Abstract
A meta-analysis was performed to assess the association between the PAI-1 -675 4G/5G polymorphism and susceptibility to diabetes mellitus (DM), diabetic nephropathy (DN), diabetic retinopathy (DR) and diabetic coronary artery disease (CAD). A literature-based search was conducted to identify all relevant studies. The fixed or random effect pooled measure was calculated mainly at the allele level to determine heterogeneity bias among studies. Further stratified analyses and sensitivity analyses were also performed. Publication bias was examined by the modified Begg’s and Egger’s test. Twenty published articles with twenty-seven outcomes were included in the meta-analysis: 6 studies with a total of 1,333 cases and 3,011 controls were analyzed for the PAI-1 -675 4G/5G polymorphism with diabetes risk, 7 studies with 1,060 cases and 1,139 controls for DN risk, 10 studies with 1,327 cases and 1,557 controls for DR and 4 studies with 610 cases and 1,042 controls for diabetic CAD risk respectively. Using allelic comparison (4G vs. 5G), the PAI-1 -675 4G/5G polymorphism was observed to have no significant association with diabetes (REM OR 1.07, 95% CI 0.96, 1.20), DN (REM OR 1.10, 95% CI 0.98, 1.25), DR (REM OR 1.09, 95% CI 0.97, 1.22) or diabetic CAD risk (REM OR 1.07, 95% CI 0.81, 1.42), and similar results were obtained in the dominant, recessive and co-dominant models. Our meta-analyses suggest that the PAI-1 -675 4G/5G polymorphism might not be a risk factor for DM, DN, DR or diabetic CAD risk in the populations investigated. This conclusion warrants confirmation by further studies.
Introduction
The plasminogen activator inhibitor-1 (PAI-1) belongs to the serine protease inhibitor superfamily, and plays a key role in the regulation of extracellular matrix degradation [1]. Studies have indicated that the increase of PAI-1 level was related to the incidence of diabetes mellitus (DM) and its complications, such as an increased risk of diabetic nephropathy (DN), diabetic retinopathy (DR) and diabetic coronary artery disease (CAD), etc [2-5].
Numerous single nucleotide polymorphisms (SNPs) have been observed in the PAI-1 gene. Some increase the t½ of PAI-1 while others inactivate it or slow down its secretion into the plasma. It has been found that the most-studied 4G/5G polymorphism (rs1799889), characterized by a single guanosine nucleotide insertion/deletion variation at -675 bp of the PAI-1 promoter, increases PAI-1 concentration or its activity in the plasma of humans without changing its t½ [6]. This polymorphism is a cause of high plasma PAI-1 level in 4G/4G allele carriers suggesting that the PAI-1 4G/5G polymorphism is a genetic risk factor for diabetes [7]. Recently many studies reported the association between the 4G/5G polymorphism of PAI-1 gene and the risk of diabetes and diabetic complications [8-27], mainly focusing on DN, DR and diabetic CAD. Despite strong functional evidence for the relevance of several studies, the results for the association with diabetes and its complications showed significant between-study variations and were inconclusive.
Considering a single study may lack the power to provide a reliable conclusion, we performed a meta-analysis on these eligible case-control studies, to investigate the precise relationship between the PAI-1 -675 4G/5G polymorphism and susceptibility to DM, DN, DR and diabetic CAD, which would have a much greater possibility of reaching reasonably strong conclusions.
Methods
This meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [28].
Search strategy
The comprehensive literature searches were carried out independently by two investigators (Kuanfeng Xu and Xiaoyun Liu) using the electronic data-bases PubMed, Embase and ScienceDirect without language restrictions, and updated on July 11, 2013. The PAI-1 -675 4G/5G polymorphism was investigated by combinations of the following search terms: ‘plasminogen activator inhibitor-1 or PAI-1’, ‘polymorphism, variant or mutation’ and ‘diabetes or diabetic complications’. We used the PubMed option ‘Related Articles’ for each study to retrieve additional potentially relevant articles, and also hand-searched the included articles to identify any other relevant citations. No restriction was set on the source of control participants (general population, clinic or hospital).
Inclusion and exclusion criteria
The inclusion and exclusion criteria were as follows: (1) each case-control study were published as an original study designed to evaluate the association; (2) numbers in case and control groups were reported for each allele or genotype; (3) case-control studies had sufficient published data to estimate an odds ratio (OR) with 95% confidence intervals (CI) or provide raw data that allowed us to calculate them; (4) only the most recent or complete study was used if the data were duplicated and had been published in several publications; (5) studies were excluded if the genotype distribution of the controls deviated from Hardy-Weinberg equilibrium (HWE); (6) the following were excluded: animal studies, review articles, abstracts, editorials, reports with incomplete data, studies based on pedigree data or prospective studies, etc.
Data extraction
Data were independently extracted by two investigators who discussed disagreements and reached a consensus on all of the items. Information extracted from each study was considered as follows: name of first author, year of publication, ethnic origin of the studied population, available number of participants in case and control groups, genotype and allele frequency by case/control status, and OR (95% CI). Not all papers reported the necessary statistics directly, so in some instances we transformed and estimated an OR from the reported data [29]. We did not define any minimum number of patients for a study to be included in our meta-analysis. In addition, all participants of the included studies provided informed consent and the studies were approved by the ethics committees of the participating institutions.
Statistical analysis
The association of the PAI-1 -675 4G/5G polymorphism with diabetes and diabetic complications was estimated by calculating the pooled OR and 95% CI in the allelic and genotypic (dominant, recessive and co-dominant model) comparisions respectively. The significance of the OR was determined by the Z test (P <0.05 was considered statistically significant). HWE of the genotype distribution of controls was tested by a goodness-of-fit χ2 analysis. The distribution was considered to be deviated from HWE at P < 0.05.
We employed the DerSimonian and Laird random effect model (REM) to bring the individual effect size estimates together, and quantified between-study heterogeneity by inconsistency index (I 2) statistic. The I 2 statistic is defined as the percentage of the observed between-study variability that is due to heterogeneity rather than chance, with high values suggesting more possible existence of heterogeneity. Potential heterogeneity between results of individual studies or in subgroups respectively by ethnicity, study design, source of controls, genotyping method, diagnostic criterion, and sample size was explored using χ2 test. Sensitivity analysis was conducted to evaluate the key studies with a substantial impact on between-study heterogeneity. Influence analysis was performed to assess the stability of the results, with a single study in the meta-analysis being deleted each time to reflect the influence of the individual data set on the pooled OR.
Publication bias was assessed by the modified Begg’s and Egger’s test [30]. The significance of the intercept was determined by the t test suggested by Egger, with p <0.10 considered representative of statistically significant publication bias. All statistical analyses were conducted using RevMan 5.0 (The Nordic Cochrane Centre, The Cochrane Collaboration) & STATA 11.0 (Stata, College Station, TX, USA). All tests were two-sided.
Results
Characteristics of study
The trial flow is summarised in Figure S1. A total of 20 published articles with 27 outcomes met the inclusion and exclusion criteria [8-27]. All were case-control studies and most were population-based. The allele and genotype distributions in the studies included are summarised in Table 1. Here 6 studies with a total of 1,333 cases and 3,011 controls were analyzed for the PAI-1 -675 4G/5G polymorphism with diabetes risk [11,12,19,22,24,27], 7 studies with 1,060 cases and 1,139 controls for this polymorphism with DN [11-14,19,23,26,], 10 studies with 1,327 cases and 1,557 controls for DR [9-11,14,15,18-21,25] and 4 studies with 610 cases and 1,042 controls for diabetic CAD risk [8,16,17,20] respectively.
Table 1. Characteristics of the PAI-1 -675 4G/5G polymorphism allelic and genotype distribution for diabetes and diabetic complications risk in studies included in the meta-analysis.
| Study details | DM & complications | Total/Genotypes(4G4G/4G5G/5G5G) | 5G allele frequency (%) | OR(95% CI) a | ||||
|---|---|---|---|---|---|---|---|---|
| Authors [ref.] | Year | Ethnicity | Cases | Controls | Cases | Controls | ||
| Mansfield MW [8] | 1995 | European | CAD | 38(20/15/3) | 122(37/67/18) | 27.6 | 42.2 | 1.91(1.09-3.36) |
| Nagi DK [9] | 1997 | Pima Indian | DR | 70(14/44/12) | 102(18/45/39) | 48.6 | 60.3 | 1.61(1.04-2.48) |
| Broch M [10] | 1998 | European | DR | 82(17/46/19) | 95(19/48/28) | 51.2 | 54.7 | 1.15(0.76-1.75) |
| Kimura H [11] | 1998 | Asian | DM | 208(64/116/28) | 177(66/80/31) | 41.3 | 40.1 | 0.95 (0.71- 1.27) |
| DN | 98(28/58/12) | 110(36/58/16) | 41.8 | 40.9 | 0.96(0.65-1.42) | |||
| DR | 110(32/62/16) | 98(32/54/12) | 42.7 | 39.8 | 0.89(0.60-1.31) | |||
| De Cosmo S b [12] | 1999 | European | DM | 311(82/156/73) | 200(54/96/50) | 48.6 | 49.0 | 1.02(0.79-1.31) |
| DN | 175(52/81/42) | 136(30/75/31) | 47.1 | 50.4 | 1.14 (0.83- 1.56) | |||
| Tarnow L b [13] | 2000 | European | DN | 197(54/104/39) | 191(63/80/48) | 46.2 | 46.1 | 1.00(0.75-1.32) |
| Wong TY [14] | 2000 | Asian | DN | 95(39/37/19) | 46(8/29/9) | 39.5 | 51.1 | 1.60(0.97-2.64) |
| DR | 84(31/38/15) | 57(16/28/13) | 40.5 | 47.4 | 1.32(0.82-2.14) | |||
| Globocnik-Petrovic M [15] | 2003 | European | DR | 124(39/58/27) | 80(25/40/15) | 45.2 | 43.8 | 0.94(0.63-1.14) |
| Lopes C [16] | 2003 | European | CAD | 229(71/114/44) | 406(106/203/97) | 44.1 | 48.9 | 1.21(0.96-1.53) |
| Petrovic D [17] | 2003 | European | CAD | 154(45/74/35) | 194(68/89/37) | 46.8 | 42.0 | 0.83(0.61-1.12) |
| Santos KG [18] | 2003 | European | DR | 99(24/41/34) | 111(22/59/30) | 55.1 | 53.6 | 0.94(0.64-1.39) |
| Liu SQ [19] | 2004 | Asian | DM | 147(42/75/30) | 26(4/16/6) | 45.9 | 53.8 | 1.37 (0.76- 2.48) |
| DN | 77(30/28/19) | 70(12/47/11) | 42.9 | 49.3 | 1.30(0.82-2.05) | |||
| DR | 56(15/26/15) | 91(27/49/15) | 50.0 | 43.4 | 0.77(0.48-1.23) | |||
| Zietz B [20] | 2004 | European | DR | 131(48/55/28) | 358(112/173/73) | 42.4 | 44.6 | 1.09(0.82-1.45) |
| CAD | 189(59/87/43) | 320(108/151/61) | 45.8 | 42.7 | 0.88(0.68-1.14) | |||
| Murata M [21] | 2004 | Asian | DR | 188(78/86/24) | 92(43/35/14) | 35.6 | 34.2 | 0.94(0.65-1.36) |
| Meigs JB [22] | 2006 | European | DM | 216(65/103/48) | 1953(529/995/429) | 46.1 | 47.4 | 1.06(0.87-1.29) |
| Martin RJ b [23] | 2007 | European | DN | 222(70/114/38) | 361(111/179/71) | 42.8 | 44.5 | 1.07(0.84-1.36) |
| Saely CH [24] | 2008 | European | DM | 148(44/78/26) | 524(148/253/123) | 43.9 | 47.6 | 1.16(0.90-1.50) |
| Ezzidi I [25] | 2009 | European | DR | 383(77/167/139) | 473(54/242/177) | 58.1 | 63.0 | 1.23(1.01-1.49) |
| Prasad P [26] | 2010 | Asian | DN | 196(57/90/49) | 225(52/117/56) | 48.0 | 50.9 | 1.12(0.86-1.74) |
| Al-Hamodi Z [27] | 2012 | Asian | DM | 303(76/151/76) | 131(30/63/38) | 50.0 | 53.1 | 1.13(0.85-1.51) |
Note: DM, diabetes mellitus; OR(95% CI), odds ratio (95% confidence intervals); CAD, coronary artery disease; DR, diabetic retinopathy; DN, diabetic nephropathy; a, data were analyzed in the risk allele (4G vs. 5G); b, the data were on the association with type 1 diabetes, others were with type 2 diabetes. The genotype distributions in the controls of all the included studies were in agreement with Hardy-Weinberg equilibrium.
Quantitative syntheses
Our meta-analysis revealed no association between the PAI-1 -675 4G/5G polymorphism and diabetes risk, either by allelic comparision (REM OR 1.07, 95% CI 0.96, 1.20), dominant (REM OR 1.16, 95% CI 0.96, 1.40), recessive (REM OR 1.04, 95% CI 0.88, 1.24) or co-dominant (REM OR 1.18, 95% CI 0.94, 1.47) models. When the study from De Cosmo S et al on type 1 diabetes (T1D) was excluded [12], the results on type 2 diabetes (T2DM) risk were consistent with above in all genetic models (allelic comparision REM OR 1.09, 95% CI 0.96, 1.23; dominant REM OR 1.18, 95% CI 0.95, 1.46; recessive REM OR 1.06, 95% CI 0.87, 1.28; co-dominant REM OR 1.21, 95% CI 0.94, 1.56). Moreover, after stratified by ethnicity, no association was observed using an allelic comparision (Asian descent REM OR 1.07, 95% CI 0.88, 1.30; European descent REM OR 1.06, 95% CI 0.90, 1.18), the similar results were obtained in other genetic models (data not shown). Results of pooled analyses are summarised in detail in Table 2 & Figure 1.
Table 2. Pooled measures for the association between the PAI-1 -675 4G/5G polymorphism and susceptibility to diabetes.
| Comparisions | Data | n | Heterogeneity | OR (95% CI) | Model | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Cases | Controls | I 2 (%) | P | |||||
| Allelic comparisions | Overall | 6 | 1333 | 3011 | 0 | 0.845 | 1.07 (0.96-1.20) | REM | 0.234 |
| Asian | 3 | 658 | 334 | 0 | 0.484 | 1.07(0.88-1.30) | REM | 0.502 | |
| European | 3 | 665 | 2677 | 0 | 0.696 | 1.06(0.90-1.18) | REM | 0.324 | |
| Dominant | Overall | 6 | 1333 | 3011 | 0 | 0.835 | 1.16(0.96-1.40) | REM | 0.123 |
| Recessive | Overall | 6 | 1333 | 3011 | 0 | 0.447 | 1.04(0.88-1.24) | REM | 0.655 |
| Co-dominant | Overall | 6 | 654 | 1508 | 0 | 0.896 | 1.18(0.94-1.47) | REM | 0.155 |
Note: Allelic comparisions, 4G vs. 5G; REM, Random effect model.
Figure 1. Pooled ORs for the association between the PAI-1 -675 4G/5G polymorphism (4G vs. 5G) and susceptibility to diabetes.
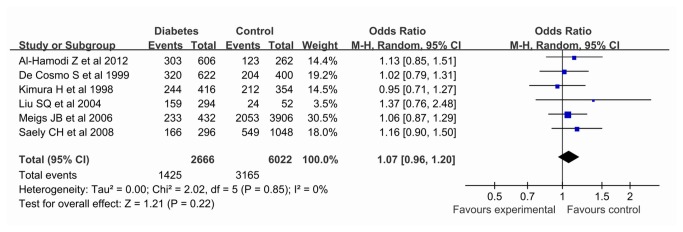
The area of the squares reflects the study-specific weight. The diamond shows the summary fixed-effects OR estimate from 6 studies.
Our meta-analysis also showed this polymorphism had no significant association with DN risk using all genetic models (allelic comparision REM OR 1.10, 95% CI 0.98, 1.25; dominant REM OR 1.06, 95% CI 0.86, 1.31; recessive REM OR 1.32, 95% CI 0.93, 1.88; co-dominant REM OR 1.23, 95% CI 0.96, 1.57). Further, no significant association was revealed using an allelic comparision on both T1D (Asian descent, REM OR 1.16, 95% CI 0.97, 1.40) and T2D risk (European descent, REM OR 1.06, 95% CI 0.91, 1.25), and the results were consistent in the dominant, recessive and co-dominant models stratified by ethnicity. Results of pooled analyses are summarised in detail in Table 3 & Figure 2.
Table 3. Pooled measures for the association between the PAI-1 -675 4G/5G polymorphism and susceptibility to diabetic nephropathy.
| Comparisions | Data | n | Heterogeneity | OR (95% CI) | Model | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Cases | Controls | I 2 (%) | P | |||||
| Allelic comparisions | Overall | 7 | 1060 | 1139 | 0 | 0.763 | 1.10(0.98-1.25) | REM | 0.103 |
| T1D | 4 | 466 | 451 | 0 | 0.425 | 1.16(0.97-1.40) | REM | 0.108 | |
| T2D | 3 | 594 | 688 | 0 | 0.831 | 1.06(0.91-1.25) | REM | 0.441 | |
| Dominant | Overall | 7 | 1060 | 1139 | 0 | 0.682 | 1.06(0.86-1.31) | REM | 0.566 |
| T1D | 4 | 466 | 451 | 0 | 0.598 | 0.94(0.68-1.30) | REM | 0.699 | |
| T2D | 3 | 594 | 688 | 0 | 0.577 | 1.16(0.88-1.53) | REM | 0.281 | |
| Recessive | Overall | 7 | 1060 | 1139 | 67.1 | 0.006 | 1.32(0.93-1.88) | REM | 0.114 |
| T1D | 4 | 466 | 451 | 71.9 | 0.014 | 1.72(0.94-3.16) | REM | 0.080 | |
| T2D | 3 | 594 | 688 | 45.6 | 0.159 | 1.04(0.74-1.46) | REM | 0.821 | |
| Co-dominant | Overall | 7 | 548 | 554 | 0 | 0.933 | 1.23(0.96-1.57) | REM | 0.107 |
| T1D | 4 | 253 | 200 | 0 | 0.721 | 1.34(0.90-1.98) | REM | 0.148 | |
| T2D | 3 | 295 | 354 | 0 | 0.898 | 1.16(0.84-1.60) | REM | 0.369 |
Note: Allelic comparisions, 4G vs. 5G; REM, Random effect model. T1D, type 1 diabetes; T2D, type 2 diabetes. Studies on T1D were all from Asian descent, and those on T2D were all from European descent.
Figure 2. Pooled ORs for the association between the PAI-1 -675 4G/5G polymorphism (4G vs. 5G) and susceptibility to diabetic nephropathy.
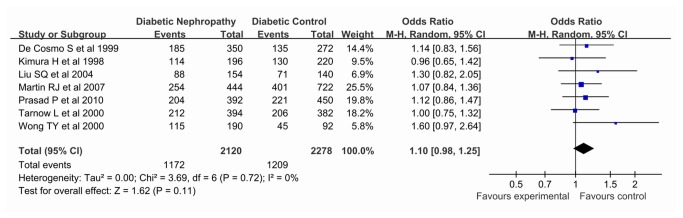
The area of the squares reflects the study-specific weight. The diamond shows the summary fixed-effects OR estimate from 7 studies.
On the association with DR risk, the 4G allele was not associated with an increased DR risk using the allelic comparision (REM OR 1.09, 95% CI 0.97, 1.22) or dominant model (REM OR 1.05, 95% CI 0.82, 1.28), the recessive (REM OR 1.18, 95% CI 0.96, 1.46) or co-dominant (REM OR 1.22, 95% CI 0.94, 1.58) model, not using model. Moreover, after stratified by ethnicity, a weak association was revealed by recessive model (REM OR 1.38, 95% CI 1.07, 1.79), but not in an allelic comparision dominant or co-dominant model in populations of European descent, and after Bonferroni correction, the P value in the recessive model were 0.06, which indicated no significant association existed in Europeans. Also our results indicated that no significant association was observed in those of Asian descent in all genetic models. Results of pooled analyses are summarised in detail in Table 4 & Figure 3.
Table 4. Pooled measures for the association between the PAI-1 -675 4G/5G polymorphism and susceptibility to diabetic retinopathy.
| Comparisions | Data | n | Heterogeneity | OR (95% CI) | Model | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Cases | Controls | I 2 (%) | P | |||||
| Allelic comparisions | Overall | 10 | 1327 | 1557 | 10 | 0.350 | 1.09(0.97-1.22) | REM | 0.160 |
| Asian | 4 | 438 | 338 | 0 | 0.443 | 0.95(0.77-1.17) | REM | 0.624 | |
| European | 5 | 819 | 1117 | 0 | 0.653 | 1.12(0.98-1.28) | REM | 0.09 | |
| Dominant | Overall | 10 | 1327 | 1557 | 36.5 | 0.116 | 1.05(0.82-1.34) | REM | 0.695 |
| Asian | 4 | 438 | 338 | 6.7 | 0.360 | 0.94(0.63-1.41) | REM | 0.765 | |
| European | 5 | 819 | 1117 | 0 | 0.626 | 0.99(0.80-1.21) | REM | 0.910 | |
| Recessive | Overall | 10 | 1327 | 1557 | 22.8 | 0.233 | 1.18(0.96-1.46) | REM | 0.128 |
| Asian | 4 | 438 | 338 | 0 | 0.560 | 0.93(0.68-1.26) | REM | 0.629 | |
| European | 5 | 819 | 1117 | 18.7 | 0.295 | 1.38(1.07-1.79) | REM | 0.015 | |
| Co-dominant | Overall | 10 | 704 | 784 | 19.6 | 0.263 | 1.22(0.94-1.58) | REM | 0.132 |
| Asian | 4 | 226 | 172 | 0 | 0.413 | 0.94(0.61-1.46) | REM | 0.779 | |
| European | 5 | 452 | 555 | 8.4 | 0.359 | 1.31(0.98-1.75) | REM | 0.068 |
Note: Allelic comparisions, 4G vs. 5G; REM, Random effect model.
Figure 3. Pooled ORs for the association between the PAI-1 -675 4G/5G polymorphism (4G vs. 5G) and susceptibility to diabetic retinopathy.
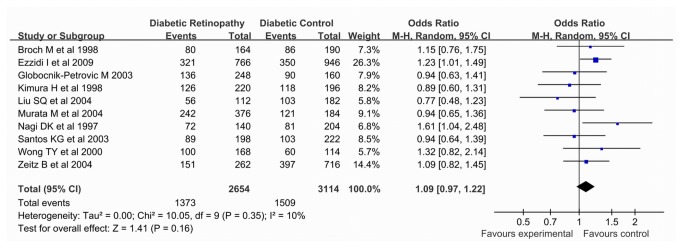
The area of the squares reflects the study-specific weight. The diamond shows the summary fixed-effects OR estimate from 10 studies.
In addition, our meta-analysis showed no significant association between the PAI-1 -675 4G/5G polymorphism and diabetic CAD in all genetic models (allelic comparision REM OR 1.07, 95% CI 0.81, 1.42; dominant REM OR 1.01, 95% CI 0.72, 1.42; recessive REM OR 1.13, 95% CI 0.76, 1.68; co-dominant REM OR 1.08, 95% CI 0.65, 1.80), which were all from European descent. Results of pooled analyses are summarised in detail in Table 5 & Figure 4.
Table 5. Pooled measures for the association between the PAI-1 -675 4G/5G polymorphism and susceptibility to diabetic coronary heart diseases.
| Comparisions | Data | n | Heterogeneity | OR (95% CI) | Model | p | |||
|---|---|---|---|---|---|---|---|---|---|
| Studies | Cases | Controls | I 2 (%) | P | |||||
| Allelic comparisions | Overall | 4 | 610 | 1042 | 69.5 | 0.054 | 1.07(0.81-1.42) | REM | 0.631 |
| Dominant | Overall | 4 | 610 | 1042 | 36.2 | 0.195 | 1.01(0.72-1.42) | REM | 0.954 |
| Recessive | Overall | 4 | 610 | 1042 | 67.4 | 0.027 | 1.13(0.76-1.68) | REM | 0.555 |
| Co-dominant | Overall | 4 | 407 | 683 | 63.0 | 0.044 | 1.08(0.65-1.80) | REM | 0.772 |
Note: Allelic comparisions, 4G vs. 5G; REM, Random effect model.
Figure 4. Pooled ORs for the association between the PAI-1 -675 4G/5G polymorphism (4G vs. 5G) and susceptibility to diabetic coronary artery disease.
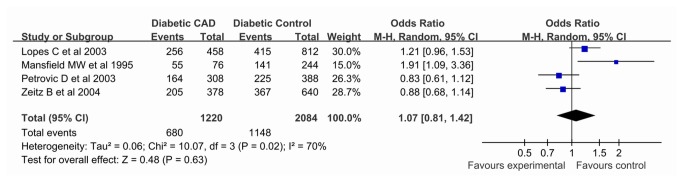
The area of the squares reflects the study-specific weight. The diamond shows the summary random-effects OR estimate from 4 studies.
Sensitivity analyses and influence analyses
As shown in Table 2-5, low or high heterogeneity in most of the inherited models was observed among studies in the overall population except for the association with diabetes risk. To identify the studies with the greatest impact on the overall between-study heterogeneity, sensitivity analyses and stratified analyses were conducted. On the association with DN risk, the heterogeneity in the recessive model was not significantly decreased after either sensitivity analysis or stratified analysis by ethnicity. On the association with DR risk, low heterogeneity existed in all genetic models. Sensitivity analysis indicated that the heterogeneity was also effectively decreased in all genetic models when excluded the study from Nagi DK et al [9]. Moreover, when the data were stratified by ethnicity, the heterogeneity in all genetic models was significantly decreased or eliminated in populations of Asian and European descent. On the association with diabetic CAD risk, the study from Mansfield MW et al [8] were mainly responsible for the observed heterogeneity, but sensitivity analysis suggested that the significant heterogeneity still existed in all genetic models when excluded this study.
To assess the degree to which each individual study affected the overall OR estimates, influence analysis was conducted by repeating the meta-analysis sequentially excluding one study at a time. No single study excessively influenced the analyses (data not shown).
Publication bias
Funnel plots and Egger’s test were performed to assess the publication bias of the literature. As expected, symmetrical funnel plots were obtained in diabetes and its complications tested in all genetic models. And Egger’s test further confirmed no publication bias for any of the polymorphisms examined, indicating that our results are statistically robust, as shown in Table S1.
Discussion
Elevated concentrations of PAI-1 have been observed consistently in blood from patients with T2D or insulin resistance [2,3]. The PAI-1 -675 4G/5G polymorphism, a single guanosine insertion/deletion, has been identified to contain an additional binding site for a DNA binding protein that may play a pivotal role as a repressor during transcription and exert the greatest impact on plasma PAI-1 concentration [6,31]. The information suggested that this polymorphism might be a genetic risk factor for diabetes. However, our meta-analysis results indicated that the PAI-1 -675 4G/5G polymorphism had no association with T2D in all genetic models. Further studies also indicated that the elevation of PAI-1 concentration correlates with complications of diabetes, including DN, DR and diabetic CAD risk [32-34]. Also studies on the PAI-1 -675 4G/5G polymorphism with the risk of diabetic complications were reported, which showed significant between-study variations and were inconclusive. Unfortunately, in our results of meta-analysis this polymorphism had no association with risk of diabetic complications in overall populations in all genetic models, given that the underlying studies were carried out in different populations, we also performed by random effects model, in which the results indicated no association in either European or Asian descendent.
Heterogeneity is potentially a significant problem when interpreting the results of any meta-analysis of genetic association studies [35]. To determine the amount of heterogeneity that existed among these variants, we did an χ2-based Q test. Our meta-analysis showed no significant between-study heterogeneity except for diabetic CAD risk in all genetic models and DN risk in the recessive model. Many of the variables that varied between the various studies might be responsible for this observed heterogeneity, including the source of the controls, sex bias, ethnicity, etc. Initial inspection of the data did not immediately identify any likely candidate variable or study that was significantly impacting on our overall results. Then we conducted sensitivity analyses by repeating the meta-analysis with one study excluded at each time [36]. The results showed that none of the individual study dramatically influenced the heterogeneity or pooled ORs.
The results of the present meta-analysis should also be interpreted within the context of its limitations. First, other potential environment-gene interactions or gene-gene interactions may well be contributors to the observed disease-effect unconformity except for ethnicity, but we had insufficient data to perform an evaluation of such interactions. Second, our meta-analysis is based on unadjusted estimates because of a lack of original data. Third, though the Egger’s test gave no publication bias, sample size is still a limitation of our meta-analysis [37], especially on the association with diabetic CAD risk.
In conclusion, our results indicate that the PAI-1 -675 4G/5G polymorphism might not be a risk factor for DM, DN, DR or diabetic CAD risk in the populations investigated. Maybe this association is not robust and could be due to chance, and additional larger studies that allow stratification for other gene-gene and gene-environment should also be conducted in future analyses.
Supporting Information
PRISMA Checklist.
(DOC)
Systematic review flow diagram n, number of studies.
(TIF)
Egger's publication bias test for the PAI-1 -675 4G/5G polymorphisms in DM, DN, DR and diabetic CAD.
(DOC)
Funding Statement
The study was supported by grants from the Natural Science Foundation of Jiangsu province (number BK2012486). A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vassalli JD, Sappino AP, Belin D (1991) The plasminogen activator/plasmin system. J Clin Invest 88: 1067-1072. doi: 10.1172/JCI115405. PubMed: 1833420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Festa A, D'Agostino R Jr, Tracy RP, Haffner SM (2002) Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 51: 1131-1137. doi: 10.2337/diabetes.51.4.1131. PubMed: 11916936. [DOI] [PubMed] [Google Scholar]
- 3. Festa A, Williams K, Tracy RP, Wagenknecht LE, Haffner SM (2006) Progression of plasminogen activator inhibitor-1 and fibrinogen levels in relation to incident type 2 diabetes. Circulation 113: 1753-1759. doi: 10.1161/CIRCULATIONAHA.106.616177. PubMed: 16585388. [DOI] [PubMed] [Google Scholar]
- 4. Gori AM, Marcucci R, Fatini C, Gensini F, Sticchi E et al. (2004) Impaired fibrinolysis in retinal vein occlusion: a role for genetic determinants of PAI-1 levels. Thromb Haemost 92: 54-60. PubMed: 15213845. [DOI] [PubMed] [Google Scholar]
- 5. Steinberger J, Daniels SR (2003) Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation 107: 1448-1453. doi: 10.1161/01.CIR.0000060923.07573.F2. PubMed: 12642369. [DOI] [PubMed] [Google Scholar]
- 6. Wiklund PG, Nilsson L, Ardnor SN, Eriksson P, Johansson L et al. (2005) Plasminogen activator inhibitor-1 4G/5G polymorphism and risk of stroke: replicated findings in two nested case-control studies based on independent cohorts. Stroke 36: 1661-1665. doi: 10.1161/01.STR.0000174485.10277.24. PubMed: 16020771. [DOI] [PubMed] [Google Scholar]
- 7. Eriksson P, Kallin B, van 't Hooft FM, Båvenholm P, Hamsten A (1995) Allele-specific increase in basal transcription of the plasminogen-activator inhibitor 1 gene is associated with myocardial infarction. Proc Natl Acad Sci U S A 92: 1851-1855. doi: 10.1073/pnas.92.6.1851. PubMed: 7892190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mansfield MW, Stickland MH, Grant PJ (1995) Environmental and genetic factors in relation to elevated circulating levels of plasminogen activator inhibitor-1 in Caucasian patients with non-insulin-dependent diabetes mellitus. Thromb Haemost 74: 842-847. PubMed: 8571308. [PubMed] [Google Scholar]
- 9. Nagi DK, McCormack LJ, Mohamed-Ali V, Yudkin JS, Knowler WC et al. (1997) Diabetic retinopathy, promoter (4G/5G) polymorphism of PAI-1 gene, and PAI-1 activity in Pima Indians with type 2 diabetes. Diabetes Care 20: 1304-1309. doi: 10.2337/diacare.20.8.1304. PubMed: 9250459. [DOI] [PubMed] [Google Scholar]
- 10. Broch M, Gutierrez C, Aguilar C, Simon I, Richart C et al. (1998) Genetic variation in promoter (4G/5G) of plasminogen activator inhibitor 1 gene in type 2 diabetes. Absence of relationship with microangiopathy. Diabetes Care 21: 463. doi: 10.2337/diacare.21.3.463a. [DOI] [PubMed] [Google Scholar]
- 11. Kimura H, Gejyo F, Suzuki Y, Suzuki S, Miyazaki R et al. (1998) Polymorphisms of angiotensin converting enzyme and plasminogen activator inhibitor-1 genes in diabetes and macroangiopathy1. Kidney Int 54: 1659-1669. doi: 10.1046/j.1523-1755.1998.00139.x. PubMed: 9844142. [DOI] [PubMed] [Google Scholar]
- 12. De Cosmo S, Margaglione M, Tassi V, Garrubba M, Thomas S et al. (1999) ACE, PAI-1, decorin and Werner helicase genes are not associated with the development of renal disease in European patients with type 1 diabetes. Diabetes/Metab Res Rev 15: 247-253. doi: 10.1002/(SICI)1520-7560(199907/08)15:4. [DOI] [PubMed] [Google Scholar]
- 13. Tarnow L, Stehouwer CD, Emeis JJ, Poirier O, Cambien F et al. (2000) Plasminogen activator inhibitor-1 and apolipoprotein E gene polymorphisms and diabetic angiopathy. Nephrol Dial Transplant 15: 625-630. doi: 10.1093/ndt/15.5.625. PubMed: 10809802. [DOI] [PubMed] [Google Scholar]
- 14. Wong TY, Poon P, Szeto CC, Chan JC, Li PK (2000) Association of plasminogen activator inhibitor-1 4G/4G genotype and type 2 diabetic nephropathy in Chinese patients. Kidney Int 57: 632-638. doi: 10.1046/j.1523-1755.2000.00884.x. PubMed: 10652041. [DOI] [PubMed] [Google Scholar]
- 15. Globocnik-Petrovic M, Hawlina M, Peterlin B, Petrovic D (2003) Insertion/deletion plasminogen activator inhibitor 1 and insertion/deletion angiotensin-converting enzyme gene polymorphisms in diabetic retinopathy in type 2 diabetes. Ophthalmologica 217: 219-224. doi: 10.1159/000068975. PubMed: 12660488. [DOI] [PubMed] [Google Scholar]
- 16. Lopes C, Dina C, Durand E, Froguel P (2003) PAI-1 polymorphisms modulate phenotypes associated with the metabolic syndrome in obese and diabetic Caucasian population. Diabetologia 46: 1284-1290. doi: 10.1007/s00125-003-1170-0. PubMed: 12856128. [DOI] [PubMed] [Google Scholar]
- 17. Petrovic D, Globocnik-Petrovic M, Peterlin B (2003) 4G4G genotype of PAI-1 gene promoter polymorphism is not associated with myocardial infarction in Caucasians with type-2 diabetes. Cardiology 100: 157-158. doi: 10.1159/000073935. PubMed: 14631138. [DOI] [PubMed] [Google Scholar]
- 18. Santos KG, Tschiedel B, Schneider J, Souto K, Roisenberg I (2003) Diabetic retinopathy in Euro-Brazilian type 2 diabetic patients: relationship with polymorphisms in the aldose reductase, the plasminogen activator inhibitor-1 and the methylenetetrahydrofolate reductase genes. Diabetes Res Clin Pract 61: 133-136. doi: 10.1016/S0168-8227(03)00112-8. PubMed: 12951282. [DOI] [PubMed] [Google Scholar]
- 19. Liu SQ, Xue YM, Yang GC, He FY, Zhao XS (2004) Relationship between plasminogen activator inhibitor-1 gene 4G/5G polymorphism and type 2 diabetic nephropathy in Chinese Han patients in Guangdong Province. Di Yi Jun Yi Xue Xue Bao 24: 904-907. [PubMed] [Google Scholar]
- 20. Zietz B, Buechler C, Drobnik W, Herfarth H, Schölmerich J et al. (2004) Allelic frequency of the PAI-1 4G/5G promoter polymorphism in patients with type 2 diabetes mellitus and lack of association with PAI-1 plasma levels. Endocr Res 30: 443-453. doi: 10.1081/ERC-200035728. PubMed: 15554360. [DOI] [PubMed] [Google Scholar]
- 21. Murata M, Maruyama T, Suzuki Y, Saruta T, Ikeda Y (2004) Paraoxonase 1 Gln/Arg polymorphism is associated with the risk of microangiopathy in Type 2 diabetes mellitus. Diabet Med 21: 837-844. doi: 10.1111/j.1464-5491.2004.01252.x. PubMed: 15270786. [DOI] [PubMed] [Google Scholar]
- 22. Meigs JB, Dupuis J, Liu C, O'Donnell CJ, Fox CS et al. (2006) PAI-1 Gene 4G/5G polymorphism and risk of type 2 diabetes in a population-based sample. Obesity (Silver Spring) 14: 753-758. doi: 10.1038/oby.2006.85. [DOI] [PubMed] [Google Scholar]
- 23. Martin RJ, Savage DA, Patterson CC, Brady HR, Maxwell AP (2007) Common polymorphisms of the PAI1 gene do not play a major role in the development of diabetic nephropathy in Type 1 diabetes. Diabet Med 24: 259-265. doi: 10.1111/j.1464-5491.2007.02087.x. PubMed: 17263760. [DOI] [PubMed] [Google Scholar]
- 24. Saely CH, Muendlein A, Vonbank A, Sonderegger G, Aczel S et al. (2008) Type 2 diabetes significantly modulates the cardiovascular risk conferred by the PAI-1 -675 4G/5G polymorphism in angiographied coronary patients. Clin Chim Acta 396: 18-22. doi: 10.1016/j.cca.2008.06.015. PubMed: 18619429. [DOI] [PubMed] [Google Scholar]
- 25. Ezzidi I, Mtiraoui N, Chaieb M, Kacem M, Mahjoub T et al. (2009) Diabetic retinopathy, PAI-1 4G/5G and -844G/A polymorphisms, and changes in circulating PAI-1 levels in Tunisian type 2 diabetes patients. Diabetes Metab 35: 214-219. doi: 10.1016/j.diabet.2008.12.002. PubMed: 19419896. [DOI] [PubMed] [Google Scholar]
- 26. Prasad P, Tiwari AK, Kumar KM, Ammini AC, Gupta A et al. (2010) Association analysis of ADPRT1, AKR1B1, RAGE, GFPT2 and PAI-1 gene polymorphisms with chronic renal insufficiency among Asian Indians with type-2 diabetes. BMC Med Genet 11: 52. doi: 10.1186/1471-2350-11-52. PubMed: 20353610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al-Hamodi Z, Saif-Ali R, Ismail IS, Ahmed KA, Muniandy S (2012) Effect of plasminogen activator inhibitor-1 and tissue plasminogen activator polymorphisms on susceptibility to type 2 diabetes in Malaysian subjects. J Biomed Biotechnol, 2012: 234937 PubMed: 22577291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8: 336-341. doi: 10.1016/j.ijsu.2010.02.007. PubMed: 20171303. [DOI] [PubMed] [Google Scholar]
- 29. Parmar MKB, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17: 2815-2834. doi: 10.1002/(SICI)1097-0258(19981230)17:24. PubMed: 9921604. [DOI] [PubMed] [Google Scholar]
- 30. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629-634. doi: 10.1136/bmj.315.7109.629. PubMed: 9310563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Naran NH, Chetty N, Crowther NJ (2008) The influence of metabolic syndrome components on plasma PAI-1 concentrations is modified by the PAI-1 4G/5G genotype and ethnicity. Atherosclerosis 196(1): 155-163. doi: 10.1016/j.atherosclerosis.2007.03.024. PubMed: 17467713. [DOI] [PubMed] [Google Scholar]
- 32. Brazionis L, Rowley K, Jenkins A, Itsiopoulos C, O'Dea K (2008) Plasminogen activator inhibitor-1 activity in type 2 diabetes: a different relationship with coronary heart disease and diabetic retinopathy. Arterioscler Thromb Vasc Biol 28: 786-791. doi: 10.1161/ATVBAHA.107.160168. PubMed: 18239151. [DOI] [PubMed] [Google Scholar]
- 33. Lassila M, Fukami K, Jandeleit-Dahm K, Semple T, Carmeliet P et al. (2007) Plasminogen activator inhibitor-1 production is pathogenetic in experimental murine diabetic renal disease. Diabetologia 50: 1315-1326. doi: 10.1007/s00125-007-0652-x. PubMed: 17415547. [DOI] [PubMed] [Google Scholar]
- 34. Seo JY, Park J, Yu MR, Kim YS, Ha H et al. (2009) Positive feedback loop between plasminogen activator inhibitor-1 and transforming growth factor-beta1 during renal fibrosis in diabetes. Am J Nephrol 30: 481-490. doi: 10.1159/000242477. PubMed: 19786738. [DOI] [PubMed] [Google Scholar]
- 35. Munafò MR, Flint J (2004) Meta-analysis of genetic association studies. Trends Genet 20: 439-444. doi: 10.1016/j.tig.2004.06.014. PubMed: 15313553. [DOI] [PubMed] [Google Scholar]
- 36. Patsopoulos NA, Evangelou E, Ioannidis JP (2008) Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 37: 1148-1157. doi: 10.1093/ije/dyn065. PubMed: 18424475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ioannidis JP, Trikalinos TA, Ntzani EE, Contopoulos-Ioannidis DG (2003) Genetic associations in large versus small studies: an empirical assessment. Lancet 361: 567-571. doi: 10.1016/S0140-6736(03)12516-0. PubMed: 12598142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)
Systematic review flow diagram n, number of studies.
(TIF)
Egger's publication bias test for the PAI-1 -675 4G/5G polymorphisms in DM, DN, DR and diabetic CAD.
(DOC)


