Abstract
Background
With smoking rates far exceeding the general population, methadone-maintained (MMT) opiate-dependent smokers experience high rates of tobacco-related health consequences. Previous treatment studies have used nicotine replacement and produced low quit rates.
Methods
We test, using a three-group randomized design, the efficacy of varenicline vs. placebo, in comparison with nicotine replacement therapy (NRT) that combines nicotine patch prescription plus ad libitum nicotine rescue, for smoking cessation. We recruited methadone-maintained smokers from nine treatment centers in southern New England and provided six months of treatment, and a minimal behavioral intervention at baseline (NCI's 5A's). Outcomes included carbon monoxide (CO) confirmed 7-day point smoking cessation prevalence at 6 months and self-reported change in mean cigarettes per day.
Results
The 315 participants had a mean age of 40, with 50% male and 79% non-Hispanic White, smoked an average of 19.6 (± 10.4) cigarettes / day, and had a mean daily methadone dose of 109 mg. Intent-to-treat analyses, with missing considered to be smoking, showed the rate of CO-confirmed 7-day abstinence at 6-months was 5.4% overall, with varenicline 3.7% compared to placebo 2.2%, and NRT 8.3% (p>.05). Adherence rates during the 7-days immediately prior to 6-month assessment were 34.2% in varenicline, 34.4% in placebo, and 48.8% in NRT. Between baseline and 6-months there was an overall self-reported mean reduction of 8.3 cigarettes / day.
Conclusion
Varenicline did not increase quit rates over placebo. Smoking cessation rates in methadone-maintained smokers are low and novel treatment strategies are required.
Keywords: Methadone, smoking cessation, varenicline, NRT, MMT
1. INTRODUCTION
Cigarette smoking is the leading preventable cause of morbidity and mortality in the US and its health consequences remain particularly high in persons with drug use disorders (McCool and Paschall Richter, 2003). With smoking rates far exceeding the general population, methadone-maintained (MMT) opiate-dependent smokers experience high rates of tobacco-related health consequences (Centers for Disease Control and Prevention, 2007; Hser et al., 1994; Hurt et al., 1996; Nahvi et al., 2006; Okoli et al., 2010; Richter et al., 2001). Across studies, at least 80% of methadone treatment participants smoke, despite being aware of the attendant health risks (Clarke et al., 2001; Richter et al., 2002, 2001).
There has been increased interest in conceptualizing smoking cessation treatment as an integral part of polydrug dependence and treatment (Karan, 1993; Orleans and Hutchinson, 1993; Richter et al., 2002; Story and Stark, 1991). Several studies have supported the dual treatment of tobacco and substance use for opioid addicts by demonstrating that nonsmokers had lower rates of cocaine and other illicit drug use, raising the possibility that quitting smoking will lead to abstinence from other drugs of abuse (Hser et al., 2001; Lemon et al., 2003). Yet, all of the smoking cessation pharmacotherapies that have been tested in opioid dependent persons have far lower quit rates than those reported in trials of non-drug users (Hurt et al., 1994; Mooney et al., 2008; Okoli et al., 2010; Reid et al., 2008; Shoptaw et al., 2002; Stead et al., 2008; Stein et al., 2006b).
Over the past decade, three randomized trials of smoking cessation programs have tested behavioral interventions and nicotine replacement therapy for MMT patients. Shoptaw et al. (2002) tested relapse prevention versus contingency management for smoking cessation during 12 weeks of nicotine replacement therapy in this population. During treatment, those receiving contingency management had higher rates of smoking abstinence (25–33% vs. 12% for patch only), but these effects were not maintained following the removal of contingencies. At 6-months, across groups, only 2–10% of participants had ceased smoking, similar to quit rates among the general population (Etter and Stapleton, 2006).
Stein et al. (2006b) randomly assigned 383 methadone-maintained participants to nicotine patch (8–12 weeks) plus either: 1) a baseline tailored brief motivational intervention, a quit date behavioral skills counseling session, and a relapse prevention follow-up session (MAX), or 2) brief advice (MIN). The seven-day point prevalence estimate of cessation was 5.2% in the MAX group, and 4.7% in the MIN group (p= .81) at 6 months.
Reid et al. (2008) randomized 225 smokers from 5 MMT programs and 2 non-methadone substance abuse treatment programs to 8 weeks of nicotine patch plus 9 sessions of group mood management and cognitive behavioral counseling prior to and during NRT, or to treatment as usual. Smoking abstinence rates were 10–11% during treatment and 5–6% at 26 weeks.
The existing studies of smoking cessation in methadone maintained person have all offered transdermal nicotine replacement alone. As a means to improve outcomes using nicotine replacement strategies, several general population studies have demonstrated incremental short-term efficacy of nicotine patch plus nicotine gum or lozenge added to suppress urges to smoke, compared with either product alone (Fagerstrom et al., 1993; Kornitzer et al., 1995; Puska et al., 1995; Stead et al., 2012), suggesting combination treatment might improve quit rates in methadone patients.
Novel treatment strategies may be required to raise quit rates in methadone-maintained smokers further. Varenicline is an α4β2 nAChR partial agonist and antagonist, where receptor stimulation is proposed to mediate the reinforcing effects of nicotine (Tapper et al., 2004). Varenicline's efficacy has been demonstrated in multiple clinical trials (Aubin et al., 2008; Nakamura et al., 2007; Niaura et al., 2006; Wang et al., 2009), but has never been tested in methadone-maintained smokers.
In the current study, we directly test varenicline versus placebo, and include a comparison condition of combination nicotine-replacement therapy, in a randomized clinical trial of smoking cessation among persons who are methadone-maintained.
2. METHODS
2.1 Case Identification and Recruitment
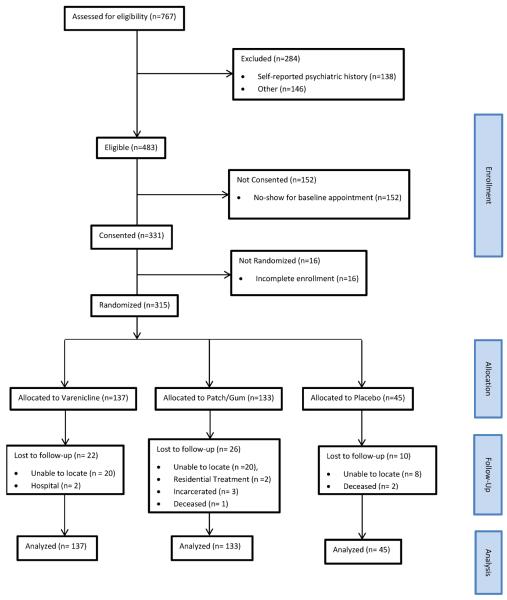
Study recruitment occurred from December, 2008 through January, 2012 at nine MMT sites in Southern New England. Potential participants were excluded if they smoked fewer than 10 cigarettes per day, had been in MMT for less than four weeks, were pregnant or nursing, or were unwilling to set a smoking quit date in the next week. The study protocol was approved by the Butler Hospital Institutional Review Board. Of 767 individuals screened for the study, 284 were ineligible (Figure 1). Of the 483 individuals who were eligible, 152 did not attend the initial study visit, resulting in 331 individuals enrolled in the protocol. Participants did not differ significantly from those ineligible and from those not enrolled based on age, gender, race or ethnicity, or mean cigarettes per day. After written informed consent, an additional 16 individuals were excluded, most often for not completing the baseline visit. The final sample consisted of 315 persons who were randomized (3:1:3) to varenicline (n=137), placebo (n=45), and combination nicotine replacement (n=133), This distribution was determined a priori (see sample size calculation in Analysis Plan below) to allow an adequately powered comparison of placebo with varenicline, while also including an NRT treatment condition similar to those tested in previous methadone trials (Stein et al., 2006b). Participants were informed of the chance of being assigned to study conditions in the consent process prior to enrollment.
Figure 1.
Consort Flow Chart detailing study enrollment, allocation, follow-up, and analysis.
2.2 Initial Assessment
Following the informed consent process at the MMT sites, participants completed a 45-minute questionnaire assessing sociodemographics, smoking history, risk behaviors, nicotine dependence (Heatherton et al., 1991), other substance use, and readiness to quit (Biener and Abrams, 1991). Research staff also assessed the participants' CO concentration via a breath sample using the Bedfont EC50 Micro III Smokelyzer (Kent, UK). Urine samples were collected for pregnancy testing. Participants were randomized to treatment after completing the baseline assessment.
2.3 Minimal Behavioral Intervention
Regardless of group assignment, participants then met with a study interventionist. At this 15-minute session, participants received standardized advice to quit smoking that followed the National Cancer Institute's 5As model for smoking cessation counseling (Fiore et al., 2008). Participants were asked to set a quit date for eight days after the baseline assessment and receipt and initiation of medication. Study visits were scheduled monthly, coinciding with refills of medication/NRT.
2.4 Pharmacotherapy
2.4.1 Varenicline Condition
Participants were instructed to begin with one capsule (0.5 mg) with food the evening of the baseline visit. This dose was continued for three days, then increased to two 0.5 mg pills a day for four days, increasing to 1 mg twice daily after one week (as per the varenicline package insert from (Pfizer, Inc.). Participants were urged to call the study staff or seek medical support if they experienced adverse effects. The importance of adherence was emphasized at all medication dispensing visits. Medication was dispensed at 4-week intervals for up to a 24-week course of therapy. Interviews at 2- and 4-week visits assessed only adherence and side effects. Participants were instructed to take varenicline for 1 week before attempting to quit smoking on day 8 of the study.
2.4.2 Varenicline-Placebo Condition
The double-blind varenicline-placebo control condition consisted of 24 weeks of placebo tablets (compounded to be identical in appearance to varenicline capsules) using an identical dosing, dispensing, and interview schedule as the active varenicline group.
2.4.3 Combination Nicotine Replacement Condition
For participants assigned to combination NRT condition, research staff dispensed the nicotine patch and described its proper use: placement, daily dosage, importance of not smoking while using the patch, and tapering of patches. Participants were urged to call if they experienced adverse effects. The importance of adherence was emphasized at all medication dispensing visits. The Nicoderm patch (GlaxoSmithKline) was given at 4-week intervals for up to 24 weeks of therapy. For participants who smoked >30 cigarettes per day the treatment began at 42 mg, and for participants smoking <30 cigarettes per day the treatment began at 21 mg.
In addition to using daily nicotine patch, participants received a four-week supply of 4 mg nicotine gum (Nicorette) at the baseline visit. Participants were instructed to chew gum when experiencing craving, and felt that they were likely to restart smoking. Patients were instructed to chew up to 1–2 pieces of gum per hour but no more than 24 pieces of gum per day. Participants were provided refills of the nicotine gum at their request at any time during the treatment phase.
2.5 Medication Recycling
We offered up to 24 weeks of medication for each condition for several reasons. First, there is data to suggest that longer treatment extends benefits without increasing risk of harm (Kornitzer et al., 1995; Tonstad et al., 2006; Williams et al., 2007). Second, we expected that many smokers would relapse after initiating treatment and might discontinue cessation treatment. Many trials end treatment participation at this point, but do not reflect how smokers quit in the real world, which is by re-trying to quit, often soon after a failed attempt (Stein et al., 2007). Therefore, we encouraged smokers with lapses to restart treatment at any point and wanted to offer an extended treatment coverage period. Research staff contacted all persons who did not come to their expected monthly medication pick-up dates to seek additional study medication when needed. At these visits, if participants reported smoking, research staff performed the 5A's counseling strategy again, and suggested restarting the assigned study medication.
2.6 Participant Retention and Follow-up
Research assessments were performed at 2 and 4 weeks (focused on side effects and adherence), and at 24 weeks after study enrollment by research assistants blinded to participant group assignment. CO breath samples and cotinine urine samples were taken at the 6-month interview when a participant in the varenicline or placebo groups reported not smoking in the prior seven days. Participants were paid $30 to complete the baseline assessment, and $40 for the 6-month assessment.
2.7 Therapist Adherence
Two 90-minute training sessions for research staff included: (1) an overview of the study, nicotine dependence, varenicline and nicotine replacement therapy, and the 5A's approach to intervention; (2) a demonstration role play of the 5A's intervention; and (3) opportunity for staff to practice the intervention using scripted role plays. A procedural manual was used during initial training sessions and monthly supervision meetings. Staff was required to complete a brief checklist to document implementation of the 5As intervention. Once the study started, all sessions were audiotaped, and a random ten percent were audited to check internal validity on an ongoing basis.
2.8 Measures of Smoking
The time line follow back (TLFB) technique (Sobell and Sobell, 1992) assessed smoking at the baseline, and smoking and medication use at the 24-week assessment.
Our primary outcome was defined as CO-confirmed (expired breath scores <8 parts per million) self-reported abstinence on the 7 days immediately prior to the 6-month assessment. Varenicline and placebo group participants who self-reported abstinence also had urine cotinine confirmation (Drug Test Systems). We also present data using 7-day self-report (not confirmed by CO testing) abstinence. We augmented our primary analyses with a third outcome, continuous abstinence from protocol day 14 through the 6-month assessment, and a fourth outcome for participants who had not quit smoking, namely reduction in the average cigarettes per day of use in the 28 days prior to the 6-month assessment.
Other covariates included age in years, gender, race, readiness to change smoking behavior using a 10-point ladder (Biener and Abrams, 1991), and CES-D to assess depressive symptoms (Irwin et al., 1999). Stata version 10.1 was used for all analyses (StataCorp, 2008).
2.9 Analysis Plan
Based on earlier results (Stein et al., 2006b), we conservatively anticipated quit rates of approximately 20% and 2.5% in the varenicline and placebo group, respectively. With a 3 to 1 allocation between these groups, to detect a difference with α/2 = .05 and power = .80, required 132 and 44 subjects, respectively. The study was not powered to detect differences between the varenicline groups and the combination NRT group.
Our primary analysis used an intent-to-treat approach, with missing individuals presumed to have continued or resumed smoking. We present means and percentages to summarize the characteristics of the cohort. Between-group differences with respect to demographic characteristics, baseline indicators of smoking duration, intensity, and level of dependence, and loss to follow-up were evaluated with ANOVA and the χ2-test for continuous and categorical indicators, respectively. We also present means and percentages to describe between group differences on 6-month smoking outcomes. Because data were collected from 9 study sites we estimated mixed logistic and linear regression models, with site as a random effect, to test the statistical significance of intervention effects. Tests of significance were two-tailed and all analyses adopted an alpha of 0.05.
3. RESULTS
Participants averaged 39.9 (± 9.7) years of age, with 49.5% males, and 79.4% non-Hispanic White (Table 1). The mean daily methadone dose at baseline was 108.7 mg (± 63.1). Participants smoked on average 19.6 (± 10.4) cigarettes / day. Thirty-six (11.4%) participants reported ever using varenicline and 183 (58.1%) reported ever using NRT. The mean CES-D score was 12.0 (± 6.2) at baseline. Fifty-eight (18.4%) persons were lost to follow-up at 6-months. The intervention arms did not differ significantly with respect to any of the background characteristics evaluated in Table 1.
Table 1.
Background Characteristics by Intervention Arm.
| INTERVENTION ARM |
|||||
|---|---|---|---|---|---|
| Full Cohort (n = 315) | NRT (n = 133) | Varenicline (n = 137) | Placebo (n = 45) | ||
|
|
|||||
| Mean (± SD) or n (%) | Mean (± SD) or n (%) | Mean (± SD) or n (%) | Mean (± SD) or n (%) | F (p = )or χ2 (p = ) | |
| Years Age | 39.9 (± 9.7) | 40.3 (± 9.3) | 39.2 (± 9.7) | 40.6 (± 10.6) | 0.59 (.558) |
| Gender (Male) | 156 (49.5%) | 65 (48.9%) | 63 (46.0%) | 28 (62.2%) | 3.61 (.164) |
| Race/Ethnicity | |||||
| Non-Hispanic White | 250 (79.4%) | 103 (77.4%) | 113 (82.5%) | 34 (75.6%) | |
| African-American | 8 (2.5%) | 3 (2.3%) | 2 (1.5%) | 3 (6.7%) | 6.83 (.337) |
| Hispanic | 38 (12.1%) | 17 (12.8%) | 17 (12.4%) | 4 (8.9%) | |
| Other | 19 (6.0%) | 10 (7.5%) | 5 (3.7%) | 4 (8.9%) | |
| Years Education | 11.8 (± 1.9) | 11.9 (± 2.2) | 11.8 (± 2.0) | 11.4 (± 1.9) | 0.85 (.428) |
| Employed (Yes) | 69 (21.9%) | 27 (20.3%) | 26 (19.0%) | 16 (35.6%) | 5.79 (.055) |
| Methadone Dose | 108.7 (± 63.1) | 109.3 (± 61.7) | 108.3 (± 62.7) | 108.0 (± 69.4) | 0.01 (.988) |
| Yrs. Regular Smoker | 23.3 (± 10.2) | 24.0 (± 9.8) | 22.8 (± 10.1) | 23.0 (± 11.3) | 0.43 (.653) |
| Fagerstrom (FTND) | 5.7 (± 2.2) | 5.6 (± 2.2) | 5.6 (± 2.1) | 6.0 ± (2.4) | 1.43 (.490) |
| Mean Cigs / Day | 19.6 (± 10.4) | 19.1 (± 7.8) | 19.5 (± 8.5) | 21.1 (± 10.4) | 0.96 (.384) |
| Ever Used Varenicline (Yes) | 36 (11.4%) | 14 (10.5%) | 18 (13.1%) | 4 (8.9%) | 0.79 (.670) |
| Ever Used NRT (Yes) | 183 (58.1%) | 81 (60.9%) | 75 (54.7%) | 27 (60.0%) | 1.13 (.570) |
| Days Smoked / 30 Days | 29.9 (± 0.7) | 29.8 (± 1.1) | 30.0 (± 0.2) | 30.0 (± 0.1) | 1.82 (.523) |
| Used Opiates (Yes) | 31 (9.8%) | 11 (8.3%) | 15 (11.0%) | 5 (11.1%) | 0.64 (.726) |
| Used Cocaine (Yes) | 27 (8.6%) | 23 (9.8%) | 14 (10.3%) | 0 (0.0%) | 4.96 (.084) |
| Mean CESD | 12.0 (± 6.2) | 12.5 (± 6.5) | 11.5 (± 6.1) | 11.5 (± 5.4) | 1.02 (.360) |
| Lost to Follow-Up | 58 (18.4%) | 26 (19.6%) | 22 (16.1%) | 10 (22.2%) | 1.05 (.590) |
Persons lost to follow-up did not differ significantly from those observed at 6-months with respect to age, ethnicity, educational attainment, mean cigarettes / day, methadone dose, or recent use of opiates. Males (25.0%) were significantly (p = .003) more likely to be lost to follow-up than females (12.0%), and persons who were employed part- or full-time (29.0%) were significantly (p = .01) more likely to be lost to follow-up than unemployed participants (15.5%).
The overall rate of early adherence to treatment, defined as the percent days persons reported using their assigned medication during the first 30 days of follow-up, was 68.5% (± 36.2). Adherence rates did not differ significantly by intervention arm (p = .49). Adherence during the 7-days immediately prior to 6-month assessment was 40.3% (± 36.2). Statistically significant (p = .003) differences in adherence rates were observed during this period; rates of adherence were 48.8% in NRT, 34.2% in varenicline, and 34.4% in placebo. With regard to the use of smoking cessation aids outside the study, the rates of any NRT use (which could be purchased over-the-counter) during the 6-months of follow-up were 11.2% in the varenicline group and 21.1% in the placebo arm.
Table 2 gives results comparing intervention arms on 4 outcomes assessed at 6-months. Only 30 (9.5%) participants reported 7-day abstinence at 6-months (Outcome 1), and we were only able to confirm abstinence (Outcome 2) for 17 (5.4%) of the participants. Between baseline and 6-months there was an overall mean reduction of 8.3 cigarettes / day (Outcome 3). Approximately 70.8% (n = 223) reported a reduction in mean cigarettes / day, 176 (55.9%) reported a mean reduction of 5 or more cigarettes / day, and 118 (37.5%) reported an absolute mean reduction of 10 or more cigarettes / day. Only 4 (1.3%) participants reported continual abstinence (Outcome 4) from day 14 through the 6-month assessment period.
Table 2.
Six-Month Smoking Outcomes by Intervention Arm.
| INTERVENTION GROUP |
|||||
|---|---|---|---|---|---|
| Full Cohort (n = 315) | NRT (n = 133) | Varenicline (n = 137) | Placebo (n = 45) | ||
|
|
|||||
| Outcome | Mean (± SD) or n (%) | Mean (± SD) or n (%) | Mean (± SD) or n (%) | Mean (± SD) or n (%) | Wald χ2 (p = ) b |
| Self-Reported 7-Day Abstinence | |||||
| Yes | 30 (9.5%) | 16 (12.0%) | 11 (8.0%) | 3 (6.7%) | 1.72 (.423) |
| No | 285 (90.5%) | 117 (88.0%) | 126 (92.0%) | 42 (93.3%) | |
| CO-Confirmed 7-Day Abstinence a | |||||
| Yes | 17 (5.4%) | 11 (8.3%) | 5 (3.7%) | 1 (2.2%) | 3.56 (.168) |
| No | 298 (94.6%) | 122 (91.7%) | 132 (96.4%) | 44 (97.8%) | |
| Change Mean Cigarettes / Day | −8.3 (± 9.3) | −7.8 (± 9.4) | −8.7 (± 9.2) | −8.5 (± 9.5) | 0.76 (.684) |
| Continuous Abstinence | |||||
| Yes | 4 (1.3%) | 2 (1.5%) | 2 (1.5%) | 0 (0.0%) | 0.00 (.999) |
| No | 311 (98.7%) | 131 (98.5%) | 135 (98.5%) | 45 (100.0%) | |
Valid CO or Cotinine tests were available for 199 participants. Participants without valid CO tests were assumed non-abstinent. Separate analysis of participants with valid CO or Cotinine tests was consistent with the results reported here (χ2 = 3.12, p = .190).
Mixed logistic and linear regression models with data collection site entered as a random effect were estimated to test the statistical significance of intervention. The intraclass correlations representing the effect of site were trivially small; analyses treating observations as independent gave results substantively and statistically consistent with those reported here.
Directionally, between-group differences in smoking outcomes tended to favor NRT, followed by varenicline (Table 2), though between group differences were substantively small and not statistically significant on any of the 4 evaluated outcomes. Logistic regression and analysis of covariance models, in which intervention effects were adjusted for baseline differences in years of regular smoking, Fagerstrom Test of Nicotine Dependence (FTND) scores, and mean cigarettes / day at baseline, were consistent with the results in Table 2.
We conducted additional analyses to assess the degree to which our results were sensitive to missing data (no Table). Under the assumption that all persons lost to follow-up were smoking abstinent 27.8%, 19.7%, and 24.4% of persons in the NRT, varenicline, and placebo arms, respectively, were smoking abstinent (p = .29). Additionally we analyzed persons with valid 6-month observations (n = 257), the rates of confirmed abstinence were 10.3%, 4.4%, and 2.9% in the NRT, varenicline, and placebo arms, respectively (p = .15).
Self-reported side effects were assessed during the initial month of follow-up (Table 3). A statistically significant between-group difference was observed with respect to reporting depressed mood or feeling sad (p = .041); with rates of endorsing the severity of this side effect as moderate or severe highest in the NRT arm. Those in the varenicline arm tended to be less likely to report feelings of anger, irritability, or frustration (p = .059), as well as feelings of anxiety (p = .057). Two participants in the varenicline arm stopped study medication due to neurobehavioral adverse effects (hearing voices, mood disturbance). Unrelated to study medication, three persons died (cerebral aneurysm, overdose, cirrhosis), two in the placebo group, one in NRT; three other persons had serious adverse events (heart attack; NRT), two with rashes (varenicline) all of whom continued in the study.
Table 3.
Percentage Reporting Moderate or Severe Side Effects in the Past 24 Hours at 1-Month by Intervention Arm.
| MEAN (SD) |
||||
|---|---|---|---|---|
| Side Effecta | NRT (n=104) | Varenicline (n=111) | Placebo (n=33) | p = b |
| Angry, irritable, frustrated | 28.9% | 16.2% | 30.3% | .059 |
| Anxious, nervous | 33.7% | 19.8% | 33.3% | .057 |
| Depressed mood, sad | 29.8% | 15.3% | 21.2% | .041 |
| Increased appetite, hungry, weight gain | 22.1% | 11.7% | 21.2% | .115 |
| Insomnia, sleep problems, awakening | 36.5% | 35.1% | 36.4% | .976 |
| Dizziness | 4.8% | 1.8% | 3.0% | .483 |
| Coughing | 20.2% | 10.8% | 6.1% | .061 |
| Dreaming or nightmares | 18.3% | 20.7% | 24.2% | .744 |
| Nausea | 8.7% | 6.3% | 6.1% | .783 |
| Headaches | 9.6% | 6.3% | 18.2% | .134 |
| Diarrhea | 0.0% | 2.7% | 3.0% | .228 |
| Skin Rash | 0.0% | 1.8% | 3.0% | .284 |
| Suicidal thinking or behavior | 1.0% | 0.9% | 0.0% | .999 |
| Dry Mouth | 16.4% | 11.7% | 12.1% | .592 |
| General muscle aches | 21.2% | 15.3% | 27.3% | .250 |
| Flushing or sweating | 28.9% | 19.8% | 33.3% | .172 |
| A change in how things taste | 13.5% | 6.3% | 3.0% | .102 |
| Confusion | 3.9% | 4.5% | 3.0% | .924 |
Respondents were asked to rate the severity of side effects they were experiencing in past 24 hours; response categories were 0 = none, 1 = slight, 2 = mild, 3 = moderate, and 4 = severe.
Mixed logistic regression models with data collection site entered as a random effect were estimated to test the statistical significance of intervention.
As an auxiliary analysis we estimated logistic regression models to evaluate potential predictors of CO-confirmed abstinence. Confirmed abstinence was not associated significantly with age (p = .506), gender (p = .527), ethnicity (p = .118), mean cigarettes / day at baseline (p = .332), baseline FTND scores (p = .833), baseline methadone dose (p = .330) or adherence (p = .974). Additionally, none of the above predictors were associated significantly with self-reported abstinence.
4. DISCUSSION
In this fourth randomized trial of smoking cessation among methadone maintained individuals and the first trial testing varenicline, we found extremely low quit rates at six months, rates comparable to findings from trials using other medications (Reid et al., 2008; Richter et al., 2005; Shoptaw et al., 2002). Varenicline did not significantly improve cessation rates compared to placebo among participants who uniformly reported being highly motivated to quit. Combination NRT also produced low quit rates, modestly higher than varenicline. The finding of low reported medication adherence during the week prior to the 6-month assessment by the majority of participants certainly contributed to these low quit rates, but continued smoking despite reported medication use in others demonstrates the difficulty of achieving abstinence in this population.
Many factors help to explain the poor treatment outcomes. Overlapping neurobiological pathways in persons with dual opioid and nicotine dependence may affect withdrawal sensitivities and craving (Frosch et al., 2000; Pontieri et al., 1996). Methadone itself may lead to increases in smoking due to more intense tobacco craving and withdrawal symptoms and decreases in respiratory symptoms such as cough (Schmitz et al., 1994; Spiga et al., 2005; Story and Stark, 1991). Individual factors certainly play a role as well. Methadone-maintained persons often have psychiatric comorbidities and high levels of stress, and use smoking as an anxiolytic or antidepressant (Hayaki et al., 2005; Khanna et al., 2010); our sample had high levels of depressive symptoms. Furthermore, methadone-maintained smokers may have low self-efficacy, and with high community norms for smoking, may have more difficulty refusing nicotine. Interestingly, compared to our earlier smoking cessation trial (Stein et al., 2006b) among methadone maintained smokers from the same region (and in some cases the same MMT programs), the average cigarettes per day at entry to the current trial was 7.4 cigarettes lower here, perhaps reflecting the increasing societal opprobrium against smoking and the rising cost of cigarettes.
As in most smoking cessation studies, we used a biological marker to confirm participants' self-reports of abstinence. We found that in this methadone maintained population, there was a discrepancy between self-report and CO confirmation of cessation rates. Across conditions, participants reported 7-day abstinence rates that were twice the CO confirmed prevalence rates, a discrepancy similar to our previous trial (Stein et al., 2006b). Participants, across conditions, reported reducing their cigarette use significantly during the intervention. However, these smoking reduction reports may be distorted, since there is no biochemical confirmation during the course of the study (just at the 6-month end point) and the abstinence self-report was inflated when there was such confirmation.
Although reported motivation to quit was extremely high, we speculate that many study participants viewed smoking reduction, rather than cessation, as an acceptable endpoint. We designed our study such that participants were not “obliged” to be abstinent at any point during the study to continue to receive medication. Indeed, we did not schedule a “quit day” appointment to confirm abstinence or discuss relapse prevention techniques because our previous trial demonstrated no benefit from such a session (Stein et al., 2006b). While we provided a prolonged duration of treatment that we believed would fit this highly dependent population with long smoking histories, such treatment did not produce positive results. Some participants may have been disappointed to receive the treatment to which they were assigned—NRT recipients might have preferred pills (even with a possibility of placebo), and varenicline recipients might have wanted NRT—lowering adherence rates. The low adherence rates at six months suggest many participants may have given up because of an inability to quit, some accumulation of side effects, or both. Medication adherence is critical for positive smoking cessation outcomes in studies involving non-drug users (Blak et al., 2010; Catz et al., 2011; Cummings et al., 1997; Halperin et al., 2009; Lam et al., 2005; Piper et al., 2009; Shiffman et al., 2008); low medication adherence has been reported in previous medication trials of MMT smokers (Frosch et al., 2002; Mooney et al., 2008; Richter et al., 2005; Stein et al., 2006a, 2007).
Two previous randomized trials compared varenicline to NRT (with brief weekly counseling sessions) in non-substance users and found no statistical difference between groups (Cahill et al., 2011; Tsukahara et al., 2010) with quit rates at 52 weeks in both studies of approximately 20%. In contrast, in a non-randomized study performed in an inner city medical clinic, quit rates using varenicline and NRT were approximately 7% after 52 weeks (Dhelaria et al., 2012). This finding, despite the limitations of a retrospective observational study, suggests that obstacles to quitting such as mental illness and substance abuse, common in urban samples, lower quit rates. The evidence that methadone and nicotine may interact to enhance the positive effects of methadone and to decrease negative feeling may in part explain the slightly higher quit rates in the combination NRT group here (Elkader et al., 2009).
One important finding in this high risk population was the low risk of medication-related adverse effects, and rare medication discontinuation due to adverse effects. Two participants in the varenicline arm stopped study medication due to neurobehavioral adverse effects, but there were no group difference in depressive symptoms across groups. Despite post-marketing surveillance noting the possibility of neuropsychiatric adverse events with varenicline, analysis of data from 10 pooled trials showed no difference in such effects compared to placebo (Tonstad et al., 2010), no difference for smokers with and without a history of psychiatric disorders (McClure et al., 2010), and no significant differences in rate of adverse medical events (Rigotti et al., 2010).
Our study had several limitations. First, our trial did not have a double-dummy design, that is, while we included a blind comparison of varenicline and placebo, we did not include an NRT placebo group. Such a design would have required a far larger sample, and we knew from our pilot work that smokers would have been less interested in participating knowing they had a greater chance of receiving inactive treatment, presenting a formidable obstacle to recruitment. With the current design, we powered the study to compare varenicline and placebo; we were too optimistic in our estimate of varenicline's effect size. Second, we measured adherence by self-report only and such reports include the possibility that a participant might have used a lower dose than prescribed (e.g., varenicline taken once a day); adherence was relatively low by six months, and we could, in future trials, yoke smoking cessation medication to methadone dispensing or introduce another means to improve adherence. Third, with self-report, participants may be inaccurate in their claims of smoking reduction, which could not be confirmed biologically. Finally, our findings may not generalize to opioid dependent persons not in MMT or to those in MMT with self-reported severe mental health disorders.
Although varenicline has been promoted as a first line therapy due to its greater effectiveness in clinical trials, we found it to be no more effective than placebo among individuals involved in MMT even when offered free-of charge, and delivered on site, eliminating two barriers to varenicline treatment that have been identified by previous investigators (Garrison and Dugan, 2009). Quit rates were disappointingly low and we identified no subgroups that had superior outcomes. The combination NRT quit rate was comparable to that found with patch NRT in earlier studies (Reid et al., 2008; Shoptaw et al., 2002; Stein et al., 2006b). While our earlier work suggested that behavioral therapy did not improve quit rates, novel forms of behavioral intervention (including group sessions, daily electronic reminders, or on-line supports) may be warranted. Furthermore, novel medication strategies are needed to achieve smoking cessation for this difficult to treat population. Our findings also suggest that a greater understanding of the physiological and psychological causes of low quit rates among opiate dependent smokers must be addressed in treatment. The health risks associated with continued smoking are high, and the challenge of achieving lasting smoking cessation among methadone maintained smokers remains daunting.
Acknowledgments
Trial registered at clinicaltrials.gov; Clinical Trial # NCT00790569
Role of Funding Source: Funding for this study was provided by the National Cancer Institute (RO1 CA129226). Dr. Stein is a recipient of a NIDA Mid-Career Investigator Award (K24 DA00512). NCI and NIDA had no further role in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Author Stein designed the study and wrote the protocol. Authors Caviness and Kurth managed the study and undertook literature searches and provided extensive editorial feedback. Authors Audet and Olson were responsible for data collection, management, and literature searches. Author Anderson undertook all statistical analyses. All authors contributed to and have approved the final manuscript.
Conflict of Interest: No conflict declared
REFERENCES
- Aubin HJ, Bobak A, Britton JR, Oncken C, Billing CB, Jr., Gong J, Williams KE, Reeves KR. Varenicline versus transdermal nicotine patch for smoking cessation: results from a randomised open-label trial. Thorax. 2008;63:717–724. doi: 10.1136/thx.2007.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The contemplation ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Blak BT, Wilson K, Metcalfe M, Maguire A, Hards M. Evaluation of varenicline as an aid to smoking cessation in UK general practice - a THIN database study. Curr. Med. Res. Opin. 2010;26:861–870. doi: 10.1185/03007990903526461. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database System.Rev. 2011:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, McAfee T, Richards J, Swan GE. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine Tob. Res. 2011;13:361–368. doi: 10.1093/ntr/ntr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention State-specific prevalence of cigarette smoking among adults and quitting among persons aged 18–35 years--United States, 2006. MMWR. 2007;56:993–996. [PubMed] [Google Scholar]
- Clarke JG, Stein MD, McGarry KA, Gogineni A. Interest in smoking cessation among injection drug users. Am. J. Addict. 2001;10:159–166. doi: 10.1080/105504901750227804. [DOI] [PubMed] [Google Scholar]
- Cummings KM, Hyland A, Ockene JK, Hymowitz N, Manley M. Use of the nicotine skin patch by smokers in 20 communities in the United States, 1992–1993. Tob. Control. 1997;6(Suppl. 2):S63–70. doi: 10.1136/tc.6.suppl_2.s63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhelaria RK, Friderici J, Wu K, Gupta E, Khan C, Rothberg MB. Effectiveness of varenicline for smoking cessation at 2 urban academic health centers. Eur. J. Intern. Med. 2012;23:461–464. doi: 10.1016/j.ejim.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Elkader AK, Brands B, Selby P, Sproule BA. Methadone-nicotine interactions in methadone maintenance treatment patients. J. Clin. Psychopharmacol. 2009;29:231–238. doi: 10.1097/JCP.0b013e3181a39113. [DOI] [PubMed] [Google Scholar]
- Etter JF, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob. Control. 2006;15:280–285. doi: 10.1136/tc.2005.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO, Schneider NG, Lunell E. Effectiveness of nicotine patch and nicotine gum as individual versus combined treatments for tobacco withdrawal symptoms. Psychopharmacol. (Berl.) 1993;111:271–277. doi: 10.1007/BF02244941. [DOI] [PubMed] [Google Scholar]
- Fiore M, Jaen C, Baker T, Bailey C, Benowitz N, Curry S, et al. Treating Tobacco Use and Dependence: 2008 Update. U.S. Dept. of Health and Human Services, Public Health Service; Rockville, MD: 2008. [Google Scholar]
- Frosch DL, Nahom D, Shoptaw S. Optimizing smoking cessation outcomes among the methadone maintained. J. Subst. Abuse Treat. 2002;23:425–430. doi: 10.1016/s0740-5472(02)00280-5. [DOI] [PubMed] [Google Scholar]
- Frosch DL, Shoptaw S, Nahom D, Jarvik ME. Associations between tobacco smoking and illicit drug use among methadone-maintained opiate-dependent individuals. Exp. Clin. Psychopharmacol. 2000;8:97–103. doi: 10.1037//1064-1297.8.1.97. [DOI] [PubMed] [Google Scholar]
- Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clin. Ther. 2009;31:463–491. doi: 10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Halperin AC, McAfee TA, Jack LM, Catz SL, McClure JB, Deprey TM, Richards J, Zbikowski SM, Swan GE. Impact of symptoms experienced by varenicline users on tobacco treatment in a real world setting. J. Subst. Abuse Treat. 2009;36:428–434. doi: 10.1016/j.jsat.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayaki J, Anderson BJ, Stein MD. Perceptions of health risk susceptibility in methadone maintained smokers. J. Addict. Dis. 2005;24:73–84. doi: 10.1300/J069v24n01_07. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hser YI, Hoffman V, Grella CE, Anglin MD. A 33-year follow-up of narcotics addicts. Arch. Gen. Psychiatry. 2001;58:503–508. doi: 10.1001/archpsyc.58.5.503. [DOI] [PubMed] [Google Scholar]
- Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotics addicts. Prev. Med. 1994;23:61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- Hurt RD, Dale LC, Fredrickson PA, Caldwell CC, Lee GA, Offord KP, Lauger GG, Marusic Z, Neese LW, Lundberg TG. Nicotine patch therapy for smoking cessation combined with physician advice and nurse follow-up. One-year outcome and percentage of nicotine replacement. JAMA. 1994;271:595–600. [PubMed] [Google Scholar]
- Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, Melton LJ., 3rd Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Arch. Intern. Med. 1999;159:1701–1704. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- Karan LD. Initial encounters with tobacco cessation on the Inpatient Substance Abuse Unit of the Medical College of Virginia. J. Subst. Abuse Treat. 1993;10:117–123. doi: 10.1016/0740-5472(93)90035-z. [DOI] [PubMed] [Google Scholar]
- Khanna N, Arnold S, Sadaphal S, Joshi A, Stewart D, Gandhi D. Nicotine dependence and depression among women smokers on methadone maintenance. Eur. J. Gen. Pract. 2010;16:222–228. doi: 10.3109/13814788.2010.516359. [DOI] [PubMed] [Google Scholar]
- Kornitzer M, Boutsen M, Dramaix M, Thijs J, Gustavsson G. Combined use of nicotine patch and gum in smoking cessation: a placebo-controlled clinical trial. Prev. Med. 1995;24:41–47. doi: 10.1006/pmed.1995.1006. [DOI] [PubMed] [Google Scholar]
- Lam TH, Abdullah AS, Chan SS, Hedley AJ. Adherence to nicotine replacement therapy versus quitting smoking among Chinese smokers: a preliminary investigation. Psychopharmacol. (Berl. 2005;177:400–408. doi: 10.1007/s00213-004-1971-y. [DOI] [PubMed] [Google Scholar]
- Lemon SC, Friedmann PD, Stein MD. The impact of smoking cessation on drug abuse treatment outcome. Addict. Behav. 2003;28:1323–1331. doi: 10.1016/s0306-4603(02)00259-9. [DOI] [PubMed] [Google Scholar]
- McClure JB, Swan GE, Catz SL, Jack L, Javitz H, McAfee T, Deprey M, Richards J, Zbikowski M. Smoking outcome by psychiatric history after behavioral and varenicline treatment. J. Subst. Abuse Treat. 2010;38:394–402. doi: 10.1016/j.jsat.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool RM, Paschall Richter K. Why do so many drug users smoke? J. Subst. Abuse Treat. 2003;25:43–49. doi: 10.1016/s0740-5472(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Mooney ME, Poling J, Gonzalez G, Gonsai K, Kosten T, Sofuoglu M. Preliminary study of buprenorphine and bupropion for opioid-dependent smokers. Am. J. Addict. 2008;17:287–292. doi: 10.1080/10550490802138814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi S, Richter K, Li X, Modali L, Arnsten J. Cigarette smoking and interest in quitting in methadone maintenance patients. Addict. Behav. 2006;31:2127–2134. doi: 10.1016/j.addbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Oshima A, Fujimoto Y, Maruyama N, Ishibashi T, Reeves KR. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin. Ther. 2007;29:1040–1056. doi: 10.1016/j.clinthera.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Niaura R, Jones C, Kirkpatrick P. Varenicline. Nat. Rev. Drug Disc. 2006;5:537–538. doi: 10.1038/nrd2088. [DOI] [PubMed] [Google Scholar]
- Okoli CT, Khara M, Procyshyn RM, Johnson JL, Barr AM, Greaves L. Smoking cessation interventions among individuals in methadone maintenance: a brief review. J. Subst. Abuse Treat. 2010;38:191–199. doi: 10.1016/j.jsat.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Orleans CT, Hutchinson D. Tailoring nicotine addiction treatments for chemical dependency patients. J. Subst. Abuse Treat. 1993;10:197–208. doi: 10.1016/0740-5472(93)90045-4. [DOI] [PubMed] [Google Scholar]
- Pfizer, Inc. How to Take Chantix. 2013 http://www.chantix.com/how-to-take.aspx. Retrieved May, 2013.
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch. Gen. Psychiatry. 2009;66:1253–1262. doi: 10.1001/archgenpsychiatry.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Puska P, Korhonen H, Vartiainen E, Urjanheimo E, Gustavsson G, Westin A. Combined use of nicotine patch and gum compared with gum alone in smoking cessation: a clinical trial in North Karelia. Tob. Control. 1995;4:231–235. [Google Scholar]
- Reid MS, Fallon B, Sonne S, Flammino F, Nunes EV, Jiang H, Kourniotis E, Lima J, Brady R, Burgess C, Arfken C, Pihlgren E, Giordano L, Starosta A, Robinson J, Rotrosen J. Smoking cessation treatment in community-based substance abuse rehabilitation programs. J. Subst. Abuse Treat. 2008;35:68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Richter KP, Ahluwalia HK, Mosier MC, Nazir N, Ahluwalia JS. A population-based study of cigarette smoking among illicit drug users in the United States. Addiction. 2002;97:861–869. doi: 10.1046/j.1360-0443.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- Richter KP, Gibson CA, Ahluwalia JS, Schmelzle KH. Tobacco use and quit attempts among methadone maintenance clients. Am. J. Public Health. 2001;91:296–299. doi: 10.2105/ajph.91.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter KP, McCool RM, Catley D, Hall M, Ahluwalia JS. Dual pharmacotherapy and motivational interviewing for tobacco dependence among drug treatment patients. J. Addict. Dis. 2005;24:79–90. doi: 10.1300/j069v24n04_06. [DOI] [PubMed] [Google Scholar]
- Richter KP, McCool RM, Okuyemi KS, Mayo MS, Ahluwalia JS. Patients' views on smoking cessation and tobacco harm reduction during drug treatment. Nicotine Tob. Res. 2002;4(Suppl. 2):S175–182. doi: 10.1080/1462220021000032735. [DOI] [PubMed] [Google Scholar]
- Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121:221–229. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JM, Grabowski J, Rhoades H. The effects of high and low doses of methadone on cigarette smoking. Drug Alcohol Depend. 1994;34:237–242. doi: 10.1016/0376-8716(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin. Ther. 2008;30:1852–1858. doi: 10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Rotheram-Fuller E, Yang X, Frosch D, Nahom D, Jarvik ME, Rawson RA, Ling W. Smoking cessation in methadone maintenance. Addiction. 2002;97:1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. discussion, 1325. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: a technique for assessing self-reported alcohol consumption. In: Allen L, editor. Measuring Alcohol Consumption. The Humana Press; New York: 1992. pp. 41–72. [Google Scholar]
- Spiga R, Martinetti MP, Meisch RA, Cowan K, Hursh S. Methadone and nicotine self-administration in humans: a behavioral economic analysis. Psychopharmacol. (Berl.) 2005;178:223–231. doi: 10.1007/s00213-004-2020-6. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10.1. College Station, TX: 2008. [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann-Boyce J, Cahill K, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database System. Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancaster T. Nicotine replacement therapy for smoking cessation. Cochrane Database System. Rev. 2008:CD000146. doi: 10.1002/14651858.CD000146.pub3. [DOI] [PubMed] [Google Scholar]
- Stein MD, Anderson BJ, Niaura R. Nicotine replacement therapy: patterns of use after a quit attempt among methadone-maintained smokers. J. Gen. Intern. Med. 2006a;21:753–757. doi: 10.1111/j.1525-1497.2006.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MD, Anderson BJ, Niaura R. Smoking cessation patterns in methadone-maintained smokers. Nicotine Tob. Res. 2007;9:421–428. doi: 10.1080/14622200701188885. [DOI] [PubMed] [Google Scholar]
- Stein MD, Weinstock MC, Herman DS, Anderson BJ, Anthony JL, Niaura R. A smoking cessation intervention for the methadone-maintained. Addiction. 2006b;101:599–607. doi: 10.1111/j.1360-0443.2006.01406.x. [DOI] [PubMed] [Google Scholar]
- Story J, Stark MJ. Treating cigarette smoking in methadone maintenance clients. J. Psychoactive Drugs. 1991;23:203–215. doi: 10.1080/02791072.1991.10472237. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science. 2004;306:1029–1032. doi: 10.1126/science.1099420. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Davies S, Flammer M, Russ C, Hughes J. Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Saf. 2010;33:289–301. doi: 10.2165/11319180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- Tsukahara H, Noda K, Saku K. A randomized controlled open comparative trial of varenicline vs nicotine patch in adult smokers: efficacy, safety and withdrawal symptoms (the VN-SEESAW study) Circ. J. 2010;74:771–778. doi: 10.1253/circj.cj-09-0803. [DOI] [PubMed] [Google Scholar]
- Wang C, Xiao D, Chan KP, Pothirat C, Garza D, Davies S. Varenicline for smoking cessation: a placebo-controlled, randomized study. Respirology. 2009;14:384–392. doi: 10.1111/j.1440-1843.2008.01476.x. [DOI] [PubMed] [Google Scholar]
- Williams KE, Reeves KR, Billing CB, Jr., Pennington AM, Gong J. A double-blind study evaluating the long-term safety of varenicline for smoking cessation. Curr. Med. Res. Opin. 2007;23:793–801. doi: 10.1185/030079907x182185. [DOI] [PubMed] [Google Scholar]