Abstract
Previous studies suggest that individuals with elevated levels of cortisol (the “stress hormone”) could be particularly resistant to treatment for depression. However, most of these studies have been conducted in the context of antidepressant medications, and no study has examined pretreatment cortisol levels as a predictor of treatment outcomes among older adults with depression in cognitive-behavioral therapy (CBT), despite the relevance of this population for such a research question. The current study includes 54 older adults with depression who provided salivary cortisol samples at baseline and completed measures of depression at pretreatment and posttreatment, following a 12-week course of CBT. Structural equation modeling results suggest that those with higher daily outputs of cortisol and flatter diurnal slopes were less likely to benefit from CBT—a finding which if replicated could have important implications for clinical practice and future research.
Keywords: HPA, Treatment Response, Psychotherapy, Biomarkers, Predictive Study
1. Introduction
Depression is one of the most commonly occurring psychological problems in late life. In particular, the prevalence of major depressive disorder (MDD) ranges from 1-4% among community dwelling elders, to 5-10% among primary care patients, to 10-12% among older adults hospitalized for medical and surgical services, to about 13% among home health care patients (Bruce et al., 2002; Blazer, 2003). Of course, these figures likely underestimate the impact of depression among older adults, as 8-16% of community dwelling elders tend to report elevated levels of depressive symptoms, which may or may not meet criteria for MDD (Blazer, 2003). Elevated levels of depression are a serious concern for older adults and have been shown to hasten the progression of a number of health conditions and also increase the likelihood of hospital admission and nursing home placement (Rumsfeld et al., 2005; van Gool et al., 2005; Harris and Cooper, 2006).
Generally speaking, meta-analyses of outcome studies for depression among older adults have revealed substantial treatment effects, with those in a treatment condition being between .61 and .78 of a standard deviation better off than controls on measures of depression (Scogin and McElreath, 1994; Engels and Vermey, 1997; Cuijpers et al., 2006). More specifically, Scogin et al. (2005) have identified six evidence-based psychological treatments for geriatric depression, three of which primarily rely on cognitive and/or behavioral strategies. Cognitive-behavioral therapy (CBT) tends to emphasize the role of unhelpful, negative thoughts in maintaining depressive symptoms and aims to gently challenge these cognitions in an effort to promote more rational thinking and improved mood. Notably, CBT treatments also tend to incorporate behavioral strategies (e.g., increasing pleasant activities, relaxation) and protocols have been developed that are specifically designed for older adults (Laidlaw et al., 2003).
Notwithstanding the efficacy of CBT for late-life depression, clinicians and researchers have noted variability in treatment response, highlighting the need for identification of predictors of outcome (Kiosses et al., 2011). Previous studies have found that treatment response is not strongly influenced by demographic factors (e.g., age, gender, education) or co-morbid anxiety disorders; although, there is some evidence suggesting that those with more severe depressive symptomatology at baseline may show greater improvements at posttreatment (Kiosses et al., 2011). Given the limited amount of information available on predictors of response to CBT for late-life depression, the present study aimed to examine cortisol as a predictor of treatment response, which has not been tested previously in the context of CBT for geriatric depression.
Cortisol, often referred to as the “stress hormone,” is one of the most widely used biomarkers of hypothalamic-pituitary-adrenocortical (HPA) axis activation, and when abnormally elevated (in the case of hypercortisolism), it can be an indicator of stress and psychiatric disturbance (Lovallo, 2005). In particular, the link between hypercortisolism and depression is well established and has been documented in numerous studies over the past four decades (Holsboer, 2000; Burke et al., 2005; Neigh and Nemeroff, 2006; Pariante and Lightman, 2008; Stetler and Miller, 2011). In these previous studies hypercortisolism has been assessed with a variety of methodologies, including measurement of 24-hour urinary cortisol excretion, cortisol diurnal rhythm, and overnight high-dose dexamethasone suppression test (for a review of these methods, see Hasinski, 1998).
When assessed at multiple times across the day, cortisol's normal diurnal rhythm (characterized by peak levels at or near wake that then sharply decline across the day) can become flattened in the context of chronic stress and/or depression, as levels of cortisol in the body remain more elevated throughout the day (Ockenfels et al. 1995; Smyth et al., 1997). Thus, a daily pattern characterized by peak levels of cortisol at wake and a steep negative slope is generally regarded as “normal;” whereas, a shallower or flatter cortisol slope is typically considered less healthy. Miller et al.'s (2007) meta-analysis supported this idea and found that, compared to non-stressed controls, chronically stressed groups generally had a dysregulated pattern of hormone secretion, with lower than normal morning output but higher than expected secretion across the rest of the day, often yielding a flattened diurnal pattern and/or higher overall output of cortisol throughout the day.
Another recent meta-analysis has shown that, when compared to non-depressed controls, older adults with depression are characterized by greater HPA activation, more so than for younger adults (Stetler and Miller, 2011)—highlighting the relevance of cortisol as a crucial biomarker for geriatric populations. Notably, normal aging has been shown in a number of studies to be associated with cortisol dysregulation, as indicated by increased corticotropin-releasing factor production (Meijer et al., 2005; Swaab et al., 2005), diminished HPA axis sensitivity to pharmacological challenge (Hatzinger et al., 2011), shorter nocturnal cortisol nadirs, and increased 24-hour cortisol levels (Van Cauter et al., 1996).
Glucocorticoids have also been implicated as contributing to brain aging (Nichols et al., 2001), and the association between hypercortisolism and cognitive impairment, across a variety of domains (e.g., executive functioning, visual-spatial abilities, working memory, delayed verbal recall), is well documented (Lupien et al., 1994; Seeman et al., 1997; Belanoff et al., 2001; Li et al., 2006; O'Hara et al., 2007; Reppermund et al., 2007; Gomez et al., 2009; Beluche et al., 2010; Franz et al., 2011). These cognitive deficits may in turn inhibit older adults' response to cognitively-based treatments (Mohlman, 2005; Mohlman and Gorman, 2005; Caudle et al., 2007). Other studies suggest that abnormal cortisol profiles may also be related to difficulties with emotion regulation (Stansbury and Gunnar, 1994; Giese-Davis et al., 2006; Lam et al., 2009) , which may further impede progress in CBT.
Indeed, clients with depression and HPA dysregulation have been shown to be particularly treatment resistant. The vast majority of these studies have been conducted in the context of antidepressant medications (Young et al., 2004; Horstmann and Binder, 2011), but several studies have also observed an association between pretreatment cortisol levels and posttreatment outcomes following a psychosocial intervention (Bockting et al., 2006; Jason et al., 2008; Yehuda et al., 2009; Otto et al., 2010; Bockting et al., 2012). In particular, some preliminary evidence suggests that hypercortisolism predicts poorer response to CBT (Thase et al., 1993; Thase et al., 1996; Thase and Friedman, 1999). For example, one study, with roughly 30 inpatients who received up to 20 sessions of CBT for depression, found that those with higher concentrations of urinary free cortisol at pretreatment were less likely to show improvements at posttreatment on self-reported and clinician administered assessments of depression (Thase et al., 1993; Thase et al., 1996). Notwithstanding these intriguing findings with a mixed age sample, the link between cortisol and treatment outcomes following CBT for older adults with depression has not been examined previously.
Considering the important role of cortisol in geriatric depression and the potential implications of HPA dysregulation for responsiveness to CBT, the present study aims to examine the link between pretreatment salivary cortisol profiles (as assessed by diurnal slopes and overall daily cortisol output) and posttreatment outcomes (assessed using self-report and clinician-rated measures) following CBT for late-life depression. It is hypothesized that those with more abnormal cortisol profiles (i.e., flatter diurnal slopes and elevated daily outputs) will exhibit poorer treatment outcomes.
2. Methods
2.1. Participants
This study draws upon data collected from 60 older adults with depression recruited as part of a larger study on predictors of response to CBT for late-life depression. In the present analysis, 54 participants were included, given that six participants did not provide two or more reliable saliva samples. These six excluded participants did not significantly differ from the rest of the sample in terms of demographic factors or baseline depressive symptoms. Participants' demographic and background information are presented in Table 1.
Table 1. Demographic and Background Information (N = 54).
| N | % | |
|---|---|---|
| Gender | ||
| Men | 21 | 38.9% |
| Women | 33 | 61.1% |
| Ethnicity/Race | ||
| Caucasian | 39 | 72.2% |
| African American | 2 | 3.7% |
| Asian American | 6 | 11.1% |
| Latino/Hispanic | 5 | 9.3% |
| Native American | 2 | 3.7% |
| Marital Status | ||
| Married | 20 | 37.7% |
| Divorced | 17 | 32.1% |
| Widowed | 8 | 15.1% |
| Separated | 3 | 5.7% |
| Single | 5 | 9.4% |
| Antidepressant Usage | ||
| Yes | 13 | 24.1% |
| No | 41 | 75.9% |
| Hormone Replacement Therapy Usage | ||
| Yes | 2 | 3.7% |
| No | 52 | 96.3% |
|
| ||
| Mean | SD | |
|
| ||
| Age | 70.2 | 7.5 |
| Years of Education | 15.0 | 2.6 |
| Pretreatment SF-12 Physical Health | 51.1 | 12.0 |
| Pretreatment SF-12 Mental Health | 34.0 | 10.4 |
| Diurnal Cortisol Area Under the Curve | 2.5 | 1.3 |
| Diurnal Cortisol Slope | -0.01 | 0.01 |
| Pretreatment CES-D | 27.4 | 9.1 |
| Posttreatment CES-D | 18.8 | 12.0 |
| Pretreatment BDI-II | 21.5 | 10.0 |
| Posttreatment BDI-II | 13.6 | 10.4 |
| Pretreatment HRSD | 14.4 | 5.6 |
| Posttreatment HRSD | 11.5 | 7.5 |
Note: SF-12 = Short Form – 12, CES-D = Center for Epidemiological Studies—Depression, BDI-II = Beck Depression Inventory II, HRSD = Hamilton Rating Scale for Depression
2.2. Procedure
Following IRB approval, participants were recruited throughout the San Francisco Bay Area, via radio, newspaper and internet advertisements, flyers, free talks in senior centers, and through referrals from other professionals. Individuals who responded to these recruitment efforts (N = 156) were preliminarily screened over the telephone using the Patient Health Questionnaire – 9 (Kroenke et al., 2001). Individuals who scored above a 5 (N = 139) were invited to complete a more thorough in-person assessment after signing a consent form.
During this in-person assessment, 60 participants were ultimately determined to be eligible for the study. In order to be eligible, individuals had to score 16 or higher on the Center for Epidemiologic Studies Depression Scale (Radloff, 1977) and meet diagnostic criteria for some type of current depressive disorder (e.g., major depression, dysthymic disorder, adjustment disorder with depressed mood), as assessed by the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998). The most common primary diagnosis was Major Depressive Disorder, Current (N = 19, 35.2%), followed by Minor Depressive Disorder (N = 13, 24.1%), Dysthymia (N = 10, 18.5%), Mixed Anxiety Depressive Disorder (N = 6, 11.1%), Adjustment Disorder with Depressed Mood (N = 4, 7.4%) and Major Depressive Disorder, Recurrent (N = 2, 3.7%).
Those who reported active suicidal ideation or plan, a history of psychosis or mania, or active substance abuse at baseline were excluded from the study, as well as any individuals who showed frank evidence of some type of dementia. To be eligible, participants also needed to have access to reliable transportation and be able to attend 12 in-person sessions. Participants who were taking antidepressants at the time of the baseline assessment were permitted to participate in the study; however, all participants were encouraged to not alter their antidepressant usage in any way while in the study.
After undergoing a baseline assessment, participants completed 12 1-hour individual sessions (across 3- to 4-months) of CBT for late-life depression, based on a previously published manual (Gallagher-Thompson and Thompson, 2010). After establishing treatment goals (session 1), this CBT protocol focused on training and practicing skills, including behavioral activation (sessions 2-5), cognitive restructuring (sessions 6-9), interpersonal effectiveness (session 10), and relaxation techniques (presented in sessions 1 and 5). The final two sessions were spent reviewing progress and constructing a maintenance plan to help solidify the progress that had been made. Notably, all of these skills have been identified as being key components of CBT in prior research with older adults (Holland and Gallagher-Thompson, 2011).
CBT was provided individually by postdoctoral and advanced pre-doctoral clinical psychology students who were trained in the protocol for 3 months and monitored (through audiotaping) for protocol adherence and CBT competency. Notably, the pre- to posttreatment effect size for the intervention was .81 on the Center for Epidemiological Studies—Depression Measure (CES-D; Radloff, 1977), .77 on the Beck Depression Inventory II (BDI II; Beck et al., 1996), and .44 on the Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960), with an average effect of .67, which is consistent with findings from other studies on the efficacy of CBT for late-life depression (Pinquart et al., 2007). Paired t tests revealed that pre- to posttreatment reductions in depressive symptomatology were statistically significant for all three of these outcome variables (with p values ranging from <.001 to .03). Following the completion of CBT, participants completed posttreatment self-report and interview-based assessments of depression. Notably, all of the participants who entered the study completed the posttreatment assessment.
2.3. Measures
2.3.1. Cortisol
Salivary cortisol was measured at baseline by instructing participants to collect saliva using an oral swab kit at wake, 5:00 PM, and 9:00 PM across two consecutive days. Participants were instructed to freeze each sample immediately after collection and return it to research staff at their next appointment, where it was then stored in a laboratory freezer before being assayed. They were also told not to eat, drink, smoke, brush their teeth, or use mouthwash in the 30 minutes before collection and not to drink alcohol during the 8-10 hours before collecting samples or during the two days of collection. Participants were asked to record the exact time of saliva collection as well as any events that might have influenced the sample (e.g., recent smoking or alcohol use) in a sample collection log. On average, saliva samples were collected at 7:14am (SD = 1.3 hours), 5:07pm (SD = 1.3 hours), and 8:12pm (SD = 3.9 hours). Out of the 327 saliva samples that were collected as part of this study, participants reported violating protocol (e.g.., drinking, smoking, and/or eating less than 30 minutes prior to using the swab) for 25 of the samples. Samples that were collected improperly were not used in this analysis.
All samples were assayed for salivary cortisol using a highly sensitive enzyme immunoassay (Salimetrics, State College, PA). The test used 25 μL of saliva per determination, has a lower limit of sensitivity of 0.003 (μg/dL, standard curve range from 0.012 (μg/dL to 3.0 μg/dL, an average intra-assay coefficient of variation of 3.5% and an average inter-assay coefficient of variation of 5.1%. Method accuracy determined by spike and recovery averaged 100.8%, and linearity determined by serial dilution averaged 91.7%. Values from matched serum and saliva samples show the expected strong linear relationship, r(47) = 0.91, p < 0.0001.
To correct for positive skewness, cortisol values were log transformed1. Data collected from the saliva samples across the two consecutive days were combined in order to allow for a more reliable estimate of each participants typical diurnal pattern (Kraemer et al., 2006). Consistent with prior recommendations (Fekedulegn et al., 2007), we derived two summary measures of diurnal cortisol that served as the primary independent variables: (1) the absolute magnitude of cortisol output was captured by the area under the curve (AUC) and (2) the pattern over time was captured by the slope of the best fitting line for the cortisol measurements. AUC was calculated using Simpson's rule for numeric integration (for a comparison of different methods of computing AUC, see Le Floch et al., 1990), and slope was represented by unstandardized beta coefficients when log-cortisol was regressed onto hours since wake for each participant.
2.3.2. Control variables
In our main analysis, several variables were also included as covariates. Specifically, demographic factors (age, gender, ethnicity/race, years of education, and marital status) as well as antidepressant medication and estrogen hormone replacement therapy usage were incorporated in the model.
Baseline assessments of general physical and mental health, as measured by the Short-Form Health Survey – 12 (SF-12; Ware et al., 1996), were also included. Notably, the mental and physical health component summary scores of the SF-12 have been shown to correlate highly with the summary scores of the longer 36-item version of this measure (r = .97 and .95, respectively) and have demonstrated strong 2-week test-retest reliability (r = .76 and .89, respectively; Ware et al., 1996). Other studies have also found that the physical health component of the SF-12 is associated with a number of physical health issues, including smoking (Haddock et al., 2003), white matter hyperintensitites (Sachdev et al., 2005), mild cognitive disorders (Sargent-Cox et al., 2010), body mass index (Finkelstein et al., 2000), and difficulties with physical functioning (Johnson and Coons, 1998). Likewise, the mental health component of the SF-12 has been shown to correlate with depression and anxiety disorders (Gill et al., 2007) as well as with psychiatric comorbidity among outpatients with depression (Rush et al., 2005).
2.3.3. Depression measures
Depression measures were administered at pre- and posttreatment and included the HRSD (Hamilton, 1960), CES-D (Radloff, 1977), and the BDI-II (Beck et al., 1996).
The HRSD is an interviewer-rated tool composed of 17 questions, eight on a five-point scale from 0 to 4 and nine on a three-point scale from 0 to 2, with higher scores representing greater severity of depressive symptoms. A recent meta-analytic study showed that the HRSD has good test-retest reliability (Mean r = .87), internal consistency (Mean α = .79), and inter-rater reliability (Mean r = .87) (Trajkovi ć et al., 2011), in addition to correlating highly with other established depression measures (Whisman et al., 1989).
The CES-D consists of 20 declarative statements regarding one's depressive symptoms to which the frequency of symptoms in the past week are indicated on a 4-point Likert-type scale ranging from 0 = Rarely or none of the time to 3 = Most or almost all the time. The CES-D has been used successfully with diverse older adult samples in numerous studies (Mui, Burnette, and Chen, 2002). For example, in geriatric samples this measure has been shown to have good internal consistency (α = .85), split-half reliability (r = .74), moderate consistency over a period of three years (r = .44), and the ability to discriminate between clinical and non-clinical groups (Chi and Boey, 1993; Callahan and Wolinsky, 1994; Boey, 1999).
The BDI-II, which is a 21-item self-report measure, was also used to assess depressive symptoms in the past two weeks. The frequency/intensity of each symptom is rated on a Likert scale ranging from 0 to 3, with higher scores indicating higher levels of depression. A recent study found that the BDI-II's internal consistency (α = .86) and convergent validity with other established measures of depression (e.g., r = .69 with the CES-D) was strong for a sample of community-dwelling older adults (Segal et al., 2008).
Response to CBT was represented by % change scores from pretreatment to posttreatment for each of the depression measures. For this sample, % change scores were the most independent of baseline depressive symptoms (p's = .07 - .87, for correlations with pretreatment values), compared to other traditional ways of representing pretreatment to posttreatment change, such as raw change scores (p's = .001 - .004, for correlations with pretreatment values).
2.4. Plan of analysis
To examine baseline diurnal cortisol (as assessed by the diurnal slope and AUC) as a predictor of depression outcomes, we first computed bivariate correlations with % change for the three depression measures as well as with demographic factors (i.e., age, gender, ethnicity/race, marital status, and years of education).
In order to provide a more complete picture of the interrelationships among these variables, we also used structural equation modeling to examine the unique contribution of baseline diurnal cortisol slope and AUC in predicting improvement in depression symptoms from baseline to posttreatment. In this model, depression outcome was modeled as a latent variable, and % improvement on the CES-D, BDI-II, and HRSD were used as the observed indicators. This model was tested with and without covariates. Covariates were modeled as correlated predictors of depression outcome. Covariates included age, gender, ethnicity/race, years of education, marital status, antidepressant usage, estrogen hormone replacement therapy usage, as well as pretreatment general mental and physical health (as assessed by the SF-12 component scales).
We relied upon a variety of fit indices to assess model fit, including the χ2 goodness-of-fit test, comparative fit index (CFI; Bentler, 1990), and RMSEA (Browne and Cudeck, 1993). CFI values > 0.90 are generally regarded as favorable (Hu and Bentler, 1999; Kline, 2005). Likewise, RMSEA values ≤ 0.05 are considered close approximate fit, values between 0.05 and 0.08 suggest reasonable fit, and values ≥ 0.10 are indicative of poor model fit (Browne and Cudeck, 1993). SEM analyses were performed in MPlus, Version 6.1 (Muthén and Muthén, 1998-2010) and parameters were estimated using a generalized least squares (GLS) procedure, which has been shown to perform well with smaller samples (Olsson et al., 2000).
3. Results
3.1. Bivariate analyses
As can be seen in Table 2, gender was significantly associated with diurnal cortisol area under the curve (p = .03) and showed a trend toward significance for diurnal cortisol slope (p = .08). In particular, men were more likely than women to show a pattern of diurnal cortisol characterized by higher overall daily production of cortisol (as assessed by area under the curve) but with a somewhat sharper negative slope. Those with better physical health also had significantly less overall daily production of cortisol (p = .03).
Table 2. Bivariate Associations of Cortisol Indicators with Depression Outcomes and Demographic Factors.
| Diurnal Cortisol Area Under the Curve | Diurnal Cortisol Slope | |
|---|---|---|
| Age | .21 | .07 |
| Gender (0 = Female, 1 = Male) | .30* | -.24† |
| Ethnicity/Race (0 = Caucasian, 1 = Ethnic/Racial Minority) | .05 | .22 |
| Marital Status (0 = Not Married, 1 = Married) | .10 | -.11 |
| Years of Education | -.13 | .03 |
| Antidepressant Usage (0 = No, 1 = Yes) | -.09 | -.14 |
| Hormone Replacement Therapy Usage (0 = No, 1 = Yes) | -.02 | -.11 |
| SF-12 Physical Health | -.29* | .03 |
| SF-12 Mental Health | .19 | -.23 |
| CES-D % Improvement | -.14 | -.26† |
| BDI-II % Improvement | -.05 | -.08 |
| HRSD % Improvement | -.24† | -.06 |
p < .05,
< .10.
SF-12 = Short Form – 12, CES-D = Center for Epidemiological Studies—Depression, BDI-II = Beck Depression Inventory II, HRSD = Hamilton Rating Scale for Depression.
With regard to the depression outcomes, there was a trend for those with a larger AUC and shallower diurnal cortisol slope to show less improvement from baseline to posttreatment. These findings approached statistical significance for diurnal cortisol slope and % improvement on the CES-D (p = .09) as well as for area under the curve and % HRSD improvement (p = .09).
3.2. Structural equation model
We first tested a model with our two summary measures of diurnal cortisol (slope and AUC) as the independent variables and depression outcome (modeled as a latent variables with % improvement on the CES-D, BDI-II, and HRSD as indicators) as the dependent variable. When tested simultaneously in this way, flatter diurnal cortisol slopes and larger areas under the curve at baseline were found to be significantly associated with worse outcomes following CBT (p = .004 and .02, respectively). Overall, this model was found to fit the data well, χ2(5) = 5.39, p = .37; CFI = 0.97; RMSEA = 0.038, 90% CI =0 .000 - 0.196.
Model fit remained strong when covariates were added to the model, χ2(23) = 21.98, p = .52; CFI = 1.00; RMSEA = 0.000, 90% CI = 0.000 - 0.1082. As can be seen in Figure 1, even in the presence of covariates, both diurnal cortisol slope and area under the curve significantly predicted depression outcome (p = .01 and .03, respectively), with more shallow slopes and larger areas under the curve being associated with a poorer response to CBT. Of the covariates included in the model, only age significantly predicted depression outcome (p = .01), with older individuals showing somewhat less favorable outcomes3.
Figure 1.
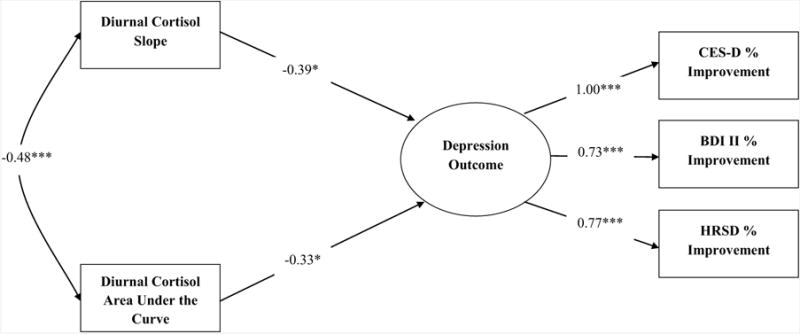
Structural equation model with cortisol indices predicting depression outcomes following a psychoeducational cognitive-behavioral intervention. Standardized estimates are presented. Demographic factors (i.e., age, ethnicity/race, gender, years of education, and marital status), medication usage (i.e., antidepressants and hormone replacement therapy), and baseline mental/physical health were included as covariates, but are not presented in the diagram.
4. Discussion
Overall, the results of this study provide preliminary support to the hypothesis that cortisol dysregulation is associated with poorer outcomes among older adults in CBT. Specifically, when examined simultaneously, more shallow slopes and larger areas under the curve were associated with a poorer response to CBT. From a methodological standpoint, these results support examining multiple summary measures of diurnal cortisol that capture both the absolute magnitude of cortisol production as well as the pattern or slope over time. In the bivariate analyses, AUC and slope for diurnal cortisol showed small to moderate associations with treatment outcome on the depression measures, with only two of the correlations approaching statistical significance. However, when these two diurnal cortisol summary measures were examined simultaneously in the SEM analysis, both were significantly associated with outcome in the expected direction, which suggests the presence of suppression effects. For example, a steep negative diurnal cortisol slope may not be as powerful of a predictor of positive outcomes, if it does not translate into lower overall cortisol output. Similarly, the relationship between high overall cortisol output and negative outcomes may not be as strong if the trajectory of the slope across the day is not taken into account. Taken together, these findings suggest that individuals who have increased cortisol output which does not resolve over the course of the day (resulting in flatter slopes) may be the poorest responders to CBT for late-life depression.
Although various treatments for depression can have a positive impact on HPA axis functioning, roughly 56% show similar coritsol profiles pre- and posttreatment regardless of symptom improvement (McKay and Zakzanis, 2010; Holland et al., 2011). With regard to older adults, specifically, it has been shown that many still show abnormal HPA functioning, even after successful treatment for depression (Beluche et al., 2009), putting them at greater risk for relapse (O'Toole et al., 1997; Zobel et al., 2001; Appelhof et al., 2006; Aubry et al., 2007; Pintor et al., 2009). Thus, the present findings suggest that depressed older adults with hypercortisolism may require treatment beyond what is traditionally offered. If our findings are replicated in controlled trials with larger, more diverse samples, researchers may begin exploring alternate treatments for depressed older adults with abnormal cortisol profiles. For example, a number of pharmacological agents that specifically target the HPA axis have been proposed (Thomson and Craighead, 2008).
With regard to future research, it is also notable that the association between HPA dysregulation and impairments in cognitive function in older adults is well documented (O'Hara et al., 2007), and many have proposed interactive and reciprocal effects between glucocorticoid function, brain aging and cognition in late life depression (Byers and Yaffe, 2011). However, the number of studies directly addressing this issue is limited, and not all studies have observed interactive effects between cortisol and depression on cognitive functioning in late-life depression. For example, Köhler and colleagues (2010) observed that white matter hyperintensities, rather than cortisol levels or brain atrophy, are associated with continuing cognitive impairments in older adults with depression. However, O'Brien and colleagues (2004) found that increased cortisol levels were significantly correlated with continuing memory deficits at a 6 months follow-up in over sixty patients with late-life depression. These findings raise the possibility that cognitive impairment associated with higher cortisol levels may mediate the poorer response to CBT that we observed among those with sustained, higher cortisol levels—a research question which should be explored in future research.
The present study is limited by a number of factors. In particular, the lack of a control group makes it difficult to discern whether or not the observed associations between cortisol and treatment outcome truly represent a diminished responsiveness to treatment among those with more abnormal cortisol profiles. We attempted to address this issue by choosing indicators of treatment response that were as independent of pretreatment depression levels as possible. However, future studies that address a similar research question and use a randomized controlled design would definitely help to clarify and confirm these findings. This study is also limited by the fact that only saliva samples were collected, and replication of these findings with other assessments of hypercortisolism (e.g., 24-hour urinary cortisol excretion, high-dose dexamethasone suppression) is warranted. In addition, it is notable that our sample size was small and made up of mostly Caucasian individuals, all of whom were over the age of 60. Participants in this study also needed to have access to transportation and be able to attend in-person sessions, which likely biased the sample toward healthier and more affluent older adults. Thus, future studies with larger, more diverse samples will help to establish the generalizability of these findings.
Notwithstanding these limitations, the present study is the first to demonstrate an association between pretreatment cortisol levels and posttreatment outcomes (when diurnal slope and AUC are examined simultaneously) among older adults in CBT for late-life depression—a finding, which if replicated, has important implications for clinical practice and future research.
Acknowledgments
This study was funded by an NIMH Exploratory/Developmental Research Grant Program (R21 MH091625-01 entitled: Predictors of Positive Outcome in Cognitive Behavior Therapy for Late Life Depression).
Footnotes
This log transformation was performed by adding 1 to the raw cortisol values and then taking the natural logarithm of that value. We added a constant (i.e., one) beforehand because many of the raw cortisol values, measured in μg/dL, were below 1. In these cases, a log transformation can yield very large negative numbers, thereby skewing the distribution further.
When the model was first run, the residual variance for % improvement for the CES-D was negative, resulting in a residual covariance matrix that was not positive definite. As a result, the residual variance for this observed indicator was constrained to zero. This change to the model had no substantive impact on any of the other parameters.
It should also be noted that we examined the interaction between diurnal cortisol AUC and slope as a predictor of treatment outcome. This interaction effect did not approach statistical significance and was, therefore, not included in the final model. We also considered the possibility that results might differ if multiple regression was used to test the hypotheses of this study, rather than SEM. These analyses showed that diurnal cortisol slope and AUC significantly predicted % improvement on the CES-D and HRSD when examined simultaneously, in the presence of the covariates. Although findings trended in the same direction for the BDI-II, the results for this measure did not reach statistical significance.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appelhof BC, Huyser J, Verweij M, Brouwer JP, van Dyck R, Fliers E, et al. Schene AH. Glucocorticoids and relapse of major depression (dexamethasone/corticotropin-releasing hormone test in relation to relapse of major depression) Biological Psychiatry. 2006;59(8):696–701. doi: 10.1016/j.biopsych.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Aubry J, Gervasoni N, Osiek C, Perret G, Rossier MF, Bertschy G, Bondolfi G. The DEX/CRH neuroendocrine test and the prediction of depressive relapse in remitted depressed outpatients. Journal of Psychiatric Research. 2007;41(3):290–294. doi: 10.1016/j.jpsychires.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the beck depression inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Belanoff JK, Gross K, Yager A, Schatzberg AF. Corticosteroids and cognition. Journal of Psychiatric Research. 2001;35(3):127–145. doi: 10.1016/S0022-3956(01)00018-8. [DOI] [PubMed] [Google Scholar]
- Beluche I, Carrière I, Ritchie K, Ancelin ML. A prospective study of diurnal cortisol and cognitive function in community-dwelling elderly people. Psychological Medicine. 2010;40:1039–1049. doi: 10.1017/S0033291709991103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beluche I, Chaudieu I, Norton J, Carrière I, Boulenger J, Ritchie K, Ancelin ML. Persistence of abnormal cortisol levels in elderly persons after recovery from major depression. Journal of Psychiatric Research. 2009;43(8):777–783. doi: 10.1016/j.jpsychires.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: Review and commentary. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2003;58(3):249–265. doi: 10.1093/gerona/58.3.M249. [DOI] [PubMed] [Google Scholar]
- Bockting CLH, Spinhoven P, Koeter MWJ, Wouters LF, Visser I, Schene AH The DELTA study group. Differential predictors of response to preventive cognitive therapy in recurrent depression: A 2-year prospective study. Psychotherapy and Psychosomatics. 2006;75:229–236. doi: 10.1159/000092893. [DOI] [PubMed] [Google Scholar]
- Bockting C, Lok A, Visser I, Assies J, Koeter M, Schene A The DELTA study group. Lower cortisol levels predict recurrence in remitted patients with recurrent depression: A 5.5 year prospective study. Psychiatry Research. 2012;200(2):281–287. doi: 10.1016/j.psychres.2012.03.044. [DOI] [PubMed] [Google Scholar]
- Boey KW. Cross-validation of a short form of the CES-D in chinese elderly. International Journal of Geriatric Psychiatry. 1999;14(8):608–617. doi: 10.1002/(SICI)1099-1166(199908)14:8<608:AID-GPS991>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Browne M, Cudeck R. Alternate ways of assessing model fit. In: Bollen K, Long J, editors. Testing structural equations models. Newbury Park, CA: Sage; 1993. pp. 1136–1162. [Google Scholar]
- Bruce ML, McAvay GJ, Raue PJ, Brown EL, Meyers BS, Keohane DJ, et al. Weber C. Major depression in elderly home health care patients. American Journal of Psychiatry. 2002;159(8):1367–1374. doi: 10.1176/appi.ajp.159.8.1367. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: A meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Byers AL, Yaffe K. Depression and risk of developing dementia. Nature Reviews Neurology. 2011;7(6):323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan CM, Wolinsky FD. The effect of gender and race on the measurement properties of the CES-D in older adults. Medical Care. 1994;32(4):341–356. doi: 10.1097/00005650-199404000-00003. [DOI] [PubMed] [Google Scholar]
- Caudle DD, Senior AC, Wetherell JL, Rhoades HM, Beck JG, Kunik ME, Stanley MA. Cognitive errors, symptom severity, and response to cognitive behavior therapy in older adults with generalized anxiety disorder. American Journal of Geriatric Psychiatry. 2007;15(8):680–689. doi: 10.1097/JGP.0b013e31803c550d. [DOI] [PubMed] [Google Scholar]
- Chi I, Boey KW. Hong kong validation of measuring instruments of mental health status of the elderly. Clinical Gerontologist. 1993;13(4):35–51. doi: 10.1300/J018v13n04_04. [DOI] [Google Scholar]
- Cuijpers P, van Straten A, Smit F. Psychological treatment of late-life depression: A meta-analysis of randomized controlled trials. International Journal of Geriatric Psychiatry. 2006;21(12):1139–1149. doi: 10.1002/gps.1620. [DOI] [PubMed] [Google Scholar]
- Engels GI, Vermey M. Efficacy of nonmedical treatments of depression in elders: A quantitative analysis. Journal of Clinical Geropsychology. 1997;3(1):17–35. [Google Scholar]
- Fekedulegn DB, Andrew ME, Burchfiel CM, Violanti JM, Hartley TA, Charles LE, Miller DB. Area under the curve and other summary indicators of repeated waking cortisol measurements. Psychosomatic Medicine. 2007;69(7):651–659. doi: 10.1097/PSY.0b013e31814c405c. [DOI] [PubMed] [Google Scholar]
- Finkelstein MM. Body mass index and quality of life in a survey of primary care patients. Journal of Family Practice. 2000;49(8):734–737. doi. [PubMed] [Google Scholar]
- Franz CE, O'Brien RC, Hauger RL, Mendoza SP, Panizzon MS, Prom-Wormley E, et al. Kremen WS. Cross-sectional and 35-year longitudinal assessment of salivary cortisol and cognitive functioning: The vietnam era twin study of aging. Psychoneuroendocrinology. 2011;36(7):1040–1052. doi: 10.1016/j.psyneuen.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher-Thompson D, Thompson LW. Treating late-life depression: A cognitive-behavioral therapy approach. New York, NY: Oxford; 2010. [Google Scholar]
- Giese-Davis J, DiMiceli S, Sephton S, Spiegel D. Emotional expression and diurnal cortisol slope in women with metastatic breast cancer in supportive-expressive group therapy: A preliminary study. Biological Psychology. 2006;73(2):190–198. doi: 10.1016/j.biopsycho.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Gill SC, Butterworth P, Rodgers B, Mackinnon A. Validity of the mental health component scale of the 12-item Short Form Health Survey (MCS-12) as measure of common mental disorders in the general population. Psychiatry Research. 2007;152(1):63–71. doi: 10.1016/j.psychres.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Gomez RG, Posener JA, Keller J, DeBattista C, Solvason B, Schatzberg AF. Effects of major depression diagnosis and cortisol levels on indices of neurocognitive function. Psychoneuroendocrinology. 2009;34(7):1012–1018. doi: 10.1016/j.psyneuen.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Haddock CK, Poston WS, Taylor JE, Conard M, Spertus J. Smoking and health outcomes after percutaneous coronary intervention. American Heart Journal. 2003;145(4):652–657. doi: 10.1067/mhj.2003.67. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;23:56–61. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris Y, Cooper JK. Depressive symptoms in older people predict nursing home admission. Journal of the American Geriatrics Society. 2006;54(4):593–597. doi: 10.1111/j.1532-5415.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- Hasinski S. Assessment of adrenal glucocorticoid function. Postgraduate Medicine. 1998;104(1):69–72. doi: 10.3810/pgm.1998.07.531. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Herzig N, Holsboer-Trachsler E. In healthy youn and elderly adults, hypothalamic-pituitary-adrenocortical axis reactivity (HPA AR) varies with increasing pharmacological challenge and with age, but not with gender. Journal of Psychiatric Research. 2011;45:1373–1380. doi: 10.1016/j.jpsychires.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Holland JM, Gallagher-Thompson D. Interventions for mental health problems in later life. In: Barlow D, editor. The Oxford handbook of clinical psychology. New York: Oxford University Press; 2011. pp. 810–836. [Google Scholar]
- Holland JM, Thompson LW, Cucciare MA, Tsuda A, Okamura H, Spiegel D, et al. Gallagher-Thompson D. Cortisol outcomes among caucasian and Latina/Hispanic women caring for a family member with dementia: A preliminary examination of psychosocial predictors and the effect of a psychoeducational intervention. Stress and Health. 2011;27:334–346. doi: 10.1002/smi.1375. [DOI] [Google Scholar]
- Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2000;23(5):477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- Horstmann S, Binder EB. Glucocorticoids as predictors of treatment response in depression. Harvard Review of Psychiatry. 2011;19:125–143. doi: 10.3109/10673229.2011.586550. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Jason LA, Torres-Harding S, Maher K, Reynolds N, Brown M, Sorenson M, Lu T. Baseline cortisol levels predict treatment outcomes in chronic fatigue syndrome nonpharmacologic clinical trial. Journal of Chronic Fatigue Syndrome. 2008;14(4):39–59. doi: 10.3109/10573320802092039. [DOI] [Google Scholar]
- Johnson JA, Coons SJ. Comparison of the EQ-5D and SF-12 in an adult U.S. sample. Quality of Life Research. 1998;7(2):155–166. doi: 10.1023/A:1008809610703. [DOI] [PubMed] [Google Scholar]
- Kiosses DN, Leon AC, Areán PA. Psychosocial Interventions for Late-life Major Depression: Evidence-Based Treatments, Predictors of Treatment Outcomes, and Moderators of Treatment Effects. Psychiatric Clinics of North America. 2011;34:377–401. doi: 10.1016/j.psc.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2nd. New York, NY: Guilford; 2005. [Google Scholar]
- Köhler S, Thomas AJ, Lloyd A, Barber R, Almeida OP, O'Brien JT. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. The British Journal of Psychiatry. 2010;196(2):143–149. doi: 10.1192/bjp.bp.109.071399. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Giese-Davis J, Yutsis M, Neri E, Gallagher-Thompson D, Taylor CB, Spiegel D. Design decisions to optimize reliability of daytime cortisol slopes in an older population. The American Journal of Geriatric Psychiatry. 2006;14(4):325–333. doi: 10.1097/01.JGP.0000201816.26786.5b. [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer R, Williams J. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw K, Thompson LW, Dick-Siskin L, Gallagher-Thompson D. Cognitive behaviour therapy with older people. New York, NY, US: John Wiley & Sons Ltd; 2003. [Google Scholar]
- Lam S, Dickerson SS, Zoccola PM, Zaldivar F. Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrinology. 2009;34(9):1355–1362. doi: 10.1016/j.psyneuen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Le Floch JP, Escuyer P, Baudin E, Baudon D, Perlemuter L. Blood glucose area under the curve: Methodological aspects. Diabetes Care. 1990;13(2):172–175. doi: 10.2337/diacare.13.2.172. [DOI] [PubMed] [Google Scholar]
- Li G, Cherrier MM, Tsuang DW, Petrie EC, Colasurdo EA, Craft S, Wilkinson CW. Salivary cortisol and memory function in human aging. Neurobiology of Aging. 2006;27(11):1705–1714. doi: 10.1016/j.neurobiolaging.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Lovallo WR. Stress and health: Biological and psychological interactions. Sage Publications; 2005. [Google Scholar]
- Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 1994;14(5 Pt 1):2893. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC, Topic B, Steenbergen PJ, Jocham G, Huston JP, Oitzl MS. Correlations between hypothalamus-pituitary-adrenal axis parameters depend on age and learning capacity. Endocrinology. 2005;146(3):1372–1381. doi: 10.1210/en.2004-0416. [DOI] [PubMed] [Google Scholar]
- McKay MS, Zakzanis KK. The impact of treatment on HPA axis activity in unipolar major depression. Journal of Psychiatric Research. 2010;44(3):183–192. doi: 10.1016/j.jpsychires.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Miller G, Chen E, Zhou E. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mohlman J. Does executive dysfunction affect treatment outcome in late-life mood and anxiety disorders? Journal of Geriatric Psychiatry and Neurology. 2005;18(2):97–108. doi: 10.1177/0891988705276061. [DOI] [PubMed] [Google Scholar]
- Mohlman J, Gorman JM. The role of executive functioning in CBT: A pilot study with anxious older adults. Behaviour Research and Therapy. 2005;43(4):447–465. doi: 10.1016/j.brat.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Mui AC, Burnette D, Chen LM. Cross-cultural assessment of geriatric depression: A review of the CES-D and GDS. In: Skinner JH, Teresi JA, Holmes D, Stahl SM, Stewart AL, editors. Multicultural measurement in older populations. New York, NY US: Springer Publishing Co; 2002. pp. 147–177. [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's guide. 6th. Los Angeles, CA: Muthén and Muthén; 1998-2010. [Google Scholar]
- Neigh GN, Nemeroff CB. Reduced glucocorticoid receptors: Consequence or cause of depression? Trends in Endocrinology and Metabolism. 2006;17(4):124–125. doi: 10.1016/j.tem.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols NR, Zieba M, Bye N. Do glucocorticoids contribute to brain aging? Brain Research Reviews. 2001;37(1):273–286. doi: 10.1016/S0165-0173(01)00131-X. [DOI] [PubMed] [Google Scholar]
- O'Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. The American Journal of Psychiatry. 2004;161(11):2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- O'Hara R, Schröder CM, Mahadevan R, Schatzberg AF, Lindley S, Fox S, Hallmayer JF. Serotonin transporter polymorphism, memory and hippocampal volume in the elderly: Association and interaction with cortisol. Molecular Psychiatry. 2007;12(6):544–555. doi: 10.1038/sj.mp.4001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockensfels M, Porter L, Smyth J, Kirschbaum C, Hellhammer D, Stone A. Effect of chronic stress associated with unemployment on salivary cortisol: Overall cortisol levels, diurnal rhythm, and acute stress reactivity. Psychosomatic Medicine. 1995;57:460–467. doi: 10.1097/00006842-199509000-00008. [DOI] [PubMed] [Google Scholar]
- Olsson UH, Foss T, Troye SV, Howell RD. The performance of ML, GLS, and WLS estimation in structural equation modeling under conditions of misspecification and nonnormality. Structural Equation Modeling. 2000;7(4):557–595. doi: 10.1207/S15328007SEM0704_3. [DOI] [Google Scholar]
- O'Toole SM, Sekula LK, Rubin RT. Pituitary-adrenal cortical axis measures as predictors of sustained remission in major depression. Biological Psychiatry. 1997;42(2):85. doi: 10.1016/S0006-3223(96)00293-4. [DOI] [PubMed] [Google Scholar]
- Otto MW, McHugh RK, Kantak KM. Combined pharmacotherapy and cognitive-behavioral therapy for anxiety disorders: Medication effects, glucocorticoids, and attenuated treatment outcomes. Clinical Psychology: Science and Practice. 2010;17(2):91–103. doi: 10.1111/j.1468-2850.2010.01198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: Classical theories and new developments. Trends in Neurosciences. 2008;31(9):464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Duberstein PR, Lyness JM. Effects of psychotherapy and other behavioral interventions on clinically depressed older adults: A meta-analysis. Aging and Mental Health. 2007;11(6):645–657. doi: 10.1080/13607860701529635. [DOI] [PubMed] [Google Scholar]
- Pintor L, Torres X, Navarro V, Martinez de Osaba M A Jesús, Matrai S, Gastó C. Prediction of relapse in melancholic depressive patients in a 2-year follow-up study with corticotropin releasing factor test. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(3):463–469. doi: 10.1016/j.pnpbp.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Reppermund S, Zihl J, Lucae S, Horstmann S, Kloiber S, Holsboer F, Ising M. Persistent cognitive impairment in depression: The role of psychopathology and altered hypothalamic-pituitary-adrenocortical (HPA) system regulation. Biological Psychiatry. 2007;62(5):400–406. doi: 10.1016/j.biopsych.2006.09.027. [DOI] [PubMed] [Google Scholar]
- Rumsfeld JS, Jones PG, Whiiley MA, Sullivan MD, Pitt B, Weintraub WS, Spertus JA. Depression predicts mortality and hospitalization in patients with myocardial infarction complicated by heart failure. American Heart Journal. 2005;150:961–967. doi: 10.1016/j.ahj.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Zimmerman M, Wisniewski SR, Fava M, Hollon SD, Warden D, et al. Trivedi MH. Comorbid psychiatric disorders in depressed outpatients: Demographic and clinical features. Journal of Affective Disorders. 2005;87(1):43–55. doi: 10.1016/j.jad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Sachdev PS, Wen W, Christensen H, Jorm AF. White matter hyperintensities are realted to physical disability and poor motor function. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(3):362–367. doi: 10.1136/jnnp.2004.042945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent-Cox K, Cherbuin N, Sachdev P, Anstey KJ. Subjective health and memory predictors of mild cognitive disorders and cognitive declin in ageing: The personality and total health (PATH) through life study. Dementia and Geriatric Cognitive Disorders. 2010;31:45–52. doi: 10.1159/000322373. [DOI] [PubMed] [Google Scholar]
- Scogin F, McElreath L. Efficacy of psychosocial treatments for geriatric depression: A quantitative review. Journal of Consulting and Clinical Psychology. 1994;62(1):69–73. doi: 10.1037//0022-006X.62.1.69. [DOI] [PubMed] [Google Scholar]
- Scogin F, Welsh D, Hanson A, Stump J, Coates A. Evidence-based psychotherapies for depression in older adults. Clinical Psychology: Science and Practice. 2005;12(3):222–237. doi: 10.1093/clipsy.bpi033. [DOI] [Google Scholar]
- Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. The Journal of Clinical Endocrinology and Metabolism. 1997;82(8):2458–2465. doi: 10.1210/jc.82.8.2458. [DOI] [PubMed] [Google Scholar]
- Segal DL, Coolidge FL, Cahill BS, O'Riley AA. Psychometric properties of the beck depression inventory II (BDI-II) among community-dwelling older adults. Behavior Modification. 2008;32(1):3–20. doi: 10.1177/0145445507303833. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Dunbar GC. The mini-international neuropsychiatric interview (M.I.N.I): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, et al. Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/S0306-4530(96)00039-X. [DOI] [PubMed] [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monographs of the Society for Research in Child Development. 1994;59(2/3):108–134. [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosomatic Medicine. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao A, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Research Reviews. 2005;4(2):141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Thase ME, Dubé S, Bowler K, Howland RH, Myers JE, Friedman E, Jarrett DB. Hypothalamic-pituitary-adrenocortical activity and response to cognitive behavior therapy in unmedicated, hospitalized depressed patients. The American Journal of Psychiatry. 1996;153(7):886. doi: 10.1176/ajp.153.7.886. [DOI] [PubMed] [Google Scholar]
- Thase ME, Friedman ES. Is psychotherapy an effective treatment for melancholia and other severe depressive states? Journal of Affective Disorders. 1999;54(1-2):1–19. doi: 10.1016/S0165-0327(99)00033-6. [DOI] [PubMed] [Google Scholar]
- Thase ME, Simons AD, Reynolds CF. Psychobiological correlates of poor response to cognitive behavior therapy: Potential indications for antidepressant pharmacotherapy. Psychopharmacology Bulletin. 1993;29(2):293–301. [PubMed] [Google Scholar]
- Thomson F, Craighead M. Innovative approaches for the treatment of depression: Targeting the HPA axis. Neurochemical Research. 2008;33(4):691–707. doi: 10.1007/s11064-007-9518-3. [DOI] [PubMed] [Google Scholar]
- Trajković G, Starčević V, Latas M, Leštarević M, Ille T, Bukumirić Z, Marinković J. Reliability of the hamilton rating scale for depression: A meta-analysis over a period of 49 years. Psychiatry Research. 2011;189(1):1–9. doi: 10.1016/j.psychres.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. Journal of Clinical Endocrinology and Metabolism. 1996;81(7):2468–2473. doi: 10.1210/jc.81.7.2468. [DOI] [PubMed] [Google Scholar]
- van Gool CH, Kempen GIJM, Penninx BWJH, Deeg DJH, Beekman ATF, van Eijk JTM. Impact of depression on disablement in late middle aged and older persons: Results from the longitudinal aging study Amsterdam. Social Science & Medicine. 2005;60(1):25–36. doi: 10.1016/j.socscimed.2004.04.021. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-item short-form health survey: Construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Strosahl K, Fruzzetti AE, Schmaling KB, Jacobson NS, Miller DM. A structured interview version of the hamilton rating scale for depression: Reliability and validity. Psychological Assessment. 1989;1(3):238–241. doi: 10.1037/1040-3590.1.3.238. [DOI] [Google Scholar]
- Yehuda R, Bierer LM, Sarapas C, Makotkine I, Andrew R, Seckl JR. Cortisol metabolic predictors of response to psychotherapy for symptoms of PTSD in survivors of the world trade center attacks on september 11, 2001. Psychoneuroendocrinology. 2009;34(9):1304–1313. doi: 10.1016/j.psyneuen.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EA, Altemus M, Lopez JF, Kocsis JH, Schatzberg AF, DeBattista C, Zubieta J. HPA axis activation in major depression and response to fluoxetine: A pilot study. Psychoneuroendocrinology. 2004;29(9):1198–1204. doi: 10.1016/j.psyneuen.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Zobel AW, Nickel T, Sonntag A, Uhr M, Holsboer F, Ising M. Cortisol response in the combined dexamethasone/CRH test as predictor of relapse in patients with remitted depression. Journal of Psychiatric Research. 2001;35(2):83–94. doi: 10.1016/S0022-3956(01)00013-9. [DOI] [PubMed] [Google Scholar]


