Abstract
Background
Substance dependence and antisocial psychopathology, such as a history of childhood conduct disorder (HCCD), are associated with impulsive or disadvantageous decision making and reduced working memory capacity (WMC). Reducing WMC via a working memory load increases disadvantageous decision making in healthy adults, but no previous studies have examined this effect in young adults with substance dependence and HCCD.
Method
Young adults with substance dependence (SubDep; n = 158, 71 female), substance dependence and HCCD (SubDep+HCCD; n = 72, 24 female), and control participants (n = 152, 84 female) completed a test of decision making (the Iowa Gambling Task; IGT) with or without a concurrent working memory load intended to tax WMC. Outcomes were i) net advantageous decisions on the IGT, and ii) preferences for infrequent- versus frequent-punishment decks.
Results
SubDep+HCCD men made fewer advantageous decisions on the IGT than control men without a load, but there were no group differences among women in that condition. Load was associated with fewer advantageous decisions for SubDep+HCCD women and control men, but not for men or women in the other groups. Participants showed greater preference for infrequent-punishment, advantageous decks under load as well.
Conclusions
There are gender differences in the effects of substance dependence, HCCD, and working memory load on decision making on the IGT. Decision making by control men and SubDep+HCCD women suffered the most under load. Load increases preferences for less-frequent punishments, similar to a delay discounting effect. Future research should clarify the cognitive and neural mechanisms underlying these effects.
Keywords: decision-making, working memory capacity, substance dependence, conduct disorder
1. Introduction
Drug and alcohol dependence are highly comorbid with antisocial psychopathology, such as a history of childhood conduct disorder (HCCD; Hasin et al., 2007; Krueger et al., 2002). Comorbid HCCD or antisociality is associated with more severe symptomatology and course of substance dependence (Finn et al., 2002; Sher and Gotham, 1999; Zucker, 1987). Impulsive decision making is a core feature of substance dependence and HCCD (Bechara et al., 2001; Bobova et al., 2009; Cantrell et al., 2008; Ernst et al., 2003; Grant et al., 2000; Kim et al., 2006; Mazas et al., 2000). Furthermore, both substance dependence and HCCD are associated with reduced working memory capacity (WMC; Bechara and Martin, 2004; Bogg and Finn, 2010; Finn et al., 2009). Lower WMC is associated with impulsive decision-making (Bechara and Martin, 2004; Endres et al., 2011; Finn et al., 2002; van der Plas et al., 2009), and reducing WMC via a working memory load increases impulsive decision making in healthy adults (Hinson et al., 2002, 2003; Hofmann et al., 2009; Ward and Mann, 2000). However, little is known about the effects of compromising WMC in those with substance dependence and HCCD. The current study was designed to investigate the associations among substance dependence, HCCD, WMC, and decision making in young adults on a version of the Iowa Gambling Task (IGT; Bechara et al., 1994) that was modified to manipulate WMC.
1.1 Assessing Decision Making with the Iowa Gambling Task (IGT)
The IGT is a card-playing task which assesses decision making under uncertainty and risk (Buelow and Suhr, 2009; see Method). For the IGT, a disadvantageous decision bias is reflected in a preference for card decks associated with high immediate wins but long-term losses. Disadvantageous decision making on the IGT has been reported in individuals with substance dependence (Bechara and Damasio, 2002; Cantrell et al., 2008; Dom et al., 2006; Fein et al., 2004) and alcohol dependence with comorbid antisocial psychopathology (Mazas et al., 2000) or HCCD (Kim et al., 2006), and may reflect a general tendency to pursue immediate rewards despite long-term negative consequences. This could contribute to the type of poor behavioral control often exhibited by individuals with substance dependence or antisocial psychopathology, such as continuing to abuse substances or engage in other risky antisocial behavior despite threats to health or freedom (Bechara, 2005; Finn, 2002).
Recent research has noted that participants generally prefer infrequent-punishment decks over the frequent-punishment ones on the IGT (infrequent punishment bias) (Chiu et al., 2008; Fridberg et al., 2010; Goudriaan et al., 2007, 2005; Upton et al., 2012). This may be related to the finding that decision-makers tend to prefer options which have been associated most frequently with positive outcomes (Barron and Erev, 2003; Erev and Barron, 2005; Hertwig et al., 2004; Yechiam et al., 2005a), and may discount infrequent punishments similar to a delay discounting effect (e.g., Weller et al., 2010). No previous studies have examined infrequent punishment bias on the IGT in the context of substance dependence, HCCD, and WMC.
1.2 Working Memory (WM), Decision Making, and Behavioral Control
Working memory (WM) is an executive attention system which facilitates the ability to maintain or suppress information and resist distraction (Engle, 2002; Finn, 2002; Kane and Engle, 2002). Increased WMC is associated with a greater capacity to shifting of attention from highly-salient, immediate outcomes to less-salient, long-term consequences (Finn, 2002; Finn et al., 2002). Both substance dependence and HCCD are associated with lower WMC in young adults, which could contribute to poorer decision making in those individuals (Bogg and Finn, 2010; Endres et al., 2011; Finn and Hall, 2004; Finn et al., 2009) and problems such as impulsivity and poor behavioral control (Finn, 2002; Finn et al., 2002; Hinson et al., 2003).
Dual-process models of self-control (Hofmann et al., 2009; Wiers et al., 2010) posit that compromising control processes, such a WMC, leads to problems with self-control in vulnerable individuals, such as those with stronger impulsivity. For instance, a WM load resulted in excessive eating only in restrained eaters (Boon et al., 2002; Ward and Mann, 2000). However, other studies suggest that this effect is more generalized and observable in healthy adults, as evidenced by less advantageous decision making on the IGT under working memory load (Hinson et al., 2002; Jameson et al., 2004; Pecchinenda et al., 2006). Those studies employed a dual-task design in which participants performed a secondary task during each decision trial, which places additional demands on the WM system (Kane and Engle, 2002). No previous studies have examined decision making under WM load in individuals with substance dependence or HCCD. Because those with substance dependence and or HCCD are inclined to toward disadvantageous decisions (i.e., vulnerable), we hypothesize that a WM load would result in larger decreases in advantageous decision making in these individuals.
1.3 Sex and Decision Making on the IGT
Some data suggest that healthy men choose more advantageously than women on the IGT (Bechara and Martin, 2004; Bolla et al., 2004; Reavis and Overman, 2001; Stout et al., 2005). A number of studies indicate that substance dependent men, especially those with comorbid antisocial psychopathology, make fewer advantageous decisions relative to control men (Grant et al., 2000; Kim et al., 2006; Mazas et al., 2000; Stout et al., 2005). Decision making among substance-dependent women has received less attention, and previous studies have produced mixed results (Bechara and Martin, 2004; Stout et al., 2005; van der Plas et al., 2009). Prior studies have not compared substance dependent women with and without comorbid antisociality in terms of decision making on the IGT.
1.4 The Present Study
The purpose of the present study was to investigate the associations among substance dependence, HCCD, WMC, and decision making in young adults. Healthy control participants and participants with substance dependence with or without HCCD completed a version of the IGT modified to manipulate WMC. We differentiate between substance dependence with and without HCCD because HCCD is associated with increased behavioral disinhibition (Finn et al., 2002) and more severe course and complications (Del Boca and Hesselbrock, 1996; Sher and Gotham, 1999; Zucker, 1987).
There were three primary hypotheses. First, substance dependence would be associated with greater preference for disadvantageous decks on the IGT. Second, based on dual process models of self-control (Hofmann et al., 2009, Wiers et al., 2010) we hypothesized that a WM load would increase disadvantageous decision-making the most in those with substance dependence and HCCD. Third, we also hypothesized that WM load would enhance the salience of frequent punishments (increasing their inhibitory influences) resulting in more choices from infrequent punishment decks (Finn, 2002). We also expected this effect to be most pronounced among participants with substance dependence and HCCD. With regard to sex differences, we hypothesized that men with substance dependence and HCCD would make less advantageous decisions compared with control men without a WM load (Grant et al., 2000; Kim et al., 2006; Mazas et al., 2000). We also expected that control men would make more advantageous decisions than control women on the IGT overall (Bechara and Martin, 2004; Bolla et al., 2004; Reavis and Overman, 2001; Stout et al., 2005).
2. Method
2.1 Participants
Participants who varied according to substance use levels and disinhibited behavioral characteristics were recruited via flyers posted around the community, as described in previous reports (Bobova et al., 2009; Cantrell et al., 2008; Finn et al., 2009). Table 1 presents the inclusion and exclusion criteria. Eligible participants were between the ages 18 and 30. The age range of 18-30 years was used because substance use disorders have the highest prevalence in this age range (Hall et al. 1999; Kessler et al., 2005) and older cohorts are more likely to be biased by morbidity and the effects of long-term chronic substance abuse on cognition. Upon arriving at the laboratory, participants provided written informed consent, were given a breath alcohol test, and were asked about their alcohol and drug use over the past 24 hours. Participants were rescheduled if their breath alcohol level was greater than 0.0%, if they reported consuming any drug within the past 12 hours, if they reported feeling hung-over, or if they appeared impaired, high, overly sleepy, or were unable to attend to questions. Most participants were white (77.2%), followed by black (8.4%), Asian (7.6%), Hispanic (6.1%), and Native American (0.3%), 44% were employed and 80 percent were students. Participants received $10.00 per hour for their participation in addition to any money won on the IGT. The local institutional review board approved all study procedures.
Table 1.
Study inclusion and exclusion criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
|
|
|
Note: HCCD = a history of childhood conduct disorder; ASPD = antisocial personality disorder, Sub Dep = Substance dependent group, SubDep+HCCD = Substance Dependent + HCCD group.
2.2 Assessment
2.2.1 Diagnostic interview
Participants were interviewed using the alcohol and other drug abuse/dependence, childhood conduct disorder, and adult antisocial behavior portions of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA; Bucholz et al., 1994) which is based on diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (APA, 1994). Participants in the present study were placed in one of three groups based upon the SSAGA: no current or past diagnosis (control; n = 152), current substance dependence without HCCD (SubDep; n = 158), and current substance dependence with HCCD (SubDep+HCCD; n = 72). Demographic and diagnostic information for each of the three groups is presented in Table 2.
Table 2. Participant Demographics, Past 3 Month Substance Use, and DSM-IV Diagnostic Status.
| Control | SubDep | SubDep+HCCD | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Male (n = 68) | Female (n = 84) | Male (n = 87) | Female (n = 71) | Male (n = 48) | Female (n = 24) | |
|
|
|
|
||||
| Age | 20.68 (1.62)a | 20.76 (1.92)a | 21.36 (2.31) | 20.68 (1.84) | 22.71 (3.24)b | 22.63 (3.89)b |
| Years of education | 14.12 (1.46)a | 14.32 (1.74)a | 14.05 (1.68) | 13.84 (1.59) | 13.16 (1.74)b | 13.08 (1.86)b |
| Estimated IQ† | 115.47 (10.86)a | 112.99 (11.73)a | 113.02 (10.98)b | 108.52 (9.71)b | 109.17 (7.61)b | 109.08 (9.84)b |
| AC total score | 32.51 (9.26)a | 30.95 (9.62)a | 29.80 (9.98)b | 29.06 (9.66)b | 27.48 (8.98)b | 29.79 (8.91)b |
| AC total score/IQ composite | 0.59 (1.55)a | 0.19 (1.76) a | 0.07 (1.75)b | -0.42 (1.601)b | -0.53 (1.35)b | -0.29 (1.49)b |
| Current student (%)† | 91 | 93 | 76 | 83 | 50 | 71 |
| Alcohol and drug use | ||||||
| Alcohol frequency | 1.03 (1.13)a | 1.25 (1.31)a | 3.20 (1.75)b | 3.21 (1.37)b | 2.63 (1.86)c | 2.29 (1.68)c |
| Alcohol quantity† | 4.93 (8.07)a | 5.63 (7.89)a | 28.29 (28.11)b | 24.25 (21.78)b | 27.15 (35.27)b | 15.88 (14.23)b |
| Marijuana frequency† | 0a | 0a | 2.78 (3.07)b | 1.68 (2.58)b | 3.46 (3.13)c | 2.50 (2.98)c |
| Other drug frequency | 0a | 0a | 0.07 (0.33)a | 0.14 (0.76)a | 0.56 (1.37)b | 0.92 (2.21)b |
| DSM diagnoses (n meeting criteria) | ||||||
| Alcohol abuse | 0 | 0 | 20 | 11 | 12 | 5 |
| Alcohol dependence | 0 | 0 | 57 | 58 | 34 | 18 |
| Marijuana abuse | 0 | 0 | 12 | 14 | 3 | 3 |
| Marijuana dependence | 0 | 0 | 51 | 22 | 36 | 14 |
| Other Drug abuse | 0 | 0 | 5 | 1 | 4 | 1 |
| Other Drug dependence | 0 | 0 | 14 | 16 | 20 | 10 |
| HCCD | 0 | 0 | 0 | 0 | 48 | 24 |
| ASPD | 0 | 0 | 0 | 0 | 36 | 14 |
Note. AC = Auditory Consonant; HCCD = a history of childhood conduct disorder; ASPD = antisocial personality disorder, Sub Dep = Substance dependent group, SubDep+HCCD = Substance Dependent + HCCD group. Substance use measures represent average weekly values for the 3 months prior to participation in the study. Values are mean (SD) unless otherwise noted. Means with different superscripted letters are significantly different, p < .05.
Significant main effect of Sex, p < .05.
2.2.2 Recent alcohol and drug use
Alcohol use was quantified as the average number of drinking days per week (frequency) and total number of drinks consumed per week (quantity) for the past 3 months. Drug use was quantified as the average frequency of use (days using per week) for marijuana, sedatives, stimulants, and opiates for the past 3 months. Table 2 shows the recent substance use for each group.
2.2.3 WMC and general intelligence
WMC was assessed using a version of the Auditory Consonant (AC) Trigram test (Brown, 1958) modified to include 3, 4, and 5 consonant letter strings, instead of only 3 consonant strings, to increase the demands on WMC (cf. Finn et al., 2009 for details on this task). For the AC test participants complete a secondary distractor task (counting backward by threes) during the primary consonant recall task requiring participants to switch their attention away from the primary task (Brown, 1958; Stuss et al., 1987).
Full-scale IQ was estimated using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). The WASI is a standard brief measure of intelligence, and has excellent reliability (rs = .97) and predictive validity for WAIS III IQ measures (Wechsler, 1999).
2.2.4 Iowa Gambling Task (IGT)
Our modified version of the original IGT (Bechara et al., 1994)was played under two conditions, with or without a WM load. The payout schedule was identicalfor both versions (Table 3). Participants began with $10.00 in fake currency and were informedthat they were going to play a card game in which the goal was to win as much money as possible, and that they would be awarded real-money equivalent to the total amount of play money earned on the task at the conclusion of the testing day. Four decks of 120 cards each (A, B, C, and D) were arranged face down in random order on a table in front of the participant. Disadvantageous decks (A and B) were associated with a net loss of $2.50 per 10 selections, while advantageous decks (C and D) were associated with a net gain of $2.50 per 10 selections. Furthermore, choices from decks A and C resulted in smaller, more frequent losses, whereas choices from decks B and D resulted in larger, less frequent losses. Following each selection the experimenter exchanged fake currency with the participant, reflecting the outcome of that selection. The task was stopped after 120 total selections.
Table 3. Payoffs for the Iowa Gambling Task (IGT).
| Deck | Win per card | Losses | Expected value per 10 selections |
|---|---|---|---|
|
|
|
|
|
| A (disadvantageous) | $1.00 | Probability = 0.5 to lose $1.50, $3.00, $2.00, $2.50, or $3.50 (frequent) | -$2.50 |
| B (disadvantageous) | $1.00 | Probability = 0.1 to lose $12.50 (infrequent) | -$2.50 |
| C (advantageous) | $0.50 | Probability = 0.5 to lose $0.75, $0.25, or $0.50 (frequent) | $2.50 |
| D (advantageous) | $0.50 | Probability = 0.1 to lose $2.50 (infrequent) | $2.50 |
Note. Payoff structures were identical in the Load and No Load conditions.
In the WM Load condition, just prior to making a choice, the experimenter read a 3-digit number and asked the participant to count aloud backwards by threes for 6 seconds. After 6 seconds, the experimenter instructed the subject to stop counting and make the next choice. As in the AC test, this manipulation placed a load upon the executive attention component of WMC by requiring participants to shift their attention from the primary decision making task to the secondary counting task. In the No Load condition, the next trial began after a delay of 8 seconds.
2.3 Data analysis
2.3.1 Dependent Measures
‘Net advantageous selections’ represents the relative preference for advantageous versus disadvantageous decks, and was calculated as the total number of selections from advantageous decks (C and D) minus the total number of selections from disadvantageous decks (A and B). ‘Infrequent punishment bias’ represents the relative preference for low punishment frequency decks versus high punishment frequency decks, and was calculated as the total number of selections from infrequent punishment decks (B and D) minus the total number of selections from frequent punishment decks (A and C).
2.3.2 Statistical analysis
ANOVA with the factors Group (3: Control, SubDep, SubDep+HCCD), Sex (2: Male, Female), and WM Load (2: No Load, Load) was used to test hypotheses regarding IGT outcomes. Because IQ and AC scores (working memory capacity) differed between groups (the controls having higher scores on each of these variables) and cognitive capacity is associated with IGT performance (Bechara and Martin, 2004), analyses of covariance (ANCOVA) were conducted to account for the influence of these factors. We conducted two separate ANCOVAs, one using two separate covariates (IQ and AC scores) and the other using a composite IQ/WMC score as in Ahn et al. (2011) calculated by summing the z-scores for each variable. (Two participants were missing data for estimated IQ and four participants were missing data for AC test scores; these data were imputed using the “multiple imputation” procedure in SPSS. All analyses were conducted using SPSS 19.0 software (SPSS, Inc.).
3. Results
3.1 IGT outcomes
3.1.1 Advantageous decision making
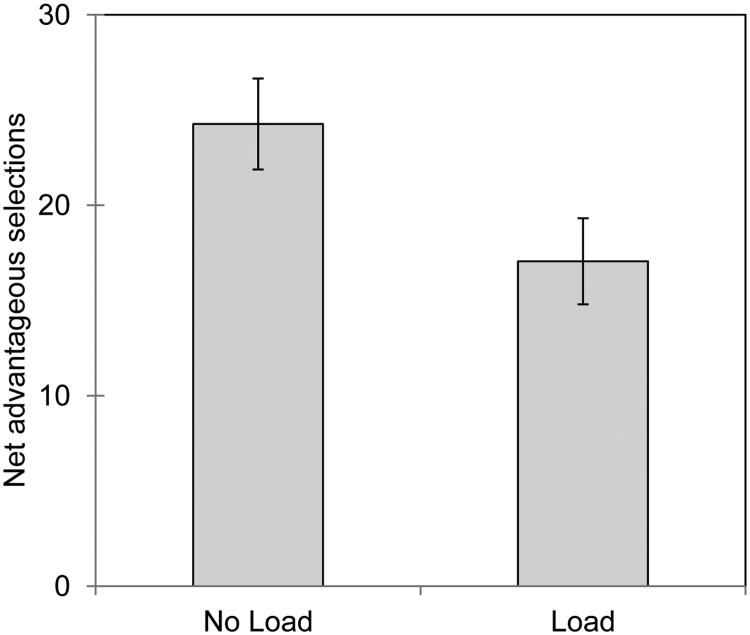
There was a significant main effect of WM Load, F(1, 370) = 8.72, p = .003, where fewer advantageous choices were mane in the Load condition compared with the No Load condition overall (Table 4, Figure 1). This effect was qualified by a significant WM Load × Group × Sex interaction for net advantageous decisions on the IGT, (2, 370) = 3.29, p = .04 (Table 4). Both of these effects remained significant in the ANCOVA with the composite score [WM main effect: F(1,368) = 9.37, p = .005, 3-way interaction: F(2,369) = 3.28, p = .04] and with IQ and AC as separate covariates, WM Load main effect: F(1,368) = 9.31, p = .005 and 3-way interaction F(2,368) = 3.0, p = 05.
Table 4. Mean (SD) IGT Net Advantageous Selections and Infrequent Punishment Bias Scores by WM Load, Group, and Gender.
| Control | SubDep | SubDep+HCCD | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Male | Female | Male | Female | Male | Female | ||
|
|
|
|
|
|
|
||
| Net advantageous selectionsa, b | No Load | 32.38 (35.31) | 26.98 (28.26) | 28.17 (40.47) | 16.82 (36.08) | 12.62 (41.51) | 34.36 (23.42) |
| Load | 15.10 (41.82) | 21.35 (31.54) | 25.25 (37.94) | 10.27 (29.90) | 14.64 (38.26) | -6.62 (30.38) | |
| Infrequent punishment bias scorec | No Load | -9.19 (40.17) | 3.12 (26.82) | -0.94 (40.12) | -1.71 (29.93) | 0.38 (29.93) | -5.27 (27.59) |
| Load | 6.26 (34.39) | 9.40 (27.22) | 14.20 (41.94) | 11.73 (29.51) | 12.09 (29.73) | 4.92 (20.39) | |
| Infrequent punishment bias – advantageous decksd | No Load | -11.00 (40.68) | 1.15 (27.30) | -8.38 (36.29) | -2.47 (23.61) | -6.08 (19.40) | -6.09 (31.64) |
| Load | 0.06 (30.81) | 5.47 (22.97) | 7.08 (38.23) | 11.84 (25.47) | 10.14 (27.73) | 2.54 (13.10) | |
| Infrequent punishment bias – disadvantageous decks | No Load | 1.81 (10.01) | 1.98 (8.37) | 7.45 (14.17) | 0.76 (13.20) | 6.46 (17.77) | 0.82 (7.59) |
| Load | 6.19 (17.36) | 3.93 (11.90) | 7.13 (13.75) | -0.11 (11.29) | 1.95 (12.63) | 2.38 (13.88) | |
Note. SubDep = Substance dependent group, SubDep+HCCD = Substance Dependent + a history of childhood conduct disorder group
Significant main effect of WM Load, p = .003.
Significant WM Load × Group × Gender interaction, p = .038.
Significant main effect of WM Load, p < .001.
Significant main effect of WM Load, p < .001.
Figure 1.
Main effect of WM load on total net advantageous selections on the IGT. Error bars represent 1 SEM.
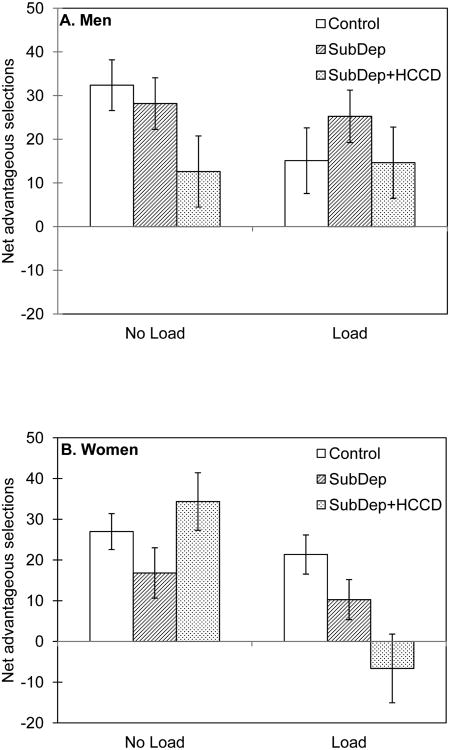
Simple main effects analyses of the 3-way interaction revealed that, for men, the SubDep+HCCD group made less advantageous decisions than controls in the No Load condition, F(1, 370) = 4.71, p = .03 (Figure 2A), however, this effect was not significant in the ANCOVAs, ps = .09 and .10, suggesting the IQ and WMC differences accounted for some of the group differences. There were no significant differences between the SubDep and SubDep+HCCD groups (p = .074), or between the control and SubDep groups (p = .59) for net advantageous decisions in the No Load condition. Furthermore, there was a significant effect of WM Load for control men (resulting in fewer advantageous decisions), F (1, 370) = 3.98, p = .05, (which remained in the ANCOVAs, ps = .05), but not in either of the substance-dependent groups (SubDep p = .70; SupDep+HCCD p = .845). Thus, decision making of control men under WM load resembled that of SubDep+HCCD men. There were no significant between-group differences for advantageous decision making among men in the Load condition (ps > .234).
Figure 2.
Mean advantageous decisions for (A) men and (B) women, by group and IGT condition. Error bars represent 1 SEM.
In contrast to the pattern shown by men, women in all three groups performed similarly on the IGT in the No Load condition (ps > .155; Figure 2B). Consistent with our general hypotheses, WM load decreased advantageous decision making in SubDep+HCCD women, relative to the No Load condition, F(1, 370) = 7.91, p = .005, but not in the control (p = .469) or SubDep (p = .703) women. In the Load condition, control women made more advantageous decisions than women in the SubDep+HCCD group, F(1, 379) = 6.17, p = .013. These two significant effects remained after the ANCOVAs, ps = .005 and .013. No other group differences were significant for women in the Load condition (ps > .142).
3.1.2 Infrequent punishment bias
There was a significant main effect of WM load condition for infrequent punishment bias scores, F(1, 370) = 10.17, p < .005, indicating that WM load was associated with greater preference for infrequent-punishment decks (Table 4). This effect remained significant when covarying out the composite score, F(1,369) = 10.9, p < .001 as well as IQ and ACT as separate covariates on the ANCOVA, F(1, 368) = 10.9, p < .001, indicating that these measures of cognitive capacity did not account for the association between WM Load and an infrequent punishment bias. A follow-up repeated measures ANOVA with WM Load as the between subjects factor, Deck Type (2: Advantageous, Disadvantageous) as the within subjects factor revealed that the main effect of load was present for advantageous decks F(1, 380) = 15.08, p < .001, but not disadvantageous decks (p = .839). Thus, WM load was associated with greater preferences for the infrequent-punishment advantageous Deck D compared with the more frequent-punishment Deck C.
4. Discussion
4.1 WM, substance dependence, sex, and disadvantageous decision making
The main finding of this study was that there were sex differences in the effects of substance dependence, HCCD, and WM load on decision making on the IGT. These data may be interpreted from the perspective of Hofmann's and Weir's dual-process model of self-control (Hofmann et al., 2009; Wiers et al., 2010), which holds that WM load will lead to increased impulsive behavior (i.e., worse decision making) in vulnerable individuals. SubDep+HCCD men had more disadvantageous decisions without the WM load, but did not show any increases in disadvantageous decisions after the load, suggesting a ceiling effect. On the other hand, without the load decision making of SubDep+HCCD women did not differ from that of control women but SubDep+HCCD women showed the largest increases in disadvantageous decisions under WM load (Figure 2B), suggesting that they may be especially prone to making impulsive decisions in situations where their WM is taxed. Control men, but not women, showed increased disadvantageous decision making under WM load (Figure 2). This suggests that control men are vulnerable to disadvantageous decision making under a WM load, while control women may be less vulnerable than control men to the negative effect of WM load upon decision making.
Although hypothetical, we offer the following explanations for the observed effects. First, our data suggest that somewhat different processes may contribute to the decision-making of the male and female participants. For instance, studies suggest that women may be more risk averse than men (Bernasek and Shwiff, 2001; Finn et al., 2002; Schubert, 2006), especially on gambling type tasks (Schubert et al., 1999) or in contexts where the negative consequence is particularly aversive (Finn et al., 2002). We propose that this risk aversion may serve as a general restraint for women, requiring a significant event or cognitive challenge to before any propensity to impulsive, risky decisions are manifest. This would partially explain the pattern for the women where only the most vulnerable participants (SubDep+HCCD) showed disadvantageous decision making after the WM load. Whether sex differences in neurocognitive mechanisms may underlie any of our observed effects is unclear. There is some suggestion that men and women different on activation of right (greater in men) and left (greater in women) prefrontal hemisphere activation during the IGT (Bolla et al., 2004; Tranel and Bechara, 2009; Tranel et al., 2002, 2005) and a WM task (Goldstein et al., 2005); however, these studies used older adult samples and their relevance to the present pattern of results is unclear. The relevance of these effects are unclear for our observed effects.
The results indicate that SubDep+HCCD men are generally less advanageous in their decision-making regardless of any challenge to WM capacity. The ANCOVA analyses suggest that the less advantageous decisions of these men are associated with their lower IQ and WMC. On the other hand, the data suggest that control men may generally have intact control processes at baseline, but a vulnerability toward disinhibited decision making under WM load. Overall, these data suggest that WM-related processes may be more involved in the pattern of decision making in the men than the women.
The association between WM load and less advantageous decision making on the IGT is consistent with data showing that reduced WMC is associated with more impulsive decision making among younger adults generally (Bechara and Martin, 2004; Bobova et al., 2009; Endres et al., 2011; Finn et al., 2002; van der Plas et al., 2009). Low WMC may contribute to impulsive decision making by limiting the individual's ability to attend to lower-salience information regarding the long-term consequences of a decision in light of a highly-salient, immediately-available reward (Finn, 2002). Thus, impulsive decision making in the context of reduced WMC could reflect a deficit in the ability to consider cognitive or motivational signals of the value of choice(s) in light of their long-term consequences (Finn, 2002; Hinson et al., 2002).
4.2 Infrequent punishment bias
For advantageous decks, WM load was associated with greater preference for the infrequent-punishment deck (Deck D) over the frequent punishment deck (Deck C). Decision-makers prefer options which are associated with infrequent losses (Barron and Erev, 2003) and discount future outcomes, whether positive or negative (Green and Myerson, 2004). Losses in the infrequent-punishment decks may be considered “delayed” because they are larger but occur infrequently (Table 2). The present data are consistent with studies showing that increased WM load results in greater discounting of delayed rewards (Hinson et al., 2003).
4.3 Limitations
The present data should be interpreted in light of some limitations. The study sample of young, fairly well-educated and intelligent adults may not generalize to older or less well-educated or intelligent individuals. The study sample of young, fairly well-educated and intelligent adults may not generalize to older or less well-educated or intelligent individuals. The SubDep group also is not representative of young adults in treatment for substance dependence. Our SubDep sample is comprised of mostly young white adults, 72% of whom are current college students. It is worth noting that substance use disorders among college students are equal to (Wu et al., 2007), or higher than (Slutske, 2005), their peers who do not attend college, but fewer college students seek treatment services for their substance-related problems (Wu et al., 2007). College students with substance use disorders are more likely to drop out (Slutske, 2005). Thus, although not representative of substance dependent individuals in treatment, substance use problems in this population represent a significant public health problem in the United States. In addition, the sample may have been biased by self-selection, and participants may have met criteria for disorders other than substance dependence and HCCD/ASPD which were not assessed in the study but which may affect decision making (e.g., OCD or pathological gambling; Buelow and Suhr, 2009). Another potential limitation is that the IGT is a complicated task which taps multiple cognitive processes related to decision making (Busemeyer and Stout, 2002; Yechiam et al., 2005b), and it is not clear from the present data which of these processes were associated with individual differences in IGT performance. Finally, participants in the study may have met criteria abuse/dependence upon multiple substances, which prevented us from speculating regarding the contributions of individual substance diagnoses to the present results.
4.4 Summary and conclusions
This study revealed sex differences in the effects of WM load, substance dependence, and HCCD on decision making on the IGT. WM load was associated with significantly less advantageous decision making in control men, but not in men with substance dependence. In contrast, for women, substance dependence and HCCD was associated with significantly fewer advantageous decisions under a WM load versus a no load condition, whereas control women were resilient to the effect of WM load on decision making. Our data also suggest that a WM load increases relative preferences for infrequent punishment, advantageous decks, similar to a delay discounting effect. Future studies should attempt to clarify the cognitive and neural mechanisms underlying association among sex, substance dependence, HCCD, WMC, and decision making on the IGT.
Acknowledgments
The authors would like to acknowledge Julie Becker, Tiffany Barrios, Samantha Grimes, Jesolyn Lucas, and Alex Miller and for their help with data collection and management.
Role of funding source: This research was supported by NIAAA R01 AA13650 to PRF and a NIAAA training grant fellowship (T32 AA007462-26) to DJF.
Footnotes
Contributors: P.R. Finn and D.J. Fridberg contributed toward the design of the study, data analysis and interpretation, and drafting of the manuscript. K.R. Gerst contributed toward data analysis and drafting of the manuscript.
Conflict of interest: All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Ahn WY, Fridberg DJ, Bishara AJ, Forsyth JK, Breier A, Busemeyer JR, Hetrick WP, Bolbecker AR, O'Donnell BF. Temporal discounting of rewards in patients with Bipolar Disorder and Schizophrenia. J Abnorm Psychol. 2011;120:911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron G, Erev I. Small feedback-based decisions and their limited correspondence to description-based decisions. J Behav Decis Mak. 2003;16:215–233. [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Martin EM. Impaired decision making related to working memory deficits in individuals with substance addictions. Neuropsychology. 2004;18:152–162. doi: 10.1037/0894-4105.18.1.152. [DOI] [PubMed] [Google Scholar]
- Bernasek A, Shwiff S. Gender, risk, and retirement. J Econ Issues. 2001;35:345–356. [Google Scholar]
- Bobova L, Finn PR, Rickert ME, Lucas J. Disinhibitory psychopathology and delay discounting in alcohol dependence: personality and cognitive correlates. Exp Clin Psychopharmacol. 2009;17:51–61. doi: 10.1037/a0014503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogg T, Finn PR. A self-regulatory model of behavioral disinhibition in late adolescence: integrating personality traits, externalizing psychopathology, and cognitive capacity. J Pers. 2010;78:441–470. doi: 10.1111/j.1467-6494.2010.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Cereb Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Brown J. Some tests of the decay of immediate memory. Q J Exp Psychol. 1958;10:12–21. [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Buelow M, Suhr J. Construct validity of the Iowa Gambling Task. Neuropsychol Rev. 2009;19:102–114. doi: 10.1007/s11065-009-9083-4. [DOI] [PubMed] [Google Scholar]
- Busemeyer JR, Stout JC. A contribution of cognitive decision models to clinical assessment: decomposing performance on the Bechara gambling task. Psychol Assess. 2002;14:253–262. doi: 10.1037//1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- Cantrell H, Finn PR, Rickert ME, Lucas J. Decision making in alcohol dependence: insensitivity to future consequences and comorbid disinhibitory psychopathology. Alcohol Clin Exp Res. 2008;32:1398–1407. doi: 10.1111/j.1530-0277.2008.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu YC, Lin CH, Huang JT, Lin S, Lee PL, Hsieh JC. Immediate gain is long-term loss: are there foresighted decision makers in the Iowa Gambling Task? Behav Brain Funct. 2008;4:13. doi: 10.1186/1744-9081-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway ARA, Kane MJ, Engle RW. Working memory capacity and its relation to general intelligence. Trends Cogn Sci. 2003;4:547–552. doi: 10.1016/j.tics.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Del Boca FK, Hesselbrock MN. Gender and alcoholic subtypes. Alcohol Health Res World. 1996;20:56–66. [PMC free article] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, Van Den Brink W, Sabbe B. Decision-making deficits in alcohol-dependent patients with and without comorbid personality disorder. Alcohol Clin Exp Res. 2006;30:1670–1677. doi: 10.1111/j.1530-0277.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Endres MJ, Rickert ME, Bogg T, Lucas J, Finn PR. Externalizing psychopathology and behavioral disinhibition: working memory mediates signal discriminability and reinforcement moderates response bias in approach–avoidance learning. J Abnorm Psychol. 2011;120:336–351. doi: 10.1037/a0022501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Curr Dir Psychol Sci. 2002;11:19–23. [Google Scholar]
- Erev I, Barron G. On adaptation, maximization, and reinforcement learning among cognitive strategies. Psychol Rev. 2005;112:912–931. doi: 10.1037/0033-295X.112.4.912. [DOI] [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn PR. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PR. Motivation, working memory, and decision making: a cognitive-motivational theory of personality vulnerability to alcoholism. Behav Cogn Neurosci Rev. 2002;1:183–205. doi: 10.1177/1534582302001003001. [DOI] [PubMed] [Google Scholar]
- Finn PR, Hall J. Cognitive ability and risk for alcoholism: short-term memory capacity and intelligence moderate personality risk for alcohol problems. J Abnorm Psychol. 2004;113:569–581. doi: 10.1037/0021-843X.113.4.569. [DOI] [PubMed] [Google Scholar]
- Finn PR, Mazas CA, Justus AN, Steinmetz J. Early-onset alcoholism with conduct disorder: go/no go learning deficits, working memory capacity, and personality. Alcohol Clin Exp Res. 2002;26:186–206. [PubMed] [Google Scholar]
- Finn PR, Rickert ME, Miller MA, Lucas J, Bogg T, Bobova L, Cantrell H. Reduced cognitive ability in alcohol dependence: examining the role of covarying externalizing psychopathology. J Abnorm Psychol. 2009;118:100–116. doi: 10.1037/a0014656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberg DJ, Queller S, Ahn WY, Kim W, Bishara AJ, Busemeyer JR, Porrino L, Stout JC. Cognitive mechanisms underlying risky decision-making in chronic cannabis users. J Math Psychol. 2010;54:28–38. doi: 10.1016/j.jmp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Anagnoson R, Breiter HC, Makris N, Goodman JM, Tsuang MT, Seidman LJ. Sex differences in prefrontal cortical brain activity during fMRI of auditory verbal working memory. Neuropsychology. 2005;19:509–519. doi: 10.1037/0894-4105.19.4.509. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Grekin ER, Sher KJ. Decision making and binge drinking: a longitudinal study. Alcohol Clin Exp Res. 2007;31:928–938. doi: 10.1111/j.1530-0277.2007.00378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Cogn Brain Res. 2005;23:137–151. doi: 10.1016/j.cogbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Green L, Myerson J. A discounting framework for choice with delayed and probabilistic rewards. Psychol Bull. 2004;130:769–792. doi: 10.1037/0033-2909.130.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Teeson M, Lynskey M, Degenhardt L. 12 month prevalence of substance use and ICD-10 substance use disorders in Australian adults. Findings from the National Survey of Mental Health and Wellbeing Addiction. 1999;94:1541–1550. [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hertwig R, Barron G, Weber EU, Erev I. Decisions from experience and the effect of rare events in risky choice. Psychol Sci. 2004;15:534–539. doi: 10.1111/j.0956-7976.2004.00715.x. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Somatic markers, working memory, and decision making. Cogn Affect Behav Neurosci. 2002;2:341–353. doi: 10.3758/cabn.2.4.341. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol Learn Mem Cogn. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Hofmann W, Friese M, Strack F. Impulse and self-control from a dual-systems perspective. Perspect Psychol Sci. 2009;4:162–176. doi: 10.1111/j.1745-6924.2009.01116.x. [DOI] [PubMed] [Google Scholar]
- Jameson T, Hinson J, Whitney P. Components of working memory and somatic markers in decision making. Psychon Bull Rev. 2004;11:515–520. doi: 10.3758/bf03196604. [DOI] [PubMed] [Google Scholar]
- Kane M, Engle R. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim YT, Lee SJ, Kim SH. Effects of the history of conduct disorder on the Iowa Gambling Tasks. Alcohol Clin Exp Res. 2006;30:466–472. doi: 10.1111/j.1530-0277.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res. 2000;24:1036–1040. [PubMed] [Google Scholar]
- Pecchinenda A, Dretsch M, Chapman P. Working memory involvement in emotion-based processes underlying choosing advantageously. Exp Psychol. 2006;53:191–197. doi: 10.1027/1618-3169.53.3.191. [DOI] [PubMed] [Google Scholar]
- Petry NM. Substance abuse, pathological gambling, and impulsiveness. Drug Alcohol Depend. 2001;63:29–38. doi: 10.1016/s0376-8716(00)00188-5. [DOI] [PubMed] [Google Scholar]
- Reavis R, Overman WH. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behav Neurosci. 2001;115:196–206. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- Schubert R. Analyzing and managing risk – on the importance of gender differences in risk attitudes. Manag Fin. 2006;32:706–715. [Google Scholar]
- Schubert R, Brown M, Gysler M, Brachinger HW. Financial decision-making: are women really more risk-averse? Am Econ Rev. 1999;89:381–385. [Google Scholar]
- Sher KJ, Gotham HJ. Pathological alcohol involvement: a developmental disorder of young adulthood. Dev Psychopathol. 1999;11:933–956. doi: 10.1017/s0954579499002394. [DOI] [PubMed] [Google Scholar]
- Slutske WS. Alcohol use disorders among US college students and their non-college-attending peers. Arch Gen Psychiatry. 2005;62:321–327. doi: 10.1001/archpsyc.62.3.321. [DOI] [PubMed] [Google Scholar]
- Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L. Gender differences in the functional organization of the brain for working memory. Neuroreport. 2000;11:2581–2585. doi: 10.1097/00001756-200008030-00046. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rock SL, Campbell MC, Busemeyer JR, Finn PR. Psychological processes underlying risky decisions in drug abusers. Psychol Addict Behav. 2005;19:148–157. doi: 10.1037/0893-164X.19.2.148. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Poirier CA. Comparison of three tests of attention and rapid information processing across six age groups. Clin Neuropsychol. 1987;1:139–152. [Google Scholar]
- Tranel D, Bechara A. Sex-related functional asymmetry of the amygdala: preliminary evidence using a case-matched lesion approach. Neurocase. 2009;15:217–234. doi: 10.1080/13554790902775492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Upton DJ, Kerestes R, Stout JC. Comparing the Iowa and Soochow gambling tasks in opiate users. Front Neurosci. 2012;6:34. doi: 10.3389/fnins.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plas EAA, Crone EA, Wildenberg WPMvd, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: a comparison of alcohol, cocaine, and methamphetamine and of men and women. J Clin Exp Neuropsychol. 2009;31:706–719. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Weller JA, Levin IP, Bechara A. Do individual differences in Iowa Gambling Task performance predict adaptive decision making for risky gains and losses? J Clin Exp Neuropsychol. 2010;32:141–150. doi: 10.1080/13803390902881926. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Ames SL, Hofmann W, Krank M, Stacey AW. Impulsivity, impulsive and reflective processes and the development of alcohol use and misuse in adolescents and young adults. Front Psychol. 2010;144:1–12. doi: 10.3389/fpsyg.2010.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu LT, Pilowsky DJ, Schlenger WE, Hasin D. Alcohol use disorders and the use of treatment services among college-age young adults. Psychiatry Serv. 2007;58:192–200. doi: 10.1176/appi.ps.58.2.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yechiam E, Barron G, Erev I. The role of personal experience in contributing to different patterns of response to rare terrorist attacks. J Conflict Resolut. 2005a;49:430–439. [Google Scholar]
- Yechiam E, Busemeyer JR, Stout JC, Bechara A. Using cognitive models to map relations between neuropsychological disorders and human decision-making deficits. Psychol Sci. 2005b;16:973–978. doi: 10.1111/j.1467-9280.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- Zucker RA. The four alcoholisms: a developmental account of the etiologic process. In: Rivers PC, editor. Nebraska Symposium on Motivation, 1987: Alcohol and Addictive Behavior. University of Nebraska Press: Lincoln; 1987. pp. 27–83. [PubMed] [Google Scholar]