Abstract
Dental-derived MSCs are promising candidates for cartilage regeneration, with high chondrogenic differentiation capacity. This property contributes to making dental MSCs an advantageous therapeutic option compared to current treatment modalities. The MSC delivery vehicle is the principal determinant for the success of MSC-mediated cartilage regeneration therapies. The objectives of this study were to: (1) develop a novel co-delivery system based on TGF-β1 loaded RGD-coupled alginate microspheres encapsulating Periodontal Ligament Stem Cells (PDLSCs) or Gingival Mesenchymal Stem Cells (GMSCs); and (2) investigate dental MSC viability and chondrogenic differentiation in alginate microspheres. The results revealed the sustained release of TGF-β1 from the alginate microspheres. After 4 weeks of chondrogenic differentiation in vitro, PDLSCs, GMSCs as well as human bone marrow mesenchymal stem cells (hBMMSC) (as positive control) revealed chondrogenic gene expression markers (Col II and Sox-9) via qPCR, as well as matrix positively stained by toluidine blue and safranin-O. In animal studies, ectopic cartilage tissue regeneration was observed inside and around the transplanted microspheres, confirmed by histochemical and immunofluorescent staining. Interestingly, PDLSCs showed more chondrogenesis than GMSCs and hBMMSCs (P<0.05). Taken together, these results suggest that RGD-modified alginate microencapsulating dental MSCs make a promising candidate for cartilage regeneration. Our results highlight the vital role played by the microenvironment, as well as value of presenting inductive signals for viability and differentiation of MSCs.
Keywords: tissue engineering, cartilage regeneration, dental mesenchymal stem cells, alginate hydrogel, RGD tripeptide
1. Introduction
Several treatment modalities have been introduced to regenerate or enhance the repair of articular cartilage, such as the grafting of autologous osteochondral tissue or the transplantation of autologous chondrocyte suspensions [1,2]. However, each of these strategies, the biological and mechanical properties of the formed tissue are inferior to those of native articular cartilage [3]. An advantageous alternative therapeutic option is regeneration of cartilage tissue using mesenchymal stem cells (MSCs). MSCs are multipotent cells that can differentiate into multiple lineages depending on the nature of the environmental signals which they receive. Specifically, MSCs undergo chondrogenesis and deposit a cartilage-specific matrix in pellet cultures and in a variety of biomaterials in the presence of appropriate growth factors. Most of the studies on chondrogenic differentiation have focused on applications using bone marrow MSCs (BMMSCs). However, it is well known that MSCs reside in a wide spectrum of post-natal tissue types including the orofacial tissues [4–6], while neural crest origin are attractive for craniofacial regenerative strategies as they might be more plastic to differentiate into craniofacial tissues [5–7]. Among the dental-derived MSCs, periodontal ligament stem cells (PDLSCs) and gingival mesenchymal stem cells (GMSCs) are of particular interest as they can be harvested easily, accessible through oral cavity and they can often be obtained as discarded biological samples in dental clinics [8,9]. Moreover, both in vitro and in vivo studies have confirmed the multilineage differentiation capabilities of these dental-derived MSCs [10,11].
However, an appropriate microenvironment and signaling molecules are required in order to effectively differentiate MSCs into chondrocytes [12]. It has been reported that growth factors such as: TGF-β1, BMP-4, and FGF-2 are often required in the process of chondrogenesis [13]. Particularly, studies have reported that transforming growth factor-beta (TGF-β) plays an important role in chondrogenesis of MSCs [14] by stimulating chondrocyte proliferation while preventing cartilage hypertrophy [15]. In addition, it is well known that the cell delivery vehicle has an important role in the in vivo performance of MSCs and the success of the regenerative therapy. Therefore, we sought to design an appropriate microenvironment by engineering the physiochemical properties of the extracellular MSC microenvironment in order to tailor the niche characteristics and direct cell phenotype through differentiation [16,17]. Hydrogel biomaterials have been widely used for cartilage tissue engineering. Among the hydrogel biomaterials, alginates, which are natural hetero-polysaccharides isolated from brown sea algae, are of particular interest due to their unique properties including injectability and biodegradability [18,19]. Alginate can provide a 3D scaffold that facilitates the spatial distribution of MSCs, thus resulting in a structural organization that resembles the native in vivo microenvironments. Moreover, alginate microspheres have been used extensively for controlled delivery of growth factors (e.g. TGF-β) making them desirable biomaterials for chondrogenesis [20,21]. In the present study, we developed a novel co-delivery system that provides a 3D architecture of RGD-coupled alginate hydrogel loaded with a TGF-β1 ligand based for microencapsulation of dental MSCs. This approach ensures optimized cartilage regeneration and provides a potential application for reconstruction of the temporomandibular joint disk and for applications in the appendicular skeleton.
2. Materials and methods
All the animal experiments in the current study were performed in accordance with the guidelines published by the Institutional Animal Care and Use Committee at the University of Southern California, and the American Association for Accreditation of Laboratory Animal Care.
2.1. Progenitor cell isolation and culture
Human PDLSCs and GMSCs were isolated and cultured according to previously published protocols by Seo et al. and Zhang et al. [9, 10]. The teeth and gingival tissues were obtained from healthy male patients (18–25 years old) undergoing third molar extractions according to IRB approval from the University of Southern California. Only subjects without any history of periodontal disease were included in this study.
For assay of colony forming units-fibroblastic (CFU-F), 0.1 × 106 cells were seeded in a culture dish and cultured for 14 days. Subsequently, the cells were stained with Toluidine Blue. A cell count of more than 50 in one colony was counted as a positive CFU-F. Passage 4 cells were used in the experiments and hBMMSCs were used as the positive control group.
2.2. Flow cytometric analysis
Approximately 5 × 105 cells at passage four were incubated with specific PE- or FITC-conjugated mouse monoclonal antibodies for human CD34 and CD45 (as negative: markers for hematopoietic stem cells); and CD73, CD105, CD 146, and CD 166 (as positive: markers for mesenchymal stem cells) (BD Biosciences, San Jose, CA), or isotype-matched control IgGs (Southern Biotechnology Associates, Birmingham, Alabama) and subjected to flow cytometric analysis using a Beckman Coulter flow cytometer (Beckman Coulter, Brea, CA) and analyzed using FACScan software (BD Biosciences).
2.3. Biomaterial fabrication and cell encapsulation
Custom-made RGD-coupled alginate with high guluronic acid content (NovaMatrix FMC Biopolymer, Norway) was utilized in this study. Alginate was purified and partially oxidized (2%) to increase its degradability according to the methods in the literature [22, 23]. Subsequently, the alginate was mixed with TGF-β1 (Abcam, Cambridge, MA) (50 μg/mL) under rigorous stirring, concentrated, and freeze-dried under reduced pressure.
PDLSC, GMSC, as well as hBMMSC (as a positive control) were encapsulated in TGF-β1 ligand (50 μg/mL) loaded alginate at a density of 2×106 cells/mL of alginate solution [24]. Microbead formation was accomplished using a microfluidic device with a two-channel fluid jacket microencapsulator equipped with a micropipette. Alginate (200 μLh−1) and soybean oil (10 mLh−1) (Sigma) were injected into the device using syringe pumps. Alginate droplets were sheared off by the soybean oil, flowed out of the device, and were added dropwise into a petri dish containing 100 mM CaCl2 solution to form microspheres. The formed constructs were incubated at 37°C for 45 min to complete cross-linking and then washed three times in non-supplemented DMEM. Alginate hydrogel without cells was used as the negative control in this study.
2.4. In vitro release study
TGF-β1 (50 μg/mL) loaded alginate microspheres were incubated in 500 mL of high-glucose DMEM supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin in 48-well plates on a rotational shaker at 37°C for one week. At each selected time interval, the medium was collected and analyzed for released TGF-β1 using an anti-human recombinant TGF-β1 ELISA kit (Abcam). At the end of release study, the residual entrapped TGF-β1 was extracted by dissolving the scaffolds in 10% sodium citrate solution in distilled water and the percentage of cumulative released TGF-β1 was measured.
2.5. Cell survival assay
Survival of the encapsulated MSCs was measured as described previously [22, 23] using calcein AM to stain live cells and ethidium bromide homodimer-1 to stain dead cells (Invitrogen, Carlsbad, CA). The percentage of live cells was measured using ImageJ software. Additionally, an MTT assay (Invitrogen) was utilized to further evaluate the metabolic activity of encapsulated MSCs according to methods previously published [25].
2.6. In vitro chondrogenic assay
Encapsulated PDLSCs and GMSCs as well as hBMMSCs (2×106 in 1 mL of alginate) were cultured with a chondrogenic medium containing DMEM with 15% FBS, 2 mM L-glutamine, 1% ITS (BD Bioscience), 100 nM Dex, 100 μM ascorbic acid, 2 mM sodium pyruvate (R&D Systems, Minneapolis, MN), 100 U/mL penicillin, and 100 μg/mL streptomycin.
Four weeks after the induction, the samples were fixed with 4% PFA, and paraffin sections were made. Chondrogenic differentiation, as indicated by matrix production, was determined by staining with 0.1% safranin-O (Sigma-Aldrich, St. Louis, MO) and 0.1% toluidine blue (Sigma-Aldrich) solution. Because the polyanions of alginate stained intensely by Safranin-O and toluidine blue resulted in a strong background, the sections were first washed with Ca2+-free PBS to remove the cross-linker Ca2+ and dissociate the alginate prior to staining.
Sections were immunolabeled using primary Sox 9 and Collagen II antibodies (Abcam) at 4°C overnight, detected using Alexa fluor conjugated secondary antibody (1:200 dilution; Invitrogen), and counterstained with DAPI. The chondrogenic assays were determined from 3 independent samples for each experimental group. Five areas were randomly selected from each sample, then the positive area in the field was calculated with NIH Image-J software (NIH, Bethesda, MD) and shown as a percentage of the area over total field area.
2.7. RNA isolation, reverse transcription and real time PCR
PDLSC, GMSC, as well as hBMMSC were encapsulated in TGF-β1 ligand (50 μg/mL) loaded alginate in presence or absence of 100 ng/ml human recombinant BMP-4 or 50 μg/mL FGF2 (both from Invitrogen) as previously described. After 2 weeks of culturing in chondrogenic media, extraction of RNA from the encapsulated cells was performed by collecting 10 alginate hydrogels and dissolving them for 15–20 min by gentle stirring in a sterile depolymerization buffer consisting of 50 mM sodium citrate dehydrate and 80 mM sodium chloride (Sigma Aldrich, St Louis, MO). Following dissolution, the decapsulated cells were centrifuged at 9400 g for 10 min and the pellet was washed with PBS and centrifuged again at 9400 g for 3 min. Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s recommendations. Single-stranded cDNA synthesis was performed with 100 ng total RNA using a Superscript III cDNA synthesis kit (Invitrogen). Data were analyzed by the 2−ΔΔCt method, with normalization to the Ct of the housekeeping gene GAPDH. Primer and probe sequences are described in Table 1.
Table 1.
Oligonucleotide primers used in RT-PCR analysis.
| Gene | Sequence | Amplication (bp) |
|---|---|---|
| Sox 9 | Sense: 5′-ACGCCGAGCTCAGCAAGA-3′ Antisense: 5′-CACGAAGGGCCGCTTCT-3′ |
289 |
| Collagen II | Sense: 5′-GGCAATAGCAGGTTCACGTACA-3′; Antisense: 5′-CGATAACAGTCTTGCCCCACTT-3′ |
827 |
| Glyceraldehyde 3-phosphate dehydrogenase (GADPH) | Sense: 5′-AGCCGCATCTTCTTTTGCGTC-3′: Antisense: 5′-TCATATTTGGCAGGTTTTT CT-3′ |
418 |
2.8. Dental MSC-mediated tissue regeneration in vivo
PDLSCs, GMSCs, and hBMMSCs were encapsulated in TGF-β1 loaded alginate systems and transplanted subcutaneously (approximately 4 × 106 cells) into the dorsal surface of 5-month-old Beige nude XID III (NU/NU) mice (Harlan, Livermore, CA; N=5 for each group). All animals were treated according to the Guidelines and Regulations for the Use and Care of Animals at University of Southern California. After 8-weeks the mice were sacrificed, the constructs and the surrounding tissue were surgically removed and analyzed using histological, histochemical and immunofluorescence staining.
2.9. Histological and histochemical assessment
For histological examination, harvested specimens were fixed in 10% formalin solution and dehydrated in an ascending series of ethanol and embedded in paraffin. Six-micrometer sections were cut using a microtome and mounted on glass slides. Four randomly selected cross-sections from each implant were stained with hematoxylin & eosin (H&E). Additionally, sections were stained with toluidine blue and with Safranin-O to identify newly synthesized cartilage ECM glycosaminoglycans (GAGs) Five areas were randomly selected from each sample, and the positive area in the field was calculated with Image-J software and shown as a percentage of the area over total field area.
2.10. Immunohistochemical and Immunofluorescence staining
For immunohistochemical analysis, the animals were scarified three weeks after transplantation and harvested specimens were prepared according to the abovementioned method. The de-paraffinized sections were washed, and non-specific endogenous peroxidase activity was quenched by immersing in 3% H2O2/methanol for 15 min. Sections were incubated with primary antibody (1:200–1:300 dilution) for 1 h. Immunohistochemistry examination was performed on sections using anti-BMP4 (Abcam, 1:200 dilution) and anti-FGF-2 (Abcam, 1:100 dilution) and counterstaining with hematoxylin.
For immunofluorescence staining, samples (retrieved after eight weeks of transplantation) were treated with 3% H2O2, followed by a blocking buffer (1% BSA and 0.25% Triton X-100 in PBS). Samples were then incubated with mouse anti-rabbit collagen II (Calbiochem, San Diego, CA) and Sox 9 (Abcam) antibodies at a dilution of 1:100 and detected using Alexa Fluor conjugated secondary antibody (1:200 dilution; Invitrogen). The samples were then counterstained with DAPI.
2.11. Statistical Analysis
Data were analyzed using an independent two–tailed Student’s t-test or analysis of variance (ANOVA) (α<0.05). The numerical data are presented as the mean ± SD.
3. Results
3.1. In vitro characterization of dental-derived MSCs
In this study the CFU-F assay was performed to assess the colony-forming ability of the newly isolated stem cells. PDLSCs and GMSCs showed significantly higher numbers of single-colony clusters (CFU-F) compared to hBMMSCs (Fig. 1a). Next, in order to identify whether the isolated cells were MSC-like, FACS analysis was performed and demonstrated that human PDLSCs and GMSCs display specific MSC markers such as CD 73, CD 105 CD 146, and CD 166, while not expressing hematopoietic lineage markers such as CD 34 and CD 45 (Fig. 1b). These data confirmed the stem cell properties for each of these types of dental-derived MSCs.
Figure 1.
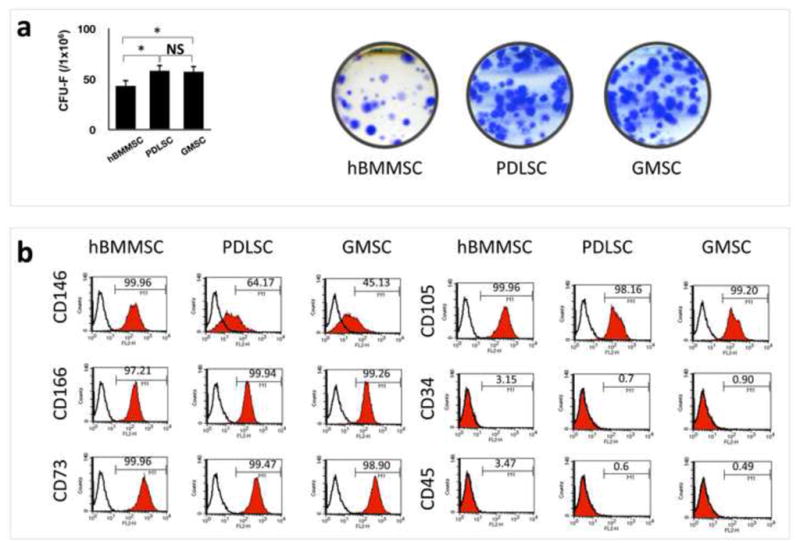
Characterization of stem cells used in this study: (a) PDLSCs and GMSCs were able to form significantly higher numbers of single colonies than BMMSCs (*P < 0.05) when 1× 106 cells were plated at a low density and cultured for 10 days. Single colonies were formed for PDLSCs, GMSCs as well as hBMMSCs after plating at low density and cultured for 10 days; (b) Expression of cell surface markers on PDLSCs, GMSCs, and hBMMSCs (passage 4) as determined by flow cytometric analysis.
3.2. Alginate microspheres as a drug delivery vehicle that maintains MSC viability
An alginate microencapsulation system was developed in this study in order to induce cartilage differentiation of PDLSCs and GMCSs. As a positive control, hBMMSCs encapsulated in alginate were used. In the current study, RGD-coupled alginate microcapsules containing stem cells had an average diameter of 327 μm (Fig 2a). Microscopic images of stem cells loaded into microcapsules showed a uniform microsphere size and cell distribution. Fig. 2b represents the cumulative release profile of TGF-β1 from the RGD-coupled alginate scaffold compared to the alginate scaffold without RGD tripeptide. The results show sustained release of TGF-β1 for up to 14-days. Also, there was no significant difference in release rates from the two scaffolds as a function of time (p-value >0.05), which both rates showing first-order kinetics.
Figure 2.
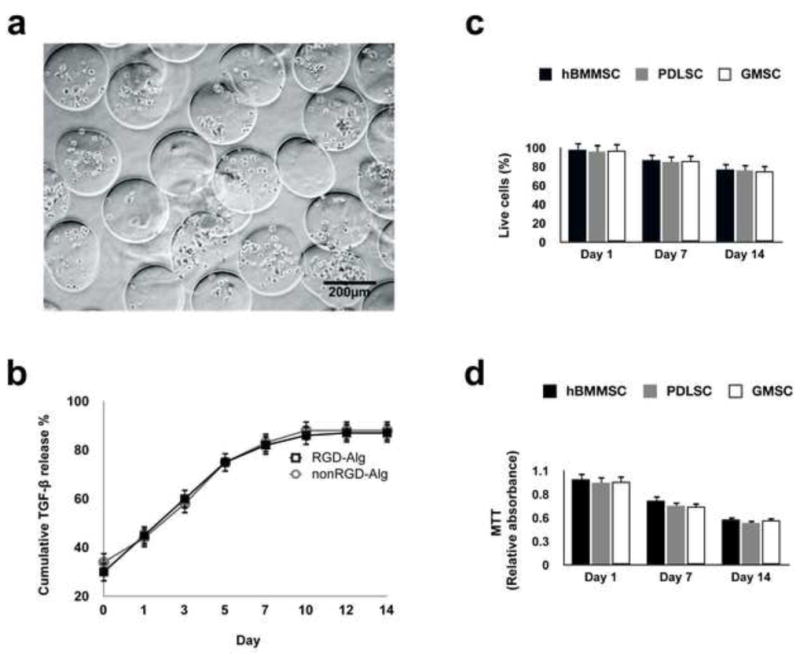
Development of microenvironment based on TGF-β1-loaded alginate hydrogel: (a) Microspheres produced by the microfluidic device. The diameter of the microspheres was between 196 μm and 581 μm (scale bar = 500 μm). (b) Characterization of the in vitro release profile of TGF-β1 loaded alginate microspheres showing sustained release of TGF-β1 from the RGD-modified alginate and the non-RGD modified alginate microspheres. Cumulative release is calculated from the total amount of TGF-β1 released after 14-days. (c) Viability of the encapsulated MSCs: percentage of live cells in RGD-coupled alginate microspheres. (d) MTT assay to determine the metabolic activity of cells. No significant difference was observed between MSC groups at each time interval.
A live-dead assay showed a high degree of viability of MSCs in alginate microspheres after four weeks of in vitro culturing (Fig. 2c). Furthermore, an MTT assay was utilized to quantitatively measure cytotoxicity and MSC viability in the alginate microencapsulation system in vitro. All the MSCs-alginate constructs showed high MTT absorbance, indicating high metabolic activity and viability of the encapsulated stem cells after up to two weeks of culturing in regular media (Fig. 2d). These results show that the viability of PDLSCs, GMSCs as well as hBMMSCs were favorably supported in our biodegradable alginate microspheres. Furthermore, MSCs encapsulated in RGD containing alginate microspheres showed significantly higher degrees of viability in comparison to MSCs encapsulated in non-RGD containing alginate microspheres after 14 days of culturing in regular culture media (supplementary Fig. 1a).
3.3. Chondrogenic differentiation of dental MSCs in vitro
After four weeks of culturing in chondrogenic induction media, the deposition of proteoglycans by either PDLSCs or GMSCs in vitro was detected using safranin-O and toluidine blue staining. Safranin-O staining was positive for PDLSCs and GMSCs, as well as hBMMSCs (Fig 3a). All the MSCs were also positive for toluidine blue (Fig. 3b). As expected, MSCs encapsulated in RGD immobilized alginate microspheres exhibited statistically greater amounts of proteoglycan deposition (by toluidine blue staining) in comparison to non-RGD immobilized counterparts (supplementary Figs. 1b and 1c). Additionally, the results of immunofluorescent staining correlated well with the histochemical staining, showing that all the tested MSCs expressed type II collagen (Col II) genes as shown by positive immunostaining (Figs. 3c). Interestingly, the semi-quantitative analysis of the specimens revealed that PDLSCs showed higher amounts of chondrogenesis than hBMMSCs and GMSCs (P-value <0.05) (Fig. 3e). No significant difference was observed between GMSCs and hBMMSCs (P>0.05). Subsequently, the expression levels of the chondrogenic genes (Sox9 and type II collagen) were confirmed and compared via RT-PCR. Sox9 is an essential factor for the initiation of the chondrogenic differentiation pathway leading to a subsequent upregulation of Col II. Sox9 shows the greatest amount of activity in proliferating chondrocytes. The results showed that all the MSC groups expressed abundant Col II and Sox 9 (Figs. 4a and b). PDLSCs showed significantly greater expression levels of Sox9 and Col II than GMSCs and hBMMSC (p<0.05). However, Sox 9 and Col II gene expression levels were similar between hBMMSCs and GMSCs (P>0.05). Further supplementation of alginate microspheres with BMP-4 or FGF2 in combination with TGF-β1, slightly increased the expression levels of Sox9 and Col II in comparison to samples loaded with only TGF-β1 (supplementary Fig. 2).
Figure 3.
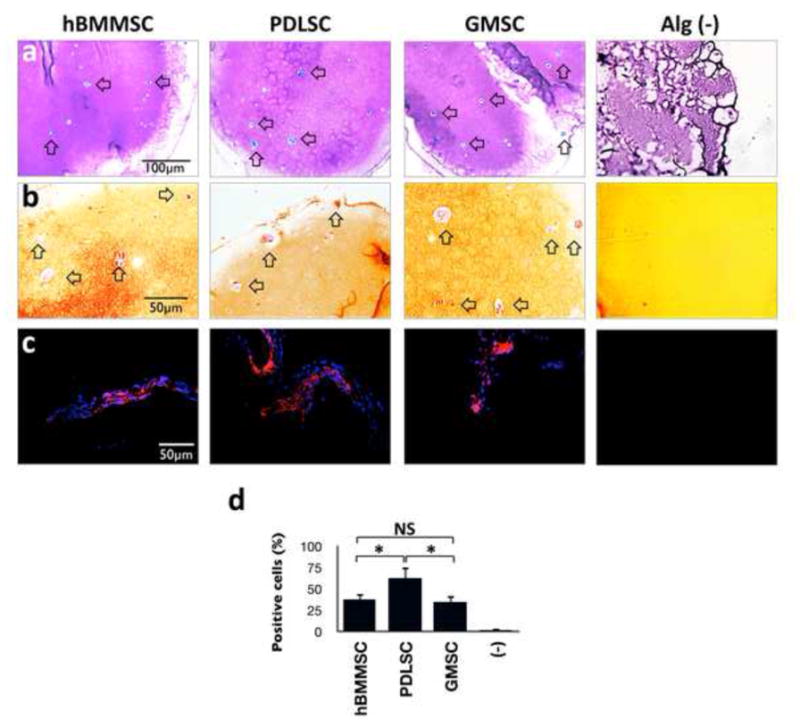
Histochemical analysis showed that RGD coupled alginate microspheres promoted spread-out cell morphology. Stem cells and cell aggregates are surrounded by toluidine blue (a) and Safranin-O (b) and strongly stained matrix, confirming the production and accumulation of cartilage extracellular matrix. (c) Immunofluorescence staining against collagen type II (Col II) antibodies confirmed the production and secretion of type-II collagen by PDLSCs and GMSCs. (d) Analysis of the percentage of cells positive for anti-Col II antibodies, showing that PDLSCs encapsulated in RGD coupled alginate presented the greatest amounts of production and secretion of collagen type II in comparison to GMSCs and hBMMSCs.
Figure 4.
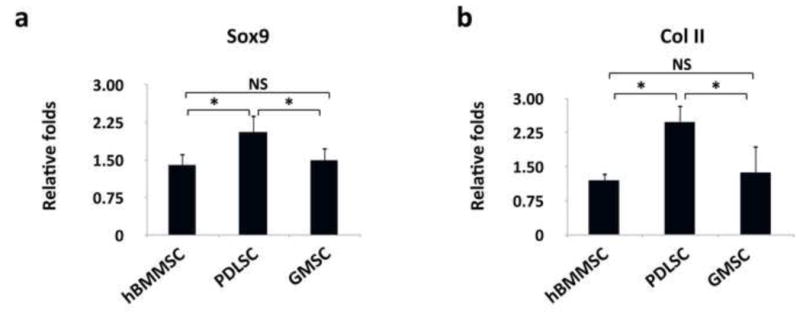
Expression level (in fold changes) of collagen type II (a) and Sox-9 (b) genes for each encapsulated stem cell population after 2-weeks of chondro-differentiation in vitro evaluated by RT-PCR. Data were normalized by the Ct of the housekeeping gene GAPDH and expressed relatively to gene expression level at day 1. *P <0.05, NS: not significant.
3.4. Dental MSCs contributed to cartilage tissue regeneration in vivo
The ability of RGD-coupled alginate microspheres loaded with TGF-β1 to support MSC chondrogenic differentiation was evaluated in an ectopic site after subcutaneous implantation into nude mice. Toluidine blue and safranin-O staining of matrix confirmed the chondrogenic differentiation of all the tested MSCs. The cells in these constructs exhibited the round morphology of committed chondrocytes. By contrast, the control group, with implants consisting of alginate hydrogel alone, failed to show any of the signs positive for chondrogenesis (Figs. 5a and b). Immunofluorescent staining for type-II collagen and Sox 9 antibodies after 8-weeks of transplantation revealed extensive production and deposition of chondrogenic tissues (Figs. 5c and 5d). The production of collagen type II was more pronounced for PDLSCs than for GMSCs and hBMMSCs (P<0.05) (Fig. 5e), while GMSCs and hBMMSCs showed the same amounts of chondrogenesis (P<0.05). H&E staining results confirmed the presence of large islands of rounded cells with human chondrocytes morphology in MSC-seeded constructs, which is not found in the cell-free transplants (Fig. 6a). To further characterize MSC-mediated chondrogenesis in vivo, immunohistochemical staining was utilized. Results revealed detection of BMP-4 and FGF-2 growth factors in the early stages of chondrogenic differentiation (after three weeks of transplantation), suggesting the important role of TGF-β1 in the process of chondrogenesis (Figs. 6b and 6c).
Figure 5.
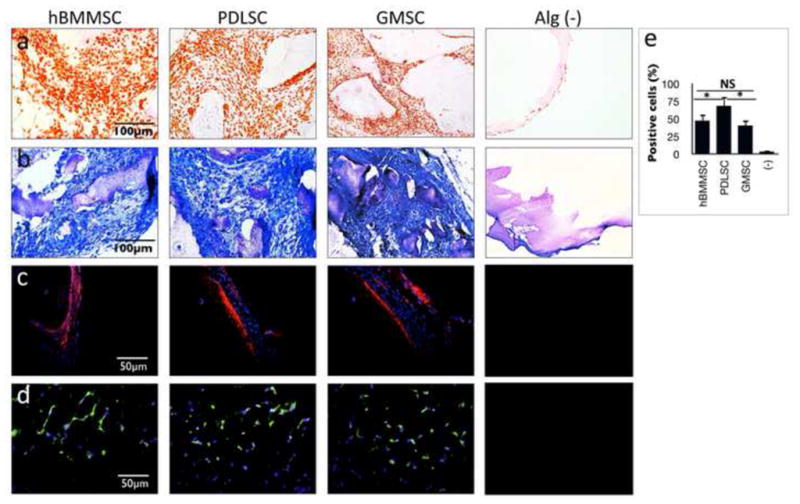
Chondrogenesis in TGF-β1 loaded alginate subcutaneously transplanted in nude mice. MSC-constructs were removed eight weeks after transplantation, sectioned, and stained with Safranin-O (a) and toluidine blue (b). Immunofluorescence staining, using antibodies against collagen type II (c) and Sox-9 (d) antibodies confirmed the production and secretion of type-II collagen. (e) Semi-quantitative analysis of the percentage of cells positive for anti-Sox9 antibodies via immunofluorescence staining images. *P <0.05, NS: not significant.
Figure 6.
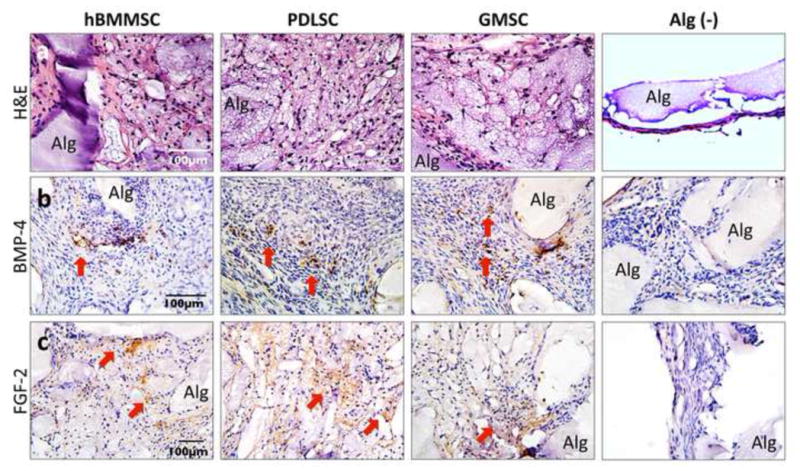
In vivo chondrogenic differentiation after subcutaneous transplantation in nude mice: (a) H&E staining showing presence of large islands of rounded cells with human chondrocytes morphology in MSC-seeded constructs, while (−) is the unseeded alginate scaffold. Immunohistochemical staining in specimens retrieved three weeks after transplantation showing positive results (red arrows) for anti-BMP-4 (b) and anti-FGF-2 (c) antibody staining, while negative control (−) failed to express any positive staining (Alg: unresorbed alginate scaffold).
4. Discussion
It is well known that the cell delivery vehicle has an important role in the in vivo performance of stem cells and the success of regenerative therapy [25]. In this study, we developed an injectable RGD-coupled alginate-hydrogel microsphere as a stem cell delivery system for potential application in cartilage tissue engineering. We demonstrated that this system supported the viability, metabolic activity, and chondrogenic differentiation of encapsulated PDLSCs and GMSCs in vitro and in vivo. More importantly, in the current study we are introduced PDLSCs and GMSCs for the first time as promising candidates for optimized cartilage regeneration with potential applications for reconstruction of the temporomandibular joint (TMJ) disk.
To our knowledge, the chondrogenesis of dental MSC has not been developed and reported in an RGD-coupled alginate microencapsulation system before. From a practical perspective, dental MSCs such as PDLSCs and GMSCs are a superior source of stem cells considering their accessibility and suitability for autologous transplantation. Studies have confirmed that both PDL and gingiva contain populations of multipotent postnatal stem cells that can readily be isolated and expanded in vitro [10,11]. As a result, human PDLSC- or GMSC-mediated tissue regeneration can be considered as a promising cellular-based treatment for cartilage tissue engineering. More importantly, considering the neural crest origin of both dental MSCs and TMJ disk cartilage [26], they can be used as a platform for functional regeneration of the TMJ disk.
The RGD-coupled-dental MSC system developed in the current study was observed strongly positively stained by Safranin-O and toluidine blue in vitro and in vivo. We observed the in vitro positive staining 4 weeks after culturing in chondrogenic culturing media. Furthermore, immunofluroscent staining demonstrated strong positive reactivity for Col II and Sox 9 antibodies around the cells both in vitro and in vivo. The synthesis of the two main matrices of articular cartilage, proteoglycan and type II collagen, demonstrates that dental MSCs embedded in alginate microspheres and induced with the addition of TGF-β1 in the medium, can effectively undergo chondrogenic differentiation. Furthermore, we report that PDLSCs have greater chondrogenic differentiation capacity than hBMMSCs.
The alginate hydrogel microencapsulation system provides a unique 3D cell delivery scaffold for cartilage tissue engineering. Studies confirm that macromolecules with molecular weights of less than 49 kDa can penetrate the pores of the alginate hydrogel microspheres to influence the cell behavior [27]. Therefore, different growth factors and proteins (e.g. TGF-β1) can enter the microspheres and regulate the proliferation and differentiation of encapsulated MSCs [28]. This and other advantages make alginate hydrogel a more desirable scaffold than other polymeric materials, such as polylactic acid, for cartilage tissue engineering [29]. Alginate hydrogel can be considered a semisolid structure, where the MSCs assumed a polygonal-rounded morphology with increasing proteoglycan and type II collagen synthesis [30].
It has been shown that several growth factors and cytokines, such as members of transforming growth factor superfamily (TGF-β1, and BMP-4), and FGF-2 have important role in chondrogenic differentiation of MSCs [31, 32]. The results of the current study, confirmed the importance of presence of these cytokines specifically TGF-β1 in the chondrogenesis of the encapsulated dental MSCs (Figs. 6b and c) showing that the provided micro milieu and the initiating signals from TGF-β1 alone might be sufficient to cause endogenous expression of BMP 4 and FGF 2 that are required for chondrogenesis.
Re’em et al. in their studies, investigated the effects of RGD peptide immobilized onto alginate scaffolds, on the chondrogenesis of human MSCs in the presence of TGF- β1. Their results confirmed that immobilized RGD did not interfere with progression of MSCs [19]. In addition, immobilized RGD considerably encouraged and accelerated the TGF- β 1-induced chondrogenesis of human MSC encapsulated in alginate scaffolds. We have previously shown that our prepared alginate scaffold has a porous structure [22] (data not shown here). This porous structure might have a critical role in the diffusion capacity of oxygen, nutrients and signaling molecules within the alginate microspheres. Therefore, one can conclude that cell matrix interactions facilitated by the immobilization of RGD tripeptide onto the alginate hydrogel scaffold with porous structure can enable the adhesion of encapsulated dental-derived MSCs to the matrix, and permit enhanced cell accessibility to the oxygen, nutrients and the growth factors (e.g. TGFb1) [20].
In the current study, stem cells were not cultured in chondrogenic media prior to surgical transplantation, and encapsulated PDLSCs and GMSCs in alginate microspheres promoted cartilage regeneration in situ. Chai et al. reported that the articulating disc and TMJ originate from cranial neural crest (CNC) cells [25]. Taking into account the fact that neural crest is the same origin of the dental MSCs used in this study, cells recovered from these origins might be considered as excellent candidates for disc and TMJ regeneration. Therefore, in future studies, the possibility of utilizing PDLSCs and GMSCs in the repair of cartilage and disk defects in TMJ in more appropriate animal models will be evaluated. The results of these endeavors will be reported in due course. More importantly, dental derived MSCs encapsulated in alginate hydrogel can be considered a promising candidate for appendicular skeleton repair and regeneration.
6. Conclusions
Altogether, our findings demonstrate the important role of the microenvironment as well as the presentation of inductive signals (TGF-β1) for viability and chondro-differentiation of dental MSCs in an RGD-modified alginate microencapsulation system. Our in vitro and in vivo studies confirmed that the proposed system comprises a promising model for high quality cartilage regeneration. We show that RGD-coupled alginate hydrogel can be used to encapsulate PDLSCs and GMSCs for chondrogenesis in an animal model. The system developed here has the advantages of providing a simple and readily manipulated means to encapsulate dental MSCs in RGD-coupled alginate hydrogel, and a 3D, injectable and biodegradable cell delivery scaffold for cartilage tissue engineering.
Supplementary Material
Acknowledgments
This work was partially supported by grants from the National Institute of Dental and Craniofacial Research, National Institutes of Health (R01DE017449 and R01 DE019932 to S.S.). The first author (A.M.) was part of NIDCR postdoctoral training grant (T90DE021982) and Provost’s Postdoctoral Scholar Research Grant by the USC Office of Postdoctoral Affairs, while another (MLS) was supported by R01 DE013045. The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this paper.
Footnotes
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–63. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 2.Redman SN, Oldfield SF, Archer CW. Current strategies for articular cartilage repair. Eur Cell Mater. 2005;9:23–32. doi: 10.22203/ecm.v009a04. [DOI] [PubMed] [Google Scholar]
- 3.Kurth T, Hedbom E, Shintani N, Sugimoto M, Chen FH, Haspl M, Martinovic S, Hunziker EB. Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthritis and Cartilage. 2007;15:1178–1189. doi: 10.1016/j.joca.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 4.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. PNAS. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: Stem cells from human exfoliated deciduous teeth. PNAS. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, et al. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod. 2008;34:166–71. doi: 10.1016/j.joen.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, et al. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37:1088–1099. doi: 10.1111/j.1600-051X.2010.01597.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, et al. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng Part A. 2010;17:1015–26. doi: 10.1089/ten.tea.2010.0140. [DOI] [PubMed] [Google Scholar]
- 9.Sonoyama W, Liu Y, Fang D, Yamaza T, Seo B-M, Zhang C, et al. Mesenchymal Stem Cell-Mediated Functional Tooth Regeneration in Swine. PLoS ONE. 2006;1:1–8. doi: 10.1371/journal.pone.0000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–1155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Shi S, Liu Y, Uyanne J, Shi Y, Shi S, et al. Mesenchymal Stem Cells Derived from Human Gingiva Are Capable of Immunomodulatory Functions and Ameliorate Inflammation-Related Tissue Destruction in Experimental Colitis. J Immunol. 2009;183:7787–7798. doi: 10.4049/jimmunol.0902318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stevens MM, Marini RP, Martin I, Langer R, Prasad Shastri V. FGF-2 enhances TGF-beta1-induced periosteal chondrogenesis. J Orthop Res. 2004;22:1114–9. doi: 10.1016/j.orthres.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Origuchi N, Ishidou Y, Nagamine T, Onishi T, Matsunaga S, Yoshida H, et al. The spatial and temporal immunolocalization of TGF-beta 1 and bone morphogenetic protein-2/-4 in phallic bone formation in inbred Sprague Dawley male rats. In Vivo. 1998;12:473–80. [PubMed] [Google Scholar]
- 15.Tchetina E, Mwale F, Poole AR. Distinct phases of coordinated early and late gene expression in growth plate chondrocytes in relationship to cell proliferation, matrix assembly, remodeling, and cell differentiation. J Bone Miner Res. 2003;18:844–51. doi: 10.1359/jbmr.2003.18.5.844. [DOI] [PubMed] [Google Scholar]
- 16.Cantu DA, Hematti P, Kao WJ. Cell encapsulating biomaterial regulates mesenchymal stromal/stem cell differentiation and macrophage immunophenotype. Stem Cells Transl Med. 2012;1:740–9. doi: 10.5966/sctm.2012-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JC, Hsu SH, Chen DC. The promotion of chondrogenesis in adipose- derived adult stem cells by an RGD-chimeric protein in 3D alginate culture. Biomaterials. 2009;30:6265–75. doi: 10.1016/j.biomaterials.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 18.Drury JL, Dennis RG, Mooney DJ. The tensile properties of alginate hydrogels. Biomaterials. 2004;25:3187–3199. doi: 10.1016/j.biomaterials.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Alsberg A, Anderson KW, Albeiruti A, Franceschi RT, Mooney DJ. Cell-interactive Alginate Hydrogels for Bone Tissue Engineering. J Dent Res. 2001;80:2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 20.Re’em T, Tsur-Gang O, Cohen S. The effect of immobilized RGD peptide in macroporous alginate scaffolds on TGFb1-induced chondrogenesis of human mesenchymal stem cells. Biomaterials. 2010;31:6746–6755. doi: 10.1016/j.biomaterials.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Bian L, Zhai DY, Tous E, Rai R, Mauck RL, Burdick JA. Enhanced MSC chondrogenesis following delivery of TGF-b3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moshaverinia A, Chen C, Akiyama K, Ansari S, Xu X, Chee W, et al. Alginate as a potential scaffold for dental-derived stem cells: An in vitro study. J Mater Sci: Mater Med. 2012;23:3041–3051. doi: 10.1007/s10856-012-4759-3. [DOI] [PubMed] [Google Scholar]
- 23.Moshaverinia A, Chen C, Akiyama K, Xu X, Chee W, Schricker SR, et al. Encapsulated Periodontal Ligament and Gingival Mesenchymal Stem Cells in 3-D Injectable Biodegradable Scaffold: A Unique Platform for Bone Tissue Engineering. J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34546. In Press. 34546. [DOI] [PubMed] [Google Scholar]
- 23.Choi YJ, Lee JY, Park JH, Park JB, Suh JS, Choi YS, Lee SJ, Chung C-P, Park YJ. The identification of a heparin binding domain peptide from bone morphogenetic protein-4 and its role on osteogenesis. Biomaterials. 2010;31:7226–7238. doi: 10.1016/j.biomaterials.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Dvir-Ginzberg M, Gamlieli-Bonshtein I, Agbaria R, Cohen S. Liver tissue engineering within alginate scaffolds: effects of cell-seeding density on hepatocyte viability, morphology, and function. Tissue Eng Part A. 2003;9:757–66. doi: 10.1089/107632703768247430. [DOI] [PubMed] [Google Scholar]
- 25.Pham QP, Kasper FK, Scott Baggett L, Raphael RM, Jansen JA, Mikos AG. The influence of an in vitro generated bone-like extracellular matrix on osteoblastic gene expression of marrow stromal cells. Biomaterials. 2008;29:2729–2739. doi: 10.1016/j.biomaterials.2008.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Chai Y, Jiang X, Ito Y, Bringas P, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- 27.Ma HL, Hung S, Lin S, Chen Y, Lo W. Chondrogenesis of human mesenchymal stem cells encapsulated in alginate beads. J Biomed Mater Res. 2003;64A:273–281. doi: 10.1002/jbm.a.10370. [DOI] [PubMed] [Google Scholar]
- 28.Guo JF, Jourdian GW, MacCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 11989;9:277–297. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- 29.Osteochondral repair using perichondrial cells. Clin Orthop Rel Res. 1997;340:220–229. doi: 10.1097/00003086-199707000-00029. [DOI] [PubMed] [Google Scholar]
- 30.Stevens MM, Qanadilo HF, Langer R, Prasad Shastri V. A rapid-curing alginate gel system: utility in periosteum-derived cartilage tissue engineering. Biomaterials. 2004;25:887–894. doi: 10.1016/j.biomaterials.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Miura Y, Fitzsimmons JS, Commisso CN, Gallay SH, O’Driscoll SW. Enhancement of periosteal chondrogenesis in vitro. Dose-response for transforming growth factor-beta 1 (TGF-beta 1) Clin Orthop Relat Res. 1994:271–280. [PubMed] [Google Scholar]
- 32.Xu J, Wang W, Ludeman M, Cheng K, Hayami T, Lotz JC, Kapila S. Chondrogenic differentiation of human mesenchymal stem cells in three-dimensional alginate gels. Tissue Eng Part A. 2008;14:667–80. doi: 10.1089/tea.2007.0272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


