Abstract
Embryo attachment and implantation is critical to successful reproduction of all eutherian mammals, including humans; a better understanding of these processes could lead to improved infertility treatments and novel contraceptive methods. Experience with assisted reproduction, especially oocyte donation cycles, has established that despite the diverse set of hormones produced by the ovary in a cycle-dependent fashion, the sequential actions of only two of them, oestrogen and progesterone, are sufficient to prepare a highly receptive endometrium in humans. Further investigation on the endometrial actions of these two hormones is currently providing significant insight into the implantation process in women, strongly suggesting that an abnormal response to progesterone underlies infertility in some patients.
Keywords: embryo implantation, endometrium, oestradiol, progesterone
Introduction
A thorough understanding of the processes governing human embryo implantation would be of significant benefit for the treatment of infertility and the development of novel contraceptives. However, implantation processes remain poorly understood, largely due to differences between humans and experimental animals and appropriate ethical, moral and legal barriers to direct examination of implanting human embryos. Despite these barriers, significant knowledge has been gained through experience with assisted reproduction coupled with application of improving analytic techniques applied to human tissues and non-human primate models.
Experience with donor oocyte IVF cycles has allowed profound clinical insights into the regulation of human endometrial receptivity. Donor oocyte cycles achieve the highest implantation rates of all assisted reproduction approaches (Sunderam et al. 2009), suggesting that the hormonal preparation of the endometrium has been well optimized (van der Linden et al. 2011). In donor oocyte cycles, the endometrium of the recipient is prepared by sequential treatment with oestrogen and progesterone, using protocols that prevent ovulation and corpus luteum formation. Notably, these protocols work just as well in a woman without ovaries. Thus, these two hormones, without any other ovarian or corpus luteum products, are sufficient for excellent preparation of human endometrium to accept an implanting embryo. Their primacy is further supported by the requirement of both hormones for pregnancy initiation and early survival in all eutherian mammals, despite major species-specific differences in ovarian and uterine anatomy and physiology. Given the critical and fundamental role that oestrogen and progesterone play in establishment of receptivity, a deep understanding of the action of these steroid hormones on the human endometrium will allow clear insight into the mechanisms determining endometrial receptivity. This review will attempt to summarize the current, albeit limited, understanding of oestrogen and progesterone action in determination of endometrial receptivity.
Molecular biology of oestrogen and progesterone action
Both oestrogen and progesterone act through specific, high-affinity, low-capacity nuclear receptors that function as ligand-activated transcription factors and chromatin modifiers to directly regulate expression of a large number of genes (Cheung and Kraus 2010; Huang et al. 2010). The products of steroid receptor-regulated genes can also act in a downstream, autocrine, paracrine or endocrine fashion to regulate expression of additional genes. It is important to recognize that some non-steroidal ligands can also bind the steroid receptors. Examples of non-steroidal ligands which act through oestrogen receptors include endogenous lipoxin A4 (LXA4), an eicosanoid produced in the endometrium (Russell et al. 2011), bisphenol A, an environmental compound (Li et al. 2012), and clomiphene citrate, a pharmaceutical agent. Thus, nuclear steroid receptors are responsible for the so-called ‘classical’ actions of oestrogen and progesterone (Figure 1).
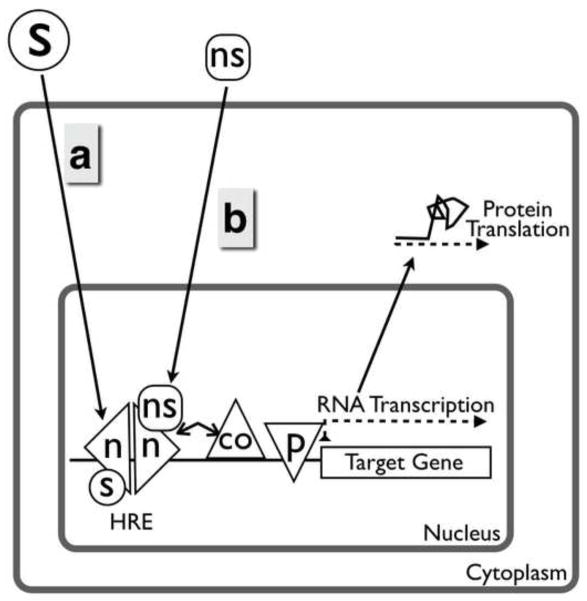
Figure 1.
Classical actions of nuclear oestrogen and progesterone receptors. (a) Steroid receptors bind steroid and then bind cognate DNA sequences. (b) Non-steroidal ligands can also act through nuclear steroid receptors. co = co-regulator; HRE = hormone response element; n = nuclear steroid receptor monomer; ns = non-steroid; p = RNA polymerase; s = steroid.
It is important to point out some significant simplifications made to improve readability in Figure 1. For example, oestrogen receptors and progesterone receptors are bound to chaperone proteins and are released from them after ligand binding. Chaperone binding may regulate steroid receptor availability and access to the nucleus, and therefore function. Another key feature of the classical actions of oestrogen and progesterone, not included in Figure 1, is that there are multiple oestrogen receptor and progesterone receptor isoforms, each having distinct actions on the genome. Differential expression of these isoforms in different cell types and physiological states results in differential effects of the steroids.
There are two nuclear oestrogen receptors – oestrogen receptor α and oestrogen receptor β – each derived from a distinct gene (ESR1 and ESR2, respectively). These genes have high sequence homology, likely resulting from an ancient gene duplication event, since homologous genes are seen in fish and amphibians as well as mammals (Katsu et al. 2008). Although similar in structure, oestrogen receptors and have distinct effects in experimental model organisms and distinct patterns of expression in human disease (Hewitt and Korach 2003). For example, overexpression of oestrogen receptor is observed in endometrioma lesions due to hypomethylation of the promoter leading to a molecular cascade resulting in inflammation and other pathophysiological changes (Bulun et al. 2010).
The progesterone receptors have at least two isoforms – progesterone receptor A and progesterone receptor B. Unlike oestrogen receptors, the progesterone receptor isoforms are derived from alternate transcription and translation start sites in a single gene (PGR; Ogle 2002; Jacobsen and Horwitz 2012). Progesterone receptor A and B are identical in structure except that the progesterone receptor B isoform contains a 164-amino acid N-terminal sequence, which is lacking in the progesterone receptor A isoform. The presence or absence of the N-terminal extension appears to be responsible for the distinct differences in progesterone receptors A and B actions. Truncated isoforms – progesterone receptor C and progesterone receptor M – that retain the progesterone-binding domain but lose the DNA-binding domain have been described as a possible suppressor of progesterone receptors A and B action, but their relevance in vivo is controversial (Wei et al. 1990; Samalecos and Gellersen 2008; Taylor et al. 2009).
A further level of complexity is seen in the interaction between steroid receptors and co-activators and co-repressors. These co-activators and repressors mediate the effects of the nuclear receptors on gene transcription (Figure 1). The expression and activity of the co-activators and co-repressors can be determined both developmentally and dynamically in the adult, providing a further basis for the pleiotropic effects of steroid hormones. In this regard, it is important to note that there are distinct mechanistic differences between mammalian species in steroid hormone and co-activator expression. For example, oestrogen receptor appears to be significantly more expressed in human endometrium as opposed to the mouse. A more extreme example is the progesterone receptor B specific co-activator, MAGEA-11, which is only present in primates and appears to play an important role in the human endometrial response to progesterone (Su et al. 2012).
The effects of progesterone via its receptor also depend on other signals and transcription factors. An indisputably critical action of progesterone on endometrial stroma is decidualization. However, full decidualization requires signalling by both progesterone receptor and cAMP (Kajihara et al. 2013). Interestingly, cAMP induces expression of many transcription factors, including FOXO1, C/EBPb (CCAAT/enhancer-binding protein b), STAT5 (signal transducers and activators of transcription 5) and HOXA11, all of which directly interact with and modulate progesterone receptor (Kajihara et al. 2013). These factors, including progesterone receptor, form multimeric complexes at promoters for genes critical to a decidualized phenotype. Without this synergistic interaction between other cellular signals and transcription factors, progesterone would not exert this important effect on endometrial stroma. Emerging data suggesting that progesterone-driven decidualization may act as a biosensor of embryo quality during early implantation is reviewed by Lucas in this issue (Lucas, 2013).
Another simplification in Figure 1 is that steroid receptors dynamically interact with chromatin in a manner regulated by chromatin remodelling, chaperones, the proteasome and binding of other transcription factors (Grontved and Hager 2012). Oestrogen receptor and progesterone receptor isoforms can only bind DNA if the chromatin structure is open enough to allow access. The areas of open and closed chromatin in a particular cell type in a particular physiological environment are yet another mechanism for tissue-specific actions of oestrogen and progesterone.
In this context, it is important to note that epigenetic mechanisms and microRNA expression may be important modifiers of progesterone action. Initial studies in humans have shown epigenetic changes with cycle phase, including alterations in DNA methyltransferase and histone-modifying enzyme expression (Guo 2012). Initial studies have also shown significant cycle-regulated changes in microRNA through the cycle (Sha et al. 2011; Altmae et al. 2013). The role of microRNA in both normal endometrium and in endometriosis are discussed in the review by Hull and Nisenblat (2013, in this issue).
In addition to their direct, genomic effects, both oestrogen and progesterone also exert rapid, ‘non-classical’ effects on the cell via action at the plasma membrane, via nuclear receptors interacting with other transcription factors or via less well-understood effects on mRNA stability (Figure 2). Oestrogen can act through both membrane-associated oestrogen receptor and a structurally unrelated, integral membrane, G-protein coupled oestrogen receptor, GPR30, to stimulate one or more cytoplasmic signalling cascades in response to oestrogen. The effects of signalling via GPR30 in the endometrium are unclear, but there is a profound cyclic regulation of this receptor (Plante et al. 2012).
Figure 2.
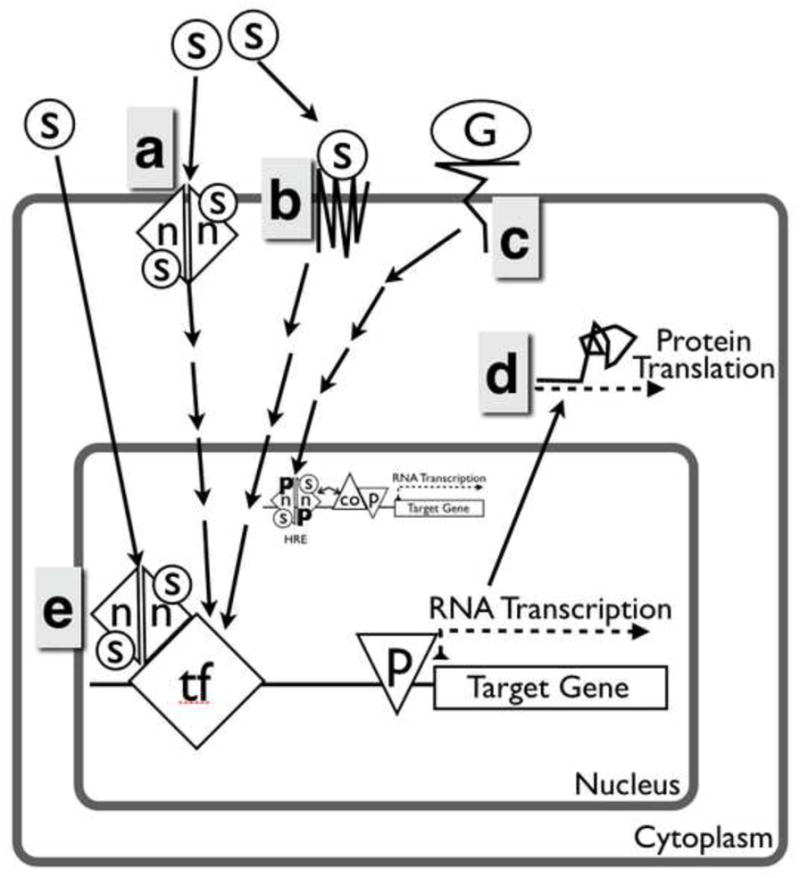
Non-classical actions of nuclear oestrogen and progesterone receptors. (a) Membrane-associated steroid receptors, either isoforms of classical receptors, or (b) unrelated transmembrane receptors recognize steroid hormones and initiate a cytoplasmic signalling cascade. (c) Growth factors signalling can act by causing post-translational modifications of nuclear steroid receptors. (d) Additionally, oestrogen and progesterone can modulate expression by altering mRNA turnover and translation. (e) Alternatively, steroids can bind classical nuclear receptors, which act by binding other proteins rather than DNA. co = co-regulator; G = growth factor; HRE = hormone response element; n = nuclear steroid receptor monomer; ns = non-steroid; p = RNA polymerase; s = steroid; TF = transcription factor.
The non-classical actions of progesterone are less-well understood, but no less complex. As mentioned above, alternative transcription start sites in PGR may result in production of progesterone receptor M or progesterone receptor C, although conflicting evidence exists regarding their relevance in vivo. A separate family of membrane progesterone receptors, mPRα (PAQR VII), mPRβ (PAQR VIII) and mPRγ (PAQR V) that are structurally unrelated to the PGR gene, can also bind progesterone and are thought to activate G-protein coupled signalling pathways (Zhu et al. 2003; Dressing et al. 2011). Significant controversy exists regarding the structure and function of this molecular family. For example, the predicted structure of PAQR family members shows eight transmembrane domains rather than the seven seen in the G-protein coupled receptor family and there is no significant sequence similarity to known G-protein coupled receptors (Moussatche et al. 2012). Furthermore, the PAQR family shows sequence motifs more closely related to alkaline ceramidases and may have similar enzymic activity (Moussatche et al. 2012). Thus, the function of the PAQR family receptors remains to be firmly established and although expression of the mRP family has been shown in the human endometrium, their role in endometrial function remains unclear (Fernandes et al. 2005). Finally, a newly described membrane channel/receptor on human spermatozoa, CatsPer, is capable of binding progesterone (and other compounds released by the cumulus–oocyte complex) and causing calcium influx (Brenker et al. 2012; Lishko et al. 2011). However, CatsPer expression appears to be sperm specific and is, therefore, unlikely to play a role in the endometrium.
Endometrial receptivity to embryo implantation exists for a brief period of time and this timing is driven by time of progesterone exposure, only after sufficient exposure to oestrogen. Given this temporally specific process, it is not surprising that expression and localization of steroid receptors and their co-regulators vary markedly in different menstrual cycle phases (Table 1). In all eutherian mammals studied, oestrogen receptor disappears from the endometrial epithelium at the time of embryo implantation (Donaghay and Lessey 2007). In the human endometrial epithelium, both oestrogen receptor and progesterone receptor immunohistochemical staining diminish markedly during the midsecretory implantation window (Lessey et al. 1988; Young and Lessey 2010). Further analysis of the mid- and late proliferative phases shows that progesterone receptors A and B are easily detected in both epithelial and stromal compartments of the human endometrium (Mote et al. 2000; Wang et al. 1998). In the secretory-phase epithelium, progesterone receptor A expression is virtually absent during the mid- and late secretory phases, while progesterone receptor B expression is maintained at low concentrations through the mid-secretory phase and falls to even lower concentrations by the late secretory phase. In the stroma, progesterone receptor A expression is significantly higher than progesterone receptor B throughout the cycle, although present in low abundance in the late secretory phase. Given the absence or paucity of oestrogen receptor and progesterone receptors A and B in the mid- to late secretory endometrial epithelium, it is likely that epithelial effects of oestrogen and progesterone during these cycle phases results from oestrogen- or progesterone-induced paracrine factors, produced in the stroma and acting on the epithelium, termed oestromedins and progestomedins. Potential human endometrial oestromedins and progestomedins include insulin-like growth factor 1 (Giudice et al. 1993), heparin-binding epidermal growth factor (Leach et al. 1999; Young et al. 2002) and fibroblast growth factor 7 (Koji et al. 1994).
Table 1.
Cyclic steroid receptor expression in the human endometrium.
| Compartment | Phase
|
|||
|---|---|---|---|---|
| Proliferative | Early secretory | Mid-secretory | Late secretory | |
| Epithelium | ||||
| Oestrogen receptor α | ++++ | ++ | − | − |
| Oestrogen receptor β | ++ | ++ | ++ | ++ |
| Progesterone receptor A | +++ | ++ | − | − |
| Progesterone receptor B | +++ | ++ | + | − |
| Stroma | ||||
| Oestrogen receptor α | +++ | ++ | − or + | − |
| Oestrogen receptor β | ++ | + | + | + |
| Progesterone receptor A | ++ | ++ | ++ | ++ |
| Progesterone receptor B | ++ | ++ | + | − |
Role of oestrogen in embryo implantation
While molecular studies of oestrogen and progesterone receptors provide the mechanistic framework for understanding endometrial function, it is the physiological and clinical studies that provide the most practical insight into implantation mechanisms. Oestrogen is essential for endometrial proliferation, as repeatedly demonstrated in humans and experimental animals lacking ovaries and those in whom oestrogen production or action has been prevented.
The role for oestrogen in the secretory phase and in implantation is less clear. In mice, oestrogen appears to be critical to support implantation and early pregnancy (Dey et al. 2004). Interestingly, the decidualized mouse endometrium appears to produce its own oestradiol and does not require corpus luteum-derived oestrogens (Das et al. 2009). As far as is known, there is no substantive data to support this pathway in human decidua.
There are, of course, many differences between human 28-day menstrual cycle and the mouse 4-day oestrus cycle, including circulating oestradiol concentrations. Mouse peak serum oestradiol concentrations in pro-oestrus are equal to or lower than typical perimenstrual nadir concentrations in the human and 10–20 times lower than peak preovulatory concentrations. However, oestrogen action in the human midsecretory phase could possibly occur through other, non-steroidal oestrogen receptor agonists. An eicosanoid, LXA4, was recently shown to bind oestrogen receptor and act as an agonist, and the biosynthetic pathway for LXA4 appears to be present in the human endometrium (Russell et al. 2011). Further work is needed, however, to determine any role that LXA4 might play in the human endometrium.
Studies in women without functional ovaries demonstrate that luteal oestrogen is not necessary for normal day-25 morphology or normal changes in oestrogen receptor and progesterone receptor immunolocalization (de Ziegler et al. 1992). Surprisingly no vaginal spotting was noted in the subjects during the 10 days of progesterone treatment without any oestrogen given. In another study employing oestrogen receptor antagonism with clomiphene begun 2 days after LH surge in a spontaneous cycle and continued until biopsy on day 13 resulted in consistently delayed histological maturation (Fritz et al. 1987). The clomiphene antagonism study findings are echoed by experiments in the bonnet macaque; in these studies, peri-implantation administration of aromatase inhibitor (fadrozole) or oestrogen antagonist (tamoxifen) markedly decreased, but did not eliminate, conception. In another primate study, this time in oophorectomized rhesus macaques, provision of progesterone alone was able to support endometrial receptivity, early post-implantation embryo development and normal pregnancy (Ghosh et al. 1994).
In order to better understand these apparently conflicting data, this study group analysed gonadotrophin-releasing hormone downregulated cycles followed by oestrogen (at varying doses) and progesterone replacement (Groll et al. 2009). Effects on endometrial histology and immunohistochemical staining for integrin subunit β, osteopontin, oestrogen receptor and progesterone receptors A and B were examined. These studies demonstrated no difference in between groups not receiving oestradiol and those receiving physiological or supraphysiological oestradiol.
It is striking that the oestrogen receptor inhibitor studies demonstrate a necessity for luteal-phase oestrogen, while progesterone (with or without oestrogen) replacement studies show no luteal-phase requirement. A possible explanation is that in studies where exogenous progesterone is given, there is sufficient extra-ovarian conversion of progesterone to oestrogen (via testosterone) to maintain endometrial function. The oestradiol antagonism and aromatase inhibition studies might provide a more profound impact by blocking oestrogen action (even that derived in the endometrium). The data in the ovariectomized rhesus macaque, however, remains remarkable, because systemic oestradiol concentrations were measured and shown to be very low, even with administration of progesterone. Taken together, the data suggest that the (human or non-human) primate endometrium appears to function normally with very low concentrations of oestradiol.
Clinical data are also mixed. It is well known that use of gonadotrophin-releasing hormone agonists or antagonists in non-donor IVF cycles results in a shortened luteal phase and possibly other qualitative luteal defects. Thus, luteal support with progesterone and sometimes oestrogen is given. Clinical outcomes are mixed demonstrating a benefit of luteal oestrogen supplementation in IVF (Farhi et al. 2000; Lukaszuk et al. 2005) or no benefit (Smitz et al. 1993; Lewin et al. 1994; Fatemi et al. 2007). The most recent systematic review suggests no overall benefit (Fatemi et al. 2007). Given the experimental results in women and monkeys with absent luteal function and the mixed evidence in clinical trials, any possible clinical benefit of luteal oestrogen support in IVF must accrue only to a small subset of patients.
Role of progesterone in embryo implantation
Progesterone is absolutely required for successful embryo implantation and pregnancy maintenance. In fact, progesterone was discovered because of its effects on the endometrium and early pregnancy survival (Allen and Doisey 1923; Allen and Corner 1929). The effects of progesterone on the endometrium were confirmed in non-human primates (Zuckerman 1937), leading Georgeanna Seeger Jones to characterize patients with possible progesterone deficiency leading to infertility (Jones 1949; Jones 1973). The concept that progesterone insufficiency will cause infertility is logically irrefutable. Progesterone is necessary for implantation and pregnancy survival and thus, at some lower threshold, there will be insufficient progesterone for these functions. However, the methods of diagnosing progesterone insufficiency (or sufficiency) and therefore its role in patients have been controversial.
There are three major contributors to the uncertainty regarding the role of luteal-phase defect in infertility. The first is that the corpus luteum releases progesterone in pulses, which are rapidly cleared from the body, resulting in marked fluctuations of progesterone serum concentrations (Filicori et al. 1984), changing as much as 6-fold within a few hours. The rapidly fluctuating concentrations preclude using individual serum progesterone measurements as a measurement of progesterone sufficiency. Secondly, there is no ‘gold standard’ marker of endometrial receptivity to embryo implantation that would allow evaluation of endometrial function outside of a conception cycle. Current progress in the identification of markers of the receptive endometrium is discussed by Salamonsen et al. (2013, in this issue). Thirdly, there are clear differences between species in the mechanisms regulating embryo implantation, but profound ethical issues prevent systematic study of human embryo and endometrial interactions in vivo.
To avoid the aforementioned barriers to understanding progesterone sufficiency in endometrial function, this study group has utilized a modelled cycle, in which progesterone concentrations are experimentally determined (Figure 3). The controlled cycles are highly similar to endometrial preparation for an oocyte donor IVF cycle, and thus should result in a highly receptive endometrium, if physiological progesterone is provided. The protocol begins with lupron downregulation, followed by transdermal oestrogen replacement at physiological concentrations, followed by oestrogen plus daily i.m. progesterone at physiological and subphysiological concentrations, and subsequent biopsy on day 10 of progesterone treatment. Using this model, endometria from healthy women exposed to physiological concentrations of progesterone (40 mg dose, steady-state concentration about 15–25 ng/ml) were compared with those exposed to subphysiological (10 mg dose, steady-state concentration about 4–6 ng/ml) and assessed histological dating of endometria, immunohistochemistry for endometrial integrins and quantitative real-time PCR analysis for nine putative functional markers (Usadi et al. 2008). However, despite a 4-fold difference in progesterone, none of the assessed markers of endometrial structure and function showed a significant difference between groups. Given the critical importance of progesterone action in the endometrium and the expectation of a dose-dependent response, a further reduction in dose will certainly have effects on both histology and gene expression. However, the data to date clearly demonstrate that progesterone concentrations in the low end of what is seen in ovulatory women do not cause profound changes in human endometrial structure or function. Thus, it would appear that, in normal women, a progesterone dose threshold can be defined, below which consistent alterations in gene expression and in histological maturation can be seen. Since this threshold concentration is below the lowest serum concentrations encountered clinically, the data strongly suggest the following two conclusions: (i) isolated progesterone deficiency is very unlikely to be a cause of infertility in couples; and (ii) normal secretory-phase endometrial structure and function in young healthy women can be achieved across a wide range of progesterone concentrations. It must be noted that these experiments were performed on young healthy women without any evidence of endometriosis or infertility.
Figure 3.
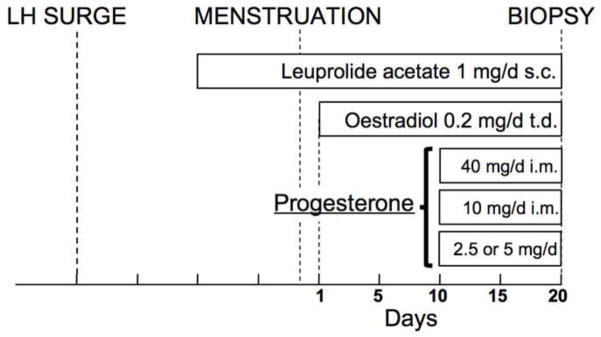
Protocol for modelled cycles (adapted from Usadi et al. 2008).
In all of the above studies, it must also be recognized that local effects of sex steroids can be strongly influenced by local metabolism. For example, a recent study examined oestrogen metabolizing enzyme concentrations in human endometrial tissue as well as serum and tissue oestradiol and oestrone concentrations (Huhtinen et al. 2012). These studies showed marked differences between serum and tissue oestradiol/oestrone ratios, which depended on cycle phase and correlated with the type of 17 -hydroxysteroid dehydrogenase expressed.
Progesterone and endometriosis
Abnormalities in endometrial oestrogen and progesterone action
It has been postulated that women with endometriosis-related infertility may be partially resistant to progesterone actions on the endometrium (Burney et al. 2007; Bulun, Cheng et al. 2010; Fazleabas 2010). Strikingly, the baboon model demonstrates that simply inducing peritoneal lesions can result in changes in progesterone action, consistent with progesterone resistance (Fazleabas, 2010). It is presumed that local inflammation is involved in the observed alterations in progesterone action, although the mechanism for this remains unclear. This hypothesis could explain why some women have persistently delayed histological maturation or persistently abnormal expression of progesterone-regulated genes. If progesterone resistance is truly present in some women, then, depending on the mechanism conferring resistance, such women might achieve normal secretory-phase structure and function with a higher progesterone dose or with treatments targeted at abnormal inflammation.
Given the known mechanisms of progesterone action, resistance might occur through a variety of means. Abnormal expression of specific progesterone receptors is one possible mechanism and women with endometriosis often show failure of mid-secretory downregulation of epithelial progesterone receptor (Lessey, Killam et al. 1988) and evidence for specific suppression of progesterone receptor B, but not progesterone receptor A, at multiple cycle phases (Attia et al. 2000). Another possible mechanism of resistance is an alteration of expression or function of progesterone receptor chaperones and co-chaperones. Overexpression of co-chaperone FKBP51 (Hubler et al. 2003) or lack of co-chaperone FKBP52 (Tranguch et al. 2005; Tranguch et al. 2006; Tranguch et al. 2007) causes progesterone resistance in experimental models. Interestingly, high FKBP51 expression appears to be responsible for the relative progesterone resistance seen in normal squirrel monkeys (Hubler, Denny et al. 2003); however it also leads to glucocorticoid and androgen resistance, which has not been described in women with endometriosis. FKBP52 gene knockout in mice leads to progesterone resistance and embryo implantation failure, which can be overcome with supplemental progesterone (Tranguch, Wang et al. 2007).
Co-regulators, which bind steroid receptors and modify their nuclear effects, are also potential modifiers of progesterone resistance. One co-activator, Hic-5, has recently been shown to be deficient in the stroma of proliferative and late-secretory endometria of women with endometriosis (Aghajanova et al. 2009), and null mutations in the progesterone receptor co-activator, steroid receptor co-activator 2 (SRC-2) cause mice to have severe defects in endometrial receptivity. KLF9 is another progesterone receptor co-regulator, whose absence in the mouse results in partial progesterone resistance, subfertility and reduced HOXA10 expression (Simmen and Simmen 2002; Simmen et al. 2002; Zhang et al. 2003). KLF9 was recently shown to be reduced in a mouse model of endometriosis (Lee et al. 2009) and in infertile women with endometriosis (Pabona et al. 2012). Whether these findings are a root cause or an effect of endometriosis remains to be evaluated, but they lend further credence to the concept of progesterone resistance.
Summary and conclusions
To summarize, although a plethora of hormones are produced by the corpus luteum, the sequential actions of oestrogen and progesterone, without any other corpus luteum hormones, are sufficient to drive a highly receptive endometrium in humans. The mechanisms by which oestrogen and progesterone act are highly complex and involve multiple nuclear receptors as well as recently described membrane receptors. Cell-type specific effects of oestrogen and progesterone depend on differential expression of receptors, chaperones and co-regulators as well as chromatin structure. The role of oestrogen in endometrial proliferation and the importance of that proliferation in embryo implantation are clear. It is also likely that a small amount of oestrogen is necessary for normal luteal-phase endometrium in humans, but the sources of oestrogenic activity and dose requirements remain unclear and the possibility remains that oestrogen or oestrogen-like substances are made locally within the endometrium.
Progesterone is absolutely necessary, during the secretory phase, to allow the endometrium to be receptive to the implanting embryo. However, evidence in normal women suggests that only a very small amount of progesterone is necessary, a concentration achieved by the vast majority or perhaps all ovulatory women. Thus, in women with otherwise normal endometrial function, only small amounts of oestrogen and progesterone appear to be required in the luteal phase for full reproductive function. There is also evidence that some women, especially those with endometriosis-related infertility, may be somewhat resistant to the actions of progesterone and it seems that some of these defects are likely to be overcome with higher concentrations of progesterone, but that hypothesis remains to be proven.
Biography

Steven L Young, MD, PhD is a tenured associate professor and board-certified obstetrician, gynaecologist and reproductive endocrinologist at the University of North Carolina School of Medicine. He is an active reproductive endocrine and infertility clinician and scientist, with scientific interests in the endometrium, endometriosis, progesterone action, and implantation. He has authored over 40 peer-reviewed articles in the medical literature as well as numerous book chapters and abstracts.
Footnotes
Declaration: The author reports no financial or commercial conflicts of interest.
Publisher's Disclaimer: This is a PDF le of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aghajanova L, Velarde MC, Giudice LC. The progesterone receptor coactivator Hic-5 is involved in the pathophysiology of endometriosis. Endocrinology. 2009;150(8):3863–3870. doi: 10.1210/en.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E, Doisey EA. An ovarian hormone: preliminary report on its localizaton, extraction and partial purification, and action in test animals. J Amer Med Asso. 1923;81:819–821. doi: 10.1001/jama.250.19.2681. [DOI] [PubMed] [Google Scholar]
- Allen WM, Corner GW. Physiology of the corpus luteum. III. Normal growth and implantation of embryos after very early ablation of the ovaries, under the influence of extracts of the corpus luteum. Am J Physiol. 1929;88:340. [Google Scholar]
- Altmae S, Martinez-Conejero JA, Esteban FJ, Ruiz-Alonso M, Stavreus-Evers A, Horcajadas JA, Salumets A. MicroRNAs miR-30b, miR-30d, and miR-494 regulate human endometrial receptivity. Reprod Sci. 2013;20(3):308–317. doi: 10.1177/1933719112453507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85(8):2897–2902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- Brenker C, Goodwin N, Weyand I, Kashikar ND, Naruse M, Krahling M, Muller A, Kaupp UB, Strunker T. The CatSper channel: a polymodal chemosensor in human sperm. EMBO J. 2012;31(7):1654–1665. doi: 10.1038/emboj.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Cheng YH, Pavone ME, Xue Q, Attar E, Trukhacheva E, Tokugawa H, Utsunomiya H, Yin P, Luo X, Lin Z, Imir G, Thung S, Su EJ, Kim JT. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Endocrinol. 2010;28:36–43. doi: 10.1055/s-0029-1242991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- Cheung E, Kraus WL. Genomic analyses of hormone signaling and gene regulation. Annu Rev Physiol. 2010;72:191–218. doi: 10.1146/annurev-physiol-021909-135840. [DOI] [PubMed] [Google Scholar]
- Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci U S A. 2009;106(30):12542–12547. doi: 10.1073/pnas.0901647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ziegler D, Bergeron C, Cornel C, Medalie DA, Massai MR, Milgrom E, Frydman R, Bouchard P. Effects of luteal estradiol on the secretory transformation of human endometrium and plasma gonadotropins. J Clin Endocrinol Metab. 1992;74:322–331. doi: 10.1210/jcem.74.2.1730810. [DOI] [PubMed] [Google Scholar]
- Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. Molecular cues to implantation. Endocr Rev. 2004;25(3):341–373. doi: 10.1210/er.2003-0020. [DOI] [PubMed] [Google Scholar]
- Donaghay M, Lessey BA. Uterine receptivity: alterations associated with benign gynecological disease. Semin Reprod Med. 2007;25(6):461–475. doi: 10.1055/s-2007-991044. [DOI] [PubMed] [Google Scholar]
- Dressing GE, Goldberg JE, Charles NJ, Schwertfeger KL, Lange CA. Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications. Steroids. 2011;76(1–2):11–17. doi: 10.1016/j.steroids.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2000;73(4):761–766. doi: 10.1016/s0015-0282(99)00632-9. [DOI] [PubMed] [Google Scholar]
- Fatemi HM, Camus M, Kolibianakis EM, Tournaye H, Papanikolaou EG, Donoso P, Devroey P. The luteal phase of recombinant follicle-stimulating hormone/gonadotropin-releasing hormone antagonist in vitro fertilization cycles during supplementation with progesterone or progesterone and estradiol. Fertil Steril. 2007;87(3):504–508. doi: 10.1016/j.fertnstert.2006.07.1521. [DOI] [PubMed] [Google Scholar]
- Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010;28(1):75–80. doi: 10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- Fernandes MS, Pierron V, Michalovich D, Astle S, Thornton S, Peltoketo H, Lam EW, Gellersen B, Huhtaniemi I, Allen J, Brosens JJ. Regulated expression of putative membrane progestin receptor homologues in human endometrium and gestational tissues. J Endocrinol. 2005;187(1):89–101. doi: 10.1677/joe.1.06242. [DOI] [PubMed] [Google Scholar]
- Filicori M, Butler JP, Crowley WF., Jr Neuroendocrine regulation of the corpus luteum in the human: evidence for pulsatile progesterone secretion. J Clin Invest. 1984;73:1638. doi: 10.1172/JCI111370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz MA, Westfahl PK, Graham RL. The effect of luteal phase estrogen antagonism on endometrial development and luteal function in women. J Clin Endocrinol Metab. 1987;65:1006. doi: 10.1210/jcem-65-5-1006. [DOI] [PubMed] [Google Scholar]
- Ghosh D, De P, Sengupta J. Luteal phase ovarian oestrogen is not essential for implantation and maintenance of pregnancy from surrogate embryo transfer in the rhesus monkey. Hum Reprod. 1994;9:629. doi: 10.1093/oxfordjournals.humrep.a138561. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Dsupin BA, Jin IH, Vu TH, Hoffman AR. Differential expression of messenger ribonucleic acids encoding insulin-like growth factors and their receptors in human uterine endometrium and decidua. J Clin Endocrinol Metab. 1993;76:1115. doi: 10.1210/jcem.76.5.8496300. [DOI] [PubMed] [Google Scholar]
- Groll JM, Usadi RS, Lessey BA, Lininger R, Young SL, Fritz MA. Effects of variations in serum estradiol concentrations on secretory endometrial development and function in experimentally induced cycles in normal women. Fertil Steril. 2009;92(6):2058–2061. doi: 10.1016/j.fertnstert.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grontved L, Hager GL. Impact of chromatin structure on progesterone receptor signaling: transition from local to global analysis. Mol Cell Endocrinol. 2012;357(1–2):30–36. doi: 10.1016/j.mce.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SW. The endometrial epigenome and its response to steroid hormones. Mol Cell Endocrinol. 2012;358(2):185–196. doi: 10.1016/j.mce.2011.10.025. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Korach KS. Oestrogen receptor knockout mice: roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction. 2003;125(2):143–149. doi: 10.1530/rep.0.1250143. [DOI] [PubMed] [Google Scholar]
- Huang P, Chandra V, Rastinejad F. Structural overview of the nuclear receptor superfamily: insights into physiology and therapeutics. Annu Rev Physiol. 2010;72:247–272. doi: 10.1146/annurev-physiol-021909-135917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144(6):2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- Huhtinen K, Desai R, Stahle M, Salminen A, Handelsman DJ, Perheentupa A, Poutanen M. Endometrial and endometriotic concentrations of estrone and estradiol are determined by local metabolism rather than circulating levels. J Clin Endocrinol Metab. 2012;97(11):4228–4235. doi: 10.1210/jc.2012-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull ML, Nisenblat V. Tissue and circulating microRNAs influence reproductive function in endometrial disease. Reprod Biomed. 2013 doi: 10.1016/j.rbmo.2013.07.012. Online XXXX. [DOI] [PubMed] [Google Scholar]
- Jacobsen BM, Horwitz KB. Progesterone receptors, their isoforms and progesterone regulated transcription. Mol Cell Endocrinol. 2012;357(1–2):18–29. doi: 10.1016/j.mce.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones GS. Some newer aspects of management of infertility. JAMA. 1949;141:1123–1129. doi: 10.1001/jama.1949.02910160013004. [DOI] [PubMed] [Google Scholar]
- Jones GS. Luteal phase insufficiency. Clin Obstet Gynecol. 1973;16:255–273. doi: 10.1097/00003081-197309000-00016. [DOI] [PubMed] [Google Scholar]
- Kajihara T, Brosens JJ, Ishihara O. The role of FOXO1 in the decidual transformation of the endometrium and early pregnancy. Med Mol Morphol 2013. 2013 Feb 5; doi: 10.1007/s00795-013-0018-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Katsu Y, Kohno S, Hyodo S, Ijiri S, Adachi S, Hara A, Guillette LJ, Jr, Iguchi T. Molecular cloning, characterization, and evolutionary analysis of estrogen receptors from phylogenetically ancient fish. Endocrinology. 2008;149(12):6300–6310. doi: 10.1210/en.2008-0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koji T, Chedid M, Rubin JS, Slayden OD, Csaky KG, Aaronson SA, Brenner RM. Progesterone-dependent expression of keratinocyte growth factor mRNA in stromal cells of the primate endometrium: Keratinocyte growth factor as a progestomedin. J Cell Biol. 1994;125:393. doi: 10.1083/jcb.125.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach RE, Khalifa R, Ramirez ND, Das SK, Wang J, Dey SK, Romero R, Armant DR. Multiple roles for heparin-binding epidermal growth factor-like growth factor are suggested by its cell-specific expression during the human endometrial cycle and early placentation. J Clin Endocrinol Metab. 1999;84(9):3355–3363. doi: 10.1210/jcem.84.9.5980. [DOI] [PubMed] [Google Scholar]
- Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod. 2009;80(1):79–85. doi: 10.1095/biolreprod.108.070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty KS., Jr Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab. 1988;67:334–340. doi: 10.1210/jcem-67-2-334. [DOI] [PubMed] [Google Scholar]
- Lewin A, Benshushan A, Mezker E, Yanai N, Schenker JG, Goshen R. The role of estrogen support during the luteal phase of in vitro fertilization-embryo transplant cycles: a comparative study between progesterone alone and estrogen and progesterone support. Fertil Steril. 1994;62(1):121–125. doi: 10.1016/s0015-0282(16)56826-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Burns KA, Arao Y, Luh CJ, Korach KS. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor alpha and beta in vitro. Environ Health Perspect. 2012;120(7):1029–1035. doi: 10.1289/ehp.1104689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, I, Botchkina L, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471(7338):387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Lucas ES. Epigenetic effects on the embryo as a result of periconceptional environment and ART. Reprod Biomed. 2013 doi: 10.1016/j.rbmo.2013.06.003. Online XXXX. [DOI] [PubMed] [Google Scholar]
- Lukaszuk K, Liss J, Lukaszuk M, Maj B. Optimization of estradiol supplementation during the luteal phase improves the pregnancy rate in women undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2005;83(5):1372–1376. doi: 10.1016/j.fertnstert.2004.11.055. [DOI] [PubMed] [Google Scholar]
- Mote PA, Balleine RL, McGowan EM, Clarke CL. Heterogeneity of progesterone receptors A and B expression in human endometrial glands and stroma. Hum Reprod. 2000;15(Suppl 3):48–56. doi: 10.1093/humrep/15.suppl_3.48. [DOI] [PubMed] [Google Scholar]
- Moussatche P, Lyons TJ. Non-genomic progesterone signalling and its non-canonical receptor. Biochem Soc Trans. 2012;40(1):200–204. doi: 10.1042/BST20110638. [DOI] [PubMed] [Google Scholar]
- Ogle TF. Progesterone-action in the decidual mesometrium of pregnancy. Steroids. 2002;67(1):1–14. doi: 10.1016/s0039-128x(01)00137-4. [DOI] [PubMed] [Google Scholar]
- Pabona JM, Simmen FA, Nikiforov MA, Zhuang D, Shankar K, Velarde MC, Zelenko Z, Giudice LC, Simmen RC. Kruppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2012;97(3):E376–392. doi: 10.1210/jc.2011-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante BJ, Lessey BA, Taylor RN, Wang W, Bagchi MK, Yuan L, Scotchie J, Fritz MA, Young SL. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod Sci. 2012;19(7):684–693. doi: 10.1177/1933719111431000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R, Gori I, Pellegrini C, Kumar R, Achtari C, Canny GO. Lipoxin A4 is a novel estrogen receptor modulator. FASEB J. 2011;25(12):4326–4337. doi: 10.1096/fj.11-187658. [DOI] [PubMed] [Google Scholar]
- Salamonsen LA, Edgell TA, Rombauts LJ. Assessing receptivity in the endometrium: the need for a rapid, non-invasive test. Reprod Biomed. doi: 10.1016/j.rbmo.2013.05.014. Online XXXX. [DOI] [PubMed] [Google Scholar]
- Samalecos A, Gellersen B. Systematic expression analysis and antibody screening do not support the existence of naturally occurring progesterone receptor (progesterone receptor)-C, progesterone receptor M, or other truncated progesterone receptor isoforms. Endocrinology. 2008;149(11):5872–5887. doi: 10.1210/en.2008-0602. [DOI] [PubMed] [Google Scholar]
- Sha AG, Liu JL, Jiang XM, Ren JZ, Ma CH, Lei W, Su RW, Yang ZM. Genome-wide identification of micro-ribonucleic acids associated with human endometrial receptivity in natural and stimulated cycles by deep sequencing. Fertil Steril. 2011;96(1):150–155. e155. doi: 10.1016/j.fertnstert.2011.04.072. [DOI] [PubMed] [Google Scholar]
- Simmen RC, Simmen FA. Progesterone receptors and Sp/Kruppel-like family members in the uterine endometrium. Front Biosci. 2002;7:d1556–1565. doi: 10.2741/simmen. [DOI] [PubMed] [Google Scholar]
- Simmen RC, Zhang XL, Michel FJ, Min SH, Zhao G, Simmen FA. Molecular markers of endometrial epithelial cell mitogenesis mediated by the Sp/Kruppel-like factor BTEB1. DNA Cell Biol. 2002;21(2):115–128. doi: 10.1089/104454902753604998. [DOI] [PubMed] [Google Scholar]
- Smitz J, Bourgain C, Van Waesberghe L, Camus M, Devroey P, Van Steirteghem AC. A prospective randomized study on oestradiol valerate supplementation in addition to intravaginal micronized progesterone in buserelin and HMG induced superovulation. Hum Reprod. 1993;8(1):40–45. doi: 10.1093/oxfordjournals.humrep.a137871. [DOI] [PubMed] [Google Scholar]
- Su S, Blackwelder AJ, Grossman G, Minges JT, Yuan L, Young SL, Wilson EM. Primate-specific Melanoma Antigen-A11 Regulates Isoform-specific Human Progesterone Receptor-B Transactivation. J Biol Chem. 2012;287(41):34809–34824. doi: 10.1074/jbc.M112.372797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderam S, Chang J, Flowers L, Kulkarni A, Sentelle G, Jeng G, Macaluso M. Assisted reproductive technology surveillance--United States, 2006. MMWR Surveill Summ. 2009;58(5):1–25. [PubMed] [Google Scholar]
- Taylor AH, McParland PC, Taylor DJ, Bell SC. The cytoplasmic 60 kDa progesterone receptor isoform predominates in the human amniochorion and placenta at term. Reprod Biol Endocrinol. 2009;7:22. doi: 10.1186/1477-7827-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A. 2005;102(40):14326–14331. doi: 10.1073/pnas.0505775102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranguch S, Smith DF, Dey SK. Progesterone receptor requires a co-chaperone for signalling in uterine biology and implantation. Reprod Biomed Online. 2006;13(5):651–660. doi: 10.1016/s1472-6483(10)60655-4. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Wang H, Daikoku T, Xie H, Smith DF, Dey SK. FKBP52 deficiency-conferred uterine progesterone resistance is genetic background and pregnancy stage specific. J Clin Invest. 2007;117(7):1824–1834. doi: 10.1172/JCI31622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usadi RS, Groll JM, Lessey BA, Lininger RA, Zaino RJ, Fritz MA, Young SL. Endometrial development and function in experimentally induced luteal phase deficiency. J Clin Endocrinol Metab. 2008;93(10):4058–4064. doi: 10.1210/jc.2008-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev. 2011;(10):CD009154. doi: 10.1002/14651858.CD009154.pub2. [DOI] [PubMed] [Google Scholar]
- Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB. ‘5’-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated. C’-receptor and unique A-receptor messages. Molecular Endocrinology. 1990;4:1833. doi: 10.1210/mend-4-12-1833. [DOI] [PubMed] [Google Scholar]
- Wang H, Critchley HO, Kelly RW, Shen D, Baird DT. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod. 1998;4(4):407–412. doi: 10.1093/molehr/4.4.407. [DOI] [PubMed] [Google Scholar]
- Young SL, Lessey BA. Progesterone function in human endometrium: clinical perspectives. Semin Reprod Med. 2010;28(1):5–16. doi: 10.1055/s-0029-1242988. [DOI] [PubMed] [Google Scholar]
- Young SL, Lessey BA, Fritz MA, Meyer WR, Murray MJ, Speckman PL, Nowicki BJ. In vivo and in vitro evidence suggest that HB-EGF regulates endometrial expression of human decay-accelerating factor. J Clin Endocrinol Metab. 2002;87(3):1368–1375. doi: 10.1210/jcem.87.3.8350. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RC. Selective interactions of Kruppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem. 2003;278(24):21474–21482. doi: 10.1074/jbc.M212098200. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA. 2003;100:2247–2242. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman S. The Menstrual Cycle of the Primates XII--The Interaction of Ovarian Hormones in the Cycle. Proceedings of the Royal Society of London, Series B, Biological Sciences. 1937;124(835):150–162. [Google Scholar]