Abstract
Aims
C-peptide is renoprotective in type 1 diabetes, however, the mechanisms of its actions are not completely understood. We hypothesized that C-peptide attenuates diabetes-associated renal microvascular injury.
Method
After 4 or 8 weeks of streptozotocin (STZ)-induced diabetes, rats received either vehicle or C-peptide in the presence of low or high doses of insulin. Urine albumin excretion (UAE) was measured prior to initiation of treatment (baseline) and 2 or 4 weeks after treatment (sacrifice). Glomerular hypertrophy, glomerular filtration rate (GFR) and renal microvascular density, quantified ex vivo by 3D micro-CT reconstruction, were measured at sacrifice.
Results
In rats receiving low doses of insulin, treatment with C-peptide reduced HbA1c levels by 24%. In these rats, the 107% increase in UAE rate from baseline to sacrifice in vehicle-treated rats was largely prevented with C-peptide. C-peptide also reduced diabetes-associated glomerular hyperfiltration by 30%, glomerular hypertrophy by 22% and increased the density of microvessels between 0–500 μm in diameter by an average of 31% compared with vehicle-treated groups. Similar renoprotective effects of C-peptide were observed in rats treated with higher doses of daily insulin, despite no differences in HbA1c levels.
Conclusions
The study suggests that C-peptide is renoprotective by preserving the integrity of the renal microvasculature irrespective of glucose regulation.
Keywords: diabetes, kidney, blood vessels, rarefaction
1. Introduction
C-peptide plays the important role of connecting the A and B chains of the pro-insulin molecule and once cleaved off, it is released from pancreatic beta-cells in equal molar amounts to insulin (Hills et al 2010; Horwitz et al 1975; Steiner 2004). Due to the high degree of structural variability between species and a lack of a conserved sequence, C-peptide has historically thought to have no functional significance independent of insulin production and has only been used clinically as a surrogate marker of insulin release and a measure of beta-cell activity in diabetes mellitus (Faber & Binder 1977; Horwitz et al 1975; Roth et al 2012; Steiner 2004). However, recent studies have shown that C-peptide may have therapeutic applications in the treatment of type 1 diabetes-associated end-organ complications, including diabetic nephropathy (Huang et al 2002; Johansson et al 2000; Nordquist et al 2007; Samnegard et al 2004; Sjoquist et al 1998). Indeed, studies have shown that acute and chronic infusion of C-peptide reduces albuminuria, proteinuria, glomerular hyperfiltration and hypertrophy in streptozotocin (STZ)-induced diabetic rats and mice or alloxan-induced diabetic mice (Huang et al 2002; Maezawa et al 2006; Nordquist et al 2009; Nordquist et al 2008; Samnegard et al 2005; Samnegard et al 2001; Sjoquist et al 1998). Furthermore, clinical studies show that long term administration of C-peptide to patients with type 1 diabetes decreases albuminuria and improves renal function (Johansson et al 2000). However, the mechanisms by which C-peptide protects the diabetic kidney are unclear.
Most efforts to examine the potential mechanisms by which C-peptide exerts some of its actions in the diabetic kidney have focused on its role in regulating microvascular changes. Specifically, several studies have shown that C-peptide reduces glomerular hyperfiltration associated with early stages of diabetes by constricting afferent arterioles (Huang et al 2002; Nordquist et al 2009; Nordquist et al 2008; Samnegard et al 2005; Samnegard et al 2001). In addition, diabetic nephropathy is also characterized by early and progressive renal microvascular rarefaction (Khavandi et al 2009; Lindenmeyer et al 2007; Maric-Bilkan et al 2012). Thus, successful therapeutic treatments for diabetic renal disease should include preservation of the renal microvascular function and architecture. Therefore, the current study was designed to test the hypothesis that C-peptide protects the kidney in STZ-induced diabetic rat by preserving the structure and integrity of the renal microvasculature.
2. Materials and methods
2.1 Study design
Effects of C-peptide in diabetic rats and non-diabetic rat in the presence of low doses of insulin
The study was performed in 12-week old male Sprague-Dawley rats (Harlan, Madison, WI) maintained on standard rat chow and tap water ad libitum. Nineteen rats were rendered diabetic with a single i.p. injection of streptozotocin (STZ, 55 mg/kg in 0.1 mM citrate buffer, pH 7.4) as previously described (Mankhey et al 2006) and given insulin (2–4 U every 3 days, Lantus, Aventis Pharmaceuticals Inc., Kansas City, MO) throughout the study to prevent excessive weight loss and mortality. Supplementation of insulin using this regimen mimics moderately controlled type 1 diabetes (Xu et al 2008). After 8 weeks of untreated diabetes (baseline), the animals were randomly divided to receive either vehicle (0.9% saline, n=9) or rat C-peptide (50 pmol × kg−1 × min−1, American Peptide Company, Sunnyvale, CA, n=10) for 4 weeks via osmotic minipumps (type 2004, Alzet, Cupertino, CA) that were implanted subcutaneously in the nape of the neck. The specificity and purity of C-peptide was verified by the manufacturer (lot # S1206011T).
In a separate experiment, eight, 12-week old non-diabetic rats were randomly divided to receive vehicle(non-diabetic, n=4) or C-peptide(non-diabetic, n=4). The treatments were administered using the same protocol as described for the diabetic rats.
At baseline and after 4 weeks of treatment with either vehicle or C-peptide (sacrifice), all animals (non-diabetic and diabetic) were placed in metabolic cages for 24 hours for urine collection for determination of urine albumin (UAE) and urine protein (UPE) excretion. One day prior to sacrifice, the animals were instrumented with catheters for measurement of blood pressure and renal function. At the time of sacrifice, the left kidney was prepared for Micro-CT, while parts of the right kidney were snap frozen in liquid nitrogen (for protein analysis) or fixed with 10% buffered formalin (for histology and immunohistochemistry). All experiments were performed according to the guidelines recommended by the National Institutes of Health and approved by the University of Mississippi Medical Center Animal Care and Use Committee.
Effects of C-peptide in diabetic rats in the absence of insulin and in the presence of high doses of insulin
In order to test whether the renoprotective effects of C-peptide are dependent on the presence of insulin or blood glucose lowering, we examined the responses to treatment with C-peptide in the absence of insulin and high doses of insulin, respectively. Sixteen male Sprague-Dawley rats were rendered diabetic as described above. After 4 weeks of untreated diabetes (note: no insulin was supplemented in these animals during this time), the animals were randomly divided into the following treatment groups: vehicle (no insulin), C-peptide (no insulin), vehicle (4 U insulin/day), C-peptide (4 U insulin/day), The duration of treatment was 2 weeks and the doses and route of administration were identical to that of Experiment 1. Prior to initiation of treatment (baseline) and after 2 weeks of treatment (sacrifice), the animals were placed in metabolic cages, following which they were instrumented with catheters for measurement of mean arterial pressure and renal function as described below.
2.2 Measurement of mean arterial pressure (MAP) and renal function
One day prior to sacrifice, the rats were anaesthetized with 3% isofluorane and the femoral artery and vein cannulated and the catheters were routed under the skin to exit the back of the neck. After an overnight recovery, MAP, glomerular filtration rate (GFR) and renal plasma flow were measured in conscious, restrained rats. Measurements of arterial pressure were taken continuously for 2h using a pressure transducer connected to a computerized data-acquisition system (PowerLab, ADInstruments, Colorado Springs, CO). Glomerular filtration rate was measured as previously described (Sawyer et al 2012). Briefly, 125I-iothalamate (10 μCi/ml) was infused at a rate of 2 ml/hr. After a 3-hour equilibration, 3 blood samples (50 μL each) were taken at 30-minute intervals. The GFR was then calculated as radioactive counts per minute (cpm) for infusate × infusion rate divided by cpm for plasma samples. Renal plasma flow was measured following infusion of PAH (2.5 mg/mL). Renal blood flow (RBF) was then calculated as RPF/1-hematocrit and renal vascular resistance (RVR) as MAP/RBF.
2.3 Measurement of blood glucose and glycated hemoglobin (HbA1C)
Blood glucose and HbA1C levels were measured in a sample of blood obtained from a tail prick using the OneTouch Ultra glucometer and the A1CNow+® monitor (Bayer, Sunnyvale, CA, according to the manufacturer’s protocol), respectively. Blood glucose levels were measured every two weeks while HbA1C levels were only measured at the time of sacrifice.
2.4 Measurements of plasma insulin and C-peptide
Plasma insulin levels were measured by ELISA (Crystal Chem Inc.; cat. no. 90060; Downers Grove, IL) and plasma C-peptide levels were measured by radioimmunoassay (cat. no. RCP-21K; Millipore, Billerica, MA), according to the manufacturer’s protocols. Both insulin and C-peptide levels were measured only at the time of sacrifice.
2.5 Measurement of urine albumin excretion (UAE) and urine protein excretion (UPE)
Urine albumin concentration was measured using the Nephrat II albumin kit (Exocell Inc., Philadelphia PA) and urine protein concentration was measured using the Pierce BCA protein assay (Thermo Scientific, Rockford, IL) following the manufacturer’s protocol. UAE and UPE were calculated as urine albumin or protein concentration multiplied by urine output.
2.6 Micro-computed tomography (micro-CT)
At the time of sacrifice, under physiological pressure, the right kidney was perfused first with saline followed by a contrast agent (radio-opaque silicone polymer containing lead chromate (Microfil MV122, Flow Tech, Inc., Carver, MA). The polymer-filled kidneys were left at 4°C overnight and then immersed in 10% buffered formalin for 72h prior to scanning. The kidneys were scanned at 0.3° increments using a micro-CT scanner, (SkyScan 1076 system, Micro Photonics Inc, PA) and the X-ray transmission images were acquired in each angle of view at a resolution of 18 μm and digitized to 16 bits gray scale. 3D volume images were reconstructed using a filtered back-projection algorithm and displayed on a computer workstation by volume rendering for display and analysis of renal microvessels using the Analyze™ (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN) software package. The cortex was tomographically divided and the spatial density and distribution of microvessels (diameters 0–500μm) and vascular volume fraction (the ratio of the sum of cross-sectional areas of all vessels and the total area of the region of interest) were calculated, as previously described (Iliescu et al 2010; Maric-Bilkan et al 2012).
2.7 Glomerular hypertrophy
The mean glomerular volume was estimated in PAS-stained sections in 40 randomly selected glomeruli in 3 sections in each animal. Glomerular profiles were traced and area estimated using image analysis software (NIS-Elements, Ver. 2.32; Nikon Instruments, Melville, NY). Glomerular volume was estimated by multiplying the mean glomerular area with the thickness of the section.
2.8 Immunohistochemistry
Immunolocalization of CD68 (marker of activated macrophages), JG-12 (endothelial cell marker) and Bax (pro-apoptotic marker) was performed on 4 μm paraffin-embedded sections as previously described in detail (Maric-Bilkan et al 2012) using the following antibodies: CD68 (1:200; mouse monoclonal; cat. no. MCA341R; Serotec, Oxford, UK), JG-12 (1:1,000, mouse monoclonal, cat. no. BMS1104, eBioscience, San Diego, CA) and Bax (1:1,000, mouse monoclonal, cat. No. Sc-7480, Santa Cruz).
The number of CD68-positive cells was assessed in 40 different fields per animal from each group and expressed per millimeter squared. The area positively stained for JG-12 immunoexpression was measured in thirty randomly selected glomeruli and/or cortical interstitial regions per section and quantified using image analysis software (NIS-Elements, Ver. 2.32; Nikon Instruments, Melville, NY). The data are expressed as % of JG-12-positive staining per selected area (glomerulus or cortical peritubular regions).
2.9 Western blotting
Renal cortical expression of VEGF, VEGFR1, VEGFR2, Bax and Bcl2 protein was performed as previously described in detail (Maric-Bilkan et al 2012) using the following antibodies: VEGF (1:2,000, rabbit polyclonal, cat. no. sc-152, Santa Cruz Biotechnology, Santa Cruz, CA), VEGFR1 (1:1,000, rabbit polyclonal, cat. no. sc-316, Santa Cruz), VEGFR2 (1:1,000, rabbit polyclonal, cat. no. sc-315, Santa Cruz), Bax (1:500, mouse monoclonal, cat. no. sc-7480, Santa Cruz) and Bcl2 (1:1,000, rabbit polyclonal, cat. No. Sc-492). Proteins were visualized by enhanced chemiluminescence (Thermo Scientific, Rockford, IL) and the densities of specific bands were quantified by densitometry using the Scion Image (version alpha 4.0.3.2) software. The membranes were then striped and re-probed with an antibody against β-actin (1:1000; mouse monoclonal; cat. no. 4970; Cell Signaling, Danvers, MA) and the densities of specific bands were then normalized to the densities of bands probed for β-actin.
For assessing expression of angiopoietin-1 protein expression, homogenates of the renal cortex were denatured at 95°C, loaded onto a 12% SDS-PAGE precast gel (Bio-Rad, Hercules, CA) and transferred to a nitrocellulose membrane. The membrane was incubated with antisera against angiopoietin-1 (1:500, rabbit polyclonal; cat. no. ab8451, Abcam, Cambridge, MA) and alpha-actinin (1:1,00, mouse monoclonal, cat. no. sc-17829, Santa Cruz) followed by appropriate fluorescently-labeled secondary antibodies. Angiopoietin-1 normalized to alpha-actinin protein abundance was measured using the LI-COR Odyssey imaging system.
2.10 Statistical Analysis
All values, except time courses, are expressed as mean SE and were analyzed using the Student’s t-test (Prism 4, Graph Pad Software, San Diego, CA). Time courses in the development of UAE, UPE and blood glucose were analyzed using repeated measures ANOVA. Post hoc comparisons after two-way ANOVA were performed using the Bonferroni post-test. Statistical significance was accepted at P<0.05.
3. Results
3.1 Effects of C-peptide on metabolic function in non-diabetic and diabetic rats treated with low doses of insulin
No differences in body weight, water or food intake were observed between the vehicle(diabetic) and C-peptide(diabetic)-treated groups (Table 1). Plasma levels of C-peptide(diabetic) were 135% higher in the C-peptide-treated compared with vehicle(diabetic)-treated animals (Table 1); however, these levels were approximately 2.5-fold lower than those observed in a non-diabetic rat (Sawyer et al 2012).
Table 1.
Metabolic and renal parameters in Diabetic rats at the time of sacrifice
| Vehicle n=9 | C-peptide n=10 | |
|---|---|---|
| Body weight (g) | 279 ± 14 | 280 ± 11 |
| Food intake (g) | 48.0 ± 1.6 | 45.0 ± 2.2 |
| Water intake (ml) | 241 ± 3 | 217 ± 14 |
| HbA1c (%) | 11.1 ± 0.2 | 9.0 ± 0.5* |
| Plasma insulin (ng/ml) | 0.20 ± 0.02 | 0.70 ± 0.14* |
| Plasma C-peptide (nmol/l) | 0.24 ± 0.03 | 0.63 ± 0.25* |
| Kidney/body weight (mg/g) | 6.03 ± 0.31 | 5.38 ± 0.28 |
| Kidney weight (g) | 1.68 ± 0.13 | 1.49 ± 0.05 |
| Urine output (ml) | 200 ± 6 | 166 ± 14 |
| MAP (mmHg) | 106 ± 3 | 110 ± 2 |
| GFR (ml/min) | 2.66 ± 0.20 | 2.07 ± 0.13* |
| RBF (ml/min) | 11.8 ± 1.4 | 11.5 ± 1.2 |
| RVR (mmHg/ml/min) | 9.63 ± 1.44 | 10.4 ± 1.23 |
Values are expressed as mean ± SE, Statistical significance was accepted at P < 0.05; *0.05 vs. vehicle
Abbreviations: Mean arterial pressure; GFR, glomerular filtration rate; RBF, renal blood flow; RVR, renal vascular resistance.
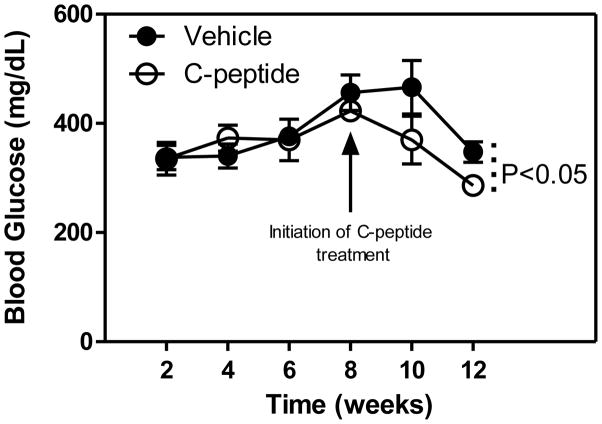
For the first 8 weeks of untreated diabetes, no differences in blood glucose levels were observed between the vehicle(diabetic) and C-peptide(diabetic)-treated groups (Fig. 1). However, at the time of sacrifice, blood glucose levels were 18% lower in the C-peptide(diabetic) compared with vehicle(diabetic)-treated group (Fig. 1). Consistent with the blood glucose lowering effect of C-peptide, there was a 24% decrease in HbA1c at the time of sacrifice. In addition, a 250% increase in plasma insulin levels were observed in the C-peptide(diabetic) compared with the vehicle(diabetic)-treated group (Table 1).
Fig. 1.
Effects of C-peptide on blood glucose levels. Values are means ± SE.
In the non-diabetic rats, no differences in any of the metabolic parameters were observed between the vehicle(non-diabetic)- and C-peptide(non-diabetic)-treated rats (Table 2).
Table 2.
Metabolic and renal parameters in Non-diabetic rats at the time of sacrifice
| Vehicle n=4 | C-peptide n=4 | |
|---|---|---|
| Body weight (g) | 321 ± 12 | 358 ± 5.2 |
| Food intake (g) | 32.0 ± 1.0 | 28.0 ± 1.1 |
| Water intake (ml) | 43.3 ± 14 | 33.3 ± 2.59 |
| HbA1c (%) | 4.13 ± 0.09 | 4.30 ± 0.07 |
| Plasma insulin (ng/ml) | 1.35 ± 0.54 | 1.75 ± 054 |
| Plasma C-peptide (nmol/l) | 1.42 ± 0.05 | 1.88 ± 0.10 |
| Kidney/body weight (mg/g) | 4.24 ± 0.18 | 3.56 ± 0.07 |
| Kidney weight (g) | 1.36 ± 0.05 | 1.27 ± 0.04 |
| Urine output (ml) | 42.5 ± 3.18 | 33.5 ± 15.9 |
| MAP (mmHg) | 111 ± 4 | 112 ± 4 |
| GFR (ml/min) | 1.19 ± 0.04 | 1.16 ± 0.03 |
| RBF (ml/min) | 14.8 ± 0.9 | 15.4 ± 0.5 |
| RVR (mmHg/ml/min) | 5.83 ± 0.35 | 5.60 ± 0.08 |
Values are expressed as mean ± SE, Statistical significance was accepted at P < 0.05; *0.05 vs. vehicle
Abbreviations: Mean arterial pressure; GFR, glomerular filtration rate; RBF, renal blood flow; RVR, renal vascular resistance.
3.2 Effects of C-peptide on MAP and renal function in non-diabetic and diabetic rats treated with low doses of insulin
While no differences in urine output, MAP, RBF or RVR were observed between the vehicle(diabetic) and C-peptide(diabetic)-treated groups, the C-peptide(diabetic)-treated animals exhibited a 30% lower GFR compared with vehicle(diabetic)-treated animals (Table 1). In addition, no differences in kidney/body weight were observed between the two treatment groups (Table 1).
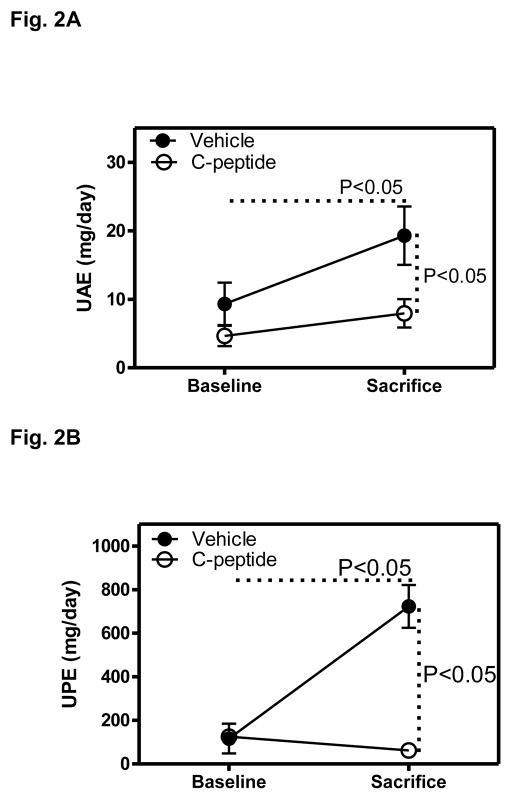
In the vehicle(diabetic)-treated group, UAE increased by 107% between baseline and the time of sacrifice, and this increase was largely prevented in the C-peptide(diabetic)-treated group, so that at the time of sacrifice, UAE was lower by 59% in the C-peptide compared with vehicle treated groups (Fig. 2A). Similarly, UPE increased by 520% between baseline and the time of sacrifice in the vehicle(diabetic)-treated group, and this increase was completely prevented in the C-peptide(diabetic)-treated group, so that at the time of sacrifice, UAE was lower by 91% in the C-peptide compared with vehicle treated groups (Fig. 2B).
Fig. 2.
Effects of C-peptide on urine albumin (UAE) and urine protein (UPE) excretion. A. UAE at baseline (after 8 weeks of untreated diabetes, i.e. prior to the initiation of treatment with either vehicle or C-peptide) and at the time of sacrifice (following 4 weeks of treatment with either vehicle or C-peptide). B. UPE at baseline and at the time of sacrifice. Values are means ± SE.
In the non-diabetic rats, no differences in MAP or renal function were observed between the vehicle(non-diabetic)- and C-peptide(non-diabetic)-treated rats (Table 2). Note: since we did not observe any effects of C-peptide treatment on either metabolic or renal parameters, we did not perform further histological, immunohistochemical or biochemical analyses in samples from these rats.
3.3 Glomerular hypertrophy and Inflammation in diabetic rats treated with low doses of insulin
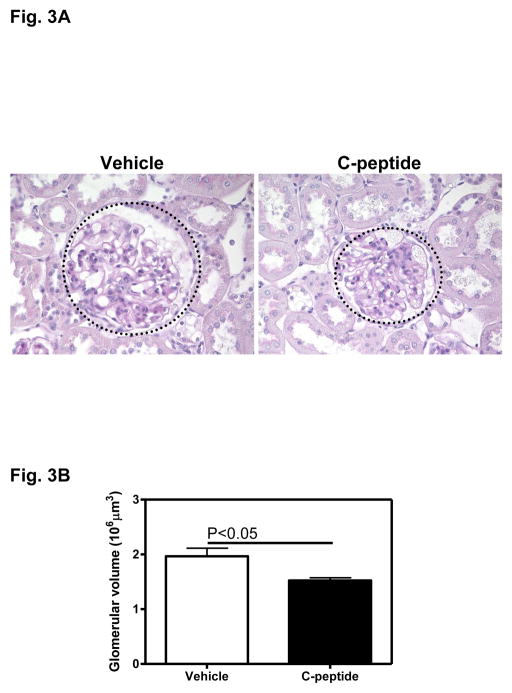
Fig. 3A depicts the diabetes-associated glomerular hypertrophy in the vehicle(diabetic)-treated group and the apparent decrease in glomerular hypertrophy following treatment with C-peptide(diabetic) (Fig. 3A). Quantitative analysis revealed a 22% decrease in glomerular volume in the C-peptide compared with the vehicle-treated group (Fig. 3B).
Fig. 3.
Effects of C-peptide on glomerular hypertrophy. A. Representative PAS-stained section. Original magnification x400. B. Quantitative analysis of mean glomerular volume. Data are expressed as means ± SE.
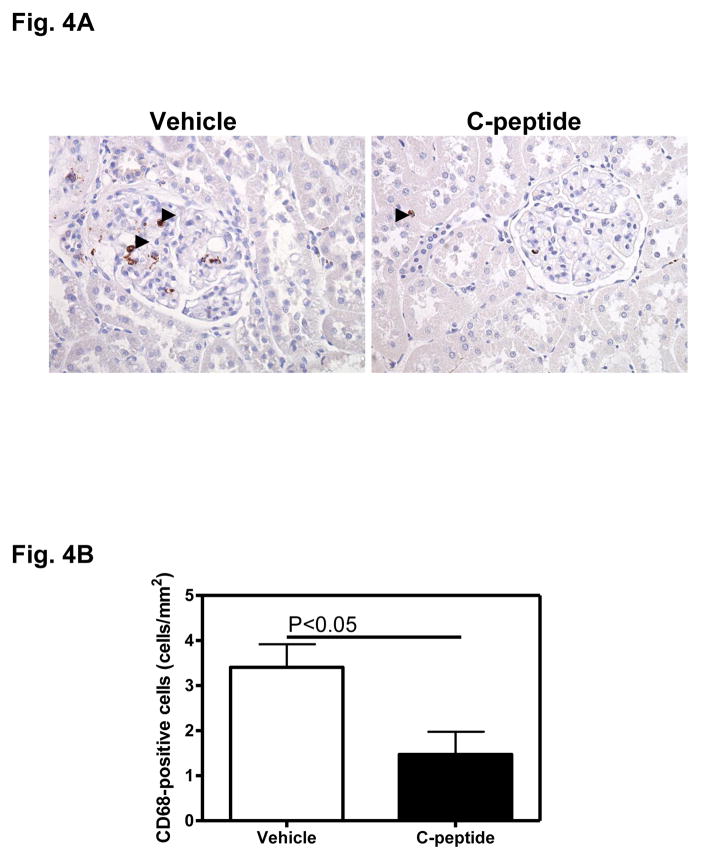
The vehicle(diabetic)-treated group showed abundant CD68-positive cells, suggesting presence of activated macrophages (Fig. 4A). Treatment with C-peptide(diabetic) appeared to reduce the abundance of CD68-positive cells throughout the renal cortex (Fig. 4A). These observations were confirmed by quantitative analysis which showed a 57% decrease in the abundance of CD68-positive cells in the C-peptide(diabetic) compared with the vehicle(diabetic)-treated group (Fig. 4B).
Fig. 4.
Effects of C-peptide on renal inflammation. A. Representative image showing immunohistochemical staining for CD68. Original magnification x400. B. Quantitative analysis of CD68-positive staining. Data are expressed as means ± SE.
3.4 Microvascular architecture in diabetic rats treated with low doses of insulin
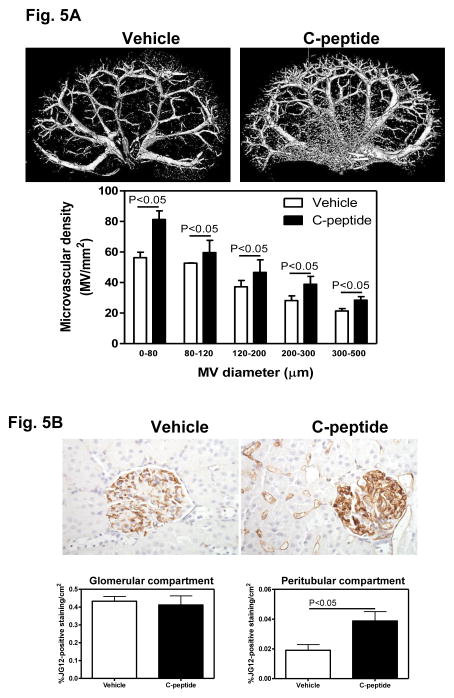
The renal microvascular architecture was visualized using micro-CT reconstruction followed by image analysis (Fig. 5A, top panel). Visually, the vehicle(diabetic)-treated group appeared to be characterized by a less dense microvascular tree compared with the C-peptide(diabetic)-treated group (Fig. 5A, top panel). This observation was confirmed by quantification of the renal microvessels which revealed a 45%, 13%, 25%, 38% and 33% increase in the density of microvessels 0–80 μm, 80–120 μm, 120–200 μm, 200–300 μm and 300–500 μm in diameter, respectively, in C-peptide(diabetic) compared with vehicle(diabetic)-treated groups (Fig. 5A, bottom panel).
Fig. 5.
Effects of C-peptide on renal microvascular architecture. A. Representative 3D micro-CT reconstruction of the renal vasculature (top panel) and quantification of the cortical microvascular density and vascular volume fraction of microvessels with diameters between 0–80, 80–120, 120–200, 200–300 and 300–500 micrometers (bottom panel). B. JG-12 immunolocalization (brown staining, top panel) and quantification of the cortical JG-12 density (bottom panel). Original magnification x400. Data are expressed as means ± SE.
To further examine the effect of C-peptide treatment on renal microvascular density, we performed immunohistochemical labeling of JG-12, a marker of endothelial cells (Fig. 5B, top panel). We performed image analysis on JG12-stained section in the glomerular and cortical peritubular compartments, separately. No differences in the density of JG12 immunostaining between vehicle(diabetic) and C-peptide(diabetic)-treated groups were observed in the glomerular compartment (Fig. 5B, bottom panel); however, there was a 103% increase in the density of JG12 immunostaining in the peritubular compartment in the C-peptide compared to the vehicle-treated group (Fig. 5B, bottom panel).
3.5 Expression of angiopoietin-1, VEGF and VEGF receptor protein in diabetic rats treated with low doses of insulin
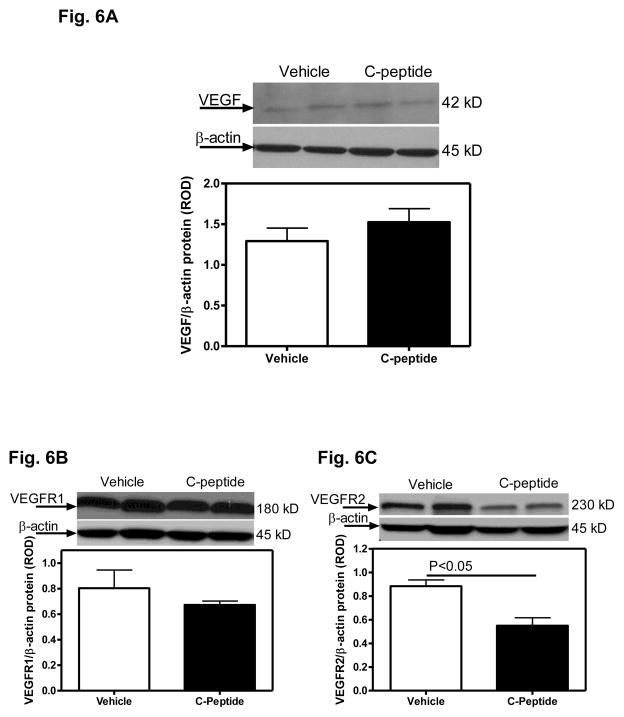
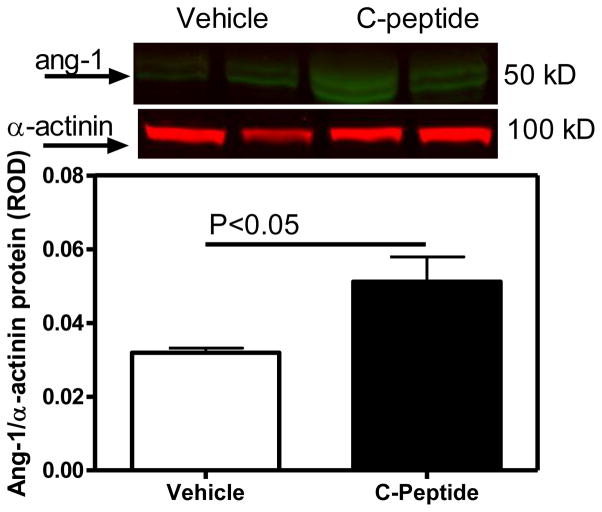
No differences in renal cortical VEGF or VEGFR1 protein expression were observed between the vehicle(diabetic) and C-peptide(diabetic)-treated groups (Fig. 6A). In contrast, the renal cortical VEGFR2 protein expression was decreased by 38% (Fig. 6B), while angiopoietin-1 protein expression increased by 60% (Fig. 7) in the C-peptide(diabetic) compared with the vehicle(diabetic)-treated group.
Fig. 6.
Effects of C-peptide on renal cortical VEGF and VEGF receptor protein expression. A. VEGF protein expression. Top: representative immunoblot of VEGF protein expression. Bottom: densitometric scans in relative optical density (ROD) expressed as a ratio of VEGF/Beta-actin. B. VEGFR1 protein expression. Top: representative immunoblot of VEGFR1 protein expression. Bottom: densitometric scans in relative optical density (ROD) expressed as a ratio of VEGFR1/Beta-actin. C. VEGFR2 protein expression. Top: representative immunoblot of VEGFR2 protein expression. Bottom: densitometric scans in relative optical density (ROD) expressed as a ratio of VEGFR2/Beta-actin. Data are expressed as means ± SE.
Fig. 7.
Effects of C-peptide on renal cortical angiopoietin-1 (ang-1) protein expression. Top: representative immunoblot of angiopoietin-1 protein expression. Bottom: densitometric scans in relative optical density (ROD) expressed as a ratio of ang-1/alpha-actinin. Data are expressed as means ± SE.
3.6 Apoptosis in diabetic rats treated with low doses of insulin
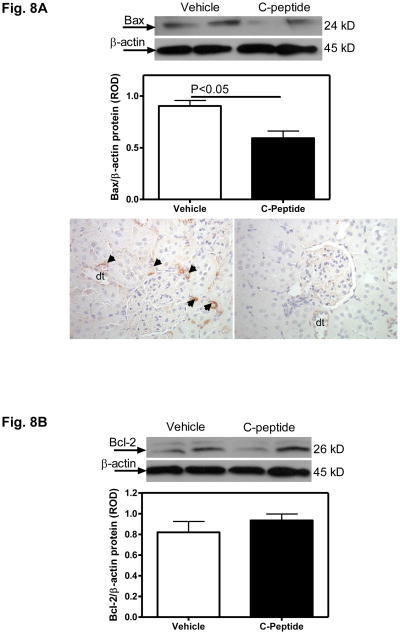
While no difference in the renal cortical Bcl2 protein expression was observed between the vehicle(diabetic) and C-peptide(diabetic)-treated groups (Fig. 8B), there was a 34% decrease in the expression of Bax protein in the renal cortex in the C-peptide compared with vehicle-treated group (Fig. 8A). This decrease in Bax expression was confirmed by immunohistochemistry, which showed an apparent decrease in the amount of staining, particularly evident in the peritubular vasculature (Fig. 7A).
Fig. 8. Effects of C-peptide on renal expression of apoptotic markers.
A. Bax protein expression. Top: representative immunoblot of Bax protein expression. Middle: densitometric scans in relative optical density (ROD) expressed as a ratio of Bax/Beta-actin. Bottom: Bax immunolocalization (brown staining) in pertibular capillaries (arrow heads) and distal tubules (dt); Original magnification x400.
B. Bcl2 protein expression. Top: representative immunoblot of Bcl2 protein expression. Bottom: densitometric scans in relative optical density (ROD) expressed as a ratio of Bcl2/Beta-actin. Data are expressed as means ± SE.
3.7 Effects of C-peptide in diabetic rats in the absence of insulin and in the presence of high doses of insulin
Similar to the effects in diabetic rats receiving low doses of insulin, treatment with C-peptide in diabetic rats not receiving insulin also reduced blood glucose (vehicle, 448±19 mg/dl; C-peptide, 386±15 mg/dl; P<0.05) and HbA1c levels (vehicle, 11.2±0.5; C-peptide, 9.32±0.8; P<0.05). In these rats, C-peptide also reduced UPE (vehicle, 10.2±1.1 % change from baseline; C-peptide, −23.2±1.2 % change from baseline; P<0.05) and GFR (vehicle, 2.84±0.1 ml/min; C-peptide, 2.12±0.3 ml/min; P<0.05). No differences in MAP (vehicle, 108±3 mmHg; C-peptide, 110±3 mmHg), plasma insulin (0.23±0.03 ng/ml; C-peptide, 0.24±0.04 ng/ml) or kidney/body weight ratio (5.45±0.25 mg/g; C-peptide, 5.38±0.13 mg/g) were observed.
In rats treated with higher doses of insulin daily, treatment with C-peptide did not affect either blood glucose (vehicle, 258±22 mg/dL; C-peptide, 284±12 mg/dL) or HbA1c levels (vehicle, 9.1±0.8; C-peptide, 8.9±0.5). Despite no effects on glucose regulation, treatment with C-peptide still reduced UPE (vehicle, 1.2±0.9 % change from baseline; C-peptide, −25.6±0.9 % change from baseline; P<0.05) and GFR (vehicle, 2.70±0.1 ml/min; C-peptide, 2.3±0.1 ml/min; P<0.05). No differences in MAP (vehicle, 111±4 mmHg; C-peptide, 112±5 mmHg), plasma insulin (0.42±0.04 ng/ml; C-peptide, 0.47±0.05 ng/ml) or kidney/body weight ratio (5.06±0.13 mg/g; C-peptide, 5.10±0.26 mg/g) were observed.
4. Discussion
The current study shows that, in animals receiving low doses of insulin, the 4-week treatment with C-peptide administered after an 8-week period of untreated diabetes, reduced UAE, glomerular hyperfiltration, glomerular hypertrophy and macrophage infiltration. Furthermore, treatment with C-peptide increased the density of renal microvessels up to 500 μm in diameter, which was accompanied by reduced renal protein expression of VEGFR2 and pro-apoptotic Bax and increased expression of angiopoietin-1. The renoprotective effects of C-peptide appear to be independent of blood glucose lowering, as similar effects were observed in animals treated without insulin or in the presence of higher doses of insulin.
The present study confirms previous reports that treatment with C-peptide is renoprotective in experimental models as well as patients with type 1 diabetes (Huang et al 2002; Johansson et al 2000; Maezawa et al 2006; Samnegard et al 2005; Samnegard et al 2001). Those studies showed that C-peptide reduces UAE and glomerular hypertrophy and normalizes glomerular hyperfiltration via afferent arteriolar constriction and net dilation of the efferent arteriole (Huang et al 2002; Johansson et al 2000; Maezawa et al 2006; Nordquist et al 2009; Nordquist et al 2008; Samnegard et al 2005; Samnegard et al 2001; Stridh et al 2009). Data from the present study not only confirm that C-peptide is renoprotective in the STZ-induced diabetic rat via similar mechanisms, but also extends those observations by showing that administration of C-peptide slows the progression of the disease in animals with already elevated albuminuria. This is of clinical relevance as it suggests that C-peptide treatment may be useful in patients with already established diabetic renal disease.
Despite the fact that previous studies have shown renoprotective effects of C-peptide in experimental models of type 1 diabetes, few have actually reported the effects of treatment with C-peptide on blood glucose levels. This is surprising given the fact that C-peptide is intimately linked to the formation of insulin and regulation of glucose utilization (Li et al 1999; Sato et al 2004). The present study shows that treatment with C-peptide either in the presence of low doses of insulin or in the absence of insulin was associated with lower HbA1C and blood glucose levels. These observations are consistent with one previous study showing augmented whole body glucose disposal rate following treatment with C-peptide (Li et al 1999). However, the majority of studies have shown no effect of C-peptide on blood glucose levels (Rebsomen et al 2006; Samnegard et al 2005; Samnegard et al 2001; Samnegard et al 2004). The disparate observations between the present and previous observations may be related to differences in the length of the treatment (acute vs. chronic), basal blood glucose levels (administration of C-peptide at the time of initiation of diabetes vs. following a period of uncontrolled diabetes), strain of animals used or even time of day when glucose levels were measured. Consistent with the effects on blood glucose, the present study shows that C-peptide also lowers HbA1c levels, indicating that the blood glucose lowering effect of C-peptide is likely a chronic event.
One possible mechanism by which C-peptide lowers blood glucose levels may be via increasing plasma insulin levels, as observed in the present performed in the presence of low doses of insulin. It is conceivable that C-peptide may increase plasma insulin levels by lowering GFR and thus reducing renal clearance. However, in experiments performed in the absence of exogenous insulin, C-peptide lowered blood glucose levels without affecting plasma insulin levels, suggesting that the ability of C-peptide to reduce blood glucose levels may be independent of insulin. Nonetheless, further investigation is warranted to examine both the mechanisms by which C-peptide both lowers blood glucose and increases plasma insulin levels.
While our data suggest that one of the potential mechanisms by which C-peptide exerts renoprotective effects is via blood glucose lowering, we also show that C-peptide exerts similar renoprotective effects even when blood glucose levels are unchanged, as in the presence of high doses of insulin. In addition, our recent study in an experimental model of chronic, non-diabetic renal disease namely the Dahl-Salt sensitive (DSS) rat fed a 2% NaCl diet, shows that administration of C-peptide is renoprotective independent of blood glucose regulation (Sawyer et al 2012). These observations support the notion that C-peptide may protect the kidney directly as well as indirectly, via glucose lowering.
Diabetes-associated glomerular hyperfiltration is accompanied by increased glomerular capillary volume, as a result of neovascularization, endothelial cell hypertrophy and hyperplasia (Nyengaard & Rasch 1993; Osterby & Nyberg 1987; Seyer-Hansen 1976). These new vessels however are structurally immature and are highly permeable to protein, which is partially responsible for the increased UAE in the early stages of the disease (Nakagawa et al 2009). Our data show that treatment with C-peptide reduces glomerular hypertrophy and UAE. While we did not observe any effect of C-peptide on the density of glomerular endothelial cells (as measured by image analysis of JG12 immunostaining), our recent study showed that C-peptide directly reduces permeability of the glomerular filtration barrier (Maric-Bilkan et al 2012). Thus, the protective effects of C-peptide on glomerular hypertrophy and UAE seem to be more related to preserving the structural integrity of the glomerular filtration surface, reducing its leakiness, rather than regulating glomerular capillary hyperplasia.
In contrast to early diabetic nephropathy, which is characterized by augmented angiogenesis, advanced stages of diabetic nephropathy are believed to be associated with progressive capillary rarefaction (Khavandi et al 2009; Lindenmeyer et al 2007). Interestingly, we recently reported that peritubular microvascular rarefaction and remodeling in fact precedes the decline in renal function in the STZ-induced diabetic rat and may initiate the events leading to the progression of diabetic renal disease (Maric-Bilkan et al 2012). The present study shows that this diabetes-associated microvascular rarefaction is largely reversed by treatment with C-peptide. Specifically, C-peptide increases the density of peritubular microvessels of 0 to 500 μm in diameter, as shown by micro-CT reconstruction and quantification of the renal microvascular architecture and confirmed by image analysis of JG-12 immunostaining. These are previously unrecognized effects of C-peptide and suggest that C-peptide may improve renal function via preserving the integrity of peritubular microvasculature.
It is believed that a major driver of angiogenesis in the diabetic kidney is VEGF, which stimulates endothelial cell proliferation, differentiation and survival, mediates endothelium-dependent vasodilatation and induces microvascular hyperpermeability (Chen & Ziyadeh 2008; Schrijvers et al 2004). However, the present study revealed no effects of C-peptide on protein expression of either VEGF or its receptor VEGFR1 protein expression, but a decrease in VEGFR2 protein expression. These data do not support the involvement of the VEGF pathway in mediating the protective effects of C-peptide on preserving the renal microvascular architecture. However, the increased expression of angiopoietin-1 following C-peptide treatment may explain the increased density of peritubular microvessels. Ang-1 includes promotion of endothelial survival, stabilization of supporting perivascular cells and inhibition of endothelial permeability (Dei Cas & Gnudi 2012). Increased angiopoietin-1 expression has also been associated with improved microvascular density and function in an experimental model of renovascular stenosis (Chade et al 2010).
The present study found that treatment with C-peptide is associated with increased renal peritubular microvascular density, despite the apparent lack of an effect on promoting microvascular angiogenesis. Thus, it is conceivable that C-peptide exerts its protective effect by preventing further loss of microvessels due to prolonged diabetes. We thus examined the effects of C-peptide on the expression of pro- and anti-apoptotic markers, Bax and Bcl2, respectively (Ghiotto et al 2010). While we found no effect of C-peptide on the expression of Bcl2, the expression of Bax was reduced, especially in the peritubular capillaries. These observations suggest that C-peptide may be protective by decreasing the expression of pro-apoptotic molecules and thus preventing the loss of peritubular renal microvessels.
One of the shortcomings of the study is that we used STZ-induced diabetic rats, which do not fully recapitulate all aspects of human diabetic nephropathy.
5. Conclusion
The current study shows that C-peptide in the STZ-induced diabetic rat is renoprotective independent of blood glucose regulation. The renoprotective effects of C-peptide were observed after a period of untreated diabetes, which supports C-peptide therapy as a potential intervention for slowing or possibly, reversing the progression of renal injury in patients with already established diabetic renal disease.
Acknowledgments
The authors would like to thank Ms. Stephanie Evans for technical assistance with paraffin sectioning and Ms. Haiyan Zhang for measurement of plasma insulin levels. This work was supported by National Institutes of Health Grants DK075832 (C. Maric-Bilkan), HL095638 (A. Chade) and HL51971 (J.E. Hall for the support of core facilities used for part of the study) as well as the Intramural Research grant C. Maric-Bilkan).
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chade AR, Zhu XY, Krier JD, Jordan KL, Textor SC, Grande JP, Lerman A, Lerman LO. Endothelial progenitor cells homing and renal repair in experimental renovascular disease. Stem Cells. 2010;28:1039–1047. doi: 10.1002/stem.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Ziyadeh FN. Vascular endothelial growth factor and diabetic nephropathy. Current Diabetes Reports. 2008;8:470–476. doi: 10.1007/s11892-008-0081-3. [DOI] [PubMed] [Google Scholar]
- Dei Cas A, Gnudi L. VEGF and angiopoietins in diabetic glomerulopathy: how far for a new treatment? Metabolism. 2012;61:1666–1673. doi: 10.1016/j.metabol.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Faber OK, Binder C. B-cell function and blood glucose control in insulin dependent diabetics within the first month of insulin treatment. Diabetologia. 1977;13:263–268. doi: 10.1007/BF01219710. [DOI] [PubMed] [Google Scholar]
- Ghiotto F, Fais F, Bruno S. BH3-only proteins: the death-puppeteer’s wires. Cytometry A. 2010;77:11–21. doi: 10.1002/cyto.a.20819. [DOI] [PubMed] [Google Scholar]
- Hills CE, Brunskill NJ, Squires PE. C-peptide as a therapeutic tool in diabetic nephropathy. American Journal of Nephrology. 2010;31:389–397. doi: 10.1159/000289864. [DOI] [PubMed] [Google Scholar]
- Horwitz DL, Starr JI, Mako ME, Blackard WG, Rubenstein AH. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. Journal of Clinical Investigation. 1975;55:1278–1283. doi: 10.1172/JCI108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DY, Richter K, Breidenbach A, Vallon V. Human C-peptide acutely lowers glomerular hyperfiltration and proteinuria in diabetic rats: a dose-response study. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2002;365:67–73. doi: 10.1007/s00210-001-0502-1. [DOI] [PubMed] [Google Scholar]
- Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrology Dialysis Transplantation. 2010;25:1079–1087. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson BL, Borg K, Fernqvist-Forbes E, Kernell A, Odergren T, Wahren J. Beneficial effects of C-peptide on incipient nephropathy and neuropathy in patients with Type 1 diabetes mellitus. Diabetic Medicine. 2000;17:181–189. doi: 10.1046/j.1464-5491.2000.00274.x. [DOI] [PubMed] [Google Scholar]
- Khavandi K, Greenstein AS, Sonoyama K, Withers S, Price A, Malik RA, Heagerty AM. Myogenic tone and small artery remodelling: insight into diabetic nephropathy. Nephrology Dialysis Transplantation. 2009;24:361–369. doi: 10.1093/ndt/gfn583. [DOI] [PubMed] [Google Scholar]
- Li L, Oshida Y, Kusunoki M, Yamanouchi K, Johansson BL, Wahren J, Sato Y. Rat C peptide I and II stimulate glucose utilization in STZ-induced diabetic rats. Diabetologia. 1999;42:958–964. doi: 10.1007/s001250051254. [DOI] [PubMed] [Google Scholar]
- Lindenmeyer MT, Kretzler M, Boucherot A, Berra S, Yasuda Y, Henger A, Eichinger F, Gaiser S, Schmid H, Rastaldi MP, Schrier RW, Schlondorff D, Cohen CD. Interstitial vascular rarefaction and reduced VEGF-A expression in human diabetic nephropathy. Journal of American Society of Nephrology. 2007;18:1765–1776. doi: 10.1681/ASN.2006121304. [DOI] [PubMed] [Google Scholar]
- Maezawa Y, Yokote K, Sonezaki K, Fujimoto M, Kobayashi K, Kawamura H, Tokuyama T, Takemoto M, Ueda S, Kuwaki T, Mori S, Wahren J, Saito Y. Influence of C-peptide on early glomerular changes in diabetic mice. Diabetes/Metabolism Research and Reviews. 2006;22:313–322. doi: 10.1002/dmrr.612. [DOI] [PubMed] [Google Scholar]
- Mankhey R, Wells CC, Bhatti F, Maric C. 17-beta estradiol supplementation reduces tubulointerstitial fibrosis by increasing MMP activity in the diabetic kidney. American Journal of Physiology- Regulatory, Intergrative and Comparative Physiology. 2006;292:R769–R777. doi: 10.1152/ajpregu.00375.2006. [DOI] [PubMed] [Google Scholar]
- Maric-Bilkan C, Flynn ER, Chade AR. Microvascular disease precedes the decline in renal function in the streptozotocin-induced diabetic rat. American Journal of Physiology- Renal Physiology. 2012;302:F308–F315. doi: 10.1152/ajprenal.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009;58:1471–1478. doi: 10.2337/db09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist L, Brown R, Fasching A, Persson P, Palm F. Proinsulin C-peptide reduces diabetes-induced glomerular hyperfiltration via efferent arteriole dilation and inhibition of tubular sodium reabsorption. American Journal of Physiology- Renal Physiology. 2009;297:F1265–F1272. doi: 10.1152/ajprenal.00228.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordquist L, Lai EY, Sjoquist M, Patzak A, Persson AE. Proinsulin C-peptide constricts glomerular afferent arterioles in diabetic mice. A potential renoprotective mechanism. American Journal of Physiology- Regulatory, Intergrative and Comparative Physiology. 2008;294:R836–R841. doi: 10.1152/ajpregu.00811.2007. [DOI] [PubMed] [Google Scholar]
- Nordquist L, Moe E, Sjoquist M. The C-peptide fragment EVARQ reduces glomerular hyperfiltration in streptozotocin-induced diabetic rats. Diabetes/Metabolism Research and Reviews. 2007;23:400–405. doi: 10.1002/dmrr.704. [DOI] [PubMed] [Google Scholar]
- Nyengaard JR, Rasch R. The impact of experimental diabetes mellitus in rats on glomerular capillary number and sizes. Diabetologia. 1993;36:189–194. doi: 10.1007/BF00399948. [DOI] [PubMed] [Google Scholar]
- Osterby R, Nyberg G. New vessel formation in the renal corpuscles in advanced diabetic glomerulopathy. Journal of Diabetes and its Complications. 1987;1:122–127. doi: 10.1016/s0891-6632(87)80069-7. [DOI] [PubMed] [Google Scholar]
- Rebsomen L, Pitel S, Boubred F, Buffat C, Feuerstein JM, Raccah D, Vague P, Tsimaratos M. C-peptide replacement improves weight gain and renal function in diabetic rats. Diabetes and Metabolism. 2006;32:223–228. doi: 10.1016/s1262-3636(07)70272-0. [DOI] [PubMed] [Google Scholar]
- Roth J, Whitford I, Dankner R, Szulc AL. How the immunoassay transformed C-peptide from a duckling into a swan. Diabetologia. 2012;55:865–869. doi: 10.1007/s00125-011-2421-0. [DOI] [PubMed] [Google Scholar]
- Samnegard B, Jacobson SH, Jaremko G, Johansson BL, Ekberg K, Isaksson B, Eriksson L, Wahren J, Sjoquist M. C-peptide prevents glomerular hypertrophy and mesangial matrix expansion in diabetic rats. Nephrology Dialysis Transplantation. 2005;20:532–538. doi: 10.1093/ndt/gfh683. [DOI] [PubMed] [Google Scholar]
- Samnegard B, Jacobson SH, Jaremko G, Johansson BL, Sjoquist M. Effects of C-peptide on glomerular and renal size and renal function in diabetic rats. Kidney International. 2001;60:1258–1265. doi: 10.1046/j.1523-1755.2001.00964.x. [DOI] [PubMed] [Google Scholar]
- Samnegard B, Jacobson SH, Johansson BL, Ekberg K, Isaksson B, Wahren J, Sjoquist M. C-peptide and captopril are equally effective in lowering glomerular hyperfiltration in diabetic rats. Nephrology Dialysis Transplantation. 2004;19:1385–1391. doi: 10.1093/ndt/gfh163. [DOI] [PubMed] [Google Scholar]
- Sato Y, Oshida Y, Han YQ, Morishita Y, Li L, Ekberg K, Jornvall H, Wahren J. C-peptide fragments stimulate glucose utilization in diabetic rats. Cellular and Molecular Life Sciences. 2004;61:727–732. doi: 10.1007/s00018-003-3460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer RT, Flynn ER, Hutchens ZM, Jr, Williams JM, Garrett MR, Maric-Bilkan C. Renoprotective effects of C-peptide in the Dahl salt-sensitive rat. A American Journal of Physiology- Renal Physiology. 2012;303:F893–F899. doi: 10.1152/ajprenal.00068.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney International. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- Seyer-Hansen K. Renal hypertrophy in streptozotocin-diabetic rats. Clinical Science and Molecular Medicine Suppl. 1976;51:551–555. doi: 10.1042/cs0510551. [DOI] [PubMed] [Google Scholar]
- Sjoquist M, Huang W, Johansson BL. Effects of C-peptide on renal function at the early stage of experimental diabetes. Kidney Int. 1998;54:758–64. doi: 10.1046/j.1523-1755.1998.00074.x. [DOI] [PubMed] [Google Scholar]
- Steiner DF. The proinsulin C-peptide--a multirole model. Experimental Diabesity Research. 2004;5:7–14. doi: 10.1080/15438600490424389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stridh S, Sallstrom J, Friden M, Hansell P, Nordquist L, Palm F. C-peptide normalizes glomerular filtration rate in hyperfiltrating conscious diabetic rats. Advances in Experimental Medicine and Biology. 2009;645:219–225. doi: 10.1007/978-0-387-85998-9_34. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wells CC, Garman JH, Asico L, Escano CS, Maric C. Imbalance in sex hormone levels exacerbates diabetic renal disease. Hypertension. 2008;51:1218–1224. doi: 10.1161/HYPERTENSIONAHA.107.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]