinical and medical factors, psychosocial functioning or baseline attitudes towards professional treatment. This finding was robust despite fewer than half of the dropouts responding to these items. Had these dropouts been forced to respond, it is likely that their responses would produce results even further away from a null hypothesis of no group differences in satisfaction since they were less likely to be satisfied than non-dropouts. Satisfaction with clinician, one of the initial predictors of overall dropping out as shown in supplemental Table 2, was significantly correlated with satisfaction with treatment. And as shown in supplemental Table 1, African Americans were significantly lower on satisfaction with clinician than whites even though they were similar on satisfaction with treatment. It is likely that the clinician is viewed as just one component of the overall treatment, which when assessed among African Americans, better predicted attrition.
Although satisfaction with clinicians and treatment was not reported in previous publications on attrition in the STAR*D study, this finding does raise the need for increased attention to be paid to certain factors within the treatment experience that increase or decrease patient satisfaction. Previous studies have alluded to perceived treatment quality as being paramount in retaining minority patients and one index of treatment quality in psychiatric settings is duration of visits, with longer visits indicative of better quality due to more communication and disclosure. Although research has indicated that vast improvements have been made in the duration of visits among African American psychiatric outpatients due to implementation of policies designed to reduce racial health disparities in treatment settings, there may be persisting disparities in communication and disclosure (16). This study did not report the race and/or gender of the clinician, both of which have been shown to influence the level of meaningful disclosure that occurs in the patient-physician dyad (14, 15). Future clinical trials involving multiple racial groups might benefit from including this information in assessment.
A discernible pattern of dropping out due to non-compliance, including non adherence to medication regimen or study procedures that occurred during the earlier phases of the study but diminished in the latter phases, was evident for both African Americans and whites. Although there were no racial group differences among the dropouts who simply failed to return to the clinic for treatment, we found that non-adherence to medication or study procedures was cited more frequently for African American than white dropouts, especially at level 1 when the difference was significant. Moreover, as noted from the regression results, medication side effects were not a significant factor in early dropout and they were not significantly associated with non-adherence to medication or procedures. It is plausible that some of the noncompliance effects found at level 1 began upon enrollment.
For example, many study subjects may have dropped out after the baseline assessments but before the first treatment phase of the study was initiated. Some research has suggested that this is a critical period because once antidepressant treatment has been initiated, the differences in compliance between African-American and Caucasian patients tend to diminish (34). Our study results showing a closing racial gap in adherence after level 1 are consistent with this idea.
A significant number of dropouts failed to return to the clinic with no other reason cited. Among African-Americans, this kind of dropping out was associated with being depressed for the first time. Although it may be tempting to think that dropouts who failed to return to the clinic can be safely ignored because the they appear to have less psychiatric and physical impairment, and they are not disproportionately represented among African Americans relative to whites, it is important to remember that a first episode of major depression is a significant risk factor for subsequent episodes, which may be more severe than the first (40). The likelihood of seeking treatment when appropriate may be decreased if the initial experience was deemed unsatisfactory. Because these dropouts also were less likely to have comorbid psychiatric disorders such as anxiety or substance dependence than other dropouts, along with higher perceived physical functioning, such individuals may not have had prior experience with professional treatment for medical or mental illness. Unrealistic expectations about the treatment experience may have resulted in dissatisfaction leading to premature termination. Although they were more likely to be married, they were also less likely to believe that their family and friends made it easier to cope with their depression. The assumption that friends and family are supportive in treatment may not always be true – particularly for mental illnesses which may invoke various forms of stigma and psychosocial burden among relatives of the sufferer. This situation may be even more pronounced in ethnic minority communities. Thus it is instructive to be aware of not just patient attitudes towards mental illness but that of those closest to them who may hinder or help the treatment process.
Some study limitations warrant caution in interpreting these findings. For example, study predictors did little to distinguish between African-American dropouts who did not adhere to medication or study procedures vs other African-American dropouts. This may have been a limitation due to lack of power from the small sample size, but also may have reflected a range of non-specific factors simultaneously responsible for this form of noncompliance.
The small sample sizes for the later dropouts also decreased the power to detect group differences on several factors. At the same time, these small numbers suggest that there are critical periods in trials by which the bulk of participants are likely to drop out. Therefore it is imperative that engagement strategies identify and focus on these critical periods, which in this trial appear to have been immediately post-enrollment and after treatment began. Although we did not have data on participants’ attitudes specifically towards antidepressants, we assume that at least initially, they were willing to use antidepressants given that this was a pharmacological study. We also did not assess whether there were differences in dropout status between those who were offered the “psychotherapy alone” condition versus those who were randomized to the medication alone or medication with psychotherapy options. While some studies have suggested that African-Americans show a preference for psychotherapy over medication (11) in treating depression, earlier findings on the STAR*D sample indicate that African-Americans were not more likely than whites to endorse a preference for the psychotherapy conditions over the medication switch/augment options (2).
Conclusion
Our findings have highlighted some important facets of the clinical trial experience among depressed African-American outpatients. First, these patients generally start the treatment with attitudes and expectations that may not significantly differ from that of whites, but there are some critical periods during which the potential for disengagement and premature termination in this group may be heightened: the period immediately following enrollment or initial assessment and the period following the onset of treatment(s). As the data from earlier studies demonstrate, participants who were able to remain in the study through Level 2 for the most part showed improvement. Strategies and resources aimed at improving treatment engagement would be best focused at these early stages.
Improvement in psychoeducation, treatment type and explicit advice from the clinician about treatment limitations could help to maintain realistic expectations on the part of the patient about the rate and occasional inconsistency of progress. Participants could be encouraged to enlist trusted family members or friends as allies, who are understanding of their illness and supportive of their treatment, and who could assist clinicians and researchers in keeping lines of communication open and facilitating alternate forms of outreach with patients between clinic visits and research assessments. Offering incentives like transportation reimbursement or home visits might be useful in some cases particularly where patients might have difficulty getting to and from appointments.
Further research on clinical trials for depression treatment among African-Americans is necessary to identify specific features in the patient-clinician relationship that predict better patient engagement and treatment satisfaction once study enrollment is completed. A focus on within-group variation can highlight areas of relative strengths or weaknesses in patient resources, clinical settings and research environments that facilitate retention of African-Americans and promote better treatment outcomes for this large and growing group of patients.
Supplementary Material
Acknowledgments
Sources of support
This study was funded by the Intramural Research Program of the National Institute of Minority Health and Health Disparities, and the National Institute of Mental Health, NIH; by K22MD006140-01 to Eleanor Murphy; a NARSAD Independent Investigator Award to Francis McMahon; and by K99MH085098-01 to Gonzalo Laje. The authors thank the STAR*D research team for acquisition of clinical data and DNA samples. Data and sample collection were funded with federal funds from the NIMH, NIH, under contract N01MH90003 to University of Texas Southwestern Medical Center at Dallas (Principal investigator, A. John Rush). The authors appreciate the support of the SingHealth/Duke-NUS Academic Medicine Research Institute Singapore and medical editing assistance of Taara Madhavan (Associate in Clinical Sciences, Duke-NUS Graduate Medical School, Singapore). The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) Study is registered at www.clinicaltrials.gov with identifier number NCT00021528. The content of this publication does not necessarily reflect the views or policies of the DHHS, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Disclosures
Drs. Murphy, Kassem, Laje, McMahon and Ms Chemerinski report no competing interests. Dr. Rush has served as an advisor, consultant, or speaker for or received research support from Advanced Neuromodulation Systems, Inc.; AstraZenica; Best Practice Project Management, Inc.; Bristol-Meyers Squibb Company; Cyberonics Inc.; Eli Lilly & Company; Forest Pharmaceuticals Inc.; Gerson Lehman Group; GlaxoSmithKline; Healthcare Technology Systems Inc.; Jazz Pharmaceuticals; Magellan Health Services; Merck & Co. Inc.; the National Institute of Mental Health; Neuronetics; Ono Pharmaceutical; Organon USA Inc.; Otsuka; Pamlab; Personality Disorder Research Corporation; Pfizer Inc.; the Robert Wood Johnson Foundation; the Stanley Medical Research Institute; the Urban Institute; and Wyeth-Ayerst Laboratories Inc. Dr. Rush has equity holdings in Pfizer Inc., and receives royalty/patent income from Guilford Publications and Healthcare Technology Systems Inc., and UT Southwestern Medical Center.
References
- 1.Lesser IM, Zisook S, Gaynes BN, Wisniewski SR, Luther JF, Fava M, et al. Effects of race and ethnicity on depression treatment outcomes: the CO-MED trial. Psychiat Serv. 2011;62(10):1167–79. doi: 10.1176/ps.62.10.pss6210_1167. [DOI] [PubMed] [Google Scholar]
- 2.Warden D, Rush AJ, Wisniewski SR, Lesser IM, Thase ME, Balasubramani GK, et al. Income and attrition in the treatment of depression: a STAR*D report. Depress Anxiety. 2009;26(7):622–33. doi: 10.1002/da.20541. [DOI] [PubMed] [Google Scholar]
- 3.Warden D, Trivedi MH, Wisniewski SR, Davis L, Nierenberg AA, Gaynes BN, et al. Predictors of attrition during initial (citalopram) treatment for depression: a STAR*D report. The American journal of psychiatry. 2007;164(8):1189–97. doi: 10.1176/appi.ajp.2007.06071225. [DOI] [PubMed] [Google Scholar]
- 4.Lesser IM, Castro DB, Gaynes BN, Gonzalez J, Rush AJ, Alpert JE, et al. Ethnicity/race and outcome in the treatment of depression: results from STAR*D. Med Care. 2007;45(11):1043–51. doi: 10.1097/MLR.0b013e3181271462. [DOI] [PubMed] [Google Scholar]
- 5.Diaz E, Woods SW, Rosenheck RA. Effects of ethnicity on psychotropic medications adherence. Community Ment Health J. 2005;41(5):521–37. doi: 10.1007/s10597-005-6359-x. [DOI] [PubMed] [Google Scholar]
- 6.Fortuna LR, Alegria M, Gao S. Retention in depression treatment among ethnic and racial minority groups in the United States. Depress Anxiety. 2010;27(5):485–94. doi: 10.1002/da.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dixon L, Lewis-Fernandez R, Goldman H, Interian A, Michaels A, Kiley MC. Adherence disparities in mental health: opportunities and challenges. J Nerv Ment Dis. 2011;199(10):815–20. doi: 10.1097/NMD.0b013e31822fed17. [DOI] [PubMed] [Google Scholar]
- 8.Anglin DM, Alberti PM, Link BG, Phelan JC. Racial differences in beliefs about the effectiveness and necessity of mental health treatment. Am J Community Psychol. 2008;42(1–2):17–24. doi: 10.1007/s10464-008-9189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anglin DM, Link BG, Phelan JC. Racial differences in stigmatizing attitudes toward people with mental illness. Psychiat Serv. 2006;57(6):857–62. doi: 10.1176/ps.2006.57.6.857. [DOI] [PubMed] [Google Scholar]
- 10.Schnittker J, Freese J, Powell B. Nature, nurture, neither, nor: Black-white differences in beliefs about the causes and appropriate treatment of mental illness. Soc Forces. 2000;78(3):1101–32. [Google Scholar]
- 11.Givens JL, Houston TK, Van Voorhees BW, Ford DE, Cooper LA. Ethnicity and preferences for depression treatment. Gen Hosp Psychiat. 2007;29(3):182–91. doi: 10.1016/j.genhosppsych.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Schnittker J. Misgivings of medicine?: African Americans’ skepticism of psychiatric medication. J Health Soc Behav. 2003;44(4):506–24. [PubMed] [Google Scholar]
- 13.Gonzalez HM, Croghan T, West B, Williams D, Nesse R, Tarraf W, et al. Antidepressant use in black and white populations in the United States. Psychiat Serv. 2008;59(10):1131–8. doi: 10.1176/appi.ps.59.10.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-centered communication, ratings of care, and concordance of patient and physician race. Ann Intern Med. 2003;139(11):907–15. doi: 10.7326/0003-4819-139-11-200312020-00009. [DOI] [PubMed] [Google Scholar]
- 15.Cooper-Patrick L, Gallo JJ, Gonzales JJ, Vu HT, Powe NR, Nelson C, et al. Race, gender, and partnership in the patient-physician relationship. JAMA: the journal of the American Medical Association. 1999;282(6):583–9. doi: 10.1001/jama.282.6.583. [DOI] [PubMed] [Google Scholar]
- 16.Olfson M, Cherry DK, Lewis-Fernandez R. Racial differences in visit duration of outpatient psychiatric visits. Arch Gen Psychiat. 2009;66(2):214–21. doi: 10.1001/archgenpsychiatry.2008.523. [DOI] [PubMed] [Google Scholar]
- 17.Rush AJ, Fava M, Wisniewski SR, Lavori PW, Trivedi MH, Sackeim HA, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Controlled clinical trials. 2004;25(1):119–42. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 18.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 19.Rush AJ, Trivedi MH, Stewart JW, Nierenberg AA, Fava M, Kurian BT, et al. Combining medications to enhance depression outcomes (CO-MED): acute and long-term outcomes of a single-blind randomized study. The American journal of psychiatry. 2011;168(7):689–701. doi: 10.1176/appi.ajp.2011.10111645. [DOI] [PubMed] [Google Scholar]
- 20.Warden D, Rush AJ, Wisniewski SR, Lesser IM, Kornstein SG, Balasubramani GK, et al. What predicts attrition in second step medication treatments for depression?: a STAR*D Report. The international journal of neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2009;12(4):459–73. doi: 10.1017/S1461145708009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schraufnagel TJ, Wagner AW, Miranda J, Roy-Byrne PP. Treating minority patients with depression and anxiety: what does the evidence tell us? Gen Hosp Psychiat. 2006;28(1):27–36. doi: 10.1016/j.genhosppsych.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Rush J. Sequenced Treatment Alternatives to Relieve Depression (STAR*D, Rev 06/28/02) 2000. pp. 1–63. [Google Scholar]
- 23.Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Brit J Psychiat. 2002;180:461–4. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- 24.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacology bulletin. 1993;29(2):321–6. [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 26.Zimmerman M, Mattia JI. The reliability and validity of a screening Questionnaire for 13 DSM-IV Axis I disorders (the Psychiatric Diagnostic Screening Questionnaire) in psychiatric outpatients. The Journal of clinical psychiatry. 1999;60(10):677–83. doi: 10.4088/jcp.v60n1006. [DOI] [PubMed] [Google Scholar]
- 27.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–6. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 28.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiat Res. 1992;41(3):237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 31.Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiat. 1988;45(8):742–7. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- 32.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biological Psychiatry. 2003;54(5):573–83. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 33.Wisniewski SR, Rush AJ, Balasubramani GK, Trivedi MH, Nierenberg AA. Self-rated global measure of the frequency, intensity, and burden of side effects. J Psychiatr Pract. 2006;12(2):71–9. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Harman JS, Edlund MJ, Fortney JC. Disparities in the adequacy of depression treatment in the United States. Psychiat Serv. 2004;55(12):1379–85. doi: 10.1176/appi.ps.55.12.1379. [DOI] [PubMed] [Google Scholar]
- 35.Magruder KM, Bichun O, Miller S, Tilley BC. Retention of under-represented minorities in drug abuse treatment studies. Clinical trials. 2009;6(3):252–60. doi: 10.1177/1740774509105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aikens JE, Nease DE, Jr, Klinkman MS. Explaining patients’ beliefs about the necessity and harmfulness of antidepressants. Ann Fam Med. 2008;6(1):23–9. doi: 10.1370/afm.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnow BA, Blasey C, Manber R, Constantino MJ, Markowitz JC, Klein DN, et al. Dropouts versus completers among chronically depressed outpatients. J Affect Disorders. 2007;97(1–3):197–202. doi: 10.1016/j.jad.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Lantz PM, House JS, Lepkowski JM, Williams DR, Mero RP, Chen J. Socioeconomic factors, health behaviors, and mortality: results from a nationally representative prospective study of US adults. JAMA: the journal of the American Medical Association. 1998;279(21):1703–8. doi: 10.1001/jama.279.21.1703. [DOI] [PubMed] [Google Scholar]
- 39.Last CG, Thase ME, Hersen M, Bellack AS, Himmelhoch JM. Patterns of attrition for psychosocial and pharmacologic treatments of depression. The Journal of clinical psychiatry. 1985;46(9):361–6. [PubMed] [Google Scholar]
- 40.Belsher G, Costello CG. Relapse after recovery from unipolar depression: A critical review. Psychological Bulletin. 1988;104 (1):84–96. doi: 10.1037/0033-2909.104.1.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.