Abstract
Molecular regulation of fatty acid desaturase (Fads) gene expression by dietary arachidonic (ARA) and docosahexaenoic acid (DHA) during early postnatal period, when the demand for long chain polyunsaturated fatty acids (LC-PUFA) is very high, has not been well defined. The objective of the current study was to determine regulation of liver Fads1, Fads2 and Fads3 classical (CS) and alternative transcripts (AT) expression by dietary ARA and DHA, within the physiological range present in human breast milk, in suckling piglets. Piglets were fed one of six milk replacer formula diets (Formula-reared groups, FR) with varying ARA and DHA content from days 3-28 of age. The ARA/DHA levels of the six formula diets were as follows (% total fatty acid, FA/FA): (A1) 0.1/1.0; (A2) 0.53/1.0; (A3-D3) 0.69/1.0; (A4) 1.1/1.0; (D2) 0.67/0.62; (D1) 0.66/0.33. The control maternal-reared (MR) group remained with the dam. Fads1 expression was not significantly different between FR and MR groups. Fads2 expression was down-regulated significantly in diets with 1:1 ratio of ARA:DHA, compared to MR. Fads2 AT1 expression was highly correlated to Fads2 expression. Fads3 AT7 was the only Fads3 transcript sensitive to dietary LC-PUFA intake and was up-regulated in the formula diets with lowest ARA and DHA content compared to MR. Thus, the present study provides evidence that the proportion of dietary ARA:DHA is a significant determinant of Fads2 expression and LC-PUFA metabolism during the early post-natal period. Further, the data suggest that Fads3 AT7 may have functional significance when dietary supply of ARA and DHA are low during early development.
Keywords: Arachidonic acid, Docosahexaenoic acid, fatty acid desaturase gene, infant nutrition, piglet
Introduction
Long chain polyunsaturated fatty acids (LC-PUFA) arachidonic acid (20:4n-6, ARA) and docosahexaenoic acid (22:6n-3, DHA) are essential for optimal health, cognition and development during fetal and early postnatal life [1, 2]. Dietary LC-PUFA intake and endogenous synthesis of LC-PUFA from precursor polyunsaturated fatty acids (PUFA) namely, linoleic acid (18:2n-6, LA) and linolenic acid (18:3n-3, LNA), are key determinants of LC-PUFA accretion during early development.
Endogenous biosynthesis of LC-PUFA from precursor PUFA requires a series of desaturation and elongation reactions. Δ6 and Δ5 desaturation are two key enzyme activities that catalyze the synthesis of LC-PUFA from PUFA by introducing cis double bonds at specific positions [3]. The Δ6-desaturase catalyzes the first and the rate limiting step in the biosynthesis of LC-PUFA from precursor PUFA [3, 4]. Fads2 and Fads1 genes, which belong to the fatty acid desaturase (Fads) gene cluster, encode for Δ6 and Δ5 desaturase, respectively [5]. Fads1 and Fads2 are clustered within the 100kb region on the long arm of human chromosome 11q12-13.1 [5],
A third member of the Fads gene family, Fads3, is a putative desaturase gene adjacent to Fads2 in a tail-to-tail orientation [5]. It codes for multiple alternative transcripts [6] and protein isoforms [7]. However, functions of Fads3 products are not known. We characterized Fads3 alternative transcripts (AT) in baboon neonates and human neuronal cells [6] and demonstrated regulation of Fads3 AT by dietary DHA intake in baboon neonates [8]. Fads3 and its AT expression are tissue specific [6, 7], are evolutionarily conserved and are widely and differentially expressed across twelve neonate baboon tissues [6, 9]. Fads3 is highly expressed at the implantation site of the embryo in mouse uterus [10] and its AT are differentially expressed in response to neuronal cell differentiation [6], suggesting that Fads3 products have distinct physiological functions. Further, recent studies have provided evidence that Fads3 may play an important role in regulating lipid metabolism [11, 12]. Thus, understanding molecular regulation of Fads3 is crucial for characterizing its physiological functions.
Many previous studies have established that Δ6 and Δ5 desaturase are regulated by dietary LC-PUFA and hormonal factors [4, 13, 14]. Δ6 and Δ5 desaturase were down-regulated by diets enriched with vegetable oils and up-regulated with PUFA deficient diets [4, 13, 15]. Thus, adequate dietary intake of preformed LC-PUFA suppresses Fads2 and Fads1 expression and endogenous LC-PUFA synthesis. However, LC-PUFA regulation of Fads gene products during the early postnatal period, when the demand for LC-PUFA is very high, has not been well studied. Recently Jacobi et al [16] showed that in suckling piglets, ARA intake at supra-physiological levels failed to down-regulate Fads2 or Fads1 in liver and actually increased their expression and LC-PUFA flux in intestine, providing evidence that dietary LC-PUFA may regulate Fads gene differently during early development. Less is known on the molecular regulation of Fads3 gene expression.
All breast milks reported to date contain ARA and DHA [1, 17]. Mean values of ARA and DHA reported in human breast milk worldwide are 0.47 ± 0.13% (range 0.24-1.0% TFA) and 0.32% ± 0.22 (range 0.06-1.4% TFA) respectively, although DHA intake varied widely depending on maternal intake [18]. Thus, the mean ratio of ARA:DHA reported in human milk is near 1.5 but varies widely based on maternal diet [18]. The current recommendations for ARA and DHA levels in formula are 0.66%,w/w ARA and 0.33%,w/w DHA [19], The regulation of Fads gene products at these physiological intake levels of ARA and DHA, within the range provided by breast milk during the early post-natal period has not been studied. Further, how ARA and DHA regulate Fads gene expression in relation to one another, i.e. with increasing doses of one of these two LC-PUFA at a constant intake of the other, has not been studied.
The suckling piglet provides a practical mode for human infant development because the metabolic responses and the genetics of fatty acid desaturases in piglets are similar to those in humans [16, 20]. Thus, this study used the neonatal piglet model to investigate molecular determinants of FADS gene expression during early development. We previously reported on a study evaluating diets of systematically altered DHA and ARA levels within the physiological range present in human milk on neonatal piglet tissue fatty acid profiles [21, 22]. Here we report on the Fads gene regulation, including selected alternative transcripts, in liver from these animals. The present study determined i) liver Fads1 and Fads2 classical transcript (CS) gene regulation, ii) liver Fads AT expression and its regulation by increasing dietary levels of ARA (0.1%-1.6% TFA) at constant DHA (approx 1.0% TFA) intake and iii) liver FADS AT expression and its regulation by increasing dietary levels of DHA (0.3-1.0% wt/wt) at constant ARA intake (approx 0.7% TFA).
Methods
Animals, Diets and Study Design
All procedures involving animals were approved by the Institutional Animal Care and Use Committee at Cornell University. Experimental design and diets were reported in detail previously [22]. Briefly, on day 3 of life domestic piglets Domestic piglets (from Yorkshire and Landrace sows bred to Hampshire boars) matched for age and sex were assigned to one of six milk replacer formula diets (FR, n=4/diet) with varying ARA/DHA ratios (Table 1) and individually housed. The seventh group was maternal-reared (MR) control group and remained with dam for the duration of the study.
Table 1.
ARA (g/100g) and DHA (g/100g) composition of FR diets and sow milk MR diet
| A1 | A2 | A3/D3 | A4 | D2 | D1 | MR | |
|---|---|---|---|---|---|---|---|
| ARA | 0.09 | 0.53 | 0.69 | 1.06 | 0.67 | 0.66 | 0.74 |
| DHA | 1.0 | 1.02 | 1.01 | 1.04 | 0.62 | 0.33 | 0.01 |
Milk replacer formula consisting of 60% experimental diet (Research Diets Inc, New Brunswick, NJ, USA) and 40% Birthright baby pig milk replacer (Ralco Nutrition, Inc, Marshall, MN) was fed to FR piglets from day 3-28 of age. Details of the nutrient and fatty acid composition of the six FR diets have been reported previously [22]. Table 1 shows the ARA (g/100g) and DHA (g/100g) composition of the milk replacer formula in the six FR diets and sow’s milk in MR group. On day 28 of age, piglets were sacrificed and liver was removed, weighed and flash frozen in RNA later (Ambion, USA) and stored at -80 C for RNA extraction and real-time PCR.
RNA Extraction and cDNA Preparation
Piglet liver samples were thawed and 30mg of tissue from each animal was homogenized (FR, n=4/diet; MR, n=4). Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA). The yield of total RNA was assessed by 260nm UV and its quality was analyzed by 260/280 nm ratios using NanoDrop One microgram total RNA was reverse transcribed into first strand cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, CA, USA). This cDNA was used as template for subsequent PCR reactions.
Semi-quantitative RT-PCR Analysis
Conventional PCR was used to amplify the classical (CS) and alternative transcripts (AT) of Fads genes. qPCR was not successful because copy number for AT is low, and primer efficiency, constrained to the spliced region, was low as well. Primer pairs specific for each transcript bridging the deleted parts of the exons were designed. 18S was used as the reference gene. PCR primers were designed using PrimerQuest software (IDT, Coralville, IA) and ordered from Integrated DNA Technologies (IDT, Coralville, IA). Primer sequences and standardized annealing temperatures of each primer pair are presented in Table 2. Initially primers were tested by polymerase chain reactions with liver cDNA as a template in a 30 μL reaction volume using a gradient thermal cycler (Eppendorf, USA), with 1 μMof each primer, 0.25 mM each of dNTPs, 3 μ of 10×PCR buffer (Life Technologies, NY), 1.5 mM MgCl2 and 1.5 U Taq polymerase (Ampli Taq II; Life Technologies, NY). PCR products were run on 2% agarose gel stained with ethidium bromide. The intensities of the expressed products were quantified densitometrically by ImageJ software (National Institutes of Health, USA). Expression levels of each transcript were normalized to expression values of control 18S gene. Each FR group’s average expression was then compared to the MR group to determine the fold change in gene expression. Statistical significance (p<0.05; p<0.10 for marginal significance) was determined by performing student’s t-test on normalized expression levels of Fads gene transcripts of each FR group compared to MR group using Microsoft Excel; linear regression was also performed in Excel.
Table 2.
Primer pair’s specific for Pig fatty acid desaturase (FADS) classical and alternative transcripts
| FADS Primers |
Forward Primer | Reverse Primer | Annealing Temperature (°C) |
|---|---|---|---|
|
| |||
| FADS1CS | TAA TGC ACC TGC TGC ACA TCC T | TAG AGG TGC TGA AGA CGG ACA | 65 |
| FADS2CS | AGG CCC AGG CTG GGT GGC TGC AG | AGT TGG CAG AGG CAC CCT TTA AG | 60 |
| FADS2AT1 | AGA AGC ACA ACC TGT CTG TTT ACA | ATG GTT CCA CCA GTT GGC AGA G | 55 |
| FADS3AT1 | AGA ATG CCC AGA GCT GGT GTC TGC A | AAG TGG TTC CAC CAG GAC TTC CTG A | 65 |
| FADS3AT4 | TGC AGT GGA CGG GTC CTG GAG AGC CA | TCG TGG CCG ATC TCC TTG GGG ATG T | 65 |
| FADS3AT6 | CTT CAT CCT GGG CCA GCT CCC AGT T | AAG AGG TGG TGC TCG ATC TGG AAG T | 62 |
| FADS3AT7 | TTC TTA TCC TAC CTC TTC CCC ACG AT | TTC ACC TCG TAG CTG AGG CCG TGC TT | 62 |
| 18S | TCC TTG GAT GTG GTA GCC GTT TCT | TCA ACT TTC GAT GGT AGT CGC CGT | 65 |
Results
Liver PUFA accretion in piglets fed increasing amounts of ARA and DHA
The liver fatty acid profiles of these piglets appear elsewhere [21]; for convenient reference the ARA and DHA compositions are presented in Table 3. Liver ARA content increased with increasing dietary ARA intake at constant DHA intake. Similarly, DHA accretion in liver reflected dietary DHA intake, with piglets fed approx 1% wt/wt DHA (diets A1-A4) having the highest DHA content. DHA content in liver decreased dose-dependently with D2 and D1 diets and was lowest in the MR diet. Liver ARA accretion was lower with increasing dietary DHA intake. However, DHA accretion in liver was not affected by increased ARA intake.
Table 3.
Liver ARA and DHA composition (g/100g of total lipids) in piglets fed varying levels of ARA and DHA from days 3-28 of age
| A1 | A2 | A3/D3 | A4 | D2 | D1 | MR | |
|---|---|---|---|---|---|---|---|
| Diet ARA/DHA | 0.1/1.0 | 0.53/1.0 | 0.69/1.01 | 1.1/1.0 | 0.67/0.62 | 0.66/0.33 | 0.74/0.01 |
|
| |||||||
| ARA (g/100g) | 13.92±0.49a | 16.14±0.87b | 16.12±1.10bc | 16.88±1.50c | 17.36±0.63c | 17.87±1.46d | 17.00±1.36bcd |
| DHA (g/100g) | 10.77±1.77a | 11.43±1.22a | 10.49±1.41a | 10.45±1.79a | 9.65±1.01b | 7.52±0.69c | 2.37±0.30d |
Values represent mean±SD (n=4); means not sharing a common superscript were significantly different (p<0.05)
Liver Fads1 and Fads2 CS expression in piglets fed increasing amounts of ARA and DHA
Figure 1 shows liver Fads1 CS expression in piglets fed varying concentrations of ARA and DHA. The mean relative expression levels of Fads1 CS showed significant inter-individual variability and were not sensitive to dietary ARA or DHA intake in piglets. Fads2 CS was consistently expressed in piglet liver and was altered by dietary ARA and DHA intake (Figure 2). Liver Fads2 CS expression was significantly down-regulated in diets with 1:1 ratio of ARA:DHA (1.1 %/1.0% and 0.67%/0.62% ARA/DHA in A4 and D2 diets) compared to MR. Further, liver Fads2 CS expression tended to be lower in all FR piglets compared to control (MR) group.
Figure 1.
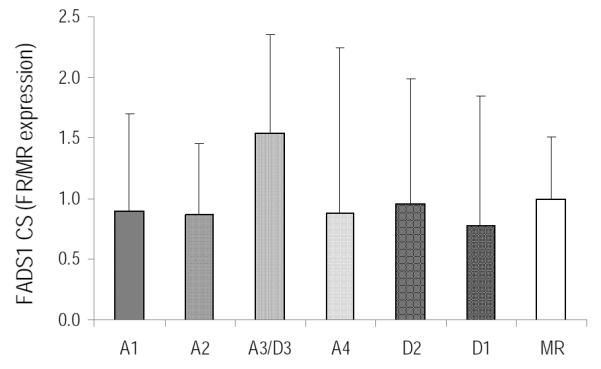
FADS1 CS liver relative expression in piglets fed six different formula diets (FR) and control (MR), n=4/treatment. Values are mean±SD. No means are different from MR control.
Figure 2.
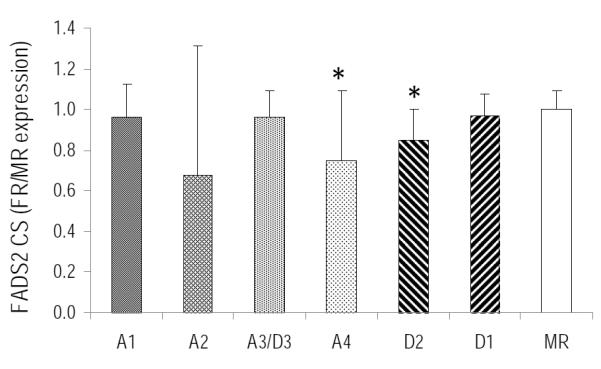
FADS2 CS liver relative expression, mean±SD. * indicates significantly different from control MR group (p<0.05)
Liver Fads AT expression in piglets fed varying amounts of ARA and DHA
Expression of liver Fads2 alternative transcript (AT1) in piglets fed varying ratios of ARA:DHA is shown in Figure 3. In general, Fads2 AT1 expression was lower in all FR groups compared to MR group (Fig 3). Fads2 AT1 was down-regulated by 48% (p=0.01) in A2 and 18%, 36% and 27% in D1 (p=0.04), D2 (p=0.002) and A4 was marginally significant (p=0.086) diets, respectively compared to MR diet. Liver Fads2 AT1 expression was highly correlated to Fads2 CS expression in piglets fed diets with varying levels of ARA and DHA (Figure 4).
Figure 3.
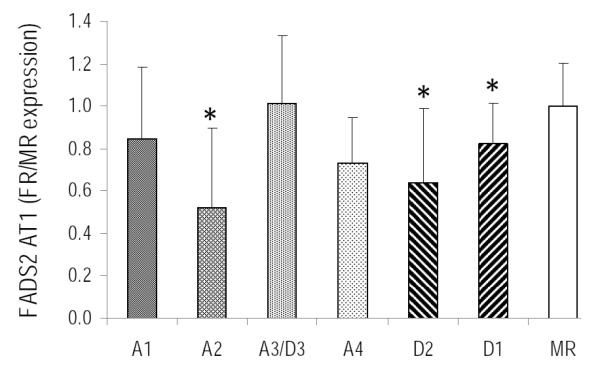
FADS2 AT1 liver relative expression, mean±SD. * indicates significantly different from control MR group (p<0.05)
Figure 4.
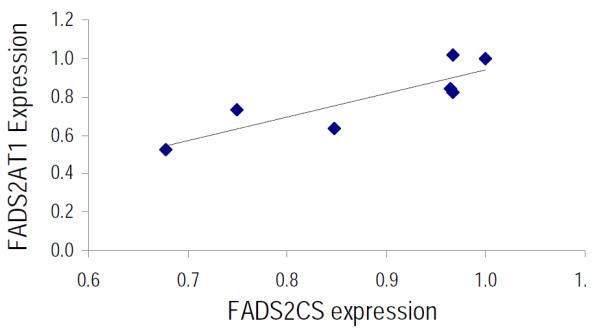
Correlation of liver FADS2 CS and FADS 2 AT1 relative expression paired by diet. The regression coefficient is significant (y = 1.24x - 0.30; r2 = 0.75; p < 0.05)
In piglets fed varying ratios of ARA:DHA, Fads3 AT1, AT4, AT6 and AT7 were consistently expressed in liver (Fig 5a-d). Liver Fads3 AT7 was sensitive to dietary ARA and DHA intake (Fig 5d). Fads3 AT7 expression in liver was significantly up-regulated in piglets fed diets A1, A2 and D1 (47%, 37% and 41% higher in piglets fed diets A1, A2 and D1, respectively), compared to MR control group. Fads3 AT1, AT4 and AT6 expression were not altered in any of the treatment groups compared to MR group (Fig 5a-c). Fads3 ATS was not detected in piglet liver for any of the dietary groups, while Fads3 AT2 and AT3 expression were detected but low across all treatments and PCR amplification was inconsistent.
Figure 5.
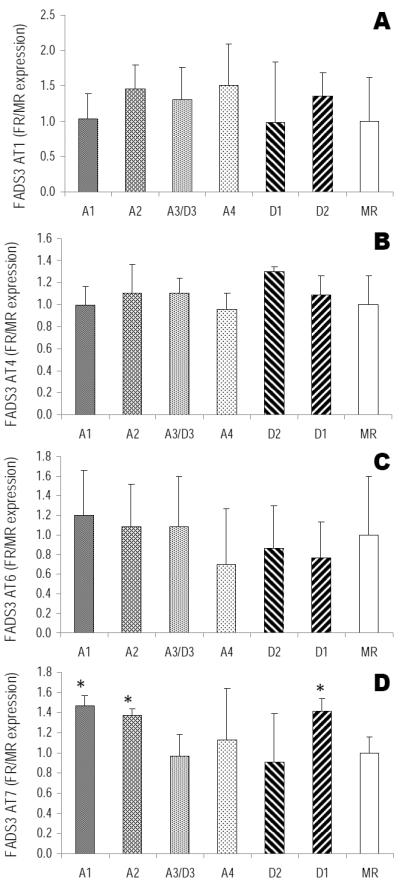
Relative expression of liver FADS3 AT in piglets fed six different formula diets (FR) and control (MR) (n=4/treatment). A) FADS3 AT1 relative expression; B) FADS3 AT4 relative expression; C) FADS3 AT6 relative expression; D) FADS3 AT7 relative expression. mean±SD. *significantly different from control MR group (p<0.05).
Discussion
Fads2 encodes for the Δ6-desaturase that catalyzes the rate limiting step in the synthesis of n-6 and n-3 LC-PUFA from 18:2n-6 and 18:3n-3, respectively [4, 5, 14]. We also recently showed that Fads2 gene product catalyzes Δ8-desaturation of 20 carbon LC-PUFA [23], The liver was the focus here because it is a predominant site of biosynthesis and supply of LC-PUFA to other tissues [24, 25],
Molecular regulation of liver Fads transcripts with increasing physiological intake of ARA (0.1-1.0% wt/wt) and DHA (0.33-1.0% wt/wt) at constant DHA and ARA intake, respectively, was determined in piglets during the early post-natal period. To our knowledge, this is the first study investigating Fads gene regulation by ARA and DHA within the physiological range present in human breast milk. Our data demonstrate that Fads2 CS expression in liver was down-regulated when dietary ARA:DHA ratio approached 1:1 in piglets. Further, Fads2 AT1 and Fads3 AT7 expression in piglets were altered by dietary ARA and DHA intake levels, providing evidence that alternative transcripts of Fads2 and Fads3 may be involved in the regulation of LC-PUFA metabolism during early postnatal period.
In piglets, liver Fads1 CS expression was not altered by dietary LC-PUFA intake with increasing doses of ARA or DHA. High inter-individual variability for Fads1 CS expression in piglets suggests that genetic factors may play a pre-dominant role. In contrast, liver Fads2 CS in piglets was sensitive to dietary ARA and DHA levels and was significantly down-regulated in the two diets that provided ARA:DHA at a ratio of 1:1 (1.1 %/1.0% and 0.67%/0.62% TFA ARA/DHA in diets A4 and D2, respectively). Further, the MR diet with minimal DHA had the highest Fads2 expression compared to all formula diets which had varying ARA and DHA levels. Liver ARA and DHA accretion of piglets fed these diets increased with higher dietary intake of ARA and DHA, respectively, and was not influenced by a 1:1 ratio of dietary ARA:DHA. However, Fads2 was significantly down-regulated in the two dietary groups that had ARA/DHA ratio of 1:1 and was not decreased in the diet that provided 0.69% ARA and 1.1% DHA (A3/D3), even though ARA and DHA levels were comparable or higher than the diet D2 (with 0.67%ARA and 0.62% DHA). Thus, this study indicates that the ratio of dietary ARA:DHA is a significant factor in the regulation of Fads2 expression and LC-PUFA metabolism during early post-natal period. It is interesting to note that global breast milk ARA and DHA means of 0.47/0.32 yielding a ratio of 1.48 has a wide range, down to at least 0.5, and has many with values above and below 1.0 [18], though DHA content of human milk varies widely dependent on maternal intake. Thus, present study suggests that dietary ARA:DHA ratio near that in human milk may be important for support of optimal LC-PUFA status in neonates, in addition to actual mass of these LC-PUFA in the diet. Whether Fads2 regulation would be altered at lower intake of ARA and DHA but with a ratio of 1:1 (e.g., at 0.3% ARA and 0.3% DHA with a ratio of 1:1) requires further investigation.
Dietary supply of preformed LC-PUFA has been reported to down-regulate Fads2 and Fads1 [4, 13]. However, a recent study in piglets fed supra-physiological levels of ARA (0-5.0% ARA wt/wt) [16] did not find any effect of dietary ARA, even at 5% intake, on liver Fads2 or Fads1 expression or on the flux of n-6 PUFA in liver. In fact, intestinal Fads2 expression and the flux of precursor EFA to LC-PUFA synthesis increased linearly with increasing intake of dietary ARA. However, the DHA levels of all ARA enriched diets used in this study was low (0.2-0.3% wt/wt) and the high EPA diet (with 5% EPA and 1.2% DHA) had lower ARA (0.4%) content [16] than the A4 and D2 diets in the present study. Thus, our data and the study by Jacobi et al [16] indicates that FADS2 expression during early post-natal period is uniquely regulated by dietary ARA and DHA and warrants further investigation.
A secondary aim of this study was to investigate Fads AT expression and regulation by dietary ARA and DHA. Our data demonstrate that Fads3 AT regulation by LC-PUFA is specific and different from Fads2 and its splice variant Fads2 AT1, despite a high degree of Fads3 sequence similarity with Fads2 [5], In piglets fed varying amounts of ARA and DHA, liver Fads2 AT1 expression was correlated to Fads2 CS, as would be expected for spliced variants arising from the same gene, and was down-regulated in diets providing ARA and DHA compared to the MR diet. In contrast, Fads3 AT7was up-regulated in diets with lower ARA content (0.1% and 0.53% in A1 and A2) or 0.33% DHA (diet D1 with the lowest DHA among the FR diets), compared to MR. This suggests that Fads3 AT7 may be uniquely sensitive to dietary ARA and DHA levels in piglets and may have a functional role when dietary intake of LC-PUFA is at the lower physiological range of LC-PUFA present in human milk.
In a recent study [26], we demonstrated that Fads1 AT1 enhanced desaturation of Fads2 CS and increased synthesis of eicosanoid precursors, the first reported function for a FADS AT. We have also previously reported up-regulation of Fads3 AT1, AT3, AT4 and AT7 at 0.71% ARA and 1.13% DHA intake (similar to diet A3/D3 in this study) in baboon neonates compared to a control group fed formula deficient in ARA and DHA [8]. In this study with neonatal piglets, we show that only Fads3 AT7 was up-regulated at lower ARA and DHA intake levels. Taken together, these data suggest that Fads3 AT regulation is species specific and the splice variants may have distinct physiological functions depending on dietary levels of ARA and DHA.
In conclusion, present study provides crucial information on molecular regulation of Fads2 by dietary LC-PUFA in piglets fed varying levels of ARA and DHA during the early post-natal period. An ARA:DHA ratio similar to human breast milk was a significant determinant of liver Fads2 expression in suckling piglets. Further, the current study provides evidence that some splice variants of liver Fads3 are uniquely regulated by dietary ARA and DHA in piglets. This observation, along with other studies demonstrating specific regulatory patterns for Fads3 and its alternative transcripts [6, 8, 9], suggest that Fads3 gene products may have important functions during early development.
Acknowledgements
The authors are grateful for the technical assistance of Lisa Furman, Peter Lawrence and Karl Roneker (Cornell University). This work was supported by DSM Food Specialties-Nutritional Products (Delft, The Netherlands and Columbia, MD). Mead Johnson Nutrition (Evansville, IN) provided oil for the experimental diets.
This project was supported in part by Award T32DK007158 from the National Institute of Diabetes and Digestive and Kidney Diseases and by R01AT007003 of the National Center for Complementary and Alternative Medicine of the NIH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ARA
arachidonic acid
- DHA
docosahexaenoic acid
- FA
fatty acid
- FAME
FA methyl ester
- FR
formula-reared
- LCPUFA
long chain polyunsaturated fatty acid
- MR
maternal-reared
- TFA
total fatty acids
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: V. Wijendran, C. Tyburczy, Ian Downs, K. S. D. Kothapalli, W. J. Park, B. S. Blank, and J. T. Brenna report no conflicts of interest. J. P. Zimmer, C. M. Butt and N. Salem, Jr. are employed by DSM Nutritional Lipids, a company that produces essential fatty acids.
References
- 1.Carlson SE. Early determinants of development: a lipid perspective. Am J Clin Nutr. 2009;89:1523S–1529S. doi: 10.3945/ajcn.2009.27113G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Innis SM. Essential fatty acid transfer and fetal development. Placenta. 2005;26(Suppl A):S70–75. doi: 10.1016/j.placenta.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Sprecher H. Biochemistry of essential fatty acids. Prog Lipid Res. 1981;20:13–22. doi: 10.1016/0163-7827(81)90009-6. [DOI] [PubMed] [Google Scholar]
- 4.Cho HP, Nakamura MT, Clarke SD. Cloning, expression, and nutritional regulation of the mammalian Delta-6 desaturase. J Biol Chem. 1999;274:471–477. doi: 10.1074/jbc.274.1.471. [DOI] [PubMed] [Google Scholar]
- 5.Marquardt A, Stohr H, White K, Weber BH. cDNA cloning, genomic structure, and chromosomal localization of three members of the human fatty acid desaturase family. Genomics. 2000;66:175–183. doi: 10.1006/geno.2000.6196. [DOI] [PubMed] [Google Scholar]
- 6.Park WJ, Kothapalli KS, Reardon HT, Kim LY, Brenna JT. Novel fatty acid desaturase 3 (FADS3) transcripts generated by alternative splicing. Gene. 2009;446:28–34. doi: 10.1016/j.gene.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedrono F, Blanchard H, Kloareg M, D'Andrea S, Daval S, Rioux V, Legrand P. The fatty acid desaturase 3 gene encodes for different FADS3 protein isoforms in mammalian tissues. J Lipid Res. 2010;51:472–479. doi: 10.1194/jlr.M000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reardon HT, Hsieh AT, Jung Park W, Kothapalli KS, Anthony JC, Nathanielsz PW, Brenna JT. Dietary long-chain polyunsaturated fatty acids upregulate expression of FADS3 transcripts. Prostaglandins Leukot Essent Fatty Acids. 2013;88:15–19. doi: 10.1016/j.plefa.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenna JT, Kothapalli KS, Park WJ. Alternative transcripts of fatty acid desaturase (FADS) genes. Prostaglandins Leukot Essent Fatty Acids. 2010;82:281–285. doi: 10.1016/j.plefa.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma XH, Hu SJ, Ni H, Zhao YC, Tian Z, Liu JL, Ren G, Liang XH, Yu H, Wan P, Yang ZM. Serial analysis of gene expression in mouse uterus at the implantation site. J. Biol. Chem. 2006;281:9351–9360. doi: 10.1074/jbc.M511512200. [DOI] [PubMed] [Google Scholar]
- 11.Plaisier CL, Horvath S, Huertas-Vazquez A, Cruz-Bautista I, Herrera MF, Tusie-Luna T, Aguilar-Salinas C, Pajukanta P. A systems genetics approach implicates USF1, FADS3, and other causal candidate genes for familial combined hyperlipidemia. PLoS Genet. 2009;5:e1000642. doi: 10.1371/journal.pgen.1000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kathiresan S, Wilier CJ, Peloso GM, Demissie S, Musunuru K, Schadt EE, Kaplan L, Bennett D, Li Y, Tanaka T, Voight BF, Bonnycastle LL, Jackson AU, Crawford G, Surti A, Guiducci C, Burtt NP, Parish S, Clarke R, Zelenika D, Kubalanza KA, Morken MA, Scott LJ, Stringham HM, Galan P, Swift AJ, Kuusisto J, Bergman RN, Sundvall J, Laakso M, Ferrucci L, Scheet P, Sanna S, Uda M, Yang Q, Lunetta KL, Dupuis J, de Bakker PI, O'Donnell CJ, Chambers JC, Kooner JS, Hercberg S, Meneton P, Lakatta EG, Scuteri A, Schlessinger D, Tuomilehto J, Collins FS, Groop L, Altshuler D, Collins R, Lathrop GM, Melander O, Salomaa V, Peltonen L, Orho-Melander M, Ordovas JM, Boehnke M, Abecasis GR, Mohlke KL, Cupples LA. Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet. 2009;41:56–65. doi: 10.1038/ng.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho HP, Nakamura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J Biol Chem. 1999;274:37335–37339. doi: 10.1074/jbc.274.52.37335. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Ann. Rev. Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 15.Melin T, Nilsson A. Delta-6-desaturase and delta-5-desaturase in human Hep G2 cells are both fatty acid interconversion rate limiting and are upregulated under essential fatty acid deficient conditions. Prostaglandins Leukot Essent Fatty Acids. 1997;56:437–442. doi: 10.1016/s0952-3278(97)90596-2. [DOI] [PubMed] [Google Scholar]
- 16.Jacobi SK, Lin X, Corl BA, Hess HA, Harrell RJ, Odle J. Dietary arachidonate differentially alters desaturase-elongase pathway flux and gene expression in liver and intestine of suckling pigs. J Nutr. 2011;141:548–553. doi: 10.3945/jn.110.127118. [DOI] [PubMed] [Google Scholar]
- 17.Innis SM. Essential fatty acids in growth and development. Prog Lipid Res. 1991;30:39–103. doi: 10.1016/0163-7827(91)90006-q. [DOI] [PubMed] [Google Scholar]
- 18.Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85:1457–1464. doi: 10.1093/ajcn/85.6.1457. [DOI] [PubMed] [Google Scholar]
- 19.FAO/WHO, Fats and Oils in Human Nutrition . Report of a Joint Expert Consultation. FAO Food and Nutrition in, FAO/WHO; Rome: 1994. [PubMed] [Google Scholar]
- 20.Innis SM. The colostrum-deprived piglet as a model for study of infant lipid nutrition. J Nutr. 1993;123:386–390. doi: 10.1093/jn/123.suppl_2.386. [DOI] [PubMed] [Google Scholar]
- 21.Tyburczy C, Kothapalli KS, Park WJ, Blank BS, Bradford KL, Zimmer JP, Butt CM, Salem N, Jr., Brenna JT. Heart arachidonic acid is uniquely sensitive to dietary arachidonic acid and docosahexaenoic acid content in domestic piglets. Prostaglandins Leukot Essent Fatty Acids. 2011 doi: 10.1016/j.plefa.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyburczy C, Kothapalli KS, Park WJ, Blank BS, Liu YC, Nauroth JM, Zimmer JP, Salem N, Jr., Brenna JT. Growth, clinical chemistry and immune function in domestic piglets fed varying ratios of arachidonic acid and DHA. Br J Nutr. 2012;107:809–816. doi: 10.1017/S000711451100359X. [DOI] [PubMed] [Google Scholar]
- 23.Park WJ, Kothapalli KS, Lawrence P, Tyburczy C, Brenna JT. An alternate pathway to long-chain polyunsaturates: the FADS2 gene product Delta8-desaturates 20:2n-6 and 20:3n-3. J Lipid Res. 2009;50:1195–1202. doi: 10.1194/jlr.M800630-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott BL, Bazan NG. Membrane docosahexaenoate is supplied to the developing brain and retina by the liver. Proc Natl Acad Sci U S A. 1989;86:2903–2907. doi: 10.1073/pnas.86.8.2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bourre JM, Piciotti M, Dumont O. Delta 6 desaturase in brain and liver during development and aging. Lipids. 1990;25:354–356. doi: 10.1007/BF02544347. [DOI] [PubMed] [Google Scholar]
- 26.Park WJ, Kothapalli KS, Reardon HT, Lawrence P, Qian SB, Brenna JT. A novel FADS1 isoform potentiates FADS2-mediated production of eicosanoid precursor fatty acids. J Lipid Res. 2012;53:1502–1512. doi: 10.1194/jlr.M025312. [DOI] [PMC free article] [PubMed] [Google Scholar]


