Abstract
We designed a phase II study evaluating the upfront combination of clofarabine and daunorubicin in acute myeloid leukemia (AML) patients ≥60 years old. The median age of the 21 patients was 69 (range 60–85) years. Fourteen patients (67%) had unfavorable risk features. The principal toxicities were grade ≥3 infections and prolonged myelosuppression. Three (14%) deaths occurred from infectious complications. Six (28.6%) patients achieved complete remission including three (21.4%) of 14 patients with unfavorable AML. The median disease-free survival was 6.8 months and the median overall survival was 11.2 months.
Keywords: elderly, AML, clofarabine, daunorubicin, clinical trial
Introduction
The outcome of acute myeloid leukemia (AML) in patients ≥60 years old remains poor with a median survival of approximately 12 months. This poor outcome is partially explained by the inability of patients to tolerate treatment and by the aggregation of unfavorable risk factors including secondary leukemia, adverse karyotypes, aberrant signaling pathways such as FLT3-internal tandem duplication (ITD), and overexpression of P-glycoprotein [1].
Clofarabine, a second generation purine nucleoside analog, was designed to overcome some of the above features. Specifically, halogenation at the 2 position of adenine and substitution of a fluorine group at the C-2 position of the arabino furanosyl moiety allows high affinity for deoxycytidine kinase, prolonged intracellular retention of clofarabine triphosphate, potent inhibition of DNA synthesis and ribonucleotide reductase, and resistance to P-glycoprotein [2]. In addition, clofarabine is active in non-dividing cells as well as cells with a low proliferation rate, enhancing potential activity against leukemic stem cells. Clofarabine is approved for pediatric relapsed/refractory acute lymphoblastic leukemia [3] but there has been significant interest in its role in AML. In particular, the combination of clofarabine and cytarabine has shown promising activity as induction therapy in newly diagnosed AML patients 50 years of age or older [4]. Since daunorubicin is also active in AML [5], we conducted a phase II study of clofarabine plus daunorubicin in newly diagnosed AML patients older than 60 years of age. The primary end point of the study was treatment response, and the secondary end points were disease-free survival (DFS) and overall survival (OS).
Patients and Methods
Patient Criteria
Newly diagnosed AML patients ≥60 years of age were eligible to participate in the study following pathological disease confirmation. Patients with acute promyelocytic leukemia with t(15;17) or other variants were excluded. Additional inclusion criteria were no previous therapy for AML with the exception of hydroxyurea, Eastern Cooperative Oncology Group performance status ≤2, left ventricular ejection fraction ≥45%, as well as adequate renal (defined as an estimated glomerular filtration rate ≥60 mL/min/1.73m2 per modified diet in renal disease equation) and hepatic (serum bilirubin ≤1.5 x the upper limit of normal; aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase ≤2.5 x the upper limit of normal) functions. Major exclusion criteria included active clinical infection including HIV positivity, and clinical evidence of central nervous system disease. Patients with a diagnosis of previous malignancy were also excluded unless they had been free of documented disease for a minimum of three years. Patients had to be without evidence of severe concurrent disease or organ dysfunction involving the heart, kidney or liver that would place the patient at undue risk from induction chemotherapy. Approval of the study was granted by the Scientific Review Committee and the Institutional Review Board of Roswell Park Cancer Institute. All patients signed informed consent prior to study initiation. The study was conducted in accordance with the basic principles of the Declaration of Helsinki. The study was registered as NCT00814164.
Karyotype analyses were performed at Roswell Park Cancer Institute. We used the European LeukemiaNet classification [6]. FLT3-ITD [7], NPM1[8] and CEPBA[9] mutations were assessed at Roswell Park Cancer Institute.
Treatment Plan
The study design consisted of three treatment cycles (one induction and up to two cycles of consolidation) unless the patient exhibited evidence of treatment failure, disease recurrence, or an unacceptable toxicity that led to treatment discontinuation. During induction, clofarabine was administered at 20 mg/m2 as a one hour intravenous (IV) infusion for five days followed three hours later (from the end of infusion) by daunorubicin 50 mg/m2 administered as IV infusion (over five minutes) given every other day for three doses (days 1, 3 and 5). Patients who achieved remission after induction were offered up to two cycles of consolidation therapy with clofarabine administered at 20 mg/m2 as a one hour IV infusion for three consecutive days followed three hours later (from the end of infusion) by daunorubicin 50 mg/m2 administered as IV infusion (over five minutes) given every other day for two doses (days 1 and 3). Prophylactic anti-bacterial, anti-fungal and anti-viral agents were utilized according to institutional guidelines.
Response Criteria
Treatment response was assessed using the International Working Group criteria [10]. Complete response (CR) was defined as recovery of normal hematopoiesis with an absolute neutrophil count ≥1.0×109/L and a platelet count ≥100x109/L and normalization of the bone marrow blasts (<5%). CR without platelet recovery was defined as CRp. If assessment of bone marrow examination following induction confirmed >5% blasts, then a second treatment cycle could be administered as re-induction but not before Cycle 1 Day 28. If the marrow assessment following induction precluded a definitive treatment decision (e.g., a hypocellular or regenerating marrow), then a repeat bone marrow examination was performed every 7–14 days through day 84 until a determination could be made. If a definitive treatment decision could not be made by day 84, then the patient was assessed as a treatment failure. If the patient met the criteria for leukemic progression, then the patient was considered a treatment failure and no further treatment cycles were administered.
Statistical Analysis
This was a phase II study in adult AML patients aged ≥60 years old. The study was planned to enroll 60 patients with an interim analysis after 30 patients. The study was prematurely halted by the sponsor due to the unfavorable outcome. Fisher’s exact test was used to study the association between categorical variables. The Wilcoxon rank sum test was used to compare the response groups in regards to numeric variables. The logistic regression model was used to test the difference between response and no-response groups for multivariate analysis. The estimated DFS and OS distributions were obtained using the Kaplan-Meier method. Using this distributional estimate, summary descriptive statistics such as the median survival and a 95% confidence interval (CI) of the median survival were obtained. Exact 95% CI using the Clopper-Pearson method was used to evaluate response rate. A 0.05 nominal significance level was used in all testing. All statistical analyses were done using SAS (version 9.3).
End Point Definitions
Overall response (OR) was defined as the sum of the number of patients with morphologic CR plus those with CRp divided by the total number of patients. Duration of remission was defined as the time from first objective documentation of CR or CRp to date of first objective documentation of disease relapse, initiation of alternative anti-leukemic therapy while in remission (including stem cell transplantation), or death due to any cause, whichever occurred first. Patients who did not respond (i.e., those who failed to achieve CR or CRp) were coded as failures at the last date on which their disease status was assessed. DFS was defined as the time from first objective documentation of CR or CRp until the date of first objective documentation of disease relapse or death due to any cause, whichever occurred first. OS was defined as the time from date of treatment initiation until date of death due to any cause.
Results
Patient Characteristics
Between December 2008 and June 2011, twenty-one patients were enrolled. The median age was 69 (range 60–85) years, and 12 (57%) were women. Nine patients (43%) were older than 70 years of age. Fourteen patients (67%) had unfavorable risk features consisting of secondary AML (all had antecedent hematologic disorders, none had therapy-related AML), presence of complex karyotype (≥3 aberrations) and/or the presence of FLT3-ITD (Table 1). All 21 patients completed one induction cycle, one patient received a second induction cycle due to residual disease, and six (29%) patients received at least one consolidation regimen.
Table 1.
Patient characteristics
| Characteristics | Number | % |
|---|---|---|
| Age | ||
| Median | 68 | |
| Range | 60–85 | |
|
| ||
| ELN Classification | ||
| Favorable | 3 | 14 |
| Intermediate-I | 5 | 24 |
| Intermediate-II | 4 | 19 |
| Adverse* | 9 | 43 |
|
| ||
| Molecular Aberrations | ||
| FLT3-ITD | 1 | 5 |
| NPM1 | 4 | 19 |
| CEBPA | 1 | 5 |
|
| ||
| White blood cell count (×109/L) | ||
| Median | 7.89 | |
| Range | 0.22–134.0 | |
|
| ||
| Secondary AML | ||
| Antecedent hematologic disorder | 10 | 48 |
| Previous treatment for another malignancy | 0 | 0 |
|
| ||
| ECOG Performance Status | ||
| 0 | 1 | 5 |
| 1 | 16 | 76 |
| 2 | 4 | 19 |
Abbreviations: AML, acute myeloid leukemia; CEBPA, CAAT/enhancer-binding protein alpha; ECOG, Eastern Cooperative Oncology Group; ELN, European LeukemiaNet; FLT3-ITD, Fms-like tyrosine kinase 3-internal tandem duplication; NPM1, nucleophosmin 1;
This cohort included one patient with both del(5) and del(7), three patients with del(5)/−5 and four patients with del(7)/−7. Six patients had complex karyotype.
Response
Among the 21 patients, eight achieved a CR/CRp to induction therapy (six CR, two CRp) for an OR rate of 38.1% (95% CI, 18.1%–61.6%) and CR rate of 28.6% (95% CI, 11.3%–52.2%) (Table 2). In multivariate analyses, the unfavorable group (defined as secondary AML, complex karyotype and FLT3-ITD) showed significant association with response when controlling for age, white blood cell count and performance status (P=0.0311); however, no significance was detected in univariate analysis. Of note, three of 14 (21.4%) AML patients with unfavorable features achieved a CR/CRp and three of nine (33%) AML patients aged 70 or above achieved CR following treatment (Table 2).
Table 2.
Response Characteristics
| Response (%) | Unfavorable AML (%) | Age ≥70 | N | |
|---|---|---|---|---|
| CR | 6 (28.6) | 3 (21.4) | 3 (33) | 6 |
| CRp | 2 (9.5) | 0 (0) | 2 (22) | 2 |
| NR | 13 (61.9) | 11 (78.6) | 4 (44) | 13 |
| Total | 21 (100) | 14 (100) | 9 (100) | 21 |
Abbreviations: AML, acute myeloid leukemia; CR, complete response; CRp, CR without platelet recovery; N, number; NR, no response;
The median DFS for the eight patients who achieved a CR/CRp to induction therapy was 6.8 (95% CI, 1.1–36.3) months (Figure 1) and median OS for the whole cohort (21 patients) was 11.2 (95% CI, 4.2–14.2) months (Figure 2). The one year OS for all 21 treated patients was 38.1% (95% CI: 18.3%, 57.8%).
Figure 1.
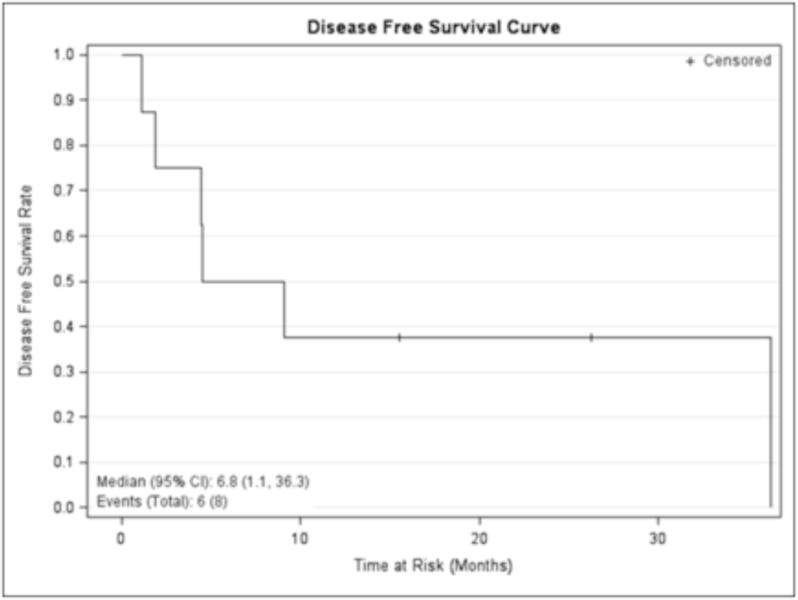
Disease-free survival of the whole cohort. Kaplan Meier curve with + denoting censored patients.
Figure 2.
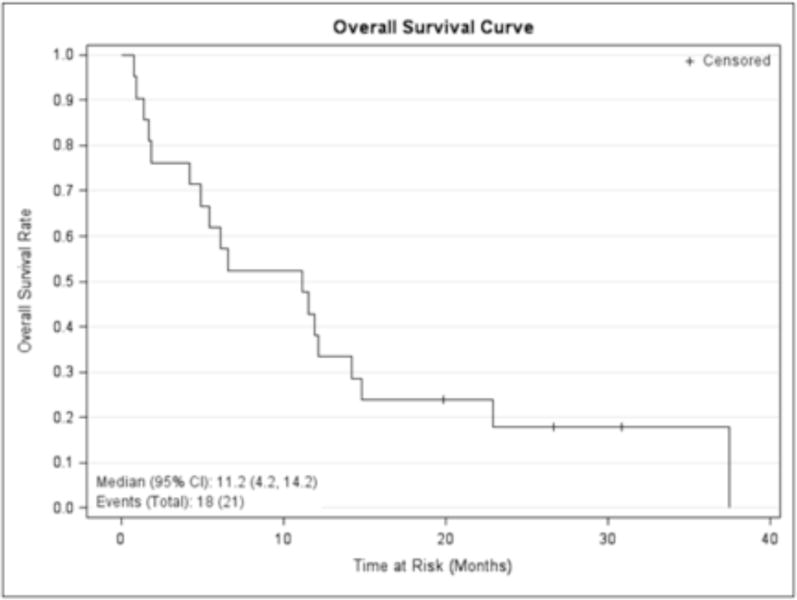
Overall survival of the whole cohort. Kaplan Meier curve with + denoting censored patients.
Safety
The most common principal severe toxicities experienced by patients were grade ≥3 infections in six (29%) patients and prolonged thrombocytopenia and neutropenia in three (14.3%) patients. Two patients (9.5%) died within 30 days, one from toxicity and one from indeterminate cause, and three (14.3%) died within 60 days, one from toxicity and two from progressive disease.
Discussion
Clofarabine is an active agent for the treatment of AML, both in the refractory/relapsed and upfront treatment setting. The combination of clofarabine with cytarabine was compared with cytarabine alone in relapsed/refractory AML patients in a large placebo-controlled, double-blind, randomized phase III study [11]. Clofarabine and cytarabine resulted in significant improvement in CR rates and event-free survival over cytarabine alone. Although OS did not differ between the two arms, the data suggested that more patients from the clofarabine and cytarabine arm who achieved CR were able to proceed onto allogeneic stem cell transplantation, which by itself could be viewed as a clinical benefit. Other recent studies of clofarabine included a randomized comparison of clofarabine as a single agent (20 mg/m2 intravenously daily for five days) versus low-dose cytarabine (20 mg twice daily subcutaneously for ten days) as first line treatment for AML patients older than 50 years of age. This study again demonstrated almost doubling of the CR rate (28% versus 13%) with clofarabine as compared with cytarabine but also did not result in improved OS [12]. The longer OS in the low-dose cytarabine arm was attributed to better survival for those who did not achieve CR. Interestingly, the initial clofarabine and cytarabine combinations administered a higher clofarabine dose as compared to the more recent trial and ours. This difference may have contributed to the relatively unfavorable outcome in the later studies.
Burnett and colleagues [13] recently evaluated the combination of clofarabine and daunorubicin using the same doses as in the current trial as compared with cytarabine (100 mg/m2 twice daily for ten days) and daunorubicin (50 mg/m2 for three days) therapy in AML patients 55 years and older [13]. Their results showed a CR rate of 57% in the clofarabine arm and 63% in the daunorubicin/cytarabine arm with a 60-day mortality of 14% and 15%. The main differences between this study and our results relate to the patient populations. In the randomized study by Burnett et al, only 17% of patients had secondary AML and only 23% had adverse karyotype as as compared with 48% and 43%, respectively, of the AML patients enrolled in our study. Furthermore, the cytogenetic classifications between the two studies differed; the randomized study used the Wheatley method [14], and the current study used the European LeukemiaNet (ELN) classification [6]. However, both included inv(3)/t(3;3), −5 or del(5q), −7, and complex karyotype as adverse karyotype. The ELN also includes t(6;9), t(v;11)(v;q23), and abn(17p) as adverse karyotype; the first two were not detected in the current cohort; abn(17p) was detected in two patients. Therefore it seems that the worse outcome reported in the current study may be attributed to a more challenging patient population. Of note, this patient population is also known to express multidrug resistance proteins such as ABCB1/MDR1 [15]. Daunorubicin is a substrate for these proteins; this may further explain the unfavorable outcome in this study.
No unexpected toxicities related to the combination of clofarabine and daunorubicin were detected, and the mortality rate was not different when compared to the randomized trial by Burnett et al [13] despite the different AML populations.
In conclusion, the study demonstrated that the combination of clofarabine and daunorubicin is safe and well-tolerated as upfront therapy of AML patients ≥ 60 years of age. However this regimen does not appear to be sufficiently active for the treatment of AML patients with unfavorable disease characteristics to warrant additional investigation in this setting. The results of a currently accruing additional randomized trial of clofarabine vs. daunorubicin and cytarabine (NCT01041703) in older AML patients will likely determine the future role of clofarabine in the armamentarium of drugs for AML therapy.
Table 3.
Adverse Events by grade
| Event | Gr 3(R)[SAE] | Gr 4(R)[SAE] | Gr 5(R)[SAE] |
|---|---|---|---|
| Blood/Bone Marrow | |||
|
| |||
| Anemia | 3 | 0 | 0 |
| Leukopenia | 0 | 2 | 0 |
|
| |||
| Neutropenia | 0 | 2 | 0 |
|
| |||
| Thrombocytopenia | 0 | 3 | 0 |
|
| |||
| Constitutional | |||
|
| |||
| Fatigue | 1 | 0 | 0 |
|
| |||
| Infection | |||
|
| |||
| Bacteremia | 4 | 0 | 0 |
|
| |||
| Cellulitis | 4 | 0 | 0 |
|
| |||
| Metabolic | |||
|
| |||
| Hyperglycemia | 1 | 0 | 0 |
|
| |||
| Neurology | |||
|
| |||
| Syncope | 1 | 0 | 0 |
|
| |||
| Pulmonary | |||
|
| |||
| Pneumonia | 3 | 0 | 0 |
|
| |||
| Syndrome | |||
|
| |||
| Tumor lysis syndrome | 2 | 0 | 0 |
Abbreviations: Gr, grade; R, related; SAE, serious adverse event;
Acknowledgments
Supported partially by a grant from the National Cancer Institute Grant CA16056 (CEV, WT, EAG, JET, JDG, LAF, ESW, MA), the Leonard S. LuVullo Endowment for Leukemia Research (MW), the Nancy C. Cully Endowment for Leukemia Research (MW), the Dennis Szefel Jr. Endowment (ESW), the Babcock Family Endowment (MW), the Heidi Leukemia Research Fund, Buffalo, NY (MW) and Genzyme (now Sanofi) (MW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contribution
C.E.V. reviewed the literature, conducted the study, contributed to the care of the patients, and wrote the manuscript; W.T. performed the statistical analysis; G.D. reviewed all diagnostic pathology material, classified the cases according to the World Health Organization classification and critically reviewed the manuscript; S.J.N.S. and A.W.B. reviewed the cytogenetic analyses; P.S. reviewed the molecular studies; J.D.G. and L.A.F served as research coordinators on this trial; E.A.G., J.E.T and E.S.W. contributed to the care of the patients, reviewed and edited the manuscript, and approved the final version; M.W. designed the study, oversaw the conduct of the study, contributed to the care of the patients, edited and reviewed the manuscript and approved the final version.
All authors approved the final version of the manuscript.
Financial disclosures: The authors have no conflict of interest.
References
- 1.Burnett A, Wetzler M, Lowenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29:487–94. doi: 10.1200/JCO.2010.30.1820. [DOI] [PubMed] [Google Scholar]
- 2.Montgomery JA, Shortnacy-Fowler AT, Clayton SD, Riordan JM, Secrist JA., 3rd Synthesis and biologic activity of 2’-fluoro-2-halo derivatives of 9-beta-D-arabinofuranosyladenine. J Med Chem. 1992;35:397–401. doi: 10.1021/jm00080a029. [DOI] [PubMed] [Google Scholar]
- 3.Kantarjian HM, Jeha S, Gandhi V, Wess M, Faderl S. Clofarabine: past, present, and future. Leukemia & lymphoma. 2007;48:1922–30. doi: 10.1080/10428190701545644. [DOI] [PubMed] [Google Scholar]
- 4.Faderl S, Verstovsek S, Cortes J, Ravandi F, Beran M, Garcia-Manero G, et al. Clofarabine and cytarabine combination as induction therapy for acute myeloid leukemia (AML) in patients 50 years of age or older. Blood. 2006;108:45–51. doi: 10.1182/blood-2005-08-3294. [DOI] [PubMed] [Google Scholar]
- 5.Yates J, Glidewell O, Wiernik P, Cooper MR, Steinberg D, Dosik H, et al. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood. 1982;60:454–62. [PubMed] [Google Scholar]
- 6.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 7.Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer research. 2001;61:7233–9. [PubMed] [Google Scholar]
- 8.Dohner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A, et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood. 2005;106:3740–6. doi: 10.1182/blood-2005-05-2164. [DOI] [PubMed] [Google Scholar]
- 9.Marcucci G, Maharry K, Radmacher MD, Mrozek K, Vukosavljevic T, Paschka P, et al. Prognostic significance of, and gene and microRNA expression signatures associated with, CEBPA mutations in cytogenetically normal acute myeloid leukemia with high-risk molecular features: a Cancer and Leukemia Group B Study. J Clin Oncol. 2008;26:5078–87. doi: 10.1200/JCO.2008.17.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Faderl S, Wetzler M, Rizzieri D, Schiller G, Jagasia M, Stuart R, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30:2492–9. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett AK, Russell NH, McMullin MF, Kell J, Ali S, Moreton P, et al. A Randomised Comparison of Clofarabine Versus Low Dose Ara-C As First Line Treatment for Older Patients with AML. ASH Annual Meeting Abstracts. 2012;120:889. [Google Scholar]
- 13.Burnett AK, Russell NH, Kell J, Kjeldsen L, Milligan D, Cahalin P, et al. A Comparison of Daunorubicin/Ara-C (DA) Versus Daunorubicin/Clofarabine (DClo) and Two Versus Three Courses of Total Treatment for Older Patients with AML and High Risk MDS: Results of the UK NCRI AML16 Trial. ASH Annual Meeting Abstracts. 2012;120:892. [Google Scholar]
- 14.Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council’s Adult and Childhood Leukaemia Working Parties. British journal of haematology. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 15.Shaffer BC, Gillet JP, Patel C, Baer MR, Bates SE, Gottesman MM. Drug resistance: still a daunting challenge to the successful treatment of AML. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2012;15:62–9. doi: 10.1016/j.drup.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


