Abstract
Neuroimmune semaphorin 4A (Sema4A) has been shown to play an important costimulatory role in T cell activation and regulation of Th1-mediated diseases such as multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE), and experimental autoimmune myocarditis (EAM). Sema4A has three functional receptors, Tim-2 expressed on CD4+ T cells, Th2 cells in particular, and Plexin B1 and D1 predominantly expressed on epithelial and endothelial cells, correspondingly. We recently showed that Sema4A has a complex expression pattern in lung tissue in a mouse model of asthma. We and others have shown that corresponding Plexin expression can be found on immune cells as well. Moreover, we demonstrated that Sema4A-deficient mice displayed significantly higher lung local and systemic allergic responses pointing to its critical regulatory role in the disease. To determine the utility of Sema4A as a novel immunotherapeutic, we introduced recombinant Sema4A protein to the allergen-sensitized WT and Sema4A−/− mice before allergen challenge. We observed significant reductions in the allergic inflammatory lung response in Sema4A-treated mice as judged by tissue inflammation including eosinophilia and mucus production. Furthermore, we demonstrated that in vivo administration of anti-Tim2 Ab led to a substantial upregulation of allergic inflammation in WT mouse lungs. These data highlight the potential to develop Sema4A as a new therapeutic for allergic airway disease.
Keywords: Asthma, mouse model, Sema4A, Tim-2, recombinant protein, in vivo
1. Introduction
Asthma affects approximately 300 million people worldwide and the number is projected to reach 400 million by 2025 [1]. Inhalations of allergen stimulates lung epithelial cells and innate immune cells such as dendritic cells (DC), nuocytes, basophils, mast cells, and macrophages [2-6]. Among lung innate cells, DC are unmatched in allergen-presenting function leading to the generation and activation of allergen-specific CD4+ T cells of the Th2 phenotype. These Th2 cells produce Th2 cytokines including, but not limited to, IL-4, -5, and -13. Th2 cytokines act on multiple cell types locally in the lungs and in draining lymph nodes to initiate and propagate the hallmark features of asthma such as pulmonary inflammation, bronchoconstriction, and mucus hypersecretion [7-11]. Asthma is a complex disease that is not yet preventable or curable [12-14]. Corticosteroids and bronchodilators are currently being used to alleviate the disease symptoms [15, 16]. To improve current treatment options and make them more specific, new pathways and therapies continue to be explored [17-20]. One such novel pathway relates to the T cell costimulatory molecule Sema4A [21, 22] which, in addition to its previously defined T cell co-stimulatory function [21, 22], also has many other cellular and tissue effects such as neuron axonal guidance [23, 24], photoreceptor survival [25, 26], and angiogenesis [27]. We previously have shown that Sema4A−/− mice display a significantly higher acute allergic inflammatory response as compared to WT mice [28]. Therefore, in contrast to its stimulatory role in Th1-mediated diseases, Sema4A plays a downregulatory role in allergic asthma severity. In our current work we reintroduced or introduced recombinant Sema4A to Sema4A−/− or WT mice before Ag challenge and observed its significant downregulatory effect on inflammation severity. This study will help to establish Sema4A as a potential drug for allergic asthma and to develop additional Sema4A-based measures for disease immunotherapy.
2. Materials and Methods
2.1. Mice
The generation and characterization of Sema4A−/− mice has been described in detail previously [22]. C57BL/6 mice (WT) were purchased from Taconic. Mice were bred and maintained under specific pathogen-free conditions within the animal facility at University of Maryland School of Medicine. All procedures on mice were performed according to the animal protocol approved by University of Maryland School of Medicine Animal Care and Use Committee. Age- and sex-matched mice were used in all experiments.
2.2. Anesthetic
Avertin in dose of 0.3 mg/kg or 2 mg/kg by i.p. injection was used as previously described [29] to anesthetize or euthanize the mice, correspondingly.
2.3. Experimental model of acute asthma
Mice were treated with chicken OVA (Sigma) as described previously [30]. Briefly, 100 μg OVA/2 mg Alum/200 μl was delivered intraperitoneally (i.p.) to WT and Sema4A−/− mice on days 0 and 5. Control mice were injected with sterile endotoxin-free PBS/Alum. On days 12 and 14, mice received a 40 min aerosol challenge of either PBS (control animals) or 1% (w/v) OVA, using Invacare Envoy aerosol compressor. Twenty-four hours after the last nebulization, the AHR in response to increasing doses of methacholine was measured as an indicator of changes in the airway resistance. Mice were euthanized 48h after the last OVA nebulization for other analyses.
2.4. Experimental model of chronic asthma
Mice were treated with OVA as described for an acute model of experimental asthma. After two OVA aerosol challenges on days 12 and 14 of the experimental protocol, mice received the additional challenges on days 21 and 28. AHR measurements were performed 24 h later and mice were euthanized for the allergic response assessments on day 30.
2.5. In vivo recombinant protein or Ab administration
Four micrograms of a recombinant endotoxin-free Sema4A (rSema4A) with N-terminal GST tag, full-length, NP_071762.2.1a.a. – 761 a.a. (Abnova or Novus Biologicals) in 50 μl of endotoxin-free sterile PBS were administered i.n. to the anaesthetized Sema4A−/− mice 24h before first OVA challenge in an acute model of asthma and to WT mice 24h before last allergen challenge in a chronic model of asthma. Fifty microliters of PBS administration per mouse served as a control.
Goat anti-mouse Tim-2 Ab (AF1885, R&D Systems) were purified from trehalose by centrifugation using Amicon Ultra 3kDa tubes (Millipore). Five hundred μg of Ab in 200 μl of PBS was administered by i.p. injection to WT mice before each allergen challenge. Goat IgG isotype injections were administered to the control group of mice.
2.6. Airway hyperreactivity measurements
AHR measurements to methacholine challenges were performed 24h after the last Ag nebulization using either non-invasive (BUXCO Electronics) or invasive (FlexiVent, SCIREQ) techniques as previously described [31, 32].
2.7. Histochemistry
The Core Facility at the Center for Vascular and Inflammatory Diseases was used for histochemistry (H&E and PAS stains) of deparaffinized lung tissues [33].
2.8. Cellular composition and cytokine-chemokine content in bronchoalveolar lavages and serum
Bronchoalveolar lavages (BAL) were performed 48h after last Ag nebulization, cells and BALF collected, cytospin made and cell counts performed as described earlier [28, 33]. Five times (5×) concentrated (Amicon Ultra 3K membranes, Millipore) BAL fluid and intact serum sample’s cytokine and chemokine levels were determined using Searchlight Proteome Array (Aushon), ELISA kits (R&D Systems), and CBA kits (552364, BD Biosciences) [28, 33]. Array and CBA data were analyzed using the ArrayVision software and FlowJo plus BD CBA softwares, correspondingly. All ELISA plates were read on the Emax Precision Microplate reader (Molecular Devices) using the manufacturer specified wavelengths for each assay.
2.9. Flow cytometry
Single cell suspensions from mouse lungs were obtained as described previously [34]. The staining of lung digest cells for FACS analysis was performed as described elsewhere. Cells were preincubated with anti-CD16/CD32 (2.4G2) mAb for blocking cell surface FcR. Anti-GR1-FITC or –PE (Ly-6G and Ly-6C) mAbs were obtained from BD Biosciences Pharmingen (San Diego, CA). FITC-conjugated Sema4A (5E3) mAb was obtained from Medical & Biological Laboratories, Japan. Rat IgG1-FITC (R3-34) was used as isotype control. Biotinylated antimouse Tim-2 Ab (RMT2-1, Biolegend) with SAV-PerCP (BD Biosciences Pharmingen) as the second step reagent were used for the cell surface Tim-2 visualization. Cells gated by forward-and side-scatter parameters were analyzed on either FACSCalibur flow cytometer using either CELLQuest or Flow Jo softwares.
2.10. Spleen mononuclear cell proliferation
Cell proliferation was measured as described [28, 33, 35] using a [H]3 incorporation assay. Briefly, single cell suspensions were prepared from spleens of chronically OVA-challenged mice on a day 5 post-challenge. Spleen MNC were plated to a density of 1 × 106 cells/200μl in 96-well tissue culture plates (Cellstar, Greiner) and stimulated with either OVA (100 mg/ml) or OVA323-339 peptide (200μg/ml; GenScript USA Inc.) as previously described [28]. After 72h of incubation, [H]3 thymidine (Perkinson-Maer) was added to the wells and plates were harvested on a Packard Filtermate harvester (Packard Instruments) 24h later.
2.11. Statistics
Data were summarized as mean ± SEM. To calculate the significance levels between the experimental groups, the Student’s t test (Microsoft Excel) and Mann-Whitney test (Prizm-4) were performed.
3. Results
3.1. Administration of Sema4A into the airways diminishes allergic lung response
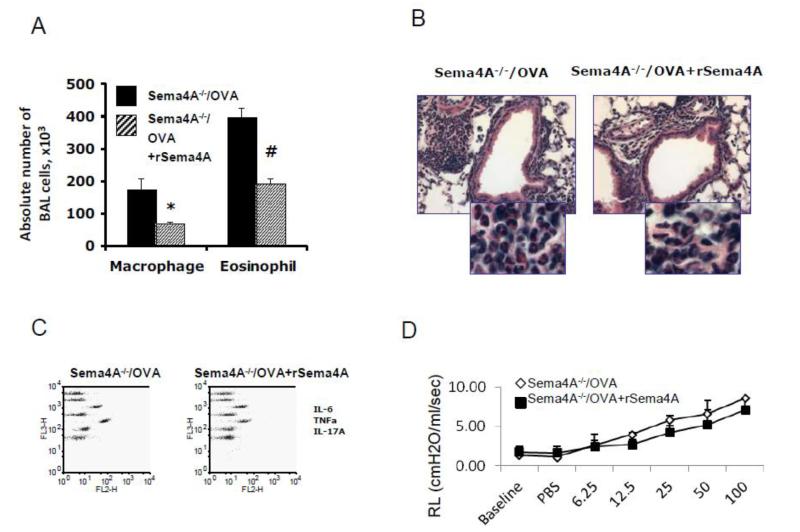
We previously demonstrated that Sema4A is weakly expressed in lung tissue and moderately upregulated by OVA [36]. Furthermore, our recent report also indicates that Sema4A reduces allergic response [28]. On the basis of these data we tested the hypothesis that introduction of recombinant Sema4A into airways would exhibit a therapeutic effect. A single low dose (4 μg/50 μl PBS/mouse) of rSema4A administration 24h before the first allergen challenge was sufficient to downregulate the numbers of BAL eosinophils (398,200 ± 29,100 vs 191,100 ± 16,000, PBS-treated vs rSema4A-treated OVA-challenged Sema4A−/− mice, p<0.003) and macrophages (173,000 ± 36,500 vs 67,800 ± 5,800, p<0.035) (Fig. 1 A). The reduced number of BAL cells correlated with reduced lung tissue inflammation (Fig. 1 B) as well as a reduction in the levels of BALF IL-6, IL-17A and TNFα (Fig. 1 C). It is important to note that that administration of rSema4A to Sema4A−/− mice exhibited a potent anti-inflammatory effect in an acute experimental allergic asthma model without affecting lung physiology (Fig. 1 D). This shows that Sema4A regulates the inflammatory arm of allergic response.
Figure 1.
Local intrarespiratory re-introduction of a recombinant Sema4A protein downregulates acute allergic airway inflammation in Sema4A−/− mice. Sema4A−/− mice were immunized with OVA as shown in Supplemental Figure S1 A. Recombinant Sema4A protein was delivered intranasally to the respiratory system 24 hours before the first OVA aerosol challenge. Note downregulatory effect of rSema4A on BAL macrophage and eosinophil numbers (A) and the extent of lung tissue inflammation (B). *,#p<0.05, macrophages and eosinophils, PBS vs rSema4A treated OVA-challenged Sema4A−/− mice, correspondingly. (C) Cytokine contents in 5× concentrated BALF were measured using the CBA proinflammatory cytokine and Th1/Th2/Th17 kits. Data are shown as the FlowJo generated dot plots for pooled samples from 2 mice in one of 2 representative experiments. (D) Single dose of rSema4A has no effect of lung resistance measured by an invasive (FlexiVent, SCIREQ) technique.
3.2. Sema4A restrains a chronic experimental model of asthma
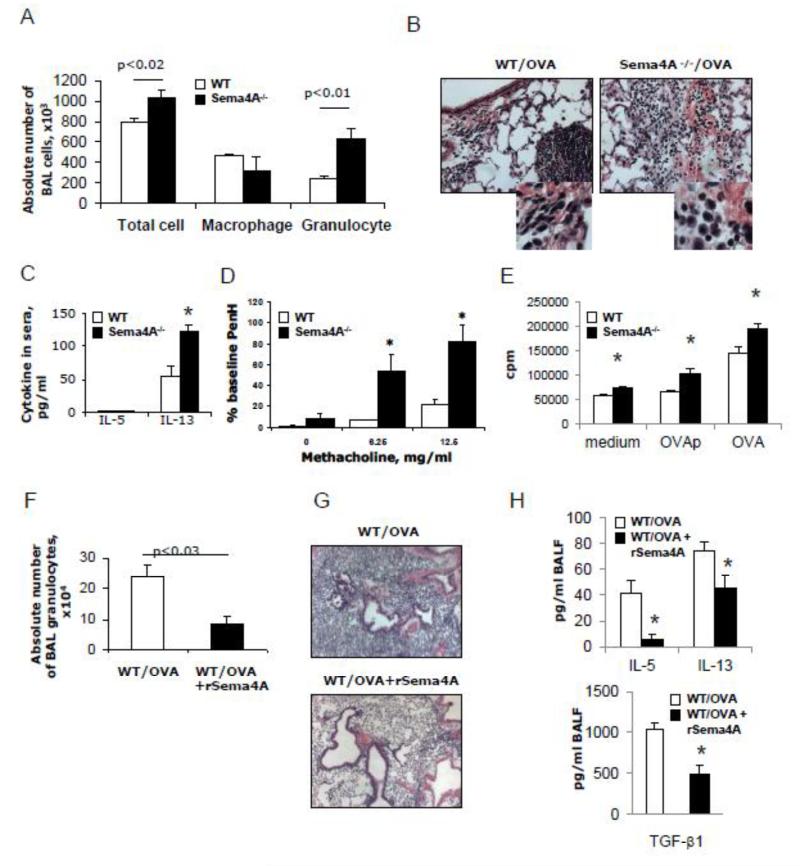
We previously reported that allergic airway response in the mouse lungs has its peaks of eosinophilia at 48h after allergen challenge, as characterized by eosinophilia, and which slowly resolves with time over a period of 6 days [37]. We included into an acute model two more allergen challenges with weekly intervals (Supplemental Figure S1 B). This let us to study the role of Sema4A in a more chronic disease setting. A heightened allergic inflammatory response was observed in the lungs of Sema4A−/− mice as compared to the similarly treated WT mice as characterized by increased numbers of granulocytes (eosinophils and neutrophils) (246,933 ± 23,403 vs 635,920 ± 101,800, WT vs Sema4A−/− mice, correspondingly, p<0.01) (Fig. 2 A). Increased lung tissue inflammatory responses in knockout mice were associated with higher sera IL-13 levels (Fig. 2 B-C). Interestingly, the difference in an index of airway obstruction, PenH, was observed between WT and Sema4A−/− mice only at the low doses (6.25 and 12.5 mg/ml) of methacholine (Fig. 2 D) whereas at the high doses the effect was not different between experimental groups (data not shown). These data need to be clarified by utilizing a more sensitive FlexiVent method [38]. Nevertheless, there is a possibility that both groups of mice experienced similar levels of respiratory distress at high doses of methacholine due to a pre-existing airway hyperreactivity as it has been shown for people with asthma [39]. Sema4A-/spleen MNC proliferated more robustly to either OVA peptide or OVA protein re-challenge as compared to WT cells (Fig. 2 E). As illustrated in Figure 2 F, the intranasal application of rSema4A to WT mice prior to a single delayed last allergen challenge in this chronic model was effective in the downregulation of OVA-induced inflammatory response. It reduced an absolute granulocyte number down from 239,033 ± 39,916 (in PBS-treated mice) to 87,500 ± 25,758 cells. This rSema4A-dependent reduction in inflammatory response in the recurrently allergen challenged WT mice was also noted on the lung tissue slides (Fig. 2 G). These data indicate that rSema4A has a potent anti-inflammatory effect in a chronic experimental allergic asthma model.
Figure 2.
Sema4A downregulates the chronic experimental allergic inflammation and Th2 cytokine production. WT and Sema4A−/− mice were immunized with OVA as shown in Supplemental Figure S1 B. (A) The average numbers (n=3-5) of BAL cells ± SEM in one of two representative experiments are shown. Statistically significant differences in absolute numbers of BAL total cells and granulocytes between WT and Sema4A−/− mice are shown. (B) Representative lung tissue histology (H&E, ×40; inserts, ×100) from OVA-challenged WT and Sema4A−/− mice. (C) Sera cytokine levels were measured using Quatikine ELISA kits. *p<0.015, WT vs Sema4A−/− mice. Of note, no detectable levels of IFNγ, IL-4, and IL-5 were found in sera of both mouse lines. (D) AHR was measured and is represented as the percent increase in PenH over a baseline (n = 2-5 mice/group from the separate 2 experiments combined). *p< 0.05, OVA-challenged WT mice versus Sema4A−/− for the corresponding doses of methacholine. (E) Sema4A deficiency leads to an upregulation of T cell proliferation to the in vitro Ag re-challenge. (F) rSema4A application to WT mice before the last allergen challenge (Supplemental Fig. S1B) leads to a downregulation of lung inflammation. (G) Representative H&E stains for tissue inflammation. Magnification used for pictures is 10x. (H) Downregulation of BALF IL-5, IL-13, and TGF-β1 cytokines by i.n. rSema4A application. p<0.05, WT/OVA mice vs WT/OVA + rSema4A mice.
3.3. Treatment with anti-Tim2 Ab increases the severity of allergic inflammatory response
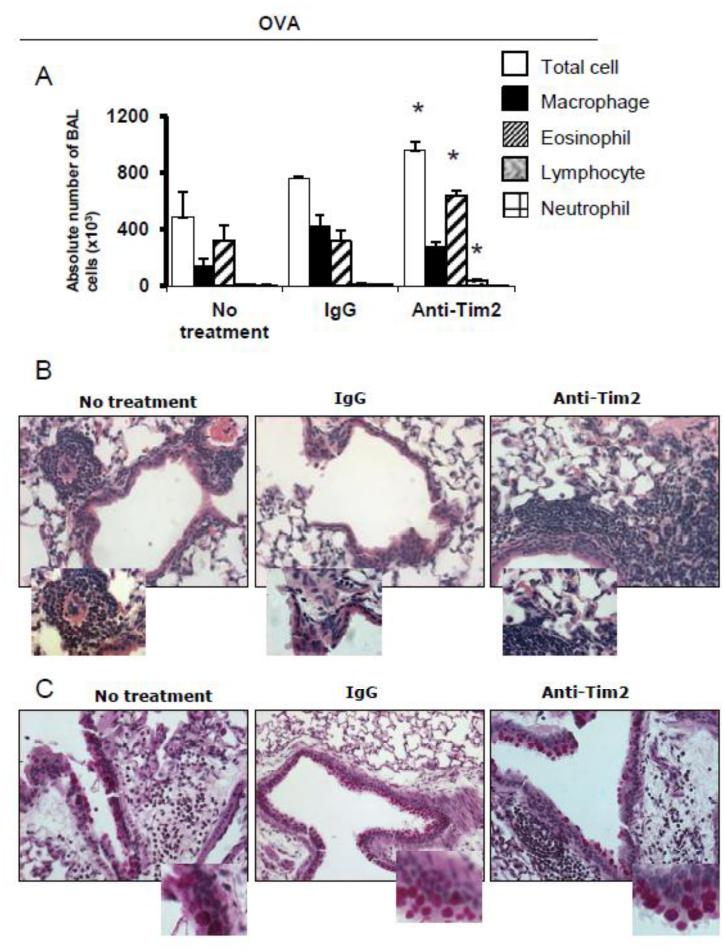
Rennert and associates reported that Tim-2−/− mice exhibited a heightened allergic airway response [40] what supports our contention that Sema4A is a negative regulator of Th2 cell activation acting through Tim-2. To determine the role that Tim-2 played in the allergen challenge phase of the inflammatory lung response, we treated WT mice with anti-Tim2 Ab 24h prior to allergen challenges and compared the lung inflammation results with those obtained using Ab-untreated mice or isotype control Ab treated mice. We have noticed a gap between heightened lung inflammation found in Ab-treated and isotype control-treated mice (Fig. 3A and B). The overall number of infiltrating cells increased from 487,500 ± 174,000 to 958,300 ± 186,200 (p<0.065) in response to anti-Tim-2 administration. However, the lung eosinophil number almost doubled in anti-Tim-2 treated mice (322,000 ± 133,600 and 642,033 ± 113,000 cells, untreated vs Ab treated WT mice, p< 0.04). Lung lymphocyte number was also affected by the in vivo interference with Tim-2 pathway (12,185 ± 3,200 vs 38,233 ± 8,800, correspondingly, p<0.04). However, the index of airway obstruction to all doses of methacholine used for challenges did not differ significantly between anti-Tim-2 treated and untreated groups (Fig. 3 D). Therefore, Sema4A-Tim-2 interaction likely plays a critical role in regulating the severity of inflammation in asthma.
Figure 3.
Systemic anti-Tim2 Ab treatment before allergen challenges upregulates the severity of allergic inflammation in WT mice. WT mice were immunized with OVA and anti-Tim2 Ab was injected i.p. 24h before allergen challenges on days 11 and 13 of experimental protocol as shown in Supplemental Figure S1 C. (A) The average numbers (n=3-5) of BAL cells ± SEM are shown. *p< 0.05, differences in absolute numbers of BAL eosinophils and lymphocytes, anti-Tim2 treated vs untreated and isotype control Ab treated WT mice. (B) Representative lung tissue histology pictures from slides (H&E, ×40; inserts, ×100) of OVA-challenged untreated, Ab and isotype control treated WT mice. (C) Representative slides of PAS-stained lung tissues (×40; inserts, ×100) of experimental groups. (D) Anti-Tim-2 Ab treatment has no effect on AHR in OVA-challenged WT mice as measured by Buxco.
3.4. Expression of Sema4A and Tim-2 on mouse lung granulocytes
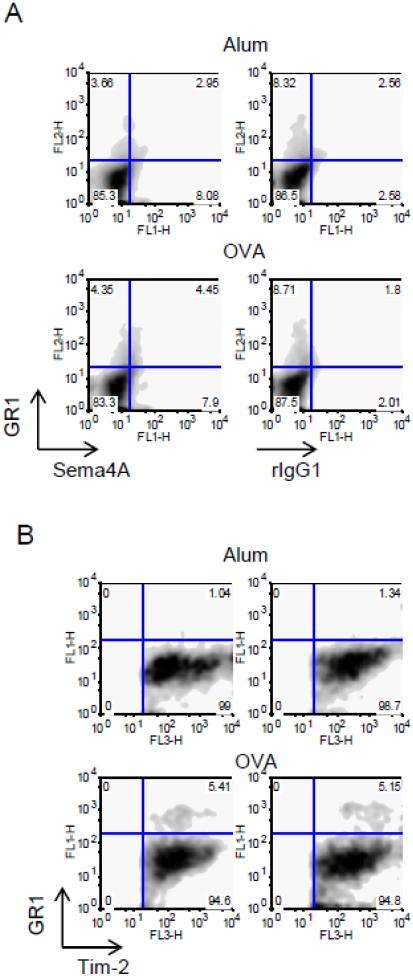
Granulocytes (neutrophils, eosinophils, basophils) play important roles in allergic asthma. GR-1 is highly expressed on neutrophils and whereas eosinophils show an intermediate level of GR-1 expression. To analyze Sema4A and Tim-2 expression on these granulocytes, we used mAb to GR-1 in flow cytometry of single cell suspensions obtained from either Alum- or OVA-treated mice. Around 50% of GR1+ cells in Alum- or OVA-treated mice co-express Sema4A (Fig. 4 A). The relative numbers of GR1+Tim2+ cells increased from 1.04-1.34% in Alum-treated WT mice to 5.15-5.41% in OVA-treated counterparts (Fig. 4 B). Therefore, Sema4A-Tim2 pathway may play a functional role in the subsets of granulocytes.
Figure 4.
Subsets of lung GR1+ cells express Sema4A and Tim-2. Single cell suspensions obtained from lungs of Alum- or OVA-treated mice were analyzed for GR1, Sema4A, and Tim-2 expression using corresponding Abs defined in Materials and Methods section. (A) Approximately a half of lung GR1+ cells co-expressed Sema4A. (B) Tim-2+ cells were selected for further analysis based on SSC-Fl3 dot plots (not shown) and then re-gated on Fl1-Fl3 density plots. Note that GR1+Tim2+ cells appear only under inflammatory lung conditions.
4. Discussion
In this study we show recombinant Sema4A protein significantly downregulates the inflammatory response observed in both acute and chronic mouse models of experimental asthma. We demonstrated here that an application of a single dose of this protein before allergen challenge significantly reduced many features of an allergic lung response such as inflammatory cell infiltration, mucus production, and inflammatory cytokine secretion. The observed in vivo critical regulatory effect of Sema4A in acute and chronic allergic responses suggests that this pathway may be used for an immunotherapeutic asthma intervention. One of such proposed intervention is related to the described here use of rSema4A instillations.
The allergic subtype of asthma is a Th2-mediated disease. Th2 cytokines (IL-4, IL-5, and IL-13) play critical role in the disease pathogenesis whereas Th1 cytokine (IFNγ) can counterbalance their effects. We found a selective upregulation of lung and serum IL-13 expression in allergen-treated Sema4A deficient mice as compared to WT mice ([28] and Fig. 2F). Moreover, BALF IL-13 levels were significantly downregulated in rSema4A-treated WT mice as compared to PBS-treated control animals (45.5 ± 19.2 vs 75.9 ± 19.6 pg/ml, correspondingly). The mechanism of a downregulatory effect of Sema4A on the in vivo IL-13 production needs to be determined. Nevertheless, our current data and previous observations [28] suggest that this effect may be associated with IL-13 production by innate immune cells such as mast cells, basophils, and nuocytes which had been shown to be the major producers of this Th2 cytokine in allergic asthma [41-43]. In addition to that, we observed a downregulatory effect of rSema4A application on lung TNFa and IL-17A production. The pathologic effects of both cytokines in allergic asthma have been shown and discussed in many recent publications [28, 44-49].
The role of Sema4A in Th17 differentiation has been recently demonstrated by Nakatsuji and associates in the experimental model of multiple sclerosis [50]. The authors have shown that patients with MS have elevated levels of Sema4A in serum. These levels were not changed by IFN-β therapy. MS patients with high Sema4A levels had a significantly higher ratio of IL-17 cells among CD4+ T cells than that in patients with low Sema4A levels. In contrast, the ratios of IL-4 and IFN-γ producing cells were not different between patients with low and high serum Sema4A. The authors suggest that Sema4A may determine the treshhold of Th17 differentiation and that elevated serum Sema4A levels reflect the underlying Th17-mediated MS pathogenesis. Moreover, the authors directly addressed the role of Sema4A in Th17 differentiation by coculturing naïve OT-II OVA-TCR CD4+ T cells with wild-type or Sema4A-deficient DC under Th17-skewing conditions in the presence of OT-II peptides. IL-17 production was considerably impaired when the naive CD4+ T cells were cocultured with Sema4A-deficient DC. Even under neutral conditions without cytokines, IL-17 production was significantly impaired in Sema4A-deficient DC and -sufficient T cell cultures. Collectively, these findings suggest that DC-derived Sema4A, in addition to Th1/Th2 cell differentiation, is also critically involved in Ag-specific Th17 differentiation in vitro and in vivo.
The utility of distinct recombinant proteins directed towards Th2 cytokines for the treatment of experimental and clinical asthma has been a subject of many studies. Recombinant IL-4Rα has shown a significant therapeutic potential in clinical trials involving patients with moderate persistent asthma [51]. The clinical perspectives of the use of recombinant Abs which target specific cytokines such as TNF-α, IL-5, IL-4 and IL-13 for asthma immunotherapy has been recently discussed by several research groups [52, 53]. However, a clinical trial with a recombinant IFN-γ in two-center randomized double-blind placebo-controlled set-up showed no effect of this Th2 response inhibiting cytokine in patients with steroid-dependent asthma [54] although it was effective in alleviating the inflammation and clinical symptoms of atopic dermatitis [55]. Interestingly, in a recent experimental model an oral administration of low doses of IL-12 plus IFN-γ has been shown to resolve the bronchial hyperresponsiveness [56] suggesting that this novel combinatory cytokine administration approach may be effective in asthmatic patients.
Recombinant human deoxyribonuclease has been recently used for a treatment of moderate to severe asthma in children [57]. This mucolitic DNAse has been administered together with standard medications and such treatment did not show any significant effect over the placebo plus standard treatment control. The authors concluded that the addition of a single dose of nebulised rhDNase to standard treatment has no beneficial effects in children with moderate to severe acute asthma. This study, however, contrasted with other study which demonstrated an efficacy of such treatment in resolution of mucus plugging and atelectasis [58]. The authors explain such differences in the treatment outcome by the lower severity of the disease and, thus, milder mucus plugging in children they had treated, as well as by a suboptimal lung deposition of rhDNase in children with bronchial obstruction resulting in its deposition in the more central airways and not reaching the peripheral airways.
We have previously demonstrated a critical role of vascular endothelial growth factor (VEGF) in asthma pathogenesis [59]. In transgenic mice, local lung VEGF expression induces inflammation, edema and mucus secretion as well as other pathological tissue remodeling relevant to human asthma. Recent study by Kim and associates has shown that insulin-like growth factor (IGF)-I is also involved in the inflammatory process associated with asthma and is able to stimulate VEGF expression [60]. The pre- or post-allergen inhalation administration of a recombinant IGF-binding protein 3 (IGFBP-3) had a significant downregulatory effect on the VEGF expression, airway inflammation, and bronchial hyper-responsiveness in the experimental model of disease. Similarly, a soluble thymic stromal lymphopoietin (TSLP) has been shown to be somewhat comparable to the effects of VEGF in the lung tissue inflammatory response [61], especially considering its activation of dendritic cells [62]. Its antagonist, TSLPR-immunoglobulin, downregulated many features of asthma pathogenesis [63]. Therefore, recombinant proteins targeting different molecules and pathways in allergic disease can be successfully used in asthmatic patients, however, carefully planned clinical trials need to be performed first.
Semaphorins represent a large family of secreted and membrane-bound glycoproteins which were originally found to be expressed in the nervous system and function as axon guidance molecules [64]. More recently they have been shown to play important roles in many physiologic and pathologic conditions such as cancer [65-67], multiple sclerosis [68], photoreceptor survival [25, 26], homeostasis of hormone system [69], and angiogenesis [27, 70, 71]. Therefore, recombinant semaphorin molecules have also been utilized for the treatments of various disease-related conditions in experimental models. For example, rSema3A has been successfully used in an ointment for a treatment of atopic dermatitis [72] where it reduced the density of immunoreactive nerve fibers in the epidermis and the numbers of inflammatory cells, such as CD4+ T cells and eosinophils. Sema3E has been shown to play an important role in bone homeostasis and rSema3E inhibited the migration of osteoblasts in a wound-healing assay and decreased the formation of multinucleated, tartrate-resistant acid phosphatase-positive osteoclasts by 81% in cultures of mouse bone marrow macrophages [73]. Therefore, rSema4E can potentially be used for a treatment of different defects in bone mineralization, differentiation, and in would healing considering its critical role in coupling osteoblast and osteoclast activity.
As shown in our previous work [28] and here there is a critical downregulatory effect of Sema4A in experimental asthma severity. Therefore, a specific stimulation of the Sema4A-receptor pathway can be used for asthma immunotherapy. As Sema4A has three functional receptors (Tim-2, Plexin B1 and Plexin D1) widely distributed in lung tissue [36], the individual contribution of each Sema4A-receptor combination in the in vitro corresponding receptor-expressing cell activation and function and in vivo specific disease modulation needs to be assessed first. Nevertheless, our data presented here and the previously published observations [40, 74] suggest a potential dominant role of Sema4A-Tim2 pathway in allergic diseases as Tim-2 deficient mice showed an exaggerated allergic inflammation. Moreover, the previously published studies on the in vivo use of anti-Tim2 Ab or Tim2-Fc fusion protein have shown a critical role of Tim-2 in Th1/Th2 differentiation in vivo. Kawamoto and associates [75] had shown that anti-Tim-2 Ab administration to the mice in early but not late phase of collagen-induced arthritis significantly exacerbated the severity of disease. The authors showed that Tim-2 expression was not found on primary activated CD4+ T cells and the anti-Tim-2 mAb treatment did not affect Th1 and Th17 responses, suggesting that the Tim-2 mAb-induced disease exacerbation does not result from inhibition of Th2 response or an augmentation of Th1 or Th17 responses. In accord to these data, Chakravarti and associates [74] demonstrated that recombinant Tim2-Ig administration to the mice significantly reduced the severity of another autoimmune disease, experimental autoimmune encephalitis. Moreover, the in vitro blockade of Tim-2 with this fusion protein led to a T cell hyperproliferation and increase of Th2 cytokine production. Therefore, Sema4A pathway(s) are essential regulatory pathway(s) in asthma and, therefore, can be targeted with small molecule activators in order to bring down the Th2-associated effect [76, 77].
It has been shown previously that Tim-2 is expressed on T cell, predominantly on Th2 cells [74]. However, we have recently demonstrated that under inflammation various cell types including macrophages, lymphocytes, and a subset of granulocytes in the lung expressed Tim-2 [36]. Moreover, we show here that the subsets of neutrophils (GR-1high) and eosinophils (GR-1intermed) in the lung express Sema4A and that a part of lung Tim-2+ cells also are GR1-positive under inflammation. This suggests that Sema4A-Tim-2 pathway may play a role in granulocyte (eosinophils, neutrophils) activation and/or migration. Indeed, the effect of other semaphorin family member, Sema7A, on neutrophil migration has been reported previously [78]. Furthermore, Sema4A protein has been recently found to be present in neutrophil granules [79]. For the protein profiling analysis neutrophil granules have been divided into AGs (primary, identified by myeloperoxidase (MPO)), SGs (secondary, identified by lactoferrin or neutrophil gelatinase-associated lipocalin (NGAL)), GGs (tertiary, identified by gelatinase), and FGs (ficolin-rich granules). This division is essential for understanding the activation and antimicrobial function of neutrophil granulocytes. Transmembrane proteins important for cell– cell interaction including Sema4A displayed peak amounts in FGs. Amount of Sema4A was also quite significant in GGs and SGs, which contained 7% and 28%, respectively. This suggests that neutrophils can potentially release Sema4A upon degranulation under inflammatory conditions. All together, the published and presented here data show a complex yet unknown role of Sema4A in granulocyte function.
Supplementary Material
Highlights.
We examined the utility of recombinant Sema4A as a novel immunotherapeutic for allergic lung inflammation
Allergic inflammatory lung response was downregulated in recombinant Sema4A-treated mice as compared to PBS-treated control mice
The in vivo administration of mAb to one of Sema4A receptors, Tim-2, upregulated allergic inflammation in WT mouse lungs pointing to a critical role of Sema4A-Tim2 pathway in allergic diseases
Acknowledgments
This work was supported by NIH grant R21AI076736 (to S.P.C.)
Abbreviations used
- Sema4A
semaphorin 4A
- rSema4A
recombinant semaphorin 4A protein
- AHR
airway hyperreactivity
- Tim-2
T cell immunoglobulin and mucin domain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bahadori K, Doyle-Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, FitzGerald JM. Economic burden of asthma: a systematic review. BMC pulmonary medicine. 2009:9, 24. doi: 10.1186/1471-2466-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18(5):684–692. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 3.van Beek AA, Knol EF, de Vos P, Smelt MJ, Savelkoul HF, van Neerven RJ. Recent developments in basophil research: do basophils initiate and perpetuate type 2 T-helper cell responses? Int Arch Allergy Immunol. 2013;160(1):7–17. doi: 10.1159/000341633. [DOI] [PubMed] [Google Scholar]
- 4.Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, Matsuda H, Matsuda A, Oboki K, Ohno T, et al. IL-33-Mediated Innate Response and Adaptive Immune Cells Contribute to Maximum Responses of Protease Allergen-Induced Allergic Airway Inflammation. J Immunol. 2013;190(9):4489–4499. doi: 10.4049/jimmunol.1201212. [DOI] [PubMed] [Google Scholar]
- 5.Herbert C, Scott MM, Scruton KH, Keogh RP, Yuan KC, Hsu K, Siegle JS, Tedla N, Foster PS, Kumar RK. Alveolar macrophages stimulate enhanced cytokine production by pulmonary CD4+ T-lymphocytes in an exacerbation of murine chronic asthma. The American journal of pathology. 2010;177(4):1657–1664. doi: 10.2353/ajpath.2010.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, Hoogsteden HC, Lambrecht BN. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. The Journal of experimental medicine. 2005;201(6):981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2(2):64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Byrne PM, Inman MD, Adelroth E. Reassessing the Th2 cytokine basis of asthma. Trends Pharmacol Sci. 2004;25(5):244–248. doi: 10.1016/j.tips.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Steinke JW, Borish L. Th2 cytokines and asthma. Interleukin-4: its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res. 2001;2(2):66–70. doi: 10.1186/rr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenfeder S, Umland SP, Cuss FM, Chapman RW, Egan RW. Th2 cytokines and asthma. The role of interleukin-5 in allergic eosinophilic disease. Respir Res. 2001;2(2):71–79. doi: 10.1186/rr41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 12.Rutishauser C, Sawyer SM, Bowes G. Quality-of-life assessment in children and adolescents with asthma. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 1998;12(2):486–494. doi: 10.1183/09031936.98.12020486. [DOI] [PubMed] [Google Scholar]
- 13.Petronella SA, Conboy-Ellis K. Asthma epidemiology: risk factors, case finding, and the role of asthma coalitions. Nurs Clin North Am. 2003;38(4):725–735. doi: 10.1016/s0029-6465(03)00099-9. [DOI] [PubMed] [Google Scholar]
- 14.Szefler SJ. Advances in pediatric asthma in 2012: moving toward asthma prevention. The Journal of allergy and clinical immunology. 2013131(1):36–46. doi: 10.1016/j.jaci.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Sloan CD, Gebretsadik T, Wu P, Mitchel EF, Hartert TV. Reactive versus Proactive Patterns of Inhaled Corticosteroid Use. Ann Am Thorac Soc. 2013;10(2):131–134. doi: 10.1513/AnnalsATS.201209-076BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conner JB, Buck PO. Improving Asthma Management: The Case for Mandatory Inclusion of Dose Counters on All Rescue Bronchodilators. The Journal of asthma: official journal of the Association for the Care of Asthma. 2013 doi: 10.3109/02770903.2013.789056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liggett SB. Bitter taste receptors on airway smooth muscle as targets for novel bronchodilators. Expert Opin Ther Targets. 2013 doi: 10.1517/14728222.2013.782395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CG, Dela Cruz CS, Herzog E, Rosenberg SM, Ahangari F, Elias JA. YKL-40, a chitinase-like protein at the intersection of inflammation and remodeling. American journal of respiratory and critical care medicine. 2012;185(7):692–694. doi: 10.1164/rccm.201202-0203ED. [DOI] [PubMed] [Google Scholar]
- 19.Papi A. A new combination therapy for asthma: bridging the gap between effectiveness in trials and clinical practice? Respir Med. 2012;106(Suppl 1):S1–3. doi: 10.1016/S0954-6111(12)00462-3. [DOI] [PubMed] [Google Scholar]
- 20.Holgate ST. Trials and tribulations in identifying new biologic treatments for asthma. Trends Immunol. 2012;33(5):238–246. doi: 10.1016/j.it.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Kumanogoh A, Marukawa S, Suzuki K, Takegahara N, Watanabe C, Ch'ng E, Ishida I, Fujimura H, Sakoda S, Yoshida K, et al. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature. 2002;419(6907):629–633. doi: 10.1038/nature01037. [DOI] [PubMed] [Google Scholar]
- 22.Kumanogoh A, Shikina T, Suzuki K, Uematsu S, Yukawa K, Kashiwamura S, Tsutsui H, Yamamoto M, Takamatsu H, Ko-Mitamura EP, et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity. 2005;22(3):305–316. doi: 10.1016/j.immuni.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Yukawa K, Tanaka T, Bai T, Ueyama T, Owada-Makabe K, Tsubota Y, Maeda M, Suzuki K, Kikutani H, Kumanogoh A. Semaphorin 4A induces growth cone collapse of hippocampal neurons in a Rho/Rho-kinase-dependent manner. International journal of molecular medicine. 2005;16(1):115–118. [PubMed] [Google Scholar]
- 24.Yukawa K, Tanaka T, Yoshida K, Takeuchi N, Ito T, Takamatsu H, Kikutani H, Kumanogoh A. Sema4A induces cell morphological changes through B-type plexin-mediated signaling. International journal of molecular medicine. 2010;25(2):225–230. [PubMed] [Google Scholar]
- 25.Toyofuku T, Nojima S, Ishikawa T, Takamatsu H, Tsujimura T, Uemura A, Matsuda J, Seki T, Kumanogoh A. Endosomal sorting by Semaphorin 4A in retinal pigment epithelium supports photoreceptor survival. Genes Dev. 2012;26(8):816–829. doi: 10.1101/gad.184481.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rice DS, Huang W, Jones HA, Hansen G, Ye GL, Xu N, Wilson EA, Troughton K, Vaddi K, Newton RC, et al. Severe retinal degeneration associated with disruption of semaphorin 4A. Invest Ophthalmol Vis Sci. 2004;45(8):2767–2777. doi: 10.1167/iovs.04-0020. [DOI] [PubMed] [Google Scholar]
- 27.Meda C, Molla F, De Pizzol M, Regano D, Maione F, Capano S, Locati M, Mantovani A, Latini R, Bussolino F, et al. Semaphorin 4A exerts a proangiogenic effect by enhancing vascular endothelial growth factor-A expression in macrophages. J Immunol. 2012;188(8):4081–4092. doi: 10.4049/jimmunol.1101435. [DOI] [PubMed] [Google Scholar]
- 28.Nkyimbeng-Takwi EH, Shanks K, Smith E, Iyer A, Lipsky MM, Detolla LJ, Kikutani H, Keegan AD, Chapoval SP. Neuroimmune semaphorin 4A downregulates the severity of allergic response. Mucosal immunology. 2012;5(4):409–419. doi: 10.1038/mi.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapoval SP, Nabozny GH, Marietta EV, Raymond EL, Krco CJ, Andrews AG, David CS. Short ragweed allergen induces eosinophilic lung disease in HLA-DQ transgenic mice. J Clin Invest. 1999;103(12):1707–1717. doi: 10.1172/JCI6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dasgupta P, Chapoval SP, Smith EP, Keegan AD. Transfer of in vivo primed transgenic T cells supports allergic lung inflammation and FIZZ1 and Ym1 production in an IL-4Ralpha and STAT6 dependent manner. BMC immunology. 2011;12:60. doi: 10.1186/1471-2172-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, Gelfand EW. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. American journal of respiratory and critical care medicine. 1997;156(3 Pt 1):766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 32.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16(11):1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chapoval SP, Dasgupta P, Smith EP, DeTolla LJ, Lipsky MM, Kelly-Welch AE, Keegan AD. STAT6 expression in multiple cell types mediates the cooperative development of allergic airway disease. J Immunol. 2011;186(4):2571–2583. doi: 10.4049/jimmunol.1002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapoval SP, Lee CG, Tang C, Keegan AD, Cohn L, Bottomly K, Elias JA. Lung vascular endothelial growth factor expression induces local myeloid dendritic cell activation. Clin Immunol. 2009;132(3):371–384. doi: 10.1016/j.clim.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chapoval SP, Neeno T, Krco CJ, Marietta EV, Harders J, David CS. HLA-DQ6 and HLA-DQ8 transgenic mice respond to ragweed allergens and recognize a distinct set of epitopes on short and giant ragweed group 5 antigens. J Immunol. 1998;161(4):2032–2037. [PubMed] [Google Scholar]
- 36.Smith EP, Shanks K, Lipsky MM, DeTolla LJ, Keegan AD, Chapoval SP. Expression of neuroimmune semaphorins 4A and 4D and their receptors in the lung is enhanced by allergen and vascular endothelial growth factor. BMC immunology. 2011;12:30. doi: 10.1186/1471-2172-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapoval SP, David CS. CD28 costimulation is critical for experimental allergic asthma in HLA-DQ8 transgenic mice. Clin Immunol. 2003;106(2):83–94. doi: 10.1016/s1521-6616(03)00002-0. [DOI] [PubMed] [Google Scholar]
- 38.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. American journal of respiratory cell and molecular biology. 2010;42(1):96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 39.Dixon C. The bronchial challenge test: a new direction in asthmatic management. Journal of the National Medical Association. 1983;75(2):199–204. [PMC free article] [PubMed] [Google Scholar]
- 40.Rennert PD, Ichimura T, Sizing ID, Bailly V, Li Z, Rennard R, McCoon P, Pablo L, Miklasz S, Tarilonte L, et al. T cell, Ig domain, mucin domain-2 gene-deficient mice reveal a novel mechanism for the regulation of Th2 immune responses and airway inflammation. J Immunol. 2006;177(7):4311–4321. doi: 10.4049/jimmunol.177.7.4311. [DOI] [PubMed] [Google Scholar]
- 41.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, Locksley RM. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(25):11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barlow JL, Bellosi A, Hardman CS, Drynan LF, Wong SH, Cruickshank JP, McKenzie AN. Innate IL-13-producing nuocytes arise during allergic lung inflammation and contribute to airways hyperreactivity. The Journal of allergy and clinical immunology. 2012;129(1):191–198. e191–194. doi: 10.1016/j.jaci.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 44.Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. The Journal of allergy and clinical immunology. 2001;108(3):430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- 45.Nembrini C, Marsland BJ, Kopf M. IL-17-producing T cells in lung immunity and inflammation. The Journal of allergy and clinical immunology. 2009;123(5):986–994. doi: 10.1016/j.jaci.2009.03.033. quiz 995-986. [DOI] [PubMed] [Google Scholar]
- 46.Doe C, Bafadhel M, Siddiqui S, Desai D, Mistry V, Rugman P, McCormick M, Woods J, May R, Sleeman MA, et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138(5):1140–1147. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan HL, Rosenthal M. IL-17 in lung disease: friend or foe? Thorax. 2013 doi: 10.1136/thoraxjnl-2013-203307. [DOI] [PubMed] [Google Scholar]
- 48.Elena Alexeevna G, Julia Anatolievna L, Taniana Vladimirovna KK, Vera Makarovna N, Sergey Vitalievich S. Levels of TNF, TNF autoantibodies, and soluble TNF receptors in patients with bronchial asthma. The Journal of asthma: official journal of the Association for the Care of Asthma. 2013 doi: 10.3109/02770903.2013.796972. [DOI] [PubMed] [Google Scholar]
- 49.Manise M, Holtappels G, Van Crombruggen K, Schleich F, Bachert C, Louis R. Sputum IgE and Cytokines in Asthma: Relationship with Sputum Cellular Profile. PloS one. 2013;8(3):e58388. doi: 10.1371/journal.pone.0058388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakatsuji Y, Okuno T, Moriya M, Sugimoto T, Kinoshita M, Takamatsu H, Nojima S, Kimura T, Kang S, Ito D, et al. Elevation of Sema4A implicates Th cell skewing and the efficacy of IFN-beta therapy in multiple sclerosis. J Immunol. 2012;188(10):4858–4865. doi: 10.4049/jimmunol.1102023. [DOI] [PubMed] [Google Scholar]
- 51.Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, Whitmore JB, Agosti JM. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. The Journal of allergy and clinical immunology. 2001;107(6):963–970. doi: 10.1067/mai.2001.115624. [DOI] [PubMed] [Google Scholar]
- 52.Thomson NC, Chaudhuri R, Spears M. Emerging therapies for severe asthma. BMC Med. 2011;9:102. doi: 10.1186/1741-7015-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atamas SP, Chapoval SP, Keegan AD. Cytokines in chronic respiratory diseases. F1000 Biol Rep. 2013;5:3. doi: 10.3410/B5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boguniewicz M, Schneider LC, Milgrom H, Newell D, Kelly N, Tam P, Izu AE, Jaffe HS, Bucalo LR, Leung DY. Treatment of steroid-dependent asthma with recombinant interferon-gamma. Clin Exp Allergy. 1993;23(9):785–790. doi: 10.1111/j.1365-2222.1993.tb00367.x. [DOI] [PubMed] [Google Scholar]
- 55.Hanifin JM, Schneider LC, Leung DY, Ellis CN, Jaffe HS, Izu AE, Bucalo LR, Hirabayashi SE, Tofte SJ, Cantu-Gonzales G, et al. Recombinant interferon gamma therapy for atopic dermatitis. J Am Acad Dermatol. 1993;28(2 Pt 1):189–197. doi: 10.1016/0190-9622(93)70026-p. [DOI] [PubMed] [Google Scholar]
- 56.Gariboldi S, Palazzo M, Zanobbio L, Dusio GF, Mauro V, Solimene U, Cardani D, Mantovani M, Rumio C. Low dose oral administration of cytokines for treatment of allergic asthma. Pulm Pharmacol Ther. 2009;22(6):497–510. doi: 10.1016/j.pupt.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 57.Boogaard R, Smit F, Schornagel R, Vaessen-Verberne AA, Kouwenberg JM, Hekkelaan M, Hendriks T, Feith SW, Hop WC, de Jongste JC, et al. Recombinant human deoxyribonuclease for the treatment of acute asthma in children. Thorax. 2008;63(2):141–146. doi: 10.1136/thx.2007.081703. [DOI] [PubMed] [Google Scholar]
- 58.Durward A, Forte V, Shemie SD. Resolution of mucus plugging and atelectasis after intratracheal rhDNase therapy in a mechanically ventilated child with refractory status asthmaticus. Crit Care Med. 2000;28(2):560–562. doi: 10.1097/00003246-200002000-00045. [DOI] [PubMed] [Google Scholar]
- 59.Lee CG, Link H, Baluk P, Homer RJ, Chapoval S, Bhandari V, Kang MJ, Cohn L, Kim YK, McDonald DM, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10(10):1095–1103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim SR, Lee KS, Lee KB, Lee YC. Recombinant IGFBP-3 inhibits allergic lung inflammation, VEGF production, and vascular leak in a mouse model of asthma. Allergy. 2012;67(7):869–877. doi: 10.1111/j.1398-9995.2012.02837.x. [DOI] [PubMed] [Google Scholar]
- 61.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. The Journal of experimental medicine. 2005;202(6):829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito T, Liu YJ, Arima K. Cellular and molecular mechanisms of TSLP function in human allergic disorders--TSLP programs the "Th2 code" in dendritic cells. Allergol Int. 2012;61(1):35–43. doi: 10.2332/allergolint.11-RAI-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang F, Huang G, Hu B, Song Y, Shi Y. A soluble thymic stromal lymphopoietin (TSLP) antagonist, TSLPR-immunoglobulin, reduces the severity of allergic disease by regulating pulmonary dendritic cells. Clin Exp Immunol. 2011;164(2):256–264. doi: 10.1111/j.1365-2249.2011.04328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kolodkin AL, Matthes DJ, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75(7):1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- 65.Song X, Zhang W, Zhang Y, Zhang H, Fu Z, Ye J, Liu L, Wu Y. Expression of semaphorin 3A and neuropilin 1 with clinicopathological features and survival in human tongue cancer. Medicina oral, patologia oral y cirugia bucal. 2012;17(6):e962–968. doi: 10.4317/medoral.18168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chakraborty G, Kumar S, Mishra R, Patil TV, Kundu GC. Semaphorin 3A suppresses tumor growth and metastasis in mice melanoma model. PloS one. 2012;7(3):33633. doi: 10.1371/journal.pone.0033633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. The Journal of biological chemistry. 2007;282(9):6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- 68.Eixarch H, Gutierrez-Franco A, Montalban X, Espejo C. Semaphorins 3A and 7A: potential immune and neuroregenerative targets in multiple sclerosis. Trends in molecular medicine. 2013;19(3):157–164. doi: 10.1016/j.molmed.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Giacobini P, Prevot V. Semaphorins in the development, homeostasis and disease of hormone systems. Seminars in cell & developmental biology; 2013. pp. 190–198. [DOI] [PubMed] [Google Scholar]
- 70.Basile JR, Barac A, Zhu T, Guan KL, Gutkind JS. Class IV semaphorins promote angiogenesis by stimulating Rho-initiated pathways through plexin-B. Cancer research. 2004;64(15):5212–5224. doi: 10.1158/0008-5472.CAN-04-0126. [DOI] [PubMed] [Google Scholar]
- 71.Zhou H, Binmadi NO, Yang YH, Proia P, Basile JR. Semaphorin 4D cooperates with VEGF to promote angiogenesis and tumor progression. Angiogenesis. 2012;15(3):391–407. doi: 10.1007/s10456-012-9268-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Negi O, Tominaga M, Tengara S, Kamo A, Taneda K, Suga Y, Ogawa H, Takamori K. Topically applied semaphorin 3A ointment inhibits scratching behavior and improves skin inflammation in NC/Nga mice with atopic dermatitis. J Dermatol Sci. 2012;66(1):37–43. doi: 10.1016/j.jdermsci.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Hughes A, Kleine-Albers J, Helfrich MH, Ralston SH, Rogers MJ. A class III semaphorin (Sema3e) inhibits mouse osteoblast migration and decreases osteoclast formation in vitro. Calcif Tissue Int. 2012;90(2):151–162. doi: 10.1007/s00223-011-9560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chakravarti S, Sabatos CA, Xiao S, Illes Z, Cha EK, Sobel RA, Zheng XX, Strom TB, Kuchroo VK. Tim-2 regulates T helper type 2 responses and autoimmunity. The Journal of experimental medicine. 2005;202(3):437–444. doi: 10.1084/jem.20050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawamoto T, Abe Y, Ito J, Makino F, Kojima Y, Usui Y, Ma J, Morimoto S, Yagita H, Okumura K, et al. Anti-T cell immunoglobulin and mucin domain-2 monoclonal antibody exacerbates collagen-induced arthritis by stimulating B cells. Arthritis research & therapy. 2011;13(2):47. doi: 10.1186/ar3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vrijens K, Lin W, Cui J, Farmer D, Low J, Pronier E, Zeng FY, Shelat AA, Guy K, Taylor MR, et al. Identification of small molecule activators of BMP signaling. PloS one. 2013;8(3):59045. doi: 10.1371/journal.pone.0059045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vingtdeux V, Chandakkar P, Zhao H, Davies P, Marambaud P. Small-molecule activators of AMP-activated protein kinase (AMPK), RSVA314 and RSVA405, inhibit adipogenesis. Mol Med. 2011;17(9-10):1022–1030. doi: 10.2119/molmed.2011.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morote-Garcia JC, Napiwotzky D, Kohler D, Rosenberger P. Endothelial Semaphorin 7A promotes neutrophil migration during hypoxia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(35):14146–14151. doi: 10.1073/pnas.1202165109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rorvig S, Ostergaard O, Heegaard NH, Borregaard N. Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. Journal of leukocyte biology. 2013 doi: 10.1189/jlb.1212619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.